Dormancy is a state of growth cessation that allows bacteria to escape the host defense system and antibiotic challenge. Understanding the mechanisms that control dormancy is of key importance for the treatment of latent infections, such as those from Mycobacterium tuberculosis. In mycobacteria, dormancy is controlled by the response regulator DevR, which responds to conditions of hypoxia. Here, we show that OsdR of Streptomyces coelicolor recognizes the same regulatory element and controls a regulon that consists of genes involved in the control of stress and development. Only the core regulon in the direct vicinity of dosR and osdR is conserved between M. tuberculosis and S. coelicolor, respectively. Thus, we show how the system has diverged from allowing escape from the host defense system by mycobacteria to the control of sporulation by complex multicellular streptomycetes. This provides novel insights into how bacterial growth and development are coordinated with the environmental conditions.
KEYWORDS: Developmental control, Streptomyces, dormancy, stress response
ABSTRACT
Two-component regulatory systems allow bacteria to respond adequately to changes in their environment. In response to a given stimulus, a sensory kinase activates its cognate response regulator via reversible phosphorylation. The response regulator DevR activates a state of dormancy under hypoxia in Mycobacterium tuberculosis, allowing this pathogen to escape the host defense system. Here, we show that OsdR (SCO0204) of the soil bacterium Streptomyces coelicolor is a functional orthologue of DevR. OsdR, when activated by the sensory kinase OsdK (SCO0203), binds upstream of the DevR-controlled dormancy genes devR, hspX, and Rv3134c of M. tuberculosis. In silico analysis of the S. coelicolor genome combined with in vitro DNA binding studies identified many binding sites in the genomic region around osdR itself and upstream of stress-related genes. This binding correlated well with transcriptomic responses, with deregulation of developmental genes and genes related to stress and hypoxia in the osdR mutant. A peak in osdR transcription in the wild-type strain at the onset of aerial growth correlated with major changes in global gene expression. Taken together, our data reveal the existence of a dormancy-related regulon in streptomycetes which plays an important role in the transcriptional control of stress- and development-related genes.
IMPORTANCE Dormancy is a state of growth cessation that allows bacteria to escape the host defense system and antibiotic challenge. Understanding the mechanisms that control dormancy is of key importance for the treatment of latent infections, such as those from Mycobacterium tuberculosis. In mycobacteria, dormancy is controlled by the response regulator DevR, which responds to conditions of hypoxia. Here, we show that OsdR of Streptomyces coelicolor recognizes the same regulatory element and controls a regulon that consists of genes involved in the control of stress and development. Only the core regulon in the direct vicinity of dosR and osdR is conserved between M. tuberculosis and S. coelicolor, respectively. Thus, we show how the system has diverged from allowing escape from the host defense system by mycobacteria to the control of sporulation by complex multicellular streptomycetes. This provides novel insights into how bacterial growth and development are coordinated with the environmental conditions.
INTRODUCTION
Complex natural habitats of bacteria call for rapid response systems to ensure adaption to often-changing environmental conditions. One prevalent mechanism that bacteria such as streptomycetes use to couple environmental stimuli to adaptive responses consists of a sensor kinase (SK) and a cognate response regulator (RR), which act as a two-component signal transduction system (TCS) (Fig. 1) (1, 2). Upon stimulation of the sensory domain of the SK by an external signal, the SK autophosphorylates itself prior to the transfer of the phosphate to a conserved His residue in the RR (1). Typically, the activity of the RR is mediated through DNA binding, although RNA and protein binding activities as well as catalytic activities have also been reported (1, 2).
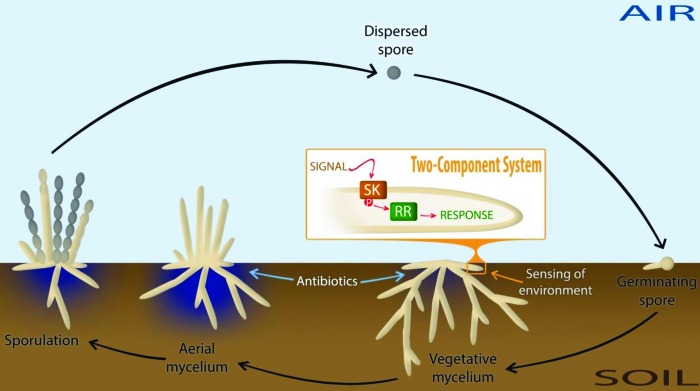
FIG 1 .
Life cycle of streptomycetes and environmental sensing of nutrients. The life cycle starts with the germination of spores, which grow out to form a branched network of vegetative hyphae. Under environmentally adverse conditions, such as nutrient depletion, streptomycetes initiate a complex developmental program whereby the vegetative mycelium serves as a substrate for a new so-called aerial mycelium. This stage of development usually corresponds with the production of secondary metabolites, such as antibiotics. Eventually, the aerial hyphae develop into chains of spores. Numerous regulatory networks exist in order to accurately sense and respond to the changing environmental conditions. Two-component systems (TCSs) couple the environmental stimulus (signal) of a sensor kinase (SK) to an adaptive response through phosphorylation of a cognate response regulator (RR), which exerts a regulatory response (usually through DNA binding), as illustrated in the orange box.
Soil-borne bacteria, such as streptomycetes, have developed intricate sensory systems to detect nutrient availability and to initiate appropriate response mechanisms. Streptomycetes are industrially important organisms and produce a wide range of natural products, including over 50% of all known antibiotics (3, 4). The bacteria have a complex mycelial lifestyle (Fig. 1) and produce a branching network of vegetative hyphae, which are compartmentalized by cross-walls, making Streptomyces a rare example of a multicellular prokaryote (5). Under environmentally adverse conditions, such as nutrient depletion, streptomycetes initiate a complex developmental program whereby the vegetative mycelium serves as a substrate for a new so-called aerial mycelium. Eventually, the outer part of the aerial hyphae develops into chains of spores (6). In turn, the spores are able to survive periods of unfavorable conditions, such as anaerobiosis (for example, as a result of heavy rainfall). Though the model organism Streptomyces coelicolor is able to survive anaerobic conditions, anaerobic growth has not been reported for this microorganism (7).
The environmental conditions of a streptomycete’s natural habitat are ever-changing, and the complexity of the signals that are received and of the responses that are transmitted is reflected in the large number of TCSs, with the genome of S. coelicolor encoding 85 sensory kinases and 79 response regulators, with 67 known sensor-regulator pairs (8). One such pair is made up of the SK SCO0203 and the RR SCO0204. Unusually, SCO0203 has a second cognate RR in addition to SCO0204, namely, the orphan response regulator SCO3818 (9). The deletion of either RR gene was shown to enhance the production of actinorhodin, the blue-pigmented antibiotic of Streptomyces coelicolor. Although no biochemical evidence was provided, it was previously suggested that sensory kinase SCO0203 may be a direct orthologue of DosT, an SK from a well-studied TCS from the pathogenic obligate aerobe Mycobacterium tuberculosis (10).
In M. tuberculosis, gradual oxygen depletion is sensed by two SKs (DosT and DevS [alternatively known as DosS]) and induces a regulon controlled by the response regulator DevR (alternatively known as DosR), which consists of some 50 genes, including universal stress proteins (USPs), nitroreductases (which allow anaerobic nitrate respiration), redox proteins, and heat shock proteins (11). It is thought that this TCS regulates the escape from the host defense system by promoting dormancy to survive anaerobic conditions, and it is likely that this nonreplicating state plays a major role in the resistance of the bacilli to antibiotics (12, 13). An orthologous oxygen-sensing mechanism in streptomycetes may be essential for the sensing of oxygen levels in soil; under conditions of oxygen depletion, the appropriate response needs to be activated to ensure survival. Alternatively, under nutrient availability (and sufficient oxygen), vegetative hyphae form a very dense mycelium, where oxygen is locally depleted, and this depletion might be regulated via SCO0203/SCO0204.
In this work, we suggest that the TCS pair SCO0203/SCO0204 regulates a dormancy-related response in S. coelicolor. Major changes are seen in the global transcription patterns of genes related to stress and development in SCO0204 null mutants. The predicted core regulon of SCO0204, which revolves around the region from SCO0167 to SCO0219 in the S. coelicolor genome, contains many dormancy regulon-related genes and is conserved between SCO0204 and the dormancy regulator, DevR, of M. tuberculosis. We show binding of SCO0204 upstream of M. tuberculosis genes that are part of the DevR regulon as well as binding to the predicted binding site in S. coelicolor, including direct binding to developmental genes (which lack a predicted binding site). The locus tags SCO0203 and SCO0204 were named osdK and osdR, respectively, to highlight their function in response to oxygen availability, stress, and development.
RESULTS
Analysis of the two-component regulatory system OsdKR.
SCO0203 (OsdK) and SCO0204 (OsdR) form a two-component regulatory system (9) and are encoded by the osdR-osdK operon. OsdK has 41% and 42% amino acid identity (57% amino acid similarity) with DevS and DosT, respectively (see Fig. S1 in the supplemental material), and it was postulated as a possible ortholog of the dormancy sensory kinases of M. tuberculosis (14). Indeed, of the 18 amino acid residues required for oxygen sensing (15, 16), 15/18 residues of DosT and 12/18 residues of DevS are conserved in OsdK (Fig. S1). The interaction between the RR DevR and its target site is known in structural detail (17). OsdR and DevR share 61% amino acid identity (79% amino acid similarity) (Fig. S1 and S2), and comparison of the residues in the DevR and OsdR proteins revealed that 11 of the 13 residues implicated in DNA binding are conserved between DevR and OsdR (Fig. S2).
Sequence alignment of the DNA binding domain of DevR with Streptomyces and Mycobacterium orthologues. The secondary-structure prediction was based on the crystal structure of DevR (PDB accession number 3C3W) and is shown on top. Conserved residues are shown in red, and boxes denote conservative substitutions. An upward arrow indicates residues involved in interactions with DNA; blue arrows indicate residues contacting nucleotide bases, and red arrows indicate residues making DNA phosphate oxygen contacts. Abbreviations: M. tuber, M. tuberculosis; Mmarin, Mycobacterium marinum; Msmeg, Mycobacterium smegmatis; Mvanba, Mycobacterium vanbaalenii; Shygro, Streptomyces hygroscopicus; Sscab, Streptomyces scabies; Scoeli, S. coelicolor; Sviola, Streptomyces violaceoruber; Ssirex, Streptomyces sp. strain Sirex. For accession numbers, see Materials and Methods. Download Figure S1, TIF file, 1 MB (1.1MB, tif) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Alignment of SCO0203 (OsdK) and SCO0204 (OsdR). Multiple alignments of OsdK with DevS and DosT of M. tuberculosis (A) and OsdR with DevR of M. tuberculosis and NarL of Pseudomonas aeruginosa (B) were created with ClustalW (digits indicate the amino acid number). The different protein domains are indicated in italic. Amino acids conserved in at least 80% of the sequences are shaded (identical amino acids are in black, and amino acids with similar properties are in gray). Important amino acids of DosT and DevS are indicated beneath and above the alignment, respectively. GAF, GAF domain; DHp, dimerization and histidine phosphotransfer domain; CA, C-terminal catalytic and ATP binding domain; REC, receiver domain; HTH_LuxR, helix-turn-helix LuxR domain; ♦, phosphorylation site; ○, RR activation; #, H-bond network from iron to the surface; *, hydrophobic space surrounding a heme; ~, contact with a propionate group heme; ●, ligand binding; ▲, surface crevice; ≈, heme binding; ■, cavity next to a ligand binding pocket. Download Figure S2, TIF file, 2.4 MB (2.5MB, tif) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
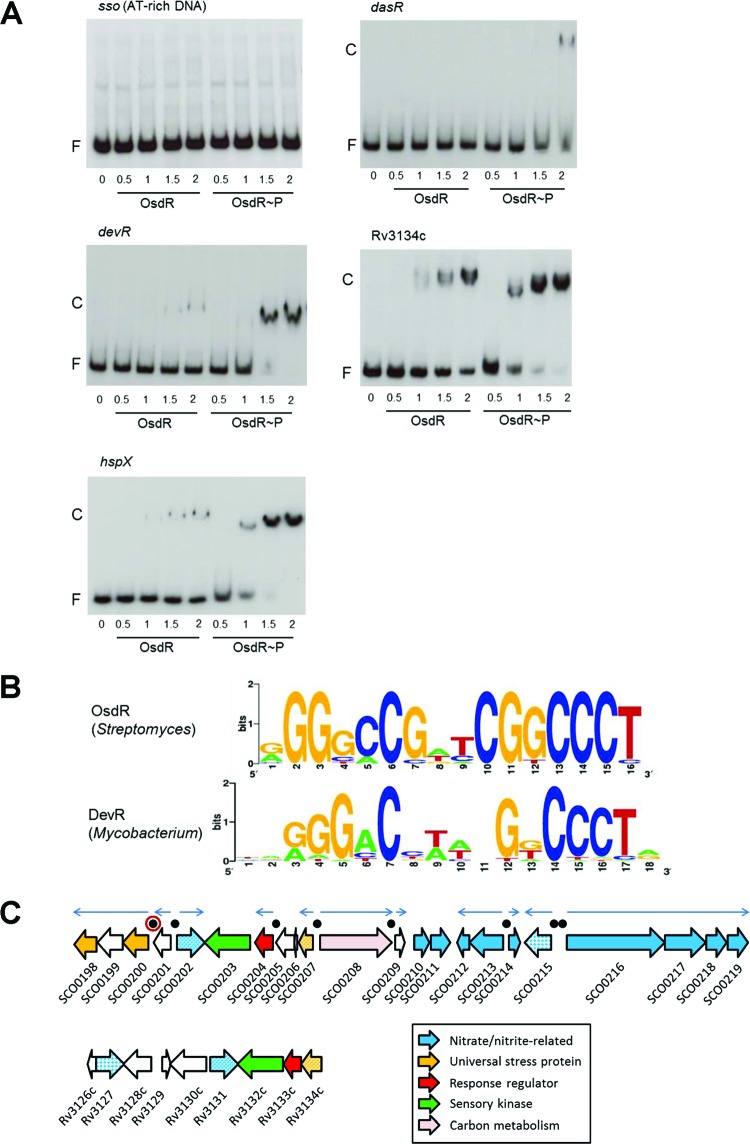
To test whether S. coelicolor OsdR could bind to the recognition site of M. tuberculosis DevR, electrophoretic mobility shift assays (EMSAs) were performed. His6-tagged OsdK and OsdR were purified, and the sensory kinase OsdK was autophosphorylated using 32P-radiolabeled ATP and then used to transphosphorylate OsdR (Fig. S3). OsdR transphosphorylation could be achieved with autophosphorylated OsdK. However, OsdR readily lost its phosphosignal in the presence of OsdK, as previously observed for DevRS/DosT. Therefore, acetyl phosphate (AcP) was used as phosphor donor (18). As probes for EMSAs we used three mycobacterial promoters that are known targets of DevR (18), namely, the promoters for devR, Rv3134c (which is located upstream of devR and encodes a universal stress domain protein), and hspX, which encodes a latency-related heat shock protein. As negative controls, DNA fragments of the upstream region of dasR of S. coelicolor and AT-rich DNA from Escherichia coli were used. OsdR bound with low affinity to the DNA fragment encompassing the dasR promoter region, while no binding was seen when AT-rich E. coli control DNA was used (Fig. 2A). Interestingly, OsdR bound well to all probes for the mycobacterial target genes (Fig. 2A). Furthermore, similar differential affinities for the three fragments were observed as described previously for DevR in M. tuberculosis (18), with stronger binding upstream of Rv3134c and hspX than to the autoregulatory site of devR. Nonphosphorylated OsdR bound significantly less efficiently to the probes. Taken together, these data strongly suggest that OsdR and the dormancy regulator DevR recognize the same upstream regulatory elements, with phosphorylation by OsdK required to enhance DNA binding.
FIG 2 .
Binding site of OsdR (SCO0204) and comparison to that of M. tuberculosis DevR. (A) EMSAs using purified His6-tagged OsdR on known targets of DevR in M. tuberculosis. Both phosphorylated OsdR (OsdR~P) and nonphosphorylated OsdR (OsdR) were used in the assays. OsdR~P was obtained after AcP phosphorylation in vitro. Numbers on the horizontal axis refer to micromolar concentrations. F, free DNA fragment; C, complexes of DNA and protein. (B) Weblogo representation of cis-regulatory elements identified upstream of osdR, identified from the upstream regions of osdR orthologues in 12 Streptomyces species (see Materials and Methods). For comparison, the upstream regulatory element recognized by DevR (97) is presented. (C) Gene synteny between the loci around osdR in S. coelicolor (top) and devR in M. tuberculosis (bottom). Functional categories are given in the figure, and black dots indicate predicted OsdR binding sites, with that of upsA (SCO0200) surrounded by a red ring. Orthologues are presented in the same colors, and when multiple genes with similar functions are present, they appear in patterns.
In vitro autophosphorylation and transphosphorylation of OsdRK. OsdK was readily phosphorylated as shown by the large band in the lane “auto.” Using autophosphorylated OsdK, OsdR was transphosphorylated as shown by the presence of a band of phosphorylated OsdR and OsdK in the lane “trans.” However, there was significant phosphosignal loss observed (a decrease in band intensity over time). The reaction mixtures were run on 3.5% acrylamide gels in 0.5× Tris-borate-EDTA (TBE) buffer. Gels were dried and then subjected to autoradiography for analysis. Download Figure S3, TIF file, 0.1 MB (140.8KB, tif) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In silico prediction of the OsdR regulon of S. coelicolor.
The OsdR consensus sequence was deduced by searching the upstream regions of osdR orthologues from 12 Streptomyces species for possible similar cis-acting regulatory elements using MEME (19). This identified a 16-nucleotide palindromic consensus sequence, 5′-AGGGCCGATCGGCCCT, which conforms well to the consensus sequence found in M. tuberculosis (Fig. 2B). The S. coelicolor genome was then scanned by PREDetector (20), using a position weight matrix (see Table S3 in the supplemental material) based on 12 predicted upstream elements as inputs. With a cutoff score of 8.0 for medium reliability (21), PREDetector identified putative binding sites for OsdR upstream of 27 transcription units. There was a total of 43 binding sites that may affect the transcription of 85 genes, which therefore may qualify as the direct OsdR response regulon (Table 1).
TABLE 1 .
Predicted binding sites for SCO0204 (OsdR) in Streptomyces coelicolor
| Locus taga |
Function | Sequence | Positionb | Scorec | Cotranscribed gene(s)d |
Function(s) |
|---|---|---|---|---|---|---|
| SCO0204c | OsdR, two-component response regulator |
AGGGCCGGTCGGCCCC | −81 | 13.74 | ||
| SCO0200c | Universal stress protein | GGGGCCGACCGTCCCT | −100 | 12.49 | SCO0199c/ SCO0198c |
Zinc-deprived alcohol dehydrogenase, universal stress protein |
| SCO0215c | Nitroreductase family protein | AGGGCCGTCCGGCCCC | −99 | 12.24 | ||
| SCO0208 | Pyruvate phosphate dikinase | CGGGCCGACCGGCCCT | −102 | 12.19 | ||
| −158 | ||||||
| SCO0207c | Universal stress protein | −144 | 10.08 | |||
| −88 | ||||||
| SCO5979 | Enoyl-CoA hydratase | CGGGACCTTCGGCCCT | −80 | 11.62 | SCO5980 | Bifunctional hydroxylase, oxidoreductase |
| SCO5978c | Hypothetical protein | −68 | ||||
| SCO2637 | Secreted serine protease | AGGGCCGGTCGGCCTT | −53 | 11.27 | ||
| SCO7188c | Subtilisin-like secreted peptidase | GGGGACGATCGTCCCC | −47 | 11.2 | ||
| SCO0039 | Hypothetical protein | AGGCCCGTTCCGCCCT | −132 | 10.86 | SCO0040/ SCO0041/ SCO0042 |
Glycosyl transferase, integral membrane protein, hypothetical protein |
| SCO0038c | Sigma factor | −130 | SCO0037c/ SCO0036c |
Sigma factor, hypothetical protein | ||
| SCO0168 | Crp-like regulatory protein | GAGGCCGGTCGGCCCT | −284 | 10.75 | ||
| GGGGCCGACGGTCCCT | −36 | 9.21 | ||||
| SCO0167c | Universal stress protein | AGGGACCTTCGGCCCC | −391 | 10.75 | ||
| −114 | 10.73 | |||||
| SCO0216 | Nitrate reductase alpha chain NarG2 | AGGGACCTTCGGCCCC | −53 | 10.73 | SCO0217/ SCO0218/ SCO0219 |
Nitrate reductase beta chain NarH2, nitrate reductase delta chain NarJ2, nitrate reductase delta chain NarI2 |
| SCO5410 | Hypothetical protein | AGGGCAGGACGGCCCT | +36 | 10.6 | ||
| SCO6041 | Protoporphyrinogen oxidase | GGGGCCGTCCGGCCCC | −51 | 10.57 | SCO6042 | Chlorite dismutase (oxygen-generating enzyme) |
| SCO6040c | Lipoprotein | −246 | SCO6039c | Flavoprotein oxidoreductase, CoA disulfide reductase |
||
| SCO3431 | EmrB/QacA subfamily transporter | GGGGCCGAACGGCCGT | +13 | 10.52 | ||
| SCO6164 | Hypothetical protein with DksA/TraR family C4 zinc finger domain |
GGGTCCGATCGGCCCG | −62 | 10.5 | ||
| SCO6163c | Sensor kinase | −334 | SCO6162c | Two-component system response regulator |
||
| SCO0517 | Possible Crp-like regulatory protein | GGGACCGACCGGCCCT | −248 | 10.49 | ||
| AGGGCCGGCCGGCCCG | −268 | 10.46 | ||||
| SCO3857 | Nosiheptide resistance regulator | GGGCCCGTTCGGCGCT | −271 | 10.34 | ||
| SCO3856c | Peptidyl-prolyl cis-trans isomerase | −66 | ||||
| SCO5251 | Puromycin N-acetyltransferase | AGGGCCGTACGGCACC | −243 | 10.31 | ||
| SCO2347 | Integral membrane protein | AGGGCCGAAAGTCCCG | −295 | 10.3 | ||
| SCO2348 | Secreted protein | −221 | ||||
| SCO0214 | Pyridoxamine 5′-phosphate oxidase | GGGGCCATCCGGCCCT | −50 | 10.18 | ||
| SCO0213c | Nitrate-nitrite transporter protein | −252 | SCO0212c | Hemerythrin cation binding domain-containing protein (oxygen transporting protein) |
||
| SCO0179c | Zinc-containing dehydrogenase | TGGGCCGGTCGGCCCC | −152 | 9.46 | ||
| SCO7021 | Secreted protein | AGGCCCGAACGGCCCA | −94 | 9.4 | SCO7022 | Hypothetical protein SC1H10.11. |
| SCO4412 | Regulatory protein | AGGGCGGAACGGCCGT | −261 | 9.34 | ||
| SCO0355 | Conserved hypothetical protein SCF41.14 |
AGGGCTGACCGGCCCG | −81 | 9.2 | SCO0356 | Probable oxidoreductase |
SCO numbers in boldface were tested by EMSA.
Position relative to the start of the gene.
The cutoff score calculated using the PREDetector algorithm and based on the position weight matrix in Table S3 in the supplemental material.
Genes known or predicted to be cotranscribed with the gene and therefore likely influenced by the regulatory element.
Eight binding sites were identified upstream of genes/operons in the vicinity of osdR, including osdR itself, controlling 20 of the 22 genes in the region between SCO0198 and SCO0219 (Fig. 2C). Comparison with the genomic region around M. tuberculosis devR revealed significant gene synteny (Fig. 2C). Of the 11 S. coelicolor genes for USP domain proteins, 8 are found in the genomic region between SCO0167 and SCO0021, and in M. tuberculosis, usp genes are part of the DevR regulon. SCO0213 to SCO0219 encode a nitrate transporter and nitrate reductase, which also prominently feature in the DevR regulon. When a lower cutoff score of 6.0 was used, PREDetector predicted a possible 27 elements in the regions SCO0167 to SCO0181 and SCO0198 to SCO0219.
Specificity analysis of OsdR binding to the predicted regulatory element of uspA.
To investigate whether OsdR binds specifically to the predicted nucleotide sequence, a 50-mer probe of the upstream region of uspA (SCO0200), centered on the predicted binding site, was used as a probe (see Table S2 in the supplemental material). Indeed, AcP-phosphorylated OsdR (OsdR~P) bound well to the DNA fragment (Fig. 3A). Some retarded DNA remained in the wells of the gel, likely due to bridging, whereas each of the monomers of the OsdR dimer bound to a different probe rather than to the same site, which can result in long concatemers, as was observed for, e.g., NagR in Bacillus subtilis (22) and DasR in S. coelicolor (23).
FIG 3 .
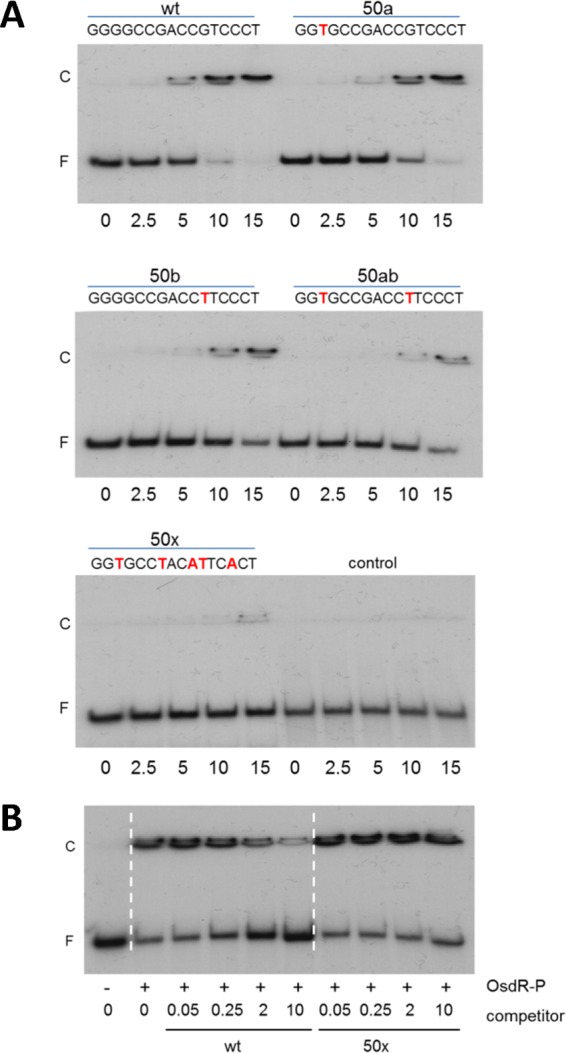
EMSAs with OsdR on a predicted S. coelicolor binding site. (A) Analysis of the OsdR binding site by mutation of highly conserved nucleotides in the uspA (SCO0200) binding site. Twenty femtomoles of a 50-mer DNA was incubated with increasing micromolar concentrations of OsdR~P. Replacements of the upsA binding site are indicated with red letters in the sequences; the 50-mer dasR fragment was used as a control. wt, wild type. (B) Competition assays using 10 µM protein and 20 fmol of labeled 50-mers centered on the uspA binding site. Increasing concentrations of the unlabeled competitor 50-mer were added, using either the wild-type uspA 50-mer or a mutated uspA 50-mer with 5 substitutions of highly conserved nucleotides (50x). − and + refer to the presence of phosphorylated OsdR (OsdR~P); competitor DNA is given in micromolar units. F, free DNA fragment; C, complexes of DNA and protein.
We then designed four mutant 50-mer probes containing single mutations (designated 50a and 50b), a double mutation (50ab), or a quintuple mutation (50x) of the most conserved nucleotides of the binding site. In line with the predicted importance of the conserved nucleotide positions in the consensus sequence (Fig. 2B), nucleotide permutations significantly decreased the binding of OsdR to the probes, such that the single G→T substitution at position 3 (50a) and the G→T substitution at position 11 (50b) lowered binding efficiency by around 50%, which was further reduced by mutating both positions (Fig. 3A). Binding was abolished when five of the conserved nucleotides were mutated (50x). We also performed a competition assay with unlabeled DNA on the radiolabeled wild-type 50-mer uspA probe. Increasing the amount of the unlabeled wild-type uspA probe strongly inhibited binding by OsdR, while addition of unlabeled competitor DNA with 5 permutations in the binding site (50x) had no effect on OsdR binding (Fig. 3B). Taken together, these experiments provide conclusive evidence that OsdR specifically recognizes the predicted regulatory element.
Verification of the regulon predictions by EMSAs.
Next we tested DNA binding by OsdR to predicted targets using EMSAs of PCR-amplified DNA probes (Table S2). These were uspA, osdR, SCO2637 (for a serine protease), and SCO2967 (for a carboxypeptidase), and the intergenic regions between the divergent genes SCO0207 and SCO0208 (for another USP domain protein and pyruvate phosphate dikinase), SCO5978 and SCO5979 (for a hypothetical protein and an enoyl coenzyme A [enoyl-CoA] hydratase), and SCO6040 and SCO6041 (for a lipoprotein and a protoporphyrinogen oxidase). All the predicted binding sites were bound by OsdR, with most probes fully bound by OsdR~P (at 1 µM), except SCO2637, which was bound with 2-fold-lower affinity (Fig. 4A). This suggests that phosphorylation (by OsdK) leads to enhanced binding of OsdR to its binding sites. The combined predictions and EMSA data reveal some 50 likely OsdR target genes or gene clusters, of which at least 13 have orthologues that are controlled by DevR in M. tuberculosis (osdR, SCO0167, SCO0198, uspA [SCO0200], and SCO0207 and genes for nitrate reductase subunits).
FIG 4 .
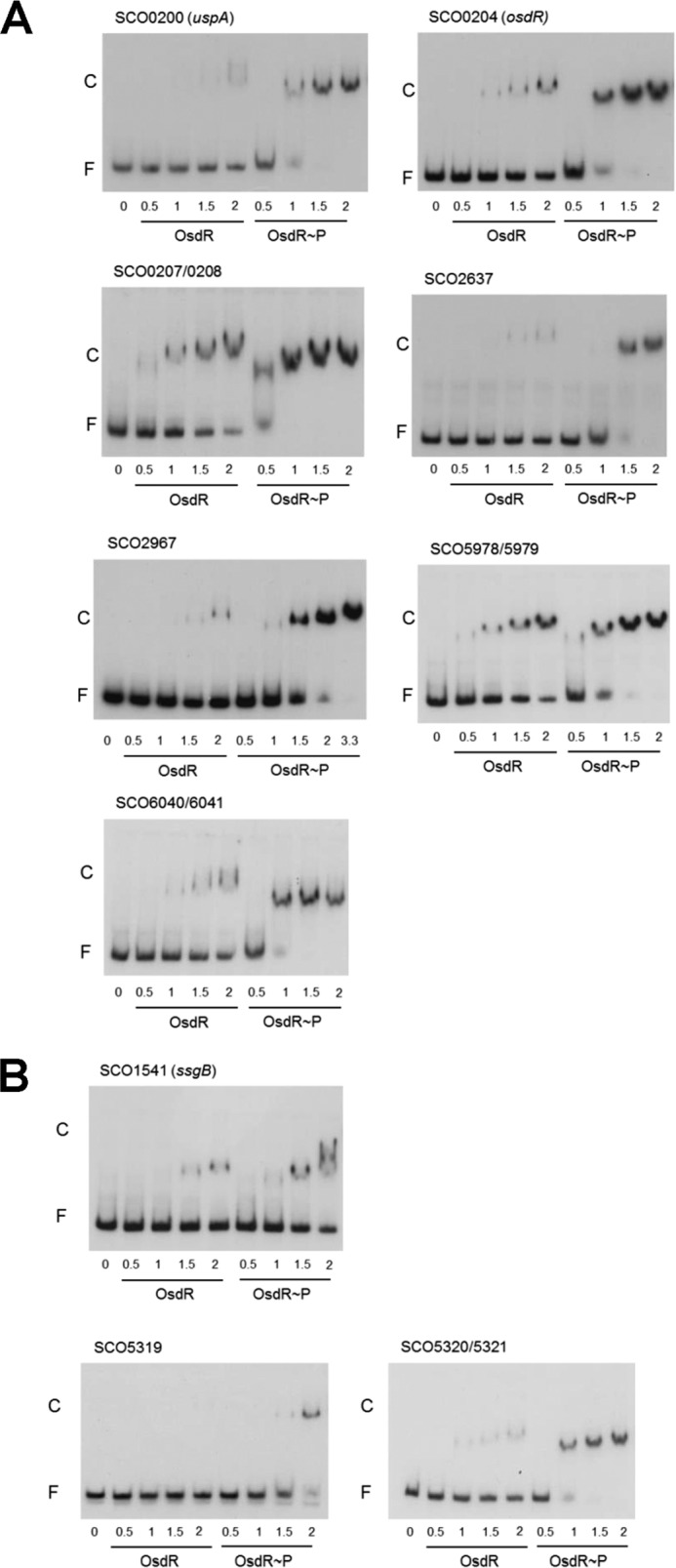
EMSAs with OsdR on selected S. coelicolor targets. (A) EMSAs were performed on DNA fragments harboring predicted binding sites upstream of the indicated genes. (B) EMSAs of ssgB (SCO1541) and whiE (SCO5319 and SCO5320-SCO5321). Concentrations of nonphosphorylated OsdR (OsdR) and phosphorylated OsdR (OsdR~P) are given in micromolar amounts. F, free DNA fragment; C, complexes of DNA and protein.
Transcriptional analysis of OsdR targets.
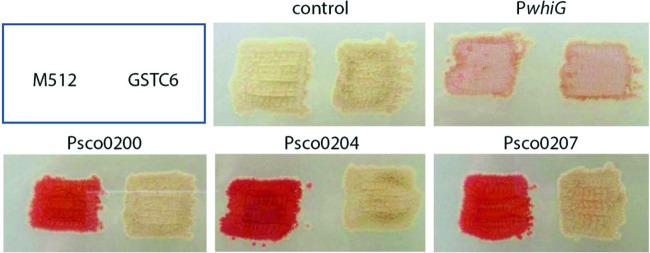
To analyze the transcriptional control by OsdR, promoter probing was performed using the Red promoter probing system (see Materials and Methods) in the nonpigmented S. coelicolor strain M512 and the M512 osdR mutant derivative GSTC6. Promoter-probe vectors harboring the upstream regions of uspA (SCO0200), osdR, and SCO0207 were introduced into S. coelicolor M512 and the mutant GSTC6, and the promoter activity was analyzed, with as a control the empty vector or the vector with the whiG promoter, which is transcribed constitutively (the developmental control of the gene product σWhiG is governed primarily at the posttranslational level). While the empty vector did not show activity and whiG transcription was not affected by the deletion of osdR, PSCO0200, PSCO0204, and PSCO0207 were all active in M512 but poorly or not expressed in the mutant (Fig. 5), strongly suggesting that the genes are transcriptionally activated by OsdR.
FIG 5 .
In vivo transcriptional analysis of OsdR targets. Promoter probing assays were performed for an analysis of the transcription of the promoters of uspA (SCO0200), osdR (SCO0204), and SCO0207 in the M512 osdR null mutant (GSTC6). As controls, the empty vector pIJ2587 and the whiG promoter were used.
Global transcription profiling of the osdR null mutant by DNA microarray analysis.
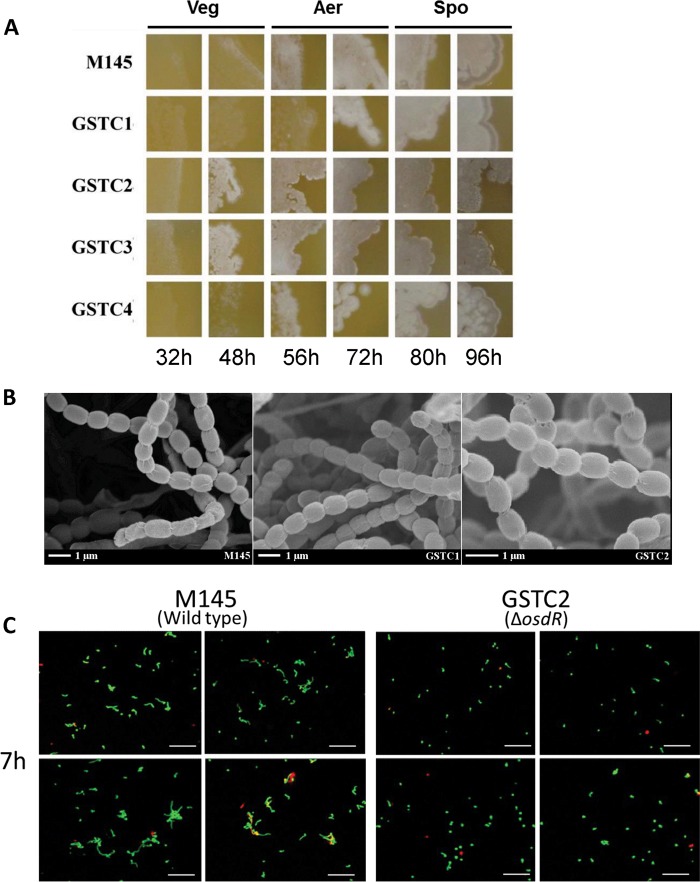
Phenotypic analysis of the M145 osdR null mutants GSTC2 and GSTC3 as well as M145 ΔosdK (GSTC1) and M145 ΔosdRK (GSTC4) on MS medium indicated earlier formation of mycelial hyphae in the osdR mutants and accelerated sporulation and enhanced production of the gray spore pigment (Fig. 6A). In the absence of both OsdR and OsdK, this phenotype was not observed. High-resolution imaging by cryo-scanning electron microscopy revealed that the spores had a normal morphology (Fig. 6B). Observation of the spores with laser confocal microscopy indicated a strong delay in the germination of spores of the osdR null mutant compared to that of spores of the parental strain (Fig. 6C). Staining of dead and viable spores showed that this delay in germination in the GSTC2 mutant was not due to extensive accumulation of dead spores, as the proportions of viable/dying spores were comparable between S. coelicolor M145 and its osdR mutant derivative GSTC2.
FIG 6 .
Phenotypic analysis of M145 OsdK and OsdR null mutants. (A) The different osdK and osdR mutants and their parent, S. coelicolor A3(2) M145, were grown on MS agar plates and monitored over time. Veg, vegetative growth; Aer, aerial growth; Spo, sporulation. (B) Phenotypic characterization of the osdK and osdR mutants and their parent, S. coelicolor M145, by cryo-scanning electron microscopy. Samples were prepared after 5 days of growth on MS. (C) Confocal fluorescence micrographs of germinating spores of S. coelicolor M145 and its osdR mutant GSTC2. Spores were inoculated onto MM agar and imaged after 7 h. Cells were stained with propidium iodide to identify dead cells (red) and with SYTO 9 green to identify living cells. GSTC1, M145 ΔosdK; GSTC2, M145 ΔosdR; GSTC3, M145 osdR in-frame deletion mutant; GSTC4, M145 osdRK double mutant.
To obtain a global overview of the effect of the deletion of osdR on transcription, microarray analysis was performed using RNA extracted from S. coelicolor M145 and its osdR null mutant GSTC2 grown on minimal medium (MM) agar plates overlaid with cellophane discs. Biomass was harvested at time points corresponding to vegetative growth (24 h), the onset of aerial growth (30 h), aerial growth (36 h), early sporulation (42 h), and sporulation (54 h) in the parental S. coelicolor M145. RNA from two independent biological replicate experiments was subsequently used as a template for cDNA synthesis/Cy3-dCTP labeling and subsequently hybridized onto oligonucleotide-based S. coelicolor whole-genome DNA microarrays (see Materials and Methods). By rank product analysis, a list of genes whose levels of expression were statistically significantly different was obtained at a percentage of false positives (PFP) of <0.01. With the additional cutoff of a minimum 2-fold change in the levels of transcription between the wild type and mutant, a list of over 800 genes whose transcription was significantly altered in the osdR null mutant was obtained (see Table S4 in the supplemental material). Classes of genes that were overrepresented were related to stress, anaerobic growth, and development. Notably, and as detailed further below, many of the genes that were differentially expressed between the wild type and osdR mutant had particularly strongly altered mRNA levels at 36 h. Suggestively, transcription of OsdR itself peaks at 36 h in wild-type cells, as shown in the present study and as established previously (see, e.g., reference 24).
Stress-related genes and the chromosomal region around osdRK.
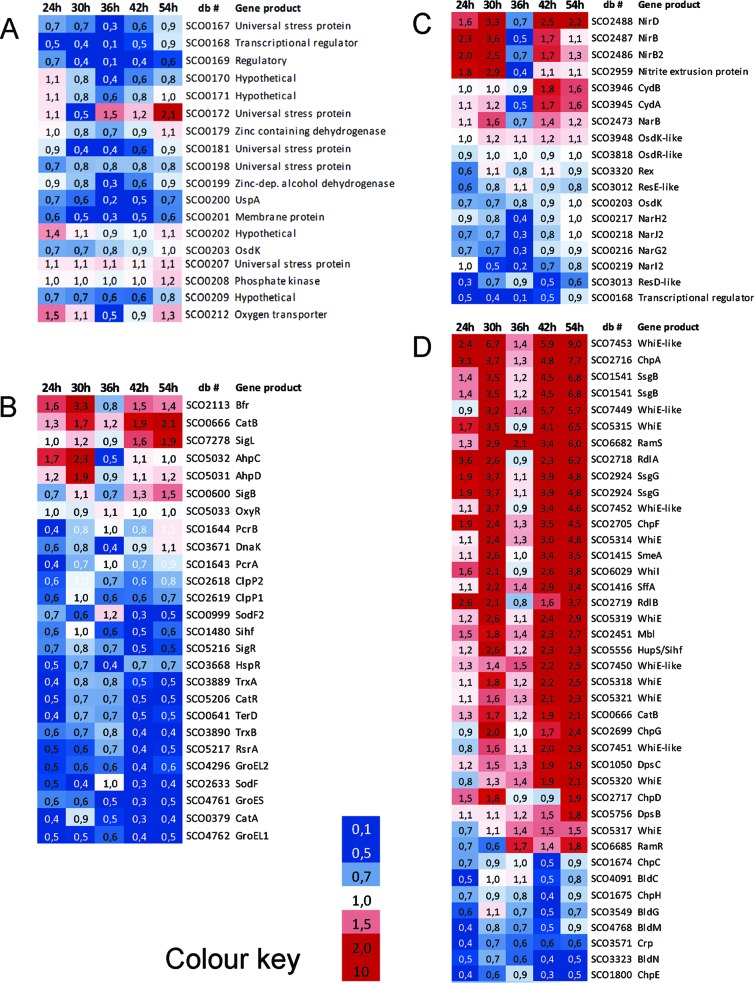
The majority of the genes encoding universal stress proteins are located in the vicinity of osdK and osdR, and several are predicted or proven members of the direct OsdR regulon (see above). Of these, SCO0167, SCO0172, SCO0181, and SCO0200 (uspA) were all downregulated at one or more time points in the mutant (Fig. 7A). The same was observed for the genes for the nitrate reductase system Nar2 (SCO0216 to SCO0219) at 36 h (Fig. 7C). S. coelicolor has three different nitrate reductases (Nar1 to -3) for anaerobic respiration, each active at different stages of development (25, 26). Genes for the two other nitrate reductase systems were not affected (see Table S4 in the supplemental material).
FIG 7 .
Heat maps of stress- and development-related genes differentially expressed between the osdR mutant and its parent, S. coelicolor M145. Transcription patterns (expressed as fold changes between the osdR mutant and the wild type) are presented for genes close to osdRK (A), stress-related genes (B), anaerobic-growth-related genes (C), and developmental genes (D). RNA was isolated from MM agar during vegetative growth (24 h), vegetative/aerial growth (30 h), aerial growth (36 h), aerial growth/early sporulation (42 h), and sporulation (54 h). Blue indicates downregulation (<0.5) and red indicates upregulation (>2.0) in the mutant; intermediate fold changes are represented in white. See Table S4 in the supplemental material. db #, database locus tag.
Deletion of osdR had a major effect on the transcription of many of the genes that were previously shown to be involved in stress management (27–31), such as the response to redox and (thiol) oxidative, osmotic, and temperature stress (Fig. 7B; see also Table S4 in the supplemental material). The σ factor gene sigL, which is involved in osmoprotection and oxidative stress (32), was upregulated, as was catB, but most of the stress-related genes were significantly downregulated. This included genes that in B. subtilis are part of the oxidative-stress response regulon (33), namely, katA, trxA, trxB, msrA, a catR/perR-like gene, and the genes for the oxidative-stress-related σ factor/anti-σ factor pair SigR/RsrA (34–36), as well as genes involved in protein degradation and folding, such as clpP1 to clpP2 (SCO2618 to SCO2619), dnaK, hspR, groEL1, groEL2, groES, genes encoding the proteasome (SCO1643 to SCO1644), and several cold shock genes. Zinc-related genes like those of the gene cluster for the zincophore coelibactin, were downregulated at all time points except 36 h, at which time levels of transcription were comparable between wild-type and osdR mutant cells (Fig. S4). Sufficient zinc is necessary for processes related to protein folding, redox balance, and oxygen stress (37–39). Similar changes in expression were observed for genes related to sulfur, cysteine synthesis, and thiol homeostasis (Fig. S4), which are involved in the management of (thiol) oxidative, redox, or osmotic stress (40, 41).
Heat maps of genes related to zinc import (A), sulfur metabolism and thiol homeostasis (B), and nitrogen metabolism (C) that are significantly differentially expressed between the osdR mutant and its parent, S. coelicolor M145. RNA was isolated from mycelium grown on MM with 1% mannitol during vegetative growth (24 h), vegetative/aerial growth (30 h), aerial growth/early sporulation (42 h), and sporulation (54 h). Only genes with a PFP value of less than 0.010 and a fold change (ΔosdR expression/M145 expression) of more than 2.0 are presented. The levels of the fold changes are indicated with colors, as represented by the scale bar. Download Figure S4, TIF file, 0.3 MB (272.8KB, tif) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Developmental control.
Major changes were observed in the global transcription profile of developmental genes, with a very distinctive pattern of upregulation of many sporulation genes in the absence of osdR at most time points, while early-developmental (bld) genes were downregulated at the same time points (Fig. 7D). Transcription of other genes, namely, ssgB, ssgG, smeA-ssfA, chpADFG, rdlAB, and sapB, all followed the same pattern, with a sharp peak at the onset of sporulation in wild-type cells and, instead, a steady increase in the mutant (see Table S5 in the supplemental material). SsgB and SsgG are members of the actinomycete-specific family of SsgA-like proteins (SALPs) (42) and determine the positions of septum sites during sporulation-specific cell division (43, 44). SmeA and SsfA are also involved in the control of septation as well as DNA segregation (45), and the rdl and chp genes encode the rodlin and chaplin proteins, respectively, which form amyloid-like structures to create a water-repellent hydrophobic sheath around aerial hyphae and spores (46–48). SapB is a lanthipeptide that acts as a signaling molecule for the onset of development (49, 50). The same transcriptional upregulation was observed for the whiE gene cluster for the spore pigment WhiE (51) and for the whiE-like gene cluster from SCO7449 to SCO7453, which also produces a spore pigment (52) (Fig. 7D; Table S4). The upregulation of sporulation genes correlates well to the accelerated development and enhanced pigmentation of osdR mutants (Fig. 6A).
Conversely, the early-developmental genes were downregulated in the osdR null mutant, including bldC, bldG, bldM, bldN, and crp, as well as chpCEH. The crp gene encodes the cAMP receptor protein that controls spore germination and early development (53, 54). The reduced expression of crp correlates with the observed strong delay in the germination of spores of the osdR null mutant (Fig. 6C). bldG encodes a developmental anti-σ factor antagonist that controls the activity of the stress σ factor σH, bldM and whiI encode orphan response regulators that control complex developmental pathways (55), and bldN encodes a σ factor that is required for the transcription of, among other genes, the chp and rdl genes (56, 57). The downregulation of chpCEH contrasts with the upregulation of the other chp genes, which is the first time that such differential regulation has been observed. Interestingly, the chpCEH genes have been shown to belong to the early chp genes and are sufficient to support aerial development, while the other chp genes as well as rdlAB are produced significantly later during development (58). This is again consistent with the concept that OsdR represses sporulation and activates early-development processes.
Differential expression at 36 h.
Interestingly, some 200 genes showed deregulated expression at the 36-h time point. These genes include 22 genes in the genomic region between SCO160 and SCO0220, as well as other members of the direct or indirect OsdR regulon that are involved in nitrogen metabolism and anaerobic respiration genes (e.g., nar2, ureAB, nirB, glnD, glnII, glnK, and draK), development (whiE and whiE-like genes, ssgB, chp, and rdl), stress management, etc. (see Table S5 in the supplemental material). These genes all showed a sharp rise or drop of transcription at 36 h in wild-type cells, with transcription recovering at 42 h, while such a sharp change in transcript levels was not seen in the osdR null mutant. The deregulated transcription of these genes in wild-type cells corresponds to a peak in osdR transcription at 36 h. A sharp peak in the expression of osdR toward the end of exponential growth in liquid cultures was observed by others, both in shake flasks (59) and in a fermentor (60). The transition from exponential to stationary phase roughly corresponds to the onset of aerial growth in surface-grown cultures. Interestingly, another peak in transcription was observed around 5 h after spore germination (61), which may correspond to OsdR’s control of early events.
Verification by RT-qPCR and EMSAs.
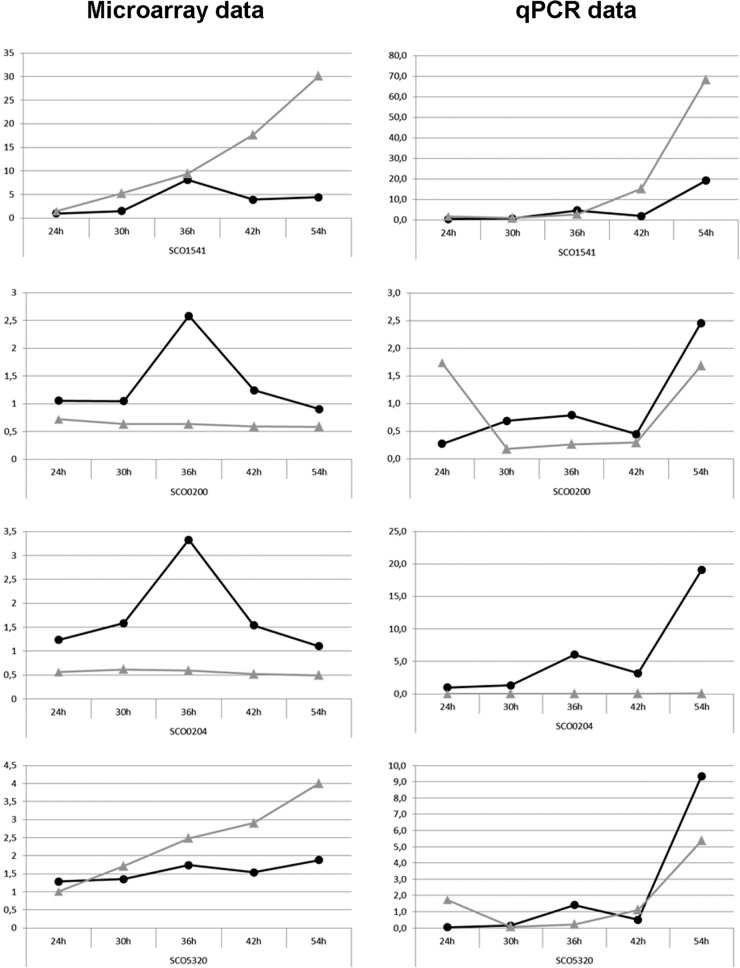
To corroborate the microarray data, reverse transcription-quantitative PCR (qPCR) analysis was performed on independent RNA samples isolated from the mycelia of S. coelicolor M145 and its osdR null mutant GSTC2 grown under the same conditions as those used to prepare RNA samples for microarray analysis. The results were normalized using rpsI (SCO4735) as the internal standard, and RNA obtained from mycelia of M145 grown for 24 h was used to normalize the results between the different qPCR runs. Similar trends in expression profiles were observed in both sets of transcript analyses (Fig. 8; see also Fig. S5 in the supplemental material). Expectedly, no osdR transcripts were detected in the osdR null mutant. The peak in the transcription of osdR after 36 h in wild-type cells, both in the microarray and in the qPCR data, again suggests that osdR plays an important regulatory role at this stage of the life cycle (Fig. 8). Downregulation of upsA (SCO0200) in the osdR mutant together with the binding of OsdR to the upstream regulatory element strongly suggests that uspA transcription is transactivated by OsdR. ssgB (SCO1541) transcription was higher in the mutant, which corresponds well with the accelerated development and enhanced spore pigmentation of GSTC2 (Fig. 6). The transcription of SCO5320 and SCO5321, which are part of the whiE gene cluster for the gray spore pigment, was increased at several time points (though whiE transcription also characteristically peaked at 36 h in the wild-type strain).
FIG 8 .
Microarray and RT-qPCR expression profiles of genes deregulated in the osdR mutant. RNAs for microarray analysis (left) and RT-qPCR (right) profiling were prepared from independent cultures. For time points, see Fig. 7. The expression profiles of the wild type (black circles) and the osdR mutant (gray triangles) from the microarray (left) and RT-qPCR (right) were compared. Genes of interest tested were SCO0200 (uspA), SCO0204 (osdR), SCO1541 (ssgB), and SCO5320 (whiE). See also Fig. S4 in the supplemental material. Note that the graphs are not at the same scale.
Microarray and RT-qPCR expression profiles of genes deregulated in the osdR mutant. RNA was isolated for microarray analysis (left) and RT-qPCR (right) profiling from independent cultures grown on MM with 1% mannitol during vegetative growth (24 h), vegetative/aerial growth (30 h), aerial growth (36 h), aerial growth/early sporulation (42 h), and sporulation (54 h). The expression profiles of the wild type (black circles) and the osdR mutant (gray triangles) over time in the microarray data (left) and the RT-qPCR data (right) were compared. Genes of interest tested were SCO2637, SCO3323 (bldN), and SCO5321 (whiE). Download Figure S5, TIF file, 0.3 MB (264.9KB, tif) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
While no regulatory elements were predicted upstream of ssgB or within the whiE cluster, EMSAs showed specific binding by phosphorylated OsdR to ssgB and to the intergenic region between genes SCO5320 and SCO5321 (Fig. 4B), while the promoters of SCO5319 and SCO5316 (the latter is not shown) were only weakly bound by OsdR in vitro. Considering the lack of binding of nonphosphorylated OsdR to the upstream regions of SCO5316 and SCO5319 and the weak binding of OsdR~P, it is unclear whether these two genes are directly controlled by OsdR in vivo.
DISCUSSION
The two-component regulatory system (TCS) formed by OsdK (SCO0203) and OsdR (SCO0204) shows significant sequence similarity to the dormancy TCS in Mycobacterium tuberculosis (16). In this work, we show not only that the OsdR binding site conforms very well to the binding site for DevR in M. tuberculosis but also that OsdR recognizes the regulatory elements upstream of key genes of the M. tuberculosis dormancy regulon and with affinities similar to those of DevR. EMSAs established OsdR binding to short, 50-bp DNA sequences containing the predicted recognition site, and the specificity was validated by the decrease in binding upon changing of one or more nucleotides of the consensus sequence. Thus, the TCS formed by OsdK and OsdR is most likely orthologous to the dormancy control system DosT/DevS/DevR in M. tuberculosis. This is further supported by gene synteny, as many genes for USP domain proteins are in the vicinity of the TCSs in the respective organisms. Despite hundreds of millions of years of evolution, some 15 targets are conserved between the DevR-controlled dormancy regulon of M. tuberculosis and the regulon predicted to be controlled by OsdR in S. coelicolor. Most of these lie in the region around osdR, namely, SCO0167, uspA, osdR, SCO0207, SCO0215, and SCO0216 to SCO0219 (narG2-narJ2).
The sensory kinase OsdK activates its cognate response regulator, OsdR, by phosphorylation and enhances its DNA binding capability, as shown by the enhanced binding of OsdR~P in the EMSAs. Combined, the in silico predictions and in vitro validation by EMSAs indicate that around 50 genes or gene clusters are controlled directly by OsdR. Analysis of the transcriptional changes in the osdR null mutant by global transcription profiling revealed the deregulation of numerous stress-related genes, including numerous stress-related genes in the region around osdR. A distinctive pattern of deregulation of developmental genes was evident, with upregulation of sporulation genes (including whiE, whiI, smeA-ssfA, rdlAB, ssgBG, ramS, and the late chp genes) and downregulation of genes involved in early development (bldC, bldG, bldM, bldN, crp, and the early chp genes), which corresponds well to the observed accelerated development of osdR mutants. The transcriptional data suggest that OsdR controls a hinge point in development. This is perhaps best illustrated by the divergent transcription of the chp genes in the osdR mutant. It has previously been shown that the chpCEH genes are expressed earlier than the other chp genes and also that the ChpCEH proteins are sufficient to form the characteristic chaplin layer on the outside the aerial hyphae and spores and to support aerial growth. BldN was previously shown to control all of the chp genes (48, 56), which does not explain the difference in chp gene expression profiles. Our data show that in the osdR null mutant, transcription of bldN and chpCEH is reduced, while the other chp genes as well as rdlAB are upregulated. Therefore, we propose that fine-tuning of chp and rdl gene expression is maintained by OsdR.
Some of the differentially expressed genes that lack an obvious consensus sequence, in particular the ssgB and genes of the whiE gene cluster, were bound by OsdR in vitro. This indicates that the OsdR regulon may be larger than anticipated, and some members of the regulon may be controlled by so-called class II binding sites, in other words, sites that do not conform to the predicted consensus sequence site. Similar duality has been shown for many other functionally diverse global regulatory networks in bacteria, including those controlled by LexA (62) and Crp (63) in E. coli, Spo0A in B. subtilis (64), CtrA in Caulobacter crescentus (65), and Crp (66), GlnR (67), PhoP (68), and DasR (69) in Streptomyces. For B. subtilis Spo0A, some 15% of the total binding sites were not bound in vitro (64).
Extensive studies of the DosT and DevS signaling systems have indicated that, during hypoxia, the dissociation of oxygen from the SKs results in the transition from the inactive to the active states of these proteins. With the initial DevR hypoxic response mediated by DosT, which has a higher dissociation constant than DevS, the response is then maintained through DevS. Differences in the local structures surrounding a heme in either SK result in different oxygen affinities (15, 16). Additionally, ascorbic acid, nitric oxide, and carbon monoxide also induce the DevR regulon (70). NO has been shown to activate DosT under aerobic conditions by displacement of oxygen (71), while DevS acts as a redox sensor of the electron transport system and a decrease activates the SK under aerobic conditions (72). The similarity of the amino acid residues involved in signal recognition by DosT/DevS and OsdK suggests that oxygen is the major candidate as a sensory signal. Indeed, Daigle and colleagues showed that osdR, as well as many genes in the genomic region around osdR, were strongly upregulated in wild-type cells under both low-oxygen conditions and when cells were grown with sodium nitroprusside, an NO donor (10). Additional evidence for the oxygen stress-related function of OsdR was provided by a study of the proteomes of large versus small pellets (73), in which oxygen depletion within large pellets—which created local anaerobic conditions—resulted in the upregulation of various proteins expressed from the OsdR-controlled SCO0168-SCO0208 genomic region (26).
In liquid-grown cultures, where S. coelicolor forms large mycelial pellets (causing oxygen transfer problems toward the center of the clump [74]), and on solid-grown cultures (7), local oxygen depletion occurs. OsdKR-mediated oxygen sensing may well be responsible for the response to microaerobic conditions, during which the bacterium switches metabolism to meet the challenge of low oxygen. Still, streptomycetes cannot grow anaerobically, despite the presence of an arsenal of genes for enzymes associated with anaerobic metabolism (75). This has previously been referred to as the “anaerobic paradox.” This is exemplified by the surprising presence of three nitrate reductases in S. coelicolor, and our work shows that one of these is directly controlled by OsdR. Alternatively, S. coelicolor may undergo a state of dormancy as a means of survival. Indeed, while S. coelicolor cannot grow in oxygen-deprived soil, it is able to survive periods of anaerobiosis in which it remains dormant (7). Sporulation is a state of dormancy, and the fact that spore germination is significantly delayed in osdR null mutants without affecting spore viability (Fig. 6C) supports the notion that osdR controls this dormancy state. This delay was corroborated independently by imaging the germination of 500 spores of the wild type and the osdR mutant using light microscopy (not shown).
The transcriptional changes at 36 h of growth in the osdR null mutant are noteworthy, and while the results need to be worked out further, they may have major implications for the control of the switch from early- to late-developmental growth. Interestingly, such a clear transition in the global transcriptional profile of S. coelicolor has been reported previously, during growth in a fermentor. Distinctive sharp increases and decreases in the transcription of many genes were observed at this time point, and importantly, this includes several genes of the OsdR regulon, namely, genes in the nitrate reductase cluster adjacent to osdR (SCO0212-SCO0220), bldN, the bldN-controlled chp genes, and several other developmental genes (60). We observed a similar distinctive change in gene expression at 36 h in surface-grown cultures of wild-type cells, with many of the genes of the OsdR regulon, as well as osdR itself, showing expression in the wild-type strain different from that in the osdR null mutant. To some extent, the data from surface- and liquid-grown cultures can be compared, with many developmental genes upregulated in liquid-grown cultures at the time corresponding to the transition from exponential to stationary growth, suggesting that the phase of growth cessation in submerged culture is comparable to the onset of development (59). Our data provide a first indication that OsdR may play a major role in mediating a switch in gene expression during the transition from normal to developmental growth. The transcription of osdR also shows a peak almost immediately after germination (61), which suggests that OsdR may play a similar role during the transition from dormancy to early growth. Such a role of OsdR in mediating a rapid and global change in gene expression requires further investigation.
In summary, the TCS OsdKR of S. coelicolor is orthologous to the dormancy TCS system of M. tuberculosis, with OsdR regulating development and stress management in S. coelicolor. The signal activating this response system is likely related to stress, such as nutrient deprivation or hypoxic stress; however, this remains to be confirmed. OsdK also partners with SCO3818 (9), which adds an extra level of complexity. This also means that deletion of osdR may not completely inactivate the OsdK-based sensory system in S. coelicolor. The system may be even more complicated, as sensory kinase SCO3948 has a higher amino acid identity to OsdK than any other SK encoded by the S. coelicolor genome. Mutational and functional analysis followed by a system-wide analysis of the effects of all possible members of the control system on global gene expression should establish the level of cross talk between the two sensory systems and how they control the stress response of the complex soil bacterium Streptomyces.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains described in this work are listed in Table S1 in the supplemental material. E. coli strains JM109 and ET12567 were grown and transformed by standard procedures (76). S. coelicolor A3(2) M145 was the parent for the osdK (GSTC1), osdR (GSTC2 and GSTC3), and osdRK (GSTC4) null mutants. S. coelicolor M512 (M145 ΔredD ΔactII-ORF4 [77]) was the parent strain for the osdR null mutant GSTC6, and M512 and GSTC6 were the hosts for promoter probing experiments (78). Preparation of protoplasts, transformations, and conjugations were performed according to routine procedures (79). R5 medium was used for regeneration of protoplasts and MS medium (79) for the selection of mutants, for the preparation of spores, and for phenotypic characterization of mutants. To obtain mycelia for transcript analysis, strains were grown on minimal medium (agar plates with mannitol [1%, wt/vol] [79]).
Bacterial strain, plasmids, and constructs. Download Table S1, PDF file, 0.4 MB (416KB, pdf) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Preparation of gene knockout constructs.
Details for all plasmids and mutants are presented in Table S1 in the supplemental material. The gene replacement strategy was as described previously (80) and used the highly unstable vector pWHM3 (81), harboring around 1,500 bp of flanking region on either side of the gene targeted for deletion, and the genes of interest were replaced by the apramycin resistance cassette aacC4 (82). PCRs were performed as previously described (83) with the oligonucleotides listed in Table S2. Plasmids pGWS378 and pGWS376 allowed gene replacement of osdK and osdR, respectively. To create an in-frame osdR deletion mutant (designated GSTC3), construct pGWS377, which carries only the flanking regions, was used for homologous recombination. Construct pGWS380 was designed for the construction of an in-frame osdRK double mutant (called GSTC4) by combining the upstream region of osdR (obtained from pGWS377) and the downstream region of osdK (obtained from pGWS378). GSTC6 (M512 ΔosdR) was created for promoter probing purposes using the same approach as for the S. coelicolor M145 osdR mutant.
Oligonucleotides. Download Table S2, PDF file, 0.5 MB (536.7KB, pdf) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Position weight matrix for the OsdR binding site. Download Table S3, PDF file, 0.3 MB (296.5KB, pdf) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Overview of microarray results. Download Table S4, PDF file, 0.1 MB (135.2KB, pdf) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Overview of genes deregulated at 36 h. Download Table S5, PDF file, 0.8 MB (891.4KB, pdf) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Protein isolation, phosphorylation of OsdR, and electrophoretic mobility shift assays.
His6-tagged OsdR and OsdK were overexpressed from plasmids pET0203 and pET0204 in E. coli BL21(DE3) (9). The plasmids were a kind gift from Weihong Jiang (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China). Proteins were isolated using Ni-nitrilotriacetic acid (NTA) chromatography as described previously (84).
In vitro autophosphorylation of 30 pmol of OsdK was performed with 32P-radiolabeled ATP as described previously (9). For transphosphorylation of OsdR, 30 pmol of OsdK was autophosphorylated in 10 µl and incubated for 20 min at 30°C. Following a chill on ice, 80 pmol of OsdR was added. In vitro phosphorylation of OsdR for electrophoretic mobility shift assays (EMSAs) was achieved using the phosphor donor acetyl phosphate (AcP) as described previously (18). EMSAs with 32P-radiolabeled probes were performed as previously described (85).
The OsdR binding site was predicted and used to scan the S. coelicolor genome by PREDetector (20). This binding sequence was investigated by binding assay experiments with wild-type and mutated 50-mers of the predicted binding site upstream of SCO0200. The most-conserved nucleotides in the predicted binding sites (Table 1 and Fig. 2B) were identified, and single (50a, 50b), double (50ab), and quintuple (50x) substitutions were introduced (for 50-mer oligomers, see Table S2 in the supplemental material).
Promoter probing.
Promoter probing experiments were performed using the redD system as described previously (78). The nonpigmented mutant S. coelicolor M512 lacks the pathway-specific activator genes actII-ORF4 and redD (77). When redD is transcribed from a promoter element cloned into the promoter-probe vector pIJ2587 (78), the RED biosynthetic pathway is activated, which can be monitored as a nondiffusible red pigment. Constructs for the redD promoter-probe system were created for the promoters of SCO0200, osdR, and SCO0207, using the whiG promoter as the control (Table S1). The promoter fragments were amplified by PCR, and EcoRI/BamHI-digested fragments were cloned into pIJ2587, resulting in the constructs pGWS345, pGWS1058, pGWS1059, pGWS1060 (for probing of whiG), SCO0200, osdR, and SCO0207.
Transcript analysis.
RNA was isolated from S. coelicolor M145 (wild-type strain) and its osdR mutant GSTC2 by harvesting biomass from cellophane disks on MM with 1% mannitol after 24, 30, 36, 42, and 54 h of growth. Total RNA was isolated as described previously (85).
Microarray analysis.
The quality and integrity of the RNA was tested with the Agilent 2100 Bioanalyzer (Agilent Technologies). The RNA was reverse transcribed into cDNA using Cy3-dCTP (http://www.surrey.ac.uk/fhms/microarrays/Downloads/Protocols/index.htm). Together with Cy5-dCTP-labeled S. coelicolor M145 genomic DNA as the common reference, the samples were hybridized onto 44,000 60-mer oligonucleotide microarray slides (86). The fluorescent signals on the slides were captured by an Agilent microarray scanner with Feature Extraction software (Agilent Technologies). Within-array normalization (global median) followed by cross-array normalization was performed in R (http://www.r-project.org) using Limma (version 2.5.0) (87, 88). Rank product analysis by means of the R packages RankProd (89) and RankProdIt (90) was applied to identify significantly differentially expressed genes (for which the probability of false prediction [PFP] value was <0.01) between the wild type and mutant at each time point.
RT-qPCR analysis.
For RT-qPCR analysis, cDNA was generated using the iScript Advanced cDNA synthesis kit (Bio-Rad Laboratories). RT-qPCRs were performed on 200 ng RNA with the iTaq universal SYBR green supermix (Bio-Rad Laboratories), using rpsI (SCO4735) as an internal control. Each reaction mixture was tested in triplicate and for normalization between different plates, with the 24-h wild-type sample as the reference. An average of all three measurements was used to calculate normalized expression.
Microscopy.
Cryo-scanning electron microscopy was performed as described previously (91) with a JEOL JSM6700F microscope. Stereomicroscopy was done using a Zeiss Lumar.V12 stereomicroscope. Confocal laser-scanning microscopy was performed with a Leica TCS-SP2 microscope and Leica confocal software. Staining of dead and viable Streptomyces filaments and spores was performed as described previously (92) using the cell-impermeable nucleic acid stain propidium iodide (for dead cells) and the green fluorescent nucleic acid stain SYTO 9 (for live cells). Samples were examined at wavelengths of 488 and 568 nm for excitation and 530 nm (green) and 630 nm (red) for emission.
Bioinformatics analysis.
Motif searching was performed with InterProScan (93) and Pfam 24.0 (94). Protein homology searches were performed using BLASTp (95). The comparative analysis of the upstream regions of OsdR orthologues was performed with MEME (19), using orthologues from S. coelicolor, S. clavuligerus, S. scabies, S. ghanaensis, S. bingchengensis, S. cattleya, S. sviceus, S. viridochromogenes, S. griseoaurentiacus, Streptoccocus sp. E14, Streptoccocus sp. TRS4, and S. hygroscopicus. The S. coelicolor genome was scanned for possible similar cis-acting regulatory elements using PREDetector (20). The consensus sequence for the predicted binding site of OsdR was visualized using WebLogo (96). The M. tuberculosis DevR binding site logo was created based on the primary DevR binding sites identified in reference 97.
Accession numbers.
The microarray expression data have been deposited in ArrayExpress (with the accession number E-MTAB-4597). The GenBank nucleotide sequence accession number of M. tuberculosis DosT is P9WGK0, and that of DevS it is NP_217648.
ACKNOWLEDGMENTS
We are grateful to Weihong Jiang for providing plasmids pET0203 and pET0204 and to Tom Ottenhoff for providing genomic DNA of M. tuberculosis Rv37.
REFERENCES
- 1.Stock AM, Robinson VL, Goudreau PN. 2000. Two-component signal transduction. Annu Rev Biochem 69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 2.Whitworth DE. 2012. Classification and organization of two-component systems, p 1–20. In Gross R, Beier D (ed), Two-component systems in bacteria. Caister Academic Press, Poole, United Kingdom. [Google Scholar]
- 3.Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Klenk HP, Clément C, Ouhdouch Y, van Wezel GP. 2016. Taxonomy, physiology, and natural products of the Actinobacteria. Microbiol Mol Biol Rev 80:1–43. doi: 10.1128/MMBR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopwood DA. 2007. Streptomyces in nature and medicine: the antibiotic makers. Oxford University Press, New York, NY. [Google Scholar]
- 5.Claessen D, Rozen DE, Kuipers OP, Søgaard-Andersen L, van Wezel GP. 2014. Bacterial solutions to multicellularity: a tale of biofilms, filaments and fruiting bodies. Nat Rev Microbiol 12:115–124. doi: 10.1038/nrmicro3178. [DOI] [PubMed] [Google Scholar]
- 6.Flärdh K, Buttner MJ. 2009. Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nat Rev Microbiol 7:36–49. doi: 10.1038/nrmicro1968. [DOI] [PubMed] [Google Scholar]
- 7.Van Keulen G, Alderson J, White J, Sawers RG. 2007. The obligate aerobic actinomycete Streptomyces coelicolor A3(2) survives extended periods of anaerobic stress. Environ Microbiol 9:3143–3149. doi: 10.1111/j.1462-2920.2007.01433.x. [DOI] [PubMed] [Google Scholar]
- 8.Hutchings MI, Hoskisson PA, Chandra G, Buttner MJ. 2004. Sensing and responding to diverse extracellular signals? Analysis of the sensor kinases and response regulators of Streptomyces coelicolor A3(2). Microbiology 150:2795–2806. doi: 10.1099/mic.0.27181-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Shu D, Chen L, Jiang W, Lu Y. 2009. Cross-talk between an orphan response regulator and a noncognate histidine kinase in Streptomyces coelicolor. FEMS Microbiol Lett 294:150–156. doi: 10.1111/j.1574-6968.2009.01563.x. [DOI] [PubMed] [Google Scholar]
- 10.Daigle F, Lerat S, Bucca G, Sanssouci É, Smith CP, Malouin F, Beaulieu C. 2015. A terD domain-encoding gene (SCO2368) is involved in calcium homeostasis and participates in calcium regulation of a DosR-like regulon in Streptomyces coelicolor. J Bacteriol 197:913–923. doi: 10.1128/JB.02278-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerasimova A, Kazakov AE, Arkin AP, Dubchak I, Gelfand MS. 2011. Comparative genomics of the dormancy regulons in mycobacteria. J Bacteriol 193:3446–3452. doi: 10.1128/JB.00179-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao MC, Rubin EJ. 2010. Letting sleeping dos lie: does dormancy play a role in tuberculosis? Annu Rev Microbiol 64:293–311. doi: 10.1146/annurev.micro.112408.134043. [DOI] [PubMed] [Google Scholar]
- 13.Martínez JL, Rojo F. 2011. Metabolic regulation of antibiotic resistance. FEMS Microbiol Rev 35:768–789. doi: 10.1111/j.1574-6976.2011.00282.x. [DOI] [PubMed] [Google Scholar]
- 14.Selvaraj S, Sambandam V, Sardar D, Anishetty S. 2012. In silico analysis of DosR regulon proteins of Mycobacterium tuberculosis. Gene 506:233–241. doi: 10.1016/j.gene.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 15.Cho HY, Cho HJ, Kim YM, Oh JI, Kang BS. 2009. Structural insight into the heme-based redox sensing by DosS from Mycobacterium tuberculosis. J Biol Chem 284:13057–13067. doi: 10.1074/jbc.M808905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Podust LM, Ioanoviciu A, Ortiz de Montellano PR. 2008. 2.3 Å X-ray structure of the heme-bound GAF domain of sensory histidine kinase DosT of Mycobacterium tuberculosis. Biochemistry 47:12523-125531. doi: 10.1021/bi8012356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wisedchaisri G, Wu M, Rice AE, Roberts DM, Sherman DR, Hol WG. 2005. Structures of Mycobacterium tuberculosis DosR and DosR-DNA complex involved in gene activation during adaptation to hypoxic latency. J Mol Biol 354:630–641. doi: 10.1016/j.jmb.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 18.Chauhan S, Tyagi JS. 2008. Cooperative binding of phosphorylated DevR to upstream sites is necessary and sufficient for activation of the Rv3134c-devRS operon in Mycobacterium tuberculosis: implication in the induction of DevR target genes. J Bacteriol 190:4301–4312. doi: 10.1128/JB.01308-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiard S, Marée R, Colson S, Hoskisson PA, Titgemeyer F, van Wezel GP, Joris B, Wehenkel L, Rigali S. 2007. PREDetector: a new tool to identify regulatory elements in bacterial genomes. Biochem Biophys Res Commun 357:861–864. doi: 10.1016/j.bbrc.2007.03.180. [DOI] [PubMed] [Google Scholar]
- 21.Rigali S, Nivelle R, Tocquin P. 2015. On the necessity and biological significance of threshold-free regulon prediction outputs. Mol Biosyst 11:333–337. doi: 10.1039/c4mb00485j. [DOI] [PubMed] [Google Scholar]
- 22.Fillenberg SB, Grau FC, Seidel G, Muller YA. 2015. Structural insight into operator dre-sites recognition and effector binding in the GntR/HutC transcription regulator NagR. Nucleic Acids Res 43:1283–1296. doi: 10.1093/nar/gku1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenconi E, Urem M, Świątek-Połatyńska MA, Titgemeyer F, Muller YA, van Wezel GP, Rigali S. 2015. Multiple allosteric effectors control the affinity of DasR for its target sites. Biochem Biophys Res Commun 464:324–329. doi: 10.1016/j.bbrc.2015.06.152. [DOI] [PubMed] [Google Scholar]
- 24.Świątek MA, Gubbens J, Bucca G, Song E, Yang YH, Laing E, Kim BG, Smith CP, van Wezel GP. 2013. The ROK family regulator Rok7B7 pleiotropically affects xylose utilization, carbon catabolite repression, and antibiotic production in Streptomyces coelicolor. J Bacteriol 195:1236–1248. doi: 10.1128/JB.02191-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer M, Alderson J, van Keulen G, White J, Sawers RG. 2010. The obligate aerobe Streptomyces coelicolor A3(2) synthesizes three active respiratory nitrate reductases. Microbiology 156:3166–3179. doi: 10.1099/mic.0.042572-0. [DOI] [PubMed] [Google Scholar]
- 26.Fischer M, Falke D, Pawlik T, Sawers RG. 2014. Oxygen-dependent control of respiratory nitrate reduction in mycelium of Streptomyces coelicolor A3(2). J Bacteriol 196:4152–4162. doi: 10.1128/JB.02202-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Facey PD, Sevcikova B, Novakova R, Hitchings MD, Crack JC, Kormanec J, Dyson PJ, Del Sol R. 2011. The dpsA gene of Streptomyces coelicolor: induction of expression from a single promoter in response to environmental stress or during development. PLoS One 6:e25593. doi: 10.1371/journal.pone.0025593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JN, Jeong Y, Yoo JS, Roe JH, Cho BK, Kim BG. 2015. Genome-scale analysis reveals a role for NdgR in the thiol oxidative stress response in Streptomyces coelicolor. BMC Genomics 16:116. doi: 10.1186/s12864-015-1311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagels M, Fuchs S, Pané-Farré J, Kohler C, Menschner L, Hecker M, McNamarra PJ, Bauer MC, von Wachenfeldt C, Liebeke M, Lalk M, Sander G, von Eiff C, Proctor RA, Engelmann S. 2010. Redox sensing by a Rex-family repressor is involved in the regulation of anaerobic gene expression in Staphylococcus aureus. Mol Microbiol 76:1142–1161. doi: 10.1111/j.1365-2958.2010.07105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bueno E, Mesa S, Bedmar EJ, Richardson DJ, Delgado MJ. 2012. Bacterial adaptation of respiration from oxic to microoxic and anoxic conditions: redox control. Antioxid Redox Signal 16:819–852. doi: 10.1089/ars.2011.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin JH, Singh AK, Cheon DJ, Roe JH. 2011. Activation of the SoxR regulon in Streptomyces coelicolor by the extracellular form of the pigmented antibiotic actinorhodin. J Bacteriol 193:75–81. doi: 10.1128/JB.00965-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee EJ, Karoonuthaisiri N, Kim HS, Park JH, Cha CJ, Kao CM, Roe JH. 2005. A master regulator sigmaB governs osmotic and oxidative response as well as differentiation via a network of sigma factors in Streptomyces coelicolor. Mol Microbiol 57:1252–1264. doi: 10.1111/j.1365-2958.2005.04761.x. [DOI] [PubMed] [Google Scholar]
- 33.Zuber P. 2009. Management of oxidative stress in Bacillus. Annu Rev Microbiol 63:575–597. doi: 10.1146/annurev.micro.091208.073241. [DOI] [PubMed] [Google Scholar]
- 34.Jung Y-G, Cho Y-B, Kim M-S, Yoo J-S, Hong S-H, Roe J-H. 2011. Determinants of redox sensitivity in RsrA, a zinc-containing anti-sigma factor for regulating thiol oxidative stress response. Nucleic Acids Res 39:7586–7597. doi: 10.1093/nar/gkr477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang JG, Paget MS, Seok YJ, Hahn MY, Bae JB, Hahn JS, Kleanthous C, Buttner MJ, Roe JH. 1999. RsrA, an anti-sigma factor regulated by redox change. EMBO J 18:4292–4298. doi: 10.1093/emboj/18.15.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MS, Dufour YS, Yoo JS, Cho YB, Park JH, Nam GB, Kim HM, Lee KL, Donohue TJ, Roe JH. 2012. Conservation of thiol-oxidative stress responses regulated by SigR orthologues in actinomycetes. Mol Microbiol 85:326–344. doi: 10.1111/j.1365-2958.2012.08115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallifidas D, Pascoe B, Owen GA, Strain-Damerell CM, Hong HJ, Paget MS. 2010. The zinc-responsive regulator zur controls expression of the coelibactin gene cluster in Streptomyces coelicolor. J Bacteriol 192:608–611. doi: 10.1128/JB.01022-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W, Bottrill AR, Bibb MJ, Buttner MJ, Paget MS, Kleanthous C. 2003. The role of zinc in the disulphide stress-regulated anti-sigma factor RsrA from Streptomyces coelicolor. J Mol Biol 333:461–472. doi: 10.1016/j.jmb.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 39.Shin JH, Jung HJ, An YJ, Cho YB, Cha SS, Roe JH. 2011. Graded expression of zinc-responsive genes through two regulatory zinc-binding sites in Zur. Proc Natl Acad Sci U S A 108:5045–5050. doi: 10.1073/pnas.1017744108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai Y, Outten FW. 2012. The E. coli SufS-SufE sulfur transfer system is more resistant to oxidative stress than IscS-IscU. FEBS Lett 586:4016–4022. doi: 10.1016/j.febslet.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paget MS, Molle V, Cohen G, Aharonowitz Y, Buttner MJ. 2001. Defining the disulphide stress response in Streptomyces coelicolor A3(2): identification of the sigmaR regulon. Mol Microbiol 42:1007–1020. doi: 10.1046/j.1365-2958.2001.02675.x. [DOI] [PubMed] [Google Scholar]
- 42.Jakimowicz D, van Wezel GP. 2012. Cell division and DNA segregation in Streptomyces: how to build a septum in the middle of nowhere? Mol Microbiol 85:393–404. doi: 10.1111/j.1365-2958.2012.08107.x. [DOI] [PubMed] [Google Scholar]
- 43.Keijser BJ, Noens EE, Kraal B, Koerten HK, van Wezel GP. 2003. The Streptomyces coelicolor ssgB gene is required for early stages of sporulation. FEMS Microbiol Lett 225:59–67. doi: 10.1016/S0378-1097(03)00481-6. [DOI] [PubMed] [Google Scholar]
- 44.Willemse J, Borst JW, de Waal E, Bisseling T, van Wezel GP. 2011. Positive control of cell division: FtsZ is recruited by SsgB during sporulation of Streptomyces. Genes Dev 25:89–99. doi: 10.1101/gad.600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ausmees N, Wahlstedt H, Bagchi S, Elliot MA, Buttner MJ, Flärdh K. 2007. SmeA, a small membrane protein with multiple functions in Streptomyces sporulation including targeting of a SpoIIIE/FtsK-like protein to cell division septa. Mol Microbiol 65:1458–1473. doi: 10.1111/j.1365-2958.2007.05877.x. [DOI] [PubMed] [Google Scholar]
- 46.Claessen D, Rink R, de Jong W, Siebring J, de Vreugd P, Boersma FG, Dijkhuizen L, Wösten HA. 2003. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev 17:1714–1726. doi: 10.1101/gad.264303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Claessen D, Wösten HA, van Keulen G, Faber OG, Alves AM, Meijer WG, Dijkhuizen L. 2002. Two novel homologous proteins of Streptomyces coelicolor and Streptomyces lividans are involved in the formation of the rodlet layer and mediate attachment to a hydrophobic surface. Mol Microbiol 44:1483–1492. doi: 10.1046/j.1365-2958.2002.02980.x. [DOI] [PubMed] [Google Scholar]
- 48.Elliot MA, Karoonuthaisiri N, Huang J, Bibb MJ, Cohen SN, Kao CM, Buttner MJ. 2003. The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes Dev 17:1727–1740. doi: 10.1101/gad.264403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kodani S, Hudson ME, Durrant MC, Buttner MJ, Nodwell JR, Willey JM. 2004. The SapB morphogen is a lantibiotic-like peptide derived from the product of the developmental gene ramS in Streptomyces coelicolor. Proc Natl Acad Sci U S A 101:11448–11453. doi: 10.1073/pnas.0404220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willey J, Santamaria R, Guijarro J, Geistlich M, Losick R. 1991. Extracellular complementation of a developmental mutation implicates a small sporulation protein in aerial mycelium formation by S. coelicolor. Cell 65:641–650. doi: 10.1016/0092-8674(91)90096-H. [DOI] [PubMed] [Google Scholar]
- 51.Kelemen GH, Brian P, Flärdh K, Chamberlin L, Chater KF, Buttner MJ. 1998. Developmental regulation of transcription of whiE, a locus specifying the polyketide spore pigment in Streptomyces coelicolor A3 (2). J Bacteriol 180:2515–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salerno P, Persson J, Bucca G, Laing E, Ausmees N, Smith CP, Flärdh K. 2013. Identification of new developmentally regulated genes involved in Streptomyces coelicolor sporulation. BMC Microbiol 13:281. doi: 10.1186/1471-2180-13-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piette A, Derouaux A, Gerkens P, Noens EE, Mazzucchelli G, Vion S, Koerten HK, Titgemeyer F, De Pauw E, Leprince P, van Wezel GP, Galleni M, Rigali S. 2005. From dormant to germinating spores of Streptomyces coelicolor A3(2): new perspectives from the crp null mutant. J Proteome Res 4:1699–1708. doi: 10.1021/pr050155b. [DOI] [PubMed] [Google Scholar]
- 54.Derouaux A, Halici S, Nothaft H, Neutelings T, Moutzourelis G, Dusart J, Titgemeyer F, Rigali S. 2004. Deletion of a cyclic AMP receptor protein homologue diminishes germination and affects morphological development of Streptomyces coelicolor. J Bacteriol 186:1893–1897. doi: 10.1128/JB.186.6.1893-1897.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Bassam MM, Bibb MJ, Bush MJ, Chandra G, Buttner MJ. 2014. Response regulator heterodimer formation controls a key stage in Streptomyces development. PLoS Genet 10:e1004554. doi: 10.1371/journal.pgen.1004554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bibb MJ, Domonkos A, Chandra G, Buttner MJ. 2012. Expression of the chaplin and rodlin hydrophobic sheath proteins in Streptomyces venezuelae is controlled by sigma(BldN) and a cognate anti-sigma factor, RsbN. Mol Microbiol 84:1033–1049. doi: 10.1111/j.1365-2958.2012.08070.x. [DOI] [PubMed] [Google Scholar]
- 57.Bibb MJ, Molle V, Buttner MJ. 2000. sigma(BldN), an extracytoplasmic function RNA polymerase sigma factor required for aerial mycelium formation in Streptomyces coelicolor A3(2). J Bacteriol 182:4606–4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Berardo C, Capstick DS, Bibb MJ, Findlay KC, Buttner MJ, Elliot MA. 2008. Function and redundancy of the chaplin cell surface proteins in aerial hypha formation, rodlet assembly, and viability in Streptomyces coelicolor. J Bacteriol 190:5879–5889. doi: 10.1128/JB.00685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang J, Lih CJ, Pan KH, Cohen SN. 2001. Global analysis of growth phase responsive gene expression and regulation of antibiotic biosynthetic pathways in Streptomyces coelicolor using DNA microarrays. Genes Dev 15:3183–3192. doi: 10.1101/gad.943401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nieselt K, Battke F, Herbig A, Bruheim P, Wentzel A, Jakobsen ØM, Sletta H, Alam MT, Merlo ME, Moore J, Omara WA, Morrissey ER, Juarez-Hermosillo MA, Rodríguez-García A, Nentwich M, Thomas L, Iqbal M, Legaie R, Gaze WH, Challis GL, Jansen RC, Dijkhuizen L, Rand DA, Wild DL, Bonin M, Reuther J, Wohlleben W, Smith MC, Burroughs NJ, Martin JF, Hodgson DA, Takano E, Breitling R, Ellingsen TE, Wellington EM. 2010. The dynamic architecture of the metabolic switch in Streptomyces coelicolor. BMC Genomics 11:10. doi: 10.1186/1471-2164-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strakova E, Bobek J, Zikova A, Vohradsky J. 2013. Global features of gene expression on the proteome and transcriptome levels in S. coelicolor during germination. PLoS One 8:e72842. doi: 10.1371/journal.pone.0072842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wade JT, Reppas NB, Church GM, Struhl K. 2005. Genomic analysis of LexA binding reveals the permissive nature of the Escherichia coli genome and identifies unconventional target sites. Genes Dev 19:2619–2630. doi: 10.1101/gad.1355605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao Z, Li F, Wu G, Zhu Y, Yu T, Yu S. 2012. Roles of hinge region, loops 3 and 4 in the activation of Escherichia coli cyclic AMP receptor protein. Int J Biol Macromol 50:1–6. doi: 10.1016/j.ijbiomac.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Molle V, Fujita M, Jensen ST, Eichenberger P, González-Pastor JE, Liu JS, Losick R. 2003. The Spo0A regulon of Bacillus subtilis. Mol Microbiol 50:1683–1701. doi: 10.1046/j.1365-2958.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- 65.Laub MT, Chen SL, Shapiro L, McAdams HH. 2002. Genes directly controlled by CtrA, a master regulator of the Caulobacter cell cycle. Proc Natl Acad Sci U S A 99:4632–4637. doi: 10.1073/pnas.062065699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gao C, Hindra, Mulder D, Yin C, Elliot MA. 2012. Crp is a global regulator of antibiotic production in Streptomyces. mBio 3:00407-12. doi: 10.1128/mBio.00407-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pullan ST, Chandra G, Bibb MJ, Merrick M. 2011. Genome-wide analysis of the role of GlnR in Streptomyces venezuelae provides new insights into global nitrogen regulation in actinomycetes. BMC Genomics 12:175. doi: 10.1186/1471-2164-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allenby NE, Laing E, Bucca G, Kierzek AM, Smith CP. 2012. Diverse control of metabolism and other cellular processes in Streptomyces coelicolor by the PhoP transcription factor: genome-wide identification of in vivo targets. Nucleic Acids Res 40:9543–9556. doi: 10.1093/nar/gks766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Świątek-Połatyńska MA, Bucca G, Laing E, Gubbens J, Titgemeyer F, Smith CP, Rigali S, van Wezel GP. 2015. Genome-wide analysis of in vivo binding of the master regulator DasR in Streptomyces coelicolor identifies novel non-canonical targets. PLoS One 10:e0122479. doi: 10.1371/journal.pone.0122479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taneja NK, Dhingra S, Mittal A, Naresh M, Tyagi JS. 2010. Mycobacterium tuberculosis transcriptional adaptation, growth arrest and dormancy phenotype development is triggered by vitamin C. PLoS One 5:e10860. doi: 10.1371/journal.pone.0010860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sousa EH, Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA. 2007. DosT and DevS are oxygen-switched kinases in Mycobacterium tuberculosis. Protein Sci 16:1708–1719. doi: 10.1110/ps.072897707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Honaker RW, Dhiman RK, Narayanasamy P, Crick DC, Voskuil MI. 2010. DosS responds to a reduced electron transport system to induce the Mycobacterium tuberculosis DosR regulon. J Bacteriol 192:6447–6455. doi: 10.1128/JB.00978-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Veluw GJ, Petrus ML, Gubbens J, de Graaf R, de Jong IP, van Wezel GP, Wösten HA, Claessen D. 2012. Analysis of two distinct mycelial populations in liquid-grown Streptomyces cultures using a flow cytometry-based proteomics approach. Appl Microbiol Biotechnol 96:1301–1312. doi: 10.1007/s00253-012-4490-5. [DOI] [PubMed] [Google Scholar]
- 74.Van Dissel D, Claessen D, Van Wezel GP. 2014. Morphogenesis of Streptomyces in submerged cultures. Adv Appl Microbiol 89:1–45. doi: 10.1016/B978-0-12-800259-9.00001-9. [DOI] [PubMed] [Google Scholar]
- 75.Borodina I, Krabben P, Nielsen J. 2005. Genome-scale analysis of Streptomyces coelicolor A3(2) metabolism. Genome Res 15:820–829. doi: 10.1101/gr.3364705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 77.Floriano B, Bibb M. 1996. afsR is a pleiotropic but conditionally required regulatory gene for antibiotic production in Streptomyces coelicolor A3(2). Mol Microbiol 21:385–396. doi: 10.1046/j.1365-2958.1996.6491364.x. [DOI] [PubMed] [Google Scholar]
- 78.Van Wezel GP, White J, Hoogvliet G, Bibb MJ. 2000. Application of redD, the transcriptional activator gene of the undecylprodigiosin biosynthetic pathway, as a reporter for transcriptional activity in Streptomyces coelicolor A3(2) and Streptomyces lividans. J Mol Microbiol Biotechnol 2:551–556. [PubMed] [Google Scholar]
- 79.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom. [Google Scholar]
- 80.Świątek MA, Tenconi E, Rigali S, van Wezel GP. 2012. Functional analysis of the N-acetylglucosamine metabolic genes of Streptomyces coelicolor and role in the control of development and antibiotic production. J Bacteriol 194:1136–1144. doi: 10.1128/JB.06370-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vara J, Lewandowska-Skarbek M, Wang YG, Donadio S, Hutchinson CR. 1989. Cloning of genes governing the deoxysugar portion of the erythromycin biosynthesis pathway in Saccharopolyspora erythraea (Streptomyces erythreus). J Bacteriol 171:5872–5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blondelet-Rouault MH, Weiser J, Lebrihi A, Branny P, Pernodet JL. 1997. Antibiotic resistance gene cassettes derived from the Omega interposon for use in E. coli and Streptomyces. Gene 190:315–317. doi: 10.1016/S0378-1119(97)00014-0. [DOI] [PubMed] [Google Scholar]
- 83.Colson S, Stephan J, Hertrich T, Saito A, van Wezel GP, Titgemeyer F, Rigali S. 2007. Conserved cis-acting elements upstream of genes composing the chitinolytic system of streptomycetes are DasR-responsive elements. J Mol Microbiol Biotechnol 12:60–66. doi: 10.1159/000096460. [DOI] [PubMed] [Google Scholar]
- 84.Mahr K, van Wezel GP, Svensson C, Krengel U, Bibb MJ, Titgemeyer F. 2000. Glucose kinase of Streptomyces coelicolor A3(2): large-scale purification and biochemical analysis. Antonie Van Leeuwenhoek 78:253–261. [DOI] [PubMed] [Google Scholar]
- 85.Rigali S, Nothaft H, Noens EE, Schlicht M, Colson S, Müller M, Joris B, Koerten HK, Hopwood DA, Titgemeyer F, van Wezel GP. 2006. The sugar phosphotransferase system of Streptomyces coelicolor is regulated by the GntR-family regulator DasR and links N-acetylglucosamine metabolism to the control of development. Mol Microbiol 61:1237–1251. doi: 10.1111/j.1365-2958.2006.05319.x. [DOI] [PubMed] [Google Scholar]
- 86.Bucca G, Laing E, Mersinias V, Allenby N, Hurd D, Holdstock J, Brenner V, Harrison M, Smith CP. 2009. Development and application of versatile high density microarrays for genome-wide analysis of Streptomyces coelicolor: characterization of the HspR regulon. Genome Biol 10:R5. doi: 10.1186/gb-2009-10-1-r5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smyth GK, Speed T. 2003. Normalization of cDNA microarray data. Methods 31:265–273. doi: 10.1016/S1046-2023(03)00155-5. [DOI] [PubMed] [Google Scholar]
- 89.Hong F, Breitling R, McEntee CW, Wittner BS, Nemhauser JL, Chory J. 2006. RankProd: a bioconductor package for detecting differentially expressed genes in meta-analysis. Bioinformatics 22:2825–2827. doi: 10.1093/bioinformatics/btl476. [DOI] [PubMed] [Google Scholar]
- 90.Laing E, Smith CP. 2010. RankProdIt: a web-interactive rank products analysis tool. BMC Res Notes 3:221. doi: 10.1186/1756-0500-3-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colson S, van Wezel GP, Craig M, Noens EE, Nothaft H, Mommaas AM, Titgemeyer F, Joris B, Rigali S. 2008. The chitobiose-binding protein, DasA, acts as a link between chitin utilization and morphogenesis in Streptomyces coelicolor. Microbiology 154:373–382. doi: 10.1099/mic.0.2007/011940-0. [DOI] [PubMed] [Google Scholar]
- 92.Tenconi E, Jourdan S, Motte P, Virolle MJ, Rigali S. 2012. Extracellular sugar phosphates are assimilated by Streptomyces in a PhoP-dependent manner. Antonie Van Leeuwenhoek 102:425–433. doi: 10.1007/s10482-012-9763-6. [DOI] [PubMed] [Google Scholar]
- 93.Zdobnov EM, Apweiler R. 2001. InterProScan—an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17:847–848. doi: 10.1093/bioinformatics/17.9.847. [DOI] [PubMed] [Google Scholar]
- 94.Finn RD, Tate J, Mistry J, Coggill PC, Sammut SJ, Hotz HR, Ceric G, Forslund K, Eddy SR, Sonnhammer EL, Bateman A. 2008. The Pfam protein families database. Nucleic Acids Res 36:D281–D288. doi: 10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Altschul SF, Wootton JC, Gertz EM, Agarwala R, Morgulis A, Schäffer AA, Yu YK. 2005. Protein database searches using compositionally adjusted substitution matrices. FEBS J 272:5101–5109. doi: 10.1111/j.1742-4658.2005.04945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res 14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chauhan S, Sharma D, Singh A, Surolia A, Tyagi JS. 2011. Comprehensive insights into Mycobacterium tuberculosis DevR (DosR) regulon activation switch. Nucleic Acids Res 39:7400–7414. doi: 10.1093/nar/gkr375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence alignment of the DNA binding domain of DevR with Streptomyces and Mycobacterium orthologues. The secondary-structure prediction was based on the crystal structure of DevR (PDB accession number 3C3W) and is shown on top. Conserved residues are shown in red, and boxes denote conservative substitutions. An upward arrow indicates residues involved in interactions with DNA; blue arrows indicate residues contacting nucleotide bases, and red arrows indicate residues making DNA phosphate oxygen contacts. Abbreviations: M. tuber, M. tuberculosis; Mmarin, Mycobacterium marinum; Msmeg, Mycobacterium smegmatis; Mvanba, Mycobacterium vanbaalenii; Shygro, Streptomyces hygroscopicus; Sscab, Streptomyces scabies; Scoeli, S. coelicolor; Sviola, Streptomyces violaceoruber; Ssirex, Streptomyces sp. strain Sirex. For accession numbers, see Materials and Methods. Download Figure S1, TIF file, 1 MB (1.1MB, tif) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Alignment of SCO0203 (OsdK) and SCO0204 (OsdR). Multiple alignments of OsdK with DevS and DosT of M. tuberculosis (A) and OsdR with DevR of M. tuberculosis and NarL of Pseudomonas aeruginosa (B) were created with ClustalW (digits indicate the amino acid number). The different protein domains are indicated in italic. Amino acids conserved in at least 80% of the sequences are shaded (identical amino acids are in black, and amino acids with similar properties are in gray). Important amino acids of DosT and DevS are indicated beneath and above the alignment, respectively. GAF, GAF domain; DHp, dimerization and histidine phosphotransfer domain; CA, C-terminal catalytic and ATP binding domain; REC, receiver domain; HTH_LuxR, helix-turn-helix LuxR domain; ♦, phosphorylation site; ○, RR activation; #, H-bond network from iron to the surface; *, hydrophobic space surrounding a heme; ~, contact with a propionate group heme; ●, ligand binding; ▲, surface crevice; ≈, heme binding; ■, cavity next to a ligand binding pocket. Download Figure S2, TIF file, 2.4 MB (2.5MB, tif) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In vitro autophosphorylation and transphosphorylation of OsdRK. OsdK was readily phosphorylated as shown by the large band in the lane “auto.” Using autophosphorylated OsdK, OsdR was transphosphorylated as shown by the presence of a band of phosphorylated OsdR and OsdK in the lane “trans.” However, there was significant phosphosignal loss observed (a decrease in band intensity over time). The reaction mixtures were run on 3.5% acrylamide gels in 0.5× Tris-borate-EDTA (TBE) buffer. Gels were dried and then subjected to autoradiography for analysis. Download Figure S3, TIF file, 0.1 MB (140.8KB, tif) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Heat maps of genes related to zinc import (A), sulfur metabolism and thiol homeostasis (B), and nitrogen metabolism (C) that are significantly differentially expressed between the osdR mutant and its parent, S. coelicolor M145. RNA was isolated from mycelium grown on MM with 1% mannitol during vegetative growth (24 h), vegetative/aerial growth (30 h), aerial growth/early sporulation (42 h), and sporulation (54 h). Only genes with a PFP value of less than 0.010 and a fold change (ΔosdR expression/M145 expression) of more than 2.0 are presented. The levels of the fold changes are indicated with colors, as represented by the scale bar. Download Figure S4, TIF file, 0.3 MB (272.8KB, tif) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Microarray and RT-qPCR expression profiles of genes deregulated in the osdR mutant. RNA was isolated for microarray analysis (left) and RT-qPCR (right) profiling from independent cultures grown on MM with 1% mannitol during vegetative growth (24 h), vegetative/aerial growth (30 h), aerial growth (36 h), aerial growth/early sporulation (42 h), and sporulation (54 h). The expression profiles of the wild type (black circles) and the osdR mutant (gray triangles) over time in the microarray data (left) and the RT-qPCR data (right) were compared. Genes of interest tested were SCO2637, SCO3323 (bldN), and SCO5321 (whiE). Download Figure S5, TIF file, 0.3 MB (264.9KB, tif) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Bacterial strain, plasmids, and constructs. Download Table S1, PDF file, 0.4 MB (416KB, pdf) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Oligonucleotides. Download Table S2, PDF file, 0.5 MB (536.7KB, pdf) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Position weight matrix for the OsdR binding site. Download Table S3, PDF file, 0.3 MB (296.5KB, pdf) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Overview of microarray results. Download Table S4, PDF file, 0.1 MB (135.2KB, pdf) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Overview of genes deregulated at 36 h. Download Table S5, PDF file, 0.8 MB (891.4KB, pdf) .
Copyright © 2016 Urem et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.