Abstract
The thiol group in cysteine residues is susceptible to several post-translational modifications (PTMs), including prenylation, nitrosylation, palmitoylation, and the formation of disulfide bonds. Additionally, cysteine residues involved in disulfide bonds are commonly reduced and alkylated prior to mass spectrometric analysis. Several of these cysteine modifications, specifically S-alkyl modifications, are susceptible to gas-phase oxidation via selective ion/ion reactions with periodate anions. Multiply protonated peptides containing modified cysteine residues undergo complex formation upon ion/ion reaction with periodate anions. Activation of the ion/ion complexes results in oxygen transfer from the reagent to the modified sulfur residue to create a sulfoxide functionality. Further activation of the sulfoxide derivative yields abundant losses of the modification with the oxidized sulfur as a sulfenic acid (namely, XSOH) to generate a dehydroalanine residue. This loss immediately indicates the presence of an S-alkyl cysteine residue, and the mass of the loss can be used to easily deduce the type of modification. An additional step of activation can be used to localize the modification to a specific residue within the peptide. Selective cleavage to create c- and z-ions N-terminal to the dehydroalanine residue is often noted. As these types of ions are not typically observed upon collision-induced dissociation (CID), they can be used to immediately indicate where in the peptide the PTM was originally located.
Keywords: ion/ion reactions, S-alkylation, prenylation, oxidation
Graphical Abstract
INTRODUCTION
The alkylation of cysteine is important from both biological and chemical standpoints.1 Prenylation is perhaps the most important biological alkylation of cysteine as it has been implicated in several cancers2–5 and other diseases,6–8 such as Hutchinson–Gilford progeria syndrome (HGPS), a genetic disease associated with premature aging in children.9–11 Protein prenylation is the addition of one of two hydrophobic moieties (farnesyl or geranylgeranyl) to the sulfur atom of a cysteine residue.2,12 Prenylated cysteine residues are typically part of a C-terminal CAAX motif, where C is cysteine, A is an aliphatic amino acid, and X is variable and determines whether the protein is farnesylated or geranylgeranylated.2,13,14 Proteins with CXC and CC motifs can also undergo prenylation.12,15 Prenylation is thought to promote protein–membrane16,17 and some protein–protein interactions.18–20 Examples of proteins with several important protein–protein and protein–membrane interactions21 that have been found in prenylated forms2,22 are Ras proteins, the most common oncoproteins.3 Inhibiting Ras farnesylation has been shown to block the signal transduction pathway and halt tumor cell growth.23,24 Other proteins known to undergo prenylation include yeast mating factors,25,26 protein kinases,27 and nuclear lamins.12,28
In addition to prenylation, S-alkylation is also important in the mass spectrometric (MS) analysis of disulfide bonds. Cysteine residues involved in disulfide bonds are commonly reduced and alkylated prior to tandem MS experiments to maximize sequence coverage. Collisional activation of disulfide bond-containing protonated peptides often does not result in cleavage of the disulfide bond, which can complicate the generation of information about residues contained within the region(s) protected by the disulfide bond. Several derivatization strategies have been developed, including protection with α-halocarbonyls (e.g., iodoacetamides),29,30 conjugation to Michael acceptors (e.g., maleimides),31,32 and aminoethylation, which is often used to convert cysteine to lysine mimics.33,34 Here, we demonstrate a robust method for the identification of S-alkyl cysteine-containing peptides and determination of the modifying alkyl group via gas-phase oxidation ion/ion reactions.
Ion/ion reactions have recently been used to carry out common solution-phase derivatizations in the gas phase.35 Benefits of this approach include significantly shorter reaction times, reduced chemical noise via mass selection of reactants, and control over the extent of modification.35 Recent examples of covalent modification ion/ion reactions include the synthesis of peptide bonds via N-hydroxysuccinimide36 and Woodward’s Reagent K37 conjugation chemistry, “Click” chemistry reactions between alkynes and azides,38 and esterification of carboxylate groups via alkyl cation transfer from fixed charge cation reagents.39
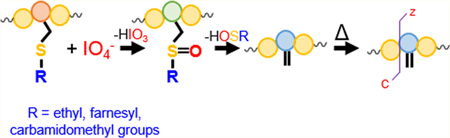
The gas-phase oxidation of methionine and tryptophan side chains,40 as well as disulfide linkages,41 via ion/ion reactions with periodate anion IO4− has been shown previously. Similar to solution-phase oxidation,42–47 methionine sulfoxide-containing peptides yielded a signature loss of 64 Da upon collisional activation,40 corresponding to the ejection of methane sulfenic acid. Furthermore, the oxidation of peptides to [M + H + O]+, [M − H]+, and M+• species via reaction with a suite of reagents derived from persulfate has been demonstrated.48 Here, we extend the approach used to oxidize methionine to S-alkyl cysteine residues because they are similar in structure. The solution-phase oxidation of various S-alkyl cysteine residues has been investigated previously and can also undergo rearrangement upon activation to lose the alkyl sulfenic acid.49–52 The oxidation of farnesylated and geranylgeranylated peptides has been investigated by Bhawal et al., who proposed the identification and differentiation of farnesylated and geranylgeranylated peptides via the signature loss observed upon collision-induced dissociation (CID) of the sulfoxide derivatives.53 The gas-phase oxidation ion/ion approach retains the benefits of the solution-phase derivatization techniques while allowing control over the extent of modification (i.e., the number of oxygen addition events) and the species subjected to oxidation. We demonstrate this oxidation with a variety of alkyl groups, including ethyl, carbamidomethyl, and farnesyl groups. Furthermore, we show that further activation of the alkyl sulfenic acid loss species results in c- and z-type ions N-terminal to the modified cysteine residue due to generation of a dehydroalanine residue. These ions can be used to indicate the presence and location of an oxidized S-alkyl cysteine.
EXPERIMENTAL SECTION
Materials
Methanol and glacial acetic acid were purchased from Mallinckrodt (Phillipsburg, NJ, USA). Sodium periodate, iodoethane, dimethylformamide (DMF), trifluoroacetic acid (TFA), n-propanol, zinc acetate (Zn(OAc)2), and trans,trans-farnesyl chloride were purchased from Sigma-Aldrich (St. Louis, MO, USA). RAKGCKGR was synthesized by CHI Scientific (Maynard, MA, USA). KGAILCGIALK was synthesized by CPC Scientific (Sunnyvale, CA, USA). A-factor was a generous gift from the Hrycyna Lab at Purdue University. The carbamidomethyl (cam)-modified BSA tryptic digest was purchased from ThermoFisher Scientific (Waltham, MA, USA). All peptide stock solutions for positive nanoelectrospray were prepared in a 49.5:49.5:1 (v/v/v) solution of methanol/water/acetic acid at an initial concentration of ~1 mg/mL and diluted 100-fold prior to use. The solution of sodium periodate (50:50 v/v methanol/water) anions was prepared at a concentration of ~1 mg/mL and diluted 10-fold prior to use.
Syntheses
S-farnesyl KGAILCGIALK was prepared according to a previously published procedure.54,55 Briefly, a solution of 1 mg of KGAILCGIALK in 600 µL of 2:1 (v/v) DMF/n-propanol was prepared. Ten microliters of farnesyl chloride was added to the peptide solution, followed by 0.8 mg of Zn(OAc)2 in 200 µL of 0.1% aqueous TFA. The reaction was allowed to proceed at room temperature while shaking for 1 h before being concentrated and reconstituted in 200 µL of 50:50 (v/v) H2O/ACN. This solution was then separated on a reverse-phase HPLC (Agilent 1100, Palo Alto, CA) using an Aquapore RP-300 column (PerkinElmer, Wellesley, MA). A linear 30 min gradient from 0 to 100% B (A: 0.1% aqueous TFA, B: 60:40:0.09 v/v/v ACN/H2O/TFA) was run with a flow rate of 1 mL/min. The collected fractions were dried under vacuum and reconstituted in 49.5:49.5:1 (v/v/v) MeOH/H2O/HOAc. For S-ethylation, RAKGCKGR (1 mg) was dissolved in 1 mL of 500 mM Tris, 25 mM iodoethane. The reaction was allowed to proceed in the dark for 30 min at room temperature.49
Mass Spectrometry
All experiments were performed on a QTRAP 4000 hybrid triple quadrupole/linear ion trap (AB Sciex, Concord, ON, Canada) previously modified for ion/ion reactions.56 Multiply protonated S-alkylated peptides were mass isolated in Q1 and injected into the q2 reaction cell followed by periodate anions via alternately pulsed nanoelectrospray ionization (nESI).57 The S-alkyl peptide cations and periodate anions were allowed to react for a mutual storage reaction time of 20–1000 ms. The ion/ion reaction products were then transferred to Q3, where the complex was subjected to further characterization via MSn and mass analysis using mass-selective axial ejection (MSAE).58
RESULTS AND DISCUSSION
Demonstration of Phenomenology with an S-Ethylated Model Peptide
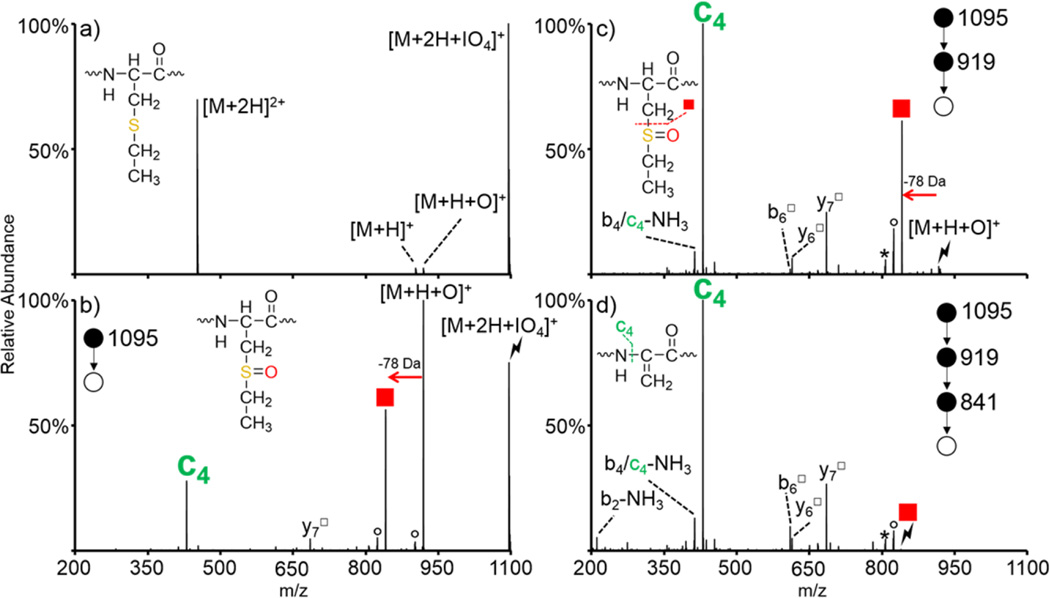
The gas-phase oxidation of S-alkyl cysteine residues is first demonstrated with ions derived from the S-ethylation of a synthetic peptide, RAKGCKGR (denoted as RAKGC(Et)KGR, structure shown in inset of Figure 1(a)), as S-ethyl cysteine both closely mimics the structure of and is isomeric with methionine. The [M + 2H]2+ species from RAKGC(Et)KGR was subjected to ion/ion reaction with IO4−, and the resulting spectrum is shown in Figure 1(a). Upon ion/ion reaction, peptide cations can either transfer a proton to the reagent anion to yield the charge-reduced peptide and neutral periodic acid (namely, HIO4) or adduct to the reagent anion to generate a long-lived electrostatic complex of the form [M + 2H + IO4]+ (monoisotopic m/z = 1095). Generation of the long-lived complex is the main product upon the reaction between RAKGC(Et)KGR [M + 2H]2+ and IO4−; proton transfer along with fragmentation of the complex (likely due to energetic transfer conditions between q2 and Q3) to generate the [M + H + O]+ (monoisotopic m/z = 919) are observed at very minor abundances. The efficiency of complex formation is a function of peptide size and, to some extent, peptide composition. Efficiencies in excess of 50% are typical for peptides with more than roughly six residues and particularly when they have at least one arginine residue. Collisional activation of the ion/ion complex between RAKGC(Et)KGR and IO4− is shown in Figure 1(b). Although proton transfer from the peptide to the periodate anion is often observed upon CID of ion/ion complexes of this type, it is not observed for the system described here; activation of the ion/ion complex predominantly results in oxygen transfer from the reagent anion to the peptide to yield the [M + H + O]+ species (Figure 1(b)). Additionally, further fragmentation of the [M+H+O]+ species is observed, as indicated by the formation of the c4 (monoisotopic m/z = 430) and y7□ (monoisotopic m/z = 685) ions (hollow square superscripts indicate fragments that have lost HOSR from oxidized S-alkyl cysteine residues to generate dehydroalanine residues) and by the loss of HOSEt from the [M + H + O]+ peak. The oxidized [M + H + O]+ species was then isolated and subjected to collisional activation (Figure 1(c)). Red squares in Figures 1–4 indicate loss of HOSR from oxidized S-alkyl cysteine residues. In the case of RAKGC(Et)-KGR, the red square indicates loss of HOSEt (78 Da) from the oxidized side-chain to generate a dehydroalanine residue (monoisotopic m/z = 841). The spectrum resulting from activation of this species is shown in Figure 1(d). The c4 ion is the most abundant ion produced upon CID of both the [M + H + O]+ (Figure 1(c)) and the [M + H + O − HOSEt]+ species (red square, Figure 1(d)). This ion corresponds to cleavage of the N–Cα bond N-terminal to the newly formed dehydroalanine residue. Doubly protonated ARAC(Et)AKA was also subjected to ion/ion reaction with IO4− (Figure S-1). Similar to the results described here for RAKGC(Et)KGR, activation of the oxidized species resulted in dominant loss of HOSEt (78 Da) to generate the dehydroalanine species (Figure S-1(b)). An additional step of activation on the HOSEt loss resulted in abundant formation of the c3 ion due to the dehydroalanine effect (Figure S-1(c)). The formation of c- and z-ions N-terminal to dehydroalanine has been observed previously upon activation of anions derived from asymmetric cleavage of disulfide bonds.59,60 Because c- and z-ions are not typically observed upon CID of protonated species, their presence could be used to easily identify and localize oxidized S-alkyl cysteines.
Figure 1.
Oxidation of doubly protonated S-ethyl RAKGCKGR upon ion/ion reaction with IO4−. (a) Ion/ion reaction between RAKGC(Et)KGR [M + 2H]2+ and IO4−. Activation of the (b) ion/ion complex, (c) [M + H + O]+ species, and (d) HOSEt loss. Insets show structures of (a) S-ethyl cysteine, (b, c) oxidized S-ethyl cysteine, and (d) dehydroalanine product formed upon loss of HOSEt. Symbols are as follows: (red squares) loss of HOSR to form the dehydroalanine product, (○) water losses, (*) ammonia losses, (lightning bolts) species subjected to CID, and (□) fragment ions that have lost the HOSR group.
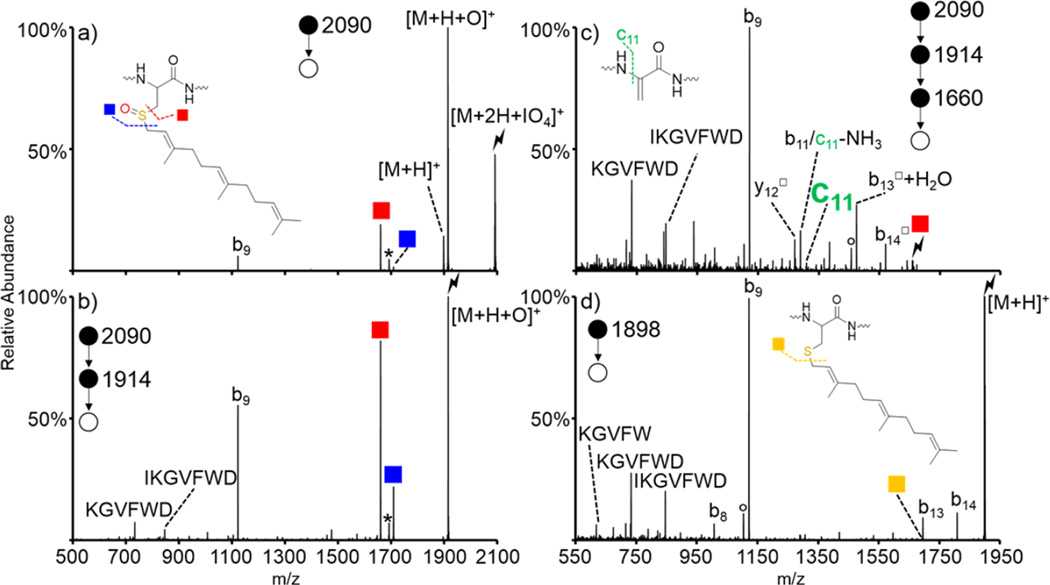
Figure 4.
Oxidation of A-factor (sequence YIIKGVFWDPAC(far)VIA) via ion/ion reaction with IO4−. Activation of (a) [M + 2H + IO4]+, (b) [M + H + O]+, and (c) dehydroalanine product formed upon HOSfar loss from the [M + H + O]+ species. The control spectrum (d) is the activation of the [M + H]+ species not subjected to ion/ion reactions. Insets show structures of (a) oxidized farnesylated cysteine and (c) the dehydroalanine residue produced upon loss of HOSfar. Symbols are as follows: (red squares) loss of HOSR to form the dehydroalanine product, (blue squares) cleavage on the other side of the oxidized sulfur atom, (yellow squares) loss of the farnesyl group in the nonoxidized species, (○) water losses, (*) ammonia losses, (lightning bolts) species subjected to CID, and (□) fragment ions that have lost the HOSR group.
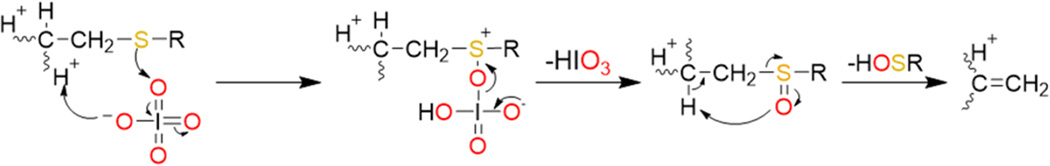
A general mechanism for the oxidation of a doubly protonated peptide containing an S-alkyl cysteine (R indicates any alkyl group) upon ion/ion reaction with periodate anion is presented in Scheme 1. The first step is presumed to proceed via nucleophilic attack by the sulfur atom on one of the neutral oxygen atoms on the periodate reagent with concurrent proton transfer from the peptide to the reagent, similar to the mechanism proposed previously for the oxidation of methionine residues via ion/ion reactions with periodate anions.40 Upon rearrangement, the S-alkyl cysteine is oxidized to the sulfoxide derivative with concurrent loss of iodic acid (HIO3). Following the loss of iodic acid, the neutral alkyl sulfenic acid is eliminated via a five-membered ring intermediate to generate the dehydroalanine residue as discussed previously by Froelich and Reid.49 Upon collisional activation, peptides containing dehydroalanine residues have been shown to undergo enhanced cleavage of the N–Cα bond of the dehydroalanine residue to create c- and z-ions.59,60
Scheme 1. Proposed Mechanism for the Ion/Ion Reaction between Periodate Anion and an S-Alkylated Cysteine Residue in a Doubly Protonated Peptide Followed by Loss of the Sulfoxide Group as HOSRa.
aR indicates an alkyl group attached to the cysteine residue. See ref 49 for further discussion of the last step to lose the alkyl sulfenic acid.
Gas-Phase Oxidation of Cam-Protected Peptides
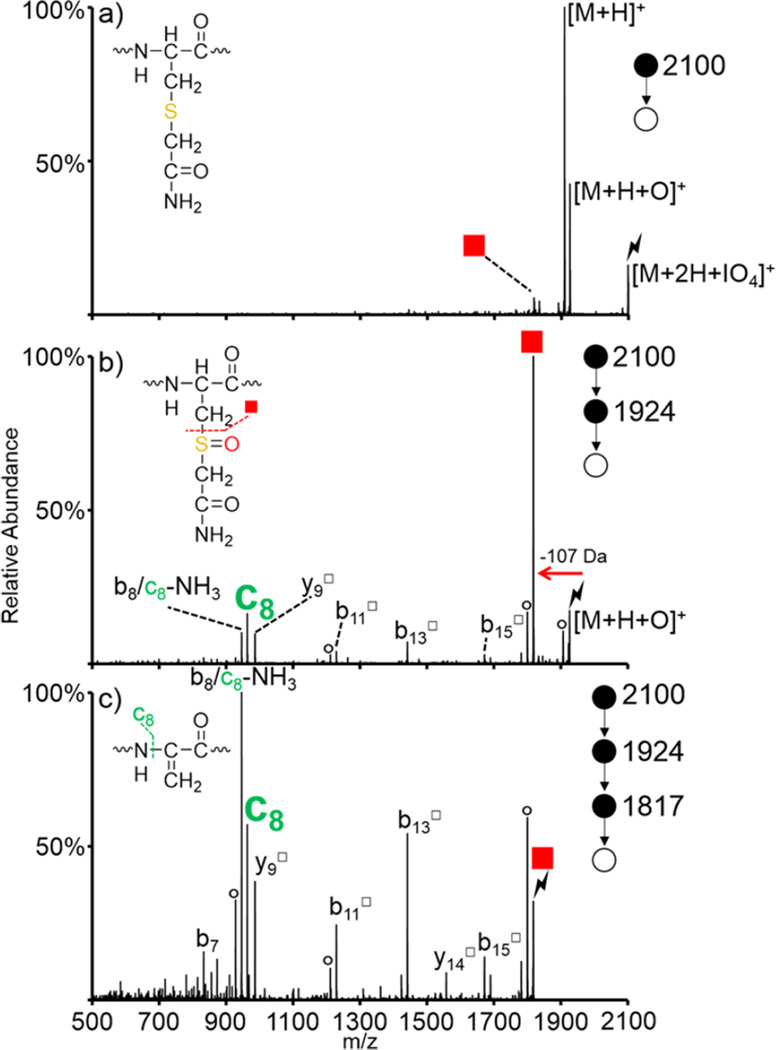
Collisional activation of protonated peptides and protonated proteins containing disulfide bonds results in fragmentation of the peptide or protein backbone; the disulfide linkage often remains intact, thus complicating analysis and inhibiting fragmentation in the regions protected by the disulfide linkages.61 For these reasons, disulfide bonds are commonly reduced and alkylated prior to analysis. One of the most common protecting reagents is iodoacetamide, which results in the formation of a stable S-carboxyamidomethylcysteine group (abbreviated as “cam”; structure shown in inset of Figure 2(a)).29,30 Here, we show that this group is susceptible to oxidation upon ion/ion reaction with periodate anion. Doubly protonated LFTFHADIC(cam)TLPDTEK, a tryptic peptide from BSA that has been protected via solution-phase reaction with iodoacetamide, was subjected to ion/ion reactions with IO4−. Activation of the long-lived complex, namely [M + 2H + IO4]+, results in both proton transfer from the peptide dication to the reagent anion to return the charge-reduced [M + H]+ peptide and oxygen transfer from the reagent to the peptide to yield the oxidized [M + H + O]+ species (monoisotopic m/z = 1924) (Figure 2(a)). (Note that the lightning bolts in all figures indicate the species that were activated in the respective CID experiments. The abundance of the residual precursor ion population is determined by the activation amplitude and time, which were not optimized here, to lead to 100% dissociation.) Isolation and activation of the [M + H + O]+ species results in dominant loss of HOSCH2CONH2 (107 Da, red square in Figure 2(b), monoisotopic m/z = 1817). The c8 ion (monoisotopic m/z = 962) is the second most abundant ion in this spectrum and is derived from secondary fragmentation of the N–Cα bond on the newly formed dehydroalanine residue. Further isolation and activation of the dehydroalanine-containing species is shown in Figure 2(c). In addition to the c8 ion, several backbone fragments are observed, both containing (b11□, b13□, b15□, and y14□) and lacking (b7) the dehydroalanine residue. The peak 17 Da below the c8 ion can correspond to either the b8 ion or an ammonia loss from the c8 ion. An additional step of CID on the c8 ion was performed and yielded highly abundant ammonia loss (Figure S-2), indicating that this peak is likely a sequential fragment from the c8 ion. These losses can be used to further confirm the location of the original S-cam cysteine residue. Doubly protonated KGAILC(cam)GIALK was also subjected to ion/ion reaction with IO4− (Figure S-3). As expected, activation of the oxidized species resulted in dominant loss of HOScam (Figure S-3(b)), and further activation of the HOScam loss resulted in dominant formation of the c5 ion due to the dehydroalanine effect (Figure S-3(c)).
Figure 2.
Oxidation of LFTFHADIC(cam)TLPDTEK via ion/ion reaction with IO4−. Activation of (a) [M + 2H + IO4]+, (b) [M + H + O]+, and (c) dehydroalanine product formed upon HOScam loss from the [M + H + O]+ species. Insets show structures of (a) cam-modified cysteine, (b) the modified side-chain upon oxidation, and (c) the dehydroalanine residue produced upon loss of HOScam. Symbols are as follows: (red squares) loss of HOSR to form the dehydroalanine product, (○) water losses, (*) ammonia losses, (lightning bolts) species subjected to CID, and (□) fragment ions that have lost the HOSR group.
Application to Prenylated Peptides
The prenylation of proteins is one of the most important post-translational lipid modifications and results in addition of one of two hydrophobic moieties (either a farnesyl or geranylgeranyl group) to the sulfur atom on a cysteine residue.1,2,12,62 Several biochemical methods, such as metabolic labeling63,64 and modification of isoprenoid groups of prenylated peptides to alkyne or azide functionalities for click chemistry reactions with modified biotin or fluorophores,65,66 have been developed to study prenylation. However, these methods are not efficient at identifying prenylation and are unable to distinguish between farnesylation and geranylgeranylation.53 Bhawal et al. recently demonstrated a method to both identify the presence of prenylated peptides and differentiate between the two different types of prenylation through the solution-phase oxidation of the prenylated peptides to the sulfoxide derivative.53 Collisional activation of these oxidized peptides then resulted in a signature loss of the prenyl sulfenic acid, the mass of which was used to distinguish between farnesylation and geranylgeranylation. Here, we extend this chemistry to the gas-phase, which allows the facile comparison of species before and after derivatization.
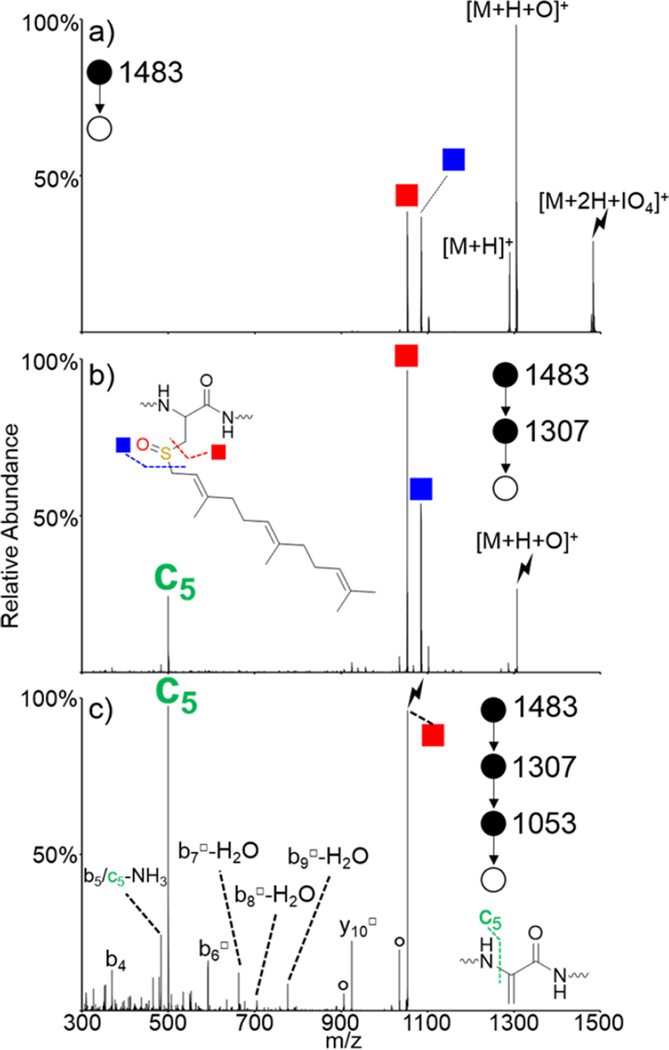
Doubly protonated S-farnesyl KGAILCGIALK (referred to as KGAILC(far)GIALK) was subjected to ion/ion reactions with periodate anion. Activation of the ion/ion complex is shown in Figure 3(a) and results in dominant formation of the oxidized species with proton transfer observed as a minor pathway. Cleavage on either side of the oxidized sulfur atom is also observed without the need for further activation. These cleavages become the dominant pathways when the [M + H + O]+ species is isolated and subjected to CID, as shown in Figure 3(b). The red square indicates the loss of HOSR (monoisotopic m/z = 1053), where R is the farnesyl group, and occurs via the mechanism shown in Scheme 1. The blue square indicates cleavage on the other side of the oxidized sulfur atom to lose the farnesyl group. This loss occurs via abstraction of one of the protons on the farnesyl chain and generates a cysteine sulfenic acid. Additionally, the c5 ion (monoisotopic m/z = 500) is present upon CID of the [M + H + O]+ species and indicates cleavage of the N–Cα bond on the newly formed dehydroalanine. This ion becomes the most dominant ion in the spectrum when the dehydroalanine-containing peptide (red square) is subjected to CID, as shown in Figure 3(c). Additionally, there are other backbone fragments both containing (b6□, b7□-H2O, b8□-H2O, b9□-H2O, and y10□) and lacking (b4, b5, and c5) the dehydroalanine residue, which can be used to further confirm the location of the modified residue.
Figure 3.
Oxidation of KGAILC(far)GIALK via ion/ion reaction with IO4−. Activation of (a) [M + 2H + IO4]+, (b) [M + H + O]+, and (c) dehydroalanine product formed upon HOSfar loss from the [M + H + O]+ species. Insets show structures of (b) oxidized farnesylated cysteine and (c) the dehydroalanine residue produced upon loss of HOSfar. Symbols are as follows: (red squares) loss of HOSR to form the dehydroalanine product, (blue squares) cleavage on the other side of the sulfur atom, (○) water losses, (*) ammonia losses, (lightning bolts) species subjected to CID, and (□) fragment ions that have lost the HOSR group.
A-factor is a yeast mating pheromone that is secreted by one of two haploid cell mating types and, along with α-factor, promotes mating and diploid formation in yeast.67 It is a peptide of the sequence YIIKGVFWDPACVIA, where the cysteine is farnesylated. Doubly protonated A-factor was subjected to ion/ion reactions with IO4−. Activation of the resulting ion/ion complex resulted in dominant formation of the oxidized [M + H + O]+ (Figure 4(a)). Some sequential fragmentation to generate cleavages on either side of the oxidized sulfur atom were observed along with the b9 fragment ion. The b9 ion corresponds to cleavage between the aspartic acid and proline residues. Facile cleavages at Asp-Pro and Asp-Xxx, where Xxx refers to other amino acids, have been investigated previously.68 It was determined that the activation energy required to cleave Asp–Xxx bonds is lower than the activation energy required to cleave the average peptide bond with the Asp–Pro bond being the most labile combination studied.68 Thus, formation of this b9 ion is an unusually facile pathway. The spectrum corresponding to isolation and activation of the [M + H + O]+ species is shown in Figure 4(b) and results in dominant loss of the HOSfar group. This cleavage is more dominant than even the b9 ion, demonstrating how favorable the sulfenic acid loss pathway is. Cleavage on the other side of the sulfur atom (namely, the side farthest from the peptide backbone) is also observed along with some internal fragments that result from cleavage of the aspartic acid–proline peptide bond and an additional peptide bond elsewhere in the peptide. Isolation and activation of the dehydroalanine residue dominantly yields the b9 ion (Figure 4(c)). The c11 ion, which is the signature cleavage indicating the presence and location of a dehydroalanine residue, is present, though at a lower abundance than observed for previous systems. This is likely due to the majority of the fragmentation occurring through the extremely low energy pathway to generate the b9 ion. There are some additional backbone fragments (b11, y12□, b13□ + H2O, b14□) that can be used to aid in the localization of the modification. Activation of protonated A-factor, which was not subjected to any ion/ion reactions, is shown in Figure 4(d). Once again, the b9 ion is highly dominant along with some sequential internal fragments. Some additional b-ions are observed (b8, b13, and b14) in lower abundance as well. Loss of the farnesyl group is extremely low in abundance (indicated by the yellow square) and is close in mass to that of the b13 ion, which could result in misidentification. It is apparent that the oxidation chemistry described here makes identification and localization of various S-alkyl cysteine PTMs facile and straightforward compared to traditional CID of the protonated species. The mass of the sulfenic acid (HOSR) loss can be used to determine the identity of the alkyl group bonded to the cysteine sulfur atom.
CONCLUSIONS
The gas-phase oxidation of various S-alkyl modifications of cysteine sulfur atoms upon ion/ion reactions with IO4− has been demonstrated. Ion/ion reactions between doubly protonated peptide cations, and IO4− results in generation of a long-lived complex of the form [M + 2H + IO4]+. Activation of this complex typically results in abundant formation of the sulfoxide derivative, namely [M + H + O]+, via oxygen transfer from the reagent to the alkylated sulfur atom. Activation of the oxidized [M + H + O]+ species results in ejection of the oxidized, alkylated sulfur atom as a sulfenic acid (HOSR, where R is an alkyl group) to generate a dehydroalanine residue in place of the S-alkyl cysteine residue. Activation of the dehydroalanine-containing species often results in cleavage of the N–Cα bond of the dehydroalanine residue to generate c- and z-type ions. Because these types of ions are rarely observed upon CID of protonated peptides, they can be used to indicate the presence and location of a dehydroalanine residue and, thus, locate the position of the original S-alkyl modification. This chemistry was illustrated here with S-ethylated, S-carbamidomethylated, and S-farnesylated peptides.
There are a number of options for how this chemistry might be implemented in a proteomics experiment, depending upon the level of automation available with the instrumentation (e.g., data-dependent scanning) and the objectives of the study. If the main objective is to focus on S-alkylated cysteines, a single data-dependent MS/MS scan could be used whereby the most abundant precursor ions are selected for interrogation. Selection of the precursor ion would be followed by an ion/ion reaction with periodate followed by a short broad-band activation step (e.g., dipolar DC, broad-band waveform, or HCD) to fragment all ions present in the post-ion/ion reaction ion population. Activation conditions could be established to fragment all of the species in the spectrum and also drive facile sequential reactions that would lead to both the sulfenic acid loss and the dehydroalanine cleavage. (Note that single frequency resonance excitation was used here to demonstrate the sequential nature of the structurally informative cleavages. The intervening isolation steps used to illustrate the chemistry here would not be needed in a proteomics workflow.) The subsequent broad-band fragmentation of the ion/ion complex as well as the first generation products would yield structural information for all components, not just the modified peptides. The majority of ion/ion peptide/periodate complexes, which would presumably not contain a modified cysteine, would fragment to give a protonated peptide. Under broad-band activation conditions, the protonated peptide (and any residual unreacted multiply protonated peptide ion population) would undergo fragmentation that could be used for peptide identification. The ion/ion MS/MS scan described above would be longer than a conventional CID MS/MS scan by the times required for anion fill and mutual storage. If an additional isolation step to throw out residual unreacted ions were to be used after the ion/ion reaction and prior to the broad-band activation step, this additional isolation step (roughly 10–30 ms) would also add to the overall time. Overall, these steps would typically add roughly 50–200 ms to a typical MS/MS scan, depending upon the times required for anion fill and mutual storage time. There are, of course, other possibilities for incorporating this chemistry into a proteomics workflow, but the procedure just described would likely require the least time. Although this method is longer than a conventional CID MS/MS scan, it is clearly executable on a chromatographic time scale.
Supplementary Material
Acknowledgments
We would like to thank Patty Wiley and the Hrycyna lab for providing the A-factor and Zhou Peng for bringing prenylation to our attention. This work was supported by the National Institutes of Health under Grant GM R37 45372. Graduate student support for A.L.P. was provided by Emerson Kampen and Bilsland Dissertation Fellowships.
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Figure S-1, summary of the oxidation of doubly protonated S-ethyl ARACAKA; Figure S-2, CID spectrum of LFTFHADIC(cam)TLPDTEK c8 ion; and Figure S-3, summary of the oxidation of doubly protonated KGAILC(cam)GIALK (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.Chalker JM, Bernardes GJL, Lin YA, Davis BG. Chemical Modification of Proteins at Cysteine: Opportunities in Chemistry and Biology. Chem. - Asian J. 2009;4:630–640. doi: 10.1002/asia.200800427. [DOI] [PubMed] [Google Scholar]
- 2.Berndt N, Hamilton AD, Sebti SM. Targeting protein prenylation for cancer therapy. Nat. Rev. Cancer. 2011;11:775–791. doi: 10.1038/nrc3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 4.Caruso MG, Notarnicola M, Bifulco M, Laezza C, Guerra V, Altomare DF, Memeo V, Lorusso D, Demma I, Di Leo A. Increased farnesyltransferase activity in human colorectal cancer: relationship with clinicopathological features and K-ras mutation. Scand. J. Gastroenterol. 2003;38:80–85. doi: 10.1080/00365520310000483. [DOI] [PubMed] [Google Scholar]
- 5.Kazi A, Carie A, Blaskovich MA, Bucher C, Thai V, Moulder S, Peng H, Carrico D, Pusateri E, Pledger WJ, Berndt N, Hamilton A, Sebti SM. Blockade of protein geranylgeranylation inhibits Cdk2-dependent p27Kip1 phosphorylation on Thr187 and accumulates p27Kip1 in the nucleus: implications for breast cancer therapy. Mol. Cell. Biol. 2009;29:2254–2263. doi: 10.1128/MCB.01029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nallan L, Bauer KD, Bendale P, Rivas K, Yokoyama K, Hornéy CP, Pendyala PR, Floyd D, Lombardo LJ, Williams DK, Hamilton A, Sebti S, Windsor WT, Weber PC, Buckner FS, Chakrabarti D, Gelb MH, Van Voorhis WC. Protein farnesyltransferase inhibitors exhibit potent antialarial activity. J. Med. Chem. 2005;48:3704–3713. doi: 10.1021/jm0491039. [DOI] [PubMed] [Google Scholar]
- 7.Walters CE, Pryce G, Hankey DJ, Sebti SM, Hamilton AD, Baker D, Greenwood J, Adamson P. Inhibition of Rho GTPases with protein prenyltransferase inhibitors prevents leukocyte recruitment to the central nervous system and attenuates clinical signs of disease in an animal model of multiple sclerosis. J. Immunol. 2002;168:4087–4094. doi: 10.4049/jimmunol.168.8.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordier BB, Ohkanda J, Liu P, Lee SY, Salazar FH, Marion PL, Ohashi K, Meuse L, Kay MA, Casey JL, Sebti SM, Hamilton AD, Glenn JS. In vivo antiviral efficacy of prenylation inhibitors against hepatitis delta virus. J. Clin. Invest. 2003;112:407–414. doi: 10.1172/JCI17704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worman HJ. Prelamin A prenylation and the treatment of progeria. J. Lipid Res. 2010;51:223–225. doi: 10.1194/jlr.E004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young SG, Yang SH, Davies BSJ, Jung HJ, Fong LG. Targeting Protein Prenylation in Progeria. Sci. Transl. Med. 2013;5:171ps3. doi: 10.1126/scitranslmed.3005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong LG, Ng JK, Meta M, Coteé N, Yang SH, Stewart CL, Sullivan T, Burghardt A, Majumdar S, Reue K, Bergo MO, Young SG. Heterozygosity for Lmna deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2004;101:18111–18116. doi: 10.1073/pnas.0408558102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang FL, Casey P. Protein Prenylation: Molecular Mechanisms and Functional Consequences. Annu. Rev. Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 13.Manolaridis I, Kulkarni K, Dodd RB, Ogasawara S, Zhang Z, Bineva G, O’Reilly N, Hanrahan SJ, Thompson AJ, Cronin N, Iwata S, Barford D. Mechanism of farnesylated CAAX protein processing by the intramembrane protease Rce1. Nature. 2013;504:301–305. doi: 10.1038/nature12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore SL, Schaber MD, Mosser SD, Rands E, O’Hara MB, Garsky VM, Marshall MS, Pompliano DL, Gibbs JB. Sequence dependence of protein isoprenylation. J. Biol. Chem. 1991;266:14603–14610. [PubMed] [Google Scholar]
- 15.Khosravi-Far R, Lutz RJ, Cox AD, Conroy L, Bourne JR, Sinensky M, Balch WE, Buss JE, Der CJ. Isoprenoid modification of rab proteins terminating in CC or CXC motifs. Proc. Natl. Acad. Sci. U. S. A. 1991;88:6264–6268. doi: 10.1073/pnas.88.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glomset JA, Farnsworth CC. Role of protein modification reactions in programming interactions between ras-related GTPases and cell membranes. Annu. Rev. Cell Biol. 1994;10:181–2085. doi: 10.1146/annurev.cb.10.110194.001145. [DOI] [PubMed] [Google Scholar]
- 17.Glomset JA, Gelb MH, Farnsworth CC. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem. Sci. 1990;15:139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- 18.Musha T, Kawata M, Takai Y. The geranylgeranyl moiety but not the methyl moiety of the smg-25A/rab3A protein is essential for the interactions with membrane and its inhibitory GDP/GTP exchange protein. J. Biol. Chem. 1992;267:9821–9825. [PubMed] [Google Scholar]
- 19.Maltese WA, Wilson AL, Erdman RA. Prenylation-dependent interaction of Rab proteins with GDP dissociation inhibitors. Biochem. Soc. Trans. 1996;24:703–708. doi: 10.1042/bst0240703. [DOI] [PubMed] [Google Scholar]
- 20.Fukada Y, Takao T, Ohguro H, Yoshizawa T, Akino T, Shimonishi Y. Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature. 1990;346:658–660. doi: 10.1038/346658a0. [DOI] [PubMed] [Google Scholar]
- 21.Hancock JF. Ras Proteins: Different Signals from Different Locations. Nat. Rev. Mol. Cell Biol. 2003;4:373–384. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- 22.Jackson JH, Cochrane CG, Bourne JR, Solski PA, Buss JE, Der CJ. Farnesol modification of Kirsten-ras exon 4B protein is essential for transformation. Proc. Natl. Acad. Sci. U. S. A. 1990;87:3042–3046. doi: 10.1073/pnas.87.8.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox AD, Der CJ. Farnesyltransferase inhibitors and cancer treatment: targeting simply Ras? Biochim. Biophys. Acta, Rev. Cancer. 1997;1333:F51–F71. doi: 10.1016/s0304-419x(97)00011-5. [DOI] [PubMed] [Google Scholar]
- 24.Gibbs JB, Oliff A, Kohl NE. Farnesyltransferase inhibitors: Ras research yields a potential cancer therapeutic. Cell. 1994;77:175–178. doi: 10.1016/0092-8674(94)90308-5. [DOI] [PubMed] [Google Scholar]
- 25.Powers S, Michaelis S, Broek D, Sonia Santa A-A, Field J, Herskowitz I, Wigler M. RAM, a gene of yeast required for a functional modification of RAS proteins and for production of mating pheromone a-factor. Cell. 1986;47:413–422. doi: 10.1016/0092-8674(86)90598-2. [DOI] [PubMed] [Google Scholar]
- 26.Anderegg RJ, Betz R, Carr SA, Crabb JW, Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J. Biol. Chem. 1988;263:18236–18240. [PubMed] [Google Scholar]
- 27.Inglese J, Glickman JF, Lorenz W, Caron MG, Lefkowitz RJ. Isoprenylation of a protein kinase. Requirement of farnesylation/alpha-carboxyl methylation for full enzymatic activity of rhodopsin kinase. J. Biol. Chem. 1992;267:1422–1425. [PubMed] [Google Scholar]
- 28.Farnsworth CC, Wolda SL, Gelb MH, Glomset JA. Human lamin B contains a farnesylated cysteine residue. J. Biol. Chem. 1989;264:20422–20429. [PMC free article] [PubMed] [Google Scholar]
- 29.Goddard DR, Michaelis L. Derivatives of Keratin. J. Biol. Chem. 1935;112:361–371. [Google Scholar]
- 30.Lundell N, Schreitmüller T. Sample preparation for peptide mapping—A pharmaceutical quality-control perspective. Anal. Biochem. 1999;266:31–47. doi: 10.1006/abio.1998.2919. [DOI] [PubMed] [Google Scholar]
- 31.Ravi S, Krishnamurthy VR, Caves JM, Haller CA, Chaikof EL. Maleimide-thiol coupling of a bioactive peptide to an elastin-like protein polymer. Acta Biomater. 2012;8:627–635. doi: 10.1016/j.actbio.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King HD, Dubowchik GM, Walker MA. Facile synthesis of maleimide bifunctional linkers. Tetrahedron Lett. 2002;43:1987–1990. [Google Scholar]
- 33.Raftery MA, Cole RD. On the Aminoethylation of Proteins. J. Biol. Chem. 1966;241:3457–3461. [PubMed] [Google Scholar]
- 34.Lindley H. A new synthetic substrate for trypsin and its application to the determination of the amino-acid sequence of proteins. Nature. 1956;178:647–648. doi: 10.1038/178647a0. [DOI] [PubMed] [Google Scholar]
- 35.Prentice BM, McLuckey SA. Gas-Phase Ion/Ion Reactions of Peptides and Proteins: Acid/Base, Redox, and Covalent Chemistries. Chem. Commun. 2013;49:947–965. doi: 10.1039/c2cc36577d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGee WA, McLuckey SA. Efficient and Directed Peptide Bond Formation in the Gas Phase via Ion/Ion Reactions. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1288–1292. doi: 10.1073/pnas.1317914111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng Z, McLuckey SA. C-terminal peptide extension via gas-phase ion/ion reactions. Int. J. Mass Spectrom. 2015;391:17–23. doi: 10.1016/j.ijms.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bu J, Pilo AL, McLuckey SA. Gas phase click chemistry via ion/ion reactions. Int. J. Mass Spectrom. 2015;390:118–123. [Google Scholar]
- 39.Gilbert JD, Prentice BM, McLuckey SA. Ion/Ion Reactions with “Onium” Reagents: An Approach for the Gas-phase Transfer of Organic Cations to Multiply-Charged Anions. J. Am. Soc. Mass Spectrom. 2015;26:818–825. doi: 10.1007/s13361-015-1077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilo AL, McLuckey SA. Oxidation of Methionine Residues in Polypeptide Ions Via Gas-Phase Ion/Ion Chemistry. J. Am. Soc. Mass Spectrom. 2014;25:1049–1057. doi: 10.1007/s13361-014-0861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilo AL, McLuckey SA. Selective Gas-Phase Ion/Ion Reactions: Enabling Disulfide Mapping via Oxidation and Cleavage of Disulfide Bonds in Intermolecularly-Linked Polypeptide Ions. Anal. Chem. 2016 Mar 15; doi: 10.1021/acs.analchem.6b01043. Submitted to. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schey KL, Finley EL. Identification of Peptide Oxidation by Tandem Mass Spectrometry. Acc. Chem. Res. 2000;33:299–306. doi: 10.1021/ar9800744. [DOI] [PubMed] [Google Scholar]
- 43.Lagerwerf FM, van de Weert M, Heerma W, Haverkamp J. Oxidation of Oxidized Methionine Peptides. Rapid Commun. Mass Spectrom. 1996;10:1905–1910. doi: 10.1002/(SICI)1097-0231(199612)10:15<1905::AID-RCM755>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 44.Reid GE, Roberts KD, Kapp EA, Simpson RJ. Statistical and Mechanistic Approaches to Understanding the Gas-Phase Fragmentation Behavior of Methionine Sulfoxide Containing Peptides. J. Proteome Res. 2004;3:751–759. doi: 10.1021/pr0499646. [DOI] [PubMed] [Google Scholar]
- 45.Qin J, Chait BT. Identification and characterization of posttranslational modifications of proteins by MALDI ion trap mass spectrometry. Anal. Chem. 1997;69:3995–4001. doi: 10.1021/ac970489n. [DOI] [PubMed] [Google Scholar]
- 46.Jiang XY, Smith JB, Abraham EC. Identification of a MS-MS fragment diagnostic for methionine sulfoxide. J. Mass Spectrom. 1996;31:1309–1310. [Google Scholar]
- 47.O’Hair RAJ, Reid GE. Neighboring group versus cis-elimination mechanisms for side chain loss from protonated methionine, methionine sulfoxide and their peptides. Eur. Mass Spectrom. 1999;5:325–334. [Google Scholar]
- 48.Pilo AL, Bu J, McLuckey SA. Transformation of [M+2H]2+ Peptide Cations to [M−H]+, [M+H+O]+, and M+• Cations via Ion/Ion Reactions: Reagent Anions Derived from Persulfate. J. Am. Soc. Mass Spectrom. 2015;26:1103–1114. doi: 10.1007/s13361-015-1125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Froelich JM, Reid GE. Mechanisms for the Proton Mobility Dependent Gas-Phase Fragmentation Reactions of S-alkyl Cysteine Sulfoxide-Containing Peptide Ions. J. Am. Soc. Mass Spectrom. 2007;18:1690–1705. doi: 10.1016/j.jasms.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 50.Steen H, Mann M. Similarity between condensed phase and gas phase chemistry: Fragmentation of peptides containing oxidized cysteine residues and its implications for proteomics. J. Am. Soc. Mass Spectrom. 2001;12:228–232. doi: 10.1016/S1044-0305(00)00219-1. [DOI] [PubMed] [Google Scholar]
- 51.Chowdhury SM, Munske GR, Ronald RC, Bruce JE. Evaluation of low energy CID and ECD fragmentation behavior of mono-oxidized thio-ether bonds in peptides. J. Am. Soc. Mass Spectrom. 2007;18:493–501. doi: 10.1016/j.jasms.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kao A, Chiu C, Vellucci D, Yang Y, Patel VR, Guan S, Randall A, Baldi P, Rychnovsky SD, Huang L. Development of a Novel Cross-Linking Strategy for Fast and Accurate Identification of Cross-linked Peptides of Protein Complexes. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M110.002212. M110.002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhawal RP, Sadananda SC, Bugarin A, Laposa B, Chowdhury SM. Mass Spectrometry Cleavable Strategy for Identification and Differentiation of Prenylated Peptides. Anal. Chem. 2015;87:2178–2186. doi: 10.1021/ac503794s. [DOI] [PubMed] [Google Scholar]
- 54.Wollack JW, Zeliadt NA, Mullen DG, Amundson G, Geier S, Falkum S, Wattenberg EV, Barany G, Distefano MD. Multifunctional Prenylated Peptides for Live Cell Analysis. J. Am. Chem. Soc. 2009;131:7293–7303. doi: 10.1021/ja805174z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xue CB, Becker JM, Naider F. Efficient Regioselective Isoprenylation of Peptides in Acidic Aqueous Solution Using Zinc Acetate as a Catalyst. Tetrahedron Lett. 1992;33:1435–1438. [Google Scholar]
- 56.Xia Y, Wu J, Londry FA, Hager JW, McLuckey SA. Mutual Storage Mode Ion/Ion Reactions in Hybrid Linear Ion Trap. J. Am. Soc. Mass Spectrom. 2005;16:71–81. doi: 10.1016/j.jasms.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 57.Liang W, Xia Y, McLuckey SA. Alternately pulsed nano-electrospray ionization/atmospheric pressure chemical ionization for ion/ion reactions in an electrodynamic ion trap. Anal. Chem. 2006;78:3208–3212. doi: 10.1021/ac052288m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Londry FA, Hager JW. Mass selective axial ion ejection from a linear quadrupole ion trap. J. Am. Soc. Mass Spectrom. 2003;14:1130–1147. doi: 10.1016/S1044-0305(03)00446-X. [DOI] [PubMed] [Google Scholar]
- 59.Chrisman PA, McLuckey SA. Dissociations of Disulfide-linked Gaseous Polypeptide/Protein Anions: Ion Chemistry with Implications for Protein Identification and Characterization. J. Proteome Res. 2002;1:549–557. doi: 10.1021/pr025561z. [DOI] [PubMed] [Google Scholar]
- 60.Wells JM, Stephenson JL, Jr, McLuckey SA. Charge dependence of protonated insulin decompositions. Int. J. Mass Spectrom. 2000;203:A1–A9. [Google Scholar]
- 61.Gorman JJ, Wallis TP, Pitt JJ. Protein Disulfide Bond Determination by Mass Spectrometry. Mass Spectrom. Rev. 2002;21:183–216. doi: 10.1002/mas.10025. [DOI] [PubMed] [Google Scholar]
- 62.Gelb MH, Brunsveld L, Hrycyna CA, Michaelis S, Tamanoi F, Van Voorhis WC, Waldmann H. Therapeutic intervention based on protein prenylation and associated modifications. Nat. Chem. Biol. 2006;2:518–528. doi: 10.1038/nchembio818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palsuledesai CC, Ochocki JD, Markowski TW, Distefano MD. A combination of metabolic labeling and 2D-DIGE analysis in response to a farnesyltransferase inhibitor facilitates the discovery of new prenylated proteins. Mol. BioSyst. 2014;10:1094–1103. doi: 10.1039/c3mb70593e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benetka W, Koranda M, Maurer-Stroh S, Pittner F, Eisenhaber F. Farnesylation or geranylgeranylation? Efficient assays for testing protein prenylation in vitro and in vivo. BMC Biochem. 2006;7:6. doi: 10.1186/1471-2091-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishimura A, Linder ME. Identification of a Novel Prenyl and Palmitoyl Modification at the CaaX Motif of Cdc42 That Regulates RhoGDI Binding. Mol. Cell. Biol. 2013;33:1417–1429. doi: 10.1128/MCB.01398-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hang HC, Wilson JP, Charron G. Bioorthogonal Chemical Reporters for Analyzing Protein Lipidation and Lipid Trafficking. Acc. Chem. Res. 2011;44:699–708. doi: 10.1021/ar200063v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michaelis S, Barrowman J. Biogenesis of the Saccharomyces cerevisiae Pheromone a-Factor, from Yeast Mating to Human Disease. Microbiol. Mol. Biol. Rev. 2012;75:626–651. doi: 10.1128/MMBR.00010-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu W, Vath JE, Huberty MC, Martin SA. Identification of the facile gas-phase cleavage of the Asp-Pro and Asp-Xxx peptide bonds in matrix assisted laser desorption time-of-flight mass spectrometry. Anal. Chem. 1993;65:3015–3023. doi: 10.1021/ac00069a014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.