Abstract
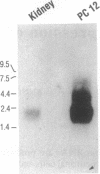
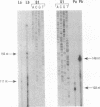
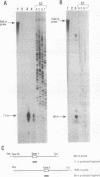
Aromatic L-amino acid decarboxylase (AADC, EC 4.1.1.28) catalyzes the decarboxylation of L-dopa to dopamine in catecholamine cells and 5-hydroxytryptophan to serotonin in serotonin-producing neurons. This enzyme is also expressed in relatively large quantities in nonneuronal tissues such as liver and kidney, where its function is unknown. Neuronal and nonneuronal tissues express AADC mRNAs with distinct 5' untranslated regions. To understand how this is accomplished at the genomic level, we have isolated rat genomic DNA encoding AADC. The organization of the AADC gene suggests that there are two separate promoters specific for the transcription of neuronal and nonneuronal forms of the AADC message. A small exon containing 68 bases of the neuronal-specific 5' end is located approximately 9.5 kilobases upstream of the translation start site, which is contained in the third exon. Approximately 7 kilobases upstream from the neuron-specific promoter is another small exon containing 71 bases of the 5' end of the nonneuronal AADC message. These data suggest that transcription initiating at distinct promoters, followed by alternative splicing, is responsible for the expression of the neuronal and nonneuronal forms of the AADC message.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert V. R., Allen J. M., Joh T. H. A single gene codes for aromatic L-amino acid decarboxylase in both neuronal and non-neuronal tissues. J Biol Chem. 1987 Jul 5;262(19):9404–9411. [PubMed] [Google Scholar]
- Ando-Yamamoto M., Hayashi H., Sugiyama T., Fukui H., Watanabe T., Wada H. Purification of L-dopa decarboxylase from rat liver and production of polyclonal and monoclonal antibodies against it. J Biochem. 1987 Feb;101(2):405–414. doi: 10.1093/oxfordjournals.jbchem.a121925. [DOI] [PubMed] [Google Scholar]
- Bouchard S., Bousquet C., Roberge A. G. Characteristics of dihydroxyphenylalanine/5-hydroxytryptophan decarboxylase activity in brain and liver of cat. J Neurochem. 1981 Sep;37(3):781–787. doi: 10.1111/j.1471-4159.1982.tb12555.x. [DOI] [PubMed] [Google Scholar]
- Boyce F. M., Beggs A. H., Feener C., Kunkel L. M. Dystrophin is transcribed in brain from a distant upstream promoter. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1276–1280. doi: 10.1073/pnas.88.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Christenson J. G., Dairman W., Udenfriend S. On the identity of DOPA decarboxylase and 5-hydroxytryptophan decarboxylase (immunological titration-aromatic L-amino acid decarboxylase-serotonin-dopamine-norepinephrine). Proc Natl Acad Sci U S A. 1972 Feb;69(2):343–347. doi: 10.1073/pnas.69.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogé F., Krieger-Poullet M., Guillemot J. C., Ferrara P., Gros F., Thibault J. Purification et séquençage partiel de la L-dopa décarboxylase de phéochromocytome de rat. C R Acad Sci III. 1989;309(14):587–592. [PubMed] [Google Scholar]
- DiMattia G. E., Gellersen B., Duckworth M. L., Friesen H. G. Human prolactin gene expression. The use of an alternative noncoding exon in decidua and the IM-9-P3 lymphoblast cell line. J Biol Chem. 1990 Sep 25;265(27):16412–16421. [PubMed] [Google Scholar]
- Dorn A., Bollekens J., Staub A., Benoist C., Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987 Sep 11;50(6):863–872. doi: 10.1016/0092-8674(87)90513-7. [DOI] [PubMed] [Google Scholar]
- Elsholtz H. P., Mangalam H. J., Potter E., Albert V. R., Supowit S., Evans R. M., Rosenfeld M. G. Two different cis-active elements transfer the transcriptional effects of both EGF and phorbol esters. Science. 1986 Dec 19;234(4783):1552–1557. doi: 10.1126/science.3491428. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S. L., Hahn M., Joh T. H. Genomic organization of the rat aromatic L-amino acid decarboxylase (AADC) locus: partial analysis reveals divergence from the Drosophila dopa decarboxylase (DDC) gene structure. Mamm Genome. 1991;1(3):145–151. doi: 10.1007/BF00351060. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Fuxe K., Goldstein M. Immunohistochemical localization of aromatic L-amino acid decarboxylase (DOPA decarboxylase) in central dopamine and 5-hydroxytryptamine nerve cell bodies of the rat. Brain Res. 1973 Apr 13;53(1):175–180. doi: 10.1016/0006-8993(73)90776-2. [DOI] [PubMed] [Google Scholar]
- Ichinose H., Kurosawa Y., Titani K., Fujita K., Nagatsu T. Isolation and characterization of a cDNA clone encoding human aromatic L-amino acid decarboxylase. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1024–1030. doi: 10.1016/0006-291x(89)91772-5. [DOI] [PubMed] [Google Scholar]
- Jaeger C. B., Albert V. R., Joh T. H., Reis D. J. Aromatic L-amino acid decarboxylase in the rat brain: coexistence with vasopressin in small neurons of the suprachiasmatic nucleus. Brain Res. 1983 Oct 16;276(2):362–366. doi: 10.1016/0006-8993(83)90748-5. [DOI] [PubMed] [Google Scholar]
- Krieger M., Coge F., Gros F., Thibault J. Different mRNAs code for dopa decarboxylase in tissues of neuronal and nonneuronal origin. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2161–2165. doi: 10.1073/pnas.88.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebacq-Verheyden A. M., Way J., Battey J. Structural characterization of a brain-specific promoter region directing transcription of the rat prepro-gastrin-releasing peptide gene. Brain Res Mol Brain Res. 1990 Apr;7(3):235–241. doi: 10.1016/0169-328x(90)90033-a. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lees-Miller J. P., Goodwin L. O., Helfman D. M. Three novel brain tropomyosin isoforms are expressed from the rat alpha-tropomyosin gene through the use of alternative promoters and alternative RNA processing. Mol Cell Biol. 1990 Apr;10(4):1729–1742. doi: 10.1128/mcb.10.4.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. A., Johnson W. A., Hirsh J. Regulated splicing produces different forms of dopa decarboxylase in the central nervous system and hypoderm of Drosophila melanogaster. EMBO J. 1986 Dec 1;5(12):3335–3342. doi: 10.1002/j.1460-2075.1986.tb04648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. K., Nagatsu T., Kato T. Aromatic L-amino acid decarboxylase activity in central and peripheral tissues and serum of rats with L-DOPA and L-5-hydroxytryptophan as substrates. Biochem Pharmacol. 1981 Mar 15;30(6):645–649. doi: 10.1016/0006-2952(81)90139-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Shirota K., Fujisawa H. Purification and characterization of aromatic L-amino acid decarboxylase from rat kidney and monoclonal antibody to the enzyme. J Neurochem. 1988 Aug;51(2):426–434. doi: 10.1111/j.1471-4159.1988.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Sumi-Ichinose C., Ichinose H., Takahashi E., Hori T., Nagatsu T. Molecular cloning of genomic DNA and chromosomal assignment of the gene for human aromatic L-amino acid decarboxylase, the enzyme for catecholamine and serotonin biosynthesis. Biochemistry. 1992 Mar 3;31(8):2229–2238. doi: 10.1021/bi00123a004. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Horio Y., Taketoshi M., Imamura I., Ando-Yamamoto M., Kangawa K., Matsuo H., Kuroda M., Wada H. Molecular cloning and sequencing of a cDNA of rat dopa decarboxylase: partial amino acid homologies with other enzymes synthesizing catecholamines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8142–8146. doi: 10.1073/pnas.86.20.8142. [DOI] [PMC free article] [PubMed] [Google Scholar]