POLICY
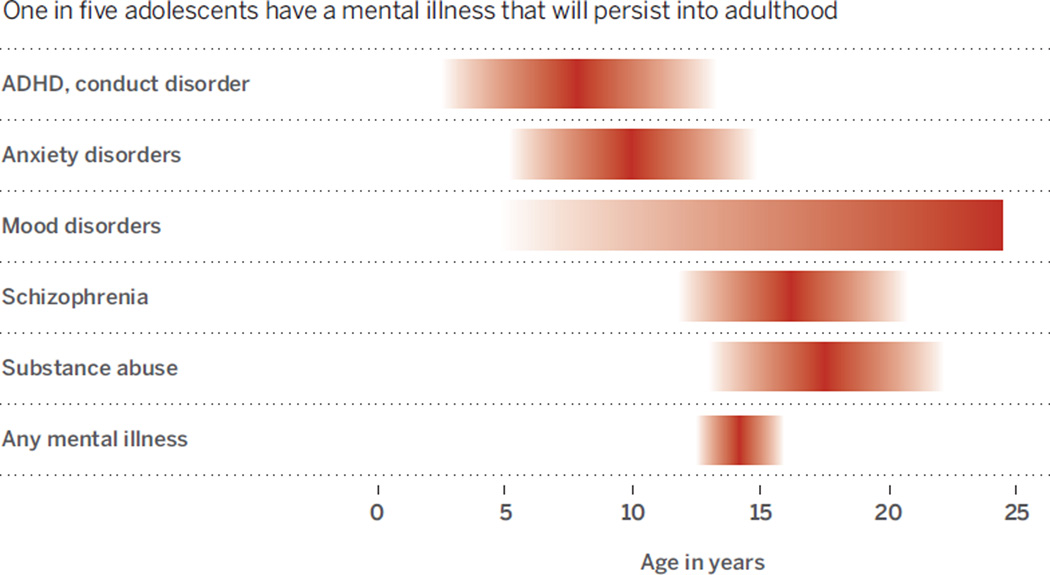
The adolescent brain is more “plastic” than it will ever be again, capable of remarkable adaptability in light of the many social, physical, sexual, and intellectual challenges that this developmental phase brings. This is also a peak time for clinical onset of most mental illnesses (see the chart) (1). One in five adolescents have a mental illness that will persist into adulthood (2). Mental illnesses that emerge before adulthood impose a 10-fold higher cost than those that emerge later in life (3). Mental health costs are the highest single source of global economic burden in the world (4).
The chronicity of adolescent-onset disorders is powerful motivation for early interventions to improve quality of life and reduce burdens on society. Yet, studies of interventions’ economic effect have not demonstrated consistent benefits, which may be due, in part, to assessment of treatments that are not biologically based and/or do not consider how neurodevelopmental changes affect long-term effectiveness (5).
Understanding neurodevelopmental changes and their roles in both emergence of mental disorders and how they affect treatment efficacy is imperative. Yet, we estimate that less than 1% of the budget of the U.S. National Institutes of Health (NIH) was directed toward adolescent brain research in fiscal year 2014 (6). We highlight opportunities and priorities for more developmentally informed research to translate basic knowledge of adolescence toward clinical applications to treat mental illness.
ADOLESCENT BRAIN DYNAMICS
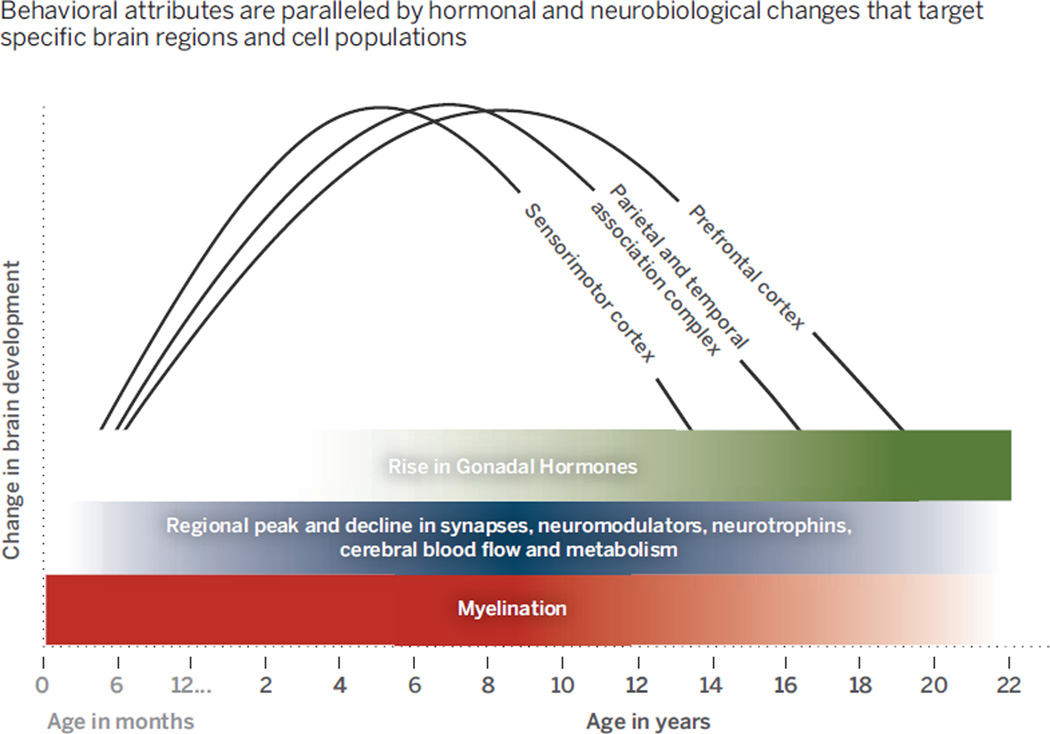
Adolescence is characterized by heightened emotional reactivity, sensitivity to peer influence, impulsivity, and novelty seeking, with a seemingly limited capacity to engage self-control to override these emotions and actions (7). These behavioral attributes are paralleled by hormonal and neurobiological changes that target specific brain regions and cell populations (see the graph) (8).
Studies in nonhuman primates show that this period is associated with overproduction, followed by selective stabilization and elimination, of principally excitatory synapses in the cortex (9) that may alter an excitatory/inhibitory balance in individual neurons and circuits. Regional changes in synaptic morphology, dendritic arborization, patterns of cortical cell firing, and availability of neurochemicals and their receptors all occur during adolescence. Changes in white matter during this time likely influence conduction of electrical impulses across the brain and (axonal) transport of cargoes essential for neurotransmission, cell metabolism, and survival.
Human-imaging studies have shown an analogous pattern in which low-level sensory and motor cortices develop earlier than do association cortices involved in rational thought and regulation of behavior (10). These regional neurochemical, structural and functional changes across development have been posited to lead to transient imbalances in functional brain circuitry during adolescence, underlying dysregulation of emotions and actions (11). Exacerbations in these imbalances by biological, environmental, and genetic factors may contribute to a risk for mental illness. A priority will be to delineate dynamics of brain development preceding, during, and following adolescence to understand the increase in mental illness during this time and how these neurobiological changes affect the type and timing of treatment of these disorders. Too often, pharmacological and behavioral treatments designed for the adult brain are imposed on the developing brain with little consideration for how neurobiological changes across time may impact the effectiveness of these treatments.
TECHNOLOGICAL ADVANCES FOR MAPPING BRAIN AND BEHAVIOR
We have unprecedented opportunities to advance understanding of the brain with novel technologies and data. These must be applied to both developing and developed brains. Techniques such as in vivo imaging of synaptic activity in deep brain structures, whole-brain clearing and imaging, and optogenetics are illuminating brain circuit dynamics in animal and cell model systems and may shed light on dysfunctional circuitry in brain disorders (12, 13).
One example in human research is the NIH Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative. This focuses largely on techniques, not yet feasible in humans, for understanding intricate workings of the brain in nonhumans. Another promising initiative is the NIH Human Connectome Project, which focuses on how brain connections underlie complex behavior (14). A priority of these efforts should be to focus on developing brain structures, connections, and functions to delineate how developmental changes,—whether fetal, infant, child, or adolescent—affect the risk for mental disorders that emerge during adolescence. Priority should be given to how these changes affect the type and timing of treatment of these disorders during these stages.
We are in an era of tremendous access to large, human-imaging data sets, novel imaging tools, and bioinformatics to guide us. Collaborative “big neuroscience” projects to map the structural and functional landscape of the human brain have been proposed and initiated around the world, but should emphasize the developing brain and changes that adolescence brings. Initiatives are under way or in conceptual stages to collect or merge large data sets in healthy and at-risk developmental populations that include psychosocial, clinical, behavioral, imaging, and/or genetic data (15). It is essential to exploit new knowledge through rigorous hypothesis-driven behavioral and brain testing. This will require support of scientific inquiries that bridge and integrate basic nonhuman and human investigations for deployment of new diagnostic tools and treatments.
In parallel with brain-imaging innovations and large data sets, systematic profiling of gene expression across regions and time points of the developing and adult human brain has revealed unforeseen spatiotemporal dynamics of the human brain transcriptome. Dramatic changes in gene expression are associated with the development of distinct brain regions and with developmental periods. Analysis of developmental transcriptome data is critical for interpreting the mechanism by which noncoding disease-associated mutations translate into clinical syndromes and for providing insights into the biology of mental illness (16). We anticipate the expansion of public data sets based on RNA sequencing of the human brain across developmental stages. A priority will be to characterize these transcriptional changes across development and translate these basic discoveries to direct novel treatments based on the age and genetic makeup of the individual.
A final example of technological developments is mobile devices. These may be used to measure fluctuations in autonomic function and arousal, location, and self-reported emotion, allowing objective assessment of individuals in the real world, in real time (17). This is in contrast to current methods that typically require individuals to self-report by reflecting back over an extended period, typically many weeks, which often does not reflect the person’s true level of functioning. The long-term potential of these technologies could provide remote anticipation of critical mental health events such as suicidality. A priority will be to optimize this technology for different age groups and integrate these data with imaging and genetic data sets to link biology with the social and physical environment to develop and deploy diagnostics and treatments.
TREATING DEVELOPING VERSUS DEVELOPED BRAINS
Adolescence is a delimited window of development when the environment has a strong influence on brain and behavior. Understanding the timetable of behavioral and brain changes could uncover patterns of potential therapeutic relevance, guiding treatments that may vary by age, and informing public health strategies and policies for modifying the environment for lasting salutary effects.
Too often, children and adolescents are lumped together in large clinical trials with little consideration for how dynamic changes in the brain across development will impact the effectiveness of treatments. This is often compounded by treatments being based on evidence from the adult brain or from one sex without appreciation for differences between the developing and developed brain or female and male brain.
Characterizing sensitive periods may allow us to apply precision medicine, directing the timing and type of interventions at the level of an individual. For example, evidence is emerging on treatment for anxiety disorders, the most common form of mental illness in young people, affecting as many as 1 in 10 (1, 2). A core symptom is difficulty recognizing when situations that have been experienced as dangerous are now safe. Exposure-based cognitive behavioral therapy is the most common treatment, based on basic principles of fear extinction learning, whereby a person is desensitized to fearful triggers through repeated exposures in a safe context.
Recent mouse and human studies indicate that adolescents have diminished fear extinction relative to younger or older age groups (18). This suggests that exposure therapies in clinical practice that build on principles of fear extinction may be less effective during adolescence than during childhood or adulthood (19). This illustrates the importance of age as a potential predictor of treatment response and even a target for novel treatments. A priority for future research will be to delineate treatments targeted to the biological state of the developing brain to maximize effectiveness.
OPPORTUNITY AND OBLIGATION
There is a tremendous opportunity to understand how sensitive windows may shift, constrict, or expand in an individual at different points in development. In parallel, it is essential that we bridge discoveries in humans and animal model systems at genetic, molecular, circuit, and behavioral levels to guide novel interventions. Together, these efforts will enhance our capacity to develop and target treatments by age, sex, and genetic makeup of the individual.
Despite the moral imperative and long-term economic benefit of improved diagnosis and treatment of mental disorders in adolescence, there has not been commensurate investment in research to bring them about. The NIH budget has not kept pace with inflation and is threatened by cutbacks. Increased commitment and resources are needed to help address our social obligation to reduce the unacceptably high burden of mental illness on youth today and to ensure a healthier tomorrow.
Emergence and peak in mental disorders during adolescence.
One in five adolescents have a mental illness that will persist into adulthood
Developmental course of brain maturation during adolescence.
Behavioral attributes are paralleled by hormonal and neurobiological changes that target specifc brain regions and cell populations
Acknowledgments
The ideas in this article were generated during a December 2013 Cold Spring Harbor Laboratory Banbury meeting on the adolescent brain, supported by the Allen Institute for Brain Science, The Lieber Institute for Brain Development, the National Institute on Alcohol Abuse and Alcoholism, and the National Institute of Mental Health, www.cshl.edu/banbury-center/banbury-reports/the-adolescent-brain-and-mental-disorders.html. The organizers and authors thank participants for contributions to the meeting and comments on this article.
REFERENCES AND NOTES
- 1.Paus T, Keshavan M, Giedd JN. Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, et al. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Economic Aspects of Mental Health in Children and Adolescents. Geneva: WHO; 2007. [Google Scholar]
- 4.Bloom DE, et al. The Global Economic Burden of Non-communicable Diseases. Geneva: World Economic Forum; 2011. [Google Scholar]
- 5.Beecham J. J. Child Psychol. Psychiatry. 2014;55:714–732. doi: 10.1111/jcpp.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.These numbers were generated from NIH RePORTER, the publicly available database on NIH-funded research, using the search terms “adolescent” and “brain” and “development”; all active grants from 1 October 2013 to 30 September 2014 (FY 2014) were searched, based on a total NIH budget of $30.1 billion.
- 7.Spear L. The Behavioral Neuroscience of Adolescence. New York: W.W. Norton; 2010. [Google Scholar]
- 8.Casey BJ, Tottenham N, Liston C, Durston S. Trends Cogn. Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Rakic P, Bourgeois JP, Goldman-Rakic PS. Prog. Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- 10.Gogtay N, et al. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casey BJ, Getz S, Galvan A. Dev. Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andermann ML, et al. Neuron. 2013;80:900–913. doi: 10.1016/j.neuron.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deisseroth K, et al. J. Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandel ER, Markram H, Matthews PM, Yuste R, Koch C. Nat. Rev. Neurosci. 2013;14:659–664. doi: 10.1038/nrn3578. [DOI] [PubMed] [Google Scholar]
- 15.These include the Pediatric Imaging, Neurocognition, and Genetics (PING) study; the Philadelphia Neurodevelopmental Cohort (PNC) study; the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA); The Human Connectome Project Lifespan Pilot Study; the Adolescent Brain Cognitive Development (ABCD) study; and the Tokyo Teen Cohort.
- 16.Tebbenkamp AT, Willsey AJ, State MW, Sestan N. Curr. Opin. Neurol. 2014;27:149–156. doi: 10.1097/WCO.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunton GF, et al. Health Psychol. 2014;33:255–263. doi: 10.1037/a0032640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pattwell SS, et al. Proc. Natl. Acad. Sci. U.S.A. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drysdale AT, et al. Biol. Psychiatry. 2014;75:e19–e20. doi: 10.1016/j.biopsych.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]