Abstract.
Pseudohypoaldosteronism type II (PHA II) is a renal tubular disease that causes hyperkalemia, hypertension, and metabolic acidosis. Mutations in four genes (WNK4, WNK1, KLHL3, and CUL3) are known to cause PHA II. We report a patient with PHA II carrying a KLHL3 mutation, who also had congenital hypopituitarism. The patient, a 3-yr-old boy, experienced loss of consciousness at age 10 mo. He exhibited growth failure, hypertension, hyperkalemia, and metabolic acidosis. We diagnosed him as having PHA II because he had low plasma renin activity with normal plasma aldosterone level and a low transtubular potassium gradient. Further investigations revealed defective secretion of GH and gonadotropins and anterior pituitary gland hypoplasia. Genetic analyses revealed a previously known heterozygous KLHL3 mutation (p.Leu387Pro), but no mutation was detected in 27 genes associated with congenital hypopituitarism. He was treated with sodium restriction and recombinant human GH, which normalized growth velocity. This is the first report of a molecularly confirmed patient with PHA II complicated by congenital hypopituitarism. We speculate that both GH deficiency and metabolic acidosis contributed to growth failure. Endocrinological investigations will help to individualize the treatment of patients with PHA II presenting with growth failure.
Keywords: pseudohypoaldosteronism type II, congenital hypopituitarism, KLHL3, growth failure
Introduction
Pseudohypoaldosteronism (PHA) is a rare renal tubular disease that can be divided into two types based on their pathogenesis. PHA type I (PHA I) is caused by abnormalities in the aldosterone receptor or epithelial sodium channel and is inherited in an autosomal dominant or recessive manner, respectively. PHA I is clinically characterized by renal salt wasting and decreased response of the aldosterone receptor or epithelial sodium channel to aldosterone. PHA type II (PHA II, also referred to as Gordon syndrome(1)) is a heterogeneous syndrome inherited in an autosomal dominant or recessive manner. The best-characterized pathogenesis of PHA II is associated with increased membrane expression of the thiazide-sensitive NaCl cotransporter (NCC) in the distal convoluted tubules, resulting in excessive sodium resorption. Excretion of potassium and hydrogen is decreased because it is coupled with sodium resorption. As a result, patients with PHA II show early-onset hypertension, hyperkalemia, and metabolic acidosis. Plasma renin levels are suppressed and aldosterone levels are variable but are relatively low given the degree of hyperkalemia. Other clinical manifestations of PHA II include growth failure, muscle weakness, periodic paralysis, skeletal abnormalities, urinary calculus, and psychomotor retardation. Electrolyte abnormalities and hypertension can be treated with thiazide.
Recently, the molecular pathogenesis of PHA II was partly elucidated. In 2001, Wilson et al. conducted positional cloning analyses of PHA II kindreds and identified mutations in the WNK family kinase genes (WNK1 and WNK4). The two kinases are involved in the plasma membrane expression of NCC (2). In 2012, Boyden et al. performed whole exome sequencing in patients with PHA II, identifying two additional causative genes (KLHL3 and CUL3) (3). The KLHL3-CUL3 complex acts as a negative regulator of WNK kinases through ubiquitination of the two kinases. Thus, mutations in KLHL3 or CUL3 are thought to increase the membrane expression of NCC via increased WNK kinases (4,5,6,7). Among the 52 PHA II kindreds, 24 showed KLHL3 mutations (eight were autosomal recessive inheritance, 16 were autosomal dominant inheritance), 17 showed CUL3 mutations, five showed WNK4 mutations, and two showed WNK1 mutations (3).
Here, we report a patient with PHA II complicated by congenital hypopituitarism carrying a KLHL3 mutation. The patient exhibited growth failure. Two possible factors, PHA II and congenital hypopituitarism, were considered as the cause(s) of the growth failure.
Case Report
The proband, a 3-yr-old boy, was the second child of healthy nonconsanguineous Japanese parents. The course of pregnancy and delivery were uneventful. He was born at gestational age 39 wk with a birth length of 51 cm (+1.0 SD) and weight of 2918 g (–0.2 SD). At age 10 mo, he experienced loss of consciousness after a 15-h fasting period and was brought to our hospital. On physical examination, short stature (68.0 cm; –2.0 SD) and normal weight (8.6 kg; –0.6 SD) were noted. He was drowsy but moved his extremities in response to verbal or physical stimulation. He had elevated blood pressure (110/55 mmHg; age-matched reference ranges 80–105/34–61) with a normal heart rate. His testes were in the scrotum and his testicular volume was 2 cm3 per testis. His stretched penile length was 3.0 cm (–0.8 SD). He had no abnormalities in deciduous teeth. Routine blood examination revealed a high serum potassium level (6.6 mM), high serum chloride level (107 mM), normal plasma glucose level (77 mg/dL), and an elevated beta-hydroxybutyrate level (1.6 mM; reference ranges 0–0.074). Mild metabolic acidosis was observed by vein blood gas analysis (pH 7.34, estimated bicarbonate level 14.8 mM). Bone age was not evaluated. He was treated with intravenous half saline including glucose, and his consciousness was normal 3 h after fluid therapy. Although hyperkalemia and hyperchloremia gradually improved, hypertension and metabolic acidosis (estimated bicarbonate levels 14.6–16.0 mM) were sustained through eight days after admission. Hyperkalemia reoccurred at home after discharge and was sustained until the introduction of sodium-intake restriction.
To clarify the etiology of metabolic acidosis, transient hyperkalemia, and hypertension, we conducted a series of renal function tests. Ultrasonography of the kidneys showed no anatomical lesions or calcification. Plasma renin activity was low (0.6 ng/mL/h; reference 1.0–3.2) with normal plasma aldosterone level (66 pg/mL; reference 66–218). Transtubular potassium gradient was low (4.5; reference >5.0), indicating that renal potassium excretion was decreased. Based on these clinical data, we diagnosed him with PHA II.
When we reviewed the growth record of the patient, we noted slow decreases in length/height and weight from age 8 mo. Serum IGF1 levels were very low (< 4 ng/mL; age-matched reference 11–149 (8)). Provocation tests for GH release revealed low GH responses to insulin, arginine, and glucagon (peak GH 0.9, 1.7, and 2.1 ng/mL, respectively). He also had a poor gonadotropin response to GnRH (peak LH 0.39 mIU/L, peak FSH 0.98 mIU/L). Responses of TSH, ACTH, and prolactin were not defective. Cranial magnetic resonance imaging revealed hypoplasia of the anterior pituitary gland. We diagnosed him with congenital hypopituitarism.
He was treated with sodium restriction (sodium intake 600 mg/day) and recombinant human GH replacement from age 14 mo. His height SD score increased gradually (93.9 cm; –0.5 SD at age 40 mo) (Fig. 1), which coincided with elevation in serum IGF1 levels. Maximum growth velocity was achieved after age 20 mo when metabolic acidosis was alleviated (Fig. 2). Hypertension was improved by age 21 mo. Metabolic acidosis was improved by age 30 mo (Fig. 2), although mild hyperkalemia (5.1–6.0 mM) was sustained. We did not use thiazide throughout the course because metabolic acidosis and hyperkalemia were mild. Loss of consciousness did not reoccur after the first admission. Psychomotor development has remained intact.
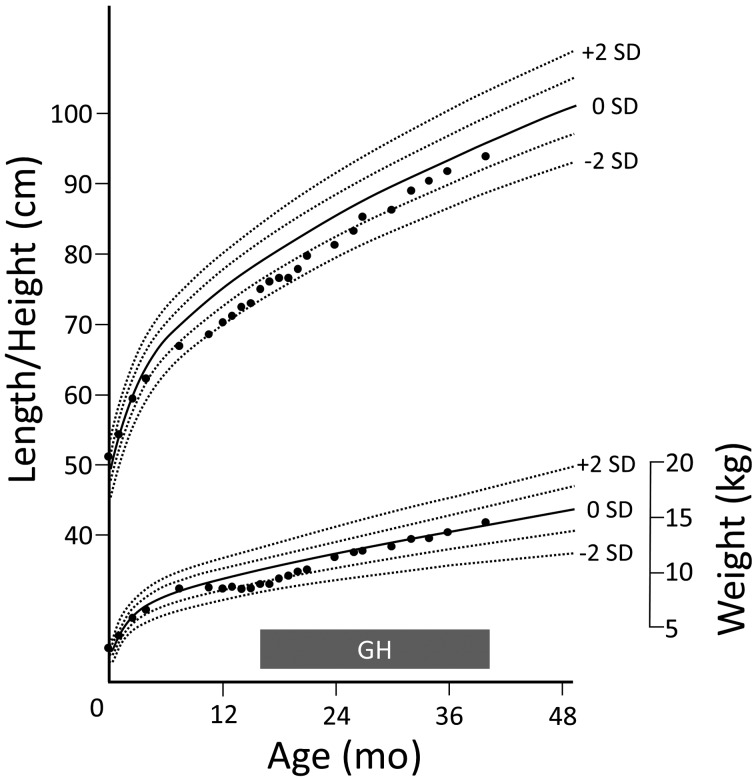
Fig. 1.
Growth chart of the patient. Prior to admission because of an episode of loss of consciousness (age 10 mo), the patient showed growth failure beginning at age 8 mo. After the initiation of treatment by GH replacement and sodium restriction at age 14 mo, he showed catch-up growth.
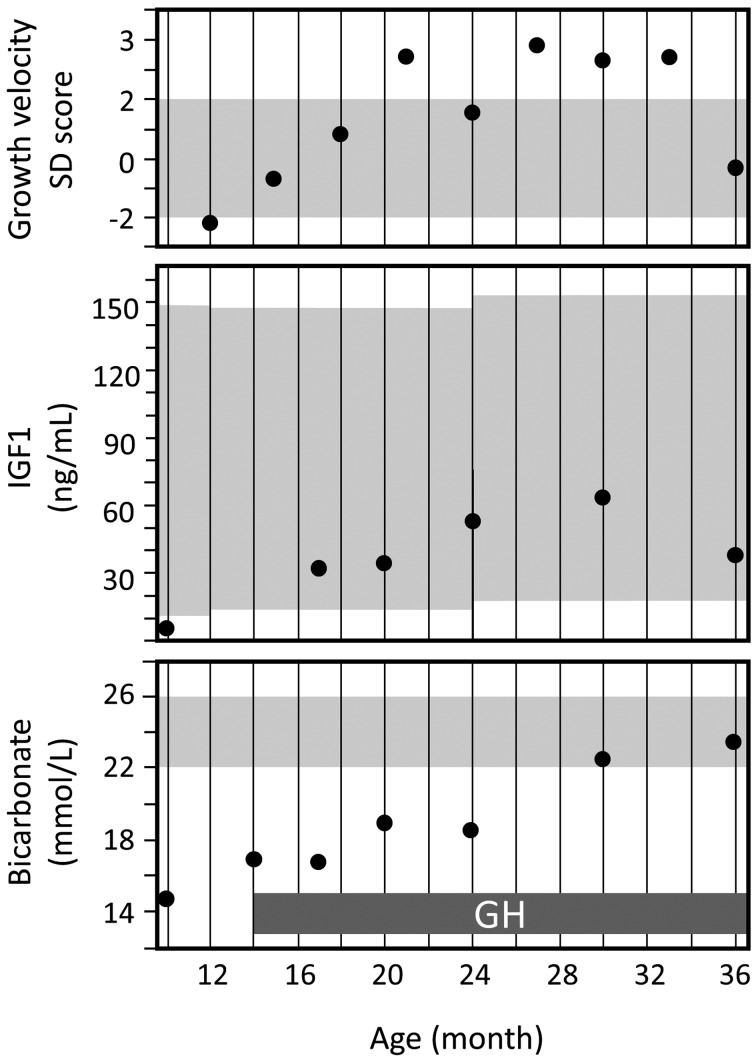
Fig. 2.
Chronological changes in growth velocity, IGF1 levels, and bicarbonate levels. Shaded areas indicate age-matched reference ranges. The recovery of growth velocity coincided with the increase in serum IGF1 levels immediately after beginning GH replacement. Metabolic acidosis was alleviated from age 20 mo. The bicarbonate level became normal by age 30 mo.
Materials and Methods
Genetic analyses
The genetic study was approved by the ethics committees of Keio University School of Medicine and Tokyo Medical and Dental University. Written informed consent for genetic analyses was obtained from the parents of the proband. Genomic DNA samples were extracted from the peripheral blood of the proband, his mother, and elder brother using standard techniques. Four causative genes for PHA II (WNK1, WNK4, KLHL3, and CUL3) were analyzed by PCR-based direct sequencing. Twenty-seven genes associated with hypopituitarism (GH1, GHRH, GHRHR, GHSR, GLI2, HESX1, LHX4, OTX2, PAXS, POU1F1, PROP1, SIX3, TBX19, CHD7, FGFR1, FSH8, TAC3, TACR3, GNRH1, GNRHR, KAL1, KISS1, KISS1R, LHB, PROK2, PROKR2, SOX2) were analyzed by next-generation targeted sequencing as previously described (9).
RNA expression analysis of KLHL3
Human pituitary gland cDNA (Human Pituitary Gland QUICK-Clone cDNA) was purchased from TaKaRa Biotechnology (Shiga, Japan). Human thymus RNA was purchased from Agilent Technologies (Santa Clara, CA, USA), and was reverse-transcribed into cDNA using the SuperScript III First-Strand Synthesis System (Life Technologies, Carlsbad, CA, USA) and an oligo-dT primer. Primer pairs each specific for KLHL3 cDNA (forward 5′-AGATGTACACACCTGCACTGACCTT-3′, reverse 5′-GACATGTTCCATCAGCTTTGC-CATG-3′) and GAPDH cDNA (TaKaRa, HA067812) were used in PCR to estimate the RNA expression levels of the two genes in the pituitary gland and thymus. Thirty-five cycles of PCR amplification were performed using ExTaq HS (TaKaRa) according to the manufacturer’s instructions. The PCR products were electrophoresed in a 1.2% agarose gel containing ethidium bromide. We detected the band using a UV transformer illuminator.
Results
Genetic analyses
Clinical diagnosis of PHA II and congenital hypopituitarism prompted us to perform genetic analyses to clarify the genetic bases of the two diseases. PCR-based direct sequencing of PHA II-associated genes revealed a heterozygous KLHL3 mutation (p.Leu387Pro), which has previously been reported in patients with PHA II (3). Family analyses unexpectedly demonstrated that the mother and elder brother carried identical mutations. Blood examination of the two family member showed that both had hyperkalemia (5.9 mM in both). Metabolic acidosis was observed only in the brother (pH 7.31, estimated bicarbonate level 21.2 mM). The mother had leg length discrepancy and persistence of deciduous teeth. The mother and brother had neither hypertension nor short stature (mother: blood pressure 110/54 mmHg and height 163 cm at age 34 yr, brother: blood pressure 79/27 mmHg and height 97.8 cm; +0.9 SD at age 3 yr). We further analyzed 27 genes associated with the hypopituitarism for the proband, but no disease-causing variation was found.
RNA expression analysis of KLHL3
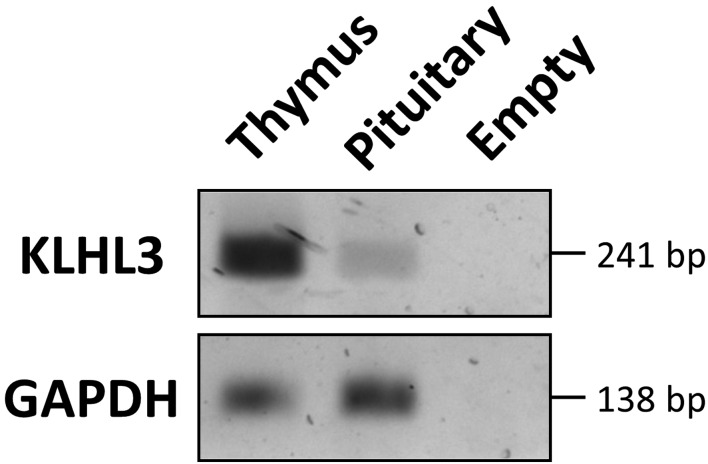
RT-PCR analysis showed that KLHL3 mRNA was expressed in the pituitary gland, although the expression level was low (Fig. 3).
Fig. 3.
RT-PCR analysis of KLHL3. KLHL3 mRNA is expressed in the human pituitary gland, although the level of expression was low. A thymus-derived sample was used as a control.
Discussion
We described a patient with PHA II carrying KLHL3 mutation, who showed growth failure at first presentation. Endocrinological investigations revealed congenital hypopituitarism affecting the secretion of GH and gonadotropins. Both GH deficiency and metabolic acidosis was suspected to be responsible for the growth failure. However, the recovery of growth velocity coincided with the increase in serum IGF1 levels, indicating that his growth was at least in part affected by GH deficiency.
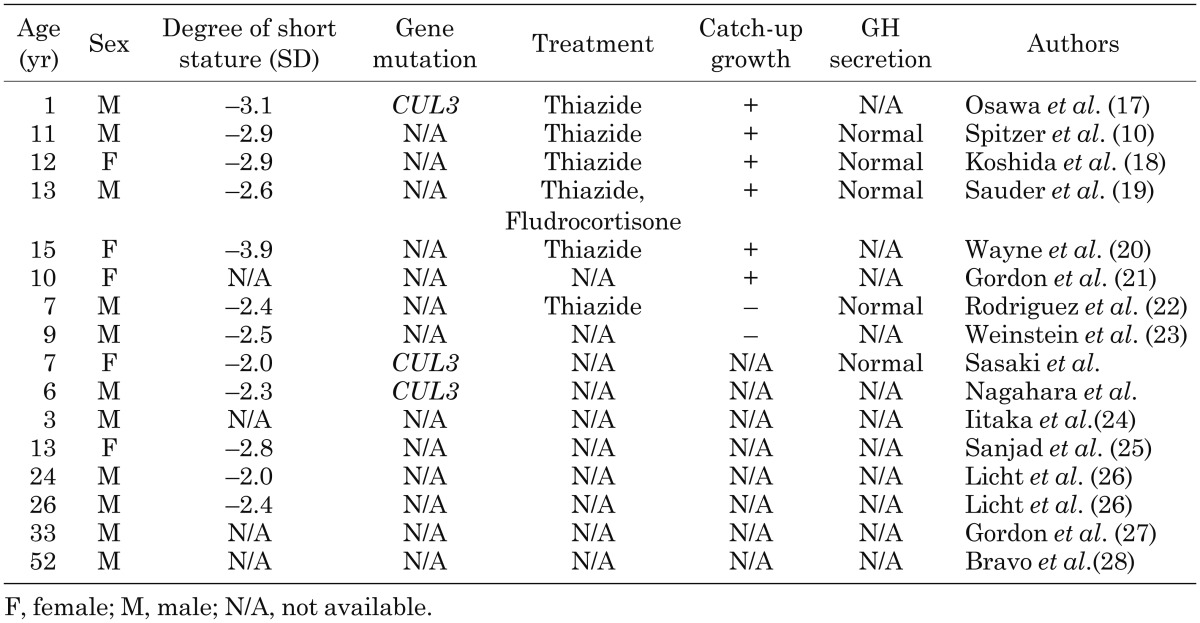
Growth failure is a PHA II-associated symptom, but the frequency has not been investigated in a structured manner. We reviewed 42 patients with PHA II whose growth records were described in the literature and found that 16 (38%) had short stature (Table 1). Six of 16 patients experienced catch-up growth after treatment with thiazide.
Table 1. Sixteen patients with pseudohypoaldosteronism type II who had short stature.
The cause(s) of growth failure in PHA II are not fully understood, although metabolic acidosis is the most plausible explanation (10). Boyden et al. reported that patients with PHA II with CUL3 mutations generally showed more severe acidosis and hyperkalemia and a greater likelihood of growth failure than patients with mutations in WNK1, WNK4, and KLHL3 (3). McSherry et al. reported that growth failure associated with renal tubular acidosis in children was reversible upon alkali treatment (11). The mechanisms of acidosis causing growth failure have been elucidated in some reports. Brungger et al. reported that the IGF1 response to GH administration was significantly blunted during acidosis, while the GH response to GH releasing factor administration was significantly enhanced (12). Mitch et al. reported that metabolic acidosis stimulated muscle protein degradation (13). A similar mechanism may explain the growth failure observed in patients with PHA II. In the present case, the recovery of growth velocity coincided with the increase in serum IGF1 levels, which began immediately after beginning GH replacement, suggesting that his growth was affected by GH deficiency. However, maximum growth velocity was achieved after age 20 months when metabolic acidosis was alleviated. This indicates that correcting the acidosis improved the actions of anabolic hormones and further increased his growth velocity.
The mother and brother of the proband did not have short stature, although they carried the identical KLHL3 mutation (p.Leu387Pro). It is clear that the complication of congenital hypopituitarism of the proband caused the differences in height among the family members. Regarding the PHA II phenotypes (e.g. hypertension, metabolic acidosis, and hyperkalemia), it is unclear which factor(s) were responsible for the differences. Farfel et al. observed no significant difference in severity of low IGF1, hyperkalemia, metabolic acidosis, and hypercalciuria in affected family members with short stature and with normal stature (14). Gordon et al. found that the difference in sodium intake appeared to cause the phenotypic differences, particularly hypertension (15).
In the present case, the patient lost consciousness on first admission. This may have been because of hypoglycemia, which was followed by a 15-h fasting period, although plasma glucose level was normal upon admission. Considering that severe hypoglycemia stimulates the secretion of counterregulatory hormones, normal plasma glucose levels at admission do not exclude the possibility of hypoglycemia as the cause of loss of consciousness.
This is the first case report of the coexistence of PHA II and congenital hypopituitarism. In the original report of the identification of the human KLHL gene, the highest KLHL3 mRNA expression was observed in the pituitary gland and cerebellum (16). Using RT-PCR, we confirmed the expression of KLHL3 mRNA in the pituitary gland. The function of KLHL3 in the pituitary gland is unknown. Considering that KLHL2 is an actin-binding protein affecting cytoskeleton dynamics in neurons, it is possible that KLHL3 also affects cell motility where it is expressed (16). However, KLHL3 mRNA expression was very low in the pituitary gland, indicating that KLHL3 mutations did not cause hypopituitarism. Additionally, no other KLHL3 mutation carriers reported so far (including the mother and brother of the proband in this report) had hypopituitarism. Thus, we speculate that the KLHL3 mutation and congenital hypopituitarism were independent occurrences. The presence of additional factor(s), such as genetic, epigenetic, and environmental factors, may be required to cause aberrant development of the anterior pituitary lobe.
In conclusion, we described a patient with PHA II carrying KLHL3 mutation, who showed growth failure and congenital hypopituitarism. Our case exemplifies the importance of endocrinological investigations in patients with PHA II with growth failure.
Acknowledgments
We thank Dr. Midori Awazu for clinical advice and all colleagues of Saitama City Hospital, Kawasaki Municipal Hospital and Shizuoka City Shimizu Hospital for helpful discussions. This study was supported by a Health and Labour Sciences Research Grant for Research on Applying Health Technology (Jitsuyoka (Nanbyo) – Ippan-014) and a grant from the Foundation for Growth Science, Japan.
References
- 1.Gordon RD. Syndrome of hypertension and hyperkalemia with normal glomerular filtration rate. Hypertension 1986;8: 93–102. doi: 10.1161/01.HYP.8.2.93 [DOI] [PubMed] [Google Scholar]
- 2.Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, et al. Human hypertension caused by mutations in WNK kinases. Science 2001;293: 1107–12. doi: 10.1126/science.1062844 [DOI] [PubMed] [Google Scholar]
- 3.Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 2012;482: 98–102. doi: 10.1038/nature10814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahle KT, Wilson FH, Leng Q, Lalioti MD, O’Connell AD, Dong K, et al. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 2003;35: 372–6. doi: 10.1038/ng1271 [DOI] [PubMed] [Google Scholar]
- 5.Mori Y, Wakabayashi M, Mori T, Araki Y, Sohara E, Rai T, et al. Decrease of WNK4 ubiquitination by disease-causing mutations of KLHL3 through different molecular mechanisms. Biochem Biophys Res Commun 2013;439: 30–4. doi: 10.1016/j.bbrc.2013.08.035 [DOI] [PubMed] [Google Scholar]
- 6.Wakabayashi M, Mori T, Isobe K, Sohara E, Susa K, Araki Y, et al. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Reports 2013;3: 858–68. doi: 10.1016/j.celrep.2013.02.024 [DOI] [PubMed] [Google Scholar]
- 7.Uchida S, Sohara E, Rai T, Sasaki S. Regulation of with-no-lysine kinase signaling by Kelch-like proteins. Biol Cell 2014;106: 45–56. doi: 10.1111/boc.201300069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isojima T, Shimatsu A, Yokoya S, Chihara K, Tanaka T, Hizuka N, et al. Standardized centile curves and reference intervals of serum insulin-like growth factor-I (IGF-I) levels in a normal Japanese population using the LMS method. Endocr J 2012;59: 771–80. doi: 10.1507/endocrj.EJ12-0110 [DOI] [PubMed] [Google Scholar]
- 9.Asakura Y, Muroya K, Hanakawa J, Sato T, Aida N, Narumi S, et al. Combined pituitary hormone deficiency with unique pituitary dysplasia and morning glory syndrome related to a heterozygous PROKR2 mutation. Clin Pediatr Endocrinol 2015;24: 27–32. doi: 10.1297/cpe.24.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spitzer A, Edelmann CM, Jr, Goldberg LD, Henneman PH. Short stature, hyperkalemia and acidosis: A defect in renal transport of potassium. Kidney Int 1973;3: 251–7. doi: 10.1038/ki.1973.38 [DOI] [PubMed] [Google Scholar]
- 11.McSherry E, Morris RC, Jr. J Clin Invest 1978;61: 509–27. doi: 10.1172/JCI108962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brüngger M, Hulter HN, Krapf R. Effect of chronic metabolic acidosis on the growth hormone/IGF-1 endocrine axis: new cause of growth hormone insensitivity in humans. Kidney Int 1997;51: 216–21. doi: 10.1038/ki.1997.26 [DOI] [PubMed] [Google Scholar]
- 13.Mitch WE, Medina R, Grieber S, May RC, England BK, Price SR, et al. Metabolic acidosis stimulates muscle protein degradation by activating the adenosine triphosphate-dependent pathway involving ubiquitin and proteasomes. J Clin Invest 1994;93: 2127–33. doi: 10.1172/JCI117208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farfel A, Mayan H, Melnikov S, Holtzman EJ, Pinhas-Hamiel O, Farfel Z. Effect of age and affection status on blood pressure, serum potassium and stature in familial hyperkalaemia and hypertension. Nephrol Dial Transplant 2011;26: 1547–53. doi: 10.1093/ndt/gfq612 [DOI] [PubMed] [Google Scholar]
- 15.Gordon RD, Ravenscroft PJ, Klemm SA, Tunny TJ, Hamlet SM. A new Australian kindred with the syndrome of hypertension and hyperkalaemia has dysregulation of atrial natriuretic factor. J Hypertens Suppl 1988;6: S323–6. doi: 10.1097/00004872-198812040-00100 [DOI] [PubMed] [Google Scholar]
- 16.Lai F, Orelli BJ, Till BG, Godley LA, Fernald AA, Pamintuan L, et al. Molecular characterization of KLHL3, a human homologue of the Drosophila kelch gene. Genomics 2000;66: 65–75. doi: 10.1006/geno.2000.6181 [DOI] [PubMed] [Google Scholar]
- 17.Osawa M, Ogura Y, Isobe K, Uchida S, Nonoyama S, Kawaguchi H. CUL3 gene analysis enables early intervention for pediatric pseudohypoaldosteronism type II in infancy. Pediatr Nephrol 2013;28: 1881–4. doi: 10.1007/s00467-013-2496-6 [DOI] [PubMed] [Google Scholar]
- 18.Koshida S. A case of renal tublar acidosis type IV. Japanese Journal of Pediatrics 2001;54: 1473–7(in Japanese). [Google Scholar]
- 19.Sauder SE, Kelch RP, Grekin RJ, Kelsch RC. Suppression of plasma renin activity in a boy with chronic hyperkalemia. Am J Dis Child 1987;141: 922–7. [DOI] [PubMed] [Google Scholar]
- 20.Wayne VS, Stockigt JR, Jennings GL. Treatment of mineralocorticoid-resistant renal hyperkalemia with hypertension (type II pseudohypoaldosteronism). Aust N Z J Med 1986;16: 221–3. doi: 10.1111/j.1445-5994.1986.tb01154.x [DOI] [PubMed] [Google Scholar]
- 21.Gordon RD, Geddes RA, Pawsey CG, O’Halloran MW. Hypertension and severe hyperkalaemia associated with suppression of renin and aldosterone and completely reversed by dietary sodium restriction. Australas Ann Med 1970;19: 287–94. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-Soriano J, Vallo A, Domínguez MJ. “Chloride-shunt” syndrome: an overlooked cause of renal hypercalciuria. Pediatr Nephrol 1989;3: 113–21. doi: 10.1007/BF00852890 [DOI] [PubMed] [Google Scholar]
- 23.Weinstein SF, Allan DM, Mendoza SA. Hyperkalemia, acidosis, and short stature associated with a defect in renal potassium excretion. J Pediatr 1974;85: 355–8. doi: 10.1016/S0022-3476(74)80115-0 [DOI] [PubMed] [Google Scholar]
- 24.Iitaka K, Watanabe N, Asakura A, Kasai N, Sakai T. Familial hyperkalemia, metabolic acidosis and short stature with normal renin and aldosterone levels. Int J Pediatr Nephrol 1980;1: 242–5. [Google Scholar]
- 25.Sanjad SA, Mansour FM, Hernandez RH, Hill LL. Severe hypertension, hyperkalemia, and renal tubular acidosis responding to dietary sodium restriction. Pediatrics 1982;69: 317–24. [PubMed] [Google Scholar]
- 26.Licht JH, Amundson D, Hsueh WA, Lombardo JV. Familiar hyperkalaemic acidosis. Q J Med 1985;54: 161–76. [PubMed] [Google Scholar]
- 27.Gordon RD, Hodsman GP. The syndrome of hypertension and hyperkalaemia without renal failure: long term correction by thiazide diuretic. Scott Med J 1986;31: 43–4. [DOI] [PubMed] [Google Scholar]
- 28.Bravo E, Textor S, Mujais S, Cotton D. Chronic hyperkalemia in siblings associated with enhanced renal chroride absorption [Abstract] Clin Res 1980;19: 161–76. [Google Scholar]