Introduction
Neonatal diabetes mellitus (NDM), characterized by hyperglycemia and the need for insulin treatment within the first 6 mo of life, is a rare monogenic form of diabetes with an estimated incidence of 1 in 90,000 neonates (1). Approximately half of NDM cases are transient and resolve at a median age of 3 mo (transient NMD: TNDM), while the remaining cases develop into a permanent form of diabetes (permanent NDM: PNDM; MIM # 606176). Adult onset non-autoimmune diabetes occurs in a significant number of patients with TNDM (2).
Most cases of TNDM (approximately 70%) are caused by abnormalities in chromosome 6q24, including paternal duplications, paternal uniparental isodisomy, and loss of methylation. In a few patients, activating mutations in the genes, which encode the two subunits of the β-cell ATP-sensitive potassium channel, i.e. ABCC8 and KCNJ11, have been reported to be associated with TNDM. Interestingly, recent studies have shown that familial analysis of TNMD with ABCC8 mutations revealed that their family members with adult onset non-autoimmune diabetes also have the same mutations (3). Here, we present a Japanese case with TNDM harboring a novel p.Glu350Asp mutation in ABCC8. Familial analysis revealed that his non-symptomatic sister and mother, other family members with adult-onset diabetes without neonatal episodes of hyperglycemia, also possessed the same mutation.
Patient Report
The propositus (III-2) is now an 8-yr-old Japanese male who was born at 40 wk of gestation after an uncomplicated pregnancy and delivery. He was the second child of nonconsanguineous healthy parents. His mother had no history of gestational diabetes. At birth, his weight was 2.974 kg (–0.1 SD). He was referred to us at 2 mo of age because of protracted and intense vomiting followed by Kussmaul breathing (respiration rate was 50/min). Blood examination showed hyperglycemia (serum glucose level was 753 mg/dL), metabolic acidosis (pH 7.26), and ketosis (serum total ketone body level, 6982 μM, acetoacetate 1455 μM, and 3-hydroxybutyrate 5527 μM). Serum C-peptide was low (below 0.5 ng/mL, Ref: 1.0–3.5 ng/mL) and autoantibodies associated with type 1 diabetes were negative. He was diagnosed as NDM and treatment with multiple daily insulin injections (initially 1.0 U/kg/day) was started immediately. Gradually, his insulin requirement decreased, and treatment was stopped at the age of 4 mo. Three months after the discontinuation of insulin, his HbA1c level was 5.5% (NGSP). The patient currently exhibits normal growth and physical development and has not experienced any delays in achieving neurodevelopmental milestones. At his last examination at the age of 8 yr and 1 mo, his height was 121.6 cm (–0.8 SD) and weight was 23.1 kg (–0.6 SD).
Mutational Analysis
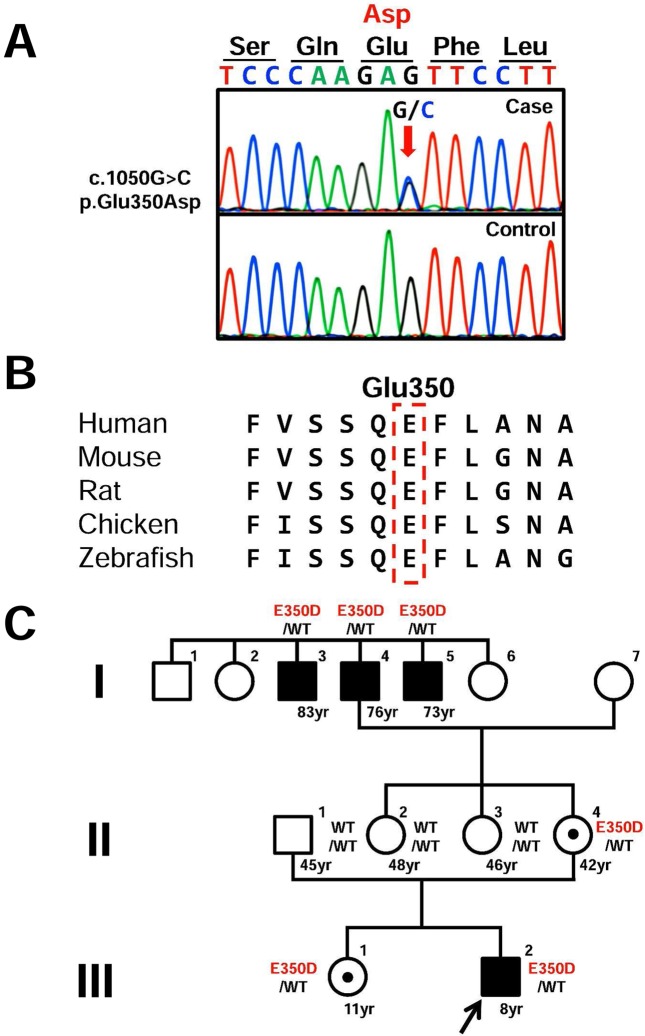
After obtaining informed consent and the approval of the Institutional Review Board of Tokyo Metropolitan Children’s Medical Center, genomic DNA was extracted from the patient’s peripheral blood leukocytes. Because ketoacidosis is not typically present in patients with 6q24 defects, we first evaluated all coding exons and flanking introns of ABCC8 and KCNJ11 by PCR-direct sequencing. We found a novel heterozygous c.1050G>C transition (p.Glu350Asp) in exon 7 of ABCC8 (Fig. 1A). This mutation was not detected in any of the 100 healthy controls or in the databases, including dbSNP, the 1,000 Genomes Project, Exome Variant Server, NHLBI Exome Sequencing Project, and Human Genetic Variation Database in Japanese. This glutamic acid is a highly evolutionarily conserved amino acid (Fig. 1B), located in the extracellular region of the transmembrane domain 1. No mutations were identified in KCNJ11.
Fig. 1.
Identification of sequence variation in ABCC8. (A) Partial sequence of PCR product. The chromatogram represents a heterozygous substitution of aspartic acid (GAC) in place of glutamic acid (GAG) at codon 350. The arrow indicates the mutated nucleotide. (B) Homology study. Glu350 is a highly evolutionarily conserved amino acid among several other species. (C) Pedigree of the patient.
Genetic analyses showed that the patient and his non-symptomatic sister and mother (III-1, II-4), his grandfather (I-4), and grandfather’s brothers with adult onset diabetes (I-3 and I-5) carried the same mutation (Fig. 1C). The patients of I-3 and I-4 received insulin treatment and the patient of I-5 received sulfonylurea treatment with good glycemic control.
Discussion
Here, we report a case of TNDM with a novel mutation in ABCC8. Although the functional consequence of this mutation has not been determined in vitro, it may cause disease as this residue is conserved across species according to several databases. To date, more than 500 mutations in ABCC8 have been reported, but the genotype-phenotype correlation remains unclear. Our finding will improve the understanding of the pathogenesis of ABCC8 mutations in monogenic diabetes.
Babenko et al. reported that heterozygous mutations in ABCC8 accounted for 12% of cases of NDM and that mutation carriers of ABCC8 may develop a monogenic form of type II diabetes with variable expression and age at onset, as was observed in our patient and his family (3). Several factors may explain this variability in the family members, such as genetic modifiers and environmental factors.
Several studies have demonstrated that sulfonylurea treatment provides effective glycemic control compared with subcutaneous insulin injection in patients with NDM harboring ATP-sensitive potassium channel mutations (4). Furthermore, transfer to sulfonylurea treatment has been successful for most patients with adult-onset diabetes or maturity-onset diabetes of the young due to ABCC8 mutation (5). Therefore, identification of ABCC8 mutations in NDM patients is useful for treatment decisions not only for the patient, but also for family members with the same mutation.
Conflict of Interest: The authors have no conflicts of interest to declare.
Acknowledgments
We thank Professor Sian Ellard (University of Exeter Medical School) for mutation screening of ABCC8 gene.
References
- 1.Grulich-Henn J, Wagner V, Thon A, Schober E, Marg W, Kapellen TM, et al. Entities and frequency of neonatal diabetes: data from the diabetes documentation and quality management system (DPV). Diabet Med 2010;27: 709–12. doi: 10.1111/j.1464-5491.2010.02965.x [DOI] [PubMed] [Google Scholar]
- 2.Polak M, Cavé H. Neonatal diabetes mellitus: a disease linked to multiple mechanisms. Orphanet J Rare Dis 2007;2: 12. doi: 10.1186/1750-1172-2-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babenko AP, Polak M, Cavé H, Busiah K, Czernichow P, Scharfmann R, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med 2006;355: 456–66. doi: 10.1056/NEJMoa055068 [DOI] [PubMed] [Google Scholar]
- 4.Aguilar-Bryan L, Bryan J. Neonatal diabetes mellitus. Endocr Rev 2008;29: 265–91. doi: 10.1210/er.2007-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman P, Flanagan SE, Edghill EL, Damhuis A, Shepherd MH, Paisey R, et al. Heterozygous ABCC8 mutations are a cause of MODY. Diabetologia 2012;55: 123–7. doi: 10.1007/s00125-011-2319-x [DOI] [PubMed] [Google Scholar]