Abstract
Cardiovascular disease (CVD) is increasingly common among women with HIV, but literature on nonlipid CVD risk factor management is lacking. We examined semiannual trends from 2006 to 2014 in hypertension treatment and control (blood pressure <140/90 mmHg), diabetes treatment and control (fasting glucose <130 mg/dL), and smoking quit rates in the Women's Interagency HIV Study. Unadjusted and adjusted Poisson regression models tested time trends and differences between HIV+ and HIV− women. Among antiretroviral therapy (ART) users, we examined the association of ART adherence and virologic suppression with each outcome. We evaluated 1636 HIV+ and 683 HIV− women, with a hypertension prevalence of 40% and 38%, respectively; diabetes prevalence of 21% and 22%; and smoking prevalence of 37% and 48%. Hypertension treatment was higher among HIV+ than HIV− women (77% vs. 67%, p < 0.001) and increased over time with no difference in trend by HIV status. Hypertension control was greater among HIV+ women (56% vs. 43%, p < 0.001) and increased over time among HIV+ but not HIV− women. Diabetes treatment was similar among HIV+ and HIV− women (48% vs. 49%) and increased over time in both groups. Diabetes control was greater among HIV+ women (73% vs. 64%, p = 0.03) and did not change over time. The percent of recent smokers who reported no longer smoking was similar between HIV+ and HIV− women (10% vs. 9%), with no differences over time. Virologic suppression was significantly associated with increased hypertension treatment and greater control. HIV+ women have better control of hypertension and diabetes than HIV− women, but many are still not at target levels.
Keywords: : cardiovascular disease, antiretroviral therapy, hypertension, diabetes mellitus, smoking, HIV-1 viral load
Introduction
Numerous reports have found that women living with HIV infection have greater cardiovascular disease (CVD) risk than HIV-uninfected women.1–3 Factors contributing to this increased risk include a high prevalence of traditional CVD risk factors such as cigarette smoking,4 potential adverse effects of antiretroviral therapy (ART),5 and immune activation and inflammation that may be present even with well-controlled HIV virema.6 Women with HIV may also have factors that impede timely access to care and use of preventive measures to minimize CVD risk,7,8 including lower socioeconomic status and higher depression rates.9
Effective management of CVD risk factors, such as the use of statins, controlling blood pressure, and smoking cessation, reduces the future risk of disease in the general population,10–12 and successful interventions likely apply to people with HIV as well.13 A major clinical trial assessing the effect of statin use on CVD outcomes among HIV-infected individuals is underway.14 However, less is known about nonlipid CVD prevention strategies and healthcare factors that may aid or impede their use in people with HIV, particularly women. Medication adherence is associated with behaviors consistent with a healthy lifestyle.15 In people with HIV, self-motivation, social support, and intentionality have been found to be important drivers of adherence.16,17 In turn, better ART adherence and subsequent virologic suppression may correlate with improved CVD risk factor management. As people living with HIV age and CVD risk factors increase,18 understanding these factors better can inform strategies recommended by providers in the course of HIV care.
We aimed to describe the degree of effective management of three major nonlipid CVD risk factors—hypertension, diabetes mellitus, and smoking—including use of medications and achieving target levels of clinical parameters, among HIV-infected women participating in the Women's Interagency HIV Study (WIHS) between 2006 and 2014, and compare time trends in these measures with those of at-risk HIV-uninfected women from the same cohort. We also assessed the association of ART adherence and virologic suppression with CVD risk factor treatment and control among HIV-infected women taking ART, hypothesizing that women with better ART outcomes were more likely to effectively manage CVD risk.
Methods
Source population
The WIHS is a longitudinal study of >4000 HIV-infected and HIV-uninfected women followed at 6-month intervals at 10 US sites, with detailed examinations, specimen collection, and structured interviews assessing health behaviors, medical history, and medication use.19,20 Women were recruited in four waves (1994–1995, 2001–2002, 2010–2012, and 2013–2015) from HIV primary care clinics, hospital-based programs, community outreach sites, women's support groups, and other locations. The demographic composition of study participants in the WIHS is representative of women in the United States living with HIV infection.21
Study population and inclusion criteria
We created three subcohorts within the WIHS of women at risk for CVD, each focusing on one of the following risk factors: hypertension, diabetes mellitus, and recent smoking. Inclusion criteria included enrollment in the WIHS before 2006 and at least one study visit between April 2006 and March 2014, during which one of these factors was reported. The hypertension subcohort comprised all person-visits within the study period associated with systolic blood pressure (SBP) ≥140 mmHg; diastolic blood pressure (DBP) ≥90 mmHg; current or prior self-report of hypertension; or current or prior self-reported use of antihypertension medications. The diabetes subcohort comprised person-visits associated with fasting blood glucose ≥126 mg/dL; hemoglobin A1c ≥6.5%; current or prior self-reported “history of high blood sugar, diabetes or sugar diabetes”; or current or prior self-reported use of antidiabetic medication. The recent smoker subcohort comprised person-visits for which the participant reported being a current smoker at the previous visit. Women could contribute >1 person-visit to each subcohort, with a maximum of 16 person-visits at risk. Because the WIHS visits occur every 6 months, this represents a maximum of 8 years at risk.
To assess the association of ART adherence and virologic suppression with effective management of each CVD risk factor, we instituted additional inclusion criteria: confirmed HIV infection before 2006 and reported use of ART at the previous study visit.
Outcomes of interest
We defined separate outcomes for each subcohort, using clinical guidelines for hypertension and diabetes relevant during the study period to define effective management. For the hypertensive subcohort, outcomes were (1) hypertensive treatment, defined as reported use of antihypertension medications within the past 6 months, and (2) hypertension control, defined as SBP <140 mmHg or DBP <90 mmHg.22 For the diabetic subcohort, outcomes were (1) diabetes treatment, defined as reported use of antidiabetic medications with the past 6 months, and (2) diabetes control, defined as a fasting glucose level <130 mg/dL.23 Fasting glucose was assessed annually instead of semiannually, and therefore, diabetes control was evaluated at 8 instead of 16 total person-visits. Because hemoglobin A1c (HgA1c), which is the usual recommended diabetes control measure since it captures longer term glycemic control,23 was only assessed at four person-visits, we conducted a secondary analysis based on these four visits, defining diabetes control as HgA1c <7%.23 For the recent smoker subcohort, the outcome of interest was smoking control, defined as reporting no smoking at the current visit. We did not include a measure of smoking treatment because the WIHS did not systematically collect data on treatments such as nicotine replacement therapy or varenicline during the study period.
Independent variables
For the examination of trends in effective CVD risk factor management over time, we were interested in comparing those with versus without HIV infection. For assessment of ART adherence and virologic suppression in relation to effective CVD risk factor management, adherence was assessed by asking the participant the percentage of time during the past 6 months that ART was taken as prescribed, based on a validated question from the AIDS Clinical Trials Group,24 categorized as 100% of the time, 95–99%, 75–94%, and <75%. We dichotomized the response to ≥95% adherence versus <95%, based on prior work that has found this level of adherence to protease inhibitor-based regimens to optimize virologic outcomes.25 We also examined an alternate definition of 100% adherence versus <100%.26 Virologic suppression was defined as an HIV-1 viral load <80 copies/mL.
Covariates
We considered the following variables as potential confounders of the association of ART adherence and virologic suppression with effective CVD risk factor management: age, race/ethnicity, calendar time, income, education, employment, insurance status, study site, CD4+ count, viral load, history of ART use, number of children, birth of child since last visit, current and past recreational drug use, current and past alcohol use, housing status, severe depressive symptoms (score ≥23) as assessed by the Center for Epidemiologic Studies Depression Scale (CES-D),27 and recruitment period.
Statistical methods
We examined time trends for each CVD risk factor management outcome based on each 6-month period between April 2006 and March 2014. At each person-visit, the numerator was the number of women with CVD risk factor management (e.g., number of women with fasting glucose level <130 mg/dL), and the denominator was the number of women at risk (e.g., number of women with diabetes). Using Poisson regression with generalized estimating equations (GEE), we tested for overall differences by HIV status and time trends for HIV-infected and HIV-uninfected women separately. We tested for differences over time by HIV status using a time*HIV status interaction term. We also examined the associations of the aforementioned covariates with each outcome in adjusted models. To further explore potential reasons for differences in CVD risk factor management by HIV serostatus, we conducted a post-hoc analysis in a subgroup of four person-visits with information on whether the participant reported having a usual source of medical care, defined as seeing “the same healthcare provider or group of providers for … medical appointments” more than half of the time in the past 6 months. Our exploratory hypothesis was that associations between HIV serostatus and CVD risk factor management would be attenuated after controlling for having a usual source of care.
To assess the association between ART adherence and CVD risk factor management outcomes, we compared outcomes between adherent and nonadherent person-visits after propensity score matching.28 We estimated the propensity score as the predicted probability of being ≥95% adherent to ART, given the aforementioned confounders, by logistic regression. Using the propensity score, we matched each adherent person-visit with a similar nonadherent person-visit, to eliminate the association between the confounding factors and ART adherence, and allowed nonadherent person-visits to be used more than once (“matching with replacement”), because most person-visits were ART adherent. We used log-binomial regression to estimate risk ratios (RRs) for each CVD risk factor management outcome at the visit following the index person-visit (i.e., 6 months after) to maintain temporality. Log-binomial regression was used instead of logistic regression to estimate the relative risk, since odds ratios overestimate the risk when the outcome is common.29 We performed similar analyses for 100% ART adherence and virologic suppression. Analyses used GEE to account for correlated data within individuals, were weighted to account for matching with replacement, and used a sandwich estimator to determine robust 95% confidence intervals (CIs).30
Fewer than 1% of participants were pregnant during study visits. We performed sensitivity analyses excluding these women. Because results were essentially the same with this exclusion criterion, we report results without this exclusion. SAS 9.3 and R 2.15.2, including the MatchIt package for propensity score matching,31 were used for analysis.
Results
Between 2006 and 2014, there were 1636 HIV+ and 683 HIV− women in the WIHS evaluated for hypertension, diabetes, or smoking status. Over the study period, we estimated a hypertension prevalence of 40% and 38% among HIV+ and HIV− women, respectively; diabetes prevalence of 21% and 22%; and recent smoking prevalence of 37% and 48%.
There were 10,546 eligible person-visits (7558 among 1039 HIV+ women, 2988 among 405 HIV− women) contributing to the hypertension subcohort; 6394 (4449 among 332 HIV+ women, 1945 among 142 HIV− women) contributing to the diabetes subcohort; and 11,258 (7291 among 806 HIV+ women, 3967 among 400 HIV− women) contributing to the smoking subcohort. Follow-up was similar between HIV+ and HIV− women. Both HIV+ and HIV− hypertensive women had a median of 7 study visits (3.5 years) during the study period. Diabetic women had a median of 8 visits for HIV+ and 7.5 visits for HIV−, while recent smokers had a median of 10 visits for HIV+ and 11 visits for HIV−.
The median age at each study visit ranged from 46 to 51 years, and black or Hispanic women comprised over 80% of each subcohort (Table 1). Over one-third had less than a high school education. Between 31% and 40% of eligible person-visits were associated with a history of injection drug use. Among HIV+ women, the median CD4+ count ranged from 465 to 540 cells/μL, and 79–83% of person-visits were associated with ART use depending on the subcohort examined. Between 87% and 94% of HIV+ visits were associated with a usual source of medical care, depending on the subcohort, compared with 59–73% of HIV− visits.
Table 1.
Women's Interagency HIV Study Subcohort Characteristics, By Subcohort and HIV Serostatus, 2006–2014
| Hypertensive participants | Diabetic participants | Recent smokers | ||||
|---|---|---|---|---|---|---|
| HIV+, % or median (IQR) | HIV−, % or median (IQR) | HIV+, % or median (IQR) | HIV−, % or median (IQR) | HIV+, % or median (IQR) | HIV−, % or median (IQR) | |
| n women | 1039 | 405 | 332 | 142 | 806 | 400 |
| n person-visits | 7558 | 2988 | 4449 | 1945 | 7347 | 3977 |
| Demographic characteristics | ||||||
| Age (median, IQR) | 51 (45–56) | 50 (44–55) | 50 (45–55) | 49 (43–55) | 48 (42–53) | 46 (38–52) |
| Race/ethnicity | ||||||
| Black (non-Hispanic) | 68 | 69 | 56 | 61 | 65 | 64 |
| Hispanic | 19 | 22 | 27 | 28 | 22 | 24 |
| White (non-Hispanic) | 10 | 6 | 13 | 6 | 11 | 9 |
| Other | 3 | 4 | 4 | 5 | 3 | 4 |
| Current income <$24,000/year | 76 | 77 | 78 | 76 | 83 | 80 |
| Education | ||||||
| Did not complete high school | 36 | 37 | 37 | 41 | 45 | 43 |
| Completed high school | 31 | 37 | 29 | 36 | 31 | 31 |
| At least some college | 34 | 26 | 34 | 24 | 24 | 25 |
| Employed | 30 | 34 | 27 | 30 | 22 | 34 |
| Had insurance | 97 | 84 | 97 | 85 | 96 | 79 |
| Behavior-related characteristics | ||||||
| History of injection drug use | 36 | 31 | 35 | 34 | 40 | 32 |
| Current recreational drug use | 20 | 28 | 16 | 21 | 31 | 40 |
| Current alcohol use | ||||||
| Abstainer | 64 | 52 | 66 | 57 | 55 | 45 |
| Light (<3 drinks/week) | 27 | 29 | 26 | 29 | 30 | 31 |
| Moderate (3–13 drinks/week) | 4 | 6 | 3 | 4 | 5 | 8 |
| Heavier (14+ drinks/week) | 6 | 14 | 5 | 10 | 10 | 16 |
| Stable housing | 89 | 77 | 91 | 78 | 83 | 73 |
| Severe depressive symptoms (CES-D ≥23) | 19 | 20 | 21 | 21 | 24 | 22 |
| Number of children (median, IQR) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 2 (1–4) |
| HIV-related and other clinical characteristics | ||||||
| CD4+ count, cells/μL (median, IQR) | 512 (319–733) | 540 (349–768) | 465 (265–688) | |||
| Suppressed HIV-1 viral load | 60 | 61 | 49 | |||
| ART use in past 6 months | 83 | 84 | 80 | |||
| ART adherence in past 6 months (among ART users) | ||||||
| 100% of the time | 49 | 53 | 47 | |||
| 95–99% of the time | 31 | 29 | 27 | |||
| 75–94% of the time | 15 | 13 | 17 | |||
| <75% of the time | 6 | 5 | 8 | |||
| Has usual source of care | 92 | 71 | 94 | 73 | 87 | 59 |
ART, antiretroviral therapy; CES-D, Center for Epidemiologic Studies Depression Scale; IQR, interquartile range.
Trends in CVD risk factor management, by HIV serostatus
Hypertensive subcohort
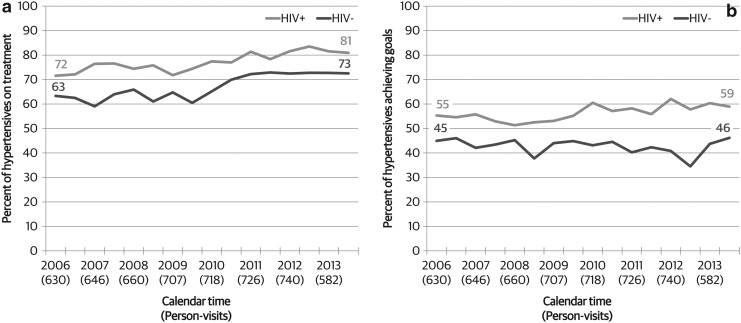
Overall, we found that antihypertensive treatment was significantly higher among HIV-infected hypertensive women than among HIV-uninfected hypertensive women (77% vs. 67%, p < 0.001, Fig. 1a). The use of antihypertension medications increased from 72% in 2006 to 81% in 2014 among HIV-infected women, and from 63% to 73% among HIV-uninfected women (both p < 0.001). There was no difference in change over time by HIV status (p = 0.71).
FIG. 1.
Trends in (a) hypertension treatment and (b) hypertension control among hypertensive WIHS participants, by HIV serostatus, 2006–2014. Hypertension control defined as systolic blood pressure <140 mmHg or diastolic blood pressure <90 mmHg. WIHS, Women's Interagency HIV Study.
Similarly, hypertension control (BP <140/90 mmHg) was higher among HIV-infected women than among HIV-uninfected women (56% vs. 43%, p < 0.001, Fig. 1b). Hypertension control increased slightly over time among HIV-infected women (55% in 2006 to 59% in 2014, p = 0.01) but not among HIV-uninfected women (45%–46%). This difference was also reflected in a significant interaction between HIV status and time for hypertension control (p = 0.048).
Diabetic subcohort
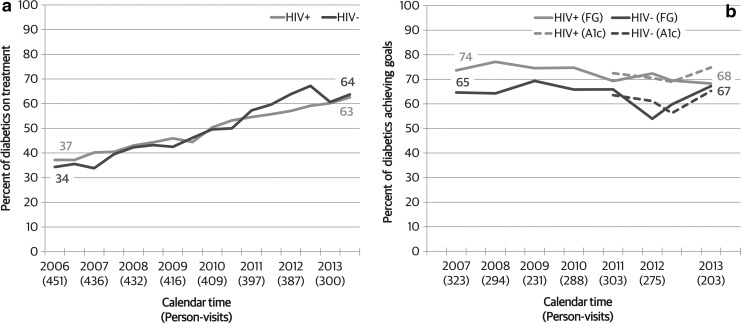
There was no difference overall in diabetes treatment comparing HIV-infected and HIV-uninfected diabetic women (48% vs. 49%, p = 0.99, Fig. 2a). Among both groups defined by HIV serostatus, use of antidiabetes medications increased over time, from 37% in 2006 to 63% among HIV-infected women, and from 34% to 64% among HIV-uninfected women (both p < 0.001). These temporal increases did not differ by HIV serostatus (p = 0.38).
FIG. 2.
Trends in (a) diabetes treatment and (b) diabetes control among diabetic WIHS participants, by HIV serostatus, 2006–2014. Diabetes control defined as fasting glucose <130 mg/dL or hemoglobin A1c <7%. WIHS, Women's Interagency HIV Study.
HIV-infected diabetic women were more likely to have fasting glucose levels within recommended levels (<130 mg/dL) compared with HIV-uninfected diabetic women (73% vs. 64%, p = 0.01, Fig. 2b). Despite increases observed in the use of diabetes medications, diabetes control did not increase over the same time period in either group (p = 0.16 among HIV-infected and p = 0.50 among HIV-uninfected). Similarly, there was no difference in change over time by HIV serostatus (p = 0.98). Patterns of diabetic control were similar when examining the subset of women with HgA1c levels available, with no significant increase over time in control defined as HgA1c <7% (p = 0.10 among HIV-infected and p = 0.27 among HIV-uninfected).
Smoking subcohort
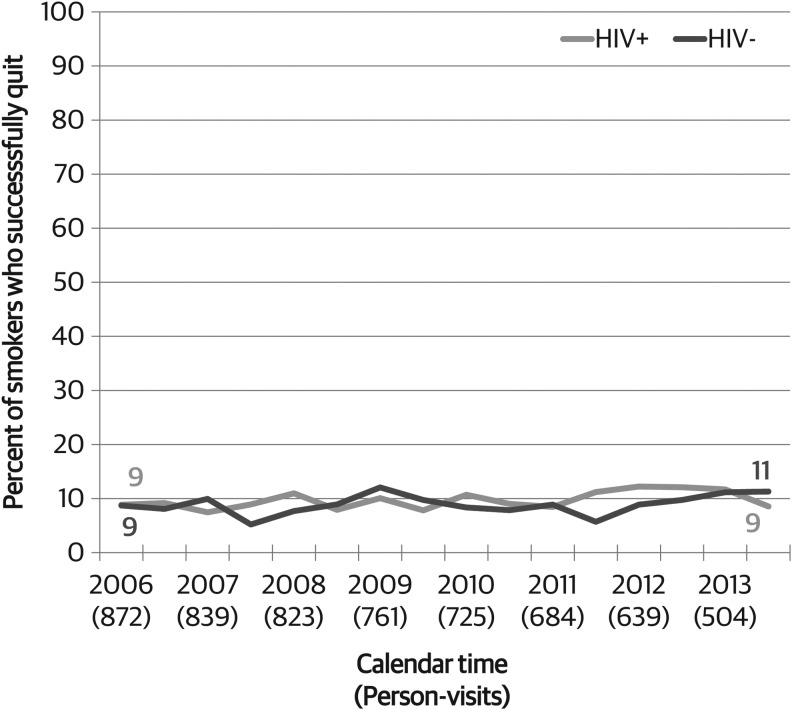
HIV-infected and HIV-uninfected women had similar smoking quit rates over the time period. Among those smoking at a given visit, the proportion who reported that they were no longer smoking at the next semiannual visit was 10% among HIV+ versus 9% among HIV− women (p = 0.33, Fig. 3). Among HIV-infected women who had reported current smoking at the previous visit, the percent who quit marginally increased from 9% in 2006 to 12% in 2014 (p = 0.08), whereas there was no change among HIV-uninfected women (p = 0.39). There was no difference in changes in quit rates over time by HIV serostatus (p = 0.67).
FIG. 3.
Trends in smoking cessation among recent current smokers in the WIHS, by HIV serostatus, 2006–2014. WIHS, Women's Interagency HIV Study.
Table 2 shows a summary of the findings regarding CVD risk factor management in this study. The significant differences that we observed by HIV serostatus in hypertension treatment, hypertension control, and diabetes control remained when models were adjusted for confounders. Of note, older age was significantly associated with improved hypertension treatment but less smoking cessation. Having insurance was significantly associated with improved hypertension treatment and control. Current recreational drug use was associated with poorer hypertension control. Both current recreational drug use and current alcohol use were associated with less smoking cessation. Other associations are shown in Table 3.
Table 2.
Summary of Findings for Nonlipid Cardiovascular Disease (CVD) Risk Factor Management Based on HIV Serostatus Among Women's Interagency HIV Study (WIHS) Participants, 2006–2014
| More favorable among HIV+ versus HIV− | Increase over time among HIV+ | Increase over time among HIV− | Difference in trend between HIV+ and HIV− | |
|---|---|---|---|---|
| Hypertension treatment | Yes | Yes | Yes | No |
| Hypertension control | Yes | Yes | No | Yes |
| Diabetes treatment | No | Yes | Yes | No |
| Diabetes control | Yes | No | No | No |
| Smoking cessation | No | No | No | No |
Table 3.
Association of HIV Serostatus and Demographic and Behavioral Characteristics with Nonlipid Cardiovascular Disease (CVD) Risk Factor Management, Women's Interagency HIV Study (WIHS) Participants, 2006–2014
| CVD risk factor management outcome | |||||
|---|---|---|---|---|---|
| Hypertension treatment, RR (95% CI) | Hypertension control, RR (95% CI) | Diabetes treatment, RR (95% CI) | Diabetes control, RR (95% CI) | Smoking cessation, RR (95% CI) | |
| HIV serostatus (vs. uninfected) | 1.10 (1.03–1.17) | 1.26 (1.14–1.39) | 0.98 (0.84–1.16) | 1.15 (1.03–1.28) | 1.08 (0.89–1.30) |
| Age (per 10 years) | 1.20 (1.16–1.23) | 1.04 (0.998–1.09) | 1.08 (0.99–1.18) | 1.04 (0.99–1.10) | 0.86 (0.79–0.95) |
| Race/ethnicity (vs. white) | |||||
| Black race | 1.09 (0.999–1.20) | 0.87 (0.79–0.97) | 1.27 (0.97–1.65) | 0.94 (0.83–1.07) | 1.01 (0.79–1.30) |
| Hispanic ethnicity | 0.98 (0.88–1.10) | 0.95 (0.83–1.08) | 1.26 (0.95–1.67) | 0.84 (0.73–0.98) | 1.04 (0.78–1.39) |
| Income >$24,000/year (vs. <$24,000/year) | 1.00 (0.96–1.04) | 0.98 (0.92–1.04) | 0.99 (0.93–1.05) | 1.02 (0.93–1.12) | 0.87 (0.73–1.03) |
| Education (vs. did not complete high school) | |||||
| Completed high school | 1.03 (0.97–1.10) | 1.04 (0.95–1.04) | 1.11 (0.93–1.31) | 1.08 (0.97–1.21) | 1.01 (0.83–1.24) |
| At least some college | 1.04 (0.98–1.12) | 1.05 (0.96–1.16) | 1.05 (0.87–1.26) | 1.03 (0.91–1.15) | 1.52 (1.23–1.87) |
| Employed (vs. unemployed) | 0.96 (0.92–0.995) | 0.96 (0.90–1.02) | 0.98 (0.92–1.05) | 0.99 (0.91–1.07) | 1.06 (0.90–1.24) |
| Insured (vs. not insured) | 1.17 (1.09–1.26) | 1.13 (1.01–1.27) | 0.97 (0.87–1.08) | 1.11 (0.97–1.26) | 0.84 (0.66–1.06) |
| Number of children (per child) | 1.01 (0.99–1.02) | 1.00 (0.98–1.02) | 1.04 (1.002–1.08) | 0.97 (0.95–0.997) | 0.96 (0.92–1.01) |
| Current recreational drug use (vs. not current) | 0.97 (0.93–1.01) | 0.89 (0.83–0.96) | 0.97 (0.90–1.04) | 1.11 (1.01–1.21) | 0.70 (0.58–0.84) |
| Ever recreational drug use (vs. never) | 1.01 (0.94–1.09) | 1.03 (0.94–1.13) | 0.95 (0.80–1.13) | 0.97 (0.86–1.10) | 0.71 (0.53–0.94) |
| Current alcohol use (vs. abstainer) | |||||
| <7 drinks/week | 1.01 (0.98–1.04) | 0.99 (0.93–1.05) | 0.96 (0.90–1.01) | 1.01 (0.94–1.10) | 0.79 (0.67–0.94) |
| 8–12 drinks/week | 1.01 (0.95–1.07) | 0.98 (0.87–1.09) | 0.96 (0.87–1.06) | 1.10 (0.93–1.30) | 0.77 (0.58–1.01) |
| >12 drinks/week | 1.00 (0.94–1.05) | 0.91 (0.81–1.02) | 0.96 (0.87–1.06) | 1.04 (0.91–1.18) | 0.52 (0.39–0.69) |
| Ever alcohol use (vs. never) | 0.96 (0.91–1.01) | 0.92 (0.85–0.99) | 0.94 (0.84–1.04) | 0.97 (0.87–1.07) | 1.18 (0.97–1.44) |
| Unstable housing (vs. stable) | 1.00 (0.96–1.04) | 1.03 (0.96–1.09) | 0.98 (0.93–1.03) | 0.98 (0.89–1.08) | 1.20 (0.99–1.44) |
| Severe depressive symptoms (CES-D ≥23) vs. CES-D <23 | 1.00 (0.96–1.03) | 1.04 (0.99–1.09) | 0.99 (0.94–1.04) | 1.01 (0.94–1.08) | 0.91 (0.78–1.06) |
Models include all factors presented in table, as well as calendar time and study site. Bold represents statistically significant associations (p < 0.05).
CES-D, Center for Epidemiologic Studies Depression Scale; CI, confidence interval; RR, risk ratio.
In exploratory analyses, we found that having a usual source of care appeared to partially mediate the associations of HIV serostatus with hypertension treatment and hypertension control, but not diabetes control. For example, after controlling for having a usual source of care, the association between HIV serostatus and hypertension control was reduced but remained statistically significant (p = 0.02).
Association of ART adherence and virologic suppression with CVD risk factor management
Within each subcohort, we examined a subset of women using ART to determine whether ART adherence and HIV virologic suppression were associated with improved CVD risk factor management. Table 4 shows the percentages of CVD risk factor management outcomes comparing ART adherent person-visits with propensity score-matched ART nonadherent person-visits. For each outcome, the ART adherent group was more likely to be managed for CVD risk factors than the ART nonadherent group. In weighted regression analyses, ≥95% ART adherence was marginally associated with improvements in diabetes control (RR 1.24, 95% CI 0.99–1.54, p = 0.06). Neither ≥95% adherence nor 100% adherence was associated with improvements in other CVD risk factor management outcomes.
Table 4.
Association of Antiretroviral Therapy (ART) Adherence and Virologic Suppression with Nonlipid Cardiovascular Disease (CVD) Risk Factor Management, Women's Interagency HIV Study (WIHS) Participants, 2006–2014
| CVD risk factor management outcome, as related to ART adherence (95% of time) | n person-visits (n women), adherent | % with outcome, adherent | n person-visits (n women), not adherent | % with outcome, not adherent | Rate ratio for adherence (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Hypertension treatment | 4193 (783) | 78 | 817 (349) | 74 | 1.03 (0.95–1.11) | 0.47 |
| Hypertension control | 4193 (783) | 69 | 817 (349) | 65 | 0.99 (0.92–1.07) | 0.82 |
| Diabetes treatment | 2593 (294) | 51 | 473 (165) | 51 | 0.98 (0.87–1.12) | 0.78 |
| Diabetes control | 1084 (254) | 75 | 193 (105) | 70 | 1.24 (0.99–1.54) | 0.06 |
| Smoking cessation | 3628 (619) | 10 | 914 (353) | 8 | 1.11 (0.87–1.42) | 0.40 |
| CVD risk factor management outcome, as related to virologic suppression | n person-visits (n women), suppressed | % with outcome, suppressed | n person-visits (n women), not suppressed | % with outcome, not suppressed | Rate ratio for suppression (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Hypertension treatment | 2654 (638) | 80 | 855 (413) | 73 | 1.07 (1.01–1.13) | 0.03 |
| Hypertension control | 2654 (638) | 72 | 855 (413) | 62 | 1.13 (1.04–1.22) | 0.003 |
| Diabetes treatment | 1506 (254) | 58 | 472 (192) | 50 | 1.08 (0.97–1.21) | 0.16 |
| Diabetes control | 627 (206) | 74 | 197 (126) | 64 | 1.11 (0.95–1.28) | 0.18 |
| Smoking cessation | 1929 (449) | 10 | 777 (366) | 9 | 1.06 (0.76–1.48) | 0.72 |
Exposed and unexposed person-visits after on 1:1 propensity score matching with replacement, accounting for age at visit, race/ethnicity, year of visit, current income, education, current employment, current insurance status, study site, CD4+ count, viral load, history of ART, number of children, change in number of children since last visit, history of recreational drug use, current recreational drug use, history of alcohol use, current alcohol use, current stable housing, depressive symptoms. Rate ratios determined by weighted log-binomial regression with robust variance.
CI, confidence interval.
We repeated these analyses examining the role of virologic suppression instead of ART adherence on CVD risk factor management. Similarly, person-visits associated with virologic suppression exhibited greater CVD risk factor treatment and control compared with matched unsuppressed person-visits (Table 4). In regression analyses, virologic suppression was most associated with improved hypertension treatment and hypertension control at the following visit. Virologic suppression among hypertensive person-visits was significantly associated with a 7% increase in the use of antihypertensive medications (RR 1.07, 95% CI 1.01–1.13, p = 0.03) and a 13% increase in hypertension control (RR 1.13, 95% CI 1.04–1.22, p = 0.003). We did not detect significant improvement in the other CVD risk factor management outcomes examined.
Discussion
Over 9 years, we found that hypertension and diabetes mellitus were better managed among HIV-infected women than HIV-uninfected women from the same socioeconomically disadvantaged communities. Better hypertension management may have stemmed at least partially from HIV-infected women being more likely to have a routine medical provider. Nonetheless, a high proportion of HIV-infected women were not optimally managed with respect to hypertension, diabetes, and smoking, suggesting the need for greater attention to these major CVD risk factors. While use of therapies to treat hypertension and diabetes increased over the time period, only small improvements in blood pressure control among hypertensive women were observed over the time period, and no concurrent improvement in glycemic control was observed in diabetic HIV-infected women. The percent of smokers who reported no longer smoking at the following visit was consistently about 10% among HIV-infected women over the 9-year period, with no improvements over time. Virologic suppression was associated with better management of hypertension, suggesting that there may be downstream benefits of consistent ART use beyond its effects on HIV virologic control.
Hypertension was relatively common in our middle-aged population, with a prevalence of about 40% among HIV-infected women between 2007 and 2014. This is substantially higher than in the early 2000s, when hypertension prevalence was first reported in the WIHS as 26% in HIV-infected women.32 The current study demonstrated suboptimal control of hypertension (56%) among HIV-infected women, despite over three-quarters of hypertensive women reporting use of antihypertensive therapy. In comparison, the general US female population has reported levels of hypertension control that increased from 51% in 2007–2008 to 56% in 2011–2012.33 We speculate that poorer levels of hypertension treatment and control that we observed among HIV-uninfected women, compared with the general population, may be due to the lower socioeconomic status of the WIHS women, which could affect access to and adherence with treatment,34 although we could not test this hypothesis. Nonetheless, the findings collectively imply that better control of hypertension is needed among all women, regardless of HIV status.
We found that having a usual source of care may explain some of the association between HIV status and hypertension control, and that among HIV-infected women, virologic suppression was associated with an improvement in hypertension treatment and control. In the general population, having a usual source of care is associated with better hypertension control35 and this relationship may be particularly strong for people with HIV, who are recommended to have HIV viral load monitoring at least every 6 months.13 In addition, the availability of similar modalities to control HIV infection and hypertension, such as single daily pill formulations, may facilitate their joint control. In contrast, diabetes management often requires more intensive action, such as self-monitoring of blood glucose and intensive insulin regimens, and smoking cessation requires behavior change. Thus, HIV-infected women may be uniquely positioned to be able to manage hypertension, given their greater likelihood of being in medical care and having comparable tasks that can be used to treat both conditions.
We found that while diabetic HIV-infected women were more likely to have glycemic control than uninfected women, this did not improve over the time period despite an increasing proportion of women on antidiabetes medication. Compared with the US diabetic population, among whom about 88% were treated between 2005 and 2010,36 the WIHS women were less likely to be on antidiabetes medication, although this gap narrowed over the period. Yet, HIV-infected women in the WIHS were more likely to have diabetic control than the general population (73% during the study, vs. 60% in 2009–2010), potentially reflecting the increased level of care that HIV-infected individuals receive.
Smoking quit rates were consistently low over the time period, with about 10% of women during each 6-month interval reporting that they had recently quit. We did not have information on the methods used to attempt smoking cessation, but the fact that quit rates were similar by HIV serostatus, in contrast to hypertension and diabetes control, suggests that there may be missed opportunities for improved smoking control among HIV-infected women given their greater use of medical care. While we did not find an association of ART adherence or virologic suppression with smoking quit rates, another WIHS study found that ART use was associated with less smoking relapse among women with sustained cessation.37 Our definition of smoking control in the current study did not take into account long-term cessation, and almost half of the women who recently quit smoking reported a smoking relapse 6 months later (data not shown). Given the substantial health benefits associated with quitting smoking, targeted interventions for HIV-infected women, potentially integrated with other behavioral interventions addressing substance use,38 should be considered to improve cessation.
Our study has several strengths. In contrast to clinic-based cohorts that collect data through routine care, the WIHS is an interval-based cohort, meaning that visits occur independently of clinical care and therefore capture behaviors (e.g., ART adherence) every 6 months that may be less likely to be reported to primary care providers. Detailed longitudinal data allowed us to report trends at a time when CVD risk factors are increasingly common in the HIV population. Over three-quarters of WIHS participants identify as black or Hispanic/Latina, which represents a similar demographic profile to women with HIV in the United States.39 Finally, we used modern statistical techniques to account for potential confounding when assessing the association of ART outcomes with CVD risk factor management. Propensity score matching minimizes potential bias from known confounders, but it does not account for unmeasured confounders.
Limitations include a lack of complete information on treatments for smoking such as pharmacological quit aids that could influence smoking cessation. Also, our study did not examine nonpharmacological lifestyle interventions such as those related to dietary patterns or physical activity.40 We also did not focus on weight management, since the relationship between HIV infection and adiposity is complex41; for example, higher body mass index may be associated with long-term advantages in immune recovery.42 Nonetheless, lifestyle factors and social determinants related to body composition greatly influence CVD prevention,43 and future studies should focus on these areas. Our measure of ART adherence relies on self-report based on 6-month recall; while self-report has been shown to have validity comparable to more costly monitoring systems such as medication event monitoring systems or unannounced pill counts,44,45 it may capture behaviors only in a broad sense. We could not define for all participants the severity of hypertension or diabetes before treatment, an important potential influence on the likelihood of controlling these risk factors, which possibly may have differed by HIV serostatus. Finally, we acknowledge that control of CVD risk factors may result from actions on the part of the treating physician (e.g., diagnosis; recommendations for behavior change and treatment), the patient (e.g., compliance with physician recommendations, medication adherence), or both, but we could not distinguish between these pathways. For example, we did not collect data on adherence to hypertension medications, which if suboptimal would help to explain why some individuals are not controlled despite being treated.
In summary, we found that hypertension and diabetes were more effectively managed among HIV-infected women than HIV-uninfected women from the same risk population, likely due, in part, to greater contact with the healthcare system. In particular, better hypertension control was observed among virologically suppressed women. Nonetheless, more than 40% of hypertensive and 25% of diabetic HIV-infected women still did not achieve target control levels, and smoking quit rates have not changed in 9 years, suggesting that CVD risk factor management remains far from optimal. HIV care providers are uniquely positioned to teach their patients about preventive strategies to reduce CVD risk and have the opportunity to intervene, given the increasing age-related risk for metabolic events among women with HIV.
Acknowledgments
Data in this article were collected by the WIHS. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). Sources of support: WIHS (Principal Investigators): Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Mary Young and Seble Kassaye), U01-AI-034994; Connie Wofsy Women's HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I–WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women's Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr 2005;39:44–54 [DOI] [PubMed] [Google Scholar]
- 2.Kaplan RC, Kingsley LA, Sharrett AR, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis 2007;45:1074–1081 [DOI] [PubMed] [Google Scholar]
- 3.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: Cross-sectional surveys. Ann Intern Med 2015;162:335–344 [DOI] [PubMed] [Google Scholar]
- 5.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med 2007;356:1723–1735 [DOI] [PubMed] [Google Scholar]
- 6.Stein JH, Hsue PY. Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA 2012;308:405–406 [DOI] [PubMed] [Google Scholar]
- 7.Aziz M, Smith KY. Challenges and successes in linking HIV-infected women to care in the United States. Clin Infect Dis 2011;52 Suppl 2:S231–S237 [DOI] [PubMed] [Google Scholar]
- 8.Hatleberg CI, Ryom L, El-Sadr W, et al. Gender differences in HIV-positive persons in use of cardiovascular disease-related interventions: D:A:D study. J Int AIDS Soc 2014;17(4 Suppl 3):19516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do AN, Rosenberg ES, Sullivan PS, et al. Excess burden of depression among HIV-infected persons receiving medical care in the United States: Data from the medical monitoring project and the behavioral risk factor surveillance system. PLoS One 2014;9:e92842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2935–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S1–S45 [DOI] [PubMed] [Google Scholar]
- 12.Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med 2008;35:158–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014;58:1–10 [DOI] [PubMed] [Google Scholar]
- 14.Gilbert JM, Fitch KV, Grinspoon SK. HIV-related cardiovascular disease, statins, and the REPRIEVE trial. Top Antivir Med 2015;23:146–149 [PMC free article] [PubMed] [Google Scholar]
- 15.Brookhart MA, Patrick AR, Dormuth C, et al. Adherence to lipid-lowering therapy and the use of preventive health services: An investigation of the healthy user effect. Am J Epidemiol 2007;166:348–354 [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, McDonald S, Kim S, Foster C, Fidler S. Importance of self-motivation and social support in medication adherence in HIV-infected adolescents in the United Kingdom and Ireland: A multicentre HYPNet study. AIDS Patient Care STDS 2015;29:354–364 [DOI] [PubMed] [Google Scholar]
- 17.Muessig KE, Panter AT, Mouw MS, et al. Medication-taking practices of patients on antiretroviral HIV therapy: Control, power, and intentionality. AIDS Patient Care STDS 2015;29:606–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks JT, Buchacz K, Gebo KA, Mermin J. HIV infection and older Americans: The public health perspective. Am J Public Health 2012;102:1516–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. Epidemiology 1998;9:117–125 [PubMed] [Google Scholar]
- 20.Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005;12:1013–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC. HIV among women. 2011. Available at: www.cdc.gov/hiv/topics/women/index.htm (Last accessed October15, 2012)
- 22.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–1252 [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association. 5. Glycemic targets. Diabetes Care 2016;39 Suppl 1:S39–S46 [DOI] [PubMed] [Google Scholar]
- 24.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. AIDS Care 2000;12:255–266 [DOI] [PubMed] [Google Scholar]
- 25.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133:21–30 [DOI] [PubMed] [Google Scholar]
- 26.Saberi P, Johnson MO, McCulloch CE, Vittinghoff E, Neilands TB. Medication adherence: Tailoring the analysis to the data. AIDS Behav 2011;15:1447–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 28.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 2006;98:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706 [DOI] [PubMed] [Google Scholar]
- 30.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: An orientation. Am J Epidemiol 2003;157:364–375 [DOI] [PubMed] [Google Scholar]
- 31.Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 2007;15:199–236 [Google Scholar]
- 32.Khalsa A, Karim R, Mack WJ, et al. Correlates of prevalent hypertension in a large cohort of HIV-infected women: Women's Interagency HIV Study. AIDS 2007;21:2539–2541 [DOI] [PubMed] [Google Scholar]
- 33.Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y, Burt V. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension 2015;65:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi T, Cocohoba J, Cohen M, et al. The impact of the AIDS Drug Assistance Program (ADAP) on use of highly active antiretroviral and antihypertensive therapy among HIV-infected women. J Acquir Immune Defic Syndr 2011;56:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinkler JM, Sugar CA, Escarce JJ, Ong MK, Mangione CM. Does age matter? Association between usual source of care and hypertension control in the US population: Data from NHANES 2007–2012. Am J Hypertens 2016;29:934–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med 2014;160:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hessol NA, Weber KM, D'Souza G, et al. Smoking cessation and recidivism in the Women's Interagency Human Immunodeficiency Virus Study. Am J Prev Med 2014;47:53–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Cleirigh C, Valentine SE, Pinkston M, et al. The unique challenges facing HIV-positive patients who smoke cigarettes: HIV viremia, ART adherence, engagement in HIV care, and concurrent substance use. AIDS Behav 2015;19:178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. HIV among women. 2013. Available at: www.cdc.gov/hiv/pdf/risk_women.pdf (Last accessed August21, 2013)
- 40.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S76–S99 [DOI] [PubMed] [Google Scholar]
- 41.Keithley JK, Duloy AM, Swanson B, Zeller JM. HIV infection and obesity: A review of the evidence. J Assoc Nurses AIDS Care 2009;20:260–274 [DOI] [PubMed] [Google Scholar]
- 42.Koethe JR, Jenkins CA, Lau B, et al. Higher time-updated body mass index: Association with improved CD4+ cell recovery on HIV treatment. J Acquir Immune Defic Syndr 2016;73:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Havranek EP, Mujahid MS, Barr DA, et al. Social determinants of risk and outcomes for cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2015;132:873–898 [DOI] [PubMed] [Google Scholar]
- 44.Deschamps AE, De Geest S, Vandamme AM, Bobbaers H, Peetermans WE, Van Wijngaerden E. Diagnostic value of different adherence measures using electronic monitoring and virologic failure as reference standards. AIDS Patient Care STDS 2008;22:735–743 [DOI] [PubMed] [Google Scholar]
- 45.Buscher A, Hartman C, Kallen MA, Giordano TP. Validity of self-report measures in assessing antiretroviral adherence of newly diagnosed, HAART-naive, HIV patients. HIV Clin Trials 2011;12:244–254 [DOI] [PMC free article] [PubMed] [Google Scholar]