Abstract
Recent data have provided evidence that microglia, the brain‐resident macrophage‐like cells, modulate neuronal activity in both physiological and pathophysiological conditions, and microglia are therefore now recognized as synaptic partners. Among different neuromodulators, purines, which are produced and released by microglia, have emerged as promising candidates to mediate interactions between microglia and synapses. The cellular effects of purines are mediated through a large family of receptors for adenosine and for ATP (P2 receptors). These receptors are present at brain synapses, but it is unknown whether they can respond to microglia‐derived purines to modulate synaptic transmission and plasticity. Here, we used a simple model of adding immune‐challenged microglia to mouse hippocampal slices to investigate their impact on synaptic transmission and plasticity at hippocampal mossy fibre (MF) synapses onto CA3 pyramidal neurons. MF–CA3 synapses show prominent forms of presynaptic plasticity that are involved in the encoding and retrieval of memory. We demonstrate that microglia‐derived ATP differentially modulates synaptic transmission and short‐term plasticity at MF–CA3 synapses by acting, respectively, on presynaptic P2X4 receptors and on adenosine A1 receptors after conversion of extracellular ATP to adenosine. We also report that P2X4 receptors are densely located in the mossy fibre tract in the dentate gyrus–CA3 circuitry. In conclusion, this study reveals an interplay between microglia‐derived purines and MF–CA3 synapses, and highlights microglia as potent modulators of presynaptic plasticity.
Keywords: A1 receptor, hippocampal synaptic plasticity, microglia, P2X4 receptor, purines
Introduction
Microglia are brain‐resident macrophage‐like cells of the central nervous system, and have been recently acknowledged as synaptic partners (Ben Achour & Pascual, 2010; Tremblay et al., 2010; Kettenmann et al., 2013). Microglia modulate neuronal activity in both physiological and pathophysiological conditions (Bessis et al., 2007; Roumier et al., 2008; Wake et al., 2009) through signalling mechanisms that are largely unknown. Purines are candidate signals, as microglia can release ATP (Pascual et al., 2012; George et al., 2015), which modulates synaptic transmission and plasticity (Neary & Zimmermann, 2009; Verkhratsky et al., 2009; Burnstock et al., 2011; Rodrigues et al., 2015).
ATP has pleiotropic effects in the brain, which depend on different types of purinergic receptors expressed by neurons and glial cells, but also on its degradation by extracellular ectonucleotidases to adenosine (Burnstock et al., 2011; Rodrigues et al., 2015). Purinergic receptors are divided into two families activated either by adenosine (P1 receptors) or by ATP (P2 receptors). P1 receptors are G‐protein‐coupled, and comprise four different subtypes: A1, A2A, A2B, and A3 (Fredholm et al., 2011). The P2 receptor family is subdivided in two subfamilies: the ionotropic P2X receptors (P2X receptors 1–7) family linked to increased cation permeability, and the metabotropic P2Y receptors (eight different subtypes) family coupled to G‐proteins (Burnstock et al., 2011).
P1 and P2 receptors are widely distributed at brain synapses. In the hippocampus, A1 receptors (A1Rs) are located at both presynaptic and postsynaptic loci (Rebola et al., 2003), whereas A2A receptors (A2ARs) are mostly presynaptic (Rebola et al., 2005), but are also present in astrocytes (Matos et al., 2012) and microglia (Rebola et al., 2011). Adenosine decreases glutamatergic transmission via presynaptic A1Rs (Dunwiddie & Masino, 2001), whereas it activates A2ARs to facilitate AMPA and N‐methyl‐d‐aspartate receptor‐mediated currents (Rebola et al., 2008; Dias et al., 2012) to modulate synaptic plasticity (Rebola et al., 2008; Costenla et al., 2011).
P2X receptors also modulate hippocampal synaptic transmission and plasticity (Pankratov et al., 2002). Presynaptic P2X2 receptors facilitate CA3 excitatory transmission onto interneurons in the CA1 region (Khakh et al., 2003), whereas P2X4 receptors (P2X4Rs) tune the N‐methyl‐d‐aspartate receptor‐dependent component of long‐term plasticity at the Schaffer–collateral CA1 synapse (Sim et al., 2006). This is in accordance with the expression of P2X2, P2X3, P2X4 and P2X6 receptor subunits by hippocampal neurons (Rodrigues et al., 2005), whereas P2X7 receptors are mostly expressed by activated glial cells (Butt, 2011).
In this study, we investigated the role of microglia–purine interactions at hippocampal synapses between mossy fibres (MFs) and CA3 pyramidal cells. MF‐CA3 synapses show prominent forms of presynaptic plasticity (Marchal & Mulle, 2004; Nicoll & Schmitz, 2005), and are instrumental in the encoding and retrieval of memories (Nakazawa et al., 2003; Bischofberger et al., 2006; Kesner, 2007). We report that ATP and adenosine, derived from lipopolysaccharide (LPS)‐challenged microglia (George et al., 2015), do modulate synaptic transmission and short‐term plasticity at MF‐CA3 synapses by acting, respectively, on P2X4Rs and A1Rs. This study reveals an interplay between microglia and MF‐CA3 synapses, and further supports microglia as true modulators of neuronal network through purinergic modulation of presynaptic function.
Materials and methods
Ethical approval
Experiments were carried out in accordance with the guidelines on the ethical use of animals from the European Communities Council Directive (86/609/EEC) and were evaluated by the Ethics commitee n°50 of Bordeaux, affiliated with the CNREEA (Centre National de Réflexion Ethique sur l'Expérimentation Animale).
Microglial cell culture and pharmacological treatment
A murine microglial cell line, N9 (a gift from C. Verderio, CNR Institute of Neuroscience, Cellular and Molecular Pharmacology, Milan, Italy), was grown in RPMI‐1640 (Sigma, St Quentin Fallavier, France) medium supplemented with 30 mm glucose (Sigma), 5% heat‐inactivated fetal bovine serum (Gibco, Invitrogen, Merelbeke, Belgium), 100 μg/mL streptomycin, and 1 U/mL penicillin (Gibco, Invitrogen). Cells were kept at 37 °C under a humidified atmosphere with 95% O2 and 5% CO2. In order to trigger microglia activation and ATP release, N9 cells were challenged with 100 ng/mL LPS (from Escherichia coli, serotype 055:B5; Sigma) for 1 h, which we have found to trigger ATP release and upregulation of A2ARs (Gomes et al., 2013).
Slice preparation
C57BL6/J mice (Janvier Labs, Le Genest St Isle, France) were killed at postnatal day (P)19–P21 by cervical dislocation. The brain was then quickly removed from the skull, and chilled in ice‐cold low‐calcium artificial cerebrospinal fluid containing 87 mm NaCl, 2.5 mm KCl, 1.25 mm NaH2PO4, 0.5 mm CaCl2, 7 mm MgCl2, 10 mm glucose, 25 mm NaHCO3, and 75 mm sucrose, adjusted to pH 7.4 by saturating with carbogen (95% O2 and 5% CO2), and with an osmolarity of 300–310 mOsm. Brains were then glued onto the stage of a vibratome (VT 1200S; Leica, Nusslich, Germany), and parasagittal hippocampal slices (320 μm) were cut. Slices were then transferred to an extracellular solution containing 129 mm NaCl, 2.5 mm KCl, 1.25 mm NaH2PO4, 2 mm CaCl2, 1 mm MgCl2, 11 mm glucose, and 25 mm NaHCO3, equilibrated with carbogen for 30 min at 33 °C and thereafter at room temperature, until being used.
Electrophysiology
The slices were submerged in a recording chamber and continuously superfused with carbogen‐saturated extracellular solution at 32–34 °C. Whole cell patch clamp recordings were made at a holding potential of −70 mV with electrodes of 3–4 MΩ. Patch clamp electrodes were pulled out from borosilicate glass (GF 150 F‐10; Clark Electromedical, Reading, UK), and filled with an internal solution containing 140 mm CsCH3SO3, 2 mm MgCl2, 4 mm NaCl, 5 mm phosphocreatine, 2 mm Na2ATP, 0.2 mm EGTA, 10 mm HEPES, and 0.33 mm GTP (pH 7.3), adjusted with CsOH. Bicuculline (10 μm) was added to the bath to inhibit GABAA receptors.
Voltage‐clamp recordings were performed on CA3 pyramidal neurons visualized with a differential interference contrast microscope (Eclipse FN‐1; Nikon, Champigny sur Marne, France) equipped with an infrared camera (VX44; Till Photonics, Gräfelfing, Germany), by use of an Axoptach‐200B amplifier (Axon Instruments, Sunnydale, CA, USA). Signals were filtered at 2 kHz and digitized at 5 kHz via a DigiData 1322A interface (Axon Instruments). Series resistance (10–20 MΩ) was monitored during the recording with a −10 mV hyperpolarizing voltage step (length, 50 ms) delivered at the beginning of each recording. Neurons were rejected if the series resistance changed by >20% during the experiment. Neurons with a holding current of >300 pA at a holding potential of −70 mV were rejected. Data were collected and analysed with pclamp software 9.2 (Axon Instruments). N9 microglia were challenged with LPS (100 ng/mL) for 1 h or not challenged, before being added to the slices. The cells were trypsinized and collected, and then added to a hippocampal slice at a density of ~100 000 cells per slice. N9 cells were allowed to adhere to the slices for 15–20 min, and the unattached cells were washed away, leaving approximately 400–500 cells stuck at the slice surface. Voltage‐clamp recordings were obtained from CA3 pyramidal cells surrounded (10–100 μm) by three to five N9 cells in close vicinity. Excitatory postsynaptic currents (EPSCs) from MF–CA3 synapses were evoked by minimal‐intensity stimulation of MFs (Marchal & Mulle, 2004; Sachidhanandam et al., 2009). A glass electrode, with a tip diameter of ~1 μm, was placed on the hilus of the dentate gyrus to stimulate MFs. The baseline stimulation frequency for all experiments was 0.1 Hz. EPSCs were identified according to the following criteria: robust low‐frequency facilitation, low release probability at low stimulation (0.1 Hz), rapid rise time of individual EPSCs, and being free of secondary peaks during decay. LCCG‐1 (10 μm), a metabotropic glutamate receptor 2 agonist, was added routinely to confirm the selective stimulation of MFs, and cells were excluded if the inhibition was <80%. Pyridoxalphosphate‐6‐azophenyl‐2′,4′‐disulfonic acid (PPADS), ivermectin, 5‐(3‐bromophenyl)‐1,3‐dihydro‐2H‐benzofuro[3,2‐e]‐1,4‐diazepin‐2‐one (5‐BDBD), apyrase, ARL67156 and SCH58261 were obtained from Tocris (Bristol, UK), and 8‐cyclopentyl‐1,3‐dipropylxanthine (DPCPX) and 2‐chloro‐N 6‐cyclopentyladenosine (CCPA) were obtained from Abcam (Cambridge, UK). The antibody used for staining P2X4Rs (Ref. HPA039494) was obtained from Atlas Antibodies (Stockholm, Sweden).
Immunohistochemistry
Mice (P19–P21) were anaesthetized by intraperitoneal administration of pentobarbital (50 mg/kg body weight), and were fixed by transcardial perfusion with 50 mL of 4% paraformaldehyde. Brains were removed and postfixed in 4% paraformaldehyde overnight, and then saturated in a solution with 30% sucrose for 24 h. They were then frozen in cold heptane, and stored at −80 °C. Brains were cut on a cryostat at −20 °C into 40‐μm‐thick coronal sections. Sections were collected into antifreeze medium, and stored at −20 °C. After a thorough wash in 0.1 m phosphate‐buffered saline (PBS) with 0.3% Triton X‐100 (PBST), free‐floating sections were incubated with 3% normal donkey serum (NDS) with 0.3% Triton X‐100 for 20 min at room temperature. Sections were then incubated overnight at 4 °C with the primary antibody anti‐P2X4R (1 : 400) in PBST–NDS solution. Slices were washed three times with 1 × PBS to rinse off the excess primary antibody before incubation for 2 h at room temperature with Alexa 488 goat anti‐rabbit (Synaptic Systems, Göttingen, Germany) at 1 : 400 in PBST–NDS solution. After being washed three times with 1 × PBS, slices were fixed with fluoromont medium containing 4′,6‐diamidino‐2‐phenylindole. Fluorescent images were acquired with a Leica DM6000 TCS SP8X microscope, with a diode laser (excitation – blue, 405 nm; white, 488 nm; and emission – green, 500–560 nm). Images were obtained by mosaic acquisition of the whole hippocampus at ×20.
Statistical analysis
Values are presented as mean ± standard error of the mean (SEM), and n indicates the number of different cells. For statistical analysis, non‐parametric tests were used. Student's t‐test was used for comparison between two groups, and one‐way anova followed by a Newman–Keuls post hoc test for comparison between more than two groups. Statistical differences were considered to be significant at P < 0.05. Note that, although the impacts of several drugs and modulators are presented as percentage values for the sake of clarity, statistical comparisons were always carried out with the absolute values. Statistical analysis was performed with prism 5.0 (GraphPad Software, La Jolla, CA, USA).
Results
LPS‐treated microglia (LPS‐microglia) increase basal synaptic transmission at MF–CA3 synapses
We first studied the effect of activated microglia on basal synaptic transmission. For this purpose, N9 microglia were first activated by pretreatment with LPS (100 ng/mL for 1 h) (Gomes et al., 2013). Activated microglia were then added to acute hippocampal slices with previously described methodology (Centonze et al., 2009). In our experimental condition, microglia fully covered the whole hippocampus and surrounded almost every CA3 pyramidal cell (Fig. 1A and B). Although microglia do not penetrate deeply into the slice, we recorded CA3 pyramidal cells surrounded (10–100 μm) by an average of three to five microglia in close vicinity, i.e. similar to the estimated density of microglia in the CA3 area (Jinno et al., 2007). Consistent with previous observations (Centonze et al., 2009), no noticeable differences in morphology between non‐treated microglia (Fig. 1A) and LPS‐microglia (Fig. 1B) were observed. However, we have not carried out detailed morphological analysis to determine whether the physical interaction between LPS‐microglia and MF–CA3 synapses is similar to the interaction between endogenous microglia and MF–CA3 synapses.
Figure 1.
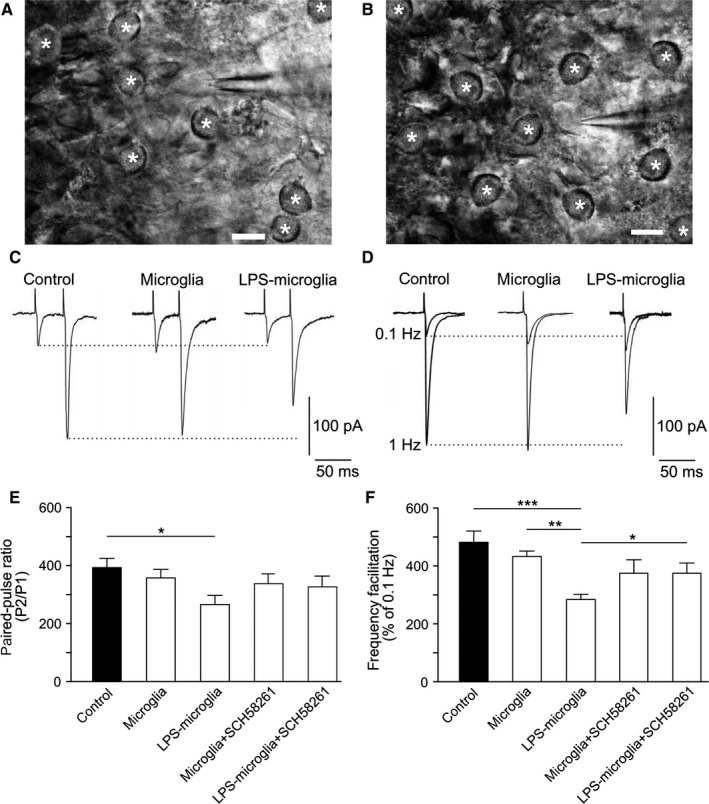
LPS‐microglia modulate short‐term synaptic plasticity via a microglial A2 AR. (A) Non‐treated microglia added to an acute hippocampal slice. A CA3 pyramidal cell was recorded in the whole cell configuration of the patch clamp technique, and is surrounded by microglia (*). Scale bar: 20 μm. (B) LPS‐microglia (*) added to an acute hippocampal slice. Scale bar: 20 μm. (C) Representative traces of paired‐pulse recordings obtained at 40 ms intervals, under different conditions. (D) Representative traces of EPSCs recorded at 0.1 Hz and 1 Hz, illustrating FF in different conditions. (E) Graphical representation of the PPR in different conditions. SCH58261 (50 nm) was added for 20 min prior to activation of microglia by LPS (control, 392 ± 32%; microglia, 357 ± 35%; LPS‐microglia, 277 ± 31%; microglia + SCH58261, 336 ± 28%; LPS‐microglia + SCH58261, 324 ± 31%; n = 9 for all conditions). (F) Graphical representation of FF in different conditions (control, 482 ± 39%; microglia, 430 ± 18%; LPS‐microglia, 281 ± 18%; microglia + SCH58261, 375 ± 39%; LPS‐microglia + SCH58261, 373 ± 27%; n = 9 for all conditions). FF and PPR protocols were performed one after another, on the same cell. Data are represented as mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 from one‐way anova followed by a Newmann–Keuls multiple comparison test.
To study basal synaptic transmission, MF‐CA3 EPSCs (MF‐EPSCs) were evoked with a minimal stimulation protocol (Marchal & Mulle, 2004). The average amplitude of MF‐EPSCs recorded at a low stimulation rate (0.1 Hz) (−73 ± 5 pA; n = 10) was consistent with previous observations (Marchal & Mulle, 2004). The addition of non‐treated microglia had no effect on basal synaptic transmission (−76 ± 12 pA; P = 0.8, n = 9) whereas the addition of LPS‐microglia increased basal synaptic transmission (−106 ± 12 pA; P < 0.05, n = 9; data not shown) (Table 1).
Table 1.
Comparison of basal transmission (BT), PPF and FF with respect to control (slice alone)
| Compared to control | Microglia | LPS‐microglia | SCH58261‐microglia | Apyrase | ARL67156 | DPCPX | CCPA | 5‐BDBD | Ivermectin | P2X4 KO |
|---|---|---|---|---|---|---|---|---|---|---|
| BT | – | ↑ | – | ↓ | – | ↑ | ↓ | ↓ | ↑ | ↓ |
| PPF | – | ↓ | – | – | – | – | – | ↓ | – | ↓ |
| FF | – | ↓ | – | – | ↓ | – | ↓ | – | ↓ | – |
For each experimental condition, values are normalized to control. ↑, increase; ↓, decrease; –, no effect.
LPS‐microglia decrease short‐term plasticity at MF‐CA3 synapses
MF‐CA3 synapses are distinct from most other synapses in the central nervous system, because they show prominent forms of presynaptic short‐term plasticity (Nicoll & Schmitz, 2005), including pronounced paired‐pulse facilitation (PPF) (two stimulations separated by 10–200 ms), represented by variation of the paired‐pulse ratio (PPR) (pulse 2/pulse 1), and frequency facilitation (FF) (tonic stimulation switching from 0.1 Hz to >1 Hz). Activity‐dependent neurotransmitter release among central nervous system synapses depends largely on the basal release probability, which is linked to the presynaptic calcium concentration. Hence, PPF at MF‐CA3 synapses has been reported to depend solely on the residual calcium concentration in the presynaptic terminal (Salin et al., 1996; Nicoll & Schmitz, 2005). MF‐EPSCs recorded from slices with added LPS‐microglia showed decreased PPF, estimated from the PPR, as compared with slices with no added microglia (LPS‐microglia, 277 ± 31%; control, 392 ± 32%; P < 0.05, n = 9; Fig. 1C and E) and slices with non‐treated microglia added (microglia, 357 ± 35%, n = 9; Fig. 1C and E). Added LPS‐microglia also significantly decreased FF as compared with control (LPS‐microglia, 281 ± 18%; control, 482 ± 39%; P < 0.001, n = 9; Fig. 1D and F) and slices with non‐treated microglia added (microglia, 430 ± 18%; P < 0.05, n = 9; Fig. 1D and F). As both PPF and FF were similar between control slices and slices with added non‐treated microglia, our results indicated that the decrease in short‐term plasticity observed with added LPS‐microglia was probably attributable to the immune challenging of microglia.
Inhibition of microglial A2ARs prevents changes in short‐term plasticity
The immune challenging of microglia with LPS (100 ng/mL) is known to drive microglia to release ATP, and this is abolished by blocking adenosine A2ARs on microglia (Gomes et al., 2013; George et al., 2015). We thus hypothesized that ATP released from LPS‐microglia was involved in the observed changes in short‐term synaptic plasticity. Taking advantage of the fact that neuronal presynaptic A2ARs do not, as such, modulate basal synaptic transmission or either PPF or FF at MF synapses (Rebola et al., 2008), we antagonized A2ARs on microglia by pre‐incubation with the A2AR antagonist SCH58261 (50 nm) for 20 min (Lopes et al., 2004) prior to activation of microglia by LPS and addition to acute slices.
The pre‐incubation of LPS‐microglia with SCH58261 significantly reduced the decrease in FF induced by the addition of LPS‐microglia (LPS‐microglia, 281 ± 18%; LPS‐microglia + SCH58261, 373 ± 27%; P < 0.05, n = 9) (Fig. 1F) but had no significant effect on the decrease in PPF (LPS‐microglia, 277 ± 31%; LPS‐microglia + SCH58261, 324 ± 31%; P = 0.65, n = 9) (Fig. 1E; Table 2). Thus, the blockade of A2ARs in LPS‐microglia alleviated some of the changes observed in MF‐CA3 short‐term synaptic plasticity. As we have previously shown that A2ARs control ATP release from microglia (George et al., 2015), this supports the hypothesis that ATP released from LPS‐microglia might impact on MF‐CA3 synaptic activity, leading to changes in short‐term synaptic plasticity.
Table 2.
Comparison of basal transmission (BT), PPF and FF with respect to LPS‐microglia (slice + LPS‐microglia)
| Compared to LPS‐microglia | SCH58261 | Apyrase | DPCPX | CCPA | 5‐BDBD | P2X4 KO |
|---|---|---|---|---|---|---|
| BT | ↓ | ↓ | ↑ | ↓ | ↓ | ↓ |
| PPF | – | ↑ | ↑ | – | – | ↑ |
| FF | ↑ | ↑ | – | – | ↑ | – |
For each experimental condition, values are normalixed to LPS‐microglia. ↑, increase; ↓, decrease; –, no effect.
LPS‐microglia regulate FF
To further validate the putative role of ATP, released by LPS‐microglia, in the observed changes in FF and PPF, we manipulated extracellular ATP by either promoting its degradation with apyrase or by blocking its extracellular degradation with ARL67156 (Frenguelli et al., 2007). Addition of apyrase (10 U/mL) decreased basal synaptic transmission at low frequency (0.1 Hz) within 5 min (control, 98 ± 1%; apyrase, 54 ± 1%; P < 0.001, n = 4) (Fig. 2A and B). In addition, apyrase abolished the decrease in FF induced by LPS‐microglia (LPS‐microglia, 281 ± 18%; LPS‐microglia + apyrase, 393 ± 24%; P < 0.01, n = 4) (Fig. 2E). Apyrase also abolished the decrease in PPF induced by LPS‐microglia (LPS‐microglia, 277 ± 31%; LPS‐microglia + apyrase, 466 ± 86%; P < 0.05, n = 4) (Fig. 2F). Conversely, ARL67156 was used in control slices (no microglia added) to determine whether increasing the endogenous levels of ATP in control conditions could mimic the alterations induced by LPS‐microglia. ARL67156 (100 μm) alone did not significantly affect the amplitude of MF‐EPSCs recorded at 0.1 Hz (control, 98 ± 1%; ARL67156, 115 ± 13%; P = 0.2, n = 4) (Fig. 2C and D), but reduced FF as LPS‐microglia did (control, 482 ± 39%; ARL67156, 183 ± 27%, P < 0.001; LPS‐microglia, 281 ± 18%, P < 0.001, n = 4) (Fig. 2G). In contrast, ARL67156 did not significantly affect PPF (control, 392 ± 32%; ARL67156, 374 ± 71%; P = 0.3, n = 3) (Fig. 2H).
Figure 2.
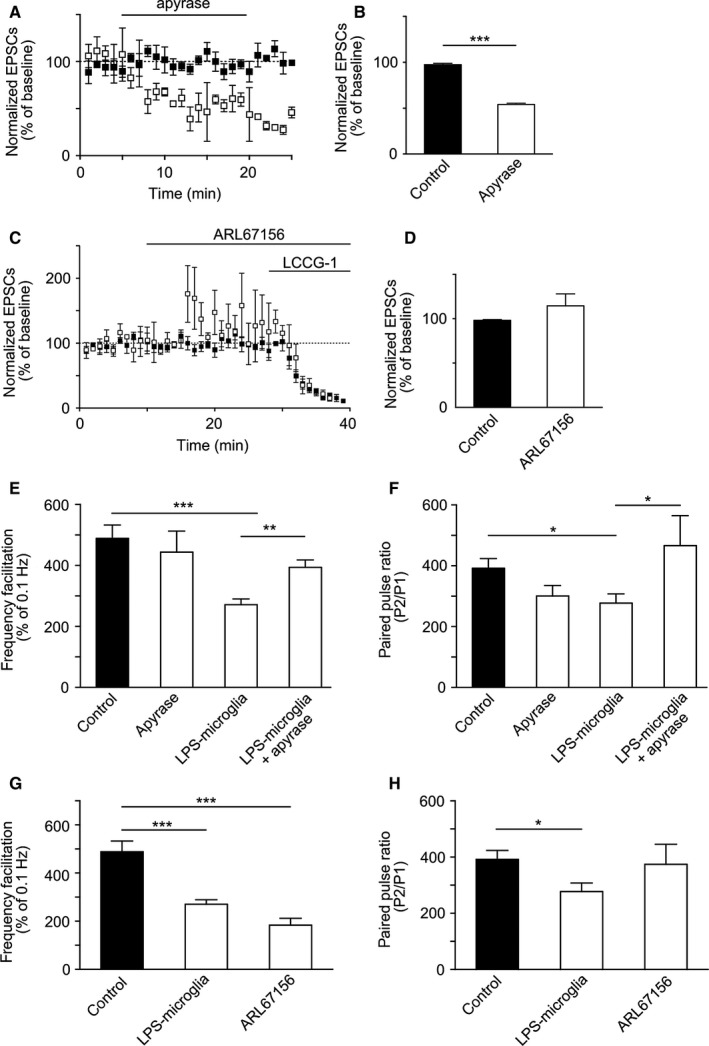
LPS‐microglia‐derived ATP modulates synaptic transmission and short‐term plasticity. (A) Summary plot of the effect of apyrase on basal synaptic transmission recorded at low frequency of stimulation (0.1 Hz). Apyrase (10 U/mL) induced a decrease in basal synaptic transmission as measured by the amplitude of EPSCs (control, black squares; apyrase, open squares). (B) Graphical representation of the basal synaptic transmission shown in A (control, 98 ± 1%, n = 9; apyrase, 54 ± 1%, n = 4). (C) Summary plot of the effect of ARL67156 (100 μm) on basal synaptic transmission recorded at low frequency of stimulation (0.1 Hz). ARL67156 did not significantly affect the amplitude of MF‐EPSCs within 10 min of addition (n = 4) (control, black squares; ARL67156, open squares). LCCG‐1 (10 μm), a metabotropic glutamate receptor 2 agonist, was added to confirm the selective stimulation of MFs. (D) Graphical representation of the basal synaptic transmission shown in C (control, 98 ± 1%, n = 9; ARL67156, 115 ± 13%, n = 4). (E) Graphical representation of FF. Apyrase abolished the effect of LPS‐microglia on FF (control, 482 ± 39%, n = 9; apyrase, 444 ± 69%, n = 4; LPS‐microglia, 281 ± 18%, n = 9; LPS‐microglia + apyrase, 393 ± 24%, n = 4). (F) Graphical representation of PPR. Apyrase abolished the reduction in PPR induced by LPS‐microglia (control, 392 ± 32%, n = 9; apyrase, 301 ± 34%, n = 4; LPS‐microglia, 277 ± 31%, n = 9; LPS‐microglia + apyrase, 466 ± 86%, n = 4). (G) Graphical representation of FF. Addition of ARL67156 (100 μm) mimicked the LPS‐microglia‐induced decrease in FF (control, 482 ± 39%, n = 9; LPS‐microglia, 281 ± 18%, n = 9; ARL67156, 183 ± 27%, n = 3). (H) Graphical representation of PPR. Increasing the extracellular level of ATP with ARL67156 (100 μm) had no effect on PPR (control, 392 ± 32%, n = 9; LPS‐microglia, 277 ± 31%, n = 9; ARL67156, 374 ± 71%, n = 3). Values in all graphs are mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 from one‐way anova followed by a Newmann–Keuls multiple comparison test.
Hence, the decrease in FF induced by LPS‐microglia was abolished by removal of extracellular ATP, whereas this decrease was mimicked by increased endogenous levels of ATP in control slices. In contrast, modulation of extracellular ATP did not have a significant effect on PPF; that is, manipulating the extracellular levels of ATP did not mimic or abolish the effects of LPS‐microglia.
Adenosine is involved in LPS‐microglia‐mediated regulation of PPF
Our data strongly suggest that ATP is the key molecule responsible for the microglia‐induced changes in presynaptic forms of short‐term plasticity. However, ATP is readily converted into adenosine by extracellular ectonucleotidases (Cunha et al., 1998), namely in synapses (Cunha, 2001), and presynaptic adenosine A1Rs are present at MF–CA3 synapses (Scanziani et al., 1992; Moore et al., 2003; Kukley et al., 2005; Rebola et al., 2008). Therefore, we investigated whether adenosine derived from the ATP released by LPS‐microglia could modulate MF–CA3 transmission.
We first studied the effects of CCPA (A1R agonist) and of DPCPX [A1R antagonist; see Sebastião et al. (2000)] on FF. As shown in Fig. 3B, DPCPX (100 nm) alone did not significantly change FF (control, 482 ± 39%; DPCPX, 426 ± 87%; P = 0.5, n = 9), in agreement with previous studies (Kukley et al., 2005; Rebola et al., 2008) [but see Moore et al. (2003)], and also had no effect on basal synaptic transmission (control, 147 ± 18%; DPCPX, 97 ± 3%; P = 0.06, n = 9; data not shown). DPCPX did not abolish the LPS‐microglia‐induced decrease in FF (LPS‐microglia, 281 ± 18%; LPS‐microglia + DPCPX, 250 ± 38%; P = 0.8, n = 9) (Fig. 3B). Furthermore, CCPA (100 nm) did not mimic the effect of LPS‐microglia on FF (CCPA, 399 ± 52%; LPS‐microglia, 281 ± 18%; P = 0.08, n = 9) (Fig. 3D). Finally, CCPA did not alter the LPS‐microglia‐induced decrease in FF (LPS‐microglia, 281 ± 18%; LPS‐microglia + CCPA, 230 ± 42%; P = 0.2, n = 9) (Fig. 3D). In contrast, DPCPX prevented the change in PPF induced by LPS‐microglia (LPS‐microglia, 277 ± 31%; LPS‐microglia + DPCPX, 383 ± 35%, P > 0.05, n = 9) (Fig. 3C). Conversely, CCPA decreased PPF when added to control slices (control, 392 ± 32%; CCPA, 269 ± 38%; P > 0.05, n = 9) (Fig. 3E), mimicking the effect of LPS‐microglia.
Figure 3.
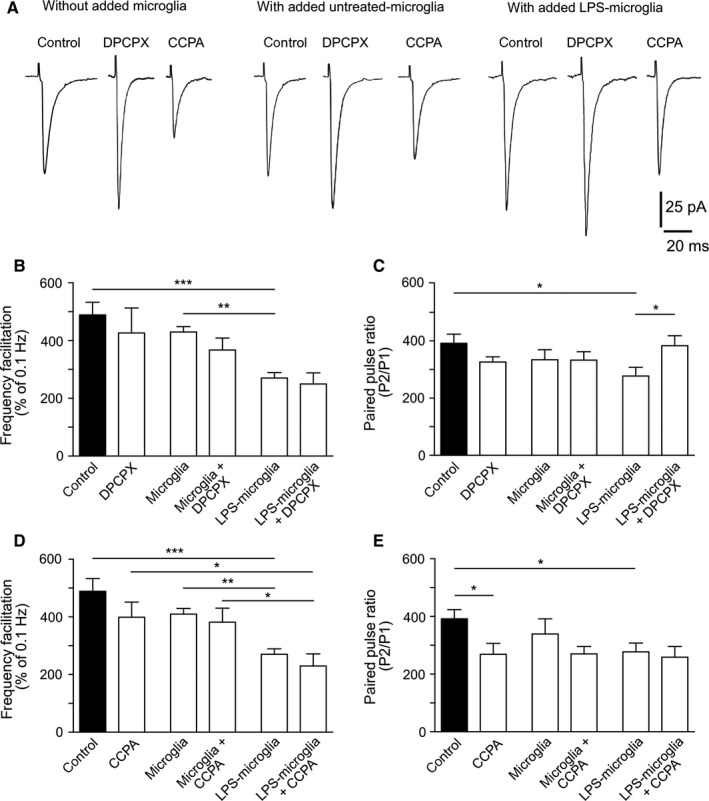
LPS‐microglia‐induced modulation of PPF involves adenosine and presynaptic A1Rs. (A) Representative traces of EPSCs triggered at a low frequency of stimulation (0.1 Hz). (B) Graphical representation of FF. Blockade of A1Rs by DPCPX (100 nm) had no effect on the LPS‐microglia‐induced change in FF (control, 482 ± 39%; DPCPX, 426 ± 87%; microglia, 430 ± 18%; microglia + DPCPX, 367 ± 41%; LPS‐microglia, 281 ± 18%; LPS‐microglia + DPCPX, 250 ± 38%; n = 9 for all conditions). (C) Graphical representation of the PPR. Blockade of A1Rs inhibited the reduction in the PPR induced by LPS‐microglia (control, 392 ± 32%; DPCPX, 326 ± 18%; microglia, 357 ± 35%; microglia + DPCPX, 333 ± 29%; LPS‐microglia, 277 ± 31%; LPS‐microglia + DPCPX, 383 ± 35%; n = 9 for all conditions). (D) Graphical representation of FF. CCPA (100 nm) did not alter the LPS‐microglia‐induced decrease in FF (control, 482 ± 39%; CCPA, 399 ± 52%; microglia, 430 ± 18%; microglia + CCPA, 382 ± 48%; LPS‐microglia, 281 ± 18%; LPS‐microglia + CCPA, 230 ± 42%; n = 9 for all conditions). (E) Graphical representation of the PPR. CCPA decreased the PPR, mimicking the effect of LPS‐microglia (control, 392 ± 32%; CCPA, 269 ± 38%; microglia, 357 ± 35%; microglia + CCPA, 270 ± 25%; LPS‐microglia, 277 ± 31%; LPS‐microglia + CCPA, 259 ± 37%; n = 9 for all conditions). Values in all graphs are mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 from one‐way anova followed by a Newmann–Keuls multiple comparison test.
Taken together, these results indicate that the level of extracellular adenosine in our experimental conditions was moderate, as estimated by the lack of a significant effect of DPCPX on basal synaptic transmission, and show that, whereas adenosine produced by the breakdown of released ATP from LPS‐microglia has no effect on FF, it modulates PPF.
P2X4Rs modulate short‐term synaptic plasticity
To further characterize the role of ATP derived from LPS‐microglia in the modulation of FF, we investigated which subtype of purinergic receptor was involved. ATP can bind to metabotropic (P2Y) and ionotropic (P2X) receptors. A role of P2X receptors has been reported in hippocampal synapses, mostly in the context of synaptic plasticity (Soto et al., 1996; Khakh et al., 1999; Pankratov et al., 2002; Priel & Silberberg, 2004; Sim et al., 2006; Pougnet et al., 2014). As shown in Fig. 4A, the effect of LPS‐microglia on FF was prevented by blocking P2X receptors with the broad‐spectrum antagonist PPADS (50 μm) (Lambrecht et al., 2002) (LPS‐microglia, 281 ± 18%; LPS‐microglia + PPADS, 427 ± 56%; P > 0.05, n = 9). In contrast, PPADS had no effect on the decrease in PPF induced by LPS‐microglia (LPS‐microglia, 277 ± 31%; LPS‐microglia + PPADS, 281 ± 22%; P = 0.9, n = 9) (Fig. 4B). These results provide evidence that P2X receptors specifically modulated FF, and not PPF.
Figure 4.
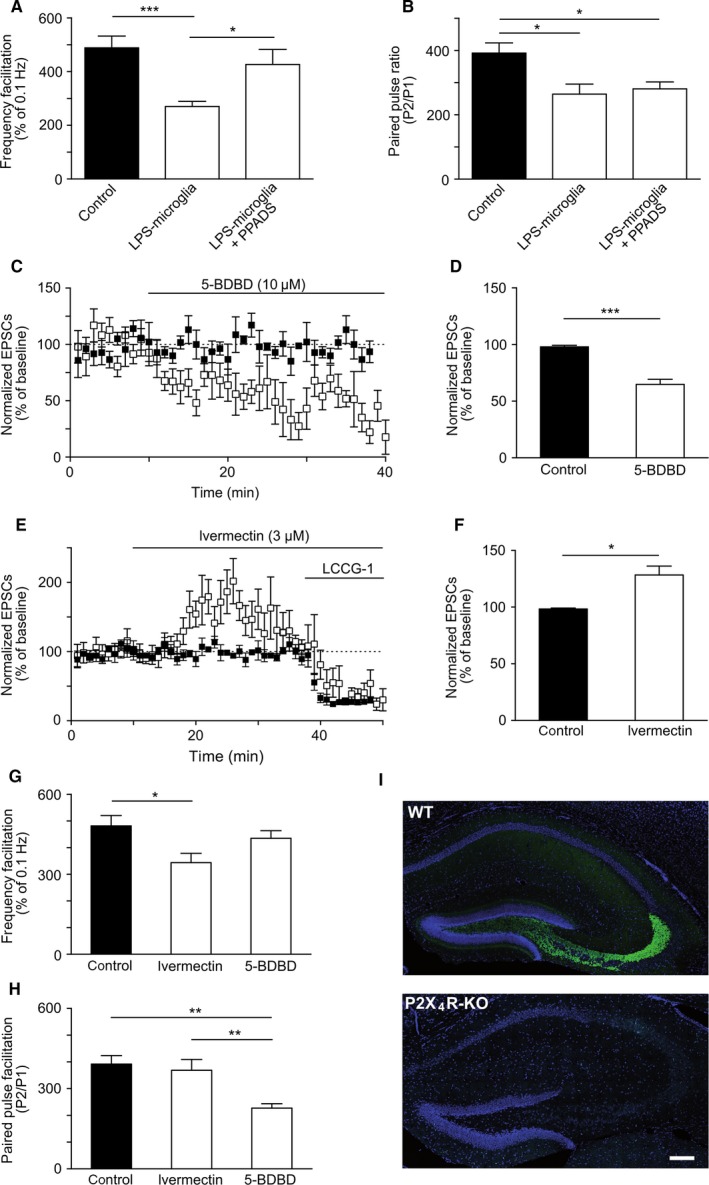
Extracellular ATP modulates basal synaptic transmission and FF via presynaptic P2X4Rs. (A) Graphical representation of FF. PPADS (50 μm) blocked the LPS‐microglia‐induced decrease in FF (control, 482 ± 39%; LPS‐microglia, 281 ± 18%; LPS‐microglia + PPADS, 427 ± 56%; n = 9 for all conditions). (B) Graphical representation of PPR. PPADS (50 μm) had no effect on the LPS‐microglia‐induced decrease in PPR (control, 392 ± 32%; LPS‐microglia, 277 ± 31%; LPS‐microglia + PPADS, 281 ± 22%; P = 0.9, n = 9 for all conditions). (C) Summary plot of the effect of 5‐BDBD (10 μm) on basal synaptic transmission. 5‐BDBD induced a decrease in basal synaptic transmission within 5 min of addition (control, black squares; 5‐BDBD, open squares). (D) Graphical representation of the basal synaptic transmission shown in C (control, 98 ± 1%; 5‐BDBD, 65 ± 4%; n = 9 for both conditions). (E) Summary plot of the effect of ivermectin (3 μm) on basal synaptic transmission. Ivermectin increased basal synaptic transmission within 10 min of addition (control, black squares; ivermectin, open squares). (F) Graphical representation of the basal synaptic transmission shown in E (control, 98 ± 1%, n = 9; ivermectin, 128 ± 7%, n = 6). The values in A, B, D and F are mean ± SEM; *P < 0.05 and ***P < 0.001, unpaired t‐test. (G) Graphical representation of FF. Ivermectin (3 μm) decreased FF, whereas 5‐BDBD (10 μm) had no effect (control, 482 ± 39%, n = 9; ivermectin, 344 ± 35%, n = 6; 5‐BDBD, 438 ± 25%, n = 9). (H) Graphical representation of PPR. 5‐BDBD (10 μm) decreased PPR, whereas ivermectin (3 μm) had no effect (control, 392 ± 32%, n = 9; ivermectin, 369 ± 40%, n = 6; 5‐BDBD, 227 ± 16%, n = 9). The values in G and H are mean ± SEM; *P < 0.05 and **P < 0.01 from one‐way anova followed by a Newmann–Keuls multiple comparison test. (I) The hippocampal MFs showed strong immunostaining for P2X4R (green) in a wild‐type (WT) mouse (upper panel), whereas it was virtually absent in a slice from a P2X4R KO mouse (lower panel). 4′,6‐Diamidino‐2‐phenylindole staining for nuclei is shown in blue. Scale bar: 200 μm.
We next investigated which subtype of P2X receptor was responsible for the effect of LPS‐microglia on FF. The presynaptic expression of P2X7Rs at MFs remains controversial (Armstrong et al., 2002; Sperlágh et al., 2002; Kukley et al., 2004; Yu et al., 2008), whereas the involvement of P2X4Rs in the modulation of synaptic strength (Baxter et al., 2011) and/or plasticity has been documented (Sim et al., 2006) at CA3–CA1 synapses. However, the presence of P2X4Rs at MF synapses remains to be studied. We first investigated whether P2X4Rs were involved in the modulation of basal synaptic transmission. Application of the selective P2X4R antagonist 5‐BDBD (10 μm) (Donnelly‐Roberts et al., 2008) decreased basal synaptic transmission at low frequency (0.1 Hz) within 5 min of its addition (control, 98 ± 1%; 5‐BDBD, 65 ± 4%; P < 0.001, n = 9) (Fig. 4C and D). Conversely, ivermectin (3 μm), a positive allosteric modulator of P2X4Rs that potentiates P2X4R currents (Khakh et al., 1999; Sim et al., 2006), increased basal synaptic transmission at low frequency (0.1 Hz) within 10 min of its addition (control, 98 ± 1%; ivermectin, 128 ± 7%; P < 0.05, n = 6) (Fig. 4E and F). Consistent with these results, basal synaptic transmission was also decreased in slices obtained from P2X4R knockout (KO) mice (control, 98 ± 1%; P2X4R KO, 85 ± 6%; P < 0.05, n = 9; data not shown). Therefore, ATP modulates basal synaptic transmission at MF‐CA3 synapses through P2X4Rs.
We then investigated the putative role of P2X4Rs in the modulation of FF. Selective blockade of P2X4Rs with 5‐BDBD on its own had no effect on FF (control, 482 ± 39%; 5‐BDBD, 438 ± 25%; P = 0.12, n = 9), whereas potentiation of P2X4Rs by ivermectin significantly decreased FF (control, 482 ± 39%; ivermectin, 344 ± 35%; P < 0.05, n = 9) (Fig. 4G). Conversely, 5‐BDBD on its own decreased PPF (control, 392 ± 32%; 5‐BDBD, 227 ± 16%; P < 0.01, n = 9), whereas ivermectin had no effect (control, 392 ± 32%; ivermectin, 369 ± 40%; P = 0.66, n = 9) (Fig. 4H).
These results provide good evidence that activation of P2X4Rs modulates FF but not PPF, and suggest a presynaptic site of action for ATP. To further strengthen the hypothesis of presynaptic localization of P2X4Rs, we used a new antibody raised against P2X4Rs and checked its specificity in slices from P2X4R KO mice. As shown in Fig. 4I, we observed strong immunostaining for P2X4R (green) on the MF tract (upper panel) that was almost completely absent in slices from P2X4R KO mice (lower panel). It is worth noting that we did not observe clear P2X4R immunoreactivity in the CA1 area, in contrast to previous reports (Rubio & Soto, 2001; Sim et al., 2006), suggesting the possibility that different antibodies may recognize different known molecular structures of homomeric P2X4Rs (Zemkova et al., 2015), different known heteromeric receptor complexes containing P2X4R subunits (Antonio et al., 2014), or P2X4Rs with different subcellular localizations (Xu et al., 2014).
P2X4Rs mediate the LPS‐microglia‐induced decrease in FF
We then investigated the possible involvement of P2X4Rs in the modulation of FF by LPS‐microglia. Blockade of P2X4Rs in hippocampal slices before the addition of LPS‐microglia prevented the decrease in FF (LPS‐microglia + 5‐BDBD, 378 ± 28%; LPS‐microglia, 281 ± 18%; P < 0.01, n = 9) (Fig. 5B) but did not alleviate the decrease in PPF (LPS‐microglia + 5‐BDBD, 242 ± 25%; LPS‐microglia, 277 ± 31%; P = 0.09, n = 9) (Fig. 5C). Because P2X4Rs are also dynamically involved in controlling endogenous microglia (Toulme & Khakh, 2012; Koizumi et al., 2013) as well as N9 microglia (Xiang & Burnstock, 2005), we investigated whether the effect of 5‐BDBD was attributable to P2X4Rs expressed at MF‐CA3 synapses or those expressed by N9 microglia added to the slice. For this purpose, LPS‐microglia were added to hippocampal slices obtained from P2X4R KO mice.
Figure 5.
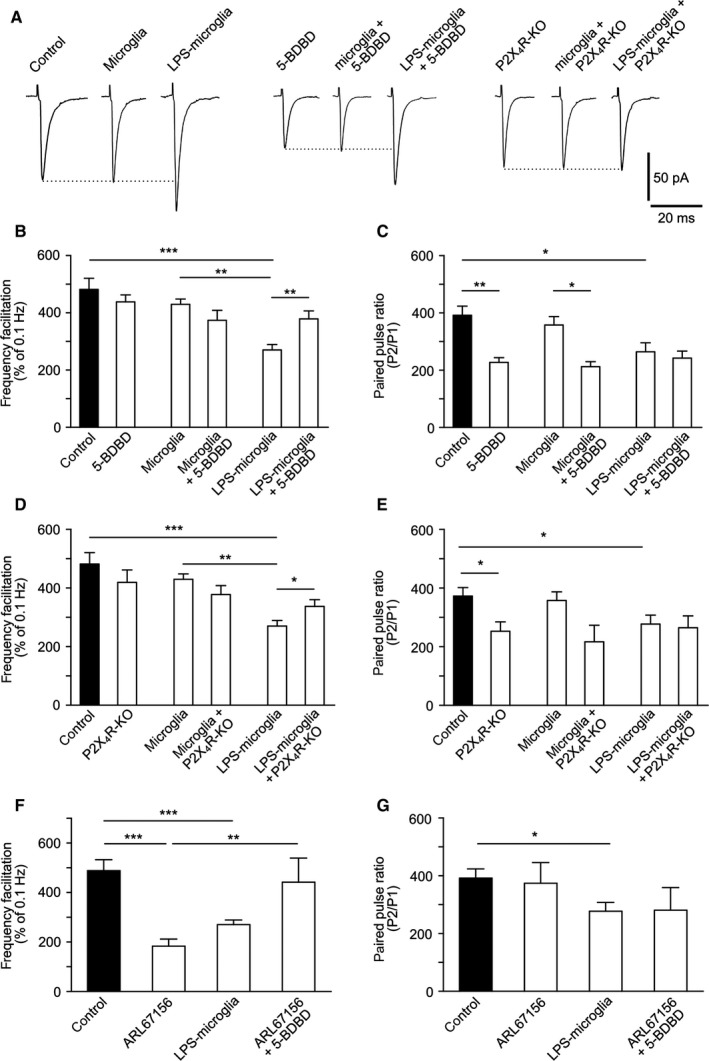
LPS‐microglia‐derived ATP regulates FF via P2X4Rs. (A) Representative traces of EPSCs triggered at low frequency of stimulation (0.1 Hz). (B) Graphical representation of FF. Blockade of P2X4Rs by 5‐BDBD (10 μm) inhibited the effect of LPS‐microglia on FF (control, 482 ± 39%; 5‐BDBD, 438 ± 25%; microglia, 430 ± 18%; microglia + 5‐BDBD, 374 ± 35%; LPS‐microglia, 281 ± 18%; LPS‐microglia + 5‐BDBD, 378 ± 28%; n = 9 for all conditions). (C) Graphical representation of the PPR. 5‐BDBD (10 μm) had no effect on the decrease in the PPR induced by LPS‐microglia, whereas it reduced it when applied alone (control, 392 ± 32%; 5‐BDBD, 227 ± 16%; microglia, 357 ± 35%; microglia + 5‐BDBD, 212 ± 18%; LPS‐microglia, 277 ± 31%; LPS‐microglia + 5‐BDBD, 242 ± 25%; n = 9 for all conditions). (D) Bar representation of FF. Genetic deletion of P2X4R (P2X4R KO) abolished the LPS‐microglia induced decrease in FF (control, 482 ± 39%; P2X4R KO, 419 ± 43%; microglia, 430 ± 18%; microglia + P2X4R KO, 377 ± 31%; LPS‐microglia, 281 ± 18%; LPS‐microglia + P2X4R KO, 337 ± 23%; n = 9 for all conditions). (E) Graphical representation of the PPR. The LPS‐microglia‐induced decrease in the PPR was preserved in P2X4R KO slices (control, 392 ± 32%; P2X4R KO, 253 ± 32%; microglia, 357 ± 35%; microglia + P2X4R KO, 216 ± 57%; LPS‐microglia, 277 ± 31%; LPS‐microglia + P2X4R KO, 265 ± 41%; n = 9 for all conditions). (F) Graphical representation of FF. Addition of ARL67156 (10 μm) mimicked the LPS‐microglia‐induced decrease in FF, whereas prior blockade of P2X4Rs by 5‐BDBD (10 μm) prevented this effect (control, 482 ± 39%, n = 9; ARL67156, 183 ± 29%, n = 3; LPS‐microglia, 281 ± 18%, n = 9; ARL67156 + 5‐BDBD, 442 ± 97%, n = 3). (G) Graphical representation of the PPR. Increasing the level of endogenous ATP with ARL67156 (10 μm) had no effect on the PPR (control, 392 ± 32%, n = 9; ARL67156, 324 ± 71%, n = 3; LPS‐microglia, 277 ± 31%, n = 9; ARL67156 + 5‐BDBD, 281 ± 78%, n = 3). Values in all graphs are mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 from one‐way anova followed by a Newmann–Keuls multiple comparison test.
The decrease in FF induced by LPS‐microglia was reduced in slices obtained from P2X4R KO mice (LPS‐microglia + P2X4R KO, 337 ± 23%; LPS‐microglia, 281 ± 18%; P < 0.05, n = 6) (Fig. 5D), whereas the decrease in PPF induced by LPS‐microglia was preserved in slices from P2X4R KO mice (LPS‐microglia + P2X4R KO, 265 ± 41%; LPS‐microglia, 277 ± 31%; P = 0.8, n = 9) (Fig. 5E).
The ectonucleotidase inhibitor ARL67156 (10 μm) mimicked the LPS‐microglia‐induced decrease in FF (control, 482 ± 39%, n = 9; LPS‐microglia, 281 ± 18%; P < 0.001, n = 9; ARL67156, 183 ± 29%; P < 0.001, n = 3) (Fig. 5F), whereas prior blockade of P2X4Rs by 5‐BDBD (10 μm) prevented this effect (ARL67156 + 5‐BDBD, 442 ± 97%; P < 0.02, n = 3) (Fig. 5F). In contrast, prior blockade of P2X4Rs by 5‐BDBD, before addition of ARL67156, had no further effect on PPF (Fig. 5G).
ATP and adenosine modulate synaptic plasticity independently of each other
Our data strongly suggest that extracellular ATP released from LPS‐microglia acts on P2X4Rs to modulate FF, and that adenosine catabolized from extracellular ATP acts on A1Rs to modulate PPF. Adenosine being the end‐product of ATP, we had to disentangle any possible indirect effects of ATP on PPF or adenosine on FF. For this purpose, FF and PPF were investigated in the presence of DPCPX in slices obtained from P2X4R KO mice. The LPS‐microglia‐induced decrease in FF (Fig. 6A and B) was reduced in P2X4R KO mice (LPS‐microglia, 281 ± 18%; LPS‐microglia + P2X4R KO, 337 ± 23%; P < 0.05, n = 9), but was preserved in the presence of DPCPX (LPS‐microglia + P2X4R KO + DPCPX, 402 ± 38%; P < 0.001, n = 5) (Fig. 6B). This indicates that A1Rs have no effect on the modulation of FF by P2X4Rs. Conversely, genetic deletion of P2X4Rs did not prevent the ability of DPCPX to block the LPS‐microglia‐induced decrease in PPF (Fig. 6C and D). Hence, ATP released by LPS‐microglia and its end‐product adenosine act independently on P2X4Rs and A1Rs, respectively, to modulate short‐term synaptic plasticity.
Figure 6.
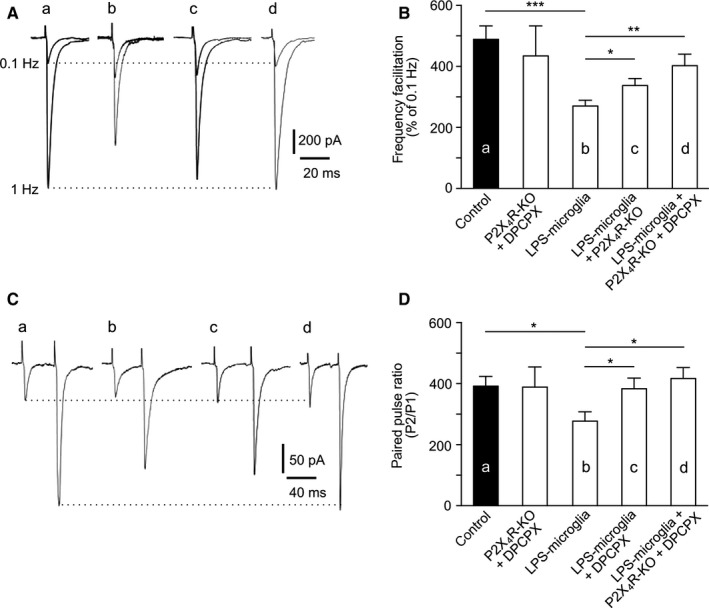
ATP and adenosine act independently on P2X4Rs and A1Rs. (A) Representative traces of EPSCs recorded at 0.1 Hz and 1 Hz, illustrating FF in different conditions. (a) Control. (b) LPS‐microglia. (c) LPS‐microglia + P2X4R KO. (d) LPS‐microglia + P2X4R KO + DPCPX. (B) Graphical representation of FF. Blockade of A1R by DPCPX (100 nm) had no effect on the inhibitory effect of P2X4R KO on the LPS‐microglia‐induced change in FF (control, 482 ± 39%, n = 9; P2X4R KO + DPCPX, 434 ± 98%, n = 5; LPS‐microglia, 281 ± 18%, n = 9; LPS‐microglia + P2X4R KO, 337 ± 23%, n = 9; LPS‐microglia + P2X4R KO + DPCPX, 402 ± 38%, n = 5). (C) Representative traces of EPSCs recorded at 0.1 Hz, illustrating the PPR in different conditions. (a) Control. (b) LPS‐microglia. (c) LPS‐microglia + DPCPX. (d) LPS‐microglia + P2X4R KO + DPCPX. (D) Graphical representation of the PPR. Genetic deletion of P2X4Rs had no effect on the DPCPX‐induced inhibition of the LPS‐microglia‐induced decrease in the PPR (control, 392 ± 32%, n = 9; P2X4R KO + DPCPX, 388 ± 66%, n = 5; LPS‐microglia, 277 ± 31%, n = 9; LPS‐microglia + DPCPX, 383 ± 35%, n = 9; LPS‐microglia + P2X4R KO + DPCPX, 417 ± 36%, n = 5). Values in all graphs are mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 from one‐way anova followed by a Neumann–Keuls multiple comparison test.
Discussion
Over the last 10 years, the notion of microglia as passive sentinels within the brain has been noticeably widened, and microglia are now acknowledged to constitute a new partner in synaptic function (Tremblay et al., 2010) [reviewed in Kettenmann et al. (2013)]. Indeed, microglia can secrete a full array of neuroactive molecules, including several proinflammatory cytokines (interleukin‐1β, interleukin‐6, and tumour necrosis factor‐α), trophic factors such as brain‐derived neurotrophic factor, and also neurotransmitters such as ATP, glycine, and glutamate [reviewed in Kettenmann et al. (2011)]. In the present study, we focused on the putative effect of microglia‐derived purines on synaptic function at MF‐CA3 synapses. In this experimental paradigm, N9 microglia were previously challenged with an immune stimulus (LPS) to induce them to release ATP (George et al., 2015), and were then added to hippocampal slices.
Combining pharmacological, electrophysiological and immunohistochemical approaches, our study demonstrated that ATP released by LPS‐microglia modulates MF‐CA3 synaptic transmission both directly via presynaptic P2X4Rs and through its metabolite adenosine, by acting on presynaptic A1Rs. Each pathway selectively modulates a different type of short‐term presynaptic plasticity at MF‐CA3 synapses, namely FF for P2X4Rs and PPF for A1Rs. This study reveals complex crosstalk between microglial release of ATP, purinergic receptor activation, and hippocampal synaptic function.
P2X4Rs modulate basal synaptic transmission
Basal synaptic transmission at MF‐CA3 synapses is governed by presynaptic intracellular calcium homeostasis and cAMP levels (Nicoll & Schmitz, 2005). Within the ionotropic P2X receptor family, P2X4Rs show the highest permeability for calcium (Soto et al., 1996; Burnstock et al., 2011), and, through this property, P2X4Rs can modulate basal synaptic transmission. In good agreement with this, the blockade of P2X4Rs by 5‐BDBD or their genetic deletion in P2X4R KO mice decreased basal synaptic transmission, whereas ivermectin, which selectively potentiates ATP‐induced currents at P2X4Rs (Khakh et al., 1999; Priel & Silberberg, 2004), increased it. As expected, the degradation of extracellular ATP by apyrase also decreased basal synaptic transmission. All together, these results suggest that extracellular ATP tonically modulates basal synaptic transmission, most likely by increasing the presynaptic calcium concentration via its influx through P2X4Rs.
Interestingly, the end‐product of ATP catabolism, i.e. adenosine, by acting on presynaptic A1Rs, also tonically decreases basal synaptic transmission (Moore et al., 2003; Kukley et al., 2005; Rebola et al., 2008), probably through the direct A1R inhibition of calcium influx via presynaptic P/Q‐type and N‐type calcium channels (Gundlfinger et al., 2007). Our results suggest that purines modulate basal synaptic transmission via a complex interplay between ATP and adenosine signalling, which produce opposite effects.
Microglia‐derived ATP impacts on FF
Owing to their unusual low initial glutamate release probability, MF‐CA3 synapses show a large facilitation of response amplitudes of up to 500–600% when the frequency of stimulation is increased from 0.1 Hz to 1 Hz. This unique form of short‐term plasticity, termed FF, was decreased by 40–50% following the addition of LPS‐microglia. Several lines of evidence clearly demonstrate that ATP produced by LPS‐microglia and acting on P2X4Rs was responsible for this effect: first, the addition of unstimulated microglia to acute slices had no effect on FF; second, the decrease in FF following the addition of LPS‐microglia was prevented by apyrase but potentiated by ARL67156; third, it was blocked by PPADS, a broad‐spectrum P2X receptor antagonist, but also by 5‐BDBD; fourth, it was mimicked by ivermectin; fifth, it was absent in P2X4R KO mice; and sixth, it was insensitive to the A1R ligands DPCPX (antagonist) and CCPA (agonist), in agreement with previous work (Rebola et al., 2008).
It is not known for certain how FF is governed at MF‐CA3 synapses, and different mechanisms have been proposed, such as the involvement of calcium/calmodulin‐dependent protein kinase II (Salin et al., 1996), presynaptic kainate receptors (Contractor et al., 2001), or presynaptic voltage‐dependent calcium channels (Gundlfinger et al., 2007). Our results show that P2X4Rs only control FF in the presence of sufficient amounts of extracellular ATP, which are probably provided by activated microglia, whereas, in the absence of LPS‐microglia, FF is insensitive to P2X4R blockade.
Microglia‐derived adenosine but not ATP impacts on PPF
The decrease in PPF induced by LPS‐microglia, which was preserved in the presence of PPADS or 5‐BDBD but also in P2X4R KO slices, strongly suggests that adenosine but not ATP was involved in this effect. Activation of A1Rs at MF‐CA3 synapses is known to inhibit synaptic transmission (Scanziani et al., 1992; Moore et al., 2003; Kukley et al., 2005), and its presynaptic localization has been confirmed by electron microscopy (Rebola et al., 2008). The blockade by DPCPX of the decrease in PPF induced by LPS‐microglia and its mimicking by CCPA are good evidence that adenosine resulting from the degradation of microglia‐derived ATP modulates PPF. Unexpectedly, the blockade of P2X4Rs by 5‐BDBD on its own also reduced PPF. This could indicate that endogenous ATP in the presence of receptors blocked by 5‐BDBD would be more readily available to be converted into adenosine, activating A1R and leading to a decrease in PPF. Hence, PPF was not affected in P2X4R KO mice, supporting our contention that the effect of 5‐BDBD involves greater availability of adenosine acting on A1Rs rather than an effect of P2X4R.
Owing to a very low initial release probability, the PPF at MF‐CA3 synapses is assumed to be directly linked to the probability of glutamate release, which is dependent on the residual calcium concentration in the presynaptic terminal (Salin et al., 1996; Nicoll & Schmitz, 2005). Therefore, one could expect that microglia‐derived adenosine acting on A1Rs by reducing calcium influx into MF terminals (Gundlfinger et al., 2007) would decrease PPF instead of increasing it. However, conflicting results have been reported for the impact of A1R activation on PPF at MF‐CA3 synapses, ranging from a reduction by half of PPF by DPCPX (Moore et al., 2003) to no effect at all (Kukley et al., 2005). These opposite results were attributed to differences in the ambient levels of adenosine in slices. Our results are consistent with a moderate level of ambient adenosine, as shown by the weak increase in basal synaptic transmission caused by DPCPX, similarly to what was previously reported (Kukley et al., 2005; Rebola et al., 2008), and by the lack of a significant effect on PPF.
Pathophysiological relevance of microglia‐derived purine modulation of MF‐CA3 synaptic plasticity
There is compelling evidence that the purinergic system is instrumental in neuroinflammation (Di Virgilio et al., 2009), which can impact on synaptic function (Ben Achour & Pascual, 2010; Pascual et al., 2012). ATP is a major trigger of microglia activation through P2X4Rs and P2X7 receptors (Di Virgilio et al., 2009; Koizumi et al., 2013), and adenosine also regulates microglia responses through the activation of A2ARs in vivo (Rebola et al., 2011), by controlling the microglial production of inflammatory cytokines, and growth factors such as BDNF and ATP (Gomes et al., 2013; George et al., 2015). By taking advantage of a simple paradigm based on the addition of activated N9 microglia releasing ATP, the present study provides evidence that microglia‐derived purines in a neuroinflammatory paradigm modulate, via presynaptic P2X4Rs and A1Rs, different forms of presynaptic short‐term plasticity at MF‐CA3 synapses. However, the simplicity of the paradigm used also introduced some limitations in the mechanistic process underlying this control of short‐term plasticity by microglia‐derived ATP. Indeed, our experimental approach does not allow us to rule out the participation of endogenous microglia, astrocytes and/or neurons in possible amplification of the N9 microglia‐derived ATP to control presynaptic short‐term plasticity at MF‐CA3 synapses. In this respect, a recent study showed that the production of tumour necrosis factor‐α and ATP by microglia was driving nearby astrocytes to release ATP, promoting, in turn, astroglial release of glutamate and a subsequent increase in CA1 synaptic transmission in acute hippocampal slices (Pascual et al., 2012). However, this documented ATP‐induced ATP release from astrocytes occurs only for ATP concentrations in the high micromolar range (Anderson et al., 2004), whereas we have previously estimated that N9 microglia release nanomolar concentrations of ATP (George et al., 2015). Certainly, future studies will be required to unravel the intricate interplay between different cell types in the purinergic control of presynaptic short‐term plasticity at MF‐CA3 synapses triggered by a neuroinflammation‐associated increase in the concentration of microglia‐derived ATP.
The CA3 subregion of the hippocampus has been reported to be involved in the encoding, storage and subsequent retrieval of memories (Nakazawa et al., 2003; Bischofberger et al., 2006; Kesner, 2007). Owing to the sparse connectivity between dentate gyrus granule cells and CA3 pyramidal neurons and the prominent ability of MF‐CA3 synapses to facilitate markedly the MF pathway has been particularly involved in the pattern separation process (Bischofberger et al., 2006; Gilbert & Kesner, 2006; Leutgeb et al., 2007; Neves et al., 2008). Therefore, a decrease in short‐term plasticity induced by neuroinflammation could impact on the pattern separation process. Relevant to this, it is well known that murine models of Alzheimer's disease, which show prominent neuroinflammation, have impaired spatial memory, as is also found in Alzheimer's patients. It would be interesting to use behavioural paradigms to test the pattern separation process in mice with conditional deletion of P2X4Rs and/or A1Rs in granule cells, in order to test the possible involvement of this subnetwork.
Acknowledgements
The authors wish to thank Catarina V. Gomes for carrying out the initial experiments to optimize the protocol for adhesion of N9 cells to hippocampal slices, François Rassendren for his generous gift of P2X4R KO mice, Virginie Labrousse for her help with the immunohistochemistry of P2X4Rs, and Eric Boué‐Grabot for his help with the pharmacology of P2X4Rs. The microscopy was done in the Bordeaux Imaging Center (BIC), a service unit of the CNRS‐Inserm and Bordeaux University, member of the national infrastructure France BioImaging.
Abbreviations
- 5‐BDBD
5‐(3‐bromophenyl)‐1,3‐dihydro‐2H‐benzofuro[3,2‐e]‐1,4‐diazepin‐2‐one
- A1R
A1 receptor
- A2AR
A2A receptor
- CCPA
2‐chloro‐N 6‐cyclopentyladenosine
- DPCPX
8‐cyclopentyl‐1,3‐dipropylxanthine
- EPSC
excitatory postsynaptic current
- FF
frequency facilitation
- KO
knockout
- LPS
lipopolysaccharide
- LPS‐microglia
lipopolysaccharide‐treated microglia
- MF
mossy fibre
- MF‐EPSC
mossy fibre‐CA3 excitatory postsynaptic current
- NDS
normal donkey serum
- P
postnatal day
- PBS
phosphate‐buffered saline
- PBST
0.1 m phosphate‐buffered saline with 0.3% Triton X‐100
- PPADS
pyridoxalphosphate‐6‐azophenyl‐2′,4′‐disulfonic acid
- PPF
paired‐pulse facilitation
- PPR
paired‐pulse ratio
- P2X4R
P2X4 receptor
- SEM
standard error of the mean
References
- Anderson, C.M. , Bergher, J.P. & Swanson, R.A. (2004) ATP‐induced ATP release from astrocytes. J. Neurochem., 88, 246–256. [DOI] [PubMed] [Google Scholar]
- Antonio, L.S. , Stewart, A.P. , Varanda, W.A. & Edwardson, J.M. (2014) Identification of P2X2/P2X4/P2X6 heterotrimeric receptors using atomic force microscopy (AFM) imaging. FEBS Lett., 588, 2125–2128. [DOI] [PubMed] [Google Scholar]
- Armstrong, J. , Brust, T. , Lewis, R. & MacVicar, B. (2002) Activation of presynaptic P2X7‐like receptors depresses mossy fiber–CA3 synaptic transmission through p38 mitogen‐activated protein kinase. J. Neurosci., 22, 5938–5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter, A.W. , Choi, S.J. , Sim, J.A. & North, R.A. (2011) Role of P2X4 receptors in synaptic strengthening in mouse CA1 hippocampal neurons. Eur. J. Neurosci., 34, 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Achour, S. & Pascual, O. (2010) Glia: the many ways to modulate synaptic plasticity. Neurochem. Int., 57, 440–445. [DOI] [PubMed] [Google Scholar]
- Bessis, A. , Béchade, C. , Bernard, D. & Roumier, A. (2007) Microglial control of neuronal death and synaptic properties. Glia, 55, 233–238. [DOI] [PubMed] [Google Scholar]
- Bischofberger, J. , Engel, D. , Frotscher, M. & Jonas, P. (2006) Timing and efficacy of transmitter release at mossy fiber synapses in the hippocampal network. Eur. J. Physiol., 453, 361–372. [DOI] [PubMed] [Google Scholar]
- Burnstock, G. , Fredholm, B. & Verkhratsky, A. (2011) Adenosine and ATP receptors in the brain. Curr. Top. Med. Chem., 11, 973–1011. [DOI] [PubMed] [Google Scholar]
- Butt, A.M. (2011) ATP: a ubiquitous gliotransmitter integrating neuron–glial networks. Semin. Cell Dev. Biol., 22, 205–213. [DOI] [PubMed] [Google Scholar]
- Centonze, D. , Muzio, L. , Rossi, S. , Cavasinni, F. , De Chiara, V. , Bergami, A. , Musella, A. , D'Amelio, M. , Cavallucci, V. , Martorana, A. , Bergamaschi, A. , Cencioni, M.T. , Diamantini, A. , Butti, E. , Comi, G. , Bernardi, G. , Cecconi, F. , Battistini, L. , Furlan, R. & Martino, G. (2009) Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J. Neurosci., 29, 3442–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor, A. , Swanson, G. & Heinemann, S. (2001) Kainate receptors are involved in short‐ and long‐term plasticity at mossy fiber synapses in the hippocampus. Neuron, 29, 209–216. [DOI] [PubMed] [Google Scholar]
- Costenla, A.R. , Diógenes, M.J. , Canas, P.M. , Rodrigues, R.J. , Nogueira, C. , Maroco, J. , Agostinho, P.M. , Ribeiro, J.A. , Cunha, R.A. & de Mendonça, A. (2011) Enhanced role of adenosine A2A receptors in the modulation of LTP in the rat hippocampus upon aging. Eur. J. Neurosci., 34, 12–21. [DOI] [PubMed] [Google Scholar]
- Cunha, R.A. (2001) Regulation of the ecto‐nucleotidase pathway in rat hippocampal nerve terminals. Neurochem. Res., 26, 979–991. [DOI] [PubMed] [Google Scholar]
- Cunha, R.A. , Sebastião, A.M. & Ribeiro, J.A. (1998) Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ecto‐nucleotidases into adenosine and channeling to adenosine A1 receptors. J. Neurosci., 18, 1987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio, F. , Ceruti, S. , Bramanti, P. & Abbracchio, M.P. (2009) Purinergic signalling in inflammation of the central nervous system. Trends Neurosci., 32, 79–87. [DOI] [PubMed] [Google Scholar]
- Dias, R. , Ribeiro, J. & Sebastião, A. (2012) Enhancement of AMPA currents and GluR1 membrane expression through PKA‐coupled adenosine A2A receptors. Hippocampus, 22, 276–291. [DOI] [PubMed] [Google Scholar]
- Donnelly‐Roberts, D. , McGaraughty, S. , Shieh, C. , Honore, P. & Jarvis, M.F. (2008) Painful purinergic receptors. J. Pharmacol. Exp. Ther., 324, 409–415. [DOI] [PubMed] [Google Scholar]
- Dunwiddie, T.V. & Masino, S.A. (2001) The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci., 24, 31–55. [DOI] [PubMed] [Google Scholar]
- Fredholm, B. , IJzerman, A. , Jacobson, K. , Linden, J. & Müller, C. (2011) International Union of Basic and Clinical Pharmacology. Nomenclature and classification of adenosine receptors – an update. Pharmacol. Rev., 63, 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenguelli, B.G. , Wigmore, G. , Llaudet, E. & Dale, N. (2007) Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J. Neurochem., 101, 1400–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, J. , Gonçalves, F.Q. , Cristóvão, G. , Rodrigues, L. , Meyer Fernandes, J.R. , Gonçalves, T. , Cunha, R.A. & Gomes, C.A. (2015) Different danger signals differently impact on microglial proliferation through alterations of ATP release and extracellular metabolism. Glia, 63, 1636–1645. [DOI] [PubMed] [Google Scholar]
- Gilbert, P. & Kesner, R. (2006) The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behav. Brain Res., 169, 142–149. [DOI] [PubMed] [Google Scholar]
- Gomes, C. , Ferreira, R. , George, J. , Sanches, R. , Rodrigues, D.I. , Gonçalves, N. & Cunha, R.A. (2013) Activation of microglial cells triggers a release of brain‐derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor‐dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J. Neuroinflamm., 10, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlfinger, A. , Bischofberger, J. , Johenning, F.W. , Torvinen, M. , Schmitz, D. & Breustedt, J. (2007) Adenosine modulates transmission at the hippocampal mossy fibre synapse via direct inhibition of presynaptic calcium channels. J. Physiol., 582, 263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno, S. , Fleischer, F. , Eckel, S. , Schmidt, V. & Kosaka, T. (2007) Spatial arrangement of microglia in the mouse hippocampus: a stereological study in comparison with astrocytes. Glia, 55, 1334–1347. [DOI] [PubMed] [Google Scholar]
- Kesner, R. (2007) Behavioral functions of the CA3 subregion of the hippocampus. Learn Memory, 14, 771–781. [DOI] [PubMed] [Google Scholar]
- Kettenmann, H. , Hanisch, U. , Noda, M. & Verkhratsky, A. (2011) Physiology of microglia. Physiol. Rev., 91, 461–553. [DOI] [PubMed] [Google Scholar]
- Kettenmann, H. , Kirchhoff, F. & Verkhratsky, A. (2013) Microglia: new roles for the synaptic stripper. Neuron, 77, 10–18. [DOI] [PubMed] [Google Scholar]
- Khakh, B.S. , Proctor, W.R. , Dunwiddie, T.V. , Labarca, C. & Lester, H.A. (1999) Allosteric control of gating and kinetics at P2X4 receptor channels. J. Neurosci., 19, 7289–7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh, B.S. , Gittermann, D. , Cockayne, D. & Jones, A. (2003) ATP modulation of excitatory synapses onto interneurons. J. Neurosci., 23, 7426–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi, S. , Ohsawa, K. , Inoue, K. & Kohsaka, S. (2013) Purinergic receptors in microglia: functional modal shifts of microglia mediated by P2 and P1 receptors. Glia, 61, 47–54. [DOI] [PubMed] [Google Scholar]
- Kukley, M. , Stausberg, P. , Adelmann, G. , Chessell, I. & Dietrich, D. (2004) Ecto‐nucleotidases and nucleoside transporters mediate activation of adenosine receptors on hippocampal mossy fibers by P2X7 receptor agonist 2′‐3′‐O‐(4‐benzoylbenzoyl)‐ATP. J. Pharmacol. Exp. Ther., 324, 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley, M. , Schwan, M. , Fredholm, B.B. & Dietrich, D. (2005) The role of extracellular adenosine in regulating mossy fiber synaptic plasticity. J. Neurosci., 25, 2832–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht, G. , Braun, K. , Damer, M. , Ganso, M. , Hildebrandt, C. , Ullmann, H. , Kassack, M.U. & Nickel, P. (2002) Structure–activity relationships of suramin and pyridoxal‐5′‐phosphate derivatives as P2 receptor antagonists. Curr. Pharm. Des., 8, 2371–2399. [DOI] [PubMed] [Google Scholar]
- Leutgeb, J. , Leutgeb, S. , Moser, M. & Moser, E. (2007) Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315, 961–966. [DOI] [PubMed] [Google Scholar]
- Lopes, L.V. , Halldner, L. , Rebola, N. , Johansson, B. , Ledent, C. , Chen, J.F. , Fredholm, B.B. & Cunha, R.A. (2004) Binding of the prototypical adenosine A2A receptor agonist CGS 21680 to the cerebral cortex of adenosine A1 and A2A receptor knockout mice. Br. J. Pharmacol., 141, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal, C. & Mulle, C. (2004) Postnatal maturation of mossy fibre excitatory transmission in mouse CA3 pyramidal cells: a potential role for kainate receptors. J. Physiol., 561, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos, M. , Augusto, E. , Santos‐Rodrigues, A. , Schwarzschild, M.A. , Chen, J.F. , Cunha, R.A. & Agostinho, P.M. (2012) Adenosine A2A receptors modulate glutamate uptake in cultured astrocytes and gliosomes. Glia, 60, 702–716. [DOI] [PubMed] [Google Scholar]
- Moore, K.A. , Nicoll, R.A. & Schmitz, D. (2003) Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc. Natl. Acad. Sci. USA, 100, 14397–14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa, K. , Sun, L. , Quirk, M. , Rondi‐Reig, L. , Wilson, M. & Tonegawa, S. (2003) Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one‐time experience. Neuron, 38, 305–315. [DOI] [PubMed] [Google Scholar]
- Neary, J. & Zimmermann, H. (2009) Trophic functions of nucleotides in the central nervous system. Trends Neurosci., 32, 189–198. [DOI] [PubMed] [Google Scholar]
- Neves, G. , Cooke, S.F. & Bliss, T.V. (2008) Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat. Rev. Neurosci., 9, 65–75. [DOI] [PubMed] [Google Scholar]
- Nicoll, R. & Schmitz, D. (2005) Synaptic plasticity at hippocampal mossy fibre synapses. Nat. Rev. Neurosci., 6, 863–876. [DOI] [PubMed] [Google Scholar]
- Pankratov, Y.V. , Lalo, U.V. & Krishtal, O.A. (2002) Role for P2X receptors in long‐term potentiation. J. Neurosci., 22, 8363–8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual, O. , Ben Achour, S. , Rostaing, P. , Triller, A. & Bessis, A. (2012) Microglia activation triggers astrocyte‐mediated modulation of excitatory neurotransmission. Proc. Natl. Acad. Sci. USA, 109, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pougnet, J. , Toulme, E. , Martinez, A. , Choquet, D. , Hosy, E. & Boué‐Grabot, E. (2014) ATP P2X receptors downregulate AMPA receptor trafficking and postsynaptic efficacy in hippocampal neurons. Neuron, 16, 417–430. [DOI] [PubMed] [Google Scholar]
- Priel, A. & Silberberg, S. (2004) Mechanism of ivermectin facilitation of human P2X4 receptor channels. J. Gen. Physiol., 123, 281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola, N. , Sebastião, A. , de Mendonça, A. , Oliveira, C. , Ribeiro, J. & Cunha, R.A. (2003) Enhanced adenosine A2A receptor facilitation of synaptic transmission in the hippocampus of aged rats. J. Neurophysiol., 90, 1295–1303. [DOI] [PubMed] [Google Scholar]
- Rebola, N. , Canas, P. , Oliveira, C. & Cunha, R. (2005) Different synaptic andsubsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience, 132, 893–903. [DOI] [PubMed] [Google Scholar]
- Rebola, N. , Lujan, R. , Cunha, R. & Mulle, C. (2008) Adenosine A2A receptors are essential for long‐term potentiation of NMDA‐EPSCs at hippocampal mossy fiber synapses. Neuron, 57, 121–134. [DOI] [PubMed] [Google Scholar]
- Rebola, N. , Simões, A.P. , Canas, P.M. , Tomé, A.R. , Andrade, G.M. , Barry, C.E. , Agostinho, P.M. , Lynch, M.A. & Cunha, R.A. (2011) Adenosine A2A receptors control neuroinflammation and consequent hippocampal neuronal dysfunction. J. Neurochem., 117, 100–111. [DOI] [PubMed] [Google Scholar]
- Rodrigues, R.J. , Almeida, T. , Richardson, P.J. , Oliveira, C.R. & Cunha, R.A. (2005) Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3 and P2X3, and inhibitory P2Y1, P2Y2 and/or P2Y4 receptors in the rat hippocampus. J. Neurosci., 27, 6286–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, R.J. , Tomé, A.R. & Cunha, R.A. (2015) ATP as a multi‐target danger signal in the brain. Front. Neurosci., 9, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumier, A. , Pascual, O. , Béchade, C. , Wakselman, S. , Poncer, J. , Réal, E. , Triller, A. & Bessis, A. (2008) Prenatal activation of microglia induces delayed impairment of glutamatergic synaptic function. PLoS One, 3, e2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio, M.E. & Soto, F. (2001) Distinct Localization of P2X receptors at excitatory postsynaptic specializations. J. Neurosci., 21, 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidhanandam, S. , Blanchet, C. , Jeantet, Y. , Cho, Y.H. & Mulle, C. (2009) Kainate receptors act as conditional amplifiers of spike transmission at hippocampal mossy fiber synapses. J. Neurosci., 29, 5000–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin, P. , Scanziani, M. , Malenka, R. & Nicoll, R. (1996) Distinct short‐term plasticity at two excitatory synapses in the hippocampus. Proc. Natl. Acad. Sci. USA, 93, 13304–13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani, M. , Capogna, M. , Gähwiler, B. & Thompson, S. (1992) Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron, 9, 919–927. [DOI] [PubMed] [Google Scholar]
- Sebastião, A.M. , Cunha, R.A. , de Mendonça, A. & Ribeiro, J.A. (2000) Modification of adenosine modulation of synaptic transmission in the hippocampus of aged rats. Br. J. Pharmacol., 131, 1629–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim, J. , Chaumont, S. , Jo, J. , Ulmann, L. , Young, M. , Cho, K. , Buell, G. , North, R. & Rassendren, F. (2006) Altered hippocampal synaptic potentiation in P2X4 knockout mice. J. Neurosci., 26, 9006–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto, F. , Garcia‐Guzman, M. , Gomez‐Hernandez, J.M. , Hollmann, M. , Karschin, C. & Stühmer, W. (1996) P2X4: an ATP‐activated ionotropic receptor cloned from rat brain. Proc. Natl. Acad. Sci. USA, 93, 3684–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperlágh, B. , Köfalvi, A. , Deuchars, J. , Atkinson, L. , Milligan, C. , Buckley, N. & Vizi, E. (2002) Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J. Neurochem., 81, 1196–1211. [DOI] [PubMed] [Google Scholar]
- Toulme, E. & Khakh, B. (2012) Imaging P2X4 receptor lateral mobility in microglia regulation by calcium and p38 MAPK. J. Biol. Chem., 287, 14734–14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, M. , Lowery, R. & Majewska, A. (2010) Microglial interactions with synapses are modulated by visual experience. PLoS Biol., 8, e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky, A. , Anderova, M. & Chvatal, A. (2009) Differential calcium signalling in neuronal–glial networks. Front Biosci., 14, 2004–2016. [DOI] [PubMed] [Google Scholar]
- Wake, H. , Moorhouse, A.J. , Jinno, S. , Kohsaka, S. & Nabekura, J. (2009) Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci., 29, 3974–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, Z. & Burnstock, G. (2005) Expression of P2X receptors on rat microglial cells during early development. Glia, 52, 119–126. [DOI] [PubMed] [Google Scholar]
- Xu, J. , Chai, H. , Ehinger, K. , Egan, T.M. , Srinivasan, R. , Frick, M. & Khakh, B.S. (2014) Imaging P2X4 receptor subcellular distribution, trafficking, and regulation using P2X4‐pHluorin. J. Gen. Physiol., 144, 81–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y. , Ugawa, S. , Ueda, T. , Ishida, Y. , Inoue, K. , Kyaw Nyunt, A. , Umemura, A. , Mase, M. , Yamada, K. & Shimada, S. (2008) Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res., 1194, 45–55. [DOI] [PubMed] [Google Scholar]
- Zemkova, H. , Khadra, A. , Rokic, M.B. , Tvrdonova, V. , Sherman, A. & Stojilkovic, S.S. (2015) Allosteric regulation of the P2X4 receptor channel pore dilation. Pflug. Arch., 467, 713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]


