Abstract
Aims
Liraglutide 3.0 mg, an acylated GLP‐1 analogue approved for weight management, lowers body weight through decreased energy intake. We conducted exposure‐response analyses to provide important information on individual responses to given drug doses, reflecting inter‐individual variations in drug metabolism, absorption and excretion.
Methods
We report efficacy and safety responses across a wide range of exposure levels, using data from one phase II (liraglutide doses 1.2, 1.8, 2.4 and 3.0 mg), and two phase IIIa [SCALE Obesity and Prediabetes (3.0 mg); SCALE Diabetes (1.8; 3.0 mg)] randomized, placebo‐controlled trials (n = 4372).
Results
There was a clear exposure–weight loss response. Weight loss increased with greater exposure and appeared to level off at the highest exposures associated with liraglutide 3.0 mg in most individuals, but did not fully plateau in men. In individuals with overweight/obesity and comorbid type 2 diabetes, there was a clear exposure–glycated haemoglobin (HbA1c) relationship. HbA1c reduction increased with higher plasma liraglutide concentration (plateauing at ∼21 nM); however, for individuals with baseline HbA1c >8.5%, HbA1c reduction did not fully plateau. No exposure–response relationship was identified for any safety outcome, with the exception of gastrointestinal adverse events (AEs). Individuals with gallbladder AEs, acute pancreatitis or malignant/breast/benign colorectal neoplasms did not have higher liraglutide exposure compared with the overall population.
Conclusions
These analyses support the use of liraglutide 3.0 mg for weight management in all subgroups investigated; weight loss increased with higher drug exposure, with no concomitant deterioration in safety/tolerability besides previously known gastrointestinal side effects.
Keywords: body weight, glucagon‐like peptide‐1, incretin, pharmacokinetic
Introduction
The actions of the glucagon‐like peptide‐1 (GLP‐1) hormone include both reduction of energy intake (via receptors in the central nervous system), and the regulation of glucose metabolism 1, 2, 3, 4, 5, 6, 7, 8. Liraglutide is an acylated analogue of GLP‐1 with an amino acid sequence 97% homologous to human GLP‐1 9. Like native GLP‐1, liraglutide lowers body weight through decreased caloric intake, while stimulating insulin secretion and reducing glucagon via a glucose‐dependent mechanism 10. As a result of its dual effects on appetite/weight and glucose regulation, liraglutide was developed for the treatment of type 2 diabetes (doses up to 1.8 mg, Victoza®; Novo Nordisk, Søborg, Denmark) and weight management (doses of 3.0 mg, Saxenda®, Novo Nordisk). Liraglutide 3.0 mg is currently approved for weight management in Australia, Canada, the European Union, Mexico and the USA.
In the liraglutide 3.0 mg clinical development programme weight loss and glycaemic improvement with liraglutide were dose‐dependent, with higher doses required for maximum weight reduction 11, 12. Women on average had a greater weight loss response with liraglutide 3.0 mg than did men; however, weight loss in men is clinically meaningful and meets US Government Food and Drug Administration (FDA) efficacy benchmarks for weight‐management products 13, 14. Data do not suggest a liraglutide dose–response with safety outcomes, with the exception of gastrointestinal (GI) side‐effects 11, 12, 13.
Intrinsic and extrinsic factors affecting clearance, and thus steady‐state plasma concentration (e.g. weight, gender), result in a range of drug exposures in individuals given the same dose; therefore, exposure–response analyses extend results of dose–response analyses (typically restricted to a few discrete dose levels) by allowing exploration of drug effects across a broad range of exposure levels. Analyses of exposure–response relationships are particularly useful when studying subpopulations with high or low exposure, allowing assessment of responses at the extremes of exposure. Previous population pharmacokinetic analysis of liraglutide 3.0 mg found that high body weight and male gender were associated with reduced drug exposure, whereas other covariates had limited effect (R. V. Overgaard, personal communication).
The present analyses explore the efficacy and safety of liraglutide 3.0 mg across a broad range of exposures using data generated during the global development programme for liraglutide 3.0 mg, and were conducted to determine whether the data support the use of this dose for all individuals covered by the weight management indication.
Subjects and Methods
Data Sources for Exposure–Response Analyses
Details of datasets used for specific analyses are summarized in Figure S1, Supporting Information. Data are derived from the safety analysis sets (SASs) generated during the following three clinical trials (two phase IIIa, one phase II), referred to as Trials 1–3 and described below.
Trial 1: SCALE Obesity and Prediabetes
Trial 1 was a phase IIIa, 56‐week, randomized, parallel‐group trial [n = 3723, SAS (NCT00422058)] which investigated the efficacy and safety of liraglutide 3.0 mg versus placebo (each as an adjunct to diet and exercise) for weight management in obese [body‐mass index (BMI) ≥ 30 kg/m2] or overweight adults with specific weight‐related comorbidity (BMI 27–29.9 kg/m2 with treated or untreated hypertension and/or dyslipidaemia) 15.
Trial 2: SCALE Diabetes
Trial 2 was a phase IIIa, 56‐week, randomized, placebo‐controlled, parallel‐group trial [n = 844, SAS (NCT01272232)] which investigated the efficacy and safety of liraglutide 3.0 and 1.8 mg in obese or overweight (BMI ≥ 27 kg/m2) adults with type 2 diabetes [glycated haemoglobin (HbA1c) 7.0–10.0% (53–85.8 mmol/mol)], treated with diet and exercise alone or in combination with up to three of metformin, sulphonylurea and/or thiazolidinedione 12.
Trial 3
Trial 3 was a phase II, 20‐week, randomized, double‐blind, placebo‐controlled, parallel‐group dose‐finding trial [n = 564, SAS (NCT00422058)] which investigated the efficacy and safety of liraglutide (1.2–3.0 mg) for weight management in obese adults (BMI ≥ 30.0 and ≤40.0 kg/m2) 11. Participants were randomized to liraglutide 1.2, 1.8, 2.4 or 3.0 mg, placebo or orlistat (3 × 120 mg daily).
For all exposure–response analyses [excluding analysis of adjudicated adverse events (AEs) of special interest], individuals were included if response data were available at baseline, and response and exposure data were available at week 12 or later (Figure S1, Supporting Information). Last observation carried forward (LOCF) was applied after week 12 for efficacy endpoints, pulse and serum calcitonin. Additional analysis of liraglutide‐exposure level was conducted for individuals with adjudicated AEs of special interest, with individuals from Trial 3 excluded as this trial did not include event adjudication.
Blood Sampling and Estimation of Liraglutide Exposure
For Trials 1 and 2, single blood samples were drawn at week 2 (during dose escalation), week 12 and week 28. Participants recorded date, time and dose of the three injections administered prior to sampling. For Trial 3 blood samples were drawn at week 20 during an oral glucose tolerance test. Liraglutide was administered in the evening; samples were drawn the following morning at start of test (time 0), and at 60 and 120 min post‐glucose load 11.
Liraglutide exposure at steady‐state, expressed as the 24‐h area under the curve (AUC), was estimated from individual blood measurements, taking into account dose level and timing of administration, using a previously described model (R. V. Overgaard, personal communication), developed and validated in accordance with guidelines from the FDA 14 and European Medicines Agency 16. Model‐derived exposure was calculated for the target dose level. For individuals dropping out during dose escalation, the exposure level was adjusted, to ensure estimated exposure levels were comparable between subjects with AEs (who could potentially drop out early), and the remaining population.
Exposure–Response Analyses
Exposure–response relationships were visualized using quantile plots with model prediction overlays. Individuals were grouped into six quantiles on the basis of liraglutide exposure (AUC); quantile 1 having the lowest, and quantile 6 the highest, exposure. Thus, six quantile estimates of exposure were plotted against any given variable of interest, and zero exposure was assigned to individuals given placebo. For each exposure quantile, the mean and 95% confidence interval of the response variable of interest (y‐axis) were plotted against the median exposure (x‐axis); thus, the response of a given variable was assessed as a function of exposure (Figures 1, 2, 3, 4 and S2–S4, Supporting Information). The median and 90% exposure range associated with each liraglutide dose were plotted parallel to the x‐axis.
Figure 1.
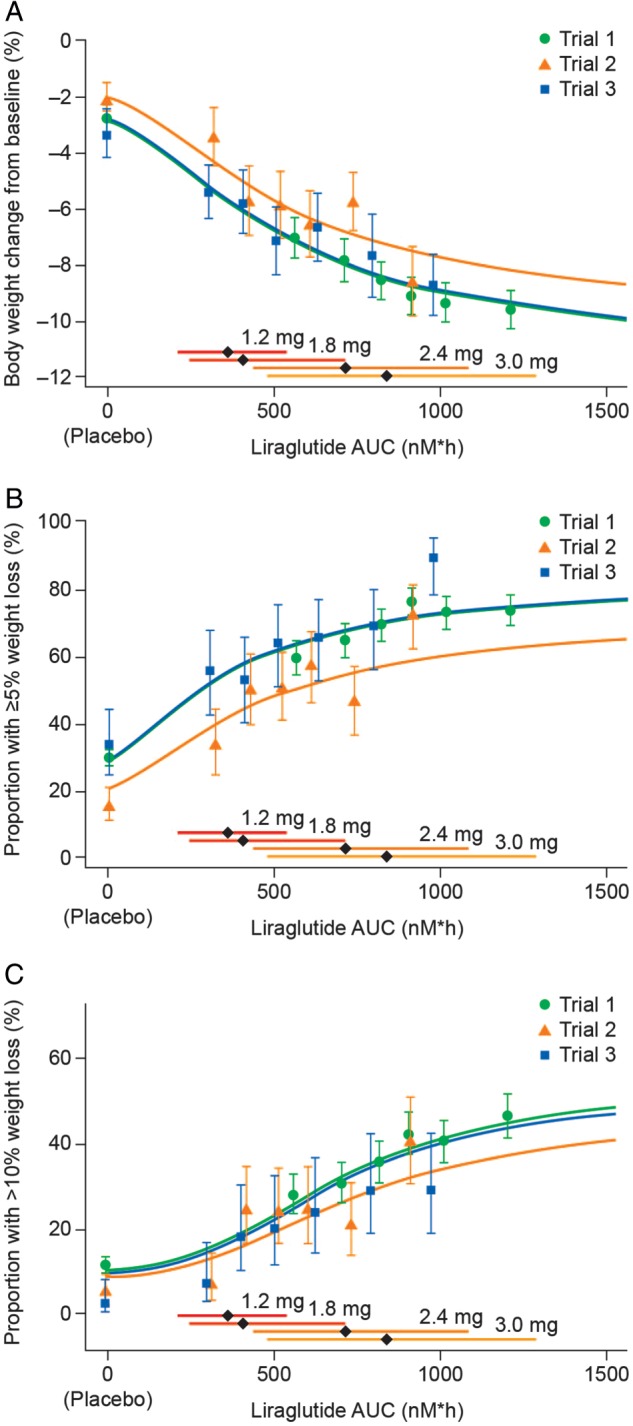
Liraglutide exposure and body weight loss. Liraglutide exposure expressed as model‐derived area under the curve (AUC) at steady‐state versus body weight loss. (A) Exposure versus body weight change from baseline; (B) exposure versus proportion of individuals with ≥5% weight loss from baseline; (C) exposure versus proportion of individuals with >10% weight loss from baseline. Data are mean values (with 95% confidence intervals) versus exposure expressed as six quantiles of area under the curve (AUC) values (plus placebo). Sigmoidal curved lines represent covariate‐adjusted model‐based estimates for each trial population. Horizontal bars with diamonds represent median and 90% exposure ranges from each dose level. Trial 1, SCALE Obesity and Prediabetes; Trial 2, SCALE Diabetes; Trial 3, the phase II trial.
Figure 2.
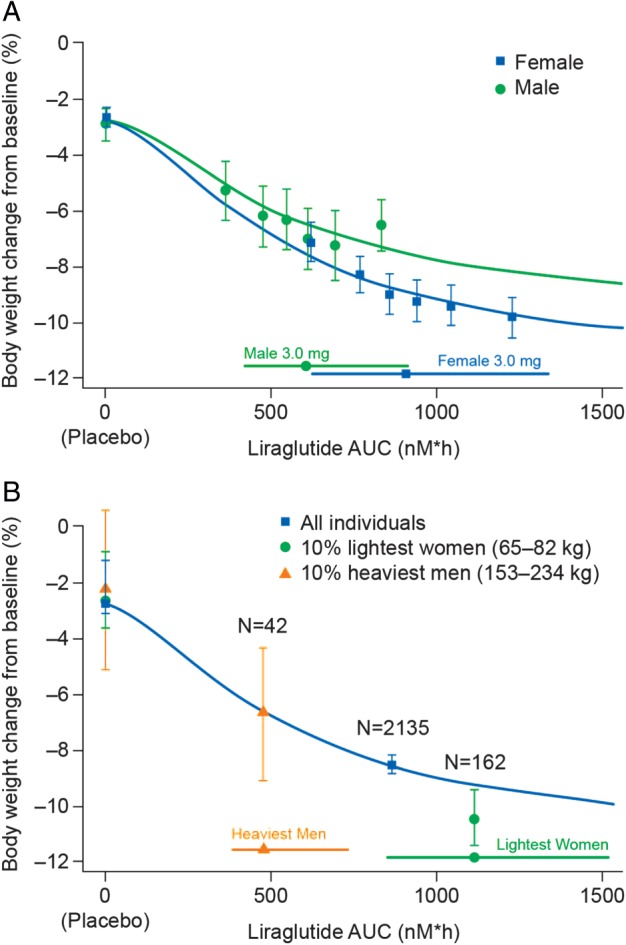
Liraglutide exposure and body weight loss in men and women. Liraglutide exposure expressed as model‐derived area under the curve (AUC) at steady state versus body weight change from baseline (A) for men and women in Trials 1–3 combined (covariate adjusted values); (B) for men and women at the extremes of exposure. Body weight data are mean values (with 95% confidence intervals). Horizontal bars with circles/squares/triangles represent median and 90% exposure ranges from each dose level (A), or from men and women at the extremes of exposure (B). Sigmoidal curved lines represent covariate‐adjusted model‐based estimates for defined populations. (A) Exposure expressed as six quantiles of AUC values (plus placebo). (B) Exposure and weight change for the placebo‐ or liraglutide‐treated heaviest men and lightest women, as well as the overall cohort mean in Trial 1. Trial 1, SCALE Obesity and Prediabetes; Trial 2, SCALE Diabetes; Trial 3, the phase II trial.
Figure 3.
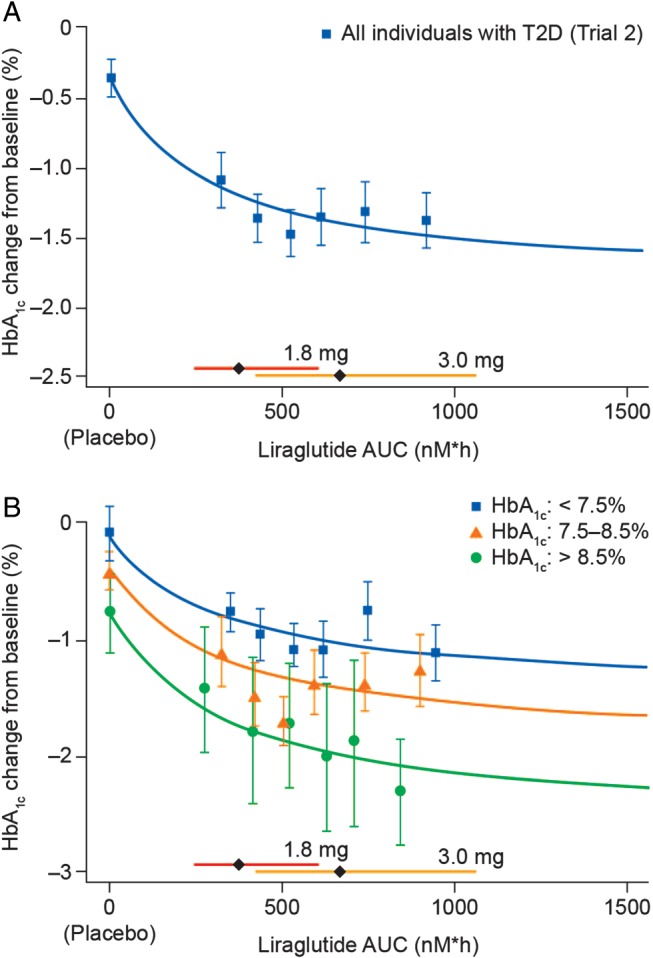
Liraglutide exposure and glycated haemoglobin (HbA1c). Exposure expressed as model‐derived area under the curve (AUC) at steady‐state versus HbA1c change from baseline (A) in individuals with overweight or obesity and type 2 diabetes (Trial 2); (B) in individuals with overweight or obesity and type 2 diabetes, stratified by baseline HbA1c (Trial 2). Data are mean values (with 95% confidence interval) versus exposure expressed as six quantiles of AUC values (plus placebo). Sigmoidal curved lines represent covariate‐adjusted model‐based estimates for each trial population. Horizontal bars with diamonds represent median and 90% exposure ranges from each dose level. T2D, type 2 diabetes.
Figure 4.
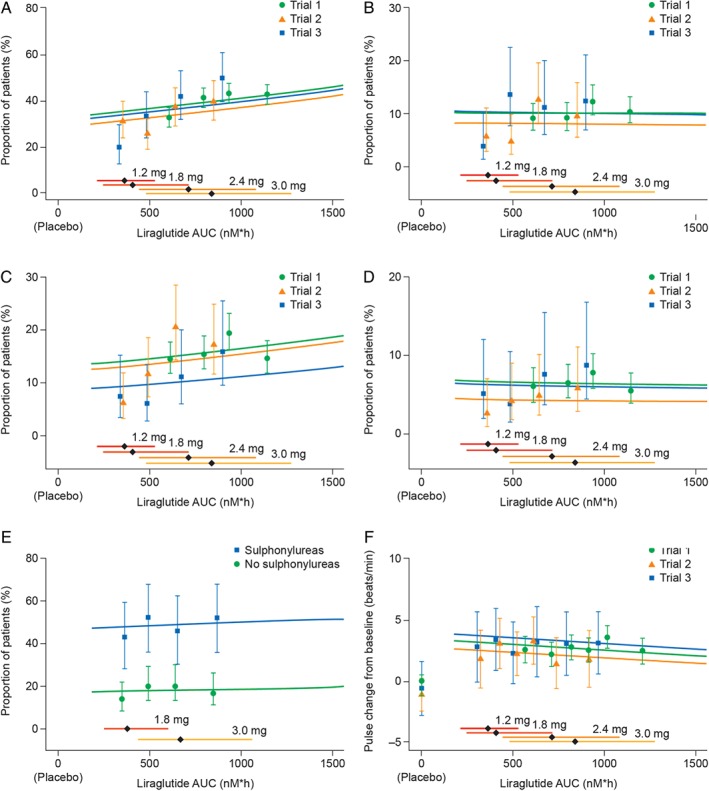
Liraglutide exposure and key safety outcomes. Liraglutide exposure expressed as model‐derived area under the curve (AUC) at steady‐state versus key safety outcomes, proportion of participants with (A) all incidences of nausea; (B) moderate–severe nausea; (C) all incidences of vomiting; (D) moderate–severe vomiting; (E) documented symptomatic hypoglycaemia (glucose ≤ 70 mg/dl); (F) change from baseline in mean resting pulse. Data are proportion of individuals who report an event at any time during the trial (A–E) or mean change in pulse from baseline (F), all with 95% confidence interval, versus exposure (in quantiles of AUC). Lines (exposure–response relationship for each trial) represent a multivariate regression analysis for the pooled data. Horizontal bars with diamonds represent median and 90% exposure ranges. Liraglutide doses were: 3.0 mg in Trial 1; 1.8 and 3.0 mg in Trial 2; 1.2 mg, 1.8 mg, 2.4 mg and 3.0 mg in Trial 3. Trial 1, SCALE Obesity and Prediabetes; Trial 2, SCALE Diabetes; Trial 3, the phase II trial.
Exposure–Response Models
Demographic characteristics were not evenly distributed between exposure quantiles, so multivariate models were used to estimate the outcome of changing exposure without a simultaneous change in participant demographics. Multivariate models included covariates previously demonstrated to influence exposure (R. V. Overgaard, personal communication). Change in body weight versus exposure was estimated using the following model:
where Emax = maximum drug effect for females; EC50 = exposure associated with half maximum effect; γ = Hill coefficient; Imale = covariate effect of gender on Emax; E 0 = placebo response for the typical subject (a normoglycaemic female aged < 70 years with baseline body weight of 100 kg); Edisease status, Emale, EBW and Eage>70 = covariate effects on the overall response in individual subgroups (baseline glycaemic status, gender, body weight, age and disease status, respectively); e = residual error. To facilitate analysis of subgroups, interaction terms (covariates for Emax) were investigated for gender, baseline body weight, age and disease status. The final model included an interaction term for gender (no other factor was found to influence percentage body weight change from baseline).
A similar equation was used for the estimation of change in HbA1c versus exposure. Linear or logistic regression models were used to explore the exposure–response relationships for safety outcomes (excluding AEs of special interest).
Subgroup Analyses
To explore potential exposure‐dependent and ‐independent contributions of demographic characteristics on weight loss, exposure–weight loss relationships were examined by baseline BMI (Figure S3, Supporting Information), gender (Figure 2A), at exposure extremes (10% lightest women; 10% heaviest males; Figure 2B) and by gender within trial (Figure 2A, B). The analysis by gender was based on data from all three trials, and adjusted for differences between trials (including baseline body weight and glycaemic status).
Analyses illustrating exposure–weight loss at exposure extremes (10% lightest women; 10% heaviest males; Figure 2B) and by BMI (Figure S3, Supporting Information) were made based on data from Trial 1 alone, to avoid potential confounding by trial and multiple doses (Trial 1 was the largest trial, and tested liraglutide 3.0 mg vs. placebo).
A mediator analysis was used to investigate the exposure–HbA1c relationship, determining the relative contribution of weight loss (Δ body weight) to the improvement in glycaemic control (Δ HbA1c) 17. The mediator analysis was conducted using a similar equation to that used for the estimation of change in HbA1c versus exposure, but with the addition of Δ body weight as an explanatory variable (mediator).
The exposure–HbA1c relationship, stratified by baseline HbA1c quartile, was investigated to determine whether individuals with poor glycaemic control could derive further glycaemic benefit (i.e. additional to that achieved with a 1.8‐mg dose) from the exposure associated with a 3.0‐mg dose (Figure 3B).
Results
Liraglutide Exposure and Body Weight Loss
Baseline characteristics of individuals included in the exposure–weight loss analysis are summarized in Table S1, Supporting Information. The percentage of body‐weight change from baseline versus liraglutide exposure is shown in Figure 1A.
There was a clear relationship between liraglutide exposure and weight loss in all three trials, and this relationship was similar in those trials that included individuals with normoglycaemia and prediabetes (Trials 1 and 3). The shape of the exposure–weight loss curve was similar for individuals with or without type 2 diabetes, although less weight loss was observed at any given exposure (including placebo) for individuals with type 2 diabetes (Figure 1A). The proportion of individuals achieving ≥5% and >10% weight loss at week 56 also showed a clear relationship with exposure (Figure 1B, C).
Although there was considerable overlap between exposure with 1.8‐ and 3.0‐mg doses (see overlapping horizontal bars above x‐axis; Figure 1), the exposure medians of both doses corresponded to the steep part of the exposure–response curve, and considerably greater weight loss was achieved with the exposure corresponding to 3.0 mg. The effect on mean body weight loss appeared to level off at the highest exposures associated with liraglutide 3.0 mg.
Liraglutide Exposure and Body Weight Loss within BMI Subgroups
Analysis of Trial 1 data stratified by baseline BMI revealed a slight decrease in liraglutide exposure with increasing BMI; however, the exposure differences across BMI subgroups were not associated with meaningful differences in body weight loss, and exposure–weight loss relationships were virtually identical for the four BMI subgroups (Figure S3, Supporting Information).
Liraglutide Exposure and Body Weight Loss in Men and Women
Exposure–weight loss relationships were observed for both men and women, and increasing exposure was associated with greater weight loss across the entire exposure range (Figure 2A). Women had greater weight loss than did men at similar exposures; ∼50% of the difference in body weight loss between men and women could be attributed to higher exposure in women. Similar results were seen when Trials 1 and 2 were analysed separately; however, an upward, leftward shift of the exposure–weight loss curves were observed for men and women with type 2 diabetes versus those without type 2 diabetes (Figure S2A, B). This shift is consistent with lower mean weight loss in individuals with type 2 diabetes 18, 19, and with lower mean exposure in these individuals (R. V. Overgaard, personal communication).
Body Weight Loss in Individuals at the Extremes of Liraglutide Exposure
The 10% lightest women [mean baseline body weight 77.1 kg (highest exposure subgroup)] achieved the greatest observed weight loss [8.1 kg (10.5%)], and the 10% heaviest men [mean baseline body weight 173.7 kg (lowest exposure subgroup)] achieved the least observed relative weight loss [11.4 kg (6.8%); Figure 2B]; the absolute mean weight loss achieved with 3.0 mg in both the high and low exposure subgroups was clinically meaningful.
Liraglutide Exposure and HbA1c Response in Individuals with Type 2 Diabetes
The reduction in HbA1c was considerably greater for all liraglutide exposure quantiles than for placebo (Figure 3A), irrespective of baseline HbA1c level (Figure 3B). The glucose‐lowering associated with liraglutide exposure plateaued above ∼500 nM × h (corresponding to a liraglutide plasma concentration of ∼21 nM; Figure 3A); the ‘plateau effect’ was least obvious in individuals with high baseline HbA1c (Figure 3B). This suggests individuals with poor glycaemic control might derive greater glycaemic benefit from the 3.0‐mg dose. A mediator analysis showed the incremental glucose‐lowering observed with exposure >500 nM × h (i.e. further HbA1c reduction associated with increasing the dose from 1.8 to 3.0 mg) could be almost entirely attributed to corresponding incremental weight loss.
Liraglutide Exposure and Safety Outcomes
The proportion of individuals reporting at least one episode of nausea (any severity) during the 56‐week treatment period increased with increasing liraglutide exposure (p value for slope = 0.04; Figure 4A); however, this was not evident when considering only moderate/severe nausea (p value for slope = 0.90; Figure 4B).
Likewise, the proportion of individuals reporting at least one episode of vomiting (any severity) during the treatment period appeared to increase with increasing exposure (Figure 4C). This relationship was not statistically significant in the combined analysis (p value for slope = 0.26) or Trial 1 (Figure 4C, p value for slope = 0.52); however, it was seen separately in both Trials 2 and 3 (Figure 4C, p values for slopes = 0.03 and 0.04, respectively). This suggests that the exposure–vomiting relationship may have been driven by exposure levels associated with doses up to and including 1.8 mg. There was no evident relationship between liraglutide exposure and moderate/severe vomiting (p‐value for slope = 0.85; Figure 4D).
There was no relationship between the liraglutide exposure associated with 1.8–3.0 mg doses and the incidence of documented symptomatic hypoglycaemia in individuals with type 2 diabetes (p‐value for slope = 0.83; Figure 4E). Although liraglutide‐treated individuals had a slight increase in mean resting pulse (∼2–3 beats per min) versus those treated with placebo, there was no exposure–response relationship between pulse and the exposure levels associated with 1.2–3.0‐mg doses (p‐value for slope = ∼0.18; Figure 4F).
Individuals with AEs of the gallbladder, acute pancreatitis, malignant neoplasms, malignant breast neoplasms or benign colorectal neoplasms did not have higher liraglutide exposure compared with the overall population (a proxy for individuals without these AEs because of the low incidence of these AEs; Figure 5).
Figure 5.
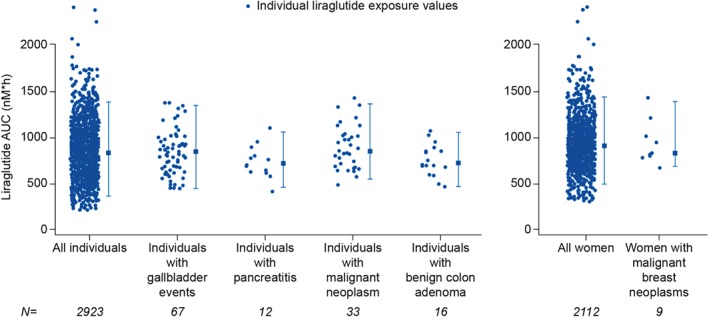
Liraglutide exposure in individuals with adverse events of special interest. Each circular data point represents an individual and the corresponding liraglutide exposure value [model‐derived area under the curve (AUC) at steady‐state for the target dose]; vertical error bars show the 95% exposure range; the square represents the median for each group. Off‐treatment events are included for all categories, with the exception of gallbladder‐related adverse events (AEs). Gallbladder‐related AEs include treatment‐emergent events [0–58 weeks (Trials 1 and 2), or within 0–21 weeks (Trial 3), or up to the end of treatment if discontinued prematurely]; adjudicated pancreatitis and neoplasms include events reported on or off‐treatment through 11 November 2013 (database lock for 120‐day safety update to US Food and Drug Administration).
Liraglutide treatment did not result in elevated serum calcitonin levels across all three trials where it was measured. The change from baseline was close to zero for all quantile estimates of exposure and the slope for calcitonin concentration versus exposure was close to zero (p = ∼0.49; Figure S4, Supporting Information).
Discussion
There was a clear exposure–response relationship between liraglutide and body weight reduction, with percentage weight loss from baseline increasing with greater exposure in all three trials. The shape of the curve describing estimated exposure–weight loss was similar across trials; however, for individuals with type 2 diabetes (Trial 2) the absolute weight loss reduction at any given exposure was lower than that in individuals with normoglycaemia or individuals with prediabetes (Trial 1), even at zero exposure (placebo; Figure 1A). Inter‐trial differences with respect to absolute weight loss may be ascribed to a trial effect, or to population differences between individuals with/without diabetes. It is established that people with diabetes have more difficulty losing weight than individuals without diabetes 17, 19, a finding previously noted in trials of other anti‐obesity medications 20, 21.
At any given exposure, women achieved more weight loss than did men (Figure S2A, Supporting Information); covariate analysis showed this effect was additional to the extra weight loss expected in women as a result of a 32% higher exposure than in men of similar body weight (R. V. Overgaard, personal communication). The exposure–weight loss response did not appear to fully plateau with exposure levels associated with the 3.0 mg dose in men (Figure S2A, Supporting Information), suggesting that more weight loss could potentially have been achieved in some men at doses above 3.0 mg, although such doses have not been clinically tested. Importantly, the 10% heaviest males subgroup [expected to have least weight loss because of the combined effects of high body weight reducing liraglutide exposure 12, and lesser weight loss response to liraglutide in men vs. women (Figure 2A)], achieved clinically significant weight loss [6.8% (11.4 kg); Figure 2B]. Also, the 10% lightest women experienced further weight loss (1.3%), compared with the overall female population [10.5% (8.1 kg) vs. 9.2% (9.3 kg); Figures 2B and S2A, Supporting Information].
In summary, exposure–weight loss analyses suggest that, of all doses tested, liraglutide 3.0 mg provides the optimum exposure for clinically relevant weight loss in all populations: men and women, the entire range of baseline body weight and people with type 2 diabetes.
The relationship between liraglutide exposure and HbA1c was analysed in individuals with type 2 diabetes (Trial 2) only. There was a clear exposure–HbA1c relationship, with the magnitude of HbA1c reduction increasing up to a plasma liraglutide concentration of ∼21 nM. This was consistent with a previous study that estimated a concentration of ∼15 nM was necessary for a full glycaemic response in individuals with similar baseline characteristics to those in Trial 2 22. In the present analysis the mean HbA1c reduction obtained with exposures associated with the 3.0 mg dose was only modestly greater than with 1.8 mg (Figure 3A); however, when stratified by baseline HbA1c, the HbA1c reduction obtained with exposure levels associated with 3.0 mg did not plateau for the highest baseline HbA1c category [>8.5% (69.4 mmol/mol)], implying that individuals with high HbA1c may derive additional glycaemic benefit from a 3.0‐mg, rather than 1.8‐mg, dose. A mediator analysis indicated that the incremental HbA1c reduction with doses above 1.8 mg, up to the maximum tested 3.0 mg, was accounted for by the concomitant reduction in body weight.
The appropriateness of a particular dose of any drug is based not only on efficacy, but also on safety and tolerability. In the present analyses, no exposure–response relationship was identified for any safety outcome, with the exception of GI AEs (nausea and vomiting), which are well‐known dose‐dependent side effects of GLP‐1 receptor agonist therapies 23, 24. We considered the incidence of GI AEs occurring at any time during treatment as a function of exposure, without accounting for the time, or duration, of occurrence. Previous studies of GLP‐1 receptor agonist therapies have shown that GI AEs, if they do occur, tend to appear early and are transient 25, 26. Although a relationship between steady‐state liraglutide exposure and nausea/vomiting at any time was identified (Figure 4A, C), this did not apply to moderate or severe events (Figure 4B, D). Furthermore, the liraglutide exposure–vomiting (any grade) relationship was stronger in those trials that included multiple dose levels (Trials 2 and 3; Figure 4C), suggesting that the relationship with exposure is stronger at the lower doses and levels off at higher doses.
Liraglutide‐treated individuals who developed infrequent but clinically significant AEs such as acute pancreatitis, gallstones, cholecystitis and breast cancer (Figure 5) did not appear to have higher exposure when compared with the overall study population. While that does not rule out a relationship with liraglutide, it is not supportive of an exposure– (or dose–) response relationship for these AEs.
Liraglutide and other GLP‐1‐based therapies increase resting pulse by ∼2–3 beats per min 27, 28, 29; however, the clinical significance of this increase remains unknown 15. A slight increase in resting pulse relative to placebo occurred with the liraglutide exposure levels associated with the 3.0‐mg dose (Figure 4F). It was reassuring, however, that no exposure–pulse relationship was evident within the investigated dose range (1.2–3.0 mg), and the magnitude of the increase in pulse was similar to that previously reported for the 1.2–1.8‐mg dose range 30. Although no liraglutide exposure–pulse relationship was evident for the investigated dose range, the lowest dose associated with increased pulse has not been established.
Limitations of the present study include the fact that analyses were based on individuals with exposure and response data available at week 12 or later, with missing response values imputed using LOCF. It is not possible to estimate how exposure–response curves may be affected if data from all individuals (e.g. including those dropping out before week 12) were included. Liraglutide was studied at a single dose level (3.0 mg, the only intended dose for weight management) in Trial 1 (the largest study in the liraglutide 3.0 mg clinical programme); consequently Trial 1 covered a limited range of exposures. Indeed, the lack of low‐dose exposure data in this trial may limit interpretation of exposure–response relationships for AEs. Specifically, while linear regression analyses were used to identify whether greater exposure was associated with more AEs, the level of exposure at which specific AEs may begin to occur could not be identified. Nonetheless, these analyses provide reassurance that the tolerability of liraglutide does not become compromised with the exposures associated with the 3.0‐mg dose. Different imputation methods affect the estimated separation between placebo and active drug 15; however, in the present study, different imputation methods (e.g. imputing dropouts with the placebo response, or analysing completers only) resulted in only modest changes to overall exposure–response relationships (data not shown).
In summary, dose selection with any drug is a complex task that requires balancing benefits with risks, and exposure–response analyses play an important role. The present analyses support the use of liraglutide 3.0 mg for weight management in all subgroups investigated, as weight loss increased with higher drug exposure, with no deterioration in safety and tolerability (beyond the known GI side‐effects of nausea and vomiting). Further, these analyses suggest that the additional weight loss associated with the exposure levels seen with 3.0‐mg dosing (compared with 1.8‐mg dosing) can translate into additional glycaemic benefits when 3.0 mg is used for weight management in individuals with obesity, high baseline HbA1c and comorbid type 2 diabetes.
Conflict of Interest
J. P. H. W. is a full time employee of the University of Liverpool, and has the following interests: AstraZeneca: advisory panel, speakers bureau, research support; Boehringer Ingelheim: speaker's bureau; advisory panel; Janssen: advisory panel, speaker's bureau; Novo Nordisk: advisory panel, speaker's bureau, research support; Menarini: advisory panel; Merck: speaker's bureau; advisory panel; Pfizer: consultant; Sanofi: advisory panel.
R. V. O, L. V. J., and C. B. J. are full‐time employees of, and share‐holders in, Novo Nordisk A/S. C. R. is a full‐time employee of University College Dublin, and has the following interests: Novo Nordisk: advisory panel; GI Dynamics: advisory panel; Fractyl: advisory panel.
All authors confirm that they meet the International Committee of Medical Journal Editors uniform requirements for authorship and that they have contributed to: critical analysis and interpretation of the data, drafting/critically revising the article and sharing in the final responsibility for the content of the manuscript and the decision to submit it for publication. R.V.O. was responsible for statistical analysis.
Supporting information
Appendix S1. Exposure–response analyses of liraglutide 3.0 mg for weight management.
Table S1. Baseline characteristics of individuals receiving liraglutide or placebo (pooled data) in exposure–response analyses.
Figure S1. Datasets used for (A) exposure–efficacy endpoint analyses and (B) exposure–safety outcome analyses.
Figure S2. Liraglutide exposure and body weight loss in men and women with and without type 2 diabetes. Liraglutide exposure expressed as model‐derived area under the curve at steady‐state versus body weight change from baseline for men and women with (A) normoglycaemia/prediabetes (Trial 1); (B) with type 2 diabetes (Trial 2).
Figure S3. Liraglutide exposure and body weight loss for body mass index (BMI) subgroups in Trial 1. Liraglutide exposure versus body weight change from baseline, stratified by baseline BMI.
Figure S4. Liraglutide exposure and change in calcitonin levels. Liraglutide exposure expressed as model‐derived area under the curve at steady‐state versus change in calcitonin levels from baseline.
Acknowledgements
The authors thank James Currie at Watermeadow Medical for medical writing, funded by Novo Nordisk. Novo Nordisk conducted the statistical analyses and reviewed the manuscript for medical accuracy. The corresponding author had full access to all the data in the analyses and all authors had final responsibility for the decision to submit for publication.
References
- 1. Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP‐1 binding sites in the rat brain: evidence that exendin‐4 is a ligand of brain GLP‐1 binding sites. Eur J Neurosci 1995; 7: 2294–2300. [DOI] [PubMed] [Google Scholar]
- 2. Turton MD, O'Shea D, Gunn I et al. A role for glucagon‐like peptide‐1 in the central regulation of feeding. Nature 1996; 379: 69–72. [DOI] [PubMed] [Google Scholar]
- 3. Flint A, Raben A, Astrup A, Holst JJ. Glucagon‐like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998; 101: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merchenthaler I, Lane M, Shughrue P. Distribution of pre‐pro‐glucagon and glucagon‐like peptide‐1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 1999; 403: 261–280. [DOI] [PubMed] [Google Scholar]
- 5. Flint A, Raben A, Ersbøll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon‐like peptide‐1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord 2001; 25: 781–792. [DOI] [PubMed] [Google Scholar]
- 6. Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J 2010; 57: 359–372. [DOI] [PubMed] [Google Scholar]
- 7. Vrang N, Larsen PJ. Preproglucagon derived peptides GLP‐1, GLP‐2 and oxyntomodulin in the CNS: role of peripherally secreted and centrally produced peptides. Prog Neurobiol 2010; 92: 442–462. [DOI] [PubMed] [Google Scholar]
- 8. Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon‐like peptide‐1 loss of function. J Neurosci 2011; 31: 3904–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knudsen LB, Nielsen PF, Huusfeldt PO et al. Potent derivatives of glucagon‐like peptide‐1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 2000; 43: 1664–1669. [DOI] [PubMed] [Google Scholar]
- 10. Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon‐like peptide 1 (7‐36 amide) in type 2 (non‐insulin‐dependent) diabetic patients. Diabetologia 1993; 36: 741–744. [DOI] [PubMed] [Google Scholar]
- 11. Astrup A, Rössner S, Van Gaal L et al, NN8022‐1807 Study Group . Effects of liraglutide in the treatment of obesity: a randomised, double‐blind, placebo‐controlled study. Lancet 2009; 374: 1606–1616. [DOI] [PubMed] [Google Scholar]
- 12. Davies MJ, Bergenstal R, Bode B et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA 2015; 314: 687–699. [DOI] [PubMed] [Google Scholar]
- 13.FDA Briefing Document for Liraglutide, NDA 206321. Endocrinologic and Metabolic Drugs Advisory Committee meeting, 11 September 2014. Available from URL: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM413317.pdf. Accessed 21 January 2016.
- 14. US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) . FDA Guidance for Industry. Population Pharmacokinetics. February 1999. Available from URL: http://www.fda.gov/downloads/Drugs/…/Guidances/UCM072137.pdf. Accessed 21 January 2016.
- 15. Pi‐Sunyer X, Astrup A, Fujioka K et al, SCALE Obesity and Prediabetes NN8022‐1839 Study Group . A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015; 373: 11–22. [DOI] [PubMed] [Google Scholar]
- 16. European Medicines Agency . Guideline on reporting the results of population pharmacokinetic analyses. London, June 2007. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003067.pdf. Accessed 21 January 2016
- 17. Baron RM, Kenny DA. The moderator‐mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986; 51: 1173–1182. [DOI] [PubMed] [Google Scholar]
- 18. Wing RR, Marcus MD, Epstein LH, Salata R. Type II diabetic subjects lose less weight than their overweight nondiabetic spouses. Diabetes Care 1987; 10: 563–566. [DOI] [PubMed] [Google Scholar]
- 19. Pi‐Sunyer FX. Weight loss in type 2 diabetic patients. Diabetes Care 2005; 28: 1526–1527. [DOI] [PubMed] [Google Scholar]
- 20. FDA Briefing Document for Contrave (bupropion/naltrexone) EMDAC meeting, 07 December 2010. Available from URL: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM235671.pdf. Accessed 21 January 2016.
- 21. FDA Briefing Document for Zimulti (rimonabant) EMDAC meeting 13 Jun 2007. Available from URL: http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007‐4306b1‐fda‐backgrounder.pdf. Accessed 21 January 2016.
- 22. Ingwersen SH, Khurana M, Madabushi R et al. Dosing rationale for liraglutide in type 2 diabetes mellitus: a pharmacometric assessment. J Clin Pharmacol 2012; 52: 1815–1823. [DOI] [PubMed] [Google Scholar]
- 23. Derosa G, Maffioli P. GLP‐1 agonists exenatide and liraglutide: a review about their safety and efficacy. Curr Clin Pharmacol 2012; 7: 214–228. [DOI] [PubMed] [Google Scholar]
- 24. Sun F, Chai S, Yu K et al. Gastrointestinal adverse events of glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a systematic review and network meta‐analysis. Diabetes Technol Ther 2015; 17: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buse JB, Rosenstock J, Sesti G et al, LEAD‐6 Study Group . Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet 2009; 374: 39–47. [DOI] [PubMed] [Google Scholar]
- 26. Lean ME, Carraro R, Finer N, NN8022‐1807 Investigators . Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non‐diabetic adults. Int J Obes (Lond) 2014; 38: 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diamant M, Van Gaal L, Stranks S et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION‐3): an open‐label randomised trial. Lancet 2010; 375: 2234–2243. [DOI] [PubMed] [Google Scholar]
- 28. Dungan KM, Povedano ST, Forst T et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet 2014; 384: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 29. Ussher JR, Drucker DJ. Cardiovascular actions of incretin‐based therapies. Circ Res 2014; 114: 1788–1803. [DOI] [PubMed] [Google Scholar]
- 30. Fonseca VA, DeVries JH, Henry RR, Donsmark M, Thomsen HF, Plutzky J. Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient‐level pooled analysis of six randomized clinical trials. J Diabetes Complications 2014; 28: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Exposure–response analyses of liraglutide 3.0 mg for weight management.
Table S1. Baseline characteristics of individuals receiving liraglutide or placebo (pooled data) in exposure–response analyses.
Figure S1. Datasets used for (A) exposure–efficacy endpoint analyses and (B) exposure–safety outcome analyses.
Figure S2. Liraglutide exposure and body weight loss in men and women with and without type 2 diabetes. Liraglutide exposure expressed as model‐derived area under the curve at steady‐state versus body weight change from baseline for men and women with (A) normoglycaemia/prediabetes (Trial 1); (B) with type 2 diabetes (Trial 2).
Figure S3. Liraglutide exposure and body weight loss for body mass index (BMI) subgroups in Trial 1. Liraglutide exposure versus body weight change from baseline, stratified by baseline BMI.
Figure S4. Liraglutide exposure and change in calcitonin levels. Liraglutide exposure expressed as model‐derived area under the curve at steady‐state versus change in calcitonin levels from baseline.


