Abstract
Background
Postoperative wound complications are common following surgical procedures. Negative‐pressure wound therapy (NPWT) is well recognized for the management of open wounds and has been applied recently to closed surgical incisions. The evidence base to support this intervention is limited. The aim of this study was to assess whether NPWT reduces postoperative wound complications when applied to closed surgical incisions.
Methods
This was a systematic review and meta‐analysis of randomized clinical trials of NPWT compared with standard postoperative dressings on closed surgical incisions.
Results
Ten studies met the inclusion criteria, reporting on 1311 incisions in 1089 patients. NPWT was associated with a significant reduction in wound infection (relative risk (RR) 0·54, 95 per cent c.i. 0·33 to 0·89) and seroma formation (RR 0·48, 0·27 to 0·84) compared with standard care. The reduction in wound dehiscence was not significant. The numbers needed to treat were three (seroma), 17 (dehiscence) and 25 (infection). Methodological heterogeneity across studies led to downgrading of the quality of evidence to moderate for infection and seroma, and low for dehiscence.
Conclusion
Compared with standard postoperative dressings, NPWT significantly reduced the rate of wound infection and seroma when applied to closed surgical wounds. Heterogeneity between the included studies means that no general recommendations can be made yet.
Short abstract
Good for closed wounds too
Introduction
Postoperative wound complications, such as infection, dehiscence, and formation of haematoma or seroma, are common complications of surgical procedures1, 2, particularly among patients with risk factors such as obesity and diabetes3, 4, 5. Postoperative wound complications may lead to increased healthcare costs due to prolonged inpatient stay, repeat surgery and the need for increased follow‐up6, 7. Wound complications may also delay recovery, increase discomfort and reduce quality of life8, 9.
Negative‐pressure wound therapy (NPWT) is usually used for the treatment of open wounds. The wound is filled with a gauze or foam and sealed with an adhesive film dressing. The dressing is then connected to a vacuum device via a drain or port. The device ensures that negative pressure is transmitted to the wound bed and removes wound fluid10. The mechanisms of action include a characteristic pattern of blood flow around the wound, reduction in tissue oedema and stimulation of granulation tissue formation10, 11, 12. In recent years, the indication for NPWT has been extended to include treatment of closed surgical incisions (incisional NPWT, iNPWT). Some of the first studies were case series13, 14 and observational studies15, 16 using one of the existing NPWT devices (VAC®; KCI, San Antonio, Texas, USA) designed for open wounds17, 18. Two simplified NPWT devices became commercially available in 2010 (Prevena™; KCI) and 2011 (PICO™; Smith & Nephew, Hull, UK). These NPWT devices consist of a single‐use battery‐powered negative‐pressure therapy device, an easy‐to‐place dressing, and either a very small and easily portable canister, or no canister at all. In the latter case, the liquid is removed by evaporation through a semipermeable dressing. The mechanisms of action of this closed incision management have been supported by biomechanical studies: increased blood flow14, 19; decreased lateral and shear stress at the suture lines with decreased risk of wound dehiscence20; and increased lymph clearance with reduced formation of haematoma/seroma21 (Fig. 1).
Figure 1.
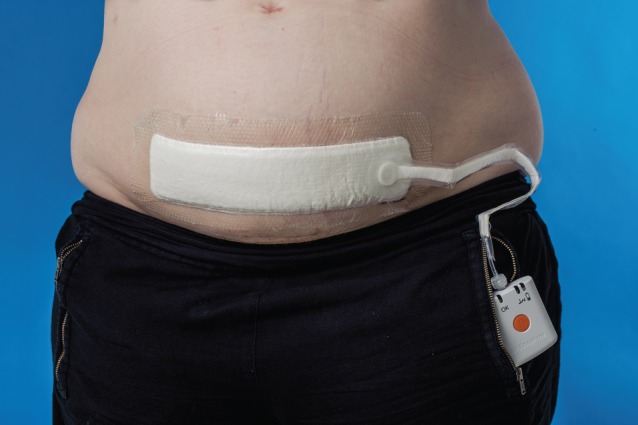
A negative‐pressure wound therapy dressing in situ
This systematic review investigated whether NPWT reduces the risk of wound complications such as wound infection, wound dehiscence and seroma when used on closed surgical incisions. It updates previous reviews22, 23, and includes data from new and unpublished trials. The quality of evidence of each outcome was evaluated thoroughly. Finally, the study applies assimilated data and includes a meta‐analysis, with the aim of providing robust evidence of the effect of NPWT on closed surgical incisions.
Watch three of the authors in a live video discussion of the results from the systematic review and the mode of action of iNPWT here.
Methods
The systematic review was conducted using well recognized methodology24 and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement25.
Eligibility criteria
Only randomized clinical trials (RCTs) comparing iNPWT with standard postoperative dressings were included. Studies that used NPWT devices designed for open wounds and surgical incisions, as well as studies that used home‐made NPWT devices, were accepted for inclusion. The standard dressings were any dressing used for surgical incisions, such as a sterile gauze dressing. The outcomes were wound complications, with wound infection, wound dehiscence and seroma as primary outcomes. No restrictions were made according to authors' definitions of outcome. Studies investigating the effect of iNPWT on other kinds of wound were excluded from the review.
Search strategy
The search strategy used the medical subject headings (MeSH) terms and free text words: ‘incisions’, ‘surgical procedures’ and ‘negative pressure wound therapy’ (Appendix S1, supporting information). The search strategy from the Cochrane Review22 was used when searching the Cochrane Library. In addition, the register ClinicalTrials.gov was searched and the authors contacted the manufacturers (KCI and Smith & Nephew) to identify ongoing trials. Finally, reference lists of identified studies, previously published systematic reviews and review articles were explored for additional references. There was no language restriction. To maintain a high sensitivity in the search strategy, no study design filters were used. One author searched the databases, assisted by a research librarian. The last literature search was performed on 1 August 2015.
Study selection and collection process
Two screening authors independently reviewed studies with full‐text assessment. From each included study, data were extracted on: the number and characteristics of the participants (including surgical procedure, and inclusion and exclusion criteria); the type of intervention (including type of NPWT device, type of dressing used in the control group for comparison, and duration of treatment in the intervention and control groups); the outcome measures, with specific definitions as reported in each study (including formation of haematoma or seroma, wound infection, dehiscence and other outcomes). If the information was inadequate, the corresponding authors were contacted for additional information.
Risk of bias in individual studies
To determine the risk of bias, two authors independently assessed the adequacy of sequence generation, concealment of allocation, blinding of outcome assessor, completeness of outcome data, and risk of selective outcomes and other bias using the Cochrane Collaboration tool26. Each item was deemed adequate, unclear or inadequate. Any disagreements between reviewers were resolved by consensus.
Synthesis of results
Data from each of the studies were organized in 2 × 2 contingency tables and used to calculate the relative risk (RR) for wound complications with associated 95 per cent c.i. The mean difference was used to summarize continuous data.
The meta‐analyses applied the random‐effects model of DerSimonian and Laird27. The random‐effects model was chosen because the studies assessed different kinds of surgical incision and therefore clinical heterogeneity was expected. Accordingly, the studies were treated as random samples, assuming that the true effect varied between studies. Study design effect and unit of analysis was taken into consideration to calculate the standard error based on the original study design (a detailed explanation can be found in Appendix S1, supporting information)26, 28. Statistical heterogeneity was examined as between‐study variation and tested using the Cochran Q test. Heterogeneity was quantified with the I 2 measuring the proportion of variation (inconsistency) in the combined estimates29. An I 2 value of 0 per cent indicates no inconsistency between the results of individual trials, and an I 2 value of 100 per cent indicates maximal inconsistency. Number needed to treat (NNT) to avoid a particular adverse outcome was calculated as 1/(ACR × (1 − RR)), where ACR is the assumed control risk26. All analyses were conducted in STATA® version 13.1 (StataCorp LP, College Station, Texas, USA). If a study was evaluated as having a high risk of bias that might influence the pooled estimates of the outcome of interest, a subgroup analysis was conducted, excluding that study.
The quality of evidence for each outcome was rated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system30. The evidence was classified as: high quality (the authors are very confident that the true effect lies close to that of the estimate of the effect); moderate quality (the authors are moderately confident in the effect estimate); low quality (the authors' confidence in the effect estimate is limited); or very low quality (the authors have very little confidence in the effect estimate)30, 31. The small study bias was assessed visually by examining the symmetry of funnel plots, and statistically as described by Egger et al.32.
Results
Study selection
The search of MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and the Cochrane Library provided a total of 1052 citations. After removal of duplicates, 872 remained. Eighty‐three full‐text papers were retrieved for inspection, of which seven were RCTs33, 34, 35, 36, 37, 38, 39. A further six unpublished studies were identified through contact with manufacturers and by searching ClinicalTrials.gov. The authors of all six unpublished studies were contacted; three responded with copies of submitted manuscripts (B. D. Crist et al. and S. Karlakki et al., personal communication) or posters (R. Galiano et al., personal communication). One study (V. Tanaydin, personal communication) was not ready for publication. A total of ten studies were included in the review (Fig. 2).
Figure 2.
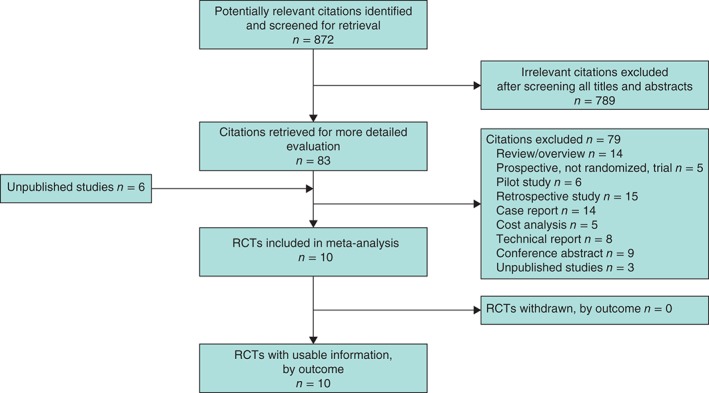
PRISMA diagram showing the selection of articles. RCT, randomized clinical trial
Study characteristics
Four studies33, 36, 37, including that of B. D. Crist et al. (personal communication), used an NPWT device (VAC®) and six34, 35, 38, 39, including those of S. Karlakki et al. and R. Galiano et al. (personal communication), employed an iNPWT device (Prevena™/PICO™). No studies of home‐made NPWT devices or studies comparing two different NPWT devices were found. One of the included studies was not reported as an RCT35. However, it was reported as a prospective trial describing a kind of randomization, and therefore is classified here as an RCT. This particular study was also categorized as an RCT in another review40. Hence, all ten studies selected for this review were rated as RCTs of NPWT versus standard postoperative dressings on closed surgical incisions. The patients were all at high risk of wound complications, due to either the surgical procedure or co‐morbidities. One study36 included both primary and delayed primary sutured incisions. In two studies33, 37 some of the patients had more than one wound treated with the intervention. Another study randomized by body part, treating each patient with both iNPWT and standard dressing (R. Galiano et al., personal communication). One study33 was stopped prematurely because of adverse reactions to iNPWT. Table 1 displays the characteristics of the included studies.
Table 1.
Characteristics of included studies
| Reference | Country | No. of incisions | Type of surgery | Treatment* | Inclusion and exclusion criteria | Outcome measures† | Follow‐up |
|---|---|---|---|---|---|---|---|
| Howell et al.33 | USA | 60 | Total knee arthroplasty | VAC® versus sterile gauze (dressing not described) (all wounds were assessed after 48 h) | Inclusion: BMI ≥ 30 kg/m2 and enoxaparin sodium for DVT | Primary: days to a dry wound (no drainage on gauze for 24 h) | 12 months |
| Exclusion: revision total knee replacement, previous knee surgery and documented diabetes | Secondary: total wound drainage, no. of gauze dressings applied to the wound, duration of hospital stay, incidence of infection, readmission | ||||||
| Pachowsky et al.34 | Germany | 19 | Total hip arthroplasty | Prevena™ versus ‘a dry wound coverage’ (dressing not described) (5 days versus n.s.) | n.s. | Primary: seroma (ultrasound imaging of the wound was used to show a seroma) | 10 days |
| Grauhan et al.35 | Germany | 150 | Median sternotomy | Prevena™ versus conventional wound dressings (dressing not described) (6–7 days versus 1–2 days) | Inclusion: BMI ≥ 30 kg/m2, and absence of preoperative signs of inflammation Exclusion: immunological disease, immunosuppressive therapy, skin disease | Primary: wound infection (defined according to US Centers for Disease Control and Prevention criteria) | 90 days |
| Secondary: dehiscence of skin or sternum | |||||||
| Masden et al.36 | USA | 81 | Primary or delayed primary closure of lower extremity or abdominal wounds | VAC® versus standard dry sterile dressing (Mepitel®) and a bacteriostatic single silver layer (Acticoat™) (all wounds assessed on day 3) | Inclusion: scheduled to undergo primary or delayed primary closure | Primary: wound infection, wound dehiscence (evaluated by a member of the research team, blinded to randomization groups) | Average of 113 days |
| Exclusion: patients allergic to tape or who could not tolerate NPWT; patients with lower‐extremity amputations distal to forefoot | |||||||
| Secondary: reoperation, duration of hospital stay | |||||||
| Stannard et al.37 | USA | 263 | High‐risk lower extremity fractures (tibial plateau, pilon, calcaneus) | VAC® versus standard postoperative dressing (dressing not described) (all wounds assessed on day 2) | Inclusion: presence of a high‐energy tibial plateau, pilon or calcaneus fractures | Primary: wound infection (defined with a combination of clinical signs and symptoms and laboratory data), wound dehiscence (defined as any separation of the incision that required wound care or reoperation) | n.s. |
| Exclusion: non‐operative or open fractures, receiving definitive surgery more than 16 days after injury, pregnant women and patients with low‐energy fractures | |||||||
| Pauser et al.38 | Germany | 21 | Hemiathroplasty | Prevena™ versus ‘a dry wound coverage’ (dressing not described) (5 days versus n.s.) | n.s. | Primary: seroma (ultrasound imaging of the wound was used to show a seroma) | 10 days |
| Nordmeyer et al.39 | Germany | 20 | Spinal fracture | PICO™ versus ‘a dry wound coverage’ (dressing not described) (5 days versus n.s.) | n.s. | Primary: seroma (ultrasound imaging of the wound was used to show a seroma) | 10 days |
| B. D. Crist et al. (personal communication) | USA | 90 | Pelvic, acetabular and hip fractures | VAC® versus a standard gauze (dressing not described) (all wounds assessed on day 2) | Inclusion: age 18 years or older, scheduled for surgical repair of pelvic and/or acetabular fracture, and subject/guardian able to provide informed consent | Primary: deep wound infection (‘Deep infection was one that went to the OR’) Secondary: wound drainage, duration of hospital stay, dressing supply costs, nursing time cost for dressing changes | 12 months |
| Exclusion: pregnancy, injury treated percutaneously without open surgery | |||||||
| S. Karlakki et al. (personal communication) | UK | 209 | Hip and knee replacement | PICO™ versus Mepore® or Tegaderm™ + Pad (3 M) (7 days versus 2 days) | Exclusion: known allergies to dressings, and those on warfarin | Primary: exudate from the surgical wound (predefined grading of wound exudate) | 6 weeks |
| Secondary: wound complications, readmissions, no. of dressing changes, overall cost‐effectiveness | |||||||
| R. Galiano et al. (personal communication) | USA, South Africa, France and the Netherlands | 398 | Bilateral breast reduction | PICO™ versus Steri‐Strip™ (7 days versus 7 days) | n.s. | Primary: delayed healing, dehiscence, wound infection (predefined description of primary outcome not stated) | 21 days |
| Secondary: scar quality | 90 days |
Text in parentheses indicates
duration of treatment before first dressing change and
predefined description of primary outcome. Mepitel™ (Mölnlycke Health Care, Gothenburg, Sweden); Acticoat™ (Smith & Nephew, Hull, UK); Mepore® (Mölnlycke Health Care); Tegaderm™ (3 M, St Paul, Minnesota, USA); Steri‐Strip™ (3 M). BMI, body mass index; DVT, deep vein thrombosis; n.s., not stated; NPWT, negative‐pressure wound therapy; OR, operating room.
Risk of bias within studies
The assessment of risk of bias within studies is shown in Table S1 (supporting information). Generally, the studies were reported incompletely (Fig. S1, supporting information). To obtain the missing information, the corresponding authors of the seven published papers were contacted. One study37 provided the information requested. One author36 did not respond to the request, but had corresponded with the authors of the Cochrane review22. Accordingly, for this specific trial, the information from the Cochrane review was used.
The included trials were of moderate methodological quality. In general, randomization and allocation concealment were not described adequately. Blinding of outcome assessment was described adequately in two studies36, 37. Three studies35, 36, including that of B. D. Crist et al. (personal communication), did not describe loss to follow‐up properly. There did not appear to be selective reporting bias. Finally, all studies, except one35, were either sponsored or had at least one consultant of a manufacturer as co‐author.
Results of individual studies
The ten included studies recruited a total of 1089 patients with 1311 incisions, of which 664 were treated with iNPWT and 647 with a standard postoperative dressing. The duration of treatment before first change of dressing varied from 2 to 7 (median 5) days in the intervention group and from 1 to 7 (median 2) days in the control group (duration was not stated in 3 studies34, 38, 39). The length of follow‐up varied from 10 days to 1 year. Only two studies (S. Karlakki et al. and R. Galiano et al., personal communication) recorded the time points of evaluation and how the outcomes were assessed after discharge. The reported outcome was wound infection in seven of ten studies (1251 incisions), wound dehiscence in four studies (892 incisions) and seroma in three (60 incisions). One study (S. Karlakki et al., personal communication; 209 incisions) reported wound exudate as a primary outcome, and another study33 (60 incisions) reported ‘days to dry wound’.
Synthesis of results
Wound infection
Wound infection was reported in seven studies33, 35, 36, 37, including the three unpublished studies. Of 634 patients treated with iNPWT, 30 (4·7 per cent) developed a wound infection, compared with 55 (8·9 per cent) of 617 in the control group (RR 0·54, 95 per cent c.i. 0·33 to 0·89) (Table 2; Fig. S2, supporting information). Two studies (reference 33 and B. D. Crist et al., personal communication) included only deep infections, and the meta‐analysis was therefore stratified by type of infection (overall and deep). Overall there was some statistical heterogeneity (I 2 = 11·1 per cent), but when stratified by type of infection, heterogeneity was reduced to 0 per cent in both subgroups. From the pooled data, the NNT to avoid one wound infection was 25 (95 per cent c.i. 17 to 93) (Table 2). The quality of evidence was downgraded to moderate, because of variability across studies and some methodological heterogeneity41. Two subgroup analyses were conducted. One omitted the study of Grauhan and colleagues35, which used alternation according to the time of operation as this can lead to selection bias42. The other subgroup analysis omitted the study that was stopped prematurely33. The pooled estimate and its 95 per cent c.i. did not change appreciably in direction or significance in either of the subanalyses (data not shown).
Table 2.
Summary of findings: incisional negative‐pressure wound therapy versus standard dressing for prevention of postoperative wound complications
| Outcome | Anticipated absolute effect (per 100)* | Relative risk | No. of incisions | Quality of evidence† | NNT | |
|---|---|---|---|---|---|---|
| Risk with standard dressing | Risk with iNPWT | |||||
| Wound infection | 9 | 5 (3, 8) | 0·54 (0·33, 0·89) | 1251 (7 RCTs) | Moderate‡ | 25 (17, 93) |
| Wound dehiscence | 20 | 14 (9, 21) | 0·69 (0·47, 1·01) | 892 (4 RCTs) | Low‡ § | 17 (10, −500) |
| Seroma | 85 | 41 (23, 71) | 0·48 (0·27, 0·84) | 40 (2 RCTs) | Moderate‡ ¶ | 3 (2, 8) |
Values in parentheses are 95 per cent c.i.
The risk in the intervention group (incisional negative‐pressure wound therapy, iNPWT) is based on the assumed risk in the comparison group (standard dressing) and the relative risk of the intervention.
Evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system30 (see text for full details);
moderate risk of bias in study;
inconsistency of direction of effect;
imprecision owing to small sample size. NNT, number needed to treat; RCT, randomized clinical trial.
Wound dehiscence
Wound dehiscence was reported in four studies that reported on sternotomy35, extremity wounds36 or fractures37, and breast reduction (R. Galiano et al., personal communication). Three studies35, 37, including that of R. Galiano et al. (personal communication), found that iNPWT reduced the risk of dehiscence, whereas one study36 found an increased risk of wound dehiscence. Overall, dehiscence occurred in 61 (13·3 per cent) of 459 patients in the iNPWT group, compared with 86 (20·0 per cent) of 433 with standard dressings (RR 0·69, 95 per cent c.i. 0·47 to 1·01; I 2 = 37·3 per cent). Owing to inconsistency in the direction of effect on wound dehiscence and a moderate risk of bias, the quality of evidence was downgraded to low43. The NNT to avoid wound dehiscence was 17; as the treatment effect was not significant, the 95 per cent c.i. became negative (10 to −500) (Table 2).
Seroma
Three studies, two on hip arthroplasty34, 38 and one on spinal fractures39, reported on seroma. The two studies on hip arthroplasty reported on the presence or absence of seroma. A seroma was present in eight (40 per cent) of 20 patients in the iNPWT group compared with 17 (85 per cent) of 20 in the standard dressing group (RR 0·48, 95 per cent c.i. 0·27 to 0·84; I 2 = 0 per cent) (Table 2 and Fig. S2, supporting information). The NNT to avoid a seroma was 3 (95 per cent c.i. 2 to 8) (Table 2). All three studies reported the volume of seroma measured by ultrasonography on day 5, and two studies34, 39 reported the volume on day 10. The mean difference between the two groups was −1·97 (95 per cent c.i. −3·13 to −0·82) and −1·44 (−3·02 to 0·13) ml respectively, favouring the iNPWT group (Fig. S3, supporting information). The same research group designed and conducted all three studies on seroma, and the studies were methodologically homogeneous. The quality of evidence was downgraded to moderate because of missing methodological information resulting in moderate risk of bias, and because of the few studies, with small sample sizes41, 44.
Other wound complications
Wound exudate was reported as a primary outcome in one study on hip and knee replacement (S. Karlakki et al., personal communication), which found that iNPWT significantly reduced the amount of wound exudate covering 50 per cent or more of the dressing. Five (4·9 per cent) of 102 patients in the intervention group and 21 (19·6 per cent) of 107 in the control group had exudate covering 50 per cent or more of the dressing. The difference was most pronounced for hip replacement, and increased with risk factors such as high body mass index (above 35 kg/m2), no drain, and wound closure with sutures. ‘Days to dry wound’ was reported in one study33 focusing on total knee arthroplasty. There was no significant difference in the days to dry wound between patients who had NPWT (4·3 (95 per cent c.i. 3·98 to 4·68) days) and those with a standard dressing (4·1 (3·79 to 4·32) days).
Three studies described the adverse effects of treatment. The first study35 stated that NPWT was well tolerated in all patients, whereas the other two studies (reference 33 and S. Karlakki et al., personal communication) found a significantly higher risk of blister formation in the iNPWT group. One study33 was stopped prematurely when 15 of 24 knee incisions treated with a iNPWT dressing developed skin blisters. The study by Karlakki and colleagues found that the blisters were minor and most pronounced around the knees.
Risk of bias across studies
As fewer than ten studies were included in each meta‐analysis, the assessment of publication bias using a funnel plot is not reported45. The meta‐analysis did, however, include studies favouring both treatments.
Discussion
This systematic review and meta‐analysis demonstrated that iNPWT reduced the rate of wound infection, seroma formation and wound exudate compared with standard postoperative dressings in surgical patients at risk of wound complications. There was no evidence that iNPWT reduces the risk of other types of wound complication. However, the diversity in clinical and methodological aspects implies that the results should be interpreted with caution, and no absolute or general recommendations can be made.
Of the ten included studies, seven had wound infection as a primary or secondary outcome. The RRs found in individual studies suggested that iNPWT increased the risk of deep wound infection (reference 33 and B. D. Crist et al., personal communication). However, this finding was based on a small number of incidents and therefore had weak statistical power. A relatively large number of patients were lost to follow‐up in the control groups in these studies. This may have resulted in an underestimation of the infection rate in the control group. In general, the included studies were small and underpowered, rendering a meta‐analysis more relevant. The follow‐up intervals and assessment timings were recorded in only two studies (S. Karlakki et al. and R. Galiano et al., personal communication), and these differed. Accordingly, small and underpowered studies are a weakness of the present meta‐analysis. Its strength is that, individually, the two largest published studies35, 37 showed significant results favouring iNPWT. Overall, iNPWT appeared to reduce the risk of wound infection, even though the quality of evidence was downgraded to moderate; this means that the true effect is likely to be close to the estimate of the effect, although there is a probability of a substantial difference30. The study by Stannard and co‐workers37 included 249 patients with 263 fractures, indicating that some patients had more than one fracture. Owing to lack of information it was not possible to adjust the design effect when synthesizing the results. The true standard error will be slightly smaller, which will result in a slightly higher weight. Accordingly, the theoretical upper limit of 95 per cent c.i. of the pooled results will increase slightly. However, given that only 5·6 per cent of the patients (14 of 249) had more than one wound, it is unlikely that the unadjusted RR would affect the pooled estimate significantly. The NNT to avoid one wound infection (25, 95 per cent c.i. 17 to 93) is relatively high considering the cost of treatment. However, the risk of developing an infection depends on the type of surgery as well as patient risk factors. Accordingly, the absolute number of patients, as well as the clinical importance, should be taken into consideration when interpreting the NNT.
Wound dehiscence can be caused by excessive tension on the wound edges, owing to the wound being located on a highly mobile or tensile area, or to wound infection. A study20 exploring the biomechanical mechanisms found that iNPWT reduced lateral tension and shared stress concentration at the sutures, which decreased the likelihood of dehiscence and possibly improved the cosmetic result. Four studies exploring the effect of iNPWT on wound dehiscence were included in the meta‐analysis. In the largest study, by R. Galiano et al. (personal communication), the patients had bilateral reduction mammoplasty and were treated with both iNPWT and a standard dressing to enable within‐patient comparisons. Furthermore, follow‐up intervals and assessment timings were well recorded. The study favouring standard dressing was the smallest (81 patients) and also had the highest number of events in both groups (30 versus 36 per cent)36. The quality of evidence was downgraded to low, indicating that confidence in the effect estimate was limited and the true effect may be substantially different from the estimate of the effect30. Accordingly, more studies are needed to examine the effect of iNPWT on wound dehiscence.
The three studies34, 38, 39 that reported on seroma formation were performed by the same research team, rendering the study designs comparable. Owing to some risk of bias within the studies, and because all three studies had a small sample size (19–21 patients), the quality of evidence was downgraded to moderate. The pooled results showed a convincing effect of iNPWT on seroma formation. The NNT to avoid one seroma (3, 95 per cent c.i. 2 to 8) suggested that iNPWT might be cost‐effective with respect to reducing the risk of seroma formation within the first 10 days after surgery. However, with a mean reduction of 1·97 ml (day 5) and 1·44 ml (day 10), the clinical relevance is questionable. A larger effect was found in an animal study21 focusing on the effect of iNPWT treatment on lymph clearance. The authors found that 4 days of treatment reduced the quantity of haematoma/seroma by a mean(s.e.m.) of 25(8) g in iNPWT sites compared with control sites. All three studies included in this review had a hospital‐based follow‐up of 10 days, which could be a weakness. Seroma volume and the duration of seroma production depend on many factors, including anatomical site, surgical procedure and the size of the defect. Many seromas might have decreased by day 10, but the total seroma production and a possible effect of iNPWT on this is difficult to assess when the patients have not been followed to the end of fluid production. Accordingly, longer follow‐up would be preferable.
The effect of iNPWT on wound exudate might be explained by the known biomechanical mechanisms that iNPWT applies to the tissue, such as a reduction in oedema, increased blood flow14, 19 and lymph clearance21. In contrast to the study on wound exudate (S. Karlakki et al., personal communication), the study that investigated ‘days to dry wounds’33 as a primary outcome found no effect of iNPWT. However, the two studies differed in size and type of iNPWT system (traditional33 versus new device) and the length of application on the incision (2 days33 versus 7 days).
Despite being RCTs, the studies in this review have weaknesses. There is some clinical and methodological heterogeneity as the studies did not assess the same surgical procedures, nor did they have exactly the same definition of the chosen outcomes. Three different devices, (PICOTM, PrevenaTM and VAC®) were used. Owing to differences in recommendations of duration of treatment, studies with 2–3 days of treatment were included together with studies with 5–7 days.
Another weakness is the duration of follow‐up, which varied from 10 days to 1 year. A surgical‐site infection is by definition incisional (superficial or deep) or organ/space infection occurring within 30 days of surgery46. The risk is prolonged to 1 year if an implant is left in place46. In addition, only two studies, by S. Karlakki et al. and R. Galiano et al. (personal communications), recorded the time intervals of evaluation and how the outcomes were assessed after discharge.
With respect to the commercially available NPWT devices used in the studies, it is a weakness that almost all included studies were either sponsored or had at least one consultant of the manufacturer as a co‐author. On the other hand, an advantage of the sponsored NPWT devices is that the results and the recommendations of the studies are easily applicable to daily clinical decisions. Furthermore, this review included studies both favouring iNPWT and those favouring standard dressings.
This systematic review and meta‐analysis has demonstrated that iNPWT reduces the rate of a number of wound complications compared with standard postoperative dressings. However, more RCTs should be performed. This is especially important for specific high‐risk surgical patients, such as those with diabetes or obesity. These trials should be suitably powered and follow international guidelines in methodology. The length of follow‐up should be 30 days when wound infection is the outcome of interest, expanded to 1 year if an implant is left in place. An economic evaluation should also be performed as part of the clinical trial.
Supporting information.
Additional supporting information may be found in the online version of this article:
Fig. S1 Bar chart of study quality (EPS file)
Fig. S2 Forest plot comparing the effect of negative‐pressure wound therapy and standard postoperative dressings on closed surgical incisions (EPS file)
Fig. S3 Forest plot comparing the effect of incisional negative‐pressure wound therapy and standard postoperative dressings on the volume of seroma (EPS file)
Fig. S4 Key messages of incisional NPWT from the systematic review and the live panel discussion (JPG file)
Table S1 Risk of bias within studies (Word document)
Appendix S1 Search strategies (Word document)
Appendix S2 Study design effect and unit of analysis (Word document)
Supporting information
Fig S1 Bar chart of study quality. Data in the stacked bars represent the number of studies meeting the quality criteria
Fig S2 Forest plot comparing the effect of negative‐pressure wound therapy (intervention) and standard postoperative dressings (control) on closed surgical incisions: A wound infection, stratified by type of infection; B wound dehiscence; C seroma formation. A DerSimonian and Laird (D‐L) random‐effects model was used for meta‐analysis. Risk ratios are shown with 95 per cent c.i.
Fig S3 Forest plot comparing the effect of incisional negative‐pressure wound therapy (intervention) and standard postoperative dressings (control) on the volume of seroma, measured by ultrasonography on days 5 and 10. A DerSimonian and Laird (D‐L) random‐effects model was used for meta‐analysis. Mean differences are shown with 95 per cent c.i.
Fig S4 Key message of incisional NPWT from the systematic review and the live panel discussion.
Table S1 Risk of bias within studies
AppendixS1 Search strategies
AppendixS2 Study design effect and unit of analysis
Acknowledgements
The authors thank C. Wu, Associate Professor at the University of Southern Denmark and Odense University Hospital, for statistical support. J. Cockwill, B. D. Crist, R. Galiano, S. Karlakki and J. Stannard kindly provided further information about their studies.
The authors are currently conducting a RCT of iNPWT that is partly funded by the manufacturer Smith & Nephew. They received an unrestricted grant of £61 093 (approximately €87 000, exchange rate 30 November 2015) and 500 PICO™ dressings free of charge.
Disclosure: The authors declare no other conflict of interest.
Presented to the 24th Conference of the European Wound Management Association, Madrid, Spain, May 2013, and to the Second Nordic Congress on Obesity in Gynaecology and Obstetrics, Middelfart, Denmark, August 2015
References
- 1. Culver DH, Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG et al Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med 1991; 91: 152S–157S. [DOI] [PubMed] [Google Scholar]
- 2. Haley RW, Culver DH, Morgan WM, White JW, Emori TG, Hooton TM. Identifying patients at high risk of surgical wound infection. A simple multivariate index of patient susceptibility and wound contamination. Am J Epidemiol 1985; 121: 206–215. [DOI] [PubMed] [Google Scholar]
- 3. Leth RA, Uldbjerg N, Norgaard M, Moller JK, Thomsen RW. Obesity, diabetes, and the risk of infections diagnosed in hospital and post‐discharge infections after cesarean section: a prospective cohort study. Acta Obstet Gynecol Scand 2011; 90: 501–509. [DOI] [PubMed] [Google Scholar]
- 4. Riou JP, Cohen JR, Johnson H Jr . Factors influencing wound dehiscence. Am J Surg 1992; 163: 324–330. [DOI] [PubMed] [Google Scholar]
- 5. Wilson JA, Clark JJ. Obesity: impediment to postsurgical wound healing. Adv Skin Wound Care 2004; 17: 426–435. [DOI] [PubMed] [Google Scholar]
- 6. Hjort A, Gottrup F. Cost of wound treatment to increase significantly in Denmark over the next decade. J Wound Care 2010; 19: 173–174, 176, 178, 180, 182, 184. [DOI] [PubMed] [Google Scholar]
- 7. Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health‐care providers in Europe. J Wound Care 2009; 18: 154–161. [DOI] [PubMed] [Google Scholar]
- 8. Andersson AE, Bergh I, Karlsson J, Nilsson K. Patients' experiences of acquiring a deep surgical site infection: an interview study. Am J Infect Control 2010; 38: 711–717. [DOI] [PubMed] [Google Scholar]
- 9. Solowiej K, Mason V, Upton D. Psychological stress and pain in wound care, part 2: a review of pain and stress assessment tools. J Wound Care 2010; 19: 110–115. [DOI] [PubMed] [Google Scholar]
- 10. Argenta LC, Morykwas MJ. Vacuum‐assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997; 38: 563–576. [PubMed] [Google Scholar]
- 11. Malmsjö M, Ingemansson R, Martin R, Huddleston E. Wound edge microvascular blood flow: effects of negative pressure wound therapy using gauze or polyurethane foam. Ann Plast Surg 2009; 63: 676–681. [DOI] [PubMed] [Google Scholar]
- 12. Malmsjö M, Ingemansson R, Martin R, Huddleston E. Negative‐pressure wound therapy using gauze or open‐cell polyurethane foam: similar early effects on pressure transduction and tissue contraction in an experimental porcine wound model. Wound Repair Regen 2009; 17: 200–205. [DOI] [PubMed] [Google Scholar]
- 13. Stannard JP, Atkins BZ, O'Malley D, Singh H, Bernstein B, Fahey M et al Use of negative pressure therapy on closed surgical incisions: a case series. Ostomy Wound Manage 2009; 55: 58–66. [PubMed] [Google Scholar]
- 14. Atkins BZ, Wooten MK, Kistler J, Hurley K, Hughes GC, Wolfe WG. Does negative pressure wound therapy have a role in preventing poststernotomy wound complications? Surg Innov 2009; 16: 140–146. [DOI] [PubMed] [Google Scholar]
- 15. Huberty SA, Gabriel A, Gupta S. Use of negative pressure therapy on closed surgical incisions. J Investig Med 2011; 59: 179. [Google Scholar]
- 16.Reddix RN Jr, Leng XI, Woodall J, Jackson B, Dedmond B, Webb LX. The effect of incisional negative pressure therapy on wound complications after acetabular fracture surgery. J Surg Orthop Adv 2010; 19: 91–97. [PubMed] [Google Scholar]
- 17. Gomoll AH, Lin A, Harris MB. Incisional vacuum‐assisted closure therapy. J Orthop Trauma 2006; 20: 705–709. [DOI] [PubMed] [Google Scholar]
- 18. DeCarbo WT, Hyer CF. Negative‐pressure wound therapy applied to high‐risk surgical incisions. J Foot Ankle Surg 2010; 49: 299–300. [DOI] [PubMed] [Google Scholar]
- 19. Malmsjo M, Huddleston E, Martin R. Biological effects of a disposable, canisterless negative pressure wound therapy system. Eplasty 2014; 14: e15. [PMC free article] [PubMed] [Google Scholar]
- 20. Wilkes RP, Kilpad DV, Zhao Y, Kazala R, McNulty A. Closed incision management with negative pressure wound therapy (CIM): biomechanics. Surg Innov 2012; 19: 67–75. [DOI] [PubMed] [Google Scholar]
- 21. Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): hematoma/seroma and involvement of the lymphatic system. Wound Repair Regen 2011; 19: 588–596. [DOI] [PubMed] [Google Scholar]
- 22. Webster J, Scuffham P, Stankiewicz M, Chaboyer WP. Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention. Cochrane Database Syst Rev 2014; (10)CD009261. [DOI] [PubMed] [Google Scholar]
- 23. Ingargiola MJ, Daniali LN, Lee ES. Does the application of incisional negative pressure therapy to high‐risk wounds prevent surgical site complications? A systematic review. Eplasty 2013; 13: e49. [PMC free article] [PubMed] [Google Scholar]
- 24. Khan KS. Systematic Reviews to Support Evidence‐based Medicine: How to Review and Apply Findings of Healthcare Research. Hodder Arnold: London, 2011. [Google Scholar]
- 25. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JPT, Green S. (eds). Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons: Chichester, 2008. [Google Scholar]
- 27. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 28. Perera R, Glasziou P. A simple method to correct for the design effect in systematic reviews of trials using paired dichotomous data. J Clin Epidemiol 2007; 60: 975–978. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J et al GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–394. [DOI] [PubMed] [Google Scholar]
- 31. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J et al GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64: 401–406. [DOI] [PubMed] [Google Scholar]
- 32. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Howell RD, Hadley S, Strauss E, Pelham FR. Blister formation with negative pressure dressings after total knee arthroplasty. Curr Orthop Pract 2011; 22: 176–179. [Google Scholar]
- 34. Pachowsky M, Gusinde J, Klein A, Lehrl S, Schulz‐Drost S, Schlechtweg P et al Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop 2012; 36: 719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grauhan O, Navasardyan A, Hofmann M, Müller P, Stein J, Hetzer R. Prevention of poststernotomy wound infections in obese patients by negative pressure wound therapy. J Thorac Cardiovasc Surg 2013; 145: 1387–1392. [DOI] [PubMed] [Google Scholar]
- 36. Masden D, Goldstein J, Endara M, Xu K, Steinberg J, Attinger C. Negative pressure wound therapy for at‐risk surgical closures in patients with multiple comorbidities: a prospective randomized controlled study. Ann Surg 2012; 255: 1043–1047. [DOI] [PubMed] [Google Scholar]
- 37. Stannard JP, Volgas DA, McGwin G III , Stewart RL, Obremskey W, Moore T et al Incisional negative pressure wound therapy after high‐risk lower extremity fractures. J Orthop Trauma 2012; 26: 37–42. [DOI] [PubMed] [Google Scholar]
- 38. Pauser J, Nordmeyer M, Biber R, Jantsch J, Kopschina C, Bail HJ et al Incisional negative pressure wound therapy after hemiarthroplasty for femoral neck fractures – reduction of wound complications. Int Wound J 2014; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nordmeyer M, Pauser J, Biber R, Jantsch J, Lehrl S, Kopschina C et al Negative pressure wound therapy for seroma prevention and surgical incision treatment in spinal fracture care. Int Wound J 2015; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karlakki S, Brem M, Giannini S, Khanduja V, Stannard J, Martin R. Negative pressure wound therapy for management of the surgical incision in orthopaedic surgery: a review of evidence and mechanisms for an emerging indication. Bone Joint Res 2013; 2: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P et al GRADE guidelines: 4. Rating the quality of evidence – study limitations (risk of bias). J Clin Epidemiol 2011; 64: 407–415. [DOI] [PubMed] [Google Scholar]
- 42. Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ et al CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg 2012; 10: 28–55. [DOI] [PubMed] [Google Scholar]
- 43. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M et al GRADE guidelines: 8. Rating the quality of evidence – indirectness. J Clin Epidemiol 2011; 64: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 44. Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D et al GRADE guidelines 6. Rating the quality of evidence – imprecision. J Clin Epidemiol 2011; 64: 1283–1293. [DOI] [PubMed] [Google Scholar]
- 45. Sterne JAC, Newton HJ, Cox NJ. Meta‐analysis in Stata: an Updated Collection from the Stata Journal. Stata Press: College Station, 2009. [Google Scholar]
- 46. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999; 27: 97–132. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1 Bar chart of study quality. Data in the stacked bars represent the number of studies meeting the quality criteria
Fig S2 Forest plot comparing the effect of negative‐pressure wound therapy (intervention) and standard postoperative dressings (control) on closed surgical incisions: A wound infection, stratified by type of infection; B wound dehiscence; C seroma formation. A DerSimonian and Laird (D‐L) random‐effects model was used for meta‐analysis. Risk ratios are shown with 95 per cent c.i.
Fig S3 Forest plot comparing the effect of incisional negative‐pressure wound therapy (intervention) and standard postoperative dressings (control) on the volume of seroma, measured by ultrasonography on days 5 and 10. A DerSimonian and Laird (D‐L) random‐effects model was used for meta‐analysis. Mean differences are shown with 95 per cent c.i.
Fig S4 Key message of incisional NPWT from the systematic review and the live panel discussion.
Table S1 Risk of bias within studies
AppendixS1 Search strategies
AppendixS2 Study design effect and unit of analysis


