Abstract
Inactivated parapoxvirus ovis (Orf virus; PPVO) recently displayed strong immunostimulating and modulating capacities in several animal models for acute and chronic virus infections through the induction of gamma interferon (IFN-γ) as a key mediator of antiviral activity. The data presented in this work demonstrate that inactivated PPVO has strong effects on cytokine secretion by human immune cells, including the upregulation of inflammatory and Th1-related cytokines (IFN-γ, tumor necrosis factor alpha [TNF-α], interleukin 6 [IL-6], IL-8, IL-12, and IL-18) as well as anti-inflammatory and Th2-related cytokines (IL-4, IL-10, and IL-1 receptor antagonist [IL-1ra]). Studies on the mechanism of action revealed virus particles to be the effective components of the preparation. The virus particles activate monocytes or other antigen-presenting cells (APC), e.g., plasmacytoid dendritic cells, through signaling over CD14 and a Toll-like receptor and the intracellular presence of certain PPVO-specific components. The activation of monocytes or APC is followed by the release of early proinflammatory cytokines (TNF-α, IL-6, and IL-8) as well as the Th1-related cytokines IL-12 and IL-18. Both IL-18 and IL-12 are involved in PPVO-mediated IFN-γ release by T cells and/or NK cells. The proinflammatory response is accompanied by the induction of anti-inflammatory and Th2-related cytokines (IL-4, IL-10, and IL-1ra), which exert a limiting efffect on the inflammatory response induced by PPVO. We conclude that the induction of a natural immune response with physiologically significant amounts of different cytokines and with antiviral potential might provide advantages over existing antiviral immunotherapies.
Parapoxvirus ovis (Orf virus; PPVO) is a member of the poxvirus family and causes pustular skin lesions in sheep, goats, and humans (20). The family of poxviruses consists of large DNA viruses with strong immunogenic properties. To evade the host immune defense, poxviruses utilize a variety of immunomodulatory cellular proteins, which enable them to replicate in spite of an active immune response (1, 16, 17, 28). Such proteins have been described for PPVO; these include a viral interleukin 10 (IL-10) homologue (11, 12), a granulocyte-macrophage colony-stimulating factor, an IL-2-inhibiting protein (8), and the product of a gene with homology to the vaccinia virus E3L gene, which counteracts interferon-induced cell resistance (18, 19, 21, 30).
The immunostimulating and modulating capacities made PPVO an interesting candidate for new antiviral strategies (36). The immunomodulatory properties of the virus could be used by means of a noninfectious preparation of inactivated PPVO to influence the clinical course of other viral infections. Thus, inactivated PPVO showed strong effects in several animal models for acute and chronic virus infections. It was effective in a guinea pig model for genital herpes disease and in a transgenic mouse model for human hepatitis B virus (HBV). In HBV-transgenic mice, PPVO led to a reduction in virus replication that was not associated with the destruction of liver cells. Furthermore, PPVO was able to reduce lethality in mice infected with herpes simplex virus. This effect also was not accompanied by a tissue-damaging inflammatory response or other side effects (36).
Investigations of the cytokine expression profiles of animals treated with PPVO implicate gamma interferon (IFN-γ) as a key mediator of the PPVO-mediated activity against HBV and herpes simplex virus. Thus, the application of PPVO first led to the release of Th1-inducing or effector cytokines, such as IL-12, IL-18, and IFN-γ. The neutralization of IFN-γ by antibodies could abolish antiviral effects in vivo. The induction of type 1 cytokines was followed by the secretion of anti-inflammatory cytokines, such as IL-4 (36). These findings indicate the induction of a limited immune response and offer an explanation for the good tolerability of the preparation in animals. The immunomodulatory properties of PPVO might be responsible for the observed antiviral effects and might contribute to the absence of systemic side effects and the absence of tissue injury that are observed after the direct administration of Th1-inducing or effector cytokines.
The aim of this study was to investigate whether the effects of PPVO can be determined under controlled conditions in human immune cells. In the work presented, we evaluated the cytokine secretion of peripheral blood cells, especially IFN-γ, which has been proved in animal models to be an important factor in transducing antiviral effects. In addition, we evaluated the mechanisms that mediate the effects of PPVO.
MATERIALS AND METHODS
Blood donors.
Blood was collected from healthy human donors after informed consent was obtained. The study was approved by the local ethics committee.
Cells.
Peripheral blood mononuclear cells (PBMC) were isolated from blood by Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation with Monovettes containing 10% citrate solution as an anticoagulant (Sarstedt, Nümbrecht, Germany) Cells were washed twice with phosphate-buffered saline (PBS) (Gibco, Eggenstein, Germany) and then cultured in RPMI 1640 medium (Biochrom KG, Berlin, Germany) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Biochrom KG), 2 mM l-glutamine (Biochrom KG), and 100 IU of penicillin-streptomycin (Gibco)/ml. Cell counts were determined by using a Neubauer chamber. Viable and dead cells were distinguished by staining with trypan blue. PBMC were used at a concentration of 106/ml. Cells were stimulated on plates. Cell viability was checked for the initial experiments after stimulation and before supernatants were collected to exclude a toxic effect of PPVO. This was done by staining cells with ethidium bromide and acridine orange at a concentration of 5 μg/ml each.
For maturation of blood monocytes to macrophages, PBMC were cultured in RPMI 1640 medium supplemented with 10% human AB serum (Sigma, Steinheim, Germany) instead of FCS. These cells were incubated before stimulation for 3 days at 37°C in 5% CO2. For whole-blood cultures, heparinized blood (15 IU/ml) collected with heparinized Vacutainers (Sarstedt) was used. The blood was diluted 1:5 with RPMI 1640 medium supplemented with 2 mM l-glutamine. Stimulation usually was performed with 500-μl portions in Eppendorf tubes.
Viruses.
PPVO was grown on a bovine kidney cell line and titrated on the same cell line. After the removal of cell debris, the harvested virus was inactivated with binary ethyleneimine and concentrated 10-fold by ultrafiltration. The virus titer of the stock preparation was approximately 105.6 50% tissue culture infective doses/ml. Vaccinia virus Lister was grown on the same bovine kidney cell line and purified and concentrated in exactly the same manner as PPVO. The virus titer of the stock preparation was approximately 105.3 tissue culture infective doses/ml. We used a well-standardized and validated procedure to obtain reproducible virus material.
Cell culture control.
As a control, supernatant from a mock-infected bovine kidney cell line which was treated in the same manner as the PPVO preparations was used.
Additional stimulants.
Concanavalin A (ConA) (lectin from Concanavalia ensiformis; Serva, Heidelberg, Germany) reconstituted in PBS was used for costimulation at a dose which led to minimal or no IFN-γ release. The concentration was determined by testing several donors and corresponded to a final concentration in PBMC of 1 μg/ml. In whole-blood cultures, this low dose varied between probands. Therefore, ConA at different concentrations (between 8 and 16 μg/ml) was used for stimulation, and then the optimal concentration was chosen. Testing of the appropriate ConA dose was repeated for every new batch of ConA and for every newly made ConA solution. Lipopolysaccharide (LPS) from Escherichia coli (Sigma), recombinant human IL-18 (R&D Systems, Abingdon, United Kingdom), recombinant human IL-12 (R&D Systems), and recombinant human tumor necrosis factor alpha (TNF-α) (R&D Systems) were also used.
Antibodies and other neutralizing substances.
The antibodies and other neutralizing substances used in this study included bactericidal permeability-increasing protein (BPI) (Xoma Corporation), zymosan A from Saccharomyces cerevisiae (Sigma), monoclonal anti-human IL-12 (p40/p70) antibody (Pharmingen), polyclonal anti-human IL-18 antibody (Tebu, Frankfurt am Main, Germany), normal rabbit immunoglobulin G as a control for anti-IL-18 (Tebu), monoclonal anti-human CD14 antibody (Immunotech), monoclonal anti-human IFN-γ antibody (Biosource, Nivelles, Belgium), and monoclonal antibody to human C3/C3b, clone 755 (Hbt; HyCult Biotechnology b.v., Uden, The Netherlands).
Assays for measurement of cytokine secretion.
The assays used for the measurement of cytokine secretion included the IFN-γ EASIA (0.9 to 30 IU/ml; (Biosource), the human IL-1ra Quantikine immunoassay (31 to 2,000 pg/ml; R&D Systems), the human IL-6 Quantikine immunoassay (3 to 300 pg/ml; R&D Systems), the human IL-12 HS Quantikine immunoassay (0.625 to 40 pg/ml; R&D Systems), the human IL-10 US ultrasensitive assay (0.78 to 50 pg/ml; Laboserv GmbH, Staufenberg, Germany), the human IL-4 US ultrasensitive assay (0.39 to 25 pg/ml; Laboserv), a human IL-18 assay (26 to 1,000 pg/ml; MBL Medical & Biological Laboratories), the Immulite TNF-α assay (2 to 1,000 pg/ml; DPC Biermann, Bad Nauheim, Germany), the Immulite IL-8 assay (6 to 7,500 pg/ml; DPC Biermann), and a human Th1/Th2 cytokine cytometric bead array (BD Biosciences Pharmingen) for the measurement of IFN-γ (0 to 10,000 pg/ml).
Real-time PCR for detection of cytokine mRNA.
Total RNA from cultured whole blood was isolated by using an Absolutely RNA RT-PCR Miniprep kit (Stratagene, La Jolla, Calif.). For cDNA synthesis, a master solution was prepared by mixing 2 μl of odT-Primer (0.1 mg/ml) (Promega, Madison, Wis.), 8 μl of 5 × first-strand buffer (GibcoBRL, Paisley, United Kingdom), 4 μl of dithiothreitol (0.1 M) (GibcoBRL), 4 μl of deoxynucleoside triphosphate (2.5 mM) (Pharmacia Biotech), 0.5 μl of RNasin RNase inhibitor (40 U/μl) (Promega, Madison, Wis.), and 2 μl of RNase-free DNase (2 U/μl) (Ambion, Huntingdon, United Kingdom). Antisense RNA dissolved in 19 μl of diethyl pyrocarbonate-treated water was reverse transcribed. The mixture was incubated at 37°C for 30 min and then for 5 min at 75°C to inactivate the DNase. Reverse transcription was started by adding 1 μl of RNasin RNase inhibitor (40 U/μl) and 1 μl of Moloney murine leukemia virus reverse transcriptase (200 U/μl) (GibcoBRL). The mixture was incubated at 42°C for 1 h. The reaction was stopped by incubation at 94°C for 10 min.
The expression of each gene was analyzed by real-time PCR with an ABI PRISM 7700 sequence detection system (TaqMan; Perkin-Elmer Applied Biosystems, Weiterstadt, Germany). The expression of the following genes was investigated: TNF-α, IFN-γ, and hypoxanthine-guanine-phosphoribosyltransferase (HPRT) (housekeeping gene). All primers and probes were designed by using Primer Express software (Perkin-Elmer) and validated. The amplification primers were designed to span the exon borders to exclude cross-reactivity with genomic DNA.
PCR was performed with a final volume of 25 μl containing 1 μl of cDNA, 12.5 μl of TaqMan universal PCR master mix (Perkin-Elmer), 1 μl of fluorogenic hybridization probe, 6 μl of primer mix, and 5.5 μl of distilled water. After an initial 2 min at 50°C for the activation of uracyl-N-glycosylase and the degradation of any preexisting contaminating RNA sequences, denaturation and hot start for AmpliTaq Gold DNA polymerase (Perkin-Elmer) were carried out at 95°C for 10 min. Amplification was carried out with a two-step PCR (40 cycles; 15 s of denaturation at 95°C and 1 min of annealing-extension at 60°C).
The cycle number at which the amplification plot crossed a fixed threshold above the baseline was defined as the threshold cycle (Ct). Because preliminary experiments demonstrated amplification efficiencies in our system of nearly 1.0 for all panels, specific gene expression was normalized to HPRT housekeeping gene expression with the formula 2−ΔCt. Relative gene expression was calculated with the formula 2−ΔΔCt, where the amount of the target gene is normalized to that of the HPRT gene: ΔΔCt is ΔCt (sample) − ΔCt (control), where ΔCt is the Ct of the target gene subtracted from the Ct of the housekeeping gene. This equation represents the normalized expression of the target gene in an unknown sample relative to the normalized expression of the control. The mean Ct values for the genes of interest and the HPRT gene were calculated from double determinations.
Substitution of plasma from whole-blood cultures.
Blood from each donor was divided into three 2-ml portions and centrifuged (10 min, 250 × g). The plasma was removed, and the cells were washed twice with RPMI 1640 medium. Plasma sample 1 remained unchanged, plasma sample 2 was heat inactivated for 30 min at 56°C, and plasma sample 3 was discarded and replaced with RPMI 1640 medium. Plasma and RPMI 1640 medium were added back to the cells, and the resulting mixture was stimulated in the same manner as whole-blood cultures.
Zymosan.
Blood from each donor was treated as described above. Plasma sample 1 remained unchanged, plasma sample 2 was heat inactivated, and plasma sample 3 was treated with zymosan. A total of 50 mg of zymosan was added to 5 ml of PBS. The mixture was boiled for 60 min before centrifugation (10 min, 750 × g) and removal of PBS. Then, plasma sample 3 was added, and plasma and zymosan were incubated for an additional 60 min at 37°C and then centrifuged (10 min, 750 × g). The plasma was removed and centrifuged over a filter in an Eppendorf tube for the complete removal of zymosan. Plasma was added back to the cells, and the resulting mixture was stimulated in the same manner as whole-blood cultures.
RESULTS
Inactivated PPVO leads to cytokine release from human immune cells. (i) Induction of IFN-γ by PPVO.
Earlier investigations of the immunomodulatory effects of inactivated PPVO were performed with various animal models. We were interested in determining whether an effect of PPVO on cytokine release could also be observed in human immune cells. First, we concentrated on the detection of IFN-γ, which was proved in animal models to be an important factor in mediating the antiviral effects of PPVO. Cultures of PBMC from several donors were stimulated for various periods with PPVO at various concentrations. Subsequently, IFN-γ levels in the supernatants were measured by an enzyme-linked immunosorbent assay (ELISA). However, only individual probands showed a slight release of IFN-γ, scarcely above the detection limit of the assay (Fig. 1). This marginal IFN-γ release reached a maximum after 4 days of stimulation and was obtained only at the highest concentration of PPVO, corresponding to a virus titer of 104.6/ml. A toxic effect of the preparation could be excluded.
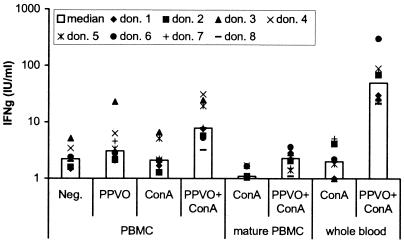
FIG. 1.
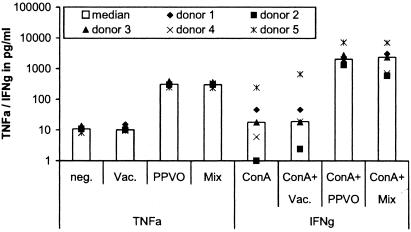
IFN-γ induction by PPVO (virus titer, 104.6/ml) in freshly stimulated PBMC (106 cells/ml), mature PBMC (106 cells/ml), and whole-blood cultures for eight donors (don.). ConA was used in PBMC at a concentration of 1 μg/ml and in whole blood at three different concentrations (8, 12, and 16 μg/ml) in parallel, because the responses to ConA differed between probands. For analysis, the optimal concentration was that which induced only a small amount of or no IFN-γ by itself. PBMC were cultured for 4 days, and whole blood was cultured for 3 days. RPMI 1640 medium served as a negative control (Neg.). The donors used for freshly stimulated PBMC, mature PBMC, and whole-blood stimulation were not identical. In mature PBMC and whole-blood cultures, no or only a small amount of IFN-γ was detectable without costimulation (data not shown). IFN-γ was significantly induced by PPVO alone (only in freshly stimulated PBMC) and by PPVO and ConA costimulation compared to the unstimulated negative control (the P value, determined by the Wilcoxon test, was <0.05).
Because in in vivo mouse infection models T cells are triggered through their T-cell receptor (TCR) by (viral) antigens, we speculated that a second stimulus might be necessary for IFN-γ induction by PPVO. From the literature, it is known that IL-12 receptors on T cells must first be upregulated by cross-linking of the TCR. We therefore selected as an additional T-cell stimulus a low dose of ConA, which alone leads to minimal or no IFN-γ release. Stimulation of PBMC cultures with PPVO was repeated in the presence of this low dose of ConA. Consistent with our hypothesis, significantly more IFN-γ was induced by simultaneous stimulation with PPVO and ConA than by stimulation with ConA or PPVO alone. Almost all probands showed a detectable IFN-γ response (Fig. 1). In addition, high responders could be distinguished from low responders.
Nevertheless, several donors still displayed marginal IFN-γ production even under these conditions. From experimental data obtained with animal models, we hypothesized that the presence of mature macrophages might be necessary. Therefore, PBMC from different persons were incubated before stimulation for 3 days with medium containing human AB serum, which contained the factors (e.g., macrophage colony-stimulating factor) that might be necessary to force macrophage maturation. The cells were stimulated with PPVO and ConA for 4 days, and IFN-γ levels in the supernatants were measured. However, this treatment caused no increase in IFN-γ induction; instead, it caused a decrease compared to freshly stimulated PBMC. Without costimulation, only two donors showed minimal IFN-γ induction (data not shown); after costimulation with ConA, a bit more IFN-γ was induced but less than in freshly stimulated PBMC (Fig. 1).
In a subsequent experiment, we examined whether the stimulation of whole-blood cultures with PPVO leads to sufficiently measurable IFN-γ release. Cultures of whole blood from several persons were stimulated with PPVO alone and together with ConA. Exclusive stimulation with PPVO caused no detectable IFN-γ release, in contrast to stimulation of PBMC (data not shown). Surprisingly, costimulation with ConA produced much more IFN-γ than was seen in PBMC cultures. All probands showed significant IFN-γ release, although the quantities varied, as was seen for PBMC cultures for individual donors (Fig. 1).
Costimulation with other substances, such as LPS and TNF-α, had no effect on IFN-γ induction by PPVO (data not shown).
(ii) Induction of other cytokines by PPVO.
Beside IFN-γ induction, it was also of interest to determine whether other cytokines are induced by PPVO. Significant differences with respect to IFN-γ release were observed between PBMC and whole-blood cultures. Therefore, the cytokine expression patterns in PBMC and whole-blood cultures were examined in parallel. Cells were stimulated with PPVO alone and costimulated with PPVO and ConA. Cytokine levels in the supernatants were measured by an ELISA. PPVO treatment alone did induce TNF-α, IL-8, IL-6, IL-10, IL-1ra, and IL-18. TNF-α, IL-8, IL-6, and IL-10 were detected in PBMC and whole-blood cultures. IL-1ra and IL-18 were detectable only in whole-blood cultures. ConA costimulation had no effect on these cytokines, except for TNF-α, the level of which was substantially increased, and for IL-18, the level of which was slightly increased. Costimulation with ConA also led to the release of IL-4 and IL-12 (biologically active heterodimer). IL-4 was released in PBMC and whole-blood cultures, while IL-12 was detectable only in whole-blood cultures (Fig. 2). A quantitative comparison of the proportions of the two antagonistically working cytokines, IL-4 and IFN-γ, showed that IFN-γ was the dominating cytokine (data not shown).
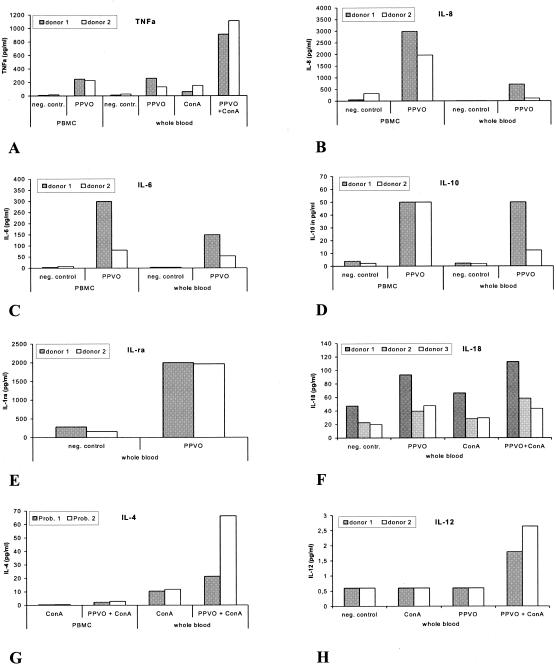
FIG. 2.
Cytokine induction by PPVO (virus titer, 104.6/ml). ConA was used in PBMC (106 cells/ml) at a concentration of 1 μg/ml and in whole blood (WB) at a concentration of 12 μg/ml. RPMI 1640 medium served as a negative (neg.) control (contr.). (A) TNF-α is induced in PBMC and WB. Costimulation with ConA caused an increase in TNF-α secretion in WB but not in PBMC. Cells were cultured for 24 h. (B) IL-8 is induced in PBMC and WB. Costimulation with ConA had no effect. Cells were cultured for 4 h. Differences between IL-8 levels in PBMC and WB might be due to high-affinity binding of IL-8 to erythrocytes. (C) IL-6 is released in PBMC and at slightly lower levels in WB. This difference might be caused by a higher level of nonspecific binding of IL-6 in WB. Cells were stimulated for 4 h. Costimulation with ConA had no effect. (D) IL-10 is induced in PBMC and WB. Cells were incubated for 24 h. Costimulation with ConA had no effect. (E) IL-1ra is induced in WB. Costimulation with ConA had no effect. Cells were cultured for 24 h. IL-1ra was not measured in PBMC. (F) Small amounts of IL-18 are induced in WB. The induction was slightly increased by ConA costimulation. Cells were incubated for 24 h. IL-18 was not measured in PBMC. (G) IL-4 is released in PBMC and at higher levels in WB with ConA costimulation. IL-4 was measured in the same supernatants as IFN-γ. PBMC were cultured for 4 days, and WB was cultured for 3 days. IFN-γ was the dominating cytokine (data not shown). (H) IL-12 is induced in small amounts but only in WB and only with ConA costimulation. Cells were incubated for 24 h.
The PPVO effect was not due to LPS contamination.
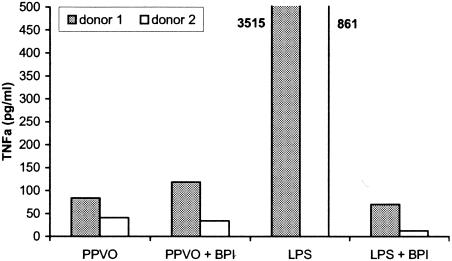
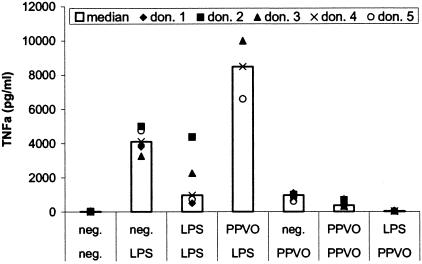
In order to exclude the possibility that LPS contamination was the reason for the induction of IFN-γ and other cytokines by the PPVO preparation, we repeated the stimulation with PPVO in the presence of BPI, which binds with a high affinity to LPS and inhibits its effect. In the presence of BPI, the stimulation of whole-blood cultures with PPVO resulted in no change in TNF-α release (Fig. 3). Therefore, the effect of PPVO was not mediated by LPS contamination.
FIG. 3.
The presence of BPI has no influence on TNF-α induction by PPVO. PBMC (106/ml) were stimulated with LPS (100 pg/ml) and PPVO (virus titer, 104.6/ml) alone or in the presence of BPI (10 μg/ml) for 24 h. BPI abolished TNF-α induction after LPS treatment but not after PPVO treatment. The TNF-α levels of the LPS-stimulated samples significantly exceeded the other values and are shown beside the columns.
TNF-α and IFN-γ secretion after PPVO stimulation is accompanied by increased levels of TNF-α and IFN-γ mRNA expression.
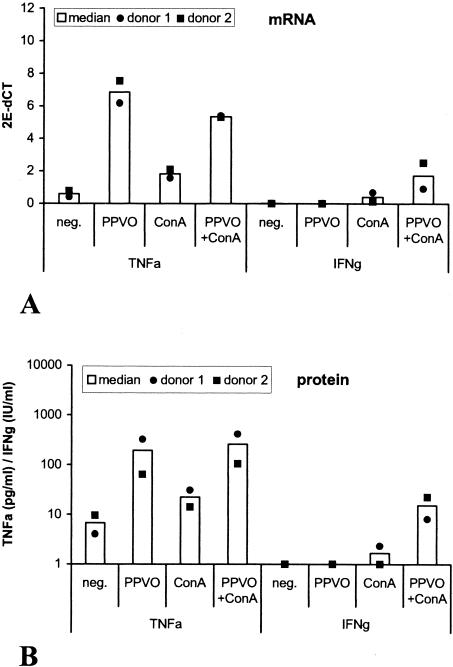
In a subsequent experiment, we investigated whether the increased release of IFN-γ and TNF-α after stimulation with PPVO is regulated at the transcriptional level or the translational level. Whole-blood cultures were stimulated with PPVO alone and costimulated with PPVO and ConA, and TNF-α and IFN-γ release in the supernatants was measured by an ELISA. Cytokine mRNA was measured in the cells by quantitative real-time reverse transcription-PCR. TNF-α and IFN-γ induction by PPVO was accompanied by increased levels of TNF-α and IFN-γ mRNA expression. TNF-α mRNA secretion and protein secretion were induced by PPVO alone. The additional costimulation with ConA resulted in a further increase in the protein level without a detectable effect on the mRNA level. The induction of both IFN-γ mRNA expression and IFN-γ protein expression occurred only after costimulation with ConA (Fig. 4). Thus, PPVO-enhanced cytokine expression seems to be regulated preferentially at the transcriptional level.
FIG. 4.
TNF-α and IFN-γ protein secretion by PPVO is accompanied by increased TNF-α and IFN-γ mRNA levels. Whole-blood cultures were stimulated with PPVO (virus titer, 104.6/ml) and ConA (12 μg/ml). RPMI 1640 medium served as a negative (neg.) control. Cells were incubated for 4 h for TNF-α and for 24 h for IFN-γ mRNA and protein detection. (A) TNF-α and IFN-γ mRNA expression after PPVO stimulation. (B) TNF-α and IFN-γ protein expression for the same donors after PPVO stimulation.
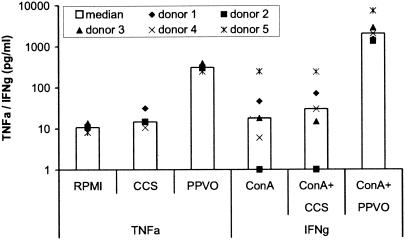
The increase in TNF-α secretion after costimulation with PPVO and ConA is only partially due to IFN-γ upregulation.
TNF-α induction by PPVO is strongly increased after costimulation with ConA. This effect could be attributed to the upregulation of IFN-γ, which occurs only in the presence of ConA. Under these conditions, IFN-γ leads to additional stimulation of monocytes that could result in an enhanced TNF-α response to PPVO. In order to investigate this hypothesis in more detail, we incubated whole-blood cultures with PPVO and ConA in the presence of a blocking antibody against IFN-γ. The antibody was administered to the cells at the same time as PPVO and ConA. Cell cultures were stimulated for 24 h, and TNF-α release in the supernatants was measured. The anti-IFN-γ antibody caused a decrease in TNF-α release by PPVO and ConA of about 20%, indicating only partial reversal of the ConA-induced enhancement of PPVO-stimulated TNF-α release (Fig. 5).
FIG. 5.
The enhanced TNF-α release by PPVO in whole-blood cultures after ConA costimulation is partially due to IFN-γ. ConA was used at a concentration of 16 μg/ml, anti-IFN-γ antibody was used at a concentration of 5 μg/ml, and the PPVO titer was 104.6/ml.
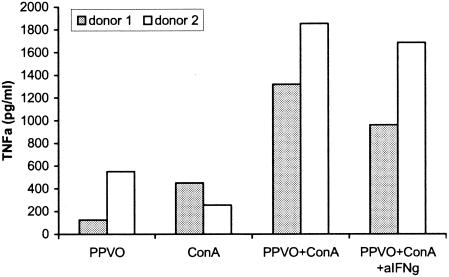
The active component of the PPVO preparation is linked to virus particles.
In subsequent studies, we investigated whether the active component of the preparation is linked to virus particles or whether it is rather a soluble factor in supernatants. The PPVO preparation was centrifuged at 4,000 × g and 17,000 × g for 30 min, and whole-blood cultures were stimulated with both supernatants and pellets. IFN-γ and TNF-α levels in the supernatants were measured by an ELISA. Even with a centrifugal force of 4,000 × g, the IFN-γ- and TNF-α-inducing components both were exclusively contained in the pellets (Fig. 6). This result indicates that the active component is linked to virus particles.
FIG. 6.
The IFN-γ- and TNF-α-inducing effects of PPVO are linked to virus particles. The PPVO titer was 104.6/ml, and ConA was used at a concentration of 12 or 16 μg/ml. The PPVO preparation was centrifuged at 4,000 × g and 17,000 × g. The resulting supernatant (su) and pellet (pe) were used for stimulation. RPMI 1640 medium served as a negative (neg.) control (contr.). Since costimulation with ConA is necessary for the induction of IFN-γ but not for the induction of TNF-α, ConA stimulation was used for IFN-γ measurement but not for TNF-α measurement. (A) The IFN-γ-inducing component of PPVO is located in the pellet after centrifugation. Cells were incubated for 3 days (B) The TNF-α-inducing component also is located in the pellet. Cells were incubated for 24 h.
This conclusion is supported by the facts that virus-free supernatants from infected and noninfected cell cultures showed no immunostimulatory activity in the murine model (36) and that supernatants from mock-infected cell cultures also had no TNF-α- or IFN-γ-inducing effect in our model (Fig. 7).
FIG. 7.
A cell culture supernatant from the same cell line as that used for growing virus has no TNF-α- or IFN-γ-inducing activity. Whole-blood cultures were stimulated with PPVO (virus titer, 104.6/ml), virus-free cell culture supernatant (CCS), and RPMI 1640 medium as a negative control for 4 h for TNF-α induction and for 2 days in the presence of ConA (1 μg/ml) for IFN-γ induction.
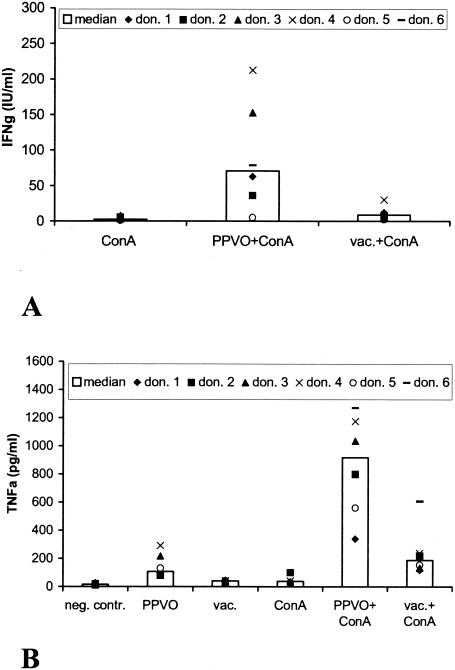
Vaccinia virus induces only minimal cytokine secretion.
In another experiment, we aimed to investigate whether the observed cytokine induction is specific for inactivated PPVO or whether a preparation of another inactivated poxvirus, vaccinia virus, would lead to a similar cytokine induction profile. Whole-blood cultures were stimulated with vaccinia virus alone and with vaccinia virus and ConA. As with PPVO, we observed a slight release of IFN-γ in the presence of ConA. However, the amount of IFN-γ secreted into the supernatants was only about 15% the amount of IFN-γ induced by PPVO. TNF-α was induced approximately threefold less by vaccinia virus than by PPVO. As with PPVO, costimulation was not necessary (Fig. 8).
FIG. 8.
Vaccinia virus induces less IFN-γ and TNF-α than does PPVO. ConA was used at a concentration of 12 or 16 μg/ml, the PPVO titer was 104.6/ml, and the vaccinia virus (vac.) titer was 105.3/ml. RPMI 1640 medium served as a negative (neg.) control (contr.). don., donor. (A) IFN-γ induction by vaccinia virus was significantly lower (the P value, determined by the Wilcoxon test, was <0.05) than that by PPVO. IFN-γ secretion induced by PPVO and vaccinia virus without ConA costimulation was below the detection limit of the assay (data not shown). (B) TNF-α induction by vaccinia virus was significantly lower (Wilcoxon test; P value, <0.05) than that by PPVO.
To confirm that the minor cytokine induction seen with the vaccinia virus preparation was indeed due to a weaker effect of virus particles and not to a different amount of a potential inhibitor in the preparation, we repeated the stimulation of whole-blood cultures with PPVO alone, with vaccinia virus alone, and with a mixture of PPVO and vaccinia virus. Mixed PPVO led to the same IFN-γ and TNF-α responses as PPVO alone (Fig. 9).
FIG. 9.
The vaccinia virus preparation is free of a contaminating cytokine-inhibiting factor. Whole-blood cultures were stimulated for 4 h for TNF-α induction and for 2 days in the presence of ConA (1 μg/ml) for IFN-γ induction with a mixture of PPVO (virus titer, 104.6/ml) and vaccinia virus (Vac.) (virus titer, 104.6/ml) and with each virus alone. neg., negative control.
A plasma factor is necessary for IFN-γ induction by PPVO.
The data so far showed considerable differences in the stimulation of PBMC and whole-blood cultures. Substantially more IFN-γ was produced in whole-blood cultures than in PBMC cultures. Therefore, we investigated which factor(s) is responsible for this discrepancy. In a comparison of the components of PBMC and whole blood, there are two major differences. First, some cells that are present in whole blood are missing in PBMC. These cells are thrombocytes, granulocytes, and erythrocytes. PBMC, in contrast, mainly consist of lymphocytes and monocytes. Second, the unchanged plasma of the donor is present in whole-blood cultures, while in PBMC cultures, inactivated FCS is used. Therefore, either the additional cell types or a factor from plasma may be essential for IFN-γ induction by PPVO.
To address this question, we carried out the following experiment. Blood from each donor was divided into three portions, and cells and plasma were separated by centrifugation. The plasma was removed, and the cells were washed twice with RPMI 1640 medium. The plasma samples were treated differently. Plasma sample 1 remained unchanged, like “normal” whole-blood cultures. Plasma sample 2 was inactivated by incubation at 56°C for 30 min, which mimics the FCS treatment used for PBMC cultures. Then, plasma samples 1 and 2 were added back to the cell cultures. Plasma sample 3 was discarded and replaced with the same quantity of RPMI 1640 medium containing heat-inactivated FCS. Thus, only changes in the plasma were made; the cellular components of the blood remained unchanged. The cultures were subsequently stimulated like whole-blood cultures with PPVO and ConA, and IFN-γ and TNF-α levels in the supernatants were determined.
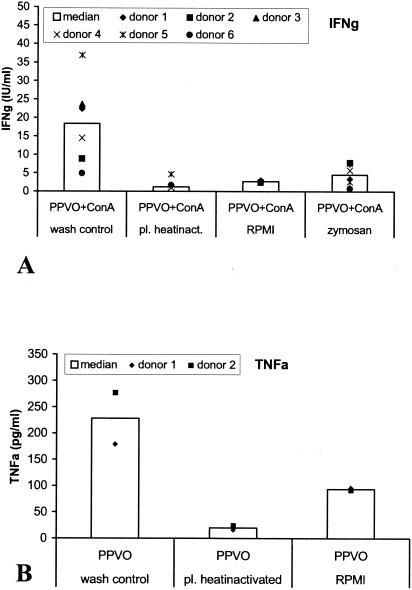
In culture 1, which corresponds to a normal whole-blood culture and serves as a wash control, IFN-γ was induced by PPVO and ConA, with no difference from other whole-blood cultures. In contrast, IFN-γ was no longer detectable in cultures with heat-inactivated plasma or in cultures in which plasma was replaced by RPMI 1640 medium with FCS (Fig. 10A). We conclude from these results that a heat-sensitive factor from plasma is needed for PPVO-mediated IFN-γ induction. This conclusion would explain why less IFN-γ is released in PBMC than in whole blood cultures. The additional cell types existing in whole blood are not important for IFN-γ induction.
FIG. 10.
A plasma factor is necessary for IFN-γ induction by PPVO. The PPVO titer was 104.6/ml, and ConA was used at a concentration of 12 μg/ml. (A) Decreased IFN-γ induction by PPVO in whole-blood cultures with heat-inactivated plasma (pl. heatinact.), with plasma replaced by RPMI 1640 medium, and with zymosan-treated plasma. Cells were costimulated with ConA and incubated for 3 days. For each category, data from six donors were available, except for replacement of plasma by RPMI 1640 medium, which was performed only for the first two donors. The decrease in IFN-γ induction was significant (the P value, determined by the Wilcoxon test, was <0.05) for cultures with heat-inactivated plasma and for those treated with zymosan. (B) Decreased TNF-α induction in whole-blood cultures with heat-inactivated plasma. TNF-α induction was not measured in cultures treated with zymosan. Cells were stimulated for 24 h.
TNF-α levels in the supernatants were also measured. In contrast to IFN-γ, TNF-α is released by PBMC. In a subsequent experiment, we investigated the influence of the plasma factor on TNF-α induction. In cell cultures with heat-inactivated plasma, TNF-α was not released. In contrast, TNF-α was induced in cultures in which plasma was replaced by RPMI 1640 medium with FCS, although to a lesser extent than in whole-blood cultures (Fig. 10B).
The plasma factor essential for IFN-γ induction is complement.
With the next experiments, we wanted to further characterize the plasma factor required for IFN-γ induction. We hypothesized that this factor might be complement because virus particles are needed for IFN-γ induction. For cellular uptake of particles, opsonization with complement might be necessary.
Blood from each donor was divided into three portions, and cells and plasma were separated by centrifugation. The plasma was removed, and the cells were washed twice with RPMI 1640 medium. Plasma sample 1 remained unchanged, plasma sample 2 was heat inactivated at 56°C for 30 min, and plasma sample 3 was incubated for 1 h at 37°C with zymosan, which then was removed by filtration. Zymosan consists of yeast particles, which are able to activate and bind complement. During the incubation of plasma with zymosan, the complement contained in the plasma is bound and later removed together with the yeast particles. After this treatment, the plasma is free of inducible C3. The plasma was added back to the cells, and the mixture was stimulated with PPVO and ConA. No IFN-γ induction was detected in these cultures after stimulation with PPVO and ConA (Fig. 10A), suggesting that complement may be important for the induction of cytokines.
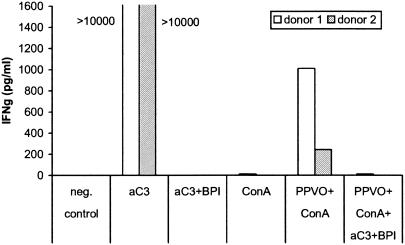
To confirm the importance of complement, we repeated the stimulation of whole-blood cultures with PPVO and ConA in the presence of a neutralizing antibody against complement factor C3. In the presence of this anti-C3 antibody, no IFN-γ induction by PPVO was detectable (Fig. 11).
FIG. 11.
Neutralization of complement factor C3 blocks IFN-γ induction by PPVO. Whole-blood cultures were stimulated for 2 days with PPVO (virus titer, 104.6/ml) and ConA (1 μg/ml) in the presence of a neutralizing anti-C3 antibody (aC3) (10 μg/ml). Because of the lack of availability of an endotoxin-free antibody preparation, we incubated the antibody prior to stimulation for 30 min with BPI (1 mg/ml) to block the effect of LPS. RPMI 1640 medium served as a negative (neg.) control.
The CD14 receptor on monocytes is involved in transducing the effects induced by PPVO.
The immune system is able to recognize characteristic structures of microorganisms through so-called pattern recognition receptors. Several recently characterized proteins, the Toll-like receptors (TLR), play an important role. So far, 10 TLR have been identified; all recognize different microbial structures. Although NF-κB seems to be activated by all TLR, different intracellular signaling pathways influence the cytokine response patterns. In the activation of these TLR, the CD14 receptor on macrophages can be involved as a coreceptor. This receptor, which has no intracellular domain, can associate with TLR to form a complex for transmembrane signaling (34, 35). CD14 plays a role in transducing the effects of different natural stimuli. Therefore, it seemed likely that it could also be involved in transducing the effects of PPVO. In order to examine this possibility, we stimulated whole-blood cultures for 4 h with LPS and PPVO in the presence of a blocking antibody against the CD14 receptor. TNF-α levels in the supernatants then were measured.
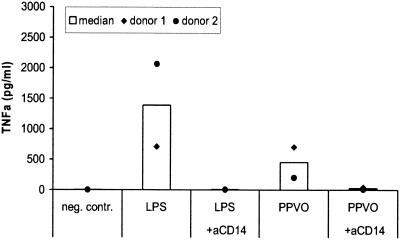
TNF-α release induced by LPS was completely blocked in the presence of the anti-CD14 antibody. The amount of TNF-α induced by PPVO also was significantly reduced in the presence of the antibody (Fig. 12). CD14 therefore is obviously important for transducing the effects of PPVO.
FIG. 12.
Blocking of the CD14 receptor leads to decreased TNF-α levels after PPVO stimulation. Whole-blood cultures were stimulated for 4 h with LPS (500 pg/ml) and PPVO (virus titer, 104.6/ml) alone or in the presence of a blocking anti-CD14 antibody (aCD14) (10 μg/ml). RPMI 1640 medium served as a negative (neg.) control (contr.).
PPVO and LPS affect each other mutually.
The signaling pathways of LPS have been relatively well examined, and it is known that LPS activates TLR4 (9). To examine the signaling pathways of PPVO, we used so-called LPS desensitization. This concept is based on the fact that cells which have been stimulated with a small quantity of LPS produce less TNF-α after renewed stimulation with LPS than cells which have not been stimulated twice. If LPS and PPVO use similar signaling pathways, cross-desensitization between LPS and PPVO might occur. PBMC were stimulated with PPVO or a low dose of LPS for 24 h, washed several times, and then stimulated again with a high dose of LPS or PPVO. After this treatment, TNF-α levels in the supernatants were determined.
Successive stimulation with the same ligand, i.e., PPVO on PPVO, like LPS on LPS, led to self-desensitization of the cells, with decreased TNF-α levels, after the second incubation. Desensitization with PPVO prior to LPS treatment did not result in a decreased response to LPS but rather in an intensified TNF-α release. However, stimulation with LPS prior to PPVO treatment almost completely abolished TNF-α release after PPVO stimulation (Fig. 13). Thus, the signaling pathways of LPS and PPVO seem not to be identical but obviously can affect each other. As signaling through TLR4 is much stronger than signaling through other TLR pathways, it seems that PPVO does not use TLR4 but crosses the pathway downstream. TLR4 activation (LPS) also induces more TNF-α release than PPVO stimulation, supporting this view.
FIG. 13.
Mutual interference of LPS and PPVO. PBMC were stimulated for 24 h with the substances noted in the upper row of the x axis, namely, RPMI 1640 medium as a negative (neg.) control, a low dose of LPS (100 pg/ml), or PPVO (virus titer, 104.6/ml). This stimulation was followed by a second stimulation for 4 h with the substances noted in the second row, namely, RPMI 1640 medium as a negative control, a high dose of LPS (100 ng/ml), or PPVO (virus titer, 104.6). For stimulation with PPVO prior to LPS and LPS prior to PPVO, the results for only three donors (don.) are shown, while for the other items, data for five donors were available.
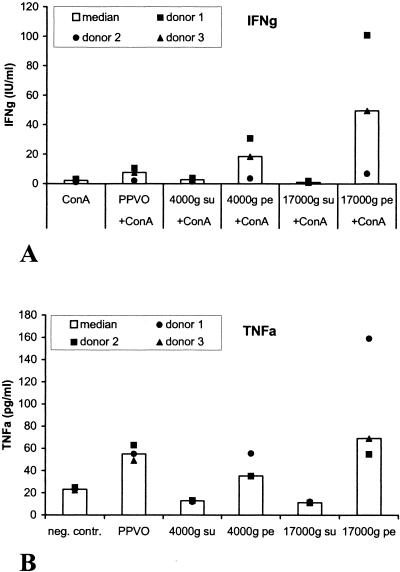
IL-12 and IL-18 are involved in IFN-γ release after PPVO stimulation.
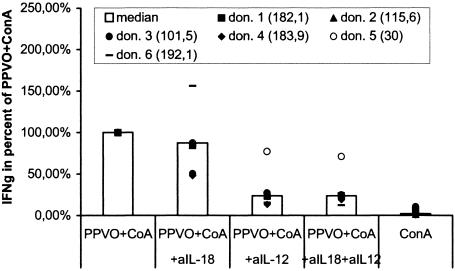
Because PPVO seems to activate monocytes through a TLR or a TLR-related pathway and also induces IL-12 and IL-18, which are both able to induce IFN-γ, it is quite possible that PPVO first leads to the release of IL-12 and IL-18, which then in turn induce IFN-γ. In order to examine the roles of both cytokines in IFN-γ induction after PPVO stimulation, whole-blood cultures were stimulated with PPVO and ConA in the presence of neutralizing antibodies against IL-12 and IL-18. Both were used at a concentration of 5 μg/ml. They were added to the cell cultures at the same time as PPVO. Isotype-matched antibodies served as a control; another control involved the administration of the antibodies to cell cultures stimulated with recombinant IL-12 and IL-18. Finally, IFN-γ levels in the supernatants were measured. The neutralization of IL-18 resulted in a decrease in IFN-γ levels of approximately 20%, while the neutralization of IL-12 caused a decrease of up to 80%. However, simultaneous neutralization of both cytokines did not lead to further inhibition. After elimination of both cytokines, approximately 20% of the initial IFN-γ was detected (Fig. 14).
FIG. 14.
Neutralization of IL-12 and IL-18 results in decreased IFN-γ secretion after PPVO stimulation. ConA was used at a concentration of 12 μg/ml, anti-IL-18 (aIL-18) and anti-IL-12 (aIL-12) antibodies were used at a concentration of 5 μg/ml each, and the PPVO titer was 104.6/ml. IFN-γ was originally measured in international units per milliliter but is shown as percentages of the values obtained with PPVO plus ConA. The absolute values for PPVO plus ConA in international units per milliliter are given in parentheses. IFN-γ secretion was significantly decreased for stimulation in the presence of the anti-IL-12 antibody and in the presence of both antibodies compared to stimulation without the neutralizing antibodies (the P value, determined by the Wilcoxon test, was <0.05). Isotype-matched control antibodies had no effect on IFN-γ induction. don., donor.
IL-10 induced by PPVO acts antagonistically to PPVO-induced IFN-γ and TNF-α.
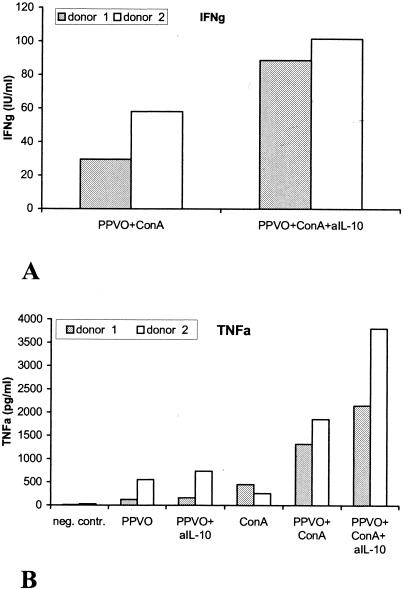
We wanted to know whether the anti-inflammatory cytokine IL-10, which is induced by PPVO, actually exerts a regulatory effect and whether IL-10 has an inhibitory effect on the induction of the inflammatory cytokines IFN-γ and TNF-α by PPVO. Whole-blood cultures were stimulated with PPVO, with or without ConA, in the presence of a blocking antibody against IL-10. An isotype-matched antibody served as a control. Both antibodies were used at a concentration of 5 μg/ml and were added to the cell cultures at the same time as PPVO. IFN-γ and TNF-α levels in the supernatants were measured by an ELISA. IFN-γ release was enhanced two- or threefold in the presence of the anti-IL-10 neutralizing antibody. TNF-α induction by PPVO alone was only marginally increased, but stimulation with PPVO and ConA together in the presence of the anti-IL-10 antibody was enhanced (Fig. 15). IL-10 obviously acts in a regulatory manner during stimulation with PPVO and limits the extent of the induced inflammatory response.
FIG. 15.
Neutralization of IL-10 in whole-blood cultures leads to increased IFN-γ and TNF-α secretion by PPVO. ConA was used at a concentration of 16 μg/ml, anti-IL-10 antibody (aIL-10) was used at a concentration of 5 μg/ml, and the PPVO titer was 104.6/ml. RPMI 1640 medium served as a negative (neg.) control (contr.). Isotype-matched control antibodies had no effect. (A) IFN-γ induction in the presence of the anti-IL-10 antibody. IFN-γ secretion in the presence of ConA or PPVO alone was under the detection limit of the ELISA (data not shown). (B) TNF-α induction in the presence of the anti-IL-10 antibody.
DISCUSSION
Inactivated parapoxvirus ovis has demonstrated antiviral effects in different animal models via induction of antiviral cytokines like IFN-γ. However, in contrast to the application of recombinant Th1 inducing (e.g., IL-12) or effector (e.g., IFN-γ) cytokines no significant side effects were observed under these experimental conditions (36). PPVO, therefore, could be an interesting candidate for novel antiviral therapies in humans. It was demonstrated recently that IFN-γ plays a key role in transducing the antiviral effects. However, since former investigations of PPVO happened so far almost exclusively in different animal models (3, 13, 29), our intention was to investigate whether PPVO has an effect on human immune cells as well and then to concentrate on the mechanism of action.
We demonstrated in this work that inactivated PPVO has a strong influence on cytokine secretion by human immune cells. Consistent with the findings in in vivo animal models, stimulation of human peripheral immune cells with PPVO led to an IFN-γ release. We found, however, that costimulation with low-dose ConA is required in ex vivo experiments with human blood leukocytes. The IFN-γ quantity varied between individual donors, whereby the IFN-γ and TNF-α high and low responders seem to be identical. Obviously exists a variability between individuals concerning the response to PPVO. In addition, we noticed differences between IFN-γ induction in whole blood and PBMC cultures in spite of the fact that cytokines are more difficult to detect in whole blood due, e.g., to nonspecific binding to erythrocytes or protease activity. Beside the IFN-γ induction, an influence of PPVO could be demonstrated for other cytokines as well. It were induced TNF-α, IL-8, IL-6, IL-1ra, IL-10, and IL-18, as well as IL-12 and IL-4 with ConA costimulation. A contamination of the preparation with endotoxin could be excluded. So PPVO evidently acts on human cells, too.
As a next step we examined the mechanisms responsible for the IFN-γ release after PPVO stimulation. First, we had to determine whether soluble proteins or particle components of the PPVO preparation could be responsible for the cytokine induction. Such a soluble protein could either be a protein encoded by the virus itself or a host cell protein induced by the virus during in vitro virus expansion. Centrifugation of the PPVO preparation revealed that already at 4,000g both the IFN-γ and the TNF-α inducing component were exclusively contained in the pellet. This indicates the effective components of the PPVO preparation are the virus particles itself and not a soluble factor in the supernatant. This corresponds with results obtained in an in vivo challenge model, in which mice are lethally infected with the Aujeszky virus, a herpes virus from the swine. PPVO has protective effects in this model and the relevant components were as well fully contained in the pellet after centrifugation (S. Friederichs, unpublished data). As active components of the virus particles viral proteins and viral DNA have to be considered. DNA can possess direct immunostimulating properties, e.g., the CpG motives of bacterial DNA (26, 27, 32, 33). In the case of PPVO, purified DNA alone could not mimic the immunomodulatory effects of the whole virus. This was shown in the in vivo Aujeszky mouse model. In addition, proteinase pretreatment of the virus destroys its efficacy (Friederichs, unpublished).
Furthermore, it revealed that for IFN-γ induction by the virus particles the presence of complement is necessary. Complement factor C3 might be required for an opsonization of the virus particles, which might facilitate their uptake by macrophages via the complement receptor. However, triggering the complement receptor by uptake of particles do not principally lead to stimulatory effects comparable with PPVO. So the virus particles possibly work intracellularly and the complement receptor only mediates the uptake of the critical components into the cell. Alternately, activated complement costimulates monocytes to produce IL-12 in response to PPVO.
In addition, it had been demonstrated that the CD14 receptor on macrophages is also involved in mediating the effects of PPVO, since blocking of the receptor with a neutralizing antibody nearly totally abolished TNF-α release after PPVO stimulation. The CD14 receptor is a pattern recognition receptor and is involved as a coreceptor in the activation of the TLR. These receptors are activated by various microbial structures, but have not been shown to bind directly to ligands, but instead to CD14 (15, 35). The CD14 receptor is able to bind different natural stimuli, e.g., LPS, mistletoe lectin, cytomegalovirus, and endogenous ligands, such as apoptotic cells (7, 15). So it is not surprising that PPVO is recognized by this receptor as well. Which TLR is concerned in detail and which intracellular signaling pathways are activated remains to be determined. Anyway, the signaling pathways of LPS and PPVO do not seem to be identical, according to the different mutual desensitization of PBMC. Under this aspect it seems rather unlikely that the effect of PPVO is mediated through TLR4 like LPS. Stimulation via TLR2, 4, and 10 induces more TNF-α compared with PPVO. IL-12 expression is hardly detectable following TLR2 stimulation. TLR10 is stimulated by CpG-DNA which was excluded as responsible compound in our system as the stimulation is not DNase sensitive. Nevertheless the TLR4 (LPS) and PPVO signaling pathways can obviously affect each other mutually. So, we presume that TLR7 might be a good candidate for the action. TLR7 is preferentially expressed on human plasmacytoid dendritic cells and B cells (24). The natural ligand of TLR7 is not known so far but imiquod, an antiviral drug, binds to TLR7 and stimulates IL-12, IFN-γ, and intermediate TNF-α—a pattern similar to the effects induced by PPVO (23). Moreover, TLR7 ligands have to go into the cell to interact and trigger TLR7 signaling which fits to our observations (22). Different cellular sources of LPS induced cytokines (monocytes and dendritic cells) and PPVO induced cytokines (plasmacytoid dendritic cells and B cells) would also explain why LPS can desensitize the PPVO response but not vice versa. Unfortunately, there are no tools for human TLR7 studies commercially available to prove this hypothesis. Regarding these results two ways seem to play a role in mediating the effects of PPVO.
In order to investigate to what extent the observed effects are specific for PPVO we examined the vaccinia virus as a further representative from the family of pox viruses. The vaccinia virus is relatively well characterized and shows some homology to the PPVO genome (31). This suggests that similar effects might be obtained as from PPVO. Indeed, the vaccinia virus was also able to induce IFN-γ and TNF-α, but at much lower quantities compared with PPVO. In agreement with these observations, the vaccinia virus had no protective effects in an in vivo Aujeszky virus mouse model (Friederichs, unpublished). These data suggest that specific structures of PPVO are important for antiviral effects that are absent in vaccinia virus.
Both binding to the CD14 receptor and an uptake of virus particles into the cell finally activates monocytes and leads to cytokine secretion. We could show the release of early proinflammatory mediators such as TNF-α, IL-6, and IL-8. All were released quickly after PPVO-stimulation and without ConA costimulation. This underlines the assumption that PPVO first leads to an activation of monocytes or other antigen-presenting cells (APC), e.g., plasmacytoid dendritic cells. For TNF-α we could show as well as for IFN-γ that the cytokine release was accompanied by an upregulation of cytokine mRNA. So PPVO leads to an increased gene transcription in activated monocytes or plasmacytoid dendritic cells. In addition it came also to a release of IL-12 and IL-18. Both are inflammatory cytokines, which are mainly produced by APC in particular in response to intracellular pathogens. IL-18 works mainly synergistically to IL-12. Both cytokines promote the differentiation of Th1-cells and can stimulate both T cells and NK cells to an IFN-γ release (14, 25, 37). IFN-γ mediates the effector functions which are characteristic for a type 1 immune response. In turn it is able to further increase the IL-12 release by macrophages and other APC. So it exists a positive feedback mechanism concerning the induction of these two cytokines. This might explain why IL-12 was measurable only with costimulation of ConA. Possibly PPVO alone leads only to an IL-12 concentration which is not detectable by ELISA but which is nevertheless biologically effective. The released IL-12 quantity is then in turn increased by IFN-γ which is induced together with ConA, so that IL-12 becomes finally measurable by an ELISA. Similar to the mechanism shown for TNF-α and IFN-γ, where the increased IFN-γ induction in the presence of ConA led to an intensified release of TNF-α by monocytes even though this was only partially due to IFN-γ.
We have demonstrated that both IL-12 and IL-18 are involved in the mediation of IFN-γ release which is induced by PPVO. In particular, IL-12 seems to play an important role, because its neutralization led to a reduction of IFN-γ production by approximately 80%. The effect of IL-18 was somewhat less pronounced. Neutralization of IL-18 led to a decrease of PPVO-mediated IFN-γ production by 20%. Since the simultaneous neutralization of IL-12 and IL-18 did not lead to a complete inhibition of IFN-γ release by PPVO, it is likely that additional mechanisms might be involved. It is known that also other cytokines (e.g., IL-15, TNF-α, and IL-1β) act synergistically with IL-12 on IFN-γ release (5).
We further addressed the question, which cells are responsible for IFN-γ production after PPVO-stimulation. Both T cells and NK cells are principally able to produce IFN-γ. Since IFN-γ induction by PPVO was possible only by simultaneous costimulation with ConA, it seems if mainly T cells are responsible for IFN-γ production. ConA is a vegetable lectine, which leads to cross-linking of the TCR and to mitogenic activation of T cells. Obviously, IL-12 and IL-18 levels induced by PPVO in monocytes are not sufficient to activate resting cells and to induce a detectable IFN-γ release. This requires in vitro the addition of a second particularly T cells activating factor. Indeed, it is well-known that additional stimulation of T cells has strong synergistic effects on the IFN-γ release by IL-12. The additional activation is in vivo very probably supplied by the infectious organism. Thus, PPVO induces IFN-γ at the site of immune activation in vivo; this provides a better pharmacokinetic profile than systemic application of IFN-γ itself. However, just as the T cells the NK cells could be responsible for the IFN-γ production as well, because it cannot be excluded that ConA has also a costimulatory effect on NK cells. On the other hand, NK cells might be secondarily activated by T cells (2, 10). This must be investigated in further experiments.
Finally, also the induction of anti-inflammatory cytokines such as IL-1ra, IL-10, and IL-4 was shown after PPVO stimulation. IL-10 is one of the most important anti-inflammatory cytokines. It is of great importance in limiting an immune response and in the prevention of tissue damage. Blocking IL-10 during stimulation with PPVO led to a significantly enhanced IFN-γ and TNF-α release and suggests that IL-10 acts regulatory in this context. IL-4 which is produced by Th2 cells is as well released and inhibits together with IL-10 the release of proinflammatory mediators. Obviously, PPVO leads not only to pure immunostimulating effects, but as well to factors, which can limit the immune response. This correlates with results obtained from in vivo experiments and could be an explanation for the good tolerability of PPVO. It could be speculated that the latter feature could be an advantage compared to antiviral therapies using single cytokines such as recombinant IL-12 (4, 6). The induction of a natural immune response with its physiological amounts of different cytokines combined with its antiviral potential might provide advantages over existing antiviral therapies.
Acknowledgments
This work was partly supported by Bayer HealthCare AG.
REFERENCES
- 1.Alcami, A., and G. L. Smith. 1995. Cytokine receptors encoded by poxviruses: a lesson in cytokine biology. Immunol. Today 16:474-478. [DOI] [PubMed] [Google Scholar]
- 2.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 3.Buttner, M., C. P. Czerny, K. H. Lehner, and K. Wertz. 1995. Interferon induction in peripheral blood mononuclear leukocytes of man and farm animals by poxvirus vector candidates and some poxvirus constructs. Vet. Immunol. Immunopathol. 46:237-250. [DOI] [PubMed] [Google Scholar]
- 4.Carr, J. A., J. Rogerson, M. J. Mulqueen, N. A. Roberts, and R. F. Booth. 1997. Interleukin-12 exhibits potent antiviral activity in experimental herpesvirus infections. J. Virol. 71:7799-7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carson, W., and M. A. Caligiuri. 1998. Interleukin-15 as a potential regulator of the innate immune response. Braz. J. Med. Biol. Res. 31:1-9. [DOI] [PubMed] [Google Scholar]
- 6.Cavanaugh, V. J., L. G. Guidotti, and F. V. Chisari. 1997. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J. Virol. 71:3236-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deane, D., C. J. McInnes, A. Percival, A. Wood, J. Thomson, A. Lear, J. Gilray, S. Fleming, A. Mercer, and D. Haig. 2000. Orf virus encodes a novel secreted protein inhibitor of granulocyte-macrophage colony-stimulating factor and interleukin-2. J. Virol. 74:1313-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobrovolskaia, M. A., and S. N. Vogel. 2002. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 4:903-914. [DOI] [PubMed] [Google Scholar]
- 10.Fehniger, T. A., W. E. Carson, and M. A. Caligiuri. 1999. Costimulation of human natural killer cells is required for interferon gamma production. Transplant. Proc. 31:1476-1478. [DOI] [PubMed] [Google Scholar]
- 11.Fleming, S. B., D. M. Haig, P. Nettleton, H. W. Reid, C. A. McCaughan, L. M. Wise, and A. Mercer. 2000. Sequence and functional analysis of a homolog of interleukin-10 encoded by the parapoxvirus orf virus. Virus Genes 21:85-95. [DOI] [PubMed] [Google Scholar]
- 12.Fleming, S. B., C. A. McCaughan, A. E. Andrews, A. D. Nash, and A. A. Mercer. 1997. A homolog of interleukin-10 is encoded by the poxvirus orf virus. J. Virol. 71:4857-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forster, R., G. Wolf, and A. Mayr. 1994. Highly attenuated poxviruses induce functional priming of neutrophils in vitro. Arch. Virol. 136:219-226. [DOI] [PubMed] [Google Scholar]
- 14.Gately, M. K., L. M. Renzetti, J. Magram, A. S. Stern, L. Adorini, U. Gubler, and D. H. Presky. 1998. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 16:495-521. [DOI] [PubMed] [Google Scholar]
- 15.Gordon, S. 2002. Pattern recognition receptors: doubling up for the innate immune response. Cell 111:927-930. [DOI] [PubMed] [Google Scholar]
- 16.Haig, D. M. 1998. Poxvirus interference with the host cytokine response. Vet. Immunol. Immunopathol. 63:149-156. [DOI] [PubMed] [Google Scholar]
- 17.Haig, D. M. 2001. Subversion and piracy: DNA viruses and immune evasion. Res. Vet. Sci. 70:205-219. [DOI] [PubMed] [Google Scholar]
- 18.Haig, D. M., and S. Fleming. 1999. Immunomodulation by virulence proteins of the parapoxvirus orf virus. Vet. Immunol. Immunopathol. 72:81-86. [DOI] [PubMed] [Google Scholar]
- 19.Haig, D. M., C. J. McInnes, J. Thomson, A. Wood, K. Bunyan, and A. Mercer. 1998. The orf virus OV20.0L gene product is involved in interferon resistance and inhibits an interferon-inducible, double-stranded RNA-dependent kinase. Immunology 93:335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haig, D. M., and A. A. Mercer. 1998. Ovine diseases. Orf. Vet. Res. 29:311-326. [PubMed] [Google Scholar]
- 21.Haig, D. M., J. Thomson, C. McInnes, C. McCaughan, W. Imlach, A. Mercer, and S. Fleming. 2002. Orf virus immuno-modulation and the host immune response. Vet. Immunol. Immunopathol. 87:395-399. [DOI] [PubMed] [Google Scholar]
- 22.Heil, F., P. Ahmad-Nejad, H. Hemmi, H. Hochrein, F. Ampenberger, T. Gellert, H. Dietrich, G. Lipford, K. Takeda, S. Akira, H. Wagner, and S. Bauer. 2003. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur. J. Immunol. 33:2987-2997. [DOI] [PubMed] [Google Scholar]
- 23.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196-200. [DOI] [PubMed] [Google Scholar]
- 24.Kadowaki, N., S. Ho, S. Antonenko, R. W. Malefyt, R. A. Kastelein, F. Bazan, and Y. J. Liu. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komastu, T., D. D. Ireland, and C. S. Reiss. 1998. IL-12 and viral infections. Cytokine Growth Factor Rev. 9:277-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieg, A. M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 27.Krieg, A. M., G. Hartmann, and A. K. Yi. 2000. Mechanism of action of CpG DNA. Curr. Top. Microbiol. Immunol. 247:1-21. [DOI] [PubMed] [Google Scholar]
- 28.Marrack, P., and J. Kappler. 1994. Subversion of the immune system by pathogens. Cell 76:323-332. [DOI] [PubMed] [Google Scholar]
- 29.Mayr, B., and A. Mayr. 1995. Present state of preclinical research on the efficacy and safety of para immunity inducers from poxviruses. A study of the literature. Tierarztl. Prax. 23:542-552. (In German.) [PubMed] [Google Scholar]
- 30.McInnes, C. J., A. R. Wood, and A. A. Mercer. 1998. Orf virus encodes a homolog of the vaccinia virus interferon-resistance gene E3L. Virus Genes 17:107-115. [DOI] [PubMed] [Google Scholar]
- 31.Mercer, A., S. Fleming, A. Robinson, P. Nettleton, and H. Reid. 1997. Molecular genetic analyses of parapoxviruses pathogenic for humans. Arch. Virol. Suppl. 13:25-34. [DOI] [PubMed] [Google Scholar]
- 32.Sparwasser, T., and G. B. Lipford. 2000. Consequences of bacterial CpG DNA-driven activation of antigen-presenting cells. Curr. Top. Microbiol. Immunol. 247:59-75. [DOI] [PubMed] [Google Scholar]
- 33.Stacey, K. J., D. P. Sester, M. J. Sweet, and D. A. Hume. 2000. Macrophage activation by immunostimulatory DNA. Curr. Top. Microbiol. Immunol. 247:41-58. [DOI] [PubMed] [Google Scholar]
- 34.Takeda, K., and S. Akira. 2001. Roles of Toll-like receptors in innate immune responses. Genes Cells 6:733-742. [DOI] [PubMed] [Google Scholar]
- 35.Underhill, D. M. 2003. Toll-like receptors: networking for success. Eur. J. Immunol. 33:1767-1775. [DOI] [PubMed] [Google Scholar]
- 36.Weber, O., A. Siegling, A. Friebe, A. Limmer, T. Schlapp, P. Knolle, A. Mercer, H. Schaller, and H. D. Volk. 2003. Inactivated parapoxvirus ovis (Orf virus) has antiviral activity against hepatitis B virus and herpes simplex virus. J. Gen. Virol. 84:1843-1852. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimoto, T., K. Takeda, T. Tanaka, K. Ohkusu, S. Kashiwamura, H. Okamura, S. Akira, and K. Nakanishi. 1998. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J. Immunol. 161:3400-3407. [PubMed] [Google Scholar]