Abstract
Objectives: The characterization and prediction of placebo response in clinical trials of youth with anxiety disorders have received little attention, despite the critical effects of placebo response rate on the success or failure of clinical trials. With this in mind, we sought to examine the factors that predict or influence placebo response in randomized controlled trials of youth with anxiety disorders.
Methods: Prospective, randomized, parallel-group controlled trials of psychopharmacologic interventions in pediatric patients with anxiety disorders were identified using a search of PubMed/Medline (1966–2015). Weighted least squares regression models and z-tests were utilized to examine the impact of continuous and categorical variables, respectively, on placebo response. These variables included demographic (e.g., age, percent white, percent female), clinical (e.g., baseline symptom severity), and trial characteristics (sample size, duration, funding). Finally, the relationship between the class of comparator medication and placebo response rate was examined using a multiple comparison for proportions test.
Results: The analyses of data from 14 trials involving 2230 patients and 9 medications reveal that higher placebo response rates were associated with a greater number of study sites (p = 0.013) and fewer patients per site (p < 0.008), while placebo dropout rates increased with more recent publication (p = 0.01) and were positively associated with the number of study visits (p < 0.02). Lower placebo response rates were associated with federally funded studies (z = −4.61, p < 0.001), studies conducted in the United States (z = 1.81, p < 0.035), and with an increased likelihood of detecting a significant effect on the primary outcome (z = 4.58, p < 0.0001). Additionally, studies, in which the majority of patients (>60%) had a diagnosis of social anxiety disorder, exhibited lower placebo response rates (p < 0.001). Finally, for trials, effect size has decreased over time (p = 0.004).
Conclusions: Important trial-specific factors affect placebo response and placebo dropout in youth with anxiety disorders and have pragmatic implications for the conduct and design of clinical trials and raise the possibility that limiting the number of sites while maximizing the number of patients per site could enhance the ability to detect medication–placebo differences.
Keywords: : placebo, anxiety disorder, antidepressant, clinical trial
Introduction
Anxiety disorders, including generalized, social, and separation anxiety disorders, are among the most common psychiatric conditions in youth (Kessler et al. 2005), frequently emerge during adolescence (Beesdo et al. 2010; Wehry et al. 2015), and are associated with significant morbidity and mortality (Sareen et al. 2005; Husky et al. 2012). However, these disorders are also amenable to both psychotherapeutic and psychopharmacologic interventions (Connolly and Bernstein 2007; Walkup et al. 2008; Strawn et al. 2015a; Strawn et al. 2015b), and long-term data suggest that successful treatment may result in sustained reductions in anxiety (Ginsburg et al. 2014).
Importantly, establishing the efficacy of psychopharmacologic treatments for pediatric anxiety disorders routinely relies on the use of double-blind, randomized placebo-controlled trials—the gold standard for evaluating medications in pediatric patients with anxiety disorders. In fact, the U.S. Food and Drug Administration (USFDA) advises, “before a new drug or biologic can be marketed, its sponsor must show through adequate and well-controlled clinical studies that it is effective. A well-controlled study permits a comparison of subjects treated with the new agent with a suitable control population so that the effect of the new agent can be determined and distinguished from other influences, such as spontaneous change, [and] placebo effects…” (United States Food and Drug Administration 2016).
Importantly, patients respond to placebo in clinical trials of mood and anxiety disorders (Walkup et al. 2008; Bridge et al. 2009; Kennard et al. 2009; March et al. 2009; Rutherford et al. 2011). Placebo response—the degree of symptomatic improvement in patients receiving placebo relative to those treated with the active medication—represents a particularly problematic issue in child and adolescent psychiatry. In this regard, some studies note placebo response rates of 40%–50% (Bridge et al. 2009; Emslie et al. 2014), although there is some suggestion that placebo response in pediatric patients may vary as a function of disorder (Cohen et al. 2010). Increasing, but variable, placebo response rates in clinical trials involving youth with anxiety disorders may result in an efficacious treatment not being statistically superior to placebo. In fact, some contend that the placebo response rate is key to detecting drug versus placebo differences within a trial and, in terms of clinical practice, to better detect those children and adolescents who would truly benefit from medication (Cohen et al. 2010). Consequently, high placebo response rates in pediatric patients may result in effective treatments being abandoned and may further limit the psychopharmacologic armamentarium for youth with anxiety disorders.
The factors that affect placebo response in adults with depressive disorders (Sneed et al. 2008; Papakostas and Fava 2009; Iovieno and Papakostas 2012; Rutherford et al. 2012; Rutherford and Roose 2013) and anxiety disorders (Khan et al. 2005; Stein et al. 2006; Feltner et al. 2009; Rutherford et al. 2015) have been extensively evaluated; however, relatively limited information is available regarding the characterization of placebo response in pediatric patients with anxiety (Cohen et al. 2010). In anxious adults, Stein et al. (2006) have observed that placebo response for generalized anxiety disorder (GAD) was greater in studies conducted in Europe and in fixed-dose studies and was affected by lower baseline symptom severity, but was unaffected by gender and age. Additionally, in reviewing placebo response in a recent meta-analysis of randomized controlled trials of antidepressants in adults with GAD, panic disorder, and social anxiety disorder, Rutherford et al. (2015) observed that placebo response rates have increased over time, are higher in patients with panic disorder (compared with those with GAD or social anxiety disorder), and are associated with decreased medication–placebo differences. However, to date, we are aware of only three prior examinations of placebo response in pediatric disorder (MDD) (Bridge et al. 2009; Cohen et al. 2010; Rutherford et al. 2011) and of only one study that included youth with anxiety disorders (Cohen et al. 2010). Bridge et al. (2009) examined 12 studies (N = 2862) of youth with MDD and noted that placebo response increased as a function of the number of study sites and in younger patients and is inversely related to depressive symptom severity at baseline. Additionally, in this examination of randomized controlled trials of antidepressants in depressed youth, the placebo response rate appeared to increase in more recent studies (Bridge et al. 2009), while one additional meta-analysis of placebo response in pediatric MDD suggests increased contact with research staff was associated with increased response in older youth. Only one study of placebo response has included pediatric patients with anxiety disorders (Cohen et al. 2010). In this study, pediatric patients with depressive disorders, anxiety disorders, and obsessive-compulsive disorder (OCD) were examined, including 10 studies of anxious youth (N = 634). Pediatric anxiety disorders—which represented a subset of this analysis—were found to have a higher response rate relative to youth with OCD, but a lower placebo response rate relative to MDD (Cohen et al. 2010). In the entire sample of patients with MDD, OCD, and non-OCD anxiety disorders, placebo response was negatively associated with Caucasian status and the percentage of male participants, while the presence of a washout decreased placebo response. However, this study was limited in its ability to examine specific factors associated with placebo response within the non-OCD anxiety disorder trials. Since the publication of this analysis, nearly 700 additional anxious youths have been studied in randomized controlled trials and, to date, the specific factors that are associated with or predict placebo response and placebo dropout rate have not been systematically examined in pediatric patients with anxiety disorders. With this in mind, we sought to examine the placebo response in pediatric patients with non-OCD anxiety disorders.
Materials and Methods
Identification of studies
The studies included were obtained through an electronic search of PubMed (1966 through November 2015), and the search was completed by two reviewers (E.T.D. and J.R.S.) and was supplemented by a search of clinicaltrials.gov. Using the following search strategy: social phobia OR social anxiety disorder OR separation anxiety disorder OR GAD OR SAD) AND (child* OR adolescent OR pediatric OR youth) AND (ssri OR ssnri OR SNRI OR fluvoxamine OR citalopram OR escitalopram OR fluoxetine OR paroxetine OR venlafaxine OR desvenlafaxine OR duloxetine OR vortioxetine OR vilazodone OR benzodiazepine OR clonazepam OR diazepam OR alprazolam OR lorazepam OR tricyclic antidepressants, limiting the results to English language articles, and age <18 years, journal articles yielded 229 entries. These results were then manually limited to randomized placebo-controlled trials and the references of all eligible trials were searched for additional clinical trials.
Studies were included if they were prospective, randomized, parallel-group placebo-controlled trials that evaluated the efficacy of a psychopharmacologic intervention in the treatment of a non-OCD anxiety disorder or the combination of these non-OCD anxiety disorders in children or adolescents and used the Clinical Global Impressions-Improvement (CGI-I) scale or other validated rating scale to measure the severity of the anxiety symptoms.
Data extraction
Study data and characteristics, including year of publication, sample size, and clinical and demographic characteristics of subjects, as well as details regarding the duration, number of visits, funding source, and study location, were entered into a database by both authors (J.R.S., E.T.D.). The active medications in the trials were classified as selective serotonin reuptake inhibitors (SSRIs), selective serotonin norepinephrine reuptake inhibitors (SSNRIs), tricyclic antidepressants, and benzodiazepines. Additionally, outcome data were extracted, including dimensional measures of anxiety symptoms and CGI-I scores.
Response criteria
Response criteria were specified a priori in the following order of preference: (1) CGI-I scores ≤2 (indicated much improved or very much improved); (2) 50% improvement on pediatric anxiety rating scale severity; and (3) global improvement or global assessment of functioning.
Statistical analyses
Descriptive statistics were used to characterize the included trials in terms of clinical and demographic factors, design factors, and treatment-specific factors. For these comparisons, t-tests and χ2 tests were used for continuous and categorical variables, respectively. Least squares regression was employed to assess continuous variables as possible predictors of placebo response and placebo dropout rates. Additionally, to control for the influence of study size, variance, and outlier effects, these least squares regression values were weighted by inverse variance. z-Tests were utilized to assess the relationship between categorical variables (e.g., study location, U.S. versus non-U.S., presence of a majority social anxiety disorder population) and placebo response, as well as placebo dropout rates.
When possible, the effect size for trials of SSRIs and SSNRIs was examined as a function of year of publication and number of sites using Spearman's rank correlation. Placebo response rates across drug classes were evaluated using a χ2 test. p Values ≤0.05 were considered statistically significant and no correction was made for multiple comparisons. Statistical analyses were performed using R (version 3.1.2).
Results
Characteristics of participants and studies included
Across the 14 studies analyzed, which were published between 1972 and 2015, 2230 anxious patients were randomized to medication or placebo and, of these, 889 received placebo. The average age was 11.6 ± 1.1 years (range 5–18 years) and the mean proportion of females was 46.8% ± 14% (range 22.7%–83%). The unweighted average placebo response rate was 36.4% (weighted placebo response rate 37.6%).
Eight of the 14 studies were federally funded, 4 were industry funded, and funding data were unavailable for 2 of these studies (Simeon et al. 1992; Berney 1981). The average number of sites per trial was 14 ± 20 and five trials (36%) were single-site studies. Federally funded studies tended to have fewer sites (federally funded: 2.25 ± 2.1 sites, industry funded: 44.3 ± 11.8 sites, p = 0.0053). Ten studies were conducted exclusively in the United States and four studies had at least one international site. Additional characteristics of the studies are described in Table 1.
Table 1.
Summary of Clinical Trial Design Features, Sample Characteristics, and Placebo Response for the Studies Included
| Study | Medication | Age range (mean) | Primary diagnosis | Duration (weeks) | N | White (%) | Female (%) | Sites | Baseline (CGI-S) | Funding | United States only | % SoP | Placebo dropout (%) | Placebo response (%) | Response criteria | Drug > placebo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gittleman-Klein et al. (1971) | Imipramine | 6–15 (10.8) | School phobia | 9 | 35 | 46 | 42 | 1 | NA | Federal | Yes | NA | 10 | 6/19 (32) | CGI-I ≤ 2 | Yes |
| Berney et al. (1981) | Clomipramine | 9–15 (NA) | School phobia | 12 | 46 | 41 | 59 | 1 | 3.8 | Yes | NA | 5 | 10/19 (53) | CGI-I ≤ 2 | No | |
| Simeon et al. (1992) | Alprazolam | 8–16 (12.6) | Overanxious or avoidant disorder | 4 | 30 | 76 | 23 | 2 | NA | No | 30 | 8 | 5/13 (38) | CGI-I ≤ 2 | No | |
| Klein et al. (1992) | Imipramine | 6–15 (9.5) | SAD | 6 | 21 | 66 | 33 | 1 | NA | Federal | Yes | 20 | 10 | 4/10 (40) | Global improvement | No |
| Rynn et al. (2001) | Sertraline | 5–17 (11.7) | GAD | 9 | 22 | 81 | 23 | 1 | 4.0 | Federal | Yes | NA | 18 | 1/11 (9.1) | CGI-I ≤ 2 | Yes |
| RUPP (2001) | Fluvoxamine | 6–17 (10.3) | Mixed | 8 | 128 | 62 | 49 | 5 | NA | Federal | Yes | 69 | 22 | 19/65 (29) | CGI-I ≤ 3 | Yes |
| Birmaher et al. (2003) | Fluoxetine | 7–17 (11.9) | Mixed | 12 | 74 | 96 | 54 | 1 | 3.9 | Federal | Yes | 54 | 16 | 13/37 (35) | CGI-I ≤ 2 | Yes |
| Wagner et al. (2004) | Paroxetine | 8–17 (13.3) | SoP | 16 | 322 | 84 | 43 | 38 | 4.4 | Federal | No | 100 | 33 | 59/154 (38) | CGI-I ≤ 2 | Yes |
| Rynn et al. (2007) | Venlafaxine | 6–17 (11.2) | GAD | 8 | 323 | 77 | 48 | 59 | 4.5 | Industry | Yes | 0 | 25 | 77/159 (48) | CGI-I ≤ 2 | Yes |
| March et al. (2007) | Venlafaxine | 8–18 (13.6) | SoP | 16 | 293 | 74 | 83 | 48 | 4.9 | Industry | Yes | 100 | 27 | 55/148 (37) | CGI-I ≤ 2 | Yes |
| Beidel et al. (2007) | Fluoxetine | 7–17 (11.6) | SoP | 12 | 139 | 78 | 47 | 2 | 4.7 | Industry | Yes | 100 | 22 | 2/32 (6.3) | CGI-I ≤ 2 | Yes |
| Walkup et al. (2008) | Sertraline | 7–17 (10.6) | Mixed | 12 | 488 | 79 | 49 | 6 | 5.1 | Industry | Yes | 83 | 16 | 18/76 (24) | CGI-I ≤ 2 | Yes |
| da Costa et al. (2013) | Clomipramine/fluoxetine | 7–17 (11.4) | Mixed | 12 | 30 | NA | 47 | 1 | 4.8 | Federal | No | NA | 18 | 7/9 (78) | CGI-I ≤ 2 | No |
| Strawn et al. (2015a) | Duloxetine | 7–17 (12.2) | GAD | 10 | 272 | 81 | 55 | 32 | 4.5 | Industry | No | 23 | 23 | 58/137 (42) | 50% improvement on PARS severity | Yes |
CGI-I, Clinical Global Impressions-Improvement; CGI-S, Clinical Global Impressions-Severity; GAD, generalized anxiety disorder; NA, not available; PARS, pediatric anxiety rating scale; SAD, separation anxiety disorder; SoP, social phobia/social anxiety disorder.
Placebo response
As shown in Table 2, a number of significant correlations between the proportion of placebo responders and specific demographic and clinical characteristics, as well as trial-related factors, were observed. Lower placebo response rates were observed in studies conducted exclusively in the United States (z = 1.81, p = 0.035), studies that received federal funding (z = 6.61, p < 0.001), and studies in which >60% of the patients met diagnostic criteria for social anxiety disorder (z = 3.69, p < 0.001).
Table 2.
Specific Factors Associated with Placebo Response in Randomized Controlled Trials of Children and Adolescents with Anxiety Disorders
| Trials included (N) | Test | Test statistic | p | |
|---|---|---|---|---|
| Demographic and clinical characteristics of patients | ||||
| Mean age | 13 | weighted LSR |
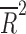 = −0.041 = −0.041 |
0.485 |
| White (%) | 13 | weighted LSR |
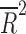 = −0.088 = −0.088 |
0.864 |
| Female (%) | 14 | weighted LSR |
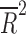 = −0.027 = −0.027 |
0.431 |
| >60% of patients with social anxiety disorder | 10 | z-test | z = 3.69 | <0.001 |
| Baseline Clinical Global Impressions-Severity Scale score | 10 | weighted LSR |
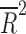 = −0.076 = −0.076 |
0.563 |
| Trial characteristics | ||||
| Total sample size | 14 | weighted LSR |
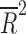 = −0.028 = −0.028 |
0.438 |
| Duration of trial | 14 | weighted LSR |
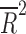 = −0.078 = −0.078 |
0.812 |
| Number of visits | 14 | weighted LSR |
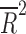 = 0.118 = 0.118 |
0.124 |
| Number of study sites | 14 | weighted LSR |
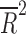 = 0.3626 = 0.3626
|
0.013 |
| Average subjects/site | 14 | weighted LSR |
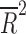 = 0.380 = 0.380
|
0.008 |
| Placebo dropout rate | 14 | weighted LSR |
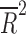 = −0.034 = −0.034 |
0.463 |
| United States only | 14 | z-test | z = 1.81 | 0.035 |
| Funding source | 12 | z-test | z = −4.61 | <0.001 |
| Year of publication | 14 | weighted LSR |
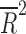 = −0.080 = −0.080 |
0.848 |
| Medication class | 13 | χ2 | χ2 = 15.0, df = 3 | 0.002 |
| Outcome | ||||
| Medication > placebo | 14 | z-test | z = 4.58 | <0.001 |
LSR, least squares regression.
Bold text denotes statistically significant findings.
Influence of study design variables on placebo response
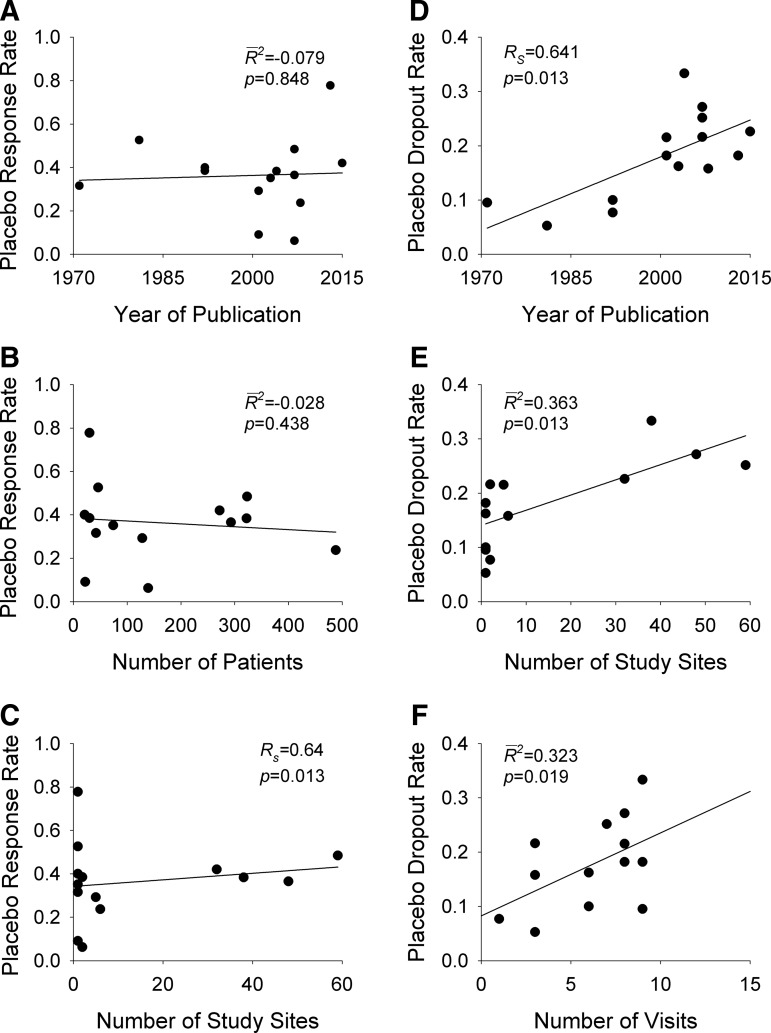
Placebo response rate was not significantly associated with the year of publication (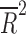 = −0.080, p = 0.848), the total number of patients randomized (
= −0.080, p = 0.848), the total number of patients randomized (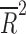 = −0.028, p = 0.4377), the duration of the trial (
= −0.028, p = 0.4377), the duration of the trial (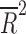 = −0.078, p = 0.812), nor the number of patient visits (
= −0.078, p = 0.812), nor the number of patient visits (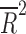 = 0.118, p = 0.124). However, the placebo response rate was significantly associated with a greater number of study sites (
= 0.118, p = 0.124). However, the placebo response rate was significantly associated with a greater number of study sites (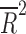 = 0.36, p = 0.013) (Table 2 and Fig. 1) and inversely associated with the average number of patients per site (p = 0.008) (i.e., as the number of patients per site increased, the placebo response rate decreased).
= 0.36, p = 0.013) (Table 2 and Fig. 1) and inversely associated with the average number of patients per site (p = 0.008) (i.e., as the number of patients per site increased, the placebo response rate decreased).
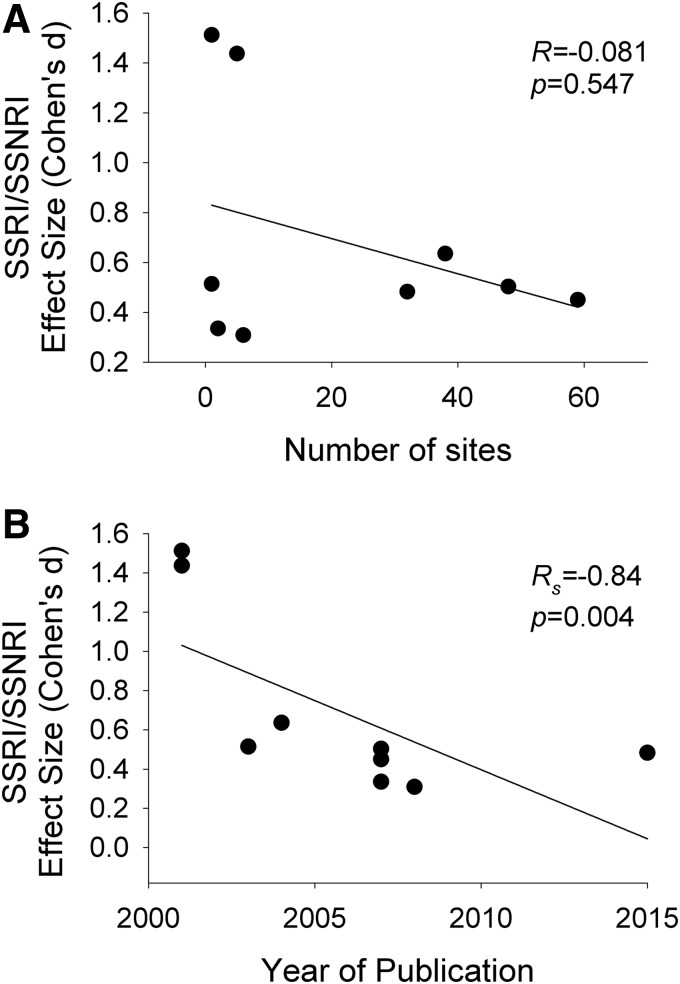
FIG. 1.
Effect size for antidepressant medications and number of sites and year of publication. The effect size of selective serotonin reuptake inhibitors (SSRIs) and selective serotonin norepinephrine reuptake inhibitors (SSNRIs) in randomized controlled trials of youth (Strawn et al. 2015b) is not associated with the number of sites (A), but is related to the year of publication (B). Lines are shown for weighted least squares regression.
Effect of study design on dropout rate
Placebo dropout rates have increased over time (Rs = 0.64, p = 0.01) and appear to increase as the number of visits increases (R2 = 0.323, p < 0.02) (Fig. 1). Additionally, placebo dropout rates increased with greater numbers of study sites (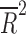 = 0.42, p = 0.007) (Fig. 1).
= 0.42, p = 0.007) (Fig. 1).
Study outcome and placebo response rate
Study outcomes supporting the efficacy of a medication—across all 14 studies—were strongly associated with higher placebo response rates (z = 4.58, p < 0.001). Additionally, the placebo response rate differed as a function of the comparison medication class (χ2 = 15.0, df = 3, p = 0.002). Specifically, studies involving SSNRIs as the comparison medication had higher placebo response rates compared with SSRIs (p < 0.05), but, in a multiple comparisons for proportions test, no other class differences were observed. Importantly, given this difference and that recent trials of SSNRIs and SSRIs have (1) utilized similar parallel-group designs, (2) have been collected within a more recent time period, and (3) all used Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994) criteria for the anxiety disorders, we utilized this relative homogeneity in design and population to perform several post hoc analyses. Among these trials (n = 9), the effect size (Strawn et al. 2015b) decreases with time (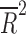 = −0.84, p = 0.004), although no association between the effect size (Cohen's d) and the number of study sites was found (p = 0.547) (Fig. 2).
= −0.84, p = 0.004), although no association between the effect size (Cohen's d) and the number of study sites was found (p = 0.547) (Fig. 2).
FIG. 2.
Placebo response and placebo dropout in clinical trials involving youth with anxiety disorders. Placebo response rates are shown in (A–C) while placebo drop rates are shown in (D–F) Lines are shown for weighted least squares regression.
Discussion
This study is the first—to our knowledge—to evaluate placebo response among clinical trials of non-OCD anxiety disorders in youth. Across 14 trials involving 2230 patients and 9 medications, higher placebo response rates were associated with a greater number of study sites, while federal funding and a U.S. setting were associated with an increased likelihood of detecting a significant effect on the primary outcome.
That the number of sites and non-federal funding predict placebo response and dropout rates is of importance particularly within the context of decreasing effect size for SSRIs and SSNRIs over time. For nearly 2 decades, the Pediatric Research Initiative Act of 1995 has increased the number of trials conducted in adolescents with anxiety disorders. The urgency to complete these companion pediatric anxiety studies to satisfy regulatory obligations increased the number of industry-funded studies, which have generally involved more sites and have also relied on more non-U.S. sites to obtain sufficient numbers of patients. The present analyses suggest that efforts to decrease placebo response should focus on reducing the number of sites and these data also raise the possibility that factors, which ostensibly do not affect placebo response, but which increase the cost of clinical trials and the amount of placebo exposure, might be modified (e.g., duration of trial, frequency of visits). It is of further interest that additional concerns have been levied against clinical trials in terms of their lack of generalizability given that the frequency of visits fails to mirror clinical practice or even clinical recommendations regarding visit frequency (Hughes et al. 2007).
Finally, our observation of placebo response being associated with the number of study sites and with industry funding—which has been observed in clinical trials of adults with anxiety disorders (Rutherford et al. 2015)—warrants further discussion. Previously it has been noted that in recent decades, the conduct of randomized controlled trials has shifted from university-based studies involving one to two sites toward larger multicenter trials, which have heavily relied on contract research organizations (CROs) and international sites. Additionally, it has been suggested that this tendency has been the result of several factors. Specifically, with regard to this trend, Rutherford et al. (2015) have noted the following: “Academic sites often entail increased time and expense associated with institutional review board approval, but commercial sites, particularly those operated by CROs, have arguably more powerful financial incentives to enroll patients, which can result in baseline score inflation by raters, followed by a rapid decline in scores once restrictive entrance criteria have been passed.”
In describing placebo response in pediatric patients with depressive disorders, patient expectancy of therapeutic improvement is believed to be the primary mechanism of placebo effects (Rutherford et al. 2011), which raises the possibility that the duration of contact and number of study visits, as well as the length of the trial, may increase placebo response rates. In fact, meta-analyses of placebo response in adults (Rutherford et al. 2009) support this hypothesis. However, youth with MDD may not exhibit a similar response and one prior study of pediatric MDD failed to observe this effect, and we have similarly not detected an effect of duration or number of visits on placebo response rates. Taken together, our findings in pediatric patients with anxiety and those of Rutherford et al. (2011) suggest that the nonspecific study effects that occur by virtue of studying a patient over time in a research study are less pronounced or even absent in youth compared with adults. Additionally, placebo response rates, which have generally been lower in youth with anxiety disorders (Pine et al. 2001; Rynn et al. 2001; Walkup et al. 2008) compared with youth with depressive disorders (DelBello et al. 2014; Emslie et al. 2014), may reflect differences in the sensitivity of patients with anxiety disorders relative to patients with depressive disorders to expectancy.
Our observation that a majority of patients having social phobia/social anxiety disorder is associated with a lower placebo response rate is of interest. In the Child/Adolescent Multimodal Study of Anxiety (CAMS) (Walkup et al. 2008), pediatric patients with a primary diagnosis of social anxiety disorder had similar responses when treated with placebo and cognitive behavioral therapy (Ginsburg et al. 2011; Compton et al. 2014), suggesting that social phobia may be less influenced by the psychological components of therapeutic interactions that may drive placebo response. However, it is noteworthy that in adults, social anxiety disorder—when co-morbid with other anxiety disorders or mood disorders—predicts a lower likelihood of response (Bruce et al. 2005). It remains unclear whether social anxiety disorder (either as a primary diagnosis or as a comorbidity) represents a more severe psychopathology or simply an inability to benefit from the psychosocial components that likely subtend a successful placebo response.
While the present study represents the first examination of predictors of placebo response within randomized controlled trials of anxious youth, it does have several important limitations. First, the number of clinical trials is relatively small (N = 14) relative to similar studies in adults with anxiety disorders (Rutherford et al., 2015), thus increasing the possibility of type II error, although it is of interest that many of the findings described herein contemporaneously parallel the trends observed in adults with anxiety disorders (Rutherford et al. 2015). Second, as has been the case with most prior evaluations of placebo response in pediatric patients (Bridge et al. 2009; Cohen et al. 2010), patient-level data are lacking, thus precluding an examination of specific factors that may influence antidepressant response in anxious youth (Compton et al. 2014; Ginsburg et al. 2014). Third, this analysis of placebo response in pediatric anxiety disorders is limited in its ability to resolve multicolinearity among variables as has been the case with other studies of placebo response. As such, the influence of multiple variables that may be interrelated (e.g., duration and number of visits) is difficult to control for in this small sample.
Conclusions
The results from our meta-regression analysis reveal placebo response to be substantial in randomized controlled trials of children and adolescents with non-OCD anxiety disorders. Moreover, placebo response rates have increased over time and for very recent trials of SSRIs and SSNRIs have been associated with a decrease in the apparent effect size of the comparator medication. Moreover, study design (e.g., number of sites, number of patients per site, and the number of visits) influences placebo response and these data raise the possibility that modification of clinical trial design could offer substantial scientific and cost advantages, while also decreasing exposure to placebo.
Clinical Significance
While the present study has clear implications for the design and interpretation of clinical trials, its findings are also of relevance to the practicing child and adolescent psychiatrist who may implicitly wish to increase the placebo components of psychopharmacologic treatment for youth with anxiety disorders. In this regard, our data do not suggest that patient age, sex, or minority status significantly affects placebo response. However, in patients with social anxiety—who may be less able to benefit from placebo—a clinician might consider more aggressive treatment (e.g., more frequent visits, earlier initiation of psychotherapy).
Acknowledgments
Mr. Dobson received support from the American Academy of Child and Adolescent Psychiatry Campaign for America's Kids and Dr. Strawn receives support from the National Institute of Mental Health (NIMH, K23MH106037).
Disclosures
Mr. Dobson has received support from the American Academy of Child and Adolescent Psychiatry (Campaign for America's Kids). Dr. Strawn has received research support from Edgemont, Eli Lilly, Shire, Forest Research Institute, Lundbeck, and the National Institute of Mental Health (NIMH). He receives royalties from Springer Publishing for two texts, has produced a rating scale training video for Neuronetics, and has received material support from Assurex.
References
- American Psychiatric Association: Diagnostic and Statistical Manual for Mental Disorders, 4th ed. (DSM-IV). Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Beesdo K, Pine DS, Lieb R, Wittchen HU: Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry 67:47–57, 2010 [DOI] [PubMed] [Google Scholar]
- Beidel DC, Turner SM, Sallee FR, Ammerman RT, Crosby LA, Pathak S: SET-C versus fluoxetine in the treatment of childhood social phobia. J Am Acad Child Adolesc Psychiatry 46:1622–1632, 2007 [DOI] [PubMed] [Google Scholar]
- Berney T, Kolvin I, Bhate SR, Garside RF, Jeans J, Kay B, Scarth L: School phobia: A therapeutic trial with clomipramine and short-term outcome. Br J Psychiatry 138:110–118, 1981 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson DA, Monk K, Kalas C, Clark DB, Ehmann M, Bridge J, Heo J, Brent DA: Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry 42:415–423, 2003 [DOI] [PubMed] [Google Scholar]
- Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA: Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry 166:42–49, 2009 [DOI] [PubMed] [Google Scholar]
- Bruce SE, Yonkers KA, Otto MW, Eisen JL, Weisberg RB, Pagano M, Shea MT, Keller MB: Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: A 12-year prospective study. Am J Psychiatry 162:1179–1187, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Consoli A, Bodeau N, Purper-Ouakil D, Deniau E, Guile J-M, Donnelly C: Predictors of placebo response in randomized controlled trials of psychotropic drugs for children and adolescents with internalizing disorders. J Child Adolesc Psychopharmacol 20:39–47, 2010 [DOI] [PubMed] [Google Scholar]
- Compton SN, Peris TS, Almirall D, Birmaher B, Sherrill J, Kendall PC, March JS, Gosch EA, Ginsburg GS, Rynn MA, Piacentini JC, McCracken JT, Keeton CP, Suveg CM, Aschenbrand SG, Sakolsky D, Iyengar S, Walkup JT, Albano AM: Predictors and moderators of treatment response in childhood anxiety disorders: Results from the CAMS trial. J Consult Clin Psychol 82:212–224, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SD, Bernstein GA: Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 46:267–283, 2007 [DOI] [PubMed] [Google Scholar]
- da Costa CZ, de Morais RM, Zanetta DM, Turkiewicz G, Lotufo Neto F, Morikawa M, Rodrigues CL, Labbadia EM, Asbahr FR: Comparison among clomipramine, fluoxetine, and placebo for the treatment of anxiety disorders in children and adolescents. J Child Adolesc Psychopharmacol 23:687–692, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelBello MP, Hochadel TJ, Portland KB, Azzaro AJ, Katic A, Khan A, Emslie G: A double-blind, placebo-controlled study of selegiline transdermal system in depressed adolescents. J Child Adolesc Psychopharmacol 24:311–317, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie GJ, Prakash A, Zhang Q, Pangallo BA, Bangs ME, March JS: A double-blind efficacy and safety study of duloxetine fixed doses in children and adolescents with major depressive disorder. J Child Adolesc Psychopharmacol 24:170–179, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltner D, Hill C, Lenderking W, Williams V, Morlock R: Development of a patient-reported assessment to identify placebo responders in a generalized anxiety disorder trial. J Psychiatr Res 43:1224–1230, 2009 [DOI] [PubMed] [Google Scholar]
- Ginsburg GS, Becker EM, Keeton CP, Sakolsky D, Piacentini J, Albano AM, Compton SN, Iyengar S, Sullivan K, Caporino N, Peris T, Birmaher B, Rynn M, March J, Kendall PC: Naturalistic follow-up of youths treated for pediatric anxiety disorders. JAMA Psychiatry 71:310–318, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg GS, Kendall PC, Sakolsky D, Compton SN, Piacentini J, Albano AM, Walkup JT, Sherrill J, Coffey KA, Rynn MA, Keeton CP, McCracken JT, Bergman L, Iyengar S, Birmaher B, March J: Remission after acute treatment in children and adolescents with anxiety disorders: Findings from the CAMS. J Consult Clin Psychol 79:806–813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CW, Emslie GJ, Crismon ML, Posner K, Birmaher B, Ryan N, Jensen P, Curry J, Vitiello B, Lopez M, Shon SP, Pliszka SR, Trivedi MH: Texas Children's Medication Algorithm Project: Update from Texas Consensus Conference Panel on Medication Treatment of Childhood Major Depressive Disorder. J Am Acad Child Adolesc Psychiatry 46:667–686, 2007 [DOI] [PubMed] [Google Scholar]
- Husky MM, Olfson M, He J, Nock MK, Swanson SA, Merikangas KR: Twelve-month suicidal symptoms and use of services among adolescents: Results from the National Comorbidity Survey. Psychiatr Serv 63:989–996, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovieno N, Papakostas GI: Correlation between different levels of placebo response rate and clinical trial outcome in major depressive disorder: A meta-analysis. J Clin Psychiatry 73:1300–1306, 2012 [DOI] [PubMed] [Google Scholar]
- Kennard BD, Silva SG, Mayes TL, Rohde P, Hughes JL, Vitiello B, Kratochvil CJ, Curry JF, Emslie GJ, Reinecke MA, March JS: Assessment of safety and long-term outcomes of initial treatment with Placebo in TADS. Am J Psychiatry 166:337–344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE: Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:617–627, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A, Kolts RL, Rapaport MH, Krishnan KRR, Brodhead AE, Browns WA: Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychol Med 35:743–749, 2005 [DOI] [PubMed] [Google Scholar]
- Klein RG, Koplewicz HS, Kanner A: Imipramine treatment of children with separation anxiety disorder. J Am Acad Child Adolesc Psychiatry 31:21–28, 1992 [DOI] [PubMed] [Google Scholar]
- March J, Silva S, Curry J, Wells K, Fairbank J, Burns B, Domino M, Vitiello B, Severe J, Riedal K, Goldman M, Feeny N, Findling R, Stull S, Baab S, Weller EB, Robbins M, Weller RA, Jessani N, Waslick B, Sweeney M, Dublin R, Walkup J, Ginsburg G, Kastelic E, Koo H, Kratochvil C, May D, LaGrone R, Vaughan B, Albano AM, Hirsch GS, Podniesinki E, Chu A, Reincecke M, Leventhal B, Rogers G, Jacobs R, Pathak S, Wells J, Lavanier SA, Danielyan A, Rohde P, Simons A, Grimm J, Frank S, Emslie G, Kennard B, Hughes C, Mayes TL, Rosenberg D, Benazon N, Butkus M, Bartoi M: The Treatment for Adolescents With Depression Study (TADS): Outcomes over 1 year of naturalistic follow-up. Am J Psychiatry 166:1141–1149, 2009 [DOI] [PubMed] [Google Scholar]
- March JS, Entusah AR, Rynn M, Albano AM, Tourian KA: A randomized controlled trial of venlafaxine ER versus placebo in pediatric social anxiety disorder. Biol Psychiatry 62:1149–1154, 2007 [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Fava M: Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol 19:34–40, 2009 [DOI] [PubMed] [Google Scholar]
- Pine DS, Walkup JT, Labellarte MJ, Riddle MA, Greenhill L, Klein R, Davies M, Sweeney M, Abikoff H, Hack S, Klee B, McCracken J, Bergman L, Piacentini J, March J, Compton S, Robinson J, O'Hara T, Baker S, Vitiello B, Ritz L, Roper M: Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med 344:1279–1285, 2001. 11323729 [Google Scholar]
- Rutherford B, Sneed JR, Roose SP: Does study design influence outcome? The effects of placebo control and treatment duration in antidepressant trials. Psychother Psychosom 78:172–81, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford BR, Bailey VS, Schneier FR, Pott E, Brwn PH, Roose SP: Influence of study design on treatment response in anxiety disorder clinical trials. Depress Anxiety 32:944–957, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford BR, Mori S, Sneed JR, Pimontel MA, Roose SP: Contribution of spontaneous improvement to placebo response in depression: A meta-analytic review. J Psychiatr Res 46:697–702, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford BR, Roose SP: A model of placebo response in antidepressant clinical trials. Am J Psychiatry 170:723–733, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford BR, Sneed JR, Tandler JM, Rindskopf D, Peterson BS, Roose SP: Deconstructing pediatric depression trials: An analysis of the effects of expectancy and therapeutic contact. J Am Acad Child Adolesc Psychiatry 50:782–795, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynn MA, Riddle MA, Yeung PP, Kunz NR: Efficacy and safety of extended-release venlafaxine in the treatment of generalized anxiety disorder in children and adolescents: Two placebo-controlled trials. Am J Psychiatry 164:290–300, 2007 [DOI] [PubMed] [Google Scholar]
- Rynn MA, Siqueland L, Rickels K: Placebo-controlled trial of sertraline in the treatment of children with generalized anxiety disorder. Am J Psychiatry 158:2008–2014, 2001 [DOI] [PubMed] [Google Scholar]
- Sareen J, Houlahan T, Cox BJ, Asmundson GJG: Anxiety disorders associated with suicidal ideation and suicide attempts in the National Comorbidity Survey. J Nerv Ment Dis 193:450–454, 2005 [DOI] [PubMed] [Google Scholar]
- Simeon JG, Ferguson HB, Knott V, Roberts N, Gauthier B, Dubois C, Wiggins D: Clinical, cognitive, and neurophysiological effects of alprazolam in children and adolescents with overanxious and avoidant disorders. J Am Acad Child Adolesc Psychiatry 31:29–33, 1992 [DOI] [PubMed] [Google Scholar]
- Sneed JR, Rutherford BR, Rindskopf D, Lane DT, Sackeim HA, Roose SP: Design makes a difference: A meta-analysis of antidepressant response rates in placebo-controlled versus comparator trials in late-life depression. Am J Geriatr Psychiatry 16:65–73, 2008 [DOI] [PubMed] [Google Scholar]
- Stein DJ, Baldwin DS, Dolberg OT, Despiegel N, Bandelow B: Which factors predict placebo response in anxiety disorders and major depression? An analysis of placebo-controlled studies of escitalopram. J Clin Psychiatry 67:1741–1746, 2006 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Prakash A, Zhang Q, Pangallo BA, Stroud CE, Cai N, Findling RL: A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry 54:283–293, 2015a [DOI] [PubMed] [Google Scholar]
- Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA: Efficacy and tolerability of antidepressants in pediatric anxiety disorders: A systematic review and meta-analysis. Depress Anxiety 32:149–157, 2015b [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Food and Drug Administration: Drug Study Designs - Information Sheet, 2016. Available at: http://www.fda.gov/RegulatoryInformation/Guidances/ucm126501.htm Accessed February17, 2016
- Wagner KD, Berard R, Stein MB, Wetherhold E, Carpenter DJ, Perera P, Gee M, Davy K, Machin A: A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry 61:1153–1162, 2004 [DOI] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B, Iyengar S, March JS, Kendall PC: Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 359:2753–2766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehry AM, Beesdo-Baum K, Hennelly MM, Connolly SD, Strawn JR: Assessment and treatment of anxiety disorders in children and adolescents. Curr Psychiatry Rep 17:52, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]