Abstract
Purpose: Purinergic receptors play a key role in the function of the lacrimal gland (LG) as P1 purinergic receptors A1, A2A, and A2B, P2X1–7 receptors, and many of the P2Y receptors are expressed.
Methods: This review examines the current knowledge of purinergic receptors in the LG as well as the signaling pathways activated by these receptors.
Results: These receptors are expressed on the acinar, ductal, and myoepithelial cells. Considerable crosstalk exists between the pathways activated by P2X7 receptors with those activated by M3 muscarinic or α1D adrenergic receptors. The mechanism of the crosstalk between P2X7 and M3 muscarinic receptors differs from that of the crosstalk between P2X7 and α1D adrenergic receptors.
Conclusions: Understanding purinergic receptors and how they modulate protein secretion could play a key role in normal and pathological responses of the LG.
Keywords: : lacrimal gland, myoepithelial cell, purinergic receptor, cellular signaling, protein secretion, cross talk of signaling pathways
Introduction
Tears are a complex fluid that consists of 3 main components. The first component is the mucins, which are produced by the conjunctival goblet cells and corneal epithelial cells. Mucins form the inner layer of the tear film and are either thought to be mixed within the aqueous layer in a gradient with the highest concentration of mucins closest to the ocular surface or form a mucous network at the ocular surface.1,2 The second major component of the tear film is the aqueous portion, which is secreted primarily by the main lacrimal gland (LG). This part of the tear film contains proteins (including dissolved mucins), water, and electrolytes.3 The third component of the tear film is the lipid layer, which is secreted by the meibomian glands. The lipids overlay the mucous and aqueous mixture.
The tears overspread the ocular surface and provide nutrients to the avascular cornea, and protect the ocular surface from the external environment. Therefore, the volume and composition of the tears are tightly controlled as any changes can be deleterious to vision and damaging to the ocular surface (the cornea and conjunctiva).
The LG is a multilobed, tubuloacinar gland with ducts that lead to the ocular surface (Fig. 1). The main functional unit within the LG is the acinus. An acinus consists of polarized acinar cells arranged around a central lumen and comprises ∼80% of the gland (Fig. 1).4,5 Receptors for neurotransmitters are found on the basolateral membranes of the acinar cell. When stimulated, these cells synthesize and secrete proteins, electrolytes, and water across the apical membrane and into the ducts. Ductal cells modify the primary fluid before releasing it onto the ocular surface. Surrounding the acinar cells are myoepithelial cells (MECs). MECs are stellate shaped cells with long branching cell processes that surround the acinar cells at the basolateral membrane.6 As MECs express α-smooth muscle actin, it is believed that they contract to help expel the secretory products out of the acini and into the ducts, but other functions are possible.6,7
FIG. 1.
Schematic diagram of the neural regulation of the LG. Sensory, afferent nerves of the cornea and conjunctiva activate the efferent parasympathetic and sympathetic nerves that innervate the LG acini. Fluid flows onto the ocular surface and are drained through the lacrimal drainage system. Reprinted from Rocha et al. Ocul. Surf. 6:162–174, 2008. LG, lacrimal gland.
Regulation of LG secretion is mainly under neural control with parasympathetic nerves predominating (Fig. 1).3 The neurotransmitters acetylcholine (Ach) and vasoactive intestinal peptide (VIP) are released from these nerves. Ach binds to M3 muscarinic receptors (M3AChRs) on the basolateral membranes of acinar cells.8 M3AChRs are classical G protein-coupled receptors (GPCRs) with 7 transmembrane spanning regions and are coupled to Gαq G proteins and phospholipase Cβ (PLCβ).9 PLCβ cleaves phosphatidylinositol 1,4-bisphosphate to produce diacylglycerol (DAG) and 1,4,5 inositol trisphosphate (InsP3) (Fig. 2).10 DAG activates protein kinase C (PKC)α, -δ, and -ɛ.11 InsP3 binds to its receptors on the endoplasmic reticulum to release Ca2+ into the cytosol, increasing the intracellular [Ca2+] ([Ca2+]i) to transduce the extracellular signal.12 M3AChRs also activate phospholipase D (PLD) and extracellular signal-regulated kinase ERK 1/2 (also known as p42/p44 mitogen activated protein kinase).13–15 Activation of ERK 1/2 attenuates LG protein secretion (Fig. 2).15
FIG. 2.
Schematic diagram of A1 and A2 adenosine receptor signal pathways and potential interactions with cholinergic pathways in the LG. Adenosine binds to the A1 and A2 adenosine receptors to potentially activate the G protein Gαs to stimulate AC and production of cAMP. cAMP in turn could activate PKA, which could lead to protein secretion. PKA could block the ERK 1/2 activity, thereby relieving its inhibition of cholinergic agonist-stimulated protein secretion to possibly cause potentiation of secretion. AC, adenylate cyclase; M3, muscarinic receptor type 3; PLCβ, phospholipase Cβ; DAG, diacylglycerol; PKA, protein kinase A; PKC, protein kinase C; InsP3, 1,4,5 inositol trisphosphate; Pyk2, a nonreceptor tyrosine kinase; Src, a nonreceptor tyrosine kinase; Raf, mitogen-activated protein kinase kinase kinase; MEK, mitogen-activated protein kinase kinase; ERK 1/2, extracellular signal-regulated kinase (also called mitogen-activated protein kinase).
VIP, which is also released by parasympathetic nerves, binds to the VIPAC1 receptor to activate adenylate cyclase (AC) to increase cAMP and increases the [Ca2+]i.16,17 VIP also blocks activation of ERK 1/2, which is likely responsible for potentiation of secretion observed with simultaneous addition of VIP and muscarinic agonists.18
While parasympathetic nerves are the predominant nerve type present in the LG, sympathetic nerves are also present.3 These nerves release the neurotransmitter norepinephrine. Norepinephrine binds to α1D-adrenergic receptors (α1D-AR) on the acinar cells to activate endothelial nitric oxide synthase to produce nitric oxide.19,20 Nitric oxide activates guanylyl cyclase to generate cGMP leading to protein secretion. While α1D-ARs also increase [Ca2+]i, there is not a concomitant increase in InsP3, and the mechanism by which they increase [Ca2+]i is not known.12,21 In addition, stimulation of α1D-ARs activates PKCɛ, which stimulates protein secretion and PKCα and -δ, which inhibit protein secretion.22 Norepinephrine can also bind to βARs, but the signaling pathways activated have not been extensively investigated in LG acini.
In addition to neural stimulation, protein secretion from acinar cells can also be stimulated by ATP and its metabolites.21,23–27 ATP is released along with the neurotransmitters Ach and norepinephrine28 and can also be released from cells by mechanical stimulation.29 ATP can then act in an autocrine manner (acting on the same cell) or paracrine manner (acting on neighboring cell). Receptors for purines belong to a class of receptors known as purinergic receptors.30 Purinergic receptors can be divided into 2 major classes, P1 and P2. There are 4 known P1 receptors, also known as adenosine receptors, A1, A2A, A2B, and A3.30 The P2 group can be subdivided into P2X and P2Y receptors. P2X receptors (P2X1–P2X7) are ATP-gated nonselective ion channels. P2Y receptors (P2Y1, -2, -4, -6, -11, -12, -13, and -14) are classical GPCRs.30
While it has been known for many years that extracellular ATP activates cation channels in the LGs,31,32 purinergic receptors have only recently been identified and the pathways activated by these receptors studied. This review will focus on current knowledge of the purinergic receptors in the LG and interactions of these pathways with other signal transduction pathways.
P1 Receptors in the LG
A1, A2A, and A2B receptors have been identified in the LGs of rabbits by reverse transcription polymerase chain reaction (RT-PCR) and immunofluorescence microscopy.23,25 The intracellular locations of A1 receptors differed slightly from the localization of the A2A and A2B. A1 was expressed almost exclusively in the basolateral membranes of acini, while A2A and A2B were found not only in the basolateral membrane but also in the cytosol.
Adenosine and the A1 agonist N6-cyclopentyladenosine (CPA) stimulated protein secretion although not to the same extent as the M3AChR agonist carbachol in cultured rabbit LG acini.25 The responses from adenosine and CPA were completely blocked by A1 antagonist 8-phenyltheophylline. Interestingly, simultaneous addition of either adenosine or CPA with carbachol (an acetylcholine analog) resulted in potentiation of secretion, that is, secretion that was greater than what would be expected from adding the 2 results.25 The potentiated response was significantly inhibited by 8-phenyltheophylline. Potentiation of secretion has been observed with other types of neurotransmitters in the LG16,33–36 and occurs when receptors activate overlapping pathways or pathways that interact with one another. Activation of multiple receptors and their signaling pathways can result in 3 different responses: (1) a less than additive response, (2) an additive response, or (3) potentiation of the response. A less than additive response indicates that the 2 signaling pathways are the same or overlap if 1 receptor inhibits the other. An additive response indicates that the 2 pathways are completely separate and there is no interaction between the 2 pathways. A potentiation of the response occurs when the 2 pathways interact synergistically to cause a response that is greater than if the 2 individual pathways were activated together. The pathway activated by the A1 receptor was not further investigated, so the point of interaction with muscarinic agonists is unknown.25
In many tissues, activation of the A1 receptor is linked to a decreased activity of AC and a decrease in cAMP. However, in the parotid gland, A1 receptors were shown to increase cAMP levels leading to amylase secretion.37 It is possible that in the LG, A1 receptors could also increase cAMP leading to inhibition of ERK 1/2. This would account for the potentiation seen with muscarinic and A1 receptors (Fig. 2). Thus the pathways activated by A1 and M3AchR agonists could interact at a step distal to the receptors at the level of ERK 1/2, however, we cannot rule out an interaction between the receptors. In contrast to the LG, A1 receptors in the parotid gland do not interact with muscarinic receptors in stimulation of amylase secretion.37
Similar to A1 agonists, the A2 agonist 5′-(N-cyclopropyl)-carboxamidoadenosine (CPCA) alone caused a small increase in protein secretion from rabbit LG acini.23 When added with carbachol, a potentiation of secretion also occurred. Using antagonists to A2A and A2B receptors, only the A2B antagonist blocked the potentiation. The A2B antagonist also blocked the potentiation of secretion with carbachol and adenosine.23 However, nature of the interactions between the 2 pathways was not investigated. A2 receptors have been shown to stimulate AC to increase cAMP. Therefore, similar to A1 receptors, A2 receptors could also inhibit ERK 1/2 to potentiate secretion in the LG (Fig. 2).
Based on these 2 studies, in the LG, the effects of adenosine on protein secretion are mediated through the A1 and A2B purinergic receptors and they can also interact with the M3AchR-stimulated pathway to cause potentiation of secretion.
P2X Receptors in the LG
The first evidence of purinergic receptors in the LG was the discovery by Sasaki and Gallacher of cation channels that were activated by ATP to increase Ca2+ influx without generation of phosphoinostides, which was consistent with P2X receptors.31 Using RT-PCR, the message of all P2X receptors have been found in rat LG.27,38 All P2X receptors, with the exception of P2X5, have been shown to be expressed in rat acinar cells by Western blot analysis and immunofluorescence microscopy.27 Therefore, it is not clear if P2X5 message is translated into protein. In human accessory LG, genes for P2X1, P2X4, and P2X5 were identified by laser microdissection and cDNA microarray analysis.39
In a cell preparation containing predominately rat LG acinar cells, ATP stimulated an increase in [Ca2+]i and protein secretion.27 Removal of extracellular Ca2+ did not have a significant effect on the plateau phase of the [Ca2+]i response indicating that P2Y receptors play a small role in ATP-stimulated increase in [Ca2+]i. To identify which P2X receptors are being activated, our laboratory took advantage of individual characteristics of each type of P2X receptor in regard to its response to divalent cations, ability to be desensitized, pH, and specific agonists/antagonists. P2X3, with a small contribution by P2X7, was found to mediate the majority of ATP response.
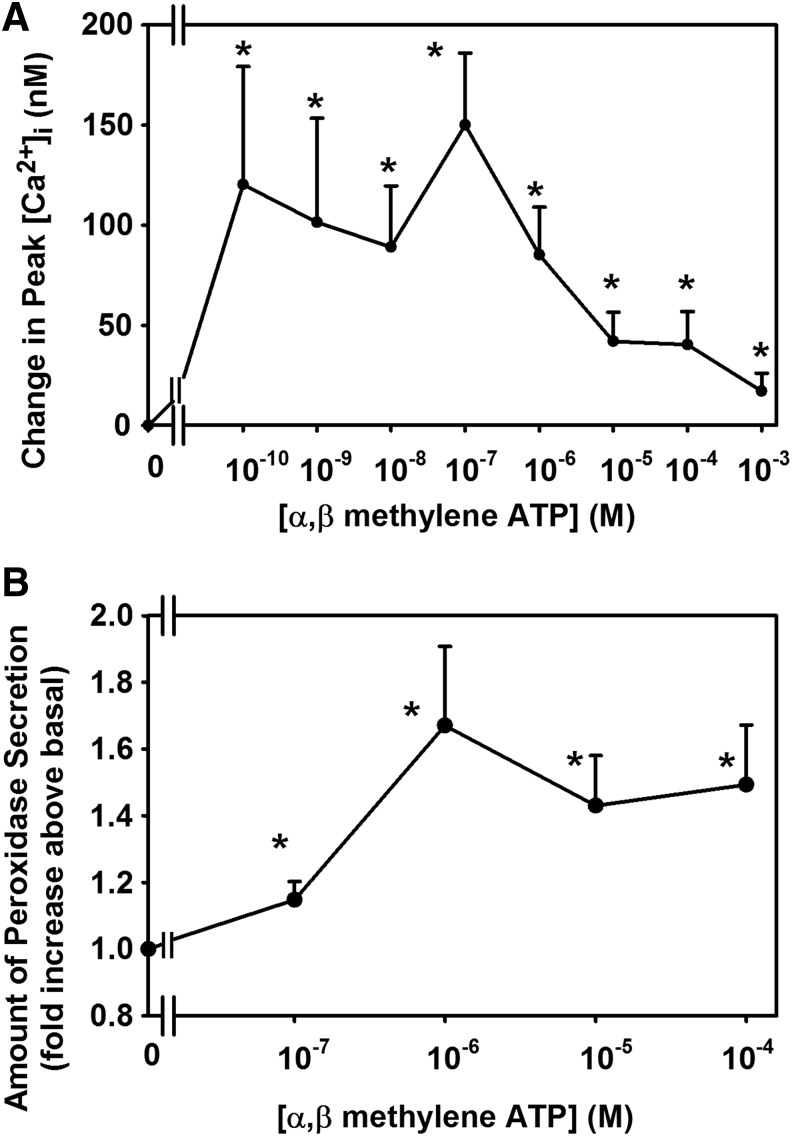
α,β Methylene ATP, a P2X3 agonist, increased [Ca2+]i and protein secretion in LG acini (Fig. 3). The intracellular Ca2+ response to α,β methylene ATP is biphasic, but the secretory response is not. A possible explanation for this discrepancy is that secretion is dependent on other signaling components in addition to the change in [Ca2+]i such as activation of PKC, ERK 1/2, and PLD. Inhibitors of P2X3 and P2X7 blocked the increase in [Ca2+]i and protein secretion in the LG acini.27
FIG. 3.
Effect of stimulation of P2X3 receptors on intracellular [Ca2+] and protein secretion. Rat LG acini were stimulated with the P2X3 receptor agonist, α,β methylene ATP, and intracellular Ca2+ (A), and protein secretion (B) was determined. Reprinted from Hodges, et al. Invest. Ophthalmol. Vis. Sci. 52:3254–3263, 2011. Copyright holder is Association for Research in Vision and Ophthalmology.
In contrast to acinar cells, a specific P2X3 agonist caused only a minor increase in [Ca2+]i in cultured rat LG MECs.40 In cultured MECs, the P2X7 receptor was identified by RT-PCR, Western blot analysis, and immunofluorescence microscopy.40 Although, the specific P2X7 receptor agonist benzoybenzoyl ATP (BzATP) increased [Ca2+]i, the majority of ATP response in MECs was mediated by P2Y receptors.40 The difference in the function of P2X7 in LG acini compared to cultured MECs suggests that the Ca2+ signal from acinar clumps was predominantly from acinar cells. This P2X7 characterization on MECs will be discussed in the P2Y section. No other P2X receptors were studied in MECs.
P2X7 is the most studied of purinergic receptors in the LG. P2X7 receptors have been found in both acinar and MECs.27,40 These receptors are present on both the apical and basolateral membranes of acinar and ductal cells (Fig. 4),27 but only on the basolateral membranes of the parotid gland acinar cells.41 In the LG, BzATP stimulates an increase in [Ca2+]i, that, was inhibited by specific P2X7 inhibitors.27 BzATP also caused an increase protein secretion and activation of ERK 1/2, both of which were inhibited by P2X7 receptor inhibitors.27
FIG. 4.
Localization of P2X7 receptors in rat LG. Immunofluorescence micrograph of the localization of P2X7 receptors is shown in (A), while phallodin conjugated to rhodamine is shown in (B). Merged image of P2X7 (green) and phallodin (red), and overlap (yellow) is shown in (C). Control peptide is shown in (D). Arrows show basal and apical membranes of acinar cells, while arrowheads indicate basal membranes of ductal cells. Magnification 200 ×, inset 400 ×. Reprinted from Hodges et al. Invest. Ophthalmol. Vis. Sci. 50:5681–5689, 2009. Copyright holder is Association for Research in Vision and Ophthalmology. Color images available online at www.liebertpub.com/jop
In acinar cells, P2X7 receptors were found to be coupled to multiple cellular pathways, namely the pathways activated by M3AchR and α1D-ARs (Fig. 5). Simultaneous activation of P2X7 receptors and M3AchR resulted in a potentiation of increase in [Ca2+]i from rat LG acini.24 However, the amount of protein secretion obtained by simultaneous addition of these 2 agonists was not potentiated, but was additive. These results indicate that while the pathways interact in the release of [Ca2+]i, there was no interaction in the pathways leading to protein secretion.
FIG. 5.

Interaction of P2X7 receptors with cholinergic and α1D-adrenergic receptors in rat LG. Ach, acetylcholine; M2Ach, muscarinic receptor type 2; M3Ach, muscarinic receptor type 3, PLC, phospholipase C; PLD, phospholipase D; α1D-AR, α1D adrenergic receptor; P2X7R, P2X7 receptor; norepi, norepinephrine; NO, nitric oxide; cGMP, cyclic guanosine monophosphate; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor. Reprinted from Sanderson et al. Exp. Eye Res. 127:270–279, 2014.
To confirm that M3AchR and P2X7 receptors use the same intracellular Ca2+ pools, 1 agonist (either carbachol or BzATP) was added to acini and the [Ca2+]i measured. The second agonist was then added and the [Ca2+]i measured. The [Ca2+]i response of the second agonist was significantly decreased compared to the response seen when the same agonist was added first, indicating that M3AchR and P2X7 use the same intracellular pool of [Ca2+]i. Furthermore, the increase in [Ca2+]i stimulated by BzATP was inhibited by a M3AchR inhibitor, while the carbachol response was not inhibited by P2X7 receptor antagonist, indicating that M3AchR activation precedes activation of the P2X7 receptors.24
In pancreatic acini, protein secretion (exocytosis) induced by G protein-coupled receptors is stimulated by increasing the [Ca2+]i in the apical microdomain. This increase allows local signaling to take place without placing a Ca2+ overload throughout the cell and to keep the increase in [Ca2+]i from interfering with the Ca2+ replenishment process that needs to take place in the basolateral region.42 ATP increases [Ca2+]i first in the basolateral region, but cholinergic agonists increase [Ca2+]i first in the apical region. If only an increase in the apical [Ca2+]i near the secretory granules is used for exocytosis of protein, then BzATP and cholinergic agonists could potentiate the increase in global Ca2+ signaling, as measured in this study without potentiating protein secretion. This suggestion is also supported by the finding that only a maximum of about 5% of secretory granules can be released upon stimulation of LG acini.
Furthermore, protein secretion in LG acini is dependent upon other intracellular signaling molecules such as PKC, in addition to intracellular Ca2+. In addition, the same intracellular Ca2+ pool used by P2X7 and M3AchR could be the apical pool surrounding the secretory granules, probably not the depletion of endoplasmic reticulum Ca2+. Further experiments are needed, however, to determine the identity of the intracellular Ca2+ pool.
PKC, the other major protein activated by M3AchR does not appear to be involved in the interactions between these 2 receptors.13 ATP release stimulated by M3AchR activation was detected only when using LG pieces, but not isolated acini. LG pieces contain myoepithelial and ductal cells and nerve endings, while acini preparation is composed of acinar and MECs. As the [Ca2+]i and protein secretion experiments were performed using acini, M3AchR on acinar cells are not using ATP to activate P2X7 receptors, but must be doing so through a different mechanism.24 In P2X7-knockout mice, Novak et al. showed that the increase in [Ca2+]i stimulated by M3AchR agonists was increased compared to wild-type mice. This observation suggests that M3AchR agonists stimulate an increase in ATP to activate P2X7 receptors, which in turn downregulate [Ca2+]i in the intact LG.43 This effect was also observed in the parotid gland, but not in the pancreas.43 Interestingly, in these mice, protein secretion stimulated by carbachol was unchanged compared to wild-type mice (Fig. 6).
FIG. 6.
Effect of absence of P2X7 receptors on protein secretion in LG acini. Acini from WT and P2X7 receptor-knockout mice (P2X7−/−) were isolated and protein secretion measured in response to the M3AchR agonist Cch or the α1DAR agonist Ph. Data are mean ± standard error of the mean of 4 individual animals. * Indicates significant difference from basal; # indicates significance from WT Ph response. Cch, carbachol; Ph, phenylephrine; WT, wild type.
P2X7 receptors also interact with the α1D-ARs, although in a very different manner than M3AchRs (Fig. 5). However, the molecular mechanism of the interaction is not known. Simultaneous addition of the 2 agonists was not different from the calculated additivity, indicating separate mechanisms to increase [Ca2+]i.21 However, an inhibitor of α1D-AR inhibited BzATP-stimulated increase in [Ca2+]i and an inhibitor of P2X7 receptor blocked phenylephrine (an α1D-adrenergic agonist)-stimulated increase in [Ca2+]i. Phenylephrine-stimulated increase in [Ca2+]i was blocked by inhibitors of PKC and calcium/calmodulin-dependent kinase, while BzATP-stimulated increase in [Ca2+]i was not inhibited by either. Despite the interaction of the α1D-AR and P2X7 receptors at the intracellular Ca2+ level, no interaction occurred with protein secretion.21
In contrast to cholinergic agonists, phenylephrine causes the release of ATP from LG pieces and acini. Also, in contrast to cholinergic agonists, protein secretion stimulated by phenylephrine in LGs of P2X7-knockout mice was significantly decreased compared to protein secretion from wild-type mice (Fig. 6).
Collectively, these data indicate that activation of the P2X7 receptor stimulates protein secretion and can augment protein secretion stimulated by neurotransmitters.
The LG is not unique in terms of overlap of signaling pathways activated by purinergic receptors and cholinergic and adrenergic receptors. In the parotid gland activating P2X4 receptors, 1 of the major P2X receptors in this gland, with a low concentration of ATP and increasing intracellular cAMP either pharmacologically or with the β-adrenergic receptor agonist isoprenaline, significantly increased the increase in [Ca2+]i from that obtained with ATP alone. Similar results were obtained with the P2X7 receptor activation, the other major P2X receptor in the parotid gland.44 Interactions with these 2 purinergic receptors and β-adrenergic receptors were mediated through both protein kinase A and Epac, and the release of Ca2+ from the endoplasmic reticulum in an InsP3-mediated manner. P2X7 antagonists in the parotid gland were also shown to decrease the rise in [Ca2+]i stimulated by the muscarinic agonist carbachol.44
In addition to release of ATP from nerve endings and cells under normal conditions, ATP is also released in stressful situations such as trauma or inflammation.45 It has been shown that ATP, through the P2X7 receptors, opens cation channels, which depolarize plasma and mitochondrial membranes creating pores, which in turn result in membrane blebbing, production of reactive oxygen species, and eventually cell death.46
While there are no studies investigating the deleterious effects of the P2X7 receptors in the LG, activation of these receptors in submandibular glands results in plasma membrane blebbing and caspase activation.45 This implies that ATP plays a duel role in cells, namely in normal homeostasis to stimulate protein secretion and an important role in inflammation. It is possible that activation of P2X7 receptors plays a role in the pathogenesis of diseases of the LG such as Sjogren's syndrome. Interestingly, surface expression of P2X7 receptors was found to be increased on peripheral blood mononuclear cells in patients with primary Sjogren's syndrome compared to controls.47 Much work remains to be done to investigate these 2 differing functions.
P2Y Receptors
One of the earliest pieces of evidence for P2Y receptors in LG acinar cells was the study by Gromada et al.,48 in which they show that addition of ATP stimulated an increase in [Ca2+]i and InsP3. The increases in both compounds were blocked by an inhibitor of PLC, implying that a GPCR was involved in these processes. In addition, the increase in both [Ca2+]i and InsP3 by ATP was regulated by PKC, in that, activation of PKC decreased and inhibition of PKC increased the responses.48 The type of P2Y receptor(s) was not identified.
Using RT-PCR, the messages for P2Y1, -2, -4, -12, -11, -13, and -14 were found to be expressed in rat LG acinar cells.38,40 In another study, P2Y2 receptor message was undetectable in rat LGs by Northern blot analysis.33 However, only P2Y1, -11, and -13 have been shown to be translated to protein as determined by Western blot analysis and immunofluorescence microscopy.40
Similar to the results obtained by Gromada et al.48 and Kamada et al.,38 we demonstrated that ATP stimulated an increase in [Ca2+]i in rat acinar cells that was blocked by a PLC inhibitor. This response was also inhibited by the general P2Y receptor antagonist reactive blue-2. In this acinar cell preparation, P2X agonists (BzATP and α,β-meATP) had only a small effect on [Ca2+]i, while the P2Y agonist 2-MeSATP, a preferential agonist for P2Y1 and -11 receptors, gave a robust increase in [Ca2+]i. UTP, a P2Y2, -4, and -6 agonist, did not have an effect on [Ca2+]i.38 These results imply that P2Y1 and -11 receptors are the predominant P2Y receptors in the LG. No further characterization of P2Y receptors in acinar cells has been done.
Only 3 studies have investigated purinergic receptors in MECs. By immunofluorescence microscopy, only P2X7 of all the P2X receptors was present in MECs in LG sections.40 For P2Y receptors, P2Y1, -11, and -16 were detected in MECs in LG sections. All of P2X and P2Y receptors identified in situ were also detected in cultured MECs. For function, ATP increased [Ca2+]i in MECs in the LG in situ38 and in cultured MECs (Fig. 7). In cultured MECs, the P2X7 agonist BzATP and the P2Y agonist UTP stimulated robust increases in [Ca2+]i (Fig. 7).40 2-MeSATP, the P2Y1 and -11 agonist, gave a substantial increase in [Ca2+]i and ATPγS, a P2Y11 agonist, had a greater effect.40
FIG. 7.
Effect of purinergic agonists on intracellular [Ca2+] from cultured rat MECs. Cultured MECs were stimulated with the P2X agonists BzATP, ATP, and α,β methylene ATP (A) and P2Y agonists UTP, MeSATP, and ATPγS (B). Reprinted from Ohtomo et al. Invest. Ophthalmol. Vis. Sci. 52:9503–9515, 2011. Copyright holder is Association for Research in Vision and Ophthalmology. BzATP, benzoylbenzoylATP; MECs, myoepithelial cells; MeSATP, methylthioATP.
In these cells, the BzATP response was neither inhibited by several P2X7 receptor antagonists nor did it have other characteristics of the P2X7 receptors such as potentiation of the response in the absence of Mg2+, dependence on extracellular Ca2+, and lack of desensitization with repeated applications. Instead, the BzATP-stimulated increase in [Ca2+]i was substantially blocked by PLC and P2Y1 inhibitors and partially blocked with P2Y11 and -13 inhibitors.40 Additional experiments comparing MECs in LG acini in situ with cultured MECs are needed, however, results obtained to date have not shown any difference in MECs in situ or in culture.
There have not been any studies to date investigating any potential interactions between P2Y and other receptors present in the LG.
In conclusion, P2X3 and P2X7 receptors play important roles in the normal function of the LG. The interactions between the major pathways activated by neurotransmitters and those activated by P2X7 receptors are likely key in modulating LG responses, while interactions between P2X3 receptors and neurotransmitters have yet to be investigated. Activation of P2X7 receptors in the LG does not appear to be harmful to the LG acini under normal nonpathological conditions, although could play a role under pathological conditions. P2Y receptors also likely play a role in the normal LG function. These receptors are predominately located on the MECs indicating that they are important in the functions of this cell type. Much work remains to be done to understand the pathways activated by purinergic receptors and their potential interactions with other stimuli of secretion in the LG.
Acknowledgment
This work was supported by NIH RO1 EY06177 and P30 EY00379035.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Dartt D.A., and Willcox M.D. Complexity of the tear film: importance in homeostasis and dysfunction during disease. Exp. Eye Res. 117:1–3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Royle L., et al. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. Glycoconj. J. 25:763–773, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Dartt D.A. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog. Retin. Eye Res. 28:155–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parod R.J., Leslie B.A., and Putney J.W., Jr. Muscarinic and alpha-adrenergic stimulation of Na and Ca uptake by dispersed lacrimal cells. Am. J. Physiol. 239:G99–G105, 1980 [DOI] [PubMed] [Google Scholar]
- 5.Herzog V., Sies H., and Miller F. Exocytosis in secretory cells of rat lacrimal gland. Peroxidase release from lobules and isolated cells upon cholinergic stimulation. J. Cell Biol. 70:692–706, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagato T., et al. A scanning electron microscope study of myoepithelial cells in exocrine glands. Cell Tissue Res. 209:1–10, 1980 [DOI] [PubMed] [Google Scholar]
- 7.Reversi A., Cassoni P., and Chini B. Oxytocin receptor signaling in myoepithelial and cancer cells. J. Mammary Gland Biol. Neoplasia. 10:221–229, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Mauduit P., Jammes H., and Rossignol B. M3 muscarinic acetylcholine receptor coupling to PLC in rat exorbital lacrimal acinar cells. Am. J. Physiol. 264(Pt 1):C1550–C1560, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Meneray M.A., Fields T.Y., and Bennett D.J. Gs and Gq/11 couple vasoactive intestinal peptide and cholinergic stimulation to lacrimal secretion. Invest. Ophthalmol. Vis. Sci. 38:1261–1270, 1997 [PubMed] [Google Scholar]
- 10.Dartt D.A., et al. Lacrimal gland inositol trisphosphate isomer and inositol tetrakisphosphate production. Am. J. Physiol. 259(Pt 1):G274–G281, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Zoukhri D., et al. Role of protein kinase C in cholinergic stimulation of lacrimal gland protein secretion. FEBS Lett. 351:67–72, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodges R.R., et al. Alpha 1-adrenergic and cholinergic agonists use separate signal transduction pathways in lacrimal gland. Am. J. Physiol. 262(Pt 1):G1087–G1096, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Zoukhri D., and Dartt D.A. Cholinergic activation of phospholipase D in lacrimal gland acini is independent of protein kinase C and calcium. Am. J. Physiol. 268(Pt 1):C713–C720, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Hodges R.R., et al. Phospholipase D1, but not D2, regulates protein secretion via Rho/ROCK in a Ras/Raf-independent, MEK-dependent manner in rat lacrimal gland. Invest. Ophthalmol. Vis. Sci. 52:2199–2210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ota I., et al. Alpha 1-adrenergic and cholinergic agonists activate MAPK by separate mechanisms to inhibit secretion in lacrimal gland. Am. J. Physiol. Cell Physiol. 284:C168–C178, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Dartt D.A., et al. Vasoactive intestinal polypeptide stimulation of protein secretion from rat lacrimal gland acini. Am. J. Physiol. 247(Pt 1):G502–G509, 1984 [DOI] [PubMed] [Google Scholar]
- 17.Hodges R.R., et al. Identification and cellular localization of the components of the VIP signaling pathway in the lacrimal gland. Adv. Exp. Med. Biol. 438:169–176, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Funaki C., Hodges R.R., and Dartt D.A. Role of cAMP inhibition of p44/p42 mitogen-activated protein kinase in potentiation of protein secretion in rat lacrimal gland. Am. J. Physiol. Cell Physiol. 293:C1551–C1560, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Hodges R.R., et al. Nitric oxide and cGMP mediate alpha1D-adrenergic receptor-Stimulated protein secretion and p42/p44 MAPK activation in rat lacrimal gland. Invest. Ophthalmol. Vis. Sci. 46:2781–2789, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L., et al. Effects of alpha1D-adrenergic receptors on shedding of biologically active EGF in freshly isolated lacrimal gland epithelial cells. Am. J. Physiol. Cell Physiol. 291:C946–C956, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dartt D.A., and Hodges R.R. Interaction of alpha1D-adrenergic and P2X(7) receptors in the rat lacrimal gland and the effect on intracellular [Ca2+] and protein secretion. Invest. Ophthalmol. Vis. Sci. 52:5720–5729, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoukhri D., et al. Lacrimal gland PKC isoforms are differentially involved in agonist-induced protein secretion. Am. J. Physiol. 272(Pt 1):C263–C269, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Carlsson S.K., et al. Adenosine A2 receptor presence and synergy with cholinergic stimulation in rabbit lacrimal gland. Curr. Eye Res. 35:466–474, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Dartt D.A., and Hodges R.R. Cholinergic agonists activate P2X7 receptors to stimulate protein secretion by the rat lacrimal gland. Invest. Ophthalmol. Vis Sci. 52:3381–3390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edman M.C., et al. Functional expression of the adenosine A1 receptor in rabbit lacrimal gland. Exp. Eye Res. 86:110–117, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Hodges R.R., et al. Identification of P2X(3) and P2X(7) purinergic receptors activated by ATP in rat lacrimal gland. Invest. Ophthalmol. Vis. Sci. 52:3254–3263, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodges R.R., et al. Characterization of P2X7 purinergic receptors and their function in rat lacrimal gland. Invest. Ophthalmol. Vis. Sci. 50:5681–5689, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson P.J., et al. Ectoenzymes control adenosine modulation of immunoisolated cholinergic synapses. Nature. 327:232–234, 1987 [DOI] [PubMed] [Google Scholar]
- 29.Fitz J.G. Regulation of cellular ATP release. Trans. Am. Clin. Climatol. Assoc. 118:199–208, 2007 [PMC free article] [PubMed] [Google Scholar]
- 30.Burnstock G. Purine and pyrimidine receptors. Cell. Mol. Life Sci. 64:1471–1483, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki T., and Gallacher D.V. Extracellular ATP activates receptor-operated cation channels in mouse lacrimal acinar cells to promote calcium influx in the absence of phosphoinositide metabolism. FEBS Lett. 264:130–134, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Vincent P. Cationic channels sensitive to extracellular ATP in rat lacrimal cells. J. Physiol. 449:313–331, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agca C., et al. Development of a novel transgenic rat overexpressing the P2Y(2) nucleotide receptor using a lentiviral vector. J. Vasc. Res. 46:447–458, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dartt D.A., et al. Role of cyclic AMP and Ca2+ in potentiation of rat lacrimal gland protein secretion. Invest. Ophthalmol. Vis. Sci. 29:1732–1738, 1988 [PubMed] [Google Scholar]
- 35.Mauduit P., Herman G., and Rossignol B. Newly synthesized protein secretion in rat lacrimal gland: post-second messenger synergism. Am. J. Physiol. 253(Pt 1):C514–C524, 1987 [DOI] [PubMed] [Google Scholar]
- 36.Mauduit P., Herman G., and Rossignol B. Protein secretion in lacrimal gland: alpha 1-beta-adrenergic synergism. Am. J. Physiol. 250(Pt 1):C704–C712, 1986 [DOI] [PubMed] [Google Scholar]
- 37.Finkelberg A., et al. Endogenous signalling system involved in parotid gland adenosine A(1) receptor-amylase release. Acta Physiol. (Oxf.). 186:29–36, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Kamada Y., et al. P2Y purinoceptors induce changes in intracellular calcium in acinar cells of rat lacrimal glands. Histochem. Cell Biol. 137:97–106, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Ubels J.L., et al. Gene expression in human accessory lacrimal glands of Wolfring. Invest. Ophthalmol. Vis. Sci. 53:6738–6747, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohtomo K., et al. Increase of intracellular Ca2+ by purinergic receptors in cultured rat lacrimal gland myoepithelial cells. Invest. Ophthalmol. Vis. Sci. 52:9503–9515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhattacharya S., et al. Distinct contributions by ionotropic purinoceptor subtypes to ATP-evoked calcium signals in mouse parotid acinar cells. J. Physiol. 590(Pt 11):2721–2737, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen O.H. Ca(2)(+) signalling in the endoplasmic reticulum/secretory granule microdomain. Cell Calcium. 58:397–404, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Novak I., Jans I.M., and Wohlfahrt L. Effect of P2X(7) receptor knockout on exocrine secretion of pancreas, salivary glands and lacrimal glands. J. Physiol. 588(Pt 18):3615–3627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattacharya S., et al. Crosstalk between purinergic receptors and canonical signaling pathways in the mouse salivary gland. Cell Calcium. 58:589–587, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods L.T., et al. P2X7 receptor activation induces inflammatory responses in salivary gland epithelium. Am. J. Physiol. Cell Physiol. 303:C790–801, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiley J.S., et al. The human P2X7 receptor and its role in innate immunity. Tissue Antigens. 78:321–332, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Xie B., et al. The expression of P2X7 receptors on peripheral blood mononuclear cells in patients with primary Sjogren's syndrome and its correlation with anxiety and depression. Clin. Exp. Rheumatol. 32:354–360, 2014 [PubMed] [Google Scholar]
- 48.Gromada J., Jorgensen T.D., and Dissing S. Role of protein kinase C in the regulation of inositol phosphate production and Ca2+ mobilization evoked by ATP and acetylcholine in rat lacrimal acini. Pflugers Arch. 429:578–586, 1995 [DOI] [PubMed] [Google Scholar]