Abstract
Objective: The neural changes underlying response to antidepressant treatment in adolescents are unknown. Identification of neural change correlates of treatment response could (1) aid in understanding mechanisms of depression and its treatment and (2) serve as target biomarkers for future research.
Method: Using functional magnetic resonance imaging, we examined changes in brain activation and functional connectivity in 13 unmedicated adolescents with major depressive disorder (MDD) before and after receiving treatment with a selective serotonin reuptake inhibitor medication for 8 weeks. Specifically, we examined brain activation during a negative emotion task and resting-state functional connectivity (RSFC), focusing on the amygdala to capture networks relevant to negative emotion. We conducted whole-brain analyses to identify how symptom improvement was related to change in brain activation during a negative emotion task or amygdala RSFC.
Results: After treatment, clinical improvement was associated with decreased task activation in rostral and subgenual anterior cingulate cortex and increased activation in bilateral insula, bilateral middle frontal cortices, right parahippocampus, and left cerebellum. Analysis of change in amygdala RSFC showed that treatment response was associated with increased amygdala RSFC with right frontal cortex, but decreased amygdala RSFC with right precuneus and right posterior cingulate cortex.
Conclusion: The findings represent a foothold for advancing understanding of pathophysiology of MDD in adolescents by revealing the critical neural circuitry changes that underlie a positive response to a standard treatment. Although preliminary, the present study provides a research platform for future work needed to confirm these biomarkers at a larger scale before using them in future target engagement studies of novel treatments.
Keywords: : adolescent, depression, fMRI, treatment response, antidepressants
Introduction
Major depressive disorder (MDD) is common in adolescents and is associated with high risk of recurrence, comorbidity, future disability, and suicide (Weissman et al. 1997; Ustün et al. 2004; Zisook et al. 2004, 2007; Kennard et al. 2009). First-line interventions for adolescent MDD include structured psychotherapies (e.g., cognitive behavioral therapy and interpersonal psychotherapy) as well as antidepressants from the selective serotonin reuptake inhibitor (SSRI) class (Aacap 1998). Unfortunately, not all adolescents respond to these standard interventions (March et al. 2006; Emslie et al. 2010; Maalouf and Brent 2012). Research is urgently needed to develop new treatments for adolescent MDD, but advancement is hindered by our limited understanding of how current treatments work.
It is important to identify the neurobiological mechanisms of known treatments such as SSRI medications so that this knowledge can be applied to novel treatment development. Although serotonin is known to be important for mood, to be widespread throughout the brain, it is unknown which specific, SSRI-induced, neural circuit alterations underlie clinical improvement of depression in adolescents. Advancement in understanding of the brain changes that accompany successful treatment could pave the way for conceptualizing novel treatments.
One strategy for gaining new insight into the neurobiology of adolescent MDD is to use a standard treatment as a probe to illuminate the pathophysiology (www.nimh.nih.gov/about/director/2014/a-new-approach-to-clinical-trials.shtml) by showing the key treatment-induced changes that lead to clinical improvement. Since MDD is characterized by abnormal neural circuitry, neuroimaging is a suitable approach to identify treatment targets, or neural biomarkers, of clinical treatment response. For example, applications of functional magnetic resonance imaging (fMRI) can yield insight into the brain response to stimuli and, when used at rest, can shed light on the functional connectivity within brain networks. These techniques have potential for providing neuroimaging biomarkers that could signal neural changes underlying clinical treatment response.
Research investigating the neural mechanisms of treatment response is still in an early stage, and the vast majority of available evidence pertains to adults. Several studies of adult MDD have suggested that after antidepressant treatment, limbic activation decreased (Sheline et al. 2001; Fu et al. 2004, 2008; Robertson et al. 2007; Fales et al. 2009). In some cases, treatment also led to increased activation in cortical areas, including the anterior cingulate cortex (ACC) (Sheline et al. 2001), middle frontal (Robertson et al. 2007), prefrontal cortex (PFC) (Fu et al. 2004; Fales et al. 2009), and precuneus (Robertson et al. 2007). Impaired fronto-limbic resting-state functional connectivity (RSFC) has been shown to partially normalize after antidepressant treatment in adults (Anand et al. 2007). Although this work in adults is relevant, there are important limits in extrapolating the findings to adolescents, given ongoing brain development during the adolescent years (Giedd et al. 1999; Casey et al. 2008; Giedd et al. 2009). Only one study has examined brain changes before and after treatment in adolescents with MDD (Tao et al. 2012). At baseline, 15 depressed adolescents showed greater limbic activation to emotion faces and greater frontal activation than healthy controls. Treatment with fluoxetine led to a decrease in both limbic and frontal activation. Contrary to predictions, these changes did not correspond with clinical treatment response.
At this early stage in the literature, studies to date have been designed to first identify an overall treatment effect on neural biomarkers, occasionally followed by post hoc investigations of the relationship between this effect and clinical treatment response. While overall treatment effects are important, given the variability across individuals in clinical treatment response, the brain changes may be different in those who have a large clinical response compared with those who have little to no clinical response. Uncovering the neural change correlates of clinical treatment response is critical to understanding the mechanisms of successful treatment. Neural change correlates underlying clinical treatment response can be identified using a whole-brain-dimensional examination of the relationship between neural change and clinical change. Neuroimaging biomarkers identified using this approach can signal the key brain changes underlying clinical treatment response. As noted above, analyses that are limited to regions of interest (ROIs) defined by baseline group differences have not been particularly fruitful to date in yielding neural change biomarkers; examination of neural changes across the whole brain allows for discovery of biomarkers that would not be identified if using a constrained approach. These biomarkers could in turn be used as neural targets in future studies to test target engagement of antidepressant treatment.
In the current study, we used both task and resting-state fMRI (rsfMRI) to examine neural circuitry in adolescents with MDD before and after 8 weeks of treatment with an SSRI. We used fMRI measurement of brain responses during a negative emotion task and measurement of RSFC with the amygdala, a brain region that is critical to processing negative emotion. Therefore, the primary study goal here was to identify the key neural changes linked with improvement in depression symptoms. Based on previous work implicating the fronto-limbic network (Mayberg 1997; Phillips et al. 2003; Price and Drevets 2010) and the default mode network (DMN) (Greicius et al. 2007; Sheline et al. 2009; Li et al. 2013; Kaiser et al. 2015) in MDD, we predicted that neural change correlates would be identified within these networks. To provide a clearer context to the neural change findings, we also conducted a straightforward pre–post change comparison analysis to identify overall treatment-related changes and, based on prior negative findings, predicted that they would not be associated with clinical improvement.
Methods
Participants
This study was approved by the University of Minnesota Institutional Review Board. Participants (or a parent if under 18 years old) provided written informed consent. Participants aged 17 years and younger provided written assent. Participants of this study represent a subset of a larger, cross-sectional neuroimaging study (NIMH K23: Fronto-Limbic Connectivity in Adolescents with Major Depression; PI:Cullen). Adolescents aged 12–19 years were recruited through community postings and referrals from local mental health services. The inclusion criteria for MDD participants required a primary diagnosis of having MDD and being medication-free (no psychotropic medications for at least 2 months before study entry). Exclusion criteria were intake of current (past 2 months) psychotropic medication; presence of a serious neurological condition, intellectual disability, pervasive developmental disorder, substance use disorder, bipolar disorder, or schizophrenia; and presence of a contraindication to MRI such as metal in the body or claustrophobia. After completing the larger study, adolescents who were currently unmedicated, diagnosed with having MDD, and indicated plans to start treatment with an antidepressant through their provider were invited to return for a second neuroimaging scan after they completed 8 weeks of antidepressant treatment.
Pre- and post-treatment clinical assessment
Pretreatment, adolescents and parents independently completed interviews with either a child psychiatrist (K.C.), a child clinical psychologist (B.K.D.), or trained doctoral students. The interview included the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (Kaufman et al. 1997) and the Children's Depression Rating Scale-Revised (CDRS-R) (Poznanski et al. 1985). Diagnosis was confirmed in a consensus meeting after the clinical evaluation based primarily on parent and child interviews (also when relevant/available, self-report scales and medical records). We also administered the Wechsler Abbreviated Scale of Intelligence (Wechsler 1999) at baseline. The Beck Depression Inventory II (BDI-II) (Beck and Steer 1996), a self-report index, was administered at the pretreatment clinical assessment and at the time of the post-treatment MRI scan (after the adolescents had completed 8 weeks of antidepressant treatment). Since the CDRS-R was not repeated post-treatment, the focus in this study is on the change in BDI-II scores. We calculated clinical depression change by subtracting the pretreatment from the post-treatment BDI-II scores. BDI-II change scores were mean deviated by subtracting the mean BDI-II change score from each individual score, thus centering the scores for the sample at zero (Poldrack et al. 2011).
Image acquisition
Participants underwent neuroimaging at baseline and after receiving 8 weeks of treatment with an SSRI antidepressant through their own provider. The MRI scan was conducted using a 3 Tesla TIM Trio scanner and 12-channel radiofrequency head coil at the University of Minnesota Center for Magnetic Resonance Research. A T1-weighted, high-resolution, magnetization-prepared, gradient echo sequence scan was obtained (repetition time = 2530 milliseconds, echo time = 3.65 milliseconds, inversion time = 1100 milliseconds, flip angle = 7°, 1-mm slices, field of view = 256, voxel size 1 × 1 × 1 mm, GRAPPA = 2, 5 minutes). For the resting-state scan, functional data were acquired using an echo planar imaging (EPI) sequence with 180 T2*-weighted whole-brain functional volumes with 34 interleaved slices of 4 mm thickness and no skip, tilted to anterior commisure-to-posterior commisure (AC-PC) alignment, repetition time = 2000 milliseconds, echo time = 30 milliseconds, flip angle = 90°, field of view = 220 mm, and 64 × 64 matrix. Participants were asked to close their eyes and stay awake. Physiological data (respiration and cardiac traces) were collected during the entire scan. For the emotion task, functional data were acquired in conjunction with the task using an EPI sequence with 197 T2*-weighted whole-brain functional volumes with 34 interleaved contiguous axial slices with 4 mm slice thickness and no skip, tilted to AC-PC −30° alignment, repetition time = 2000 milliseconds, echo time = 28 milliseconds; flip angle = 80°, field of view = 200 mm, and matrix = 64 × 64. The emotional face-matching task (Hariri et al. 2002) was implemented using E-Prime Software. A mirror attached to the head coil allowed participants to see images projected onto a screen inside the bore of the MRI scanner. Participants used a button box to match the image presented at the top row with one of the two stimuli in the bottom row. There were two types of stimuli: affective and neutral. The task used angry and fearful faces (Ekman and Friesen 1976) for the affective stimuli. Participants were presented with six images of each gender and emotion and instructed to match faces with corresponding emotional expressions (fear or anger). Neutral stimuli included geometric shapes such as circles and horizontal and vertical ellipses. The task consisted of thirteen 24-second counterbalanced blocks (three fixation, five shapes, and five facial expressions) for an approximate duration of 6.5 minutes. Finally, we collected a field map using acquisition parameters that were compatible with the rsfMRI acquisition.
Neuroimaging data preprocessing
Anatomic imaging preprocessing
The T1 data, including brain extraction and parcellation, were processed using FreeSurfer (version 5.3; http://surfer.nmr.mgh.harvard.edu) into a standard set of anatomically based regions of white and gray matter. FreeSurfer output was visually inspected; any identified errors were manually corrected on a section-by-section basis. The remaining steps of the pipeline were repeated once the corrections were satisfactory. No corrections were required near the amygdala. The processed T1 data were registered to the rsfMRI data using bbregister (https://surfer.nmr.mgh.harvard.edu/fswiki/bbregister).
Task fMRI preprocessing and activation analysis
Preprocessing of task fMRI data was conducted using software tools from the FMRIB software library (FSL; http://fsl.fmrib.ox.ac.uk) version 4.1.8. Preprocessing steps included motion correction, brain extraction, high-pass temporal filtering, prewhitening, regression of motion parameters, spatial smoothing using a Gaussian kernel of full-width at half-maximum of 5 mm, and registration to Montreal Neurological Institute (MNI) standard space. To measure brain activation in response to the task, we conducted a regression of the task model onto the fMRI data at each voxel of the brain using FSL FEAT with two explanatory variables (emotion and shape) and the motion parameters during preprocessing as covariates of no interest. An additional covariate of no interest was any volume with more than 1.5 mm change in any direction relative to the proceeding volume. For each subject, z-score maps for the emotion minus shape contrast were used for subsequent analyses described below.
rsfMRI preprocessing and RSFC analysis
Image processing was conducted using tools from the FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) as well as customized tools developed in MatLab (MathWorks; www.mathworks.com/products/matlab/). Initial fMRI processing steps included brain extraction and motion correction. A denoising procedure was applied incorporating RETROICOR (Glover et al. 2000) to remove physiological noise caused by cardiac and respiratory cycles, as well as any linear trends. Correction for magnetic field inhomogeneity-induced geometric distortion was conducted using the field map. FreeSurfer-generated ROIs for lateral ventricles (cerebrospinal fluid) and white matter were aligned with rsfMRI data. Mean time series within these ROIs were extracted. We performed a regression of all other voxels' time series on eight nuisance variables: white matter time series, cerebrospinal fluid time series, and the six motion parameters. Data scrubbing was performed following the method of Power and colleagues, excluding any volume with a value for the temporal derivative of time courses' root mean squared head motion variance value exceeding 8 and/or a framewise-dependent value exceeding 0.5, along with the previous volume and the two following volumes. We excluded scans from analyses that had greater than 30% of volumes excluded (n = 2, both post-treatment scans). RSFC of left and right amygdalae was measured using a seed-based approach. We used anatomically based ROIs to avoid misregistration errors. We registered each individual's FreeSurfer-based right and left amygdala ROIs to their preprocessed rsfMRI data, and extracted the mean time series of voxels in these regions. These time series were used as primary regressors in general linear model analyses (separately for left and right) of all other voxel time series, resulting in whole-brain amygdala RSFC maps. Additional processing steps included prewhitening, spatial smoothing (5-mm kernel), and registration to anatomic data and standard space (MNI 152). Amygdala RSFC maps for the left and right amygdalae were highly similar to each other; following our previous work (Cullen et al. 2014) and the work of others (Luking et al. 2011), we conducted a second-level analysis to average the right and left amygdala RSFC maps. These mean amygdala RSFC z-score maps were used for subsequent analyses (described below).
Measurement of association between neural change and clinical improvement
Emotion task
To identify the key changes in brain activation associated with clinical change, we first calculated change in whole-brain task activation for each individual by using fslmaths to subtract the pretreatment from the post-treatment z-score maps representing the emotion minus shape contrast (derived as described above). This yielded a whole-brain map representing change in task activation for each person. We then conducted a voxelwise regression analysis using AFNI 3dRegAna using the demeaned BDI-II change scores as the main regressor to measure the association between change in total BDI-II scores and change in whole-brain task activation after treatment (the result of the post-minus-pre z-map subtraction). Age, intelligence quotient (IQ), and sex were included as regressors of no interest in the model.
Amygdala RSFC
To identify the key changes in amygdala RSFC associated with clinical change, we conducted analyses similar to the task fMRI analyses above. Since two of the rsfMRI post-treatment scans were excluded due to excessive motion (see above), these analyses were conducted with n = 11. We calculated change in amygdala RSFC by subtracting the pretreatment mean amygdala RSFC z-score maps from the post-treatment mean amygdala RSFC z-score maps using fslmaths. This yielded a whole-brain map representing change in amygdala RSFC for each person. We then conducted a voxelwise regression analysis using AFNI 3dRegAna to measure the association between change in mean amygdala RSFC (the result of the post-minus-pre subtraction) and change in total BDI-II scores. As above, age, sex, and IQ were included as regressors of no interest in the model.
Analysis of overall pre–post-treatment neural changes
To identify overall neural changes after 8 weeks of antidepressant treatment, we conducted voxel-level analyses in AFNI (using 3dttest++) to test pre versus post neural changes, adjusting for age, sex, and IQ as covariates, using the z-score maps that resulted from the first-level analyses of emotion task and amygdala RSFC analyses.
Correction for multiple comparisons
As a final step for all analyses, AlphaSim was used to determine the minimum cluster size using individual voxel threshold of p < 0.005, and the cluster size corresponding to alpha <0.01 was selected. We then used AFNI 3dmerge to threshold the image using this cluster size and p < 0.01 to determine the final cluster results described below.
Results
Demographic and clinical characteristics
Thirteen adolescents completed both pre- and post-treatment clinical assessment and MRI scanning. Demographic and clinical information is presented in Table 1. Participants were predominantly female (71%), Caucasian (79%), and right-handed (93%). Seventy-one percent of MDD adolescents had a current comorbid psychiatric disorder. Among those with psychiatric comorbidity, generalized anxiety disorder was the most prevalent (n = 5), followed by attention-deficit/hyperactivity disorder (n = 3). Medications prescribed to the adolescents between the neuroimaging scans included fluoxetine (n = 8) and sertraline (n = 5). Mean change in depression symptoms (post-treatment BDI-II minus pretreatment BDI-II scores) was −15.2 (standard deviation = 16.3).
Table 1.
Demographic and Clinical Data
| Demographic characteristics | |
|---|---|
| N | 13 |
| Age (mean years ± SD) | 15.5 ± 2.3 |
| Sex (male/female) | 4/9 (69% female) |
| Right-handed, n (%) | 12 (92) |
| Ethnicity, n (%) | |
| Caucasian | 13 (100) |
| Hispanic | 4 (31) |
| Current comorbidities, n (%) | 9 (69) |
| Attention-deficit/hyperactivity disorder | 3 (23) |
| Generalized anxiety disorder | 4 (31) |
| Post-traumatic stress disorder | 1 (8) |
| Social anxiety | 1 (8) |
| Dysthymia | 1 (8) |
| Specific phobia | 1 (8) |
| Anxiety NOS | 1 (8) |
| Medication history | |
| Med-naive, n (%) | 11 (85) |
| Past antidepressant use, n (%) | 2 (14) |
| Past stimulant use | 0 |
| Past antipsychotic use | 1 (7) |
| Illness history, description, etc. | |
| Duration of illness (mean months ± SD) | 15.5 ± 16.1 |
| Global assessment of functioning (mean ± SD) | 55 ± 7 |
| Positive family history | 11 (85%, n = 13) |
| Clinical severity | |
| Baseline CDRS-R (T-scores mean ± SD) | 73.2 ± 7.3a |
| Baseline BDI-II (mean ± SD) | 28.7 ± 9.3 (n = 13) |
| Post-treatment BDI-II | 13.5 ± 14.0 |
There were missing data for one subject on these measures.
BDI-II, Beck Depression Inventory II; CDRS-R, Children's Depression Rating Scale-Revised; SD, standard deviation.
Relationship between improvement in depression symptoms and neural change
Emotion task
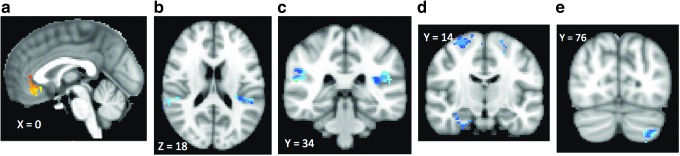
As shown in Figure 1 and Table 2, greater improvement in depression symptoms was associated with greater decreases in task activation in a large cluster, including bilateral rostral and subgenual ACC (Brodmann area [BA] 32 and 25) and inferior frontal cortex (BA 11). Increased brain activation in right parahippocampus, bilateral insula (BA 13), bilateral middle frontal cortices (BA 8), and left cerebellum was associated with clinical improvement.
FIG. 1.
Relationship between change in brain activation to negative emotion after SSRI treatment and improvement in depression symptoms. Clusters with warm colors indicate brain regions where greater improvement in depression symptoms was associated with greater decreases in task activation. These regions include bilateral rostral and subgenual ACC (BA 32 and 25) and inferior frontal cortex (BA 11). Clusters with cool colors indicate brain regions where improvement in depression symptoms was associated with an increase in brain activation (right parahippocampus, bilateral insula (BA 13), bilateral middle frontal cortices (BA 8), and left cerebellum. (a–e) Correspond to descriptions in Table 2. ACC, anterior cingulate cortex; BA, Brodmann area; SSRI, selective serotonin reuptake inhibitor.
Table 2.
Neural Change Correlates of Overall Clinical Improvement After Selective Serotonin Reuptake Inhibitor Treatment
| fMRI Type | Positive correlates (decreases in neural index correlate with decrease in total BDI-II scores after treatment) | Negative correlates (increases in neural index correlate with decreases in BDI-II scores after treatment) |
|---|---|---|
| Emotion task | Anterior cingulate cortex (BA 24, 32), bilateral subcallosal cortex (BA 25), and right inferior frontal gyrus (BA 11) (Fig. 1a) | Bilateral insula (BA 13) (Fig. 1b) Right parahippocampal cortex (Fig. 1c, d) Bilateral middle frontal cortex (BA 6) (Fig. 1d) Left cerebellum (Fig. 1e) |
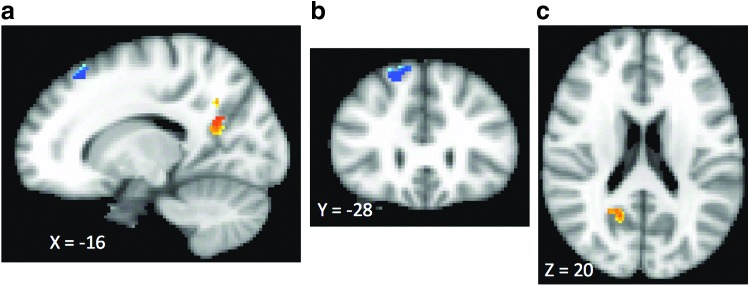
| Resting-state amygdala functional connectivity | Right precuneus and posterior cingulate precuneus (BA 31) (Fig. 2a, c) | Right superior frontal cortex (BA 8) (Fig. 2b, c) |
BA, Brodmann area; BDI-II, Beck Depression Inventory II; fMRI, functional magnetic resonance imaging.
Amygdala RSFC
As shown in Figure 2 and Table 2, this analysis revealed that decreases in severity of depression as measured by the BDI-II were associated with increased amygdala RSFC with right medial and middle frontal gyri (BA 8) and with decrease in amygdala RSFC with right posterior cingulate/precuneus (BA 31).
FIG. 2.
Relationship between change in amygdala RSFC after SSRI treatment and improvement in depression symptoms. Clusters with warm colors indicate brain regions where greater improvement in depression symptoms was associated with greater decreases in amygdala RSFC. One warm cluster was observed in the right posterior cingulate/precuneus (BA 31). Clusters with cool colors indicate brain regions where improvement in depression symptoms was associated with an increase in amygdala RSFC. These regions include right medial and middle frontal gyri (BA 8). (a–c) Correspond to descriptions in Table 2. BA, Brodmann area; RSFC, resting-state functional connectivity SSRI, selective serotonin reuptake inhibitor.
Overall changes after treatment (unrelated to clinical response)
No clusters met statistical significance from the pre–post-treatment analyses for either the emotion task data or the amygdala RSFC data.
Discussion
In this study, we examined neural circuitry changes associated with clinical improvement from treatment with an SSRI antidepressant in adolescents with MDD. Our neuroimaging tools were designed to assess neural systems relevant to emotion processing—specifically, we measured brain activation in response to negative emotion stimuli and RSFC of the amygdala, a region known to be important for processing negative emotion (Phelps and LeDoux 2005) and centrally implicated in MDD (Drevets 2003). A key strength of this study is its focus on adolescents. Adolescent depression has been understudied and requires research attention separate from adults because of neurodevelopmental differences. Advances in understanding mechanisms of depression and its treatment mechanisms are critically important, given that improved treatment during the early stage of illness could restore healthy development and circumvent life-long disability and suicide. Another key strength of this research is our utilization of dimensional analyses to examine relationships between clinical change and neural change across the whole brain. We found that the degree of clinical treatment response in these adolescents was associated with specific changes in brain responses to the negative emotion task (decreased activation in rostral/subgenual ACC, increased activation in bilateral insula, right parahippocampus, bilateral middle frontal cortices, and cerebellum), and changes in amygdala RSFC (increased with right frontal cortex, decreased with precuneus and posterior cingulate cortex). These results provide preliminary evidence for neural biomarkers of clinical treatment response.
The brain regions in which change was associated with clinical improvement fall within two networks that have been previously implicated in MDD: the fronto-limbic network (Mayberg 1997; Phillips et al. 2003) and the DMN (Greicius et al. 2007; Zhu et al. 2012; Li et al. 2013). While the implication of these networks in general is consistent with previous work, the specific neural change biomarkers reported here, which are identified by virtue of their correlation with clinical response, have not previously been reported as most literature to date involves overall pre- versus post-treatment changes. In this study, we discuss how our findings fit into and advance the existing literature. In contrast to some prior publications, our pre–post analyses did not reveal any changes that survived our criteria for correction for multiple comparisons that would indicate an overall SSRI effect. However, our primary analyses focused on neural correlates of clinical treatment response, as opposed to pre–post neural changes. Since the two fMRI approaches used in this study provide complementary information, we discuss the findings separately.
Emotion task neural correlates of clinical improvement
Using a standard emotion face-matching task, one of our key findings was that improvement in depression was associated with decreased activation in rostral and subgenual ACC. The ACC, especially the subcallosal portion, has been consistently implicated in MDD (Mayberg et al. 2005; Drevets et al. 2008; Hamani et al. 2011). Our findings are consistent with that of Mayberg and colleagues who previously reported that metabolism (measured using positron emission tomography) in the subcallosal cortex decreases after treatment with fluoxetine in responders, but not in nonresponders (Mayberg et al. 2000).
In this study, we found that improved depression symptoms after SSRI treatment were associated with increased activation in response to negative emotion in the bilateral insula cortex. Although the insula has been repeatedly implicated in prediction of antidepressant response (McGrath et al. 2013; Rizvi et al. 2013), to our knowledge, change in insula activation due to treatment has not been reported as a depression response correlate. To our knowledge, change in insula activation due to treatment has not been reported as a depression response correlate in adolescents. However, a recent meta-analysis of the neural effects of psychotherapy and pharmacological treatments in MDD found consistent activation of the right posterior insula, which was specific to pharmacological interventions (primarily SSRIs) (Boccia et al. 2015). The insula is known to be critically important for interoception and emotional processing and to have strong reciprocal connections with the amygdala (Craig 2002; Nieuwenhuys 2012). Abnormally low insula activation in response to negative emotion faces has previously been reported in adolescents with depression (Hall et al. 2014; Henje Blom et al. 2015). It may be that successful antidepressant treatment serves to enhance insular activation in response to negative emotion stimuli, alleviating dysfunctional emotion processing within the salience network.
Additional and unexpected regions that were found in our study, in which increased activation to the task after treatment was associated with clinical improvement, include the right parahippocampal gyrus and bilateral premotor cortex (BA 6). The parahippocampal gyrus is a part of the limbic system and is a brain region important for processing memory. The direction of our findings is in contrast with previous work in adults treated with fluoxetine, where response was characterized by a decrease in parahippocampal metabolism (Mayberg et al. 2000). However, baseline metabolism and activation to negative emotion are not completely synonymous. In addition, since our sample was in adolescents and not adults, the difference in pattern could have a developmental explanation. The premotor cortex is a brain region important for planning movements; the significance of its change in activation to negative emotion in association with clinical improvement is unclear. Future research is required to replicate the findings of this study; these preliminary results could suggest that future work investigating these regions incorporating behavioral measures to assess memory and motor planning may be fruitful.
Amygdala RSFC neural correlates of clinical improvement
Our finding that a positive clinical treatment response was associated with an increase in amygdala-frontal RSFC supports existing theories that depression involves a disconnection within fronto-limbic circuitry. Previous research in adults suggested that antidepressant treatment leads to an increase in corticolimbic connectivity (either resting-state or functional connectivity during a negative emotion task) (Anand et al. 2005, 2007; Chen et al. 2008). In one of these studies, the degree of increased connectivity between amygdala and ACC during a task that involved negative pictures was associated with the degree of clinical improvement (Anand et al. 2005). It may be that when successful, SSRI medications serve to restore RSFC between amygdala and frontal areas, promoting more effective regulation of negative effect during rest.
In contrast, treatment improvement was associated with a decrease in amygdala-precuneus/posterior cingulate RSFC. The posterior cingulate and the precuneus are of the DMN, a group of regions (also involving medial temporal lobe) that are more active during rest than during a task (Raichle et al. 2001). The midline cortical regions of the DMN mediate self-referential processing (Northoff and Bermpohl 2004). During rest, there may be excessive involvement of subgenual ACC in the DMN (Greicius et al. 2007; Li et al. 2013) and precuneus in the DMN (Li et al. 2013; van de Ven et al. 2013). In our prior work, we found that amygdala RSFC with a nearby region (more superior precuneus) was higher in adolescents with MDD in comparison with controls (Cullen et al. 2014). Abnormal amygdala-posterior cingulate/precuneus (PCC)/PC connectivity has also been found in other populations, such as adults with a history of child abuse (van der Werff et al. 2013), children of mothers with depression and/or a history of childhood depression (Luking et al. 2011), and adolescents with bipolar disorder (Singh et al. 2015). Taken together, one interpretation of our findings here is that hyperconnectivity between the amygdala, a region that is critical for response to negative emotional stimuli, and the brain regions that are more active during rest may contribute to a more stressful baseline state and that SSRI treatment may, in part, lead to improved depression symptoms by attenuating that connection.
Limitations
This study had several limitations that should be considered when interpreting the findings. First, although the sample size is similar to other studies that have explored treatment effects in adults (Anand et al. 2005, 2007; Kozel et al. 2011; Yang et al. 2014; Wang et al. 2015), this is a small sample of adolescents with MDD. These results therefore require replication in a larger sample. Second, our study design was naturalistic, in that we did not control the type of antidepressant medication or the dose that the adolescents received between scans. Although both of the antidepressants received by adolescents during the treatment period were in the SSRI class, they received different medications within this class (fluoxetine and sertraline) and a range of different doses. It is possible that there are subtle differences in neural mechanisms for the different SSRI medications, or that different doses of specific medications might have greater impact on neural circuitry. Future work confirming neural biomarkers of treatment response to SSRI medications should incorporate standardized treatment across participants. Third, we relied on self-report assessment to measure clinical change. While the BDI-II is a reliable measure in adolescents (Osman et al. 2004), inclusion of a clinician-administered measure such as the CDRS-R at the post-treatment assessment would have provided additional and important information about clinical change. Fourth, since the only clinical measure that we collected at the post-treatment scanning session was the BDI-II, we are unable to conclude whether the identified change correlates represent markers of improvement in other forms of psychopathology such as anxiety. Finally, the lack of a placebo group in this study precludes any conclusion about whether the neural correlates identified here are specific to SSRI drug effect versus a more general signal of treatment response. Future work, including a placebo, is needed to confirm drug-specific effects.
Conclusion
In conclusion, we report neural correlates of treatment response to SSRI antidepressant treatment in adolescents with MDD. While preliminary, these findings suggest that key changes in areas within fronto-limbic and DMNs may underlie treatment response to these medications. These findings provide new insights into the neural circuits implicated in adolescent depression and how a standard treatment (SSRIs) impacts these circuits to cause improvement in symptoms. Our preliminary evidence of neuroimaging biomarkers of treatment response in adolescents can provide a platform for future work confirming these biomarkers in standard treatments and then using these biomarkers to test novel treatments in adolescents with depression.
Clinical Significance
Adolescent depression is a significant public health problem. Not all adolescents respond to currently-available treatments. Research is needed to identify disease mechanisms biomarkers of treatment response. This will pave the way for future research developing novel, neuroscience-based interventions for adolescents with depression.
Acknowledgments
The authors would like to thank the adolescents and families who participated in this study. The authors would like to acknowledge the Minnesota Supercomputing Institute, which provided the computing resources for the storage of neuroimaging data and analyses described in the study.
Disclosures
No competing financial interests exist.
References
- AACAP: Practice parameters for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry 37 (10 Supplement): 63S–83S, 1998 [DOI] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Gardner K, Lowe MJ: Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: An fMRI study. J Neuropsychiatry Clin Neurosci 19:274–282, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Yu Li, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ: Antidepressant effect on connectivity of the mood-regulating circuit: An fMRI study. Neuropsychopharmacology 30:1334–1344, 2005 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA: Beck Depression Inventory–Revised. San Antonio (TX), Harcourt Brace, 1996 [Google Scholar]
- Boccia M, Piccardi L, Guariglia P: How treatment affects the brain: Meta-analysis evidence of neural substrates underpinning drug therapy and psychotherapy in major depression. Brain Imaging Behav 2015. [Epub ahead of print]; DOI: 10.1007/s11682-015-9429-x [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA: The adolescent brain. Ann N Y Acad Sci 1124:111–126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-H, Suckling J, Ooi C, Fu CHY, Williams SCR, Walsh ND, Mitterschiffthaler MT, Pich EM, Bullmore ED: Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology 33:1909–1918, 2008 [DOI] [PubMed] [Google Scholar]
- Craig AD: How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666, 2002 [DOI] [PubMed] [Google Scholar]
- Cullen R, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, Lim KO: Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry 71:1138–1147, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC: Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci 985:420–444, 2003 [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M: The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 13:663–681, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV: Pictures of Facial Affect. Palo Alto, California: Consulting Psychologists Press; 1976 [Google Scholar]
- Emslie GJ, Mayes T, Porta G, Vitiello B, Clarke G, Wagner KD, Asarnow JR, Spirito A, Birmaher B, Ryan N, Kennard B, DeBar L, McCracken J, Strober M, Onorato M, Zelazny J, Keller M, Iyengar S, Brent D: Treatment of resistant depression in adolescents (TORDIA): Week 24 outcomes. Am J Psychiatry 167:782–791, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun Ma, Mathews J, Snyder AZ, Sheline YI: Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord 112:206–211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET: Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry 61:877–889, 2004 [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, Donaldson C, Suckling J, Andrew C, Steiner H, Murray RM: Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiatry 64:505–512, 2008 [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK: Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry 48:465–470, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL: Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci 2:861–863, 1999 [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D: Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44:162–167, 2000 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF: Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62:429–437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LMJ, Klimes-Dougan B, Hunt RH, Thomas KM, Houri A, Noack E, Mueller BA, Lim KO, Cullen KR: An fMRI study of emotional face processing in adolescent major depression. J Affect Disord 168C:44–50, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM: The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry 69:301–308, 2011 [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR: Serotonin transporter genetic variation and the response of the human amygdala. Science 297:400–403, 2002 [DOI] [PubMed] [Google Scholar]
- Henje Blom E, Connolly CG, Ho TC, LeWinn KZ, Mobayed N, Han L, Paulus MP, Wu J, Simmons AN, Yang TT: Altered insular activation and increased insular functional connectivity during sad and happy face processing in adolescent major depressive disorder. J Affect Disord 178:215–223, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA: Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72:603–611, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Kennard BD, Silva SG, Tonev S, Rohde P, Hughes JL, Vitiello B, Kratochvil CJ, Curry JF, Emslie GJ, Reinecke M, March J: Remission and recovery in the treatment for adolescents with depression study (TADS): Acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry 48:186–195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel FA, Rao U, Lu H, Nakonezny PA, Grannemann B, McGregor T, Croarkin PE, Mapes KS, Tamminga CA, and Trivedi MH: Functional connectivity of brain structures correlates with treatment outcome in major depressive disorder. Front Psychiatry 2:7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Liu L, Friston KJ, Shen H, Wang L, Zeng L-L, Hu D: A Treatment-resistant default mode subnetwork in major depression. Biol Psychiatry 74:48–54, 2013 [DOI] [PubMed] [Google Scholar]
- Luking KR, Repovs G, Belden AC, Gaffrey MS, Botteron KN, Luby JL, Barch DM: Functional connectivity of the amygdala in early-childhood-onset depression. J Am Acad Child Adolesc Psychiatry 50:1027–1041.e3, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf FT, Brent DA: Child and adolescent depression intervention overview: What works, for whom and how well? Child Adolesc Psychiatr Clin N Am 21:299–312, viii, 2012 [DOI] [PubMed] [Google Scholar]
- March J, Silva S, Vitiello B: The treatment for adolescents with depression study (TADS): Methods and message at 12 weeks. J Am Acad Child Adolesc Psychiatry 45:1393–1403, 2006 [DOI] [PubMed] [Google Scholar]
- Mayberg HS: Limbic-cortical dysregulation: Depression. J Neuropsychiatry 9:471–481, 1997 [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA: Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol Psychiatry 48:830–843, 2000 [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH: Deep brain stimulation for treatment-resistant depression. Neuron 45:651–660, 2005 [DOI] [PubMed] [Google Scholar]
- McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Craddock RC, Mayberg HS: Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry 70:821–829, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R: The insular cortex: A review. Prog Brain Res 195:123–163, 2012 [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F: Cortical midline structures and the self. Trends Cogn Sci 8:102–107, 2004 [DOI] [PubMed] [Google Scholar]
- Osman A, Kopper BA, Barrios F, Gutierrez PM, Bagge CL: Reliability and validity of the beck depression inventory—II with adolescent psychiatric inpatients. Psychol Assess 16:120–132, 2004 [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE: Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 48:175–187, 2005 [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R: Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry 54:515–528, 2003 [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA, Nichols TE: Handbook of Functional MRI Data Analysis. New York: Cambridge University Press, 2011 [Google Scholar]
- Poznanski EO, Freman LN, Mokros HB: Children's depression rating scale-revised. Psychopharmacol Bull 21:979–989, 1985 [Google Scholar]
- Price JL, Drevets WC: Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL: A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SJ, Salomons TV, Konarski JZ, Downar J, Giacobbe P, McIntyre RS, Kennedy SH: Neural response to emotional stimuli associated with successful antidepressant treatment and behavioral activation. J Affect Disord 151:573–581, 2013 [DOI] [PubMed] [Google Scholar]
- Robertson B, Wang L, Diaz MT, Aiello M, Gersing K, Beyer J, Mukundan S, McCarthy G, Doraiswamy PM: Effect of bupropion extended release on negative emotion processing in major depressive disorder: A pilot functional magnetic resonance imaging study. J Clin Psychiatry 68:261–267, 2007 [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA: Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry 50:651–658, 2001 [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME: The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A 106:1942–1947, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Kelley RG, Chang KD, Gotlib IH: Intrinsic amygdala functional connectivity in youth with bipolar I disorder. J Am Acad Child Adolesc Psychiatry 54:763–770, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Calley CS, Hart J, Mayes TL, Nakonezny PA, Lu H, Kennard BD, Tamminga CA, Emslie GJ: Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. Am J Psychiatry 169:381–388, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustün TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJL: Global burden of depressive disorders in the year 2000. Br J Psychiatry 184:386–392, 2004 [DOI] [PubMed] [Google Scholar]
- van der Werff SJA, Pannekoek JN, Veer IM, van Tol M-J, Aleman A, Veltman DJ, Zitman FG, Rombouts SARB, Elzinga BM, van der Wee NJA: Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychol Med 43:1825–1836, 2013 [DOI] [PubMed] [Google Scholar]
- van de Ven V, Wingen M, Kim P, Kuypers C, Ramaekers JG, Formisano E: Escitalopram decreases cross-regional functional connectivity within the default-mode network. PLoS One 8:e68355, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xia M, Li K, Zeng Y, Su Y, Dai W, Zhang Q, Jin Z, Mitchell PB, Yu X, He Y, Si T: The Effects of antidepressant treatment on resting-state functional brain networks in patients with major depressive disorder. Hum Brain Mapp 36:768–778, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D: Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio (TX): American Psychological Association, 1999 [Google Scholar]
- Weissman MM, Warner V, Wickramaratne P, Moreau D, Olfson M: Offspring of depressed parents 10 years later. Arch Gen Psychiatry 54:932–940, 1997 [DOI] [PubMed] [Google Scholar]
- Yang R, Zhang H, Wu X, Yang J, Ma M, Gao Y, Liu H, Li S: Hypothalamus-anchored resting brain network changes before and after sertraline treatment in major depression. BioMed Res Int 2014:915026, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, Yao S: Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry 71:611–617, 2012 [DOI] [PubMed] [Google Scholar]
- Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani GK, Fava M, Gilmer WS, et al. : Effect of age at onset on the course of major depressive disorder. Am J Psychiatry 164:1539–1546, 2007 [DOI] [PubMed] [Google Scholar]
- Zisook S, Rush AJ, Albala A, Alpert J, Balasubramani GK, Fava M, Husain M, Sackeim H, Trivedi M, Wisniewski S: Factors that differentiate early vs. later onset of major depression disorder. Psychiatry Res 129:127–140, 2004 [DOI] [PubMed] [Google Scholar]