To the Editor
Sterile alpha motif domain– and HD domain–containing protein 1 (SAMHD1) is a cellular dNTP triphosphohydrolase (dNTPase) that restricts HIV-1 replication in myeloid cells and resting CD4+ T cells by degrading dNTPs and limiting viral reverse transcription1–5. Purified recombinant SAMHD1 also has exonuclease activity when synthetic nucleic acids or HIV-1 gag and tat RNAs transcribed in vitro are used as substrates6. Ryoo et al.7 recently suggested that SAMHD1 restricts HIV-1 infection through its ribonuclease (RNase) activity by cleaving the viral RNA genome. By using SAMHD1 mutants purported to specifically retain dNTPase (SAMHD1Q548A) or RNase (SAMHD1D137N) activities, Ryoo et al.7,8 proposed that the RNase activity of SAMHD1, but not its dNTPase activity, is essential for HIV-1 restriction in non-dividing cells. They also suggested that SAMHD1 phosphorylation at T592 negatively regulated its RNase activity7.
To extend these findings7, we measured HIV-1 protein synthesis and virion production in the presence of SAMHD1 when the requirement for intracellular dNTP-dependent HIV-1 reverse transcription was bypassed. We co-transfected an HIV-1 proviral DNA plasmid (pNL4-3) with a plasmid expressing wild-type (WT) SAMHD1 or a phospho-ablative, but dNTPase-active, mutant (SAMHD1T592A; refs. 9–11) into human embryonic kidney (HEK) 293T cells and assessed intracellular HIV-1 Gag protein synthesis and viral particle release in the supernatants. This transfection-based HIV-1 production is independent of reverse transcription requiring intracellular dNTPs as precursors of viral DNA synthesis, but is dependent on HIV-1 mRNA–mediated gene expression. Intracellular HIV-1 Gag protein levels, p24 capsid levels in released HIV-1 virions and infectivity were not reduced by the ectopic expression of WT SAMHD1 or SAMHD1T592A mutant (Supplementary Fig. 1), which suggests that SAMHD1 cannot inhibit HIV-1 production after the reverse transcription step, regardless of its phosphorylation at T592. These findings are consistent with our previous results showing that the expression of WT SAMHD1 or of the SAMHD1T592A mutant in dividing cells does not restrict HIV-1 infection11,12. Our results suggest that SAMHD1 does not have broad nuclease activity, but do not rule out a specific nucleolytic interaction between SAMHD1 and incoming HIV-1 genomic RNA (gRNA).
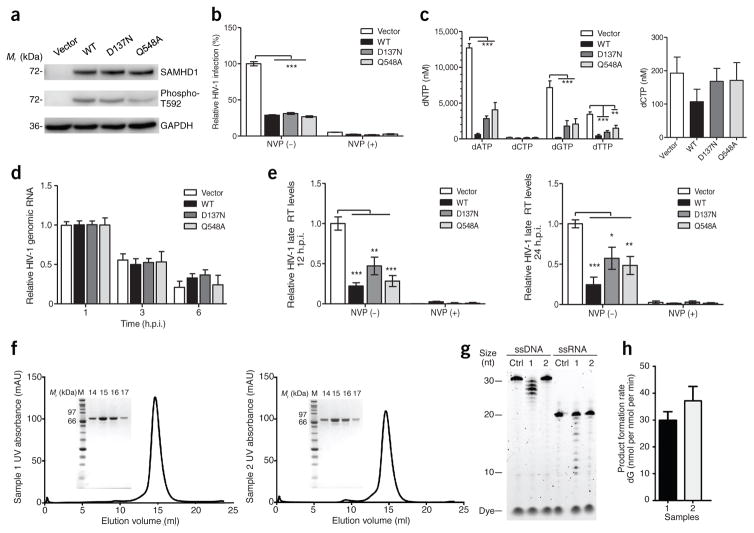
Given the preponderance of previous data implicating the dNT-Pase activity of SAMHD1 as its primary antiviral mechanism, we reproduced the key experiments of Ryoo et al.7. Their conclusion that SAMHD1 restricts HIV-1 through RNase activity was based on the differential activity of the SAMHD1 mutants, SAMHD1D137N and SAMHD1Q548A (ref. 7). We independently generated these two mutant constructs and confirmed the expected mutations by DNA sequencing to ensure that there were no other disabling mutations in the constructs. We then examined the HIV-1 restriction and intracellular dNTP regulation by these mutants and WT SAMHD1 by following the protocol of Ryoo et al.7. Our results show that both the SAMHD1D137N and SAMHD1Q548A mutants were expressed at similar levels to that of WT SAMHD1, and each efficiently restricted HIV-1 infection and decreased dATP, dGTP and dTTP levels in phorbol 12-myristate 13-acetate (PMA)-differentiated U937 cells (Fig. 1a–c). SAMHD1 expression did not significantly decrease dCTP levels, as compared to vector control cells (Fig. 1c, right), probably owing to the different biosynthesis pathway of dCTP. Notably, Ryoo et al.7 showed only dCTP levels, but not dATP, dGTP or dTTP levels, in SAMHD1-expressing or control cells.
Figure 1.
SAMHD1-mediated HIV-1 restriction in cells does not involve RNase activity. (a–e) Both D137N and Q548A mutants of SAMHD1 restrict HIV-1 infection in PMA-differentiated U937 cells by decreasing viral cDNA synthesis, but not viral genomic RNA (gRNA). U937 cell lines stably expressing WT and mutant SAMHD1 were differentiated with PMA (30 ng/ml) for 20 h. The reverse transcriptase inhibitor nevirapine (NVP) was used as a control. (a) Lysates from PMA-differentiated cells were collected for immunoblotting to confirm SAMHD1 expression and T592-phosphorylated SAMHD1 (phospho-T592). GAPDH was used as a loading control. Blot is representative of three independent experiments. (b) PMA-differentiated cells were infected with single-cycle HIV-1-Luc/VSV-G at a multiplicity of infection (MOI) of 1. At 24 h postinfection (h.p.i.), luciferase assay was used to measure HIV-1 infectivity. Graph depicts relative percentage of luciferase per 10 μg of protein, with the vector set as 100%. Data are presented as mean ± s.e.m. of six independent experiments with two biological replicates per experiment. Statistical analysis was performed using the one-way ANOVA with Dunnett’s correction, ***P < 0.001. (c) Decreased dNTP levels of PMA-differentiated U937 cell lines expressing WT or mutant SAMHD1. Data are presented as means ± s.e.m. of two independent experiments with two biological replicates per experiment. The average concentrations of dNTPs (left) and dCTP (right) in different cell lines are shown. Statistical analysis was performed using the Kruskal–Wallis one-way ANOVA. (d) SAMHD1 does not degrade HIV-1 gRNA in PMA-differentiated U937 cells. The levels of HIV-1 gRNA were measured at 1, 3 and 6 h.p.i., as described at an MOI of 1. Data are presented as mean ± s.e.m. of three independent experiments with two biological replicates per experiment and depicted as relative to HIV-1 gRNA, with the time point 1 h.p.i. set as 1. RNA samples without reverse transcription were used as negative controls and showed no detection of HIV-1 gRNA (data not shown). (e) The expression of WT or mutant SAMHD1 reduces HIV-1 late reverse transcription (RT) products in PMA-differentiated U937 cells at 12 and 24 h.p.i. The levels of HIV-1 late RT products were measured by qPCR. Data are presented as means ± s.e.m. (n = 4 experiments for 12 h.p.i. and n = 6 experiments for 24 h.p.i.). Statistical analysis was performed using the one-way ANOVA with Dunnett’s correction, ***P < 0.001, **P < 0.01, *P < 0.05. (f–h) Characterization of SAMHD1 enzymatic activities in vitro. (f) The purity of recombinant WT SAMHD1 from different batches of preparations (sample 1 and sample 2), as demonstrated by analytical size-exclusion chromatography and SDS–PAGE. SAMHD1 (150 μl) at 2 mg/ml was applied to a Superdex 200 10/300 GL column. Fraction numbers on the SDS–PAGE indicate elution volumes. (g) Moderate exonuclease activities on both ssDNA and ssRNA were observed for sample 1; only background-level activities were detected for sample 2. Ctrl indicates a control without protein. The nuclease-activity assays were performed at 37 °C for 1 h, with 1 μM of SAMHD1 and 1 μM of ssDNA or ssRNA in the presence of 5 mM Mg2+. (h) The dNTPase activity of SAMHD1 proteins (0.5 μM) was assayed with 1 mM dGTP from 5–15 min. The amount of dG products generated in the reactions was quantified by HPLC. Error bars represent s.e.m. from triplicate experiments.
Previous studies have used a SAMHD1D137A mutant to explore the effects of the dGTP binding site (D137) on the dNTPase activity, tetramer formation and HIV-1 restriction of SAMHD1 (refs. 13–15). The SAMHD1D137A mutant has no detectable dNTPase activity or HIV-1 restriction in vitro, owing to its inability to form a stable tetramer14,15. It is possible that the SAMHD1D137N mutant might be stabilized in cells to remain in a tetrameric form, thus maintaining its ability to reduce intracellular dNTP levels and restrict HIV-1 infection. It is also possible that an in vitro dNTPase assay using purified recombinant SAMHD1 proteins might not fully reflect the dNTPase activity of SAMHD1 in PMA-differentiated U937 cells. However, these possibilities remain to be examined to explain how the SAMHD1D137N mutant restricts HIV-1 infection if its dNTPase activity is impaired. In contrast to the results of Ryoo et al.7, the SAMHD1D137N and SAMHD1Q548A mutants in our experiments do not have differing anti-HIV-1 activities, and neither lacks the ability to lower cellular dNTP levels.
Ryoo et al.7 reported an approximately twofold decrease in HIV-1 gRNA levels in PMA-differentiated U937 cells expressing WT SAMHD1 or SAMHD1D137N, but not the SAMHD1Q548A mutant, as compared to the control cells at 3 h and 6 h postinfection (h.p.i.), which suggests SAMHD1-mediated HIV-1 gRNA degradation7. By contrast, we detected comparable levels of HIV-1 gRNA in PMA-differentiated U937 cells expressing SAMHD1 (WT, SAMHD1D137N and SAMHD1Q548A mutants) and the vector control cells at 1, 3 and 6 h.p.i., respectively (Fig. 1d), showing that, in our study, SAMHD1 cannot degrade HIV-1 gRNA during early infection. To further support our findings, we measured the levels of HIV-1 late reverse transcription products in infected cells at 12 and 24 h.p.i, which represent viral cDNA synthesis dependent on the intracellular dNTP pool. We found that the expression of WT SAMHD1, SAMHD1D137N and SAMHD1Q548A mutants significantly reduced HIV-1 late reverse transcription products as compared to the vector control cells (Fig. 1e), correlating well with a reduced intracellular dNTP pool (Fig. 1c). Thus, in our study, these two mutants of SAMHD1 cannot distinguish its dNTPase and RNase functions, and dNTP depletion accounts for SAMHD1-mediated HIV-1 restriction.
Seamon et al.16 reported that trace exonuclease activities of recombinant SAMHD1 arise from contaminants during purification. To clarify whether recombinant SAMHD1 has a nuclease activity, we tested its ability to degrade single-stranded (ss) DNA and ssRNA substrates using stringently purified full-length recombinant SAMHD1. Purified SAMHD1 (Fig. 1f) exhibited a weak in vitro nuclease activity on both synthesized random ssDNA and ssRNA in some protein preparations (Fig. 1g, sample 1), whereas only a background level of nuclease activity was observed for other independent protein preparations under identical conditions (Fig. 1g, sample 2). In all preparations, SAMHD1 had robust dNTPase activity, as expected (Fig. 1h). The observed activity on ssDNA and ssRNA resulted in products with distinct cleavage patterns, similar to previous observations6,7,16. However, the inconsistency of the RNase activity of SAMHD1 between different protein preparations suggests that the RNase activity is likely to be associated with contaminants, consistent with the results by Seamon et al.16.
The ability of SAMHD1 to restrict HIV-1 replication is thought to be through its dNTPase activity because the addition of exogenous deoxynucleosides rescues HIV-1 infectivity in SAMHD1-expressing cells5,17 and the anti-HIV activity of SAMHD1 is reversible17. These results suggest that dNTP depletion is the primary mechanism of SAMHD1-mediated HIV-1 restriction, and argues against a nucleolytic mechanism. Our data and two recent studies18,19 do not support the conclusion that the RNase activity of SAMHD1 is involved in its anti-HIV-1 activity7, but are in agreement with its dNTPase activity as mediating HIV-1 restriction.
The discrepancy between our results and those of Ryoo et al.7 might be due to different approaches, although we repeated HIV-1 infection and RT–qPCR experiments using the same methods (Fig. 1d)7. One difference was our use of the HIV-1-derived pLenti-puro vector to stably express high levels of SAMHD1 in PMA-differentiated U937 cells in HIV-1 restriction assays11, whereas Ryoo et al. used a murine stem cell virus–based pMSCV-puro vector7. We could not express high levels of SAMHD1 proteins in U937 cells using the pMSCV-puro vector (Supplementary Fig. 2). This might be due to lower expression driven by the long terminal repeat promoter of the pMSCV-puro vector, as compared to the cytomegalovirus promoter driving higher SAMHD1 expression from the pLenti vector. Previous studies showed that the efficiency of SAMHD1-mediated HIV-1 restriction in PMA-differentiated U937 cells is dependent on the levels of SAMHD1 expression14,20. Notably, the SAMHD1 expression levels in U937 cells were lower than the endogenous SAMHD1 levels in primary monocyte-derived macrophages from a healthy donor (Supplementary Fig. 3), which suggests that SAMHD1 expressed from the pLenti vector in U937 cells is not excessive. Different HIV-1 reporter vectors or other reagents used in these studies might also contribute to the discrepancy of the results. Overall, our data suggest that intracellular dNTP reduction by SAMHD1 is the primary mechanism of HIV-1 restriction in nondividing cells.
ONLINE METHODS
Cell culture
Human embryonic kidney 293T (HEK293T) cells and monocytic U937 cells were obtained from the American Type Culture Collection (ATCC) and maintained as described11. U937 cells stably expressing WT, mutant SAMHD1D137N, SAMHD1Q548A or SAMHD1T592A were generated by spinoculation of U937 cells with concentrated lentiviral vectors, and then selected with puromycin (0.8 μg/ml), as described11. All cell lines tested negative for mycoplasma contamination using a PCR-based universal mycoplasma detection kit (ATCC). Human peripheral blood mononuclear cells were isolated from the buffy coat of healthy donors, as previously described11. CD14+ monocytes were isolated separately from peripheral blood mononuclear cells by using anti-CD14-coated magnetic beads and cultured in RPMI medium with 10% FBS, as described21. To generate monocyte-derived macrophages, isolated monocytes were cultured in the presence of macrophage colony-stimulating factor (M-CSF; 50 ng/ml) (PeproTech) for 7 d, as described21.
Plasmids, transfection and HIV-1 production assay
The pLenti-puro plasmids encoding hemagglutinin (HA)-SAMHD1 and the empty vector were previously described5. Plasmids expressing SAMHD1 mutants (SAMHD1D137N, SAMHD1Q548A or SAMHD1T592A) were generated using a Quickchange mutagenesis kit (Agilent Technologies)11. HEK293T cells (6 × 105) were co-transfected with the HIV-1 proviral DNA pNL4-3 (1 μg) and increasing amounts of SAMHD1 WT- or SAMHD1T592A mutant-expressing plasmid (0.5, 1.0 and 1.5 μg) using calcium phosphate11. The total amount of DNA transfected was maintained at 2.5 μg per sample by using an empty vector. Transfection media was replaced with fresh media at 18 h after transfection. After another 24 h, cell supernatants containing released virions were clarified by passing them through a 0.45-micron filter, and pelleted via ultracentrifugation at 28,000 rpm for 1.5 h through a 25%-sucrose cushion. The pelleted virions were lysed in 1% Triton X-100 for analysis by immunoblotting to determine the levels of HIV-1 Gag or p24 capsid (CA). After the removal of virus containing cell supernatant, the transfected HEK293T cells were treated with subtilisin (1 mg/ml) for 15 min to remove plasma-membrane-bound viral particles. Cells were lysed in 1%-Triton-X-100-containing lysis buffer and the expression levels of SAMHD1 WT and SAMHD1T592A, as well as those of HIV-1 Gag and p24 CA, were detected by immunoblotting. T592-phosphorylated SAMHD1 (phospho-T592) was determined by immunoblotting using specific antibodies11. The relative infectivity of HIV-1 virions generated from transfected HEK293T cells expressing SAMHD1 was determined using a reporter cell line, as described11.
Immunoblotting and antibodies
Cells were harvested at 24 h after transfection or as specifically indicated, and lysed with cell lysis buffer (Cell Signaling) containing protease inhibitor cocktail (Sigma-Aldrich). Cell lysates were prepared for immunoblotting11, and the blots were detected using antibodies specific to HA (Covance, Ha.11 clone 16B12) at a 1:1,000 dilution, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (AbD serotec, AHP1628T) at a 1:3,000 dilution. Monoclonal antibody to HIV-1 p24 Gag (clone #24–2) was obtained from the NIH AIDS Reagent Program (cat. # 6457) and prepared at a 1:1,000 dilution. Polyclonal SAMHD1-specific antibodies (Abcam, ab67820) were used at a 1:1,000 dilution for immunoblotting, as described12. Rabbit antibodies specific for T592 phospho-SAMHD1 (ProSci, #8005) were used at a dilution of 1:1,000, as previously described22. Immunoblotting images were captured and analyzed using the Luminescent Image analyzer (LAS 4000), as described11. ImageJ software was used to calculate the densitometry of cellular Pr55 gag, virion p24 CA and GAPDH bands in three independent experiments. Validation for all antibodies is provided on the manufacturers’ websites.
HIV-1 infection assays
U937 cells were transduced with pLenti-puro based lentiviral vectors that do not express (vector control) or express WT, mutant SAMHD1D137N or SAMHD1Q548A to generate puromycin-resistant stable cell lines, as described11. Each cell line was differentiated with PMA (30 ng/ml) for 20 h, as described7, and subsequently infected with HIV-1-Luc (luciferase reporter virus) pseudotyped with vesicular stomatitis virus G protein (VSV-G)11. Cells were pre-treated with DMSO control or neviraprine (NVP, 10 μM) for 1 h before HIV-1 infection. DMSO or NVP was maintained in the culture medium for the duration of the infection assay.
Intracellular dNTP measurement
For dNTP analysis and quantification, U937 cells were lysed, and extracted samples were processed for the single-nucleotide incorporation assay, as described23.
Quantitative real-time PCR (qPCR) for HIV-1 genomic RNA and cDNA measurement
U937 cells expressing WT SAMHD1 and mutants and the vector control cells were differentiated using 30 ng/ml PMA for 20 h and then infected with HIV-1-Luc/VSV-G at a MOI of 1. All virus stocks were treated with DNase I (40 U/ml; Ambion) before infection to avoid plasmid DNA contamination. Total RNA was isolated from the cells at 1, 3 and 6 h.p.i. using an RNeasy kit (Qiagen), and cDNA was synthesized using the SuperScript III First-Strand Synthesis System (ThermoFisher Scientific). The levels of HIV-1 genomic RNA from infected U937 cells were measured by SYBR-green based pPCR using the method and HIV-1 gag-specific primers described by Ryoo et al.7. RNA samples without reverse transcription were used as negative controls. The levels of HIV-1 late reverse transcription products in infected U937 cells were quantified by SYBR-green based pPCR analysis using primers (5′-GACATAGCAGGAACTA CTAGTACCC-3′ and 5′-GGTCCTTGTCTTATGTCCAGAATGC-3′) and methods previously described12,24. Briefly, genomic DNA (50 ng) from HIV-1 infected U937 cells were used as input for the detection of late reverse transcription products. GAPDH was used for additional input control. Genomic DNA from HIV-1 infected cells at 12 and 24 h.p.i. was isolated using a DNeasy Blood and Tissue kit (Qiagen). NVP treatment was used as a negative control of HIV-1 infection.
Protein expression and purification
WT full-length SAMHD1 was cloned into a pET28a expression vector with a 6 × His-tag at the N terminus and expressed in Escherichia coli. SAMHD1 proteins were purified using a nickel-nitrilotriacetic acid (Ni-NTA) affinity column. The eluted peak fractions were collected and dialyzed into the assay buffer, as previously described25, and then further purified with size-exclusion chromatography.
Analytical size-exclusion chromatography
Purified samples of SAMHD1 constructs (2 mg/ml, 200 μl) were loaded onto an analytical Superdex 200 10/300 GL column (GE Healthcare), which is pre-equilibrated in assay buffer. The elution profiles (UV absorbance at 280 nm) were recorded.
SAMHD1 dNTPase activity assays
SAMHD1 dNTPase activity assay was performed with 50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 5 mM MgCl2, 0.5 mM TCEP and 1 mM substrate dGTP in a 500-μl reaction volume. Reactions were initiated at 37 °C by the addition of purified SAMHD1 samples to obtain a final concentration of 500 nM. Aliquots of reactions were collected at various times points and terminated by fivefold dilution into ice-cold buffer containing 10 mM EDTA, which was followed by spinning through an Amicon Ultra 0.5 ml 10-kDa filter (Millipore) at 16,000g for 20 min. Deproteinized samples were analyzed by HPLC using a Synergi C18 Column 150 × 4.6 mm (Phenomenex), pre-equilibrated in 20 mM ammonium acetate, pH 4.5 (buffer A). Samples were eluted with a methanol (buffer B) gradient over 14 min at a flow rate of 1 ml/min, and the elution profiles (UV absorption at 260 nm) were recorded.
SAMHD1 nuclease-activity assays
The exonuclease activity assays were performed in 100-μl reaction mixtures containing 1 μM synthesized 5′ fluorescence-labeled 30-nt ssDNA (5′-/56-FAM/TAGAAAGGGA-GATTCTAAGA-GGAAAGGTGA-3′) or 20-nt ssRNA (5′-/56-FAM/ rCrUrCrCrArUrCrArCrC rCrUrCrCrArUrCrArCrC-3′) substrates (Integrated DNA Technologies) and 1-μM purified recombinant proteins in the assay buffer at 37 °C. 25-μl aliquots of the reactions were terminated after 1 h by the addition of 7 μl of 6 × DNA loading dye (New England Biolabs). The products were separated in 15% poly-acrylamide gels containing 8 M urea and buffered with 0.5× Tris-borate–EDTA and then pictured by ChemiDoc MP gel imaging system (Bio-Rad).
Statistical analysis
The data were analyzed using one-way ANOVA with Dunnett’s correction, Kruskal–Wallis test, or Student’s t-test, as specified in the figure legends.
Supplementary Material
Acknowledgments
This work was supported by NIH grants AI104483 and CA181997 (L.W.), AI114826 (J.S.Y.), AI102778 (Y.X.), AI120845 (X.J.) and AI049781 and GM104198 (B.K.). The authors thank N. Landau for the pLenti-puro plasmids, and J. Hach and S.H. Kim for technical assistance. The antibody to HIV-1 p24 Gag (clone #24-2) and nevirapine were obtained from the NIH AIDS Reagent Program.
Footnotes
AUTHOR CONTRIBUTIONS
J.M.A., C.S.G., S.d.S., J.S.Y., Y.X. and L.W. designed the study and wrote the manuscript. J.M.A., C.S.G., S.d.S., C.T., X.J. and C.S. performed the experiments and analyses. J.M.A, C.S.G., S.d.S., J.S.Y., Y.X., B.K. and L.W. analyzed the data. All authors discussed the data and approved the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
References
- 1.Laguette N, et al. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hrecka K, et al. Nature. 2011;474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldauf HM, et al. Nat Med. 2012;18:1682–1687. doi: 10.1038/nm.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Descours B, et al. Retrovirology. 2012;9:87. doi: 10.1186/1742-4690-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lahouassa H, et al. Nat Immunol. 2012;13:223–228. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beloglazova N, et al. J Biol Chem. 2013;288:8101–8110. doi: 10.1074/jbc.M112.431148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryoo J, et al. Nat Med. 2014;20:936–941. doi: 10.1038/nm.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi J, Ryoo J, Oh C, Hwang S, Ahn K. Retrovirology. 2015;12:46. doi: 10.1186/s12977-015-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White TE, et al. Cell Host Microbe. 2013;13:441–451. doi: 10.1016/j.chom.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welbourn S, Dutta SM, Semmes OJ, Strebel K. J Virol. 2013;87:11516–11524. doi: 10.1128/JVI.01642-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.StGelais C, et al. J Virol. 2014;88:5834–5844. [Google Scholar]
- 12.StGelais C, et al. Retrovirology. 2012;9:105. [Google Scholar]
- 13.Goldstone DC, et al. Nature. 2011;480:379–382. doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 14.Yan J, et al. J Biol Chem. 2013;288:10406–10417. doi: 10.1074/jbc.M112.443796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu C, et al. Nat Commun. 2013;4:2722. doi: 10.1038/ncomms3722. [DOI] [PubMed] [Google Scholar]
- 16.Seamon KJ, Sun Z, Shlyakhtenko LS, Lyubchenko YL, Stivers JT. Nucleic Acids Res. 2015;43:6486–6499. doi: 10.1093/nar/gkv633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann H, et al. J Virol. 2013;87:11741–11750. doi: 10.1128/JVI.02002-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welbourn S, Strebel K. Virology. 2016;488:271–277. doi: 10.1016/j.virol.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wittmann S, et al. Retrovirology. 2015;12:103. doi: 10.1186/s12977-015-0229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji X, et al. Nat Struct Mol Biol. 2013;20:1304–1309. doi: 10.1038/nsmb.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong C, Kwas C, Wu L. J Virol. 2009;83:3518–3527. doi: 10.1128/JVI.02665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, et al. Virology. 2016;487:273–284. doi: 10.1016/j.virol.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond TL, et al. J Biol Chem. 2004;279:51545–51553. doi: 10.1074/jbc.M408573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.St Gelais C, Roger J, Wu L. AIDS Res Hum Retroviruses. 2015;31:806–816. doi: 10.1089/aid.2014.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji X, Tang C, Zhao Q, Wang W, Xiong Y. Proc Natl Acad Sci USA. 2014;111:E4305–E4314. doi: 10.1073/pnas.1412289111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.