Abstract
Adenovirus (Ad) is used extensively for construction of viral vectors, most commonly with deletion in its E1 and/or E3 genomic regions. Previously, our attempts to insert envelope proteins (Env) of HIV-1 into such vectors based on chimpanzee-derived Ad (AdC) viruses were thwarted. Here, we describe that genetic instability of an E1- and E3-deleted AdC vector of serotype C6 expressing Env of HIV-1 can be overcome by reinsertion of E3 sequences with anti-apoptotic activities. This partial E3 deletion presumably delays premature death of HEK-293 packaging cell lines due to Env-induced cell apoptosis. The same partial E3 deletion also allows for the generation of stable glycoprotein 140 (gp140)- and gp160-expressing Ad vectors based on AdC7, a distinct AdC serotype. Env-expressing AdC vectors containing the partial E3 deletion are genetically stable upon serial cell culture passaging, produce yields comparable to those of other AdC vectors, and induce transgene product–specific antibody responses in mice. A partial E3 deletion thereby allows expansion of the repertoire of transgenes that can be expressed by Ad vectors.
Keywords: : HIV-1, vaccine, genetic stability, immune responses
Introduction
Replication-defective adenovirus (Ad) vectors efficiently deliver transgenes to multiple cell types.1,2 They trigger a potent inflammatory response,3,4 which initiates adaptive immune responses to the transgene product and the antigens of the Ad vector.5 This has led to the development of different Ad serotypes as vaccine carriers for a plethora of antigens derived from viruses,6–10 bacteria,11,12 parasites,13,14 or tumor cells,15–17 which have consistently induced potent and sustained B and T cell responses to the transgene products.
In most Ad vaccine vectors, the E1 and E3 domains are deleted from the genome. The E1 deletion renders Ad vectors replication defective and reduces synthesis of other Ad antigens. E3 encodes a number of polypeptides that have anti-apoptotic activity or allow the virus to escape immune surveillance.18,19 The E3 domain, a region that is nonessential for viral replication, is generally deleted to allow for the insertion of lengthy transgene expression cassettes without exceeding the genome size of wild-type virus, as this could potentially result in genetic instability of Ad vectors.20
Ad vectors have been explored extensively as vaccine carriers for antigens of human immunodeficiency virus (HIV)-1,21–25 the causative agent of the acquired immunodeficiency syndrome (AIDS). While initial efforts focused on expression of internal antigens for the induction of CD8+ T cell responses against HIV-1, efforts have since shifted toward expression of the envelope protein (Env). The heavily glycosylated and highly variable Env of HIV-1 forms trimers composed of glycoprotein (gp) 120 and gp41 on the surface of the virion. These complexes allow HIV-1 to attach to its receptors and coreceptors and are thereby essential for CD4+ cell infection by HIV-1. Env is not only the sole target of HIV-1-specific neutralizing antibodies26 but also binds nonneutralizing antibodies, which may provide protection against infection as was shown by antibodies directed to the V2 loop of HIV-1 Env.27,28 The Env protein of HIV-1 is therefore considered to be an essential component of an HIV vaccine.
Previously, we attempted to insert gp140—a truncated form of gp160 lacking the transmembrane and cytoplasmic domains—of HIV-1 clade C into an E1- and E3-deleted chimpanzee-derived Ad (AdC) vector of serotype S-AdV-23, from here on referred to as AdC6. The complete E3 deletion was designed to remove E3 open reading frames (ORFs) 3–9. We were unable to rescue the virus indicative of its genetic instability. We reasoned that this might have been caused by premature apoptosis of the packaging cells due to high levels of gp140.29 We inserted the gp140 expression cassette into an E1-deleted viral molecular clone that contained the E3 domain. The rescued vector and target genome was initially changeable, however on subsequent passages remained stable. Restriction enzyme digestion indicated that the initial changes reflected deletions in part of the E3 domain. We sequenced the E3 domain of the mutated vector and then reinserted the E3 sequences that the vector had maintained (i.e., E3 ORFs 8 and 9) into the E1- and fully E3-deleted viral molecular clone, thereby creating a vector with a partial E3 deletion. Vectors with this partial E3 deletion rescued easily and were genetically stable over 12 serial passages upon insertion of the HIV-1 clade C gp140 or gp160 expression cassettes. Another chimpanzee Ad vector based on serotype S-AdV-24, termed AdC7, also stably expressed these two versions of HIV-1 Env when engineered with a similar partial E3 deletion. AdC6 and AdC7 vectors expressing gp140 or gp160 induced antibodies to HIV-1 Env. Humoral responses were higher in groups immunized with vectors expressing gp140.
Overall, our results show that genetic instability of Ad vectors caused by toxic transgene products can be overcome by the maintenance of selected E3 ORFs, specifically the regions encoding viral anti-apoptotic polypeptides.
Materials and Methods
Development of viral molecular clones
The vaccines are based on molecular clones of two chimpanzee adenoviruses, AdC6 and AdC7, also termed SAdV-23 and SAdV-24. Viruses were obtained from ATCC: AdC6 (ATCC VR-592, Genbank accession: AY530877); AdC7 (ATCC VR-593, Genbank accession: AY530878). Wild-type AdC6 and AdC7 were propagated in HEK-293 cells and purified by CsCl gradient centrifugation, followed by viral genomic DNA purification as previously described.30
We generated three viral molecular clones, the first has the entire E3 domain, the second has a complete deletion of the E3 domain and a partial deletion of the 5′ part of U, and the third has a partial E3 deletion. Generation of E1-deleted viral molecular clones containing full-length E3 based on the genome of wild-type virus has been described elsewhere.30
To generate a complete E3 deletion, the original plasmid of pAdC6 was digested with Sbf I, resulting in pC6 Sbf I; pC6 Sbf I was digested with Swa I and Eco47 III to generate pAdC6-E3 deleted.
As the vector containing full-length E3 and the gp140 expression cassette lost part of the E3 sequence upon passaging, we sequenced the E3 domain of the mutated viral clone and found that ORF 1 and 9 had remained intact, while ORF 2 and 8 had been partially lost; the other E3 ORFs had been deleted completely. The partial E3 deletions spanned from bp 27835 to 31052. We amplified the remaining E3 fragment by PCR with the forward primer 5′-CCATGGTGGCGCAGCTGAC-3′, and the reverse primer 5′-ATTTAAATCATCATCAATATGATCTTTA-3′. The PCR product was cloned into the pAdC6 E3-deleted plasmid using Nco I and Swa I restriction enzymes resulting in pC6 020-E3. Finally, both plasmids of the original pAdC6 and pC6 020-E3 were cut by Sbf I and ligated to generate the viral molecular clone of E1- and partially E3-deleted AdC6.
To create a partially E3-deleted AdC7 molecular clone, a similar cloning strategy was adopted. The E1-deleted AdC7 clone (pAdC7-E1 minus) was first generated in pBR322 as backbone plasmid. The fragment of Avr II (5.8 kb, from 23363 to 29172) was cloned into pSL1180 resulting in pC7-AvrII, which was digested by Nru I and ligated back to obtain the pC7-E3 minus. Finally, the backbone plasmid of pAdC7-E1 minus was digested with Avr II. pC7-E3 minus as insert donor was cut by Spe I and Avr II. The two fragments were ligated, resulting in the viral molecular clone of E1- and partially E3-deleted AdC7.
We inserted the gp140- and gp160-expression cassette from pShuttle vectors30 into the viral molecular clones using the I-CeuI and PI-ScaI sites present up- and downstream of the expression cassette and inserted into the molecular clones that contained the same restriction sites in the deleted E1. The pShuttle vectors have the human cytomegalovirus (CMV) promoter, the transgene (gp140 or gp160), and the bovine growth hormone poly A sequence. Viral vectors were rescued, expanded, purified, and quality controlled.30 The ratios of virus particles (vps) to infectious units were determined as described and ranged from ∼1:250 to 1:850 (AdC6-gp140, 1:467; AdC6-gp160, 1:854; AdC7-gp140, 1:493; AdC7-gp160, 1:275).
Expression of AdC6/AdC7 HIV-1 gp140/HIV-1 gp160
AdC6 or AdC7 virus expressing HIV-1 clade C gp140 or gp160 were tested by Western blots. Briefly, HEK-293 cells were infected with 1010 vp of the AdC vectors per well in a six-well plate overnight at 37°C with 5% CO2. After the infection, cells were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed using RIPA lysis buffer for 1 hour on ice. The cell supernatant was collected by centrifuging the lysate at 15,000 rpm for 30 minutes at 4°C. The concentration of total lysate was determined using Pierce BCA kit, and 40 μg of protein was loaded onto the SDS gel. The proteins were transferred to polyvinylidene difluoride membrane and blocked for 30 minutes with 5% milk powder in PBS containing 0.1% Tween. The membrane was probed overnight at 4°C with 1:1000 dilution of the ID6 mouse HIV-1 gp120 monoclonal antibody (obtained through the NIH AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health). After the incubation the membrane was washed thrice with PBST and further incubated with secondary anti-mouse immunoglobulin G (IgG) horseradish peroxidase antibody (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) at 1:20,000 dilution for 1 h. Following incubation the membrane was washed and developed using SuperSignal™ West Pico substrate (Thermo Fisher Scientific, Pittsburgh, PA). The membrane was stripped and re-probed with mouse monoclonal anti-ß-actin-peroxidase antibody, clone AC-15 (Sigma-Aldrich, St. Louis, MD) for loading control at 1:20,000 dilution.31,32
Mice
Female 6- to 8-week old CD-1 mice were purchased from the Charles River Laboratory and housed at the Animal Facility of the Wistar Institute. All experiments were performed in accordance with institutionally approved animal protocols.
Immunization of mice
Groups of 5 mice were immunized intramuscularly with AdC6 or AdC7 vectors expressing either gp140 or gp160 of HIV-1 clade C. The groups were injected with vectors diluted to 109–1011 vp in sterile PBS, while control mice were injected with PBS only. At 9 weeks after the prime, mice that received the 1010 vp prime were boosted with the same dose of a heterologous vector expressing the same transgene.
Serum collection
Blood was collected from individual mice at 2, 4, and 8 weeks after prime, and 8 weeks after the boost by submandibular bleeding. Samples were incubated at room temperature for 2 h and centrifuged at 5500 g for 10 min for separation of serum. Sera were stored at −20°C.
Enzyme-Linked Immunosorbent Assay
Round-bottom ELISA plates were coated with 150 ng of recombinant gp140 of HIV-1 clade C strain DU172 per well and incubated overnight at 4°C. The plates were washed with 0.05% PBS-Tween and blocked overnight at 4°C with 3% bovine serum albumin (BSA) in PBS-T. After a PBS-T wash, two-fold serial dilutions of sera starting at 1:200 were added to the plates and incubated for 2 hours at room temperature. The plates were washed with PBS-T and Alkaline phosphatase (AP)-conjugated anti-mouse IgG diluted to 1:30,000 was added to the plates for 1 h at room temperature. At the end of the incubation, the plates were washed, developed (phosphatase substrate dissolved in diethanolamine buffer), and read 30 min later at 405 nm. All samples were run in duplicate.
Affinity of transgene product–specific antibodies
Round-bottom ELISA plates were coated with 150 ng of recombinant gp140 of HIV-1 clade C strain DU172 per well and incubated overnight at 4°C. The plates were washed with 0.05% PBS-T and blocked overnight at 4°C with 3% BSA in PBS-T. After a PBS-T wash, serum diluted to 1:300 or 1:200 was added to the blocked plates and incubated for 2 h at room temperature. The plates were washed with PBS-T and eluted for 15 min with nine 2-fold serial dilutions of NH4SCN starting at 5 M. Control wells were incubated with PBS. The plates were washed with PBS-T and AP-conjugated anti-mouse IgG diluted to 1:30,000 was added to the plates for 1 hour at room temperature. At the end of the incubation, the plates were washed, developed (phosphatase substrate dissolved in diethanolamine buffer), and read 30 minutes later at 405 nm. All samples were run in duplicate.
Antibody isotyping
ELISA assays were used to characterize the isotypes of transgene-specific antibodies in sera from immunized mice. Round-bottomed ELISA plates were coated with 150 ng of recombinant gp140 of HIV-1 clade C strain DU172 and incubated overnight at 4°C. The plates were washed with PBS and blocked overnight at 4°C with 3% BSA in PBS. After a PBS wash, sera were diluted to 1:300 in blocking buffer and added to the coated plates for 2 h at room temperature. The plates were washed with PBS and six rabbit anti-mouse Ig antibodies directed to IgG1, IgG2a, IgG2b, IgG3, IgA, or IgM (Calbiochem Hybridoma Subisotyping Kit) were added to the plates for 1 hour at room temperature. The plates were washed with PBS and AP-conjugated goat anti-rabbit IgG diluted to 1:30,000 was applied to the plates for 1 hour at room temperature. At the end of the incubation, the plates were washed, developed (phosphatase substrate dissolved in diethanolamine buffer), and read 30 minutes later at 405 nm. All samples were run in duplicate.
Detection of V1/V2 loop–specific antibodies
Round-bottomed ELISA plates were coated with 600 ng/well of a HIV-1 V2 peptide (corresponding to HIV-1 Clade C strain DU172) and incubated overnight at 4°C. The plates were washed with PBS and blocked overnight at 4°C with 8% BSA in PBS. After a PBS wash, 2-fold serial dilutions of sera starting at 1:200 were incubated on the coated plates for 2 hours at room temperature. The plates were washed with PBS and AP-conjugated anti-mouse IgG diluted to 1:30,000 was applied to the plates for 1 hour at room temperature. At the end of the incubation, the plates were washed, developed (phosphatase substrate dissolved in diethanolamine buffer), and read 30 minutes later at 405 nm. All samples were run in duplicate.
Statistical analyses
For differences in antibody responses, the area under the curve for antibody titers in individual mice was calculated. Significant differences between groups were then determined by one-way ANOVA. Differences in isotypes were determined by two-way ANOVA. Differences in affinity were tested for by multiple t-tests with Holm-Sidak correction for type 1 errors.
Results
AdC6 and AdC7 vectors partially E3-deleted stably express gp140 or gp160 of HIV-1 clade C
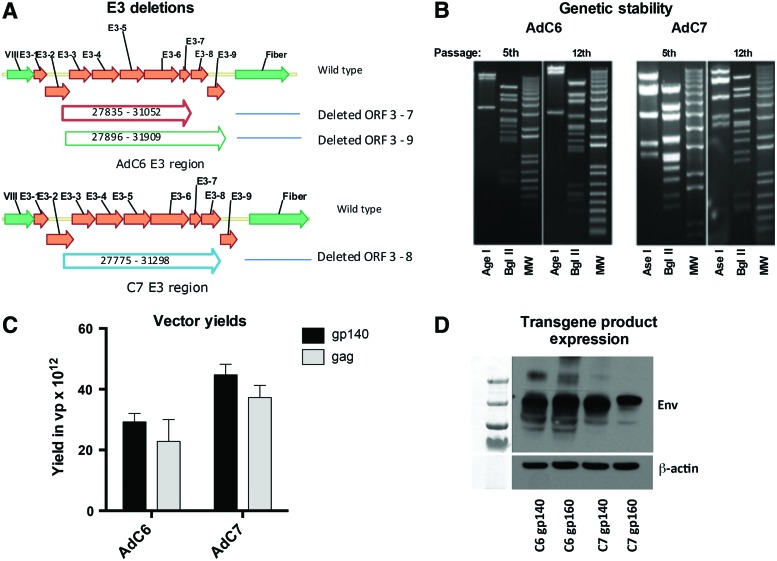
We previously developed viral molecular clones for E1- and E3-deleted AdC6 vectors. We successfully inserted a number of different transgene cassettes, which upon rescue in HEK-293 cells resulted in genetically stable vectors.33–35 Nevertheless, our initial attempts to generate AdC6 vectors expressing the gp140 of HIV-1 clade C envelope repeatedly failed. We reasoned that the toxicity of gp140 of HIV-1 might result in genetic instability and that this could be addressed by reinserting specific sequences of E3 encoding polypeptides with anti-apoptotic activity. In AdC6 wild-type virus (accession number AY530877), the E3 domain spans from base pairs (bp) 27110 to 31864. In our first generation of E3-deleted AdC6 vectors, E3 ORFs 3–9, in addition to part of the sequence encoding protein U, were deleted of 4013 bp by the use of restriction enzymes that cut at convenient sites (bp 27896–31909). Deletion of sequences encoding protein U in Ad vectors of human serotype 5 had been previously shown to impair the organization of replication centers late during infection, while vectors with a U exon deletion displayed a mild growth defect.36 Nevertheless, the partial U deletion was unlikely to cause the genetic instability of AdC6 vectors as other transgenes were well tolerated and neither rendered the vectors genetically unstable nor reduced yields.
We reconstructed the vector by reinserting E3 ORFs 8 and 9, and the missing part of the sequence encoding protein U, resulting in a new vector with a deletion of bp 27835-31052 (3218 bp deletion) corresponding to the sequences of wild-type virus (Fig. 1A). We then inserted expression cassettes containing gp140 or gp160 of HIV-1 clade C isolate Du422, which originated from a recently infected individual. Both vectors were isolated successfully, and upon 12 serial passages their genomes maintained the expected banding pattern upon restriction enzyme digest (Fig. 1B). We then developed an E1-deleted AdC7 vector with a similar E3 deletion of bp 27775–31298 (3523 bp) (Fig. 1A) and inserted gp140 or gp160 of Du172, another early clade C HIV-1 isolate. Again, these two vectors were rescued successfully and were genetically stable upon 12 serial passages in HEK-293 cells (Fig. 1B). Our attempts to generate fully E3-deleted AdC7 vectors expressing gp140 of HIV-1 repeatedly failed. Growth characteristics of the partially E3-deleted AdC vectors expressing Env of HIV-1 were similar to those observed for partially E3-deleted AdC vectors expressing other transgene products (Fig. 1C). Western blots conducted on cell lysates from HEK-293 cells infected with AdC6gp140, AdC6gp160, AdC7gp140, or AdC7gp160 showed expression of the transgene product (Fig. 1D).
Figure 1.
Partially E3-deleted adenovirus C (AdC) vectors are genetically stable and express the transgene product in transfected HEK-293 cells. (A) indicates the deletions. (B) shows the results of the restriction enzyme digest of the viral DNA isolated after an early (5th) or late (12th) passage. The molecular weight ladder (MW) is shown on the right of each gel. (C) shows vector vields for partially-(gp140) or E3-deleted (gag) AdC vectors. (D) shows Western blots for the AdC-Env vectors conducted with lysates of infected HEK-293 cells. Color images available online at www.liebertpub.com/hgtb
AdC6 and AdC7 vectors expressing Env of HV-1 induce antibody responses in mice
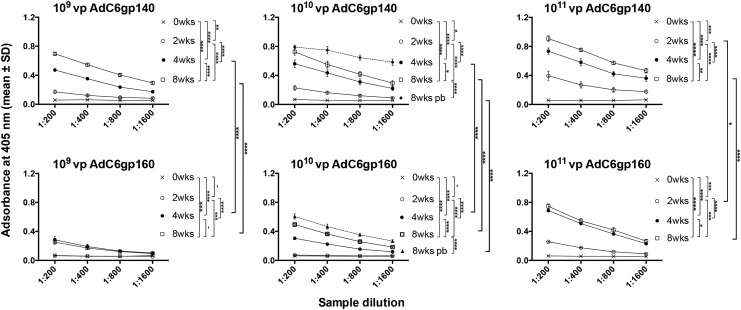
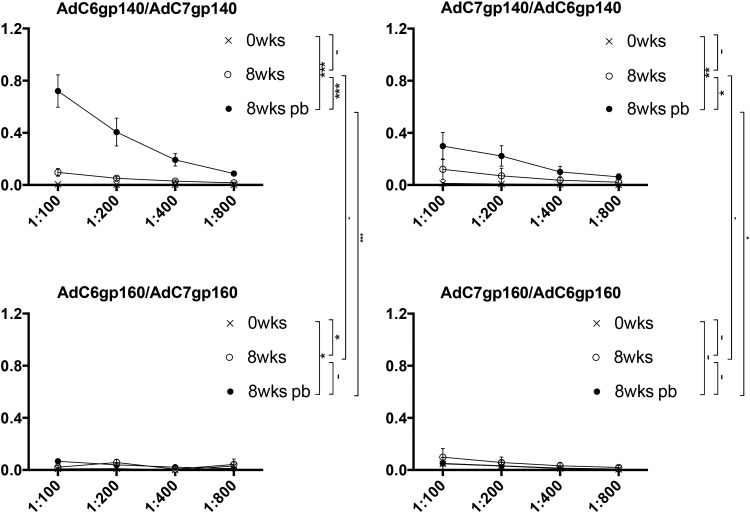
To further ensure that the HIV-1 Env-expressing AdC vectors containing the partial E3 deletion were functional, we immunized mice with three doses (109–1011 vp) of the four different constructs. Mice were bled at baseline and then in 2-week intervals for the next 8 weeks. Mice that received the 1010 vp dose were boosted at 9 weeks after the initial vaccination. Mice primed with AdC6 were boosted with the same dose of the AdC7 vector expressing the same transgene product and vice versa. As shown in Fig. 2, the AdC6gp140 vector induced an antibody response to HIV-1 Env in a dose-dependent fashion, with increases in humoral responses seen till week 8. A booster dose with a heterologous vector induced a further increase in antibody titers. The AdC6gp160 vector was less immunogenic and mice did not seroconvert until 4 weeks after immunization. The boost caused a significant increase in antibodies to Env.
Figure 2.
AdC6-induced envelope protein (Env)–specific antibody responses. Groups of 5 mice were immunized with the vectors at the indicated doses and antibodies to the transgene product were measured in individual serially diluted sera harvested at baseline or 2, 4, or 8 weeks after immunization. Mice that received the 1010 virus particle (vp) dose were boosted with AdC7 vectors expressing the same transgene used for priming and sera were tested at 8 weeks after the boost (8wks pb). The graphs show adsorbance values as means with standard error of the mean (SEM) for serially diluted sera. The lines next to the legends show significant differences between the indicated groups by ANOVA. The statistical analyses were performed on area under the curve for each serum. –p > 0.05; *0.05 ≥ p > 0.01; **0.015 ≥ p > 0.001; ***0.001 ≥ p > 0.0001; ****p ≤ 0.00001.
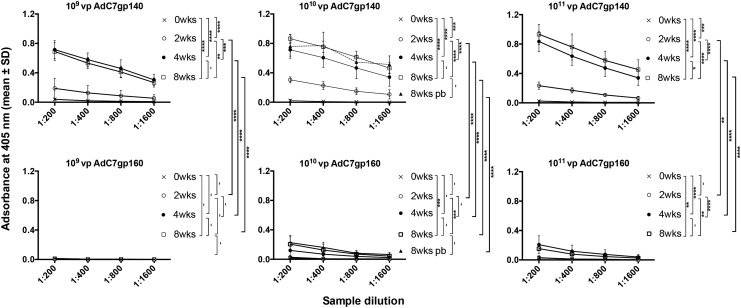
The AdC7 vectors showed a different pattern (Fig. 3). Responses to AdC7gp140 at the two lower doses peaked earlier by week 4 and there were no significant differences between responses at weeks 4 and 8. The booster immunization failed to increase transgene product–specific antibody titers. The AdC7gp160 vector was poorly immunogenic, with no detectable response elicited at the 109 vp vector dose. Responses to the two higher doses were low and came up with a delay as compared with those to the AdC6 vector expressing the same transgene product. The booster immunization with AdC6gp160 failed to increase antibody titers.
Figure 3.
AdC7-induced Env-specific antibody responses. The graphs show results for sera from AdC7-immunized mice (as described for AdC6 in Fig. 2).
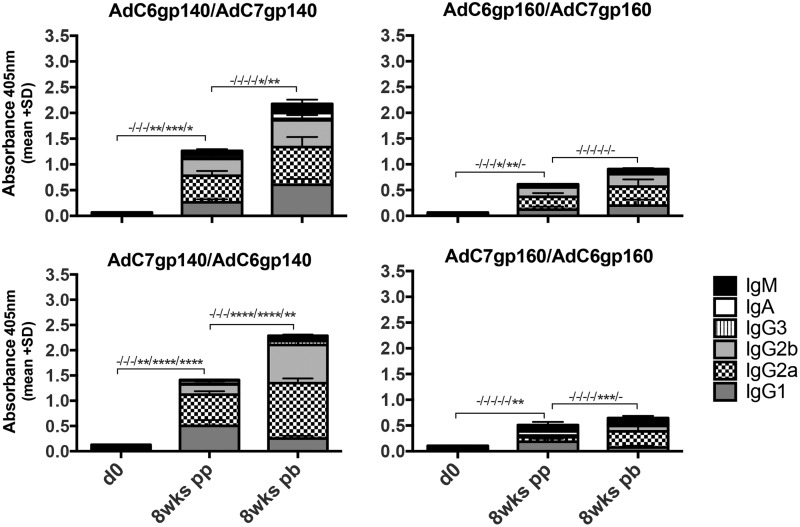
Env-specific antibodies at 8 weeks after the first immunization or booster dose were mainly of the IgG1 and IgG2a/b isotypes indicative of the mixed Th1/Th2 responses. Differences between the groups were subtle (Fig. 4).
Figure 4.
Isotypes of vaccine-induced antibodies to Env. Stacked columns show mean adsorbance + SEM of Env-specific antibodies in sera of mice harvested at baseline, 8 weeks post priming (8 wks pp) or 8 weeks post boosting (8 wks pb). Lines with -/* indicate significant differences between the indicated groups, as in Fig 2. From left to right -/* are organized following the top-to-bottom list of the isotypes listed in the right lower corner of the graph.
We tested sera of mice immunized with 1010vp of AdC vectors expressing gp140 or gp160 for antibodies to the V2 loop of HIV-1 by a peptide ELISA at baseline and at 8 weeks after the prime and the boost. AdC6gp140 and AdC7gp140 vectors induced antibodies to the V2 loop by 8 weeks after priming, which increased after the heterologous boost (Fig. 5). Increases in response were more substantial for the V2 loop than observed for the antibodies against the whole protein (Figs. 2,3). The antibody response to the V2 loop of Env was marginal after immunization with the AdC6gp160 or AdC7gp160 vectors.
Figure 5.
AdC-induced antibodies to the V2 loop. The graphs show adsorbance values as means with SEM for serially diluted sera from the same mice shown in Figs. 2 and 3. Sera harvested at baseline, at 8 weeks after priming, or at 8 weeks after the boost were tested. Lines with -/* show significant differences between the indicated groups again comparing area under the curve of individual sera.
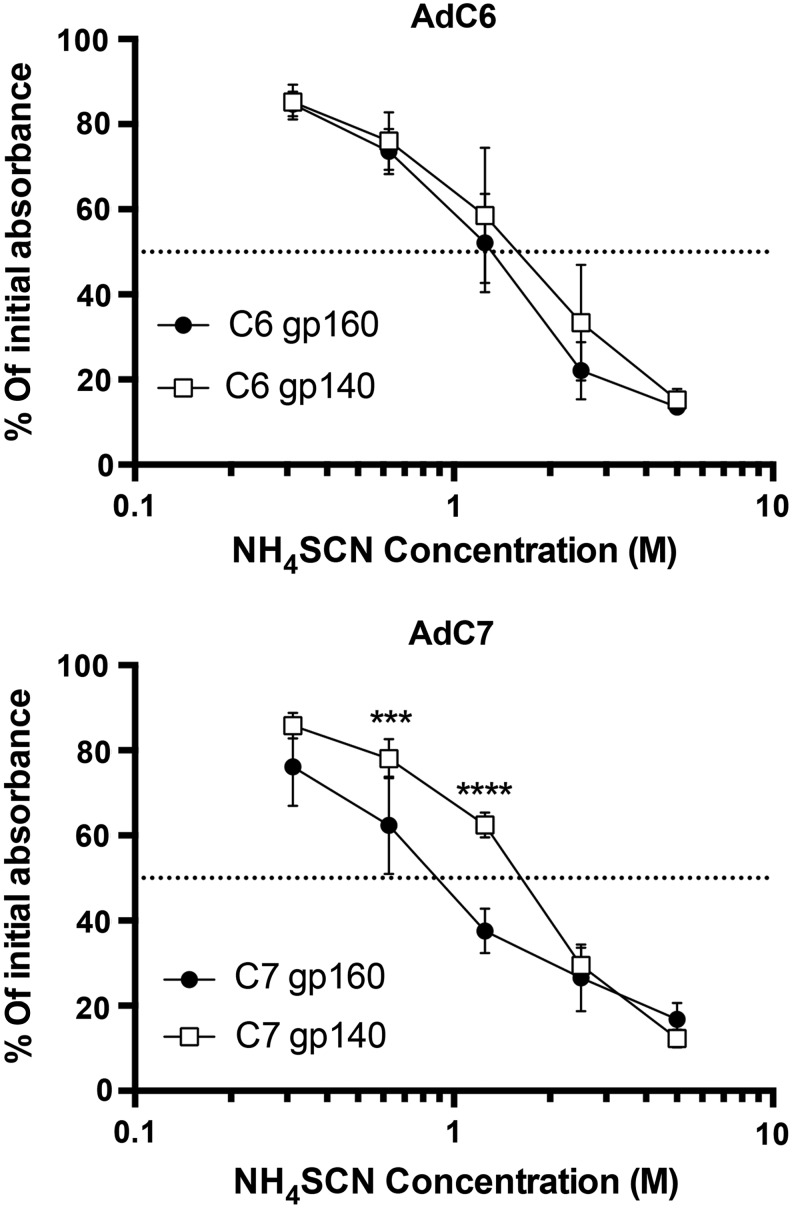
Antibodies collected at 8 weeks after priming with 1010 vp of AdC6 or AdC7 were tested for their affinity by an ammonium thiocyanate replacement assay. As shown in Fig. 6, antibodies induced by AdC6gp140 and AdC6gp160 had comparable affinity; antibodies to AdC7gp140 had superior affinity compared with antibodies elicited by AdC7gp160.
Fig 6.
Affinity of AdC-induced antibodies. The graphs show the results of a NH4SCN replacement assay conducted with a 1:200 dilution of sera from individual mice that had been immunized 8 weeks previously. Data are shown as percent adsorption in the presence of various concentrations of NH4SCN compared with adsorption without NH4SCN. The statistical analysis compared results at the same NH4SCN concentrations between the two groups in each graph by multiple t-tests with Holms-Sidak correction. Symbols are as in Fig. 2.
Overall these results show that AdC vectors expressing sequences of HIV-1 Env are immunogenic. Detectable humoral responses were higher upon immunization with gp140 cassette-expressing vectors, and induction of antibodies to the V2 loop of Env requires a prime-boost regimen with the viral vectors.
Discussion
E1-deleted Ad vectors are exceptionally well suited as vaccine carriers. After a single dose, they induce immune responses to the transgene product, which are exceptionally sustained as Ad vectors persist at low levels in a transcriptionally active form in T cells.37 This is a particularly interesting trait for vaccines that aim to induce antibody responses, as protection depends on levels of antibodies present in the circulation or at mucosal surfaces. Clinical experiences with Ad vectors have shown them to be well tolerated at doses needed to induce transgene product–specific immune responses.7,9,24 We developed AdC vectors to circumvent preexisting neutralizing antibodies, which are commonly found in humans to human Ad serotypes.38,39 Ad-neutralizing antibodies reduce uptake of the corresponding Ad vectors and hence their ability to induce transgene product–specific immune responses. Neutralizing antibodies to AdC6 and AdC7 are rare in humans residing in the US or Asia, and lower in sub-Saharan Africans than antibodies against other Ad vectors currently in testing.38,39
Although numerous transgene products have been successfully expressed by Ad vectors, a few are problematic by either resulting in poor growth or genetic instability of the recombinant Ad vectors; neither is acceptable for clinical development. This can be circumvented by using inducible expression systems.40,41 Alternatively, as we show here, it can be addressed at least for some transgenes by an incomplete E3 deletion, which preserves some of the ORFs that encode polypeptides that interfere with activation of apoptosis pathway in packaging cell lines that express E1 in trans.42,43 We assume that this prolongs the lifespan of the transduced packaging cells thus allowing for replicating Ad virus particles to mature before the cells die. Unfortunately, as we could not generate E1- and fully E3-deleted AdC vectors expressing gp140 or gp160, we were unable to pinpoint if indeed the reinserted E3 ORFs prevented premature apoptosis of the packaging cell lines. Using AdC6 vectors with full or partial E3 deletion expressing a nontoxic protein such as green fluorescent protein failed to indicate that cells transduced with the partially E3-deleted vector had a survival advantage (data not shown), but as green fluorescent protein is not particularly toxic, these results may not apply to AdC vectors expressing HIV-1 Env.
Using the partial E3 deletion we were able to generate AdC6 vectors expressing gp140 or gp160 of different isolates of HIV-1 clade C. We were also able to rescue partially E3-deleted AdC7 vector expressing the same transgenes. In contrast, fully E3-deleted AdC7 vectors with these transgenes failed to yield infectious virus. Partially E3-deleted AdC6 and AdC7 vectors expressing HIV Env vectors were genetically stable over 12 passages, and yields as well as ratios of virus particles to infectious units were comparable to those of other vectors with a more complete E3 deletion. We have since generated additional recombinant E1- and partially E3-deleted AdC vectors that were difficult to rescue in a version with the more complete E3 deletion, such as vectors expressing the rabies virus glycoprotein confirming that this approach has general value.
Vectors were immunogenic although antibody responses to gp160-expressing vectors were consistently lower than those to gp140. This may in part relate to the experimental design where antibodies were tested on plates coated with gp140. As is typical for antibodies to the Env of HIV-1, antibody responses developed rather slowly.44 The AdCgp140 vectors also induced antibodies to the V2 loop, which have been shown to provide increased resistance to HIV-1 acquisition in the RV144 trial.27 This phase 3 trial tested a vaccine regimen in which poxvirus vectors expressing gag, pol, and env were combined with gp120 protein boosts.45 A potent response to the V2 loop by the AdCgp140 vectors required a prime-boost regimen that disproportionally increased antibodies of this specificity.
Our experimental design favored detection of antibodies to Du172. AdC7 vectors expressed gp140 or gp160 of Du172 while AdC6 vectors expressed gp140 or gp160 of Du422. We selected Env sequences from two distinct clade C viruses to broaden responses after a prime boost. Antibodies were then tested on plates coated with a baculovirus-derived gp140 of Du172 or peptides representing the V1/V2 sequences of Du172, which in this domain only shows 70% homology with Du422. We thus expected to detect more potent antibody responses in mice immunized with AdC7 vectors expressing the homologous protein. This was not the case, indicating that antibodies were either highly cross-reactive or that the AdC6 vectors were intrinsically more potent. Testing on gp140 or peptides of Du172 may explain the results of the prime-boost experiments where mice primed with AdC6 showed increases in antibody titers upon boosting with AdC7, while in the reverse regimen the second immunization failed to increase antibody responses to protein and was less effective to augment titers to the V2 loop. Priming drives maturation of naïve B cells into short- and long-lived antibody-secreting cells and memory B cells. A second immunization with the same or a related antigen primarily stimulates the memory B cells rather than a distinct set of naïve mature B cells, a phenomenon known as antigenic sin. In the AdC6/AdC7 regimen, the boost preferentially expanded and affinity-matured antibody response to Du172, while the AdC7/AdC6 regimen would have promoted antibodies to Du422, which may have escaped detection.
In summary, results presented here show that a partial E3 deletion promotes genetic stability of Ad vectors carrying transgenes that would otherwise not be tolerated.
Acknowledgments
We wish to thank C. Cole for help with preparing the manuscript. Funding for this project was provided by the NIAID/ IPCAVD U19 AI074078.
Author Disclosure
No competing financial interests exist.
References
- 1.Benihoud K, Yeh P, Perricaudet M. Adenovirus vectors for gene delivery. Curr Opin Biotechnol 1999;10:440–447 [DOI] [PubMed] [Google Scholar]
- 2.Appaiahgari MB, Vrati S. Adenoviruses as gene/vaccine delivery vectors: promises and pitfalls. Expert Opin Biol Ther 2015;15:337–351 [DOI] [PubMed] [Google Scholar]
- 3.Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther 2004;15:1157–66 [DOI] [PubMed] [Google Scholar]
- 4.Hensley SE, Giles-Davis W, McCoy KC, et al. Dendritic cell maturation, but not CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J Immunol 2005;175:6032–6041 [DOI] [PubMed] [Google Scholar]
- 5.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther 2004;10:616–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang ZQ, Greenberg L, Ertl HC, et al. Protection of non-human primates against rabies with an adenovirus recombinant vaccine. Virology 2014;450–451:243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes E, Folgori A, Capone S, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med 2012;4:115ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiang K, Ying G, Yan Z, et al. Progress on adenovirus-vectored universal influenza vaccines. Hum Vaccin Immunother 2015;11:1209–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Santis O, Audran R, Pothin E, et al. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis 2016;16:311–320 [DOI] [PubMed] [Google Scholar]
- 10.Khanam S, Pilankatta R, Khanna N, et al. An adenovirus type 5 (AdV5) vector encoding an envelope domain III-based tetravalent antigen elicits immune responses against all four dengue viruses in the presence of prior AdV5 immunity. Vaccine 2009;27:6011–6021 [DOI] [PubMed] [Google Scholar]
- 11.Deng G, Li W, Wu X, et al. Immunogenicity and protective efficacy of a recombinant adenoviral based vaccine expressing heat-stable enterotoxin (STa) and K99 adhesion antigen of enterotoxigenic Escherichia coli in mice. Mol Immunol 2015;68:684–691 [DOI] [PubMed] [Google Scholar]
- 12.Alyahya SA, Nolan ST, Smith CM, et al. Immunogenicity without efficacy of an adenoviral tuberculosis vaccine in a stringent mouse model for immunotherapy during treatment. PLoS One 2015;10:e0127907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teixeira LH, Tararam CA, Lasaro MO, et al. Immunogenicity of a prime-boost vaccine containing the circumsporozoite proteins of Plasmodium vivax in rodents. Infect Immun 2014;82:793–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasconcelos JR, Dominguez MR, Neves RL, et al. Adenovirus vector-induced CD8+ T effector memory cell differentiation and recirculation, but not proliferation, are important for protective immunity against experimental Trypanosoma cruzi infection. Hum Gene Ther 2014;25:350–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Ertl HC. The effect of adjuvanting cancer vaccines with herpes simplex virus glycoprotein D on melanoma-driven CD8+ T cell exhaustion. J Immunol 2014;193:1836–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Zhang J, Wu Y, et al. Mannan-modified adenovirus targeting TERT and VEGFR-2: A universal tumour vaccine. Sci Rep 2015;5:11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez-Gutierrez JG, Elpek KG, Montes de Oca-Luna R, et al. Vaccination with an adenoviral vector expressing calreticulin-human papillomavirus 16 E7 fusion protein eradicates E7 expressing established tumors in mice. Cancer Immunol Immunother 2007;56:997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Windheim M, Hilgendorf A, Burgert HG. Immune evasion by adenovirus E3 proteins: exploitation of intracellular trafficking pathways. Curr Top Microbiol Immunol 2004;273:29–85 [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein DL, Toth K, Doronin K, et al. Functions and mechanisms of action of the adenovirus E3 proteins. Int Rev Immunol 2004;23:75–111 [DOI] [PubMed] [Google Scholar]
- 20.Bett AJ, Prevec L, Graham FL. Packaging capacity and stability of human adenovirus type 5 vectors. J Virol 1993;67:5911–5921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santra S, Sun Y, Korioth-Schmitz B, et al. Heterologous prime/boost immunizations of rhesus monkeys using chimpanzee adenovirus vectors. Vaccine 2009;27:5837–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang X, Casimiro DR, Schleif WA, et al. Vectored Gag and Env but not Tat show efficacy against simian-human immunodeficiency virus 89.6P challenge in Mamu-A*01-negative rhesus monkeys. J Virol 2005;79:12321–12331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barouch DH, Alter G, Broge T, et al. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 2015;349:320–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayton EJ, Rose A, Ibrahimsa U, et al. Safety and tolerability of conserved region vaccines vectored by plasmid DNA, simian adenovirus and modified vaccinia virus ankara administered to human immunodeficiency virus type 1-uninfected adults in a randomized, single-blind phase I trial. PLoS One 2014;9:e101591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008;372:1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saunders KO, Pegu A, Georgiev IS, et al. Sustained delivery of a broadly neutralizing antibody in nonhuman primates confers long-term protection against simian/human immunodeficiency virus infection. J Virol 2015;89:5895–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karasavvas N, Billings E, Rao M, et al. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses 2012;28:1444–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon SN, Doster MN, Kines RC, et al. Antibody to the gp120 V1/V2 loops and CD4+ and CD8+ T cell responses in protection from SIVmac251 vaginal acquisition and persistent viremia. J Immunol 2014;193:6172–6183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi A, Lee RT, Mohl J, et al. Genetic signatures of HIV-1 envelope-mediated bystander apoptosis. J Biol Chem 2014;289:2497–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou D, Zhou X, Bian A, et al. An efficient method of directly cloning chimpanzee adenovirus as a vaccine vector. Nat Protoc 2010;5:1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez-Roman VR, Cao C, Bai Y, et al. Phage-display mimotopes recognizing a biologically active anti-HIV-1 gp120 murine monoclonal antibody. J Acquir Immune Defic Syndr 2002;31:147–153 [DOI] [PubMed] [Google Scholar]
- 32.Dickey C, Ziegner U, Agadjanyan MG, et al. Murine monoclonal antibodies biologically active against the amino region of HIV-1 gp120: isolation and characterization. DNA Cell Biol 2000;19:243–252 [DOI] [PubMed] [Google Scholar]
- 33.Leskowitz R, Fogg MH, Zhou XY, et al. Adenovirus-based vaccines against rhesus lymphocryptovirus EBNA-1 induce expansion of specific CD8+ and CD4+ T cells in persistently infected rhesus macaques. J Virol 2014;88:4721–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cervasi B, Carnathan DG, Sheehan KM, et al. Immunological and virological analyses of rhesus macaques immunized with chimpanzee adenoviruses expressing the simian immunodeficiency virus Gag/Tat fusion protein and challenged intrarectally with repeated low doses of SIVmac. J Virol 2013;87:9420–9430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto AR, Fitzgerald JC, Giles-Davis W, et al. Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J Immunol 2003;171:6774–6779 [DOI] [PubMed] [Google Scholar]
- 36.Tollefson AE, Ying B, Doronin K, et al. Identification of a new human adenovirus protein encoded by a novel late l-strand transcription unit. J Virol 2007;81:12918–12926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatsis N, Fitzgerald JC, Reyes-Sandoval A, et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood 2007;110:1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Xiang ZQ, Li Y, et al. Adenovirus-based vaccines: comparison of vectors from three species of Adenoviridae. J Virol 2010;84:10522–10532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiang Z, Li Y, Cun A, et al. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg Infect Dis 2006;12:1596–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massie B, Couture F, Lamoureux L, Mosser DD, Guilbault C, Jolicoeur P, Bélanger F, Langelier Y. Inducible overexpression of a toxic protein by an adenovirus vector with a tetracycline-regulatable expression cassette. J Virol 1998;72:2289–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saydaminova K, Ye X, Wang H, Richter M, Ho M, Chen H, Xu N, Kim JS, Papapetrou E, Holmes MC, Gregory PD, Palmer D, Ng P, Ehrhardt A, Lieber A. Efficient genome editing in hematopoietic stem cells with helper-dependent Ad5/35 vectors expressing site-specific endonucleases under microRNA regulation. Mol Ther Methods Clin Dev 2015;1:14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burgert HG, Blusch JH. Immunomodulatory functions encoded by the E3 transcription unit of adenoviruses. Virus Genes 2000;21:13–25 [PubMed] [Google Scholar]
- 43.Benedict CA1, Norris PS, Prigozy TI, Bodmer JL, Mahr JA, Garnett CT, Martinon F, Tschopp J, Gooding LR, Ware CF. Three adenovirus E3 proteins cooperate to evade apoptosis by tumor necrosis factor-related apoptosis-inducing ligand receptor-1 and −2. J Biol Chem 2001;276:3270–3278 [DOI] [PubMed] [Google Scholar]
- 44.Alter G, Moody MA. The humoral response to HIV-1: new insights, renewed focus. J Infect Dis 2010;202:S315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karasavvas N, Billings E, Rao M, et al. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses 2012;28:1444–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]