Abstract
We have reported that of the 10 commonly used AAV serotype vectors, AAV6 is the most efficient in transducing primary human hematopoietic stem/progenitor cells (HSPCs). However, the transduction efficiency of the wild-type (WT) AAV6 vector varies greatly in HSPCs from different donors. Here we report two distinct strategies to further increase the transduction efficiency in HSPCs from donors that are transduced less efficiently with the WT AAV6 vectors. The first strategy involved modifications of the viral capsid proteins where specific surface-exposed tyrosine (Y) and threonine (T) residues were mutagenized to generate a triple-mutant (Y705 + Y731F + T492V) AAV6 vector. The second strategy involved the use of ex vivo transduction at high cell density. The combined use of these strategies resulted in transduction efficiency exceeding ~90% in HSPCs at significantly reduced vector doses. Our studies have significant implications in the optimal use of capsid-optimized AAV6 vectors in genome editing in HSPCs.
Genetically-modified autologous hematopoietic stem/progenitor cells (HSPCs) transplantation is the most promising therapeutic strategy to treat inherited genetic diseases, such as β-globin disorders1, leukodystrophies2,3, severe combined immunodeficiency4, Wiskott-Aldrich syndrome5, as well as acquired diseases such as AIDS6. Recent remarkable progress in genome editing tools include zinc-finger nucleases (ZFNs), transcription activator like effector nucleases (TALENs), and the RNA-guided clustered regulatory interspaced short palindromic repeat (CRISPR)/Cas9 endonucleases. These targeted nucleases further expand the application of ex vivo editing of therapeutic genes into patient HSPCs7. Most of these methods require the delivery of extracellular DNA into HSPCs, the efficiency of which is frequently sub-optimal. In the present study, we describe a novel, adeno-associated virus (AAV) vector-based DNA delivery strategy to overcome the current drawbacks that limit the clinical use of HSPCs gene editing methods.
The current drawbacks of HSPCs gene editing using viral methods such as chimeric adenoviral vectors, retroviral vectors, or integrase-defective lentiviral vectors (IDLV) include inefficient gene delivery, cytotoxicity, or DNA insertion. For example in a phase I/II clinical trial, a self-inactivating lentiviral vector achieved genetic modification in only 9–14% of blood cells after transplant2. Similarly, in laboratory, lentiviral transduction can produce no more than 22% transgene expression even in the presence of the best chemical helper8. Although it has now become feasible to achieve significantly higher transduction efficacy in HSPCs because of lentiviral vector optimization9 and good manufacturing process10, the high rate of stable transduction is also concomitant with a high vector genome copy number per cell, which, raises safety concerns because lentiviral genome, unlike the AAV genome, is associated with high risk of insertional mutagenesis. Non-integrative lentiviral vectors are safer but still limited by lower level of gene expression than other systems11. Cytotoxicity concerns also remains on transduced HSPCs. For example, the combination of the adenoviral vector and a potential enhancer, protein kinase C, resulted in >25% gene disruption, however, it is less tolerated in HSPCs than in other cell types12. Theoretically, the presence of any DNA material may have a chance to insert into the host genome. Insertion is a potential problem as extended expression of the nuclease may increase off-targeting. In addition, the nature of viral-based vectors, such as lentiviral, retroviral, and adenoviral vectors, enhance the random insertional activities that may lead to altered gene expression. For example, in a recent clinical trial using retroviral vectors, >140,000 unambiguous integration sites and a polyclonal pattern of hematopoiesis were revealed in all patients, which led to acute leukemia in 7 of 10 patients13. However, editing for therapeutic purposes in HSPCs will be done with non-integrative vectors. Although the probability of IDLV vectors to integrate in a cell is much lower than for normal integrative lentiviral vectors, and AAV vectors have been shown to undergo integration, albeit at a much lower frequency, the rates of integration for IDLV and AAV vectors remain to be directly compared.
Although vectors based on AAV2 serotype have been used for transduction of primary human HSPCs, the efficiency has been reported to be low14,15,16. Based on our initial studies, in which we mutagenized the surface-exposed tyrosine (Y) residues on AAV2 capsids, and observed a significant increase in their transduction efficiency17,18, the transduction efficiency of primary human HSPCs could be further increased with the capsid-modified AAV2 vectors19. As additional AAV serotypes became available, we systematically evaluated the transduction efficiency of the 10 most commonly used AAV serotype vectors in primary human HSPCs, and observed that the AAV6 serotype vectors were the most efficient20, and that the transduction efficiency of capsid-modified AAV6 vectors could be increased significantly21.
In our present studies, we describe two additive strategies to augment the transduction efficiency of AAV6 serotype vectors in primary human CD34+ cells, as well as propose a hypothetical model to help explain the underlying molecular mechanism of the observed increase in transduction. Our studies have not only contributed to a broader understanding of the AAV-host cell interactions, but have also led to the development of novel AAV6 serotype vectors for their optimal use in genome editing in primary human hematopoietic stem/progenitor cells.
Results
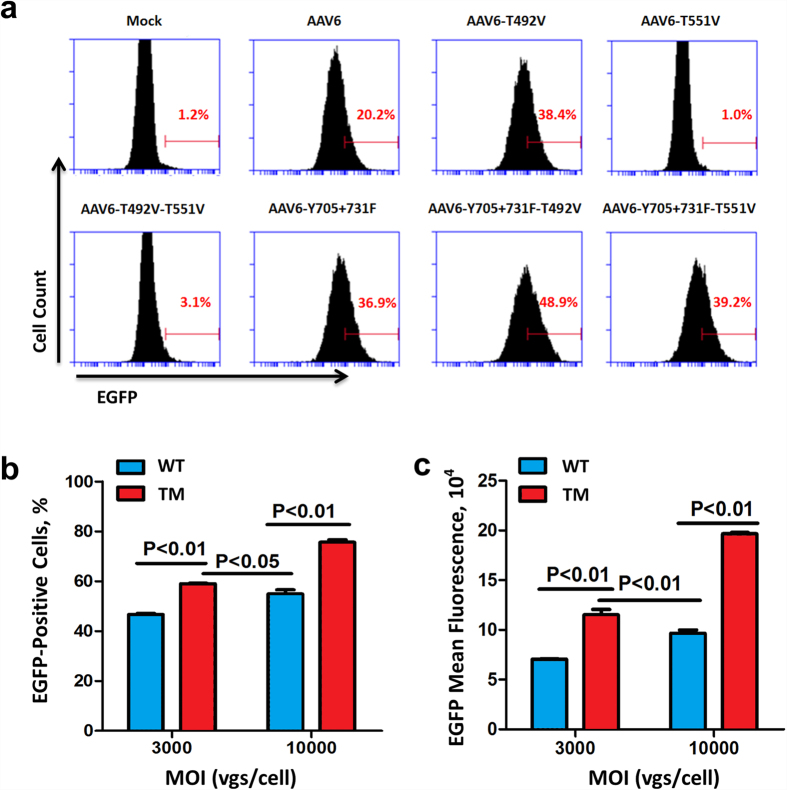
Our previous efforts have identified recombinant AAV serotype 6 (rAAV6) with high tropism for human HSPCs20,21. We have also reported that the transduction efficiency ranged between ~0–50% (n = 12 donors) for AAV2, and ~6–87% (n = 11 donors) for AAV6 in HSPCs, respectively15,20. Although we hypothesized that such a wide range of donor variation is due to different levels of expression of the putative receptors and/or co-receptors on these cells, we were able to achieve significant increase in the transduction efficiency of both AAV2 and AAV6 vectors using site-directed mutagenesis of surface-exposed tyrosine (Y) residues. We therefore wished to examine whether the transduction efficiency of AAV6 vectors could be further improved by additional capsid modifications involving mutagenesis of surface-exposed serine (S) and threonine (T) residues, and various permutations and combinations thereof22. To this end, a number of such mutant capsids were generated, and self-complementary AAV6 (scAAV6) vectors containing a chicken beta actin promoter/CMV enhancer driving an enhanced green fluorescence protein gene (CBAp-EGFP) were produced as previously reported23. Using K562 cells, frequently used as a model for hematopoietic cell transduction studies, indicated that among all AAV6 capsid variants tested, a triple-mutant (TM) AAV6-Y705 + 731F + T492V (TM-AAV6) vector was identified as the most efficient (Fig. 1a). The transduction efficiency of the TM-AAV6 vectors was also consistently significantly higher than its wild-type (WT) counterpart not only in K562 cells, but also in primary human bone marrow-derived HSPCs obtained from a normal volunteer donor (Fig. 1b,c). Similar results were obtained with HSPCs obtained from one additional donor (Supplementary Table 1, Donor 2). No variability in expression levels under different transduction conditions was observed using flow cytometry or fluorescence microscopy.
Figure 1. Transduction efficiency of various capsid-modified AAV6 vectors in human hematopoietic cells.
(a) Human erythroleukemia cells, K562, were either mock-transduced, or transduced with the wild-type (WT) or various indicated capsid-modified scAAV6-CBAp-EGFP vectors at an MOI of 3,000 vgs/cell. Transgene expression was analyzed by flow cytometry 48 hrs post-transduction. (b) Primary human bone marrow-derived CD34+ cells from a single individual donor were transduced in triplicate with the WT or the Y705 + 731F + T492V triple-mutant (TM) scAAV6-CBAp-EGFP vectors at MOIs of 3,000 or 10,000 vgs/cell. Transgene expression was analyzed by flow cytometry 48 hrs post-transduction. (c) Mean fluorescence intensity of transgene expression. Error bars represent standard deviations (SD).
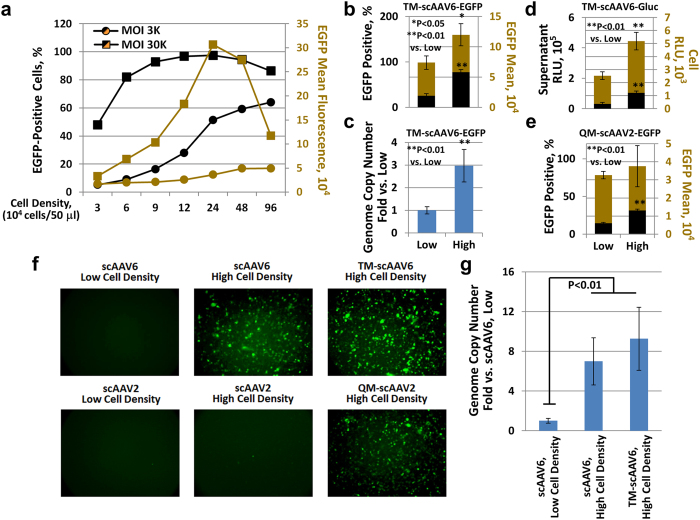
During the course of these studies, we also observed that the transduction efficiency of the WT AAV6 vectors could be significantly increased if transductions were carried at high cell density, both at low and high multiplicities of infection (MOIs), in contrast to previously published studies, in which transductions were carried out at cell densities ranging from 7.0 × 105–1.1 × 106 cells/ml14,15,16,19,20,21,24. The results, as analyzed by flow cytometry 48 hrs post-transduction, indicated that, compared to the conventionally used cell densities, increased cell density, up to 1.0 × 107 cells/ml, dramatically enhanced the scAAV6-mediated transgene expression, in both the EGFP-positivity and EGFP mean fluorescence intensity (Fig. 2a), presumably due to the increased probability of more efficient vector attachment to the cell receptor and/or co-receptor. Next, K562 cells were transduced with the optimized TM-scAAV6-CBAp-EGFP vectors either at low-density (1 × 106 cells/ml) or high-density (1 × 107 cells/ml). Whereas only ~25% of K562 cells were transduced at low-density, the transduction efficiency at high-density increased up to 77%, and the EGFP mean value increased to 160% (Fig. 2b,c). The enhancement of transgene expression also correlated with a significantly increased intra-cellular viral genome copy number (Fig. 2c), as determined by qPCR of total DNA isolated 2 hrs post-transduction25. Similar results were obtained with the TM-scAAV6 vectors expressing the Gaussia luciferase (Gluc) transgene (Fig. 2d), as well as when the optimized AAV2-CBAp-EGFP vectors containing the quadruple mutation (Y444 + 500 + 730F + T491V; QM-scAAV2) vectors, which we have previously identified to be the most efficient vector, albeit in human cell lines in vitro, and in murine hepatocytes in vivo26, were used (Fig. 2e). Similar results were also obtained when these serotype vectors were used to transduce HSPCs from a donor, which are transduced extremely poorly under conventional conditions (Fig. 2f). The enhanced transgene expression mediated by TM-scAAV6 vectors observed under high cell density conditions also correlated well with increased internalized vector genome copy number/cell (Fig. 2g) as well as with ssAAV6 vectors (Supplementary Fig. 1a).
Figure 2. Transduction efficiency of AAV vectors in human hematopoietic cells at various cell densities.
(a) K562 cells were transduced at various indicated cell densities at MOIs of 3,000 or 30,000 vgs/ml with WT scAAV6-CBAp-EGFP vectors. Transgene expression was analyzed by FACS 48 hrs post-transduction. (b) K562 cells were also transduced at low (1 × 106/mL) or high (1 × 107/mL) cell densities with 3,000 vgs/cell of TM scAAV6-CBAp-EGFP vectors, and transgene expression and mean fluorescence intensity were determined as described above. (c) The vector genome copy numbers/cell were determined 2 hrs post-vector transduction by qPCR and data were normalized to β-actin DNA copy number. (d) K562 cells were transduced at low or high cell densities with TM-scAAV6-CBAp-Gluc vectors, and luciferase expression was determined in the culture supernatants. (e) K562 cells were transduced at low or high cell densities with QM-scAAV2-CBAp-EGFP vectors, and transgene expression and mean fluorescence intensity were determined as described above. (f) Primary human bone marrow-derived CD34+ cells were transduced at low or high densities with indicated AAV6 or AAV2 vectors, EGFP-expressing cells were visualized under a fluorescence microscope 48 hrs post-transduction. (g) The vector genome copy numbers/cell were determined as described above.
To further corroborate our hypothesis, K562 cells were transduced and later concentrated or diluted. That the initial cell-cell contact was critical in achieving high-efficiency transduction, was further corroborated by experiments in which cells were transduced at low-density, and subsequently pooled together and centrifuged to reach high-density, and conversely, cells were transduced at high-density, and soon after transduction, were diluted to low-density (Supplementary Fig. 2a). The elevated transduction was observed only under the latter condition (Supplementary Fig. 2b). The increased transduction efficiency at high-density also correlated well with the vector genomes entering the cells, as determined by qPCR analyses (Supplementary Fig. 1a). In the second set of experiments, a fixed number of K562 cells were infected with viral vectors in various volumes for 2 hrs and subsequently diluted in the same volume of 2 ml (Supplementary Fig. 3a). Once again, the increased transduction efficiency was observed only under the condition of high cell density (Supplementary Fig. 3b,c), accompanied with a significantly increased intra-cellular viral genome copy numbers (Supplementary Fig. 3d).
We next extended these studies to include two additional human hematopoietic cell lines, M07e and Raji, which express low to extremely low levels of HSPG, the primary receptor for AAV2, and consequently, are transduced extremely poorly by AAV2 vectors27. As can be seen in Supplementary Fig. 4a,b, under the condition of high cell density, significantly enhanced transduction of M07e cells, but not Raji cells, was observed, since M07e cells do express high levels of AAV2 co-receptor, fibroblast growth factor receptor 1 (Supplementary Fig. 4c), but not Raji cells, which express undetectable levels of both HSPG and FGFR127. To address the possibility whether alternative receptors/co-receptors were being used under the condition of high cell density, K562 cells were transduced with scAAV2 vectors in the absence or the presence of heparin, which is known to compete for AAV2 cellular entry. As can be seen in Supplementary Fig. 4d,e, heparin at 5 μg/ml significantly reduced the transduction efficiency of scAAV2 vectors under the condition of high cell density for each of the cell types tested. These results strongly suggest that the putative receptors/co-receptors for viral entry remain unaltered under the condition of high cell density.
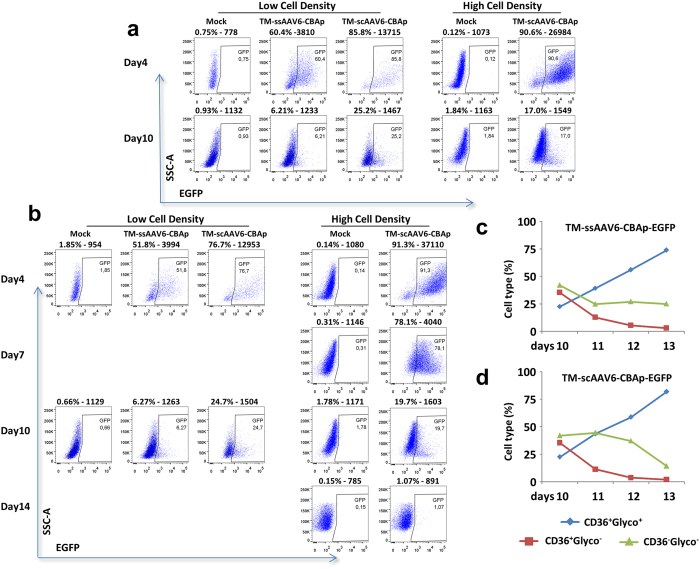
We further evaluated the efficacy of AAV vector-mediated transduction of primary HSPCs derived from bone marrow (BM) as well as from umbilical cord blood (CB). BM-derived CD34+ cells from individual donors (or a mixture from 10 donors) were purchased from a commercial source (AllCells, LLC, Alameda, CA, USA), and were used to transduce with different scAAV-CBAp-EGFP vectors at an MOI of 10,000 vgs/cell without fetal bovine serum (FBS). Transgene expression was evaluated by flow cytometry 48 hrs post-transduction. As shown in Supplementary Table 1, consistent with our previously published studies, whereas scAAV6 vectors transduced human HSPCs more efficiently than scAAV2 vectors, capsid modification on both vectors further enhanced their transduction efficiency. The transgene expression at high cell density was consistently higher than that at low cell density. The increased transduction efficiency in human HSPCs at high cell density also correlated with a significantly increased intra-cellular viral genome copy number 2 hrs post-viral transduction (Supplementary Fig. 1b). However, the extent of transgene expression declined over time, and in none of the cell populations tested, the viral genome copy number was above the detection limit of qPCR 14 days post-transduction (data not shown). Similar results were obtained with CB-derived CD34+ cells, except that the overall transduction efficiency was consistently higher than that in BM-derived CD34+ cells. In the first set of experiments, CD34+ cells were transduced at low and high cell densities with TM-scAAV6-CBAp-EGFP and TM-ssAAV6-CBAp-EGFP vectors at an MOI of 20,000 vgs/cell without FBS, followed by switch to FBS-containing expansion medium and cultured for 10 days, and transgene expression was evaluated by FACS at 4 and 10 days post-transduction (Fig. 3a). It is evident that at day 4, scAAV6 vectors were more efficient than ssAAV6 vectors, but at high cell density, there was only a modest enhancement in EGFP-positivity. Again, the transgene expression diminished over time. In the second set of experiments, CB-derived CD34+ cells were transduced at low and high cell densities with TM-scAAV6-CBAp-EGFP vectors as described above, except that at day 10, cells were switched to an erythroid differentiation medium and cultured for 4 additional days. EGFP expression was determined by flow cytometry at day 4, day 7, day 10, and day 14 (Fig. 3b). As can be seen, once again, at day 4, scAAV6 vectors were more efficient than ssAAV6 vectors. At high cell density, there was a modest enhancement in EGFP-positivity, but transgene expression diminished over time, and eventually resulted in less than 1% EGFP-positive cells 14 days post-transduction, which is within the variation of mock-transduced cells (0.14–1.84%), corroborating again the loss of vector genome, as determined by qPCR assays described above, and hence, lack of stable integration in HSPCs. Staining with hCD36-PE and hGlycophorin A-FITC showed the typical induction of erythroid cell differentiation, which was unaltered following vector transduction (Fig. 3c,d). At the end of the culture period, most cells were GlycoA+, indicating that none of erythroid-differentiated cells expressed the transgene.
Figure 3. Transduction efficiency of TM-ssAAV6 and TM-scAAV6 vectors in primary human CD34+ cells.
(a) Primary human cord blood-derived CD34+ cells were either mock-transduced, or transduced at day 0 at low (0.5 × 106 cells/ml,) or high (1 × 107 cells/ml) cell density with 20,000 vgs/cell of the indicated AAV6 vectors in serum free XVIVO20 medium. Two hrs later, cells were diluted to 5 × 105 cells/mL and switched to the expansion medium (IMDM + FBS + SCF + IL3 + Epo+ Dexamethasone + β-estradiol + β-mercapthoethanol). EGFP expression was determined by flow cytometry at day 4 and day 10 post-transduction. (b) Following mock-transduction, or transduction of CD34+ cells as described above, cells were switched to the expansion medium for 10 days, and cultured in an erythroid differentiation medium (IMDM + BSA + Insulin + Transferrin + Epo) for an additional four days. EGFP expression was determined by flow cytometry. (c,d) Vector-transduced CD34+ cells cultured in the differentiation medium were stained with hCD36-PE and hGlycophorin A−FITC and analyzed by flow cytometry for the following: non-erythroid (CD36−/glycoA−), and erythroid cells (CD36+/GlycoA+) from day 10 to day 14.
Discussion
Although AAV vectors have been successfully used in transducing a wide variety of cells and tissues, primary hematopoietic stem/progenitor cells (HSPCs) have proven to be particularly difficult to transduce despite sustained efforts by us and others spanning nearly three decades. However, most of those studies were carried out with AAV2 serotype vectors. With the subsequent availability of a number of additional serotypes, we were able to identify AAV6 as the most efficient in transducing HSPCs20,21. Despite this advance, we also observed a significant donor-variation ranging between 6–87%. Although this could be due to differences in various steps in the life cycle of AAV6 vectors in HSPCs from different donors, we chose to focus on the initial virus-host cell interaction, primarily involving the putative cellular receptors and co-receptors for AAV6. Although epidermal growth factor receptor (EGFR) has been identified to be the cellular receptor for AAV628, those studies were not performed with hematopoietic cells. In addition, we have previously reported that K562 cells, which are known to lack expression of EGFR, are efficiently transduced by AAV6 vectors, and that pre-treatment of primary human CD34+ cells with EGF has no effect on the transduction efficacy of AAV6 vectors21. Thus, the precise identity of the receptor and co-receptor for AAV6 in HSPCs remains elusive. Based on our observation of significantly increased transduction under the conditions of high cell density, we hypothesized that a close cell-cell contact facilitates more efficient vector binding and entry into cells grown in suspension culture, presumably because of the close proximity of the receptor and the co-receptor on neighboring cells, and AAV6 vector bound to the receptor on one cell utilizes the co-receptor on the neighboring cell to gain entry in the latter, and vice versa, thus leading to increased transduction. Indeed, using AAV2 vectors, for which the cellular receptors and co-receptors have been well characterized, we were able to corroborate our hypothesis to a certain extent.
Clearly, additional studies are warranted, but we believe that our data have revealed a novel mechanism, which AAV vectors exploit to gain entry into target cells. For example, adherent cells such as HEK293, are readily transduced by AAV2 vectors because these cells abundantly express the cell surface receptor, heparan sulfate proteoglycan (HSPG), and one of the co-receptors, human fibroblast growth factor receptor 1 (FGFR1) for AAV2. However, K562 cells are not transduced as efficiently, although these cells, grown in suspension, express both HSPG and FGFR1 only at modestly lower levels. We reasoned that the lack of proximity of HSPG and FGFR1 on K562 cells might account for the suboptimal transduction of these cells, and hypothesized that if the transduction was performed at high cell density, presumably allowing for HSPG on one cell to come in close proximity to FGFR1 on the neighboring cell, then AAV2 bound to HSPG on one cell could utilize FGFR1 on the neighboring cell to gain entry in the latter, and vice versa, thus leading to increased transduction which was observed experimentally.
We also believe that our studies described here have practical utility. For example, recent development of targeted nucleases provides a means to achieve precise genome editing in stem cells, including human HSPCs. However, all these strategies require the delivery of DNA materials. Although the expression of targeted nucleases could be achieved by mRNA delivery, a homologous donor DNA template must also be introduced. An exception is the CRISPR/Cas9 system that utilizes a short guide RNA and RNA-guided nuclease29. Nevertheless, the majority of research still uses DNA delivery systems, such as plasmids and viral vectors to express the short guide RNA, due to the low efficiency of RNA delivery in human HSPCs30. Thus, the off-target activities as well as random insertion of the delivered DNA constitute significant safety hurdles to overcome31. While our current studies were in progress, three independent groups not only corroborated our previously published studies20,21, but also reported successful transduction of primary human CD34+ cells using the WT AAV6 vectors for genome editing32,33,34, except that MOIs ranging from 100,000–200,000 vgs/cell were used to achieve ~40–55% transduction efficiency. Using capsid-optimized AAV6 vectors and transduction conditions described here, at an MOI of 20,000 vgs/cell, transduction efficiency exceeding 90% can now routinely be achieved, which is significantly higher than any known vector system currently in use. Thus, our studies have significant implications in highly efficient gene therapy and genome editing with capsid-optimized AAV6 vectors involving primary human HSPCs.
Methods
Cells and reagents
Human embryonic kidney cell line, HEK293, and human erythroleukemia cell line, K562, were purchased from the American Type Culture Collection (Manassas, VA). The human lymphoblastoid cell line, Raji, and the human megakaryocytic leukemia cell line, M07e, were obtained from Drs Z.A. Brahmi and H.E. Broxmeyer (Indiana University School of Medicine, Indianapolis, IN), respectively. Monolayer cultures of HEK293 were maintained in Dulbecco’s-modified Eagle’s medium (DMEM), and suspension cultures of K562, M07e, and Raji were maintained in Iscove’s-modified Dulbecco’s medium (IMDM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics (Lonza, Basel, Switzerland). Primary human bone marrow-derived CD34+ cells were purchased from AllCells (Alameda, CA). Human cord blood cells were collected by the Assistance Publique – Hôpitaux de Paris (AP-HP), according to French bioethics law 2011-814. The research project for cell usage was approved by the scientific council of Fondation Générale de Santé (FGDS) and AP-HP. Written informed consents were obtained from mothers of the newborn.
AAV vector production
The ssAAV and scAAV plasmids containing the CBAp-EGFP transgene expression cassette have been described previously35. All AAV vectors were produced using the polyethelenimine-mediated triple-plasmid transfection method36. Briefly, HEK293 cells (Agilent Technologies) were harvested 72 hrs post-transfection, and lysed by 3 rounds of freeze-thaw, and digested with Benzonase (Invitrogen, Grand Island, NY). Cell debris was removed by centrifugation. AAV vectors were purified by iodixanol (Sigma, St. Louis, MO) gradient ultracentrifugation, followed by ion exchange chromatography using HiTrap SP/Q HP columns (GE Healthcare, Piscataway, NJ), washed with phosphate-buffered saline (PBS) and concentrated by centrifugation using centrifugal spin concentrators with 150 K molecular weight cutoff. Titers were determined by Quantitative real-time PCR assays as previously described37.
AAV transduction
The culture conditions for K562, Raji, and M07e cell have been described previously27. AAV transductions were performed in FBS-free medium for 2 hrs. Cells were then switched to FBS-containing medium for growth. Primary human HSPCs were purchased from AllCells Technologies (Emeryville, CA). Cord blood CD34+ cells were thawed and immediately transduced with AAV vectors at 20,000 vgs/cell in serum free XVIVO20 medium. Two hrs post-transduction, cells were switched to the expansion medium (IMDM, FBS, SCF, IL3, Epo, Dexamethasone, β-estradiol, β-mercapthoethanol) and grown at 5 × 105 cells/mL. At day 10, cells were switched to the erythroid differentiation medium (IMDM, BSA, Insulin, Transferrin, Epo). All transgene expressions were determined either by fluorescence microscopy or by flow cytometry, as described previously20,21.
Erythroid expansion of CD34+ cells
Ten days after culturing in the expansion medium, cells were stained with hCD36-PE and hGlycophorin A-FITC showing the typical profile of expanded cells in this amplification medium: non-erythroid (CD36−/glycoA−), and erythroid cells (immature: CD36+/GlycoA−; early mature: CD36high/GlycoAmed and late mature: CD36med/glycoAhigh). Stained cells were subjected to flow cytometry assays.
Viral genome copy number determination
Total cellular DNA was isolated using the PureLink® Genomic DNA Kits (ThermoFisher Scientific, MA) in accordance with the manufacturer’s protocol. DNA concentration was determined by Nanodrop (ThermoFisher Scientific, MA). One hundred ng of DNA from each sample was used as the template material for quantitative real-time PCR. The EGFP vector genome copy numbers were determined and normalized by the results of human β-actin gene in each sample.
Heparin competition assays
Cells were transduced with scAAV2-CBAp-EGFP vectors at an MOI of 5,000 vgs/cell. Vectors were premixed with vehicle control or 5 μg/ml heparin sodium salt (Sigma, St. Louis, MO). Cells were switched to heparin-free complete DMEM 2 hrs post-transduction and then were analyzed for EGFP levels by flow cytometry 72 hrs post-transduction.
Additional Information
How to cite this article: Ling, C. et al. High-Efficiency Transduction of Primary Human Hematopoietic Stem/Progenitor Cells by AAV6 Vectors: Strategies for Overcoming Donor-Variation and Implications in Genome Editing. Sci. Rep. 6, 35495; doi: 10.1038/srep35495 (2016).
Supplementary Material
Acknowledgments
This work was supported in part by Bankhead-Coley New Investigator Research Grant and Alex’s Lemonade Stand Young Investigator Award (to C.L.); a Chaire Industrielle Grant from France’s Agence Nationale pour la Recherche (ANR) (to P.L.); and Public Health Service Grants R21 EB-015684 and R01 HL-097088 from the National Institutes of Health; a grant from the Children’s Miracle Network; and support from the Kitzman Foundation (to A.S.). We thank the “Fondation Générale de Santé” (FGDS) and the “Assistance Publique-Hôpitaux de Paris (AP-HP)” for collecting and testing cord blood cells. Z.Y. was supported by a Fellowship Award from the Government of China. We thank Prof. Changquan Ling for his continued support.
Footnotes
A.S. holds issued patents related to AAV vectors that have been licensed to various AAV gene therapy companies. P.L. and E.P. have financial relationships with bluebird bio, Inc. All other authors declare no competing interests.
Author Contributions C.L. and A.S. conceived the studies. C.L., Z.Y., M.T., M.C.Y., P.L., E.P. and A.S. designed the experiments. Z.Y., K.B. and C.L. performed the experiments. C.L., E.P. and A.S. wrote the manuscript, with comments and suggestions from all co-authors.
References
- Cavazzana-Calvo M. et al. Transfusion independence and HMGA2 activation after gene therapy of human beta-thalassaemia. Nature 467, 318–322 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N. et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 326, 818–823 (2009). [DOI] [PubMed] [Google Scholar]
- Biffi A. et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 341, 1233158 (2013). [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. et al. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 363, 355–364 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiuti A. et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 341, 1233151 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiusto D. L. et al. RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci Transl Med 2, 36ra43 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T., Gersbach C. A. & Barbas C. F. 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31, 397–405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. M. et al. High-throughput screening identifies compounds that enhance lentiviral transduction. Gene Ther 21, 1008–1020 (2014). [DOI] [PubMed] [Google Scholar]
- Negre O. et al. Preclinical evaluation of efficacy and safety of an improved lentiviral vector for the treatment of beta-thalassemia and sickle cell disease. Curr Gene Ther 15, 64–81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten O. W. et al. Large-scale manufacture and characterization of a lentiviral vector produced for clinical ex vivo gene therapy application. Hum Gene Ther 22, 343–356 (2011). [DOI] [PubMed] [Google Scholar]
- Joglekar A. V. et al. Integrase-defective lentiviral vectors as a delivery platform for targeted modification of adenosine deaminase locus. Mol Ther 21, 1705–1717 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. et al. Genomic editing of the HIV-1 coreceptor CCR5 in adult hematopoietic stem and progenitor cells using zinc finger nucleases. Mol Ther 21, 1259–1269 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun C. J. et al. Gene therapy for Wiskott-Aldrich syndrome–long-term efficacy and genotoxicity. Sci Transl Med 6, 227ra233 (2014). [DOI] [PubMed] [Google Scholar]
- Zhou S. Z. et al. Adeno-associated virus 2-mediated high efficiency gene transfer into immature and mature subsets of hematopoietic progenitor cells in human umbilical cord blood. J Exp Med 179, 1867–1875 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnazhagan S. et al. Adeno-associated virus type 2-mediated transduction in primary human bone marrow-derived CD34+ hematopoietic progenitor cells: donor variation and correlation of transgene expression with cellular differentiation. J Virol 71, 8262–8267 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S. et al. Transduction of primitive human marrow and cord blood-derived hematopoietic progenitor cells with adeno-associated virus vectors. Blood 93, 1882–1894 (1999). [PubMed] [Google Scholar]
- Zhong L. et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA 105, 7827–7832 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markusic D. M. et al. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther 18, 2048–2056 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss M. A. et al. Enhanced long-term transduction and multilineage engraftment of human hematopoietic stem cells transduced with tyrosine-modified recombinant adeno-associated virus serotype 2. Hum Gene Ther 21, 1129–1136 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L. et al. Optimizing the transduction efficiency of capsid-modified AAV6 serotype vectors in primary human hematopoietic stem cells in vitro and in a xenograft mouse model in vivo. Cytotherapy 15, 986–998 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L. et al. High-efficiency transduction of primary human hematopoietic stem cells and erythroid lineage-restricted expression by optimized AAV6 serotype vectors in vitro and in a murine xenograft model in vivo. PLoS One 8, e58757 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A. Adeno-Associated Virus: The Naturally Occurring Virus Versus the Recombinant Vector. Hum Gene Ther 27, 1–6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. N. et al. Pristimerin enhances recombinant adeno-associated virus vector-mediated transgene expression in human cell lines in vitro and murine hepatocytes in vivo. J Integr Med 12, 20–34 (2014). [DOI] [PubMed] [Google Scholar]
- Smith L. J. et al. Gene transfer properties and structural modeling of human stem cell-derived AAV. Mol Ther 22, 1625–1634 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. H. et al. Cytotoxic genes from traditional Chinese medicine inhibit tumor growth both in vitro and in vivo. J Integr Med 12, 483–494 (2014). [DOI] [PubMed] [Google Scholar]
- Aslanidi G. V. et al. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: the final threshold? PLoS One 8, e59142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K. et al. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med 5, 71–77 (1999). [DOI] [PubMed] [Google Scholar]
- Weller M. L. et al. Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nat Med 16, 662–664 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendel A. et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat Biotechnol (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F. & Grimm D. CRISPR genome engineering and viral gene delivery: a case of mutual attraction. Biotechnol J 10, 258–272 (2015). [DOI] [PubMed] [Google Scholar]
- Gammon K. Gene therapy: editorial control. Nature 515, S11–S13 (2014). [DOI] [PubMed] [Google Scholar]
- Sather B. D. et al. Efficient modification of CCR5 in primary human hematopoietic cells using a megaTAL nuclease and AAV donor template. Sci Transl Med 7, 307ra156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. Homology-driven genome editing in hematopoietic stem and progenitor cells using ZFN mRNA and AAV6 donors. Nat Biotechnol 33, 1256–1263 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ravin S. S. et al. Targeted gene addition in human CD34 hematopoietic cells for correction of X-linked chronic granulomatous disease. Nat Biotechnol 34, 424–431 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W. et al. A simple method to increase the transduction efficiency of single-stranded adeno-associated virus vectors in vitro and in vivo. Hum Gene Ther 22, 633–640 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B. et al. A concept of eliminating nonhomologous recombination for scalable and safe AAV vector generation for human gene therapy. Nucleic Acids Res 41, 6609–6617 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. et al. Efficient production of dual recombinant adeno-associated viral vectors for factor VIII delivery. Hum Gene Ther Methods 25, 261–268 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.