Summary
A combined increase in seawater [CO2] and [H+] was recently shown to induce a shift from photosynthetic HCO3 − to CO2 uptake in Emiliania huxleyi. This shift occurred within minutes, whereas acclimation to ocean acidification (OA) did not affect the carbon source.
To identify the driver of this shift, we exposed low‐ and high‐light acclimated E. huxleyi to a matrix of two levels of dissolved inorganic carbon (1400, 2800 μmol kg−1) and pH (8.15, 7.85) and directly measured cellular O2, CO2 and HCO3 − fluxes under these conditions.
Exposure to increased [CO2] had little effect on the photosynthetic fluxes, whereas increased [H+] led to a significant decline in HCO3 − uptake. Low‐light acclimated cells overcompensated for the inhibition of HCO3 − uptake by increasing CO2 uptake. High‐light acclimated cells, relying on higher proportions of HCO3 − uptake, could not increase CO2 uptake and photosynthetic O2 evolution consequently became carbon‐limited.
These regulations indicate that OA responses in photosynthesis are caused by [H+] rather than by [CO2]. The impaired HCO3 − uptake also provides a mechanistic explanation for lowered calcification under OA. Moreover, it explains the OA‐dependent decrease in photosynthesis observed in high‐light grown phytoplankton.
Keywords: calcification, CO2‐concentrating mechanism, life‐cycle stages, membrane‐inlet mass spectrometry, ocean acidification, pH, photosynthesis
Introduction
Coccolithophores are unicellular calcareous algae that take a dual role in global carbon cycling. During photosynthesis, carbon dioxide (CO2) is fixed into organic matter, leading to a net decrease in dissolved inorganic carbon (DIC) and CO2 from seawater. In the process of calcification, calcium carbonate (CaCO3) is precipitated, which results in lowered DIC and alkalinity, thus elevated CO2 levels. Emiliania huxleyi is the most abundant coccolithophore in the present‐day ocean with a distribution from tropical to subpolar waters (Winter et al., 2013). The species is able to form extensive blooms (Brown & Yoder, 1994; Sadeghi et al., 2012), which are often associated with a shallow mixed‐layer depth and high irradiances (Nanninga & Tyrrell, 1996; Raitsos et al., 2006). As one of the most important pelagic calcifiers, E. huxleyi has been a major focus of oceanographic research over the last decades, in particular with respect to ocean acidification (OA; e.g. Rost & Riebesell, 2004; Raven & Crawfurd, 2012).
As the ocean takes up anthropogenic CO2, levels of HCO3 − and CO2 increase, whereas pH and levels of CO3 2− decrease (Wolf‐Gladrow et al., 1999). These changes in carbonate chemistry are often summarized as OA, but strictly speaking this phenomenon comprises carbonation (i.e. increased [CO2] and [HCO3 −]) as well as acidification (i.e. increased [H+]/lowered pH). With a few exceptions, investigations of OA effects on E. huxleyi and other coccolithophores showed stimulated or unaffected production rates of particulate organic carbon (POC, i.e. biomass), with concomitantly impaired or unaffected production rates of particulate inorganic carbon (PIC, i.e. CaCO3; see Raven & Crawfurd, 2012, for overview). Some of the observed diversity in the OA responses could be attributed to genetic variability; but more importantly environmental factors such as irradiance were shown to modulate OA effects (Nielsen, 1997; Zondervan et al., 2002; van de Poll et al., 2007; Feng et al., 2008; Rokitta & Rost, 2012; Sett et al., 2014; Xu & Gao, 2015). OA responses are typically measured after acclimation to altered conditions over several generations, allowing cells to adjust their metabolism. A study by Barcelos e Ramos et al. (2010) demonstrated that the OA‐induced changes in cellular POC and PIC production are already evident after a few hours, indicating that OA effects are relatively immediate. In order to identify the drivers causing the OA responses in E. huxleyi, Bach and co‐workers disentangled the effects of carbonation and acidification by acclimating cells to artificial carbonate chemistry conditions (Bach et al., 2011, 2013). In these experiments, POC and PIC production were shown to be stimulated by carbonation, but inhibited by acidification.
In order to improve our understanding of E. huxleyi's response to OA, it is important to assess which cellular processes are affected by carbonation, acidification or the combination of both. Emiliania huxleyi is known to use CO2 and HCO3 − as external inorganic carbon (Ci) sources of photosynthesis, but the estimated proportions of CO2 uptake differ between studies and depend on the applied methods and assay conditions (e.g. Sikes et al., 1980; Herfort et al., 2002; Trimborn et al., 2007; Kottmeier et al., 2014). The increase in POC production after acclimation to OA is often attributed to the higher aqueous CO2 levels, which are thought to directly increase the diffusive CO2 supply at the CO2‐fixing enzyme Ribulose‐1,5‐bisphosphate‐carboxylase/‐oxygenase (RubisCO; Raven & Johnston, 1991; Rokitta & Rost, 2012; Stojkovic et al., 2013). A recent study demonstrated that the fraction of photosynthetic CO2 uptake relative to active HCO3 − uptake is indeed strongly increased under high [CO2]/low pH (Kottmeier et al., 2014). This switch in the Ci source occurred at short timescales of seconds to minutes, whereas the acclimation to OA did not significantly affect the Ci source. Thus, the beneficial OA effect seems to be directly caused by the changing carbonate chemistry rather than by changes in the expression of genes related to the CO2‐concentrating mechanism (CCM). The inhibitory effect of OA on PIC production is often attributed to changes in electrochemical gradients under high [H+] and the associated costs of H+ removal (Anning et al., 1996; Berry et al., 2002; Suffrian et al., 2011; Taylor et al., 2011). Tracer studies found HCO3 − to be the major external Ci source for calcification (Paasche, 1964; Sikes et al., 1980; Buitenhuis et al., 1999; Herfort et al., 2002; Rost et al., 2002), and it was suggested that increased H+ levels also affect HCO3 − uptake mechanisms (Fukuda et al., 2014). Despite the gained knowledge on cellular processes, relatively little is known about the differential effects of carbonation and acidification on photosynthesis, calcification and their underlying Ci supply.
In order to investigate the drivers causing the immediate shifts in the photosynthetic Ci source under high [CO2]/low pH (Kottmeier et al., 2014), here we measured the photosynthetic oxygen (O2) and Ci fluxes in direct response to carbonation, acidification and the combination of both. To this end, we acclimated both life‐cycle stages of E. huxleyi to present‐day carbonate chemistry and exposed them to a matrix of two DIC levels (1400 and 2800 μmol kg−1) and two pH values (8.15 and 7.85), yielding three different CO2 concentrations (~10, 20 and 40 μmol kg−1; Fig. 1). To further address the effect of energization, cells were acclimated to low and high photon flux densities (PFD; 50 and 400 μmol photons m−2 s−1), and fluxes were measured at two different PFD (180 and 700 μmol photons m−2 s−1).
Figure 1.
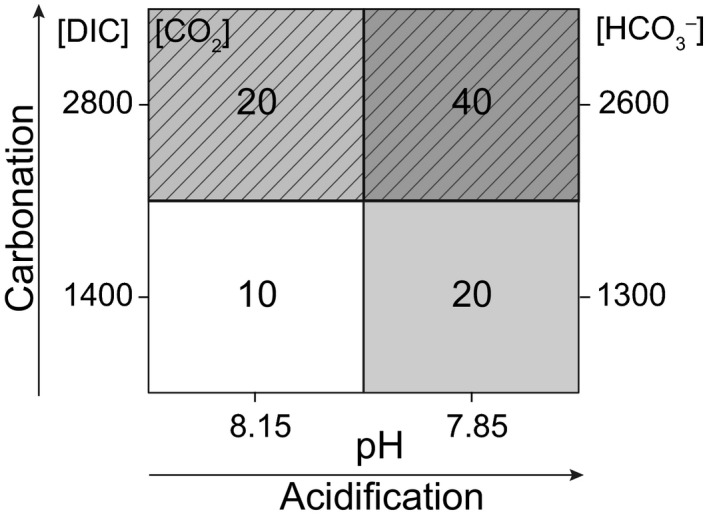
Decoupled carbonate chemistry during mass spectrometric measurements of cellular O2 and external inorganic carbon (Ci) fluxes in Emiliania huxleyi. Applied conditions were: low dissolved inorganic carbon (DIC)/high pH (LDICH pH; white); high DIC/high pH (HDICH pH; dashed, light grey); low DIC/low pH (LDICL pH; light grey); high DIC/low pH (HDICL pH; dashed, dark grey). All concentrations are given in μmol kg−1.
Materials and Methods
Culture conditions
The calcifying diplont (diploid life‐cycle stage) Emiliania huxleyi (Lohmann) Hay and Mohler, strain RCC 1216, and its noncalcifying haplont, RCC 1217, were acclimated to low and high light levels (LL, 50 ± 30 μmol photons s−1 m−2; HL, 400 ± 30 μmol photons s−1 m−2) under present‐day carbonate chemistry and a 16 h : 8 h, light : dark‐cycle. Light was provided by daylight lamps (FQ 54W/965HO; OSRAM, Munich, Germany) and adjusted by measuring photon flux densities (PFD) inside water‐containing culturing bottles with a Walz Universal Light meter (ULM 500; Walz, Effeltrich, Germany) using a 4π‐sensor (US‐SQS/L).
Cells were grown as dilute‐batch cultures in sterile‐filtered North Sea seawater (0.2 μm, Sartobran 300; Sartorius AG, Gottingen, Germany) enriched with phosphate and nitrate (~7 and ~100 μmol kg−1, respectively) as well as vitamins and trace metals according to F/2 (Guillard and Ryther, 1962). Culturing was performed in sterilized, gas‐tight 2‐l borosilicate bottles (Duran Group, Mainz, Germany), which were placed on roller tables to enable an homogenous cell suspension. Growth temperature was 15 ±1°C and was monitored by an Almemo 28–90 data logger (Ahlborn, Holzkirchen, Germany).
In all treatments, acclimations were performed under a CO2 partial pressure (pCO2) of 380 μatm (38.5 Pa), representing near‐present‐day conditions. The pCO2 was adjusted by pre‐aerating culture media with humidified, 0.2‐μm‐filtered air (Midisart 2000, PTFE; Sartorius AG), containing the desired pCO2. The gas mixture was created by a gas flow controller (CGM 2000; MCZ Umwelttechnik, Bad Nauheim, Germany) using pure CO2 (Air Liquide, Dusseldorf, Germany) and CO2‐free air (Air purification system; Parker, Kaarst, Germany). During the acclimation, head space inside the culture bottles was minimized to avoid outgassing effects. Carbonate chemistry was monitored based on total alkalinity (TA) measurements by potentiometric titration (Dickson, 1962; TitroLine alpha plus, measurement reproducibility ± 7 μmol kg−1; Schott Instruments, Mainz, Germany) and colorimetric DIC measurements with a QuAAtro autoanalyzer (measurement reproducibility ± 5 μmol kg−1; Seal Analytical, Norderstedt, Germany) in sterile‐filtered samples with the method of Stoll et al. (2001). Calculations of the carbonate system (CO2sys; Pierrot et al., 2006) were based on TA and DIC (Supporting Information Table S1). To monitor potential drifts of the carbonate chemistry on a daily basis, potentiometric measurements of pHNBS were performed with a Metrohm pH meter (826 pH mobile; Metrohm, Filderstadt, Germany) with an electrode containing an integrated temperature sensor (Aquatrode Plus with Pt 1000, measurement reproducibility ± 0.01 pH units).
Cell growth was monitored by daily cell counting with a Coulter Counter (Beckman‐Coulter, Fullerton, CA, USA) and specific growth constants μ (d−1) were determined as μ = (loge c 1 – loge c 0) Δt−1 (c 1 and c 0, cell concentrations (cells ml−1); Δt, time interval (d)). In both life‐cycle stages, μ was more or less equal and significantly reduced in the low‐light acclimations (~0.7 d−1), confirming a light limitation in this treatment (Table S1). In the high‐light treatment, μ (~1.1 d−1) was at the upper range of previously reported growth constants for the same strain (Langer et al., 2009; Rokitta & Rost, 2012).
Mass spectrometric flux measurements
Photosynthetic and respiratory O2 and Ci fluxes were measured with a mass spectrometer (Isoprime, GV Instruments, Manchester, UK) that was coupled to a cuvette via a gas‐permeable PTFE membrane (0.01 mm). This membrane‐inlet mass spectrometry (MIMS) technique uses the chemical disequilibrium between CO2 and HCO3 − during steady‐state photosynthesis to distinguish CO2 and HCO3 − uptake across the plasmalemma. Estimates of these fluxes were made following the equations of Badger et al. (1994). To include the process of calcification, we followed modifications introduced by Schulz et al. (2007).
The MIMS signals were calibrated for [CO2] by known additions of NaHCO3 into phosphoric acid (0.2 N), ensuring that all added DIC was quantitatively converted to CO2. Baseline values were obtained by adding sodium hydroxide (0.25 mmol l−1) into DIC‐free media, ensuring that any residual DIC was converted CO3 2−. Calibration for [O2] was obtained by equilibrating medium with air (21% O2), followed by the addition of sufficient amounts of sodium dithionite (Merck) to quantitatively scavenge O2 (0% O2). MIMS signals were translated into [O2] by applying the O2 solubility constants of seawater (Weiss, 1970). All O2 signals were furthermore corrected for the machine‐inherent consumption.
Experiments were performed with cells in their exponential growth phase with maximal cell concentrations of 5 × 104 cells ml−1 within 6–10 h after the start of the light period. Before the measurements, cells were concentrated to 4–10 × 106 cells ml−1 at acclimation temperature by gentle vacuum filtration over polycarbonate filters (Isopore TSTP, 3 μm or RTTP, 1.2 μm; Isopore membranes, Merck, Darmstadt, Germany). In this process, the medium was successively exchanged with pH‐buffered DIC‐free culture medium (50 mM N,N‐bis(2‐hydroxyethyl)‐glycine, BICINE; pHNBS of 7.85 or 8.15), and 8 ml were placed into an temperature‐controlled MIMS cuvette in the dark. Subsequently, 25 μmol kg−1 membrane‐impermeable dextrane‐bound sulfonamide (DBS; Synthelec, Lund, Sweden) was added, inhibiting any external carbonic anhydrase. Samples were continuously stirred to keep the cell suspension homogeneously mixed. To disentangle carbonate chemistry in the cuvette, inorganic carbon was added as ~1400 or ~2800 μmol kg−1 NaHCO3 to the DIC‐free medium, buffered at a pH of 8.15 or 7.85, yielding four different carbonate chemistry conditions (Fig. 1; Table 1): ‘Low DIC/High pH’ (LDICHpH), ‘High DIC/High pH’ (HDICHpH), ‘Low DIC/Low pH ‘(LDICLpH) and ‘High DIC/Low pH’ (HDICLpH). For each carbonate chemistry condition, photosynthetic and respiratory O2 and Ci fluxes were measured in consecutive light‐dark intervals (6 min per step), at two different light levels (180 and 700 μmol photons m−2 s−1).
Table 1.
Carbonate chemistry during mass measurements of O2 and inorganic carbon (Ci) fluxes in Emiliania huxleyi
| Acclimation | Carbonate chemistry | LDICHpH | HDICHpH | LDICLpH | HDICLpH |
|---|---|---|---|---|---|
| 2N LL | [CO2] | 12.4 ± 0.6 | 22.0 ± 0.8 | 24.0 ± 1.5 | 43.7 ± 1.8 |
| [HCO3 −] | 1370 ± 70 | 2500 ± 90 | 1420 ± 80 | 2590 ± 110 | |
| [H+] | 9.5 ± 0.2 | 9.5 ± 0.2 | 18.4 ± 0.3 | 18.4 ± 0.3 | |
| 2N HL | [CO2] | 13.6 ± 0.9 | 22.7 ± 1.0 | nd | 47.4 ± 2.0 |
| [HCO3 −] | 1560 ± 110 | 2730 ± 130 | nd | 2730 ± 110 | |
| [H+] | 9.1 ± 0.3 | 8.9 ± 0.3 | nd | 18.8 ± 0.3 | |
| 1N LL | [CO2] | 10.6 ± 0.5 | 20.5 ± 0.3 | 17.1 ± 0.5 | 40.9 ± 2.8 |
| [HCO3 −] | 1200 ± 50 | 2400 ± 30 | 1050 ± 30 | 2460 ± 160 | |
| [H+] | 9.2 ± 0.1 | 9.2 ± 0.1 | 17.4 ± 0.5 | 18.1 ± 0.0 | |
| 1N HL | [CO2] | 10.5 ± 0.6 | 18.6 ± 1.4 | 17.9 ± 1.8 | 32.5 ± 2.5 |
| [HCO3 −] | 1150 ± 70 | 2160 ± 130 | 1090 ± 120 | 1940 ± 200 | |
| [H+] | 9.3 ± 0.1 | 9.2 ± 0.1 | 17.5 ± 0.4 | 18.1 ± 0.5 |
Concentrations of CO2 (μmol kg−1), HCO3 − (μmol kg−1) and H+ (nmol kg−1) were assessed by means of mass spectrometry (n = 3; ± SD).
2N LL/HL, diploid life‐cycle stage acclimated to low/high light; 1N LL/HL, haploid life‐cycle stage acclimated to low/high light; LDICHpH, low dissolved inorganic carbon (DIC)/high pH; HDICHpH, high DIC/high pH; LDICLpH, low DIC/low pH; HDICLpH, high DIC/low pH; nd, not determined.
Calculations of oxygen and carbon fluxes
Oxygen fluxes
Net photosynthesis (Phot, μmol kg−1 min−1) and respiration (Resp, μmol kg−1 min−1) were deduced from steady‐state O2 fluxes in the light and dark, respectively (Badger et al., 1994):
| (Eqn 1) |
| (Eqn 2) |
Carbonate chemistry before light (BL)
For the calculation of the Ci fluxes, carbonate chemistry before and after the light phase was determined. [CO2]BL (μmol kg−1) could be directly taken from measured signals, whereas [HCO3 −]BL (μmol kg−1) was calculated according to Badger et al. (1994):
| (Eqn 3) |
(dCO2/dt BL, steady‐state CO2 evolution in the dark (μmol kg−1 min−1); k + and k — , effective rate constants for the conversion of CO2 to HCO3 − (min−1) and vice versa; RQ, respiratory quotient of 1 (Burkhardt et al., 2001; Rost et al., 2007)). Following Schulz et al. (2007), we applied the calculated effective rate constants derived from the measured pH, temperature and salinity in our assays:
| (Eqn 4) |
| (Eqn 5) |
([H+] and [OH−], concentrations of hydrogen and hydroxide ions, respectively (mol kg−1); k −1 , k +1 , k −4 and k +4, rate constants (Zeebe & Wolf‐Gladrow, 2001)). To assess [H+], known [DIC] (μmol kg−1) was added to cell‐free medium. From the resulting increase in [CO2] (μmol kg−1), the ratio of [DIC] : [CO2] and thus [H+] could be derived (Zeebe & Wolf‐Gladrow, 2001):
| (Eqn 6) |
( and , stoichiometric equilibrium constants (Roy et al., 1993)). [DIC]BL was derived as the sum of the Ci species, where carbonate ions ([CO3 2−]BL) can be assumed to be in equilibrium with [HCO3 −]BL (Schulz et al., 2006):
| (Eqn 7) |
The constant r hereby represents the pH‐dependent ratio between [HCO3 −] and [CO3 2−] (Zeebe & Wolf‐Gladrow, 2001; Schulz et al., 2007), which is defined as:
| (Eqn 8) |
Carbonate chemistry at the end of light (EL)
[CO2]EL (μmol kg−1) was directly obtained from measurements, whereas [HCO3 −]EL (μmol kg−1) was derived following Schulz et al. (2007):
| (Eqn 9) |
[DIC]consumed represents the concentration of DIC that was consumed in the course of the light interval and was defined as the sum of DIC used for photosynthesis ([O2]evolved/PQ, μmol kg−1) and for calcification ([DIC]CaCO3, μmol kg−1):
| (Eqn 10) |
[O2]evolved hereby represents the concentration of O2 that evolved over the course of the light phase and PQ is the photosynthetic quotient of 1.1 (Burkhardt et al., 2001; Rost et al., 2007). [DIC]CaCO3 was constrained by the measured ratio of particulate inorganic to organic carbon (PIC : POC) of the calcifying diploid life‐cycle stage under similar light treatments (1.4 at low‐light and 0.8 at high‐light acclimations; Rokitta & Rost, 2012) and was assumed to scale linearly with [O2]evolved (e.g. Paasche, 1999). Additionally, calcite production was normalized to the photoperiod (Schulz et al., 2007):
| (Eqn 11) |
In the noncalcifying haploid stage, PIC : POC was set to zero. Sensitivity analyses in which the PIC : POC were allowed to vary within typical uncertainties, revealed negligible effects on the calculated carbonate chemistry and photosynthetic fluxes.
Carbon fluxes
Knowing the carbonate chemistry, total net CO2 uptake (CO2uptotal, μmol kg−1 min−1) was inferred directly from the steady‐state CO2 drawdown in the light following Badger et al. (1994):
| (Eqn 12) |
Total CO2 uptake can be divided into one part used for photosynthesis (CO2upPS, μmol kg−1 min−1) and another part used for calcification (CO2upCaCO3 , μmol kg−1 min−1). As HCO3 − is the major external Ci source for calcification, we assumed that only 20% of calcification is supplied by external CO2 (Sikes et al., 1980; Paasche, 2001; Rost et al., 2002). Overall calcification was constrained by photoperiod‐normalized PIC : POC ratios and was assumed to scale linearly with the photosynthetic oxygen evolution:
| (Eqn 13) |
Please note that, similar to PIC : POC ratios, errors in the assumption of the CO2 usage for calcification can affect the estimated photosynthetic fluxes by relative constant and small offsets, but do not change the overall observed regulation patterns in response to carbonate chemistry. Accounting for the CO2 uptake for calcification, CO2upPS could be calculated as:
| (Eqn 14) |
Photosynthetic HCO3 − uptake (HCO3 −up, μmol kg−1 min−1) was estimated as the difference between photosynthetic net Ci fixation (calculated as Phot PQ−1) and net CO2 uptake for photosynthesis:
| (Eqn 15) |
Knowing the photosynthetic net CO2 uptake, its fraction of the overall net photosynthetic Ci uptake ( ; cf. Kottmeier et al., 2014) was derived as:
| (Eqn 16) |
Rate normalization
All rates were normalized to the amount of chlorophyll a (Chla) in the concentrated samples. Known amounts of cell suspension were filtered onto cellulose nitrate filters (0.45 μm; Sartorius, Gottingen, Germany) that were instantly frozen in liquid nitrogen. After extraction in 90% acetone, Chla content was determined fluorimetrically (TD‐700 fluorometer; Turner Designs, Sunnyvale, CA, USA) following the protocol of Knap et al. (1996).
Statistics
All experiments were carried out in biological triplicates. Fluxes estimated for the different carbonate chemistry conditions and at the same incoming PFD were tested pairwise for significant differences applying two‐sided t‐tests. Effects were called significant when P‐values were ≤ 0.05. In the figures, such significant differences were indicated by different lower‐case characters (e.g. a and b). Values denoted by two letters (e.g. ab) represent data that are not significantly different from a or b.
Results
In the following, we describe treatment‐specific differences in short‐term responses to altered carbonate chemistry and light. For clarity, only the fluxes of the diplont are shown in Figs 2, 3. Fluxes of the haplont are given in Table 2.
Figure 2.
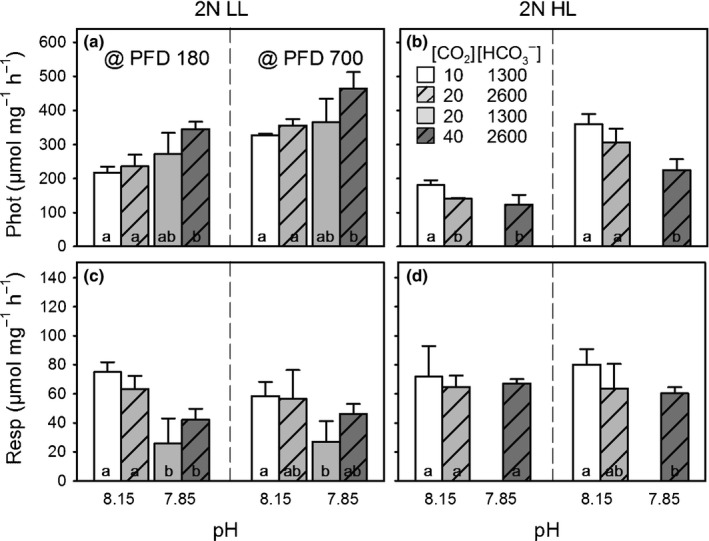
Short‐term modulations in photosynthetic and respiratory O2 fluxes of Emiliania huxleyi in response to low dissolved inorganic carbon (DIC)/high pH (LDICH pH; white bars), as well as carbonation (HDICH pH; dashed, light grey bars), acidification (LDICL pH; light grey bars) and the combination of both (HDICL pH; dashed, dark grey bars): Chla‐normalized photosynthetic net O2 evolution (Phot; a, b) and respiration (Resp; c, d) were measured at low and high photon flux densities (PFD; 180 and 700 μmol photons m−2 s−1). Data are shown for the diploid life‐cycle stage acclimated to low and high light (2N LL, 2N HL). Note: in 2N HL, no data for the LDICL pH condition were obtained. Error bar indicate mean ± SD (n = 3). Different lower‐case characters indicate significant differences between the fluxes obtained at different carbonate chemistry conditions and same PFD.
Figure 3.
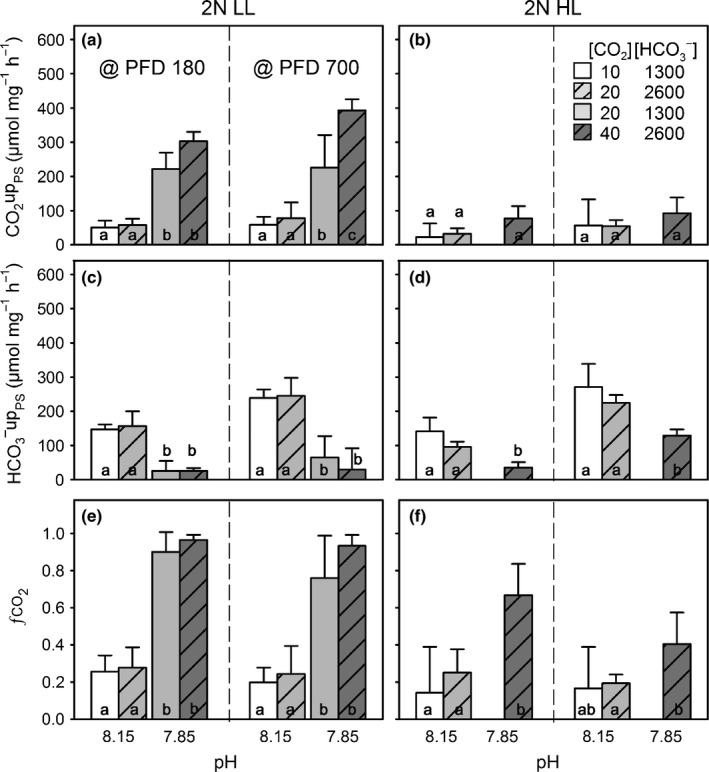
Short‐term modulations in external inorganic carbon (Ci) fluxes of Emiliania huxleyi in response to low dissolved inorganic carbon (DIC)/high pH (LDICH pH; white bars), as well as carbonation (HDICH pH; dashed, light grey bars), acidification (LDICL pH; light grey bars) and the combination of both (HDICL pH; dashed, dark grey bars): Chla‐normalized photosynthetic net CO 2 uptake (CO 2uptPS; a, b), photosynthetic HCO3 − uptake (HCO3 −uptPS; c, d) and the fraction of overall photosynthetic net Ci uptake that is covered by net CO 2 uptake (; e, f) were measured at low and high photon flux densities (PFD; 180 and 700 μmol photons m−2 s−1). Data are shown for the diploid life‐cycle stage acclimated to low and high light (2N LL, 2N HL). Note: in 2N HL, no data for the LDICL pH condition were obtained. Error bar indicate mean ± SD (n = 3). Different lower‐case characters indicate significant differences between the fluxes obtained at different carbonate chemistry conditions and same PFD.
Table 2.
Short‐term modulations in photosynthetic O2 and inorganic carbon (Ci) fluxes of Emiliania huxleyi in response to low dissolved inorganic carbon (DIC) and high pH (LDICHpH), as well as carbonation (HDICHpH), acidification (LDICLpH) and the combination (HDICLpH)
| Acclimation | PFD | Carbonate chemistry | Phot (μmol mg−1 h−1) | Resp(μmol mg−1 h−1) | CO2uptPS(μmol mg−1 h−1) | HCO3 −uptPS(μmol mg−1 h−1) |
|
|
|---|---|---|---|---|---|---|---|---|
| 2N LL | 180 | LDICHpH | 217 ± 17 | 75 ± 7 | 51 ± 20 | 147 ± 15 | 0.26 ± 0.09 | |
| HDICHpH | 236 ± 34 | 63 ± 9 | 58 ± 19 | 156 ± 43 | 0.28 ± 0.11 | |||
| LDICLpH | 271 ± 63 | 26 ± 17 | 221 ± 49 | 25 ± 29 | 0.90 ± 0.11 | |||
| HDICLpH | 345 ± 22 | 42 ± 8 | 303 ± 28 | 11 ± 9 | 0.96 ± 0.03 | |||
| 700 | LDICHpH | 327 ± 5 | 59 ± 10 | 59 ± 1 | 239 ± 1 | 0.20 ± 0.08 | ||
| HDICHpH | 355 ± 19 | 57 ± 20 | 78 ± 1 | 245 ± 1 | 0.24 ± 0.15 | |||
| LDICLpH | 365 ± 69 | 27 ± 14 | 226 ± 1 | 64 ± 1 | 0.76 ± 0.23 | |||
| HDICLpH | 464 ± 48 | 46 ± 7 | 393 ± 1 | 29 ± 1 | 0.93 ± 0.06 | |||
| 2N HL | 180 | LDICHpH | 181 ± 14 | 72 ± 21 | 23 ± 39 | 141 ± 40 | 0.14 ± 0.25 | |
| HDICHpH | 141 ± 2 | 65 ± 8 | 32 ± 17 | 96 ± 15 | 0.25 ± 0.13 | |||
| LDICLpH | nd | nd | nd | nd | nd | |||
| HDICLpH | 124 ± 28 | 67 ± 3 | 77 ± 36 | 35 ± 16 | 0.67 ± 0.17 | |||
| 700 | LDICHpH | 360 ± 30 | 80 ± 11 | 56 ± 77 | 271 ± 68 | 0.17 ± 0.22 | ||
| HDICHpH | 307 ± 40 | 64 ± 17 | 55 ± 18 | 224 ± 23 | 0.19 ± 0.05 | |||
| LDICLpH | nd | nd | nd | nd | nd | |||
| HDICLpH | 225 ± 32 | 60 ± 4 | 93 ± 46 | 129 ± 18 | 0.40 ± 0.17 | |||
| 1N LL | 180 | LDICHpH | 147 ± 22 | 38 ± 8 | 1 ± 8 | 131 ± 14 | 0.00 ± 0.06 | |
| HDICHpH | 173 ± 69 | 29 ± 7 | 28 ± 26 | 129 ± 39 | 0.15 ± 0.10 | |||
| LDICLpH | 202 ± 29 | 56 ± 12 | 58 ± 16 | 126 ± 19 | 0.31 ± 0.07 | |||
| HDICLpH | 185 ± 79 | 29 ± 3 | 55 ± 83 | 113 ± 17 | 0.24 ± 0.33 | |||
| 700 | LDICHpH | 223 ± 32 | 35 ± 4 | −6 ± 8 | 207 ± 23 | −0.03 ± 0.04 | ||
| HDICHpH | 240 ± 56 | 29 ± 5 | 7 ± 21 | 210 ± 41 | 0.02 ± 0.09 | |||
| LDICLpH | 286 ± 23 | 48 ± 11 | 26 ± 17 | 225 ± 25 | 0.11 ± 0.07 | |||
| HDICLpH | 273 ± 23 | 32 ± 4 | 38 ± 52 | 210 ± 37 | 0.14 ± 0.19 | |||
| 1N HL | 180 | LDICHpH | 119 ± 13 | 69 ± 4 | −33 ± 7 | 141 ± 11 | −0.31 ± 0.09 | |
| HDICHpH | 113 ± 36 | 54 ± 8 | −12 ± 26 | 114 ± 6 | −0.18 ± 0.30 | |||
| LDICLpH | 136 ± 19 | 67 ± 19 | 0 ± 17 | 124 ± 24 | 0.00 ± 0.14 | |||
| HDICLpH | 148 ± 34 | 79 ± 20 | −20 ± 17 | 154 ± 44 | −0.14 ± 0.10 | |||
| 700 | LDICHpH | 246 ± 13 | 68 ± 8 | −38 ± 10 | 261 ± 6 | −0.17 ± 0.05 | ||
| HDICHpH | 196 ± 33 | 55 ± 9 | −29 ± 24 | 207 ± 24 | −0.18 ± 0.15 | |||
| LDICLpH | 236 ± 25 | 56 ± 8 | −5 ± 8 | 208 ± 33 | −0.02 ± 0.03 | |||
| HDICLpH | 274 ± 68 | 75 ± 9 | −23 ± 2 | 272 ± 60 | −0.10 ± 0.03 |
Chla‐ normalized photosynthetic net O2 evolution (Phot) and respiration (Resp), photosynthetic net CO2 uptake (CO2uptPS), photosynthetic HCO3 − uptake (HCO3 −uptPS) and the fraction of overall photosynthetic net Ci uptake that is covered by net CO2 uptake () were measured at low and high photon flux densities (PFD; 180 vs 700 μmol photons m−2 s−1; n = 3; ± SD).
2N LL/HL, diploid life‐cycle stage acclimated to low light/high light; 1N LL/HL, haploid life‐cycle stage acclimated to low/high light; LDICHpH, low DIC/high pH; HDICHpH, high DIC/high pH; LDICLpH, low DIC/low pH; HDICLpH, high DIC/low pH; nd, not determined.
Oxygen fluxes
In both life‐cycle stages and light acclimations, net photosynthesis increased under increasing incoming light (Fig. 2a,b; PFD 180 vs 700), whereas dark respiration was generally independent of the light levels applied before the dark phase (Fig. 2c,d). The dependency on carbonate chemistry was stage and acclimation‐light specific (Fig. 2a–d; Table 2).
In the diplont acclimated to low light (2N LL), net photosynthesis was significantly stimulated under combined carbonation and acidification (HDICLpH; Fig. 2a). This increase could not be attributed exclusively to carbonation or acidification, but appeared to be a product of both. Respiration in 2N LL decreased under HDICLpH (significantly only at PFD 180). This effect seemed to be driven by acidification, because the rates decreased significantly under both low‐pH conditions, but not with carbonation (Fig. 2c).
In the diplont acclimated to high light (2N HL), net photosynthesis was significantly impaired under HDICLpH (Fig. 2b). Also this effect seemed to be caused by carbonation and acidification together, although the drivers could not be identified statistically due to the lack of the LDICLpH data (Fig. 1; Tables 1, 2). Respiration in 2N HL was largely unaffected by carbonate chemistry (Fig. 2d).
In contrast to the diplont, photosynthesis and respiration in the low‐ and high‐light acclimated haplont (1N LL, 1N HL) were insensitive to the applied carbonate chemistry (Table 2).
Carbon fluxes
In both life‐cycle stages and light acclimations, the higher Ci demands imposed by the higher incoming light levels during measurements were in most cases covered by additional HCO3 − uptake, whereas photosynthetic net CO2 uptake was largely unaffected by the incoming light (Fig. 3a–d; Table 2; PFD 180 vs PFD 700). The dependency of Ci fluxes on carbonate chemistry was clearly stage and acclimation‐light specific (Fig. 3; Table 2).
In 2N LL, the photosynthetic net CO2 uptake increased significantly under HDICLpH at both applied light levels, and these higher fluxes seemed to be driven mainly by acidification because CO2 uptake was strongly increased under both low‐pH conditions (Fig. 3a). Carbonation at high pH did not stimulate the CO2 uptake, whereas carbonation at low pH (LDICLpH vs HDICLpH) additionally increased CO2 uptake. HCO3 − uptake in 2N LL decreased significantly under HDICLpH (Fig. 3c). This decrease was clearly driven by acidification because HCO3 − uptake was decreased under both low‐pH conditions, independent of carbonation. The described opposing short‐term regulation of CO2 and HCO3 − uptake under HDICLpH caused significant shifts in from ~0.3 to ~0.9 (Fig. 3e).
In 2N HL, photosynthetic net CO2 uptake was relatively unaffected by carbonate chemistry (Fig. 3b). Similar to the low‐light acclimated cells, HCO3 − uptake for photosynthesis in 2N HL decreased significantly under HDICLpH, presumably also driven by acidification (Fig. 3d). As a consequence of the relatively constant net CO2 uptake and the decreased HCO3 − uptake, increased significantly from ~0.2 to ~0.7 at a PFD of 180, whereas the increase was insignificant at 700 μmol photons m−2 s−1 (Fig. 3f).
In 1N LL, photosynthetic net CO2 uptake was close to zero and the photosynthetic HCO3 − uptake clearly dominated the Ci fluxes (Table 2). Both CO2 and HCO3 − fluxes were unaffected by carbonate chemistry, resulting in constant and low values (~0.1 on average; Table 2). In 1N HL, photosynthetic net CO2 uptake was negative, reflecting a net CO2 efflux alongside a high HCO3 − uptake (Table 2). Also here, CO2 and HCO3 − fluxes were unaffected by carbonate chemistry, resulting in constant and negative values (~−0.1).
Discussion
In this study, we investigated Emiliana huxleyi's photosynthetic O2 and Ci fluxes and their short‐term modulations in response to changing carbonate chemistry and light. In the diploid life‐cycle stage (diplont), cellular fluxes were shown to be highly sensitive and to rapidly respond to the applied conditions. In the haploid stage (haplont), cellular fluxes were rather constant, even across large changes in carbonate chemistry.
H+‐driven increase in CO2 uptake stimulates photosynthesis in low‐light acclimated diplonts
In the low‐light acclimated diplont (2N LL), rates of photosynthetic O2 evolution were in a similar range as measured earlier under comparable conditions (Nielsen, 1995; Rokitta & Rost, 2012). They stayed relatively constant under carbonation or acidification alone, but were strongly stimulated by combined carbonation and acidification (Fig. 2a). Ocean acidification (OA) has earlier been shown to affect cellular fluxes rapidly (Barcelos e Ramos et al., 2010; Kottmeier et al., 2014). An immediate stimulation in photosynthesis, if maintained over longer timescales, could therefore also explain the increase in particulate organic carbon (POC) production that is typically observed in OA‐acclimated coccolithophores (Raven & Crawfurd, 2012). Even though the applied carbonate chemistry matrix generally allowed for the distinction between the effects of carbonation and acidification, their differential effects were not evident from the observed O2 fluxes (Fig. 2a). Only by measuring the underlying Ci acquisition was it possible to identify the drivers behind the photosynthetic responses (Fig. 3a,c): Net CO2 uptake was strongly promoted under acidification as well as under combined carbonation and acidification (Fig. 3a), whereas HCO3 − uptake was strongly downscaled under these conditions (Fig. 3c). As the stimulation in net CO2 uptake under combined carbonation and acidification exceeded the impairing effect on HCO3 − uptake, the overall photosynthetic Ci uptake and consequently photosynthetic O2 evolution were increased under these conditions (Fig. 2a).
The transition from the active HCO3 − to diffusive CO2 uptake under short‐term acidification is in line with Kottmeier et al. (2014), who observed that E. huxleyi increases the relative fraction of CO2 usage when being exposed to high [CO2]/low pH over short timescales. Here we show that this shift is caused by a combination of increased CO2 uptake and decreased HCO3 − uptake. The increased CO2 usage is likely to decrease the energy demand of the cell, because transport of HCO3 − is considered more costly due to the molecule's negative charge and the large hydration envelope, properties that require an active transport (Burkhardt et al., 2001; Beardall & Raven, 2004; Holtz et al., 2015b). Indeed, we found stimulated photosynthesis and decreased respiration rates under acidification despite the same incoming light (Fig. 2a,c), indicating not only a more efficient CO2 supply at RubisCO, but also altered energy allocations under these conditions. Such energy reallocations under OA have earlier been attributed to shifts from reductive towards oxidative pathways (Rokitta et al., 2012).
The H+‐driven stimulation in CO2 uptake contradicts the ‘fertilizing effect’ of CO2 that is typically ascribed to OA. Contrary to the common notion that CO2 uptake for photosynthesis benefits from carbonation, it was here promoted mainly by acidification, at least over the short timescales applied. The higher CO2 uptake under combined carbonation and acidification compared to acidification alone indicated that high H+ levels generally increase the cellular CO2 uptake capacity. Yet, the higher CO2 availability was able to stimulate its uptake even further – carbonation and acidification acted synergistically. The H+‐driven decrease in cellular HCO3 − uptake, which occurred independent of the applied dissolved inorganic carbon (DIC) levels, indicated that the HCO3 − transport capacity is generally downscaled under acidification. Carbonation alone had no effect on HCO3 − uptake, suggesting that the transporters are substrate‐saturated at the applied [HCO3 −] (~1300 and 2600 μmol kg−1). This is in line with a study by Rost et al. (2006) who measured the short‐term DIC‐dependency of photosynthesis at constant pH and showed that HCO3 − uptake in E. huxleyi was substrate‐saturated even below [HCO3 −] of ~500 μmol kg−1.
The H+‐dependent regulations in Ci fluxes are likely to be similar after acclimation. Bach et al. (2011), for instance, acclimated E. huxleyi to carbonate chemistry conditions in which either CO2 or pH varied independently. They could show that POC production increases with DIC if pH is buffered to ~8.0, but it decreases with increasing DIC if pH decreases concomitantly. This suggests that the negative H+ effects on HCO3 − uptake are retained after acclimation. However, in contrast to our study, where no short‐term carbonation effects were measured, Bach and coworkers found stimulated POC and particulate inorganic carbon (PIC) production after acclimation to carbonation. Consequently, the cells were able to increase their Ci uptake when being exposed to these conditions over longer timescales, possibly by expressing more HCO3 − transporters. In order to examine how acclimation affects the sensitivity towards changing carbonate chemistry, future studies should investigate which short‐term effects manifest over longer timescales.
The strong H+ effects on CO2 and HCO3 − uptake rates, observed in the current study, must originate from processes at the cell membrane or inside the cell, such as electrochemical gradients, enzyme activities and Ci speciation (Mackinder et al., 2010; Suffrian et al., 2011; Taylor et al., 2011). The stimulated net CO2 uptake under acidification could, for example, be explained by pH‐dependent differences in membrane morphology (Leung et al., 2012), which may also affect the CO2 permeability. It could also be caused by pH‐dependent regulations of intracellular fluxes, for instance due to different enzyme activities, which may lead to a stronger inward CO2 gradient. The decreased HCO3 − uptake under acidification is apparently caused by a direct H+‐driven inhibition of HCO3 − transporters at the plasmalemma or chloroplast membrane. The diplont E. huxleyi expresses AE1 and AE2‐type Cl−/HCO3 − transporters of the Solute Carrier 4 (SLC4) family (Herfort et al., 2002; von Dassow et al., 2009; Mackinder et al., 2011; Rokitta et al., 2011; Bach et al., 2013). This enzyme family is well investigated in the context of renal acid/base regulation in mammals, where the activity of the anion exchangers has indeed been shown to be modulated by pH (Alper, 2006).
H+‐driven decrease in HCO3 − uptake causes carbon‐limitation in high‐light acclimated diplonts
In high‐light acclimated diploid cells (2N HL), photosynthesis was inhibited under combined carbonation and acidification (Fig. 2b). This finding seems puzzling at first because in low‐light acclimated cells (2N LL), the same carbonate chemistry had a pronounced beneficial effect on photosynthesis (Fig. 2a). However, light‐dependent modulations in the sensitivity towards carbonate chemistry are well in line with other studies (e.g. Kranz et al., 2010; Gao et al., 2012; Rokitta & Rost, 2012; Jin et al., 2013; Hoppe et al., 2015). Rokitta & Rost (2012), for example, found that POC production in E. huxleyi is strongly stimulated when acclimated to OA and sub‐saturating light, but is relatively unaffected by OA under high light intensities.
Based on our flux measurements, we are able to provide an explanation for such differential OA sensitivities: In contrast to the low‐light acclimated cells, where CO2 uptake was strongly stimulated when being exposed to acidified conditions, CO2 uptake in the high‐light acclimated cells remained unaffected (Fig. 3b). Similar to the low‐light acclimated cells, HCO3 −uptake in the high‐light acclimated cells was impaired under acidification (Fig. 3d). As a result, the overall Ci uptake and consequently photosynthetic O2 evolution were significantly decreased (Fig. 2b). The inability of high‐light acclimated cells to increase CO2 uptake may not only be the reason for the Ci shortage under short‐term acidification, but also explains why photosynthesis is often not stimulated after acclimation to OA (Raven & Crawfurd, 2012). However, a detrimental H+ effect on photosynthesis in E. huxleyi has not yet been observed after acclimation, indicating that either the decrease in HCO3 − uptake is less pronounced, or the increase CO2 uptake is more pronounced when cells are exposed to acidified conditions over an extended period of time.
The reduced capability of high‐light acclimated cells to increase CO2 uptake under acidification may derive from adjustments of their CO2‐concentrating mechanism (CCM) to the higher acclimation irradiance. Emiliana huxleyi was shown to increase HCO3 − uptake with increasing irradiance during flux measurements (Fig. 3d; 180 vs 700 PFD). This may indicate that high‐light acclimated cells also used a higher fraction of HCO3 − under the conditions, at which they were cultured (Rost et al., 2006). Cells operating CCMs that are based predominantly on HCO3 − uptake need to reduce the diffusive losses and therefore downregulate their CO2 permeability, for example by altering chloroplast morphology (Sukenik et al., 1987).
Recent studies on the combined effects of OA and light indicate that similar mechanisms, as here observed for E. huxleyi, also apply to other phytoplankton taxa. In diatoms, for example, growth was shown to increase significantly when cultured under OA and sub‐saturating light, whereas these responses were reversed under high light (Gao et al., 2012). Besides light intensity, light fluctuations also have been shown to significantly modulate OA effects (Jin et al., 2013; Hoppe et al., 2015). Hoppe and coworkers, for example, observed that photosynthesis stayed constant under OA and constant light, but decreased under OA and dynamic light. According to our data, OA may generally lower the HCO3 − uptake capacity of phytoplankton. Although this is apparently not detrimental under low and stable light conditions, the impaired HCO3 − uptake seems to have severe consequences under high and dynamic light conditions. Under the latter conditions, the phytoplankton cells are dependent primarily on HCO3 − transport, because the high Ci demand under high light and the varying Ci demand under dynamic light cannot be covered or adjusted fast enough by diffusive CO2 uptake. Owing to the impairment of HCO3 − transporters, these cells are thus more prone to Ci shortage at RubisCO under OA, even though external substrate concentrations are slightly elevated. When RubisCO becomes Ci‐limited, the Calvin Cycle is a weaker electron sink, which can cause energetic overloads and higher costs associated with dissipation of energy and repair mechanisms (van de Poll et al., 2007; Gao et al., 2012; Jin et al., 2013; Hoppe et al., 2015). Thus, the high H+‐driven decrease in cellular HCO3 − uptake can explain why the energy transfer efficiency from photochemistry to biomass production is reduced under OA in combination with high or dynamic light conditions (Gao et al., 2012; Hoppe et al., 2015).
Haplonts are insensitive to carbonate chemistry
The comparison of the two life‐cycle stages of E. huxleyi revealed that their modes of Ci acquisition strongly diverge. Photosynthetic and respiratory O2 fluxes in the haploid stage did not respond to the short‐term changes in carbonate chemistry (Table 2). Also CO2 and HCO3 − uptake were not affected by carbonation or acidification. This agrees with the results of acclimation studies that often found no or few changes in POC production and other cellular processes under OA (Rokitta & Rost, 2012; Kottmeier et al., 2014). The fact that HCO3 − uptake was unaffected by external H+ levels implies that the HCO3 − uptake mechanism of the haplont is different from the one of the diplont (Table 2). Indeed, there are transcriptomic datasets demonstrating that the two life‐cycle stages express different isoforms of HCO3 − transporters of the SLC4 family (von Dassow et al., 2009; Mackinder et al., 2011; Rokitta et al., 2011). Also, the haplont was shown to express stage‐specific subunits of a vacuolar H+ ATPase and other stage‐specific ion transporters, e.g. a Ca2+ ⁄ H+ antiporters, which may further explain the differential sensitivity towards H+ levels (von Dassow et al., 2009; Rokitta et al., 2011, 2012).
The consistently high HCO3 − usage of the haplont was not in line with the results of a 14C disequilibrium method, which estimated generally higher CO2 contributions and a strong dependency on [CO2]/pH (Kottmeier et al., 2014). This discrepancy may be attributed to the different key assumptions of the MIMS and/or the 14C disequilibrium methods. Regarding the MIMS method, we tested the consequences of potential offsets in key assumptions (e.g. variations in rate constants, PIC : POC, or photosynthetic quotient (PQ)) and found that typical uncertainties cannot explain the strong deviations between the methods. In contrast to the MIMS approach, the 14C disequilibrium technique does not yield actual CO2 and HCO3 − uptake rates, but estimates the relative CO2 uptake for photosynthesis (Lehman, 1971; Espie & Colman, 1986; Elzenga et al., 2000; Kottmeier et al., 2014). In this method, is assessed based on the curvature of the cellular photosynthetic 14C incorporation during a transient isotopic 14CO2 disequilibrium in the medium. In order to estimate , the 14C‐incorporation is fitted with a model that is based on a number of parameters (Lehman, 1971; Espie & Colman, 1986). Some of these parameters, including kinetic constants, decay rates and the height of isotopic disequilibria remain error‐afflicted and are currently being re‐evaluated (S. Thoms et al. unpublished). Until these methodological discrepancies are better understood, the conflicting results for the haploid stage remain puzzling.
Impaired HCO3 − uptake under acidification may affect calcification
Although the strong negative H+ effects on photosynthetic HCO3 − uptake have not explicitly been described before, negative H+ effects on calcification are often discussed (Taylor et al., 2011; Fukuda et al., 2014; Bach et al., 2015; Cyronak et al., 2015). These inhibitory effects have often been attributed to changes in electrochemical gradients and the associated costs of H+ removal (Mackinder et al., 2010; Raven, 2011; Suffrian et al., 2011; Taylor et al., 2011). In agreement with Fukuda et al., 2014, we here found strong evidence that acidification impairs the HCO3 − uptake. Assuming that high H+ levels affect the transport of HCO3 − across the plasmalemma, the decreased uptake would not only influence photosynthesis, but also calcification. Based on flux measurements of this study, we illustrated the presumed cellular Ci fluxes in response to typical OA scenarios under different light acclimations (Fig. 4).
Figure 4.
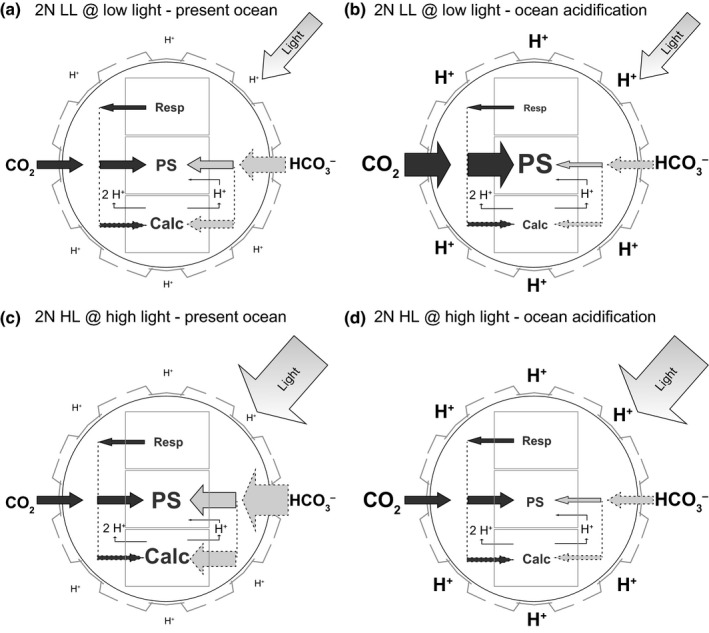
Schematic illustration of the ocean acidification (OA)‐dependent regulations in external inorganic carbon (Ci) fluxes of diploid, low‐light acclimated (2N LL; a, b) and high‐light acclimated (2N HL; c, d) Emiliania huxleyi under acclimation light. Sizes of arrows with solid lines reflect the measured photosynthetic and respiratory fluxes of CO 2 and HCO3 −. Sizes of arrows with dashed lines reflect estimated fluxes. (a) Low‐light acclimated cells mainly use external HCO3 − as photosynthetic substrate under acclimation pH and achieve similar rates of calcification (Calc) and photosynthesis (PS). (b) When exposing low‐light acclimated cells to OA, cells increase CO 2 uptake for photosynthesis, whereas HCO3 − uptake is downscaled due to the increased H+ levels. If HCO3 − fluxes into photosynthesis and calcification were downscaled to the same degree, photosynthesis would disproportionally increase over calcification. (c) High‐light acclimated cells perform higher rates of photosynthesis under acclimation light than low‐light acclimated cells. The increased Ci demand is covered by additional HCO3 − uptake. (d) When exposing high‐light acclimated cells to OA, they are not able to increase CO 2 uptake rates, but nevertheless experience the H+‐driven decrease in HCO3 − uptake. As a consequence of the decreased overall Ci supply, photosynthesis and presumably calcification experience Ci shortage and thus decrease.
As HCO3 − fluxes into POC and PIC are similar in magnitude, calcification may serve intrinsic pH regulation (Sikes et al., 1980; Price et al., 2008; Raven, 2011). More specifically, when HCO3 − is used for photosynthesis, one H+ is consumed per fixed CO2, and when HCO3 − is used for calcification, one H+ is released per produced CaCO3 (Fig. 4; Holtz et al., 2015a). The additional photosynthetic CO2 uptake observed under acidification does not interfere with such a pH‐homeostatic behaviour. Thus, independent of the external carbonate chemistry and light conditions, the need to exchange H+ with the environment seems to be generally lower in the calcifying diplont of E. huxleyi (Fig. 4). This could provide the calcifying stage with an advantage over noncalcifying HCO3 − users (such as the haplont), which have to assure constant H+ uptake to compensate for alkalization during HCO3 −‐based photosynthesis (Raven, 1986, 2011). This advantage may add to the diplont's success under bloom conditions, where seawater H+ levels can become low.
Conclusions
In this study, we reveal a strong H+‐driven regulation of photosynthetic Ci fluxes of E. huxleyi that contradicts the commonly assumed ‘fertilizing effect’ of CO2. At typical present‐day conditions, HCO3 − was shown to be the major photosynthetic Ci source of both life‐cycle stages. High H+ levels were shown to rapidly inhibit the HCO3 − uptake and concomitantly to stimulate the CO2 uptake. This H+‐dependent inhibition in HCO3 − uptake serves as a mechanistic explanation for the typical OA‐dependent decline in calcification of coccolithophores and other marine calcifiers. Such an inhibition may be widespread among various phytoplankton taxa and also elucidates how the light‐use efficiency can decrease when phytoplankton communities are grown under OA in combination with high or fluctuating light intensities. Future research should investigate whether similar H+‐dependent flux regulations are also evident when cells are acclimated to altered conditions.
Author contributions
D.M.K., S.D.R. and B.R. planned and designed the research. D.M.K. performed experiments and analysed the data. D.M.K., S.D.R. and B.R. interpreted the data and wrote the manuscript.
Supporting information
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Table S1 Acclimation carbonate chemistry
Acknowledgements
We thank Klaus‐Uwe Richter for the technical support and Dieter Wolf‐Gladrow, Silke Thoms, Lena Holtz and Clara Hoppe for the constructive comments on this manuscript. We also gratefully acknowledge the feedback of the two anonymous reviewers. S.D.R. and B.R. received funding from the German Federal Ministry for Education and Research (BMBF) under grant no. 031A518C (ZeBiCa2) and 03F0655B (Bioacid II).
References
- Alper SL. 2006. Molecular physiology of SLC4 anion exchangers. Experimental Physiology 91: 153–161. [DOI] [PubMed] [Google Scholar]
- Anning T, Nimer NA, Merrett MJ, Brownlee C. 1996. Costs and benefits of calcification in coccolithophorids. Journal of Marine Systems 9: 45–56. [Google Scholar]
- Bach LT, Mackinder LC, Schulz KG, Wheeler G, Schroeder DC, Brownlee C, Riebesell U. 2013. Dissecting the impact of CO2 and pH on the mechanisms of photosynthesis and calcification in the coccolithophore Emiliania huxleyi . New Phytologist 199: 121–134. [DOI] [PubMed] [Google Scholar]
- Bach LT, Riebesell U, Gutowska MA, Federwisch L, Schulz KG. 2015. A unifying concept of coccolithophore sensitivity to changing carbonate chemistry embedded in an ecological framework. Progress in Oceanography 135: 125–138. [Google Scholar]
- Bach LT, Riebesell U, Schulz KG. 2011. Distinguishing between the effects of ocean acidification and ocean carbonation in the coccolithophore Emiliania huxleyi . Limnology and Oceanography 56: 2040–2050. [Google Scholar]
- Badger MR, Palmqvist K, Yu JW. 1994. Measurement of CO2 and HCO3 − fluxes in cyanobacteria and microalgae during steady‐state photosynthesis. Physiologia Plantarum 90: 529–536. [Google Scholar]
- Barcelos e Ramos J, Müller MN, Riebesell U. 2010. Short‐term response of the coccolithophore Emiliania huxleyi to abrupt changes in seawater carbon dioxide concentrations. Biogeosciences 7: 177–186. [Google Scholar]
- Beardall J, Raven JA. 2004. The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia 43: 26–40. [Google Scholar]
- Berry L, Taylor AR, Lucken U, Ryan KP, Brownlee C. 2002. Calcification and inorganic carbon acquisition in coccolithophores. Functional Plant Biology 29: 289–299. [DOI] [PubMed] [Google Scholar]
- Brown CW, Yoder JA. 1994. Coccolithophorid blooms in the global ocean. Journal of Geophysical Research‐Oceans 99: 7467–7482. [Google Scholar]
- Buitenhuis ET, De Baar HJW, Veldhuis MJW. 1999. Photosynthesis and calcification by Emiliania huxleyi (Prymnesiophyceae) as a function of inorganic carbon species. Journal of Phycology 35: 949–959. [Google Scholar]
- Burkhardt S, Amoroso G, Riebesell U, Sultemeyer D. 2001. CO2 and HCO3 − uptake in marine diatoms acclimated to different CO2 concentrations. Limnology and Oceanography 46: 1378–1391. [Google Scholar]
- Cyronak T, Schulz KG, Jokiel PL. 2015. The Omega myth: what really drives lower calcification rates in an acidifying ocean. ICES Journal of Marine Science: Journal du Conseil, fs075. doi: 10.1093/icesjms/fsv075. [Google Scholar]
- von Dassow P, Ogata H, Probert I, Wincker P, Da Silva C, Audic S, Claverie J‐M, De Vargas C. 2009. Transcriptome analysis of functional differentiation between haploid and diploid cells of Emiliania huxleyi, a globally significant photosynthetic calcifying cell. Genome Biology 10: R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson AG. 1981. An exact definition of total alkalinity and a procedure for the estimation of alkalinity and total inorganic carbon from titration data. Deep‐Sea Research Part II: Topical Studies in Oceanography 28: 609–623. [Google Scholar]
- Elzenga JTM, Prins HBA, Stefels J. 2000. The role of extracellular carbonic anhydrase activity in inorganic carbon utilization of Phaeocystis globosa (Prymnesiophyceae): a comparison with other marine algae using the isotopic disequilibrium technique. Limnology and Oceanography 45: 372–380. [Google Scholar]
- Espie GS, Colman B. 1986. Inorganic carbon uptake during photosynthesis – a theoretical analysis using the isotopic disequilibrium technique. Plant Physiology 80: 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Warner ME, Zhang Y, Sun J, Fu FX, Rose JM, Hutchins DA. 2008. Interactive effects of increased pCO2, temperature and irradiance on the marine coccolithophore Emiliania huxleyi (Prymnesiophyceae). European Journal of Phycology 43: 87–98. [Google Scholar]
- Fukuda SY, Suzuki Y, Shiraiwa Y. 2014. Difference in physiological responses of growth, photosynthesis and calcification of the coccolithophore Emiliania huxleyi to acidification by acid and CO2 enrichment. Photosynthesis Research 121: 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K, Xu J, Gao G, Li Y, Hutchins DA, Huang B, Wang L, Zheng Y, Jin P, Cai X et al 2012. Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nature Climate Change 2: 519–523. [Google Scholar]
- Guillard RRL, Ryther JH. 1962. Studies of marine planktonic diatoms. I. Cyclothella nana Hustedt and Detonula confervacea Cleve. Canadian Journal of Microbiology 8: 229–239. [DOI] [PubMed] [Google Scholar]
- Herfort L, Thake B, Roberts J. 2002. Acquisition and use of bicarbonate by Emiliania huxleyi . New Phytologist 156: 427–436. [DOI] [PubMed] [Google Scholar]
- Holtz LM, Wolf‐Gladrow DA, Thoms S. 2015a. Numerical cell model investigating cellular carbon fluxes in Emiliania huxleyi . Journal of Theoretical Biology 364: 305–315. [DOI] [PubMed] [Google Scholar]
- Holtz LM, Wolf‐Gladrow DA, Thoms S. 2015b. Simulating the effects of light intensity and carbonate system composition on particulate organic and inorganic carbon production in Emiliania huxleyi . Journal of Theoretical Biology 372: 192–204. [DOI] [PubMed] [Google Scholar]
- Hoppe CJM, Holtz L‐M, Trimborn S, Rost B. 2015. Ocean acidification decreases the light use efficiency in an Antarctic diatom under dynamic but not constant light. New Phytologist 207: 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Gao K, Villafane VE, Campbell DA, Helbling EW. 2013. Ocean acidification alters the photosynthetic responses of a coccolithophorid to fluctuating ultraviolet and visible radiation. Plant Physiology 162: 2084–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knap A, Michaels A, Close A, Ducklow H, Dickson A. 1996. Protocols for the joint global ocean flux study (JGOFS) core measurements. JGOFS, Reprint of the IOC Manuals and Guides No. 29, UNESCO 1994 19: 1–170. [Google Scholar]
- Kottmeier DM, Rokitta SD, Tortell PD, Rost B. 2014. Strong shift from HCO3 − to CO2 uptake in Emiliania huxleyi with acidification: new approach unravels acclimation versus short‐term pH effects. Photosynthesis Research 121: 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz SA, Levitan O, Richter KU, Prasil O, Berman‐Frank I, Rost B. 2010. Combined effects of CO2 and light on the N2‐fixing cyanobacterium Trichodesmium IMS101: physiological responses. Plant Physiology 154: 334–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G, Nehrke G, Probert I, Ly J, Ziveri P. 2009. Strain‐specific responses of Emiliania huxleyi to changing seawater carbonate chemistry. Biogeosciences 6: 2637–2646. [Google Scholar]
- Lehman JT. 1971. Enhanced transport of inorganic carbon into algal cells and its implications for the biological fixation of carbon. Journal of Phycology 14: 33–44. [Google Scholar]
- Leung C‐Y, Palmer LC, Qiao BF, Kewalramani S, Sknepnek R, Newcomb CJ, Greenfield MA, Vernizzi G, Stupp SI, Beyzyk MJ et al 2012. Molecular crystallization controlled by pH regulates mesoscopic membrane morphology. ACS Nano 6: 10901–10909. [DOI] [PubMed] [Google Scholar]
- Mackinder L, Wheeler G, Schroeder D, von Dassow P, Riebesell U, Brownlee C. 2011. Expression of biomineralization‐related ion transport genes in Emiliania huxleyi . Environmental Microbiology 13: 3250–3265. [DOI] [PubMed] [Google Scholar]
- Mackinder L, Wheeler G, Schroeder D, Riebesell U, Brownlee C. 2010. Molecular mechanisms underlying calcification in coccolithophores. Geomicrobiology Journal 27: 585–595. [Google Scholar]
- Nanninga HJ, Tyrrell T. 1996. Importance of light for the formation of algal blooms by Emiliania huxleyi . Marine Ecology Progress Series 136: 195–203. [Google Scholar]
- Nielsen MV. 1995. Photosynthetic characteristics of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae) exposed to elevated concentrations of dissolved inorganic carbon. Journal of Phycology 31: 715–719. [Google Scholar]
- Nielsen MV. 1997. Growth, dark respiration and photosynthetic parameters of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae) acclimated to different day length‐irradiance combinations. Journal of Phycology 33: 818–822. [Google Scholar]
- Paasche E. 1964. A tracer study of the inorganic carbon uptake during coccolith formation and photosynthesis in the coccolithophorid Coccolithus huxleyi. Lund, Sweden: Scandinavian Society for Plant Physiology. [Google Scholar]
- Paasche E. 1999. Reduced coccolith calcite production under light‐limited growth: a comparative study of three clones of Emiliania huxleyi (Prymnesiophyceae). Phycologia 38: 508–516. [Google Scholar]
- Paasche E. 2001. A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification–photosynthesis interactions. Phycologia 40: 503–529. [Google Scholar]
- Pierrot D, Lewis E, Wallace D. 2006. MS Excel program developed for CO2 system calculations. ORNL/CDIAC‐105. Oak Ridge, TN, USA: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy. [Google Scholar]
- van de Poll WH, Visser RJ, Buma AG. 2007. Acclimation to a dynamic irradiance regime changes excessive irrandiance sensitivity of Emiliania huxleyi and Thalassiosira weissflogii . Limnology and Oceanography 52: 1430–1438. [Google Scholar]
- Price GD, Badger MR, Woodger FJ, Long BM. 2008. Advances in understanding the cyanobacterial CO2‐concentrating‐mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. Journal of Experimental Botany 59: 1441–1461. [DOI] [PubMed] [Google Scholar]
- Raitsos D, Lavender S, Pradhan Y, Tyrrell T, Reid P, Edwards M. 2006. Coccolithophore bloom size variation in response to the regional environment of the subarctic North Atlantic. Limnology and Oceanography 51: 2122–2130. [Google Scholar]
- Raven J. 1986. Biochemical disposal of excess H+ in growing plants? New Phytologist 104: 175–206. [Google Scholar]
- Raven J. 2011. Effects on marine algae of changed seawater chemistry with increasing atmospheric CO2 . Biology and Environment: Proceedings of the Royal Irish Academy 111 B: 1–17. [Google Scholar]
- Raven J, Crawfurd K. 2012. Environmental controls on coccolithophore calcification. Marine Ecology Progress Series 470: 137–166. [Google Scholar]
- Raven J, Johnston A. 1991. Mechanisms of inorganic carbon acquisition in marine phytoplankton and their implications for the use of other resources. Limnology and Oceanography 36: 1701–1714. [Google Scholar]
- Rokitta S, De Nooijer L, Trimborn S, De Vargas C, Rost B, John U. 2011. Transcriptome analyses reveal differential gene expression patterns between life‐cycle stages of Emiliania huxleyi (Haptophyta) and reflect specialization to different ecological niches. Journal of Phycology 47: 829–838. [DOI] [PubMed] [Google Scholar]
- Rokitta S, John U, Rost B. 2012. Ocean acidification affects redox‐balance and ion‐homeostasis in the life‐cycle stages of Emiliania huxleyi . PLoS ONE 7: e52212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokitta S, Rost B. 2012. Effects of CO2 and their modulation by light in the life‐cycle stages of the coccolithophore Emiliania huxleyi . Limnology and Oceanography 57: 607–618. [Google Scholar]
- Rost B, Kranz SA, Richter KU, Tortell PD. 2007. Isotope disequilibrium and mass spectrometric studies of inorganic carbon acquisition by phytoplankton. Limnology and Oceanography – Methods 5: 328–337. [Google Scholar]
- Rost B, Riebesell U. 2004. Coccolithophores and the biological pump: responses to environmental changes In: Thierstein HR, Young JR, eds. Coccolithophores – from molecular processes to global impact. Heidelberg, Germany: Springer, 76–99. [Google Scholar]
- Rost B, Riebesell U, Sültemeyer D. 2006. Carbon acquisition of marine phytoplankton: effect of photoperiod length. Limnology and Oceanography 51: 12–20. [Google Scholar]
- Rost B, Zondervan I, Riebesell U. 2002. Light‐dependent carbon isotope fractionation in the coccolithophorid Emiliania huxleyi . Limnology and Oceanography 47: 120–128. [Google Scholar]
- Roy RN, Roy LN, Vogel KM, Portermoore C, Pearson T, Good CE, Millero J, Campbell DM. 1993. The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45°C. Marine Chemistry 44: 249–267. [Google Scholar]
- Sadeghi A, Dinter T, Vountas M, Taylor B, Altenburg‐Soppa M, Bracher A. 2012. Remote sensing of coccolithophore blooms in selected oceanic regions using the PhytoDOAS method applied to hyper‐spectral satellite data. Biogeosciences 9: 2127–2143. [Google Scholar]
- Schulz KG, Riebesell U, Rost B, Thoms S, Zeebe RE. 2006. Determination of the rate constants for the carbon dioxide to bicarbonate inter‐conversion in pH‐buffered seawater systems. Marine Chemistry 100: 53–65. [Google Scholar]
- Schulz KG, Rost B, Burkhardt S, Riebesell U, Thoms S, Wolf‐Gladrow DA. 2007. The effect of iron availability on the regulation of inorganic carbon acquisition in the coccolithophore Emiliania huxleyi and the significance of cellular compartmentation for stable carbon isotope fractionation. Geochimica et Cosmochimica Acta 71: 5301–5312. [Google Scholar]
- Sett S, Bach LT, Schulz KG, Koch‐Klavsen S, Lebrato M, Riebesell U. 2014. Temperature modulates coccolithophorid sensitivity of growth, photosynthesis and calcification to increasing seawater pCO2 . PLoS ONE 9: e88308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikes CS, Roer RD, Wilbur KM. 1980. Photosynthesis and coccolith formation: inorganic carbon sources and net inorganic reaction of deposition. Limnology and Oceanography 25: 248–261. [Google Scholar]
- Stojkovic S, Beardall J, Matear R. 2013. CO2‐concentrating mechanisms in three southern hemisphere strains of Emiliania huxleyi . Journal of Phycology 49: 670–679. [DOI] [PubMed] [Google Scholar]
- Stoll MHC, Bakker K, Nobbe GH, Haese RR. 2001. Continuous‐flow analysis of dissolved inorganic carbon content in seawater. Analytical Chemistry 73: 4111–4116. [DOI] [PubMed] [Google Scholar]
- Suffrian K, Schulz KG, Gutowska MA, Riebesell U, Bleich M. 2011. Cellular pH measurements in Emiliania huxleyi reveal pronounced membrane proton permeability. New Phytologist 190: 595–608. [DOI] [PubMed] [Google Scholar]
- Sukenik A, Bennett J, Falkowski P. 1987. Light‐saturated photosynthesis – limitation by electron transport or carbon fixation. Biochimica et Biophysica Acta 891: 205–215. [Google Scholar]
- Taylor AR, Chrachri A, Wheeler G, Goddard H, Brownlee C. 2011. A voltage‐gated H+ channel underlying pH homeostasis in calcifying coccolithophores. PLOS Biology 9: e1001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimborn S, Langer G, Rost B. 2007. Effect of varying calcium concentrations and light intensities on calcification and photosynthesis in Emiliania huxleyi . Limnology and Oceanography 52: 2285–2293. [Google Scholar]
- Weiss RF. 1970. The solubility of nitrogen, oxygen and argon in water and seawater. Deep‐Sea Research 17: 721–735. [Google Scholar]
- Winter A, Henderiks J, Beaufort L, Rickaby REM, Brown CW. 2013. Poleward expansion of the coccolithophore Emiliania huxleyi . Journal of Plankton Research 36: 316–325. [Google Scholar]
- Wolf‐Gladrow DA, Riebesell U, Burkhardt S, Bijma J. 1999. Direct effects of CO2 concentration on growth and isotopic composition of marine plankton. Tellus Series B – Chemical and Physical Meteorology 51: 461–476. [Google Scholar]
- Xu K, Gao K. 2015. Solar UV Irradiances modulate effects of ocean acidification on the coccolithophorid Emiliania huxleyi . Photochemistry and Photobiology 91: 92–101. [DOI] [PubMed] [Google Scholar]
- Zeebe RE, Wolf‐Gladrow DA. 2001. CO2 in seawater: equilibrium, kinetics, isotopes. Amsterdam, the Netherlands: Elsevier Science B.V. [Google Scholar]
- Zondervan I, Rost B, Riebesell U. 2002. Effect of CO2 concentration on the PIC/POC ratio in the coccolithophore Emiliania huxleyi grown under light‐limiting conditions and different daylengths. Journal of Experimental Marine Biology and Ecology 272: 55–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Table S1 Acclimation carbonate chemistry


