Abstract
Background
To improve care and control for patients with adult-onset asthma, a better understanding of determinants of their risk and outcomes is important. We investigated how associations between asthma, asthma control and obesity may be modified by patient demographic characteristics.
Methods
This retrospective study of adults enrolled in several health plans across the U.S. (n = 2,860,305) examined the interacting effects of obesity, age, race, and sex on adult-onset asthma and asthma control. Multivariable adjusted Cox and logistic regression models estimated hazard ratios (HR), and 95 % confidence intervals (CI) for the associations between body mass index (BMI) and study outcomes, and interactions of BMI with demographic characteristics.
Results
Compared with individuals who had a BMI <25 kg/m2, the hazard of adult-onset asthma progressively increased with increasing BMI, from a 12 % increase among persons with a BMI of 25.0–29.9 kg/m2 (HR 1.12, 95 % CI 1.10, 1.14) to an almost 250 % increase among persons with a BMI ≥50 kg/m2 (HR 2.49, 95 % CI 2.38, 2.60). The magnitude of the association between obesity and asthma risk was greater for women (compared with men) and lower for Blacks (compared with non-Hispanic Whites). Among individuals with asthma, obesity was associated with poorly controlled and high-risk asthma.
Conclusions
The present study demonstrates that the magnitude of the associations between obesity and adult-onset asthma incidence and control are modified by race, age, and sex. Understanding the role of obesity in the development of adult-onset asthma will help to improve asthma treatment algorithms and to develop targeted interventions.
Keywords: Asthma, Adult-onset, Obesity, Race, Sex, Ethnicity
Background
Asthma is a chronic inflammatory disorder of the airways that involves a complex interaction of airflow obstruction, bronchial hyperresponsiveness, and underlying inflammation [1]. The prevalence of asthma in the United States is about 9 % in women and 7 % in men [2]. While asthma often starts in early childhood, adult-onset asthma is often associated with poor control, more symptoms, and higher medication needs than pediatric-onset asthma [3]. To improve care and control for patients with adult-onset asthma, a better understanding of determinants of their risk and outcomes is important.
While several previous studies have shown that obesity is a risk factor for asthma [4], recent studies also indicate that patients with asthma who are obese may represent a clinically distinct subset of patients [5]. Compared to patients with asthma who are normal or overweight, patients with asthma who are obese have more severe and frequent respiratory symptoms, greater exacerbation rates and reduced asthma-related quality of life [6]. Because most studies have not distinguished between pediatric and adult-onset asthma, little is known about the risk and outcomes of adult-onset asthma, the role of obesity in asthma development, and how the risk of asthma among the individuals who are obese may be modified by other factors such as race/ethnicity and sex. Understanding the role of obesity in developing adult-onset asthma and achieving control across heterogeneous groups of patients will help to improve asthma control algorithms and to develop targeted interventions. This knowledge will foster future patient-centered outcome studies to better understand patient barriers to asthma control among patients with asthma who are affected by obesity.
In this large, population-based cohort study using electronic health record (EHR) data of 2.8 million individuals who were members of nine different health plans across the United States, we examined the association between body weight and the incidence of adult-onset asthma. Among adults with incident adult-onset asthma, secondary outcomes such as poorly controlled asthma with or without high asthma medication ratio (AMR) as indicator for difficulties to achieve asthma control, and high-risk asthma, were examined. In addition, we examined race and sex as potential modifiers of the effect of body weight on asthma risk and outcomes.
Patients and methods
Study setting
This study was conducted by the patient outcomes research to advance learning (PORTAL) network. The health care systems in the PORTAL network collectively include 11 million members, as previously described [7, 8]. In brief, PORTAL includes Kaiser Permanente, Group Health Cooperative, HealthPartners, and Denver Health. The cohort is racially and socioeconomically diverse, and generally representative of the underlying populations of the health plans’ service regions [9, 10]. All PORTAL sites contributed data for the present study except HealthPartners.
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of Kaiser Permanente Southern California (KPSC) as the lead site. The IRBs at the other sites reviewed the protocol and subsequently ceded review to the KPSC IRB.
Study design and population
We performed a retrospective cohort study using the PORTAL overweight/obesity cohort, which includes over 5 million adults with a BMI ≥23.0 kg/m2 in 2012–2013. A detailed description of the cohort is provided elsewhere [8]. Briefly, the PORTAL overweight/obesity cohort consisted of individuals who were 18 years of age and older when entering the cohort, had 1 year of continuous membership in any of the contributing health plans prior to entering the cohort and had at least one outpatient medically-related visit with a biologically plausible recorded measure of weight and height with a BMI of ≥23.0 kg/m2 at any time between 2012 and 2013 (n = 5,293,458).
The study has a mixed longitudinal (primary study outcome) and cross-sectional (secondary study outcomes) design (Fig. 1). A cohort baseline date was assumed on January 1, 2011. To exclude the possibility of preexisting asthma based on at least 2 years of medical history, we restricted the original PORTAL cohort to individuals who were also enrolled in one of the health plans in 2009 and 2010 (n = 4,568,725). We excluded patients who were not at least 18 years of age at cohort baseline (n = 331,504) or who did not have a measure of weight, height, or BMI in the EHR within a year prior to the cohort baseline (n = 817,674). We then excluded individuals with a history of asthma and other chronic pulmonary diseases (Table 1; n = 559,242). The final analytic cohort consisted of 2,860,305 individuals.
Fig. 1.
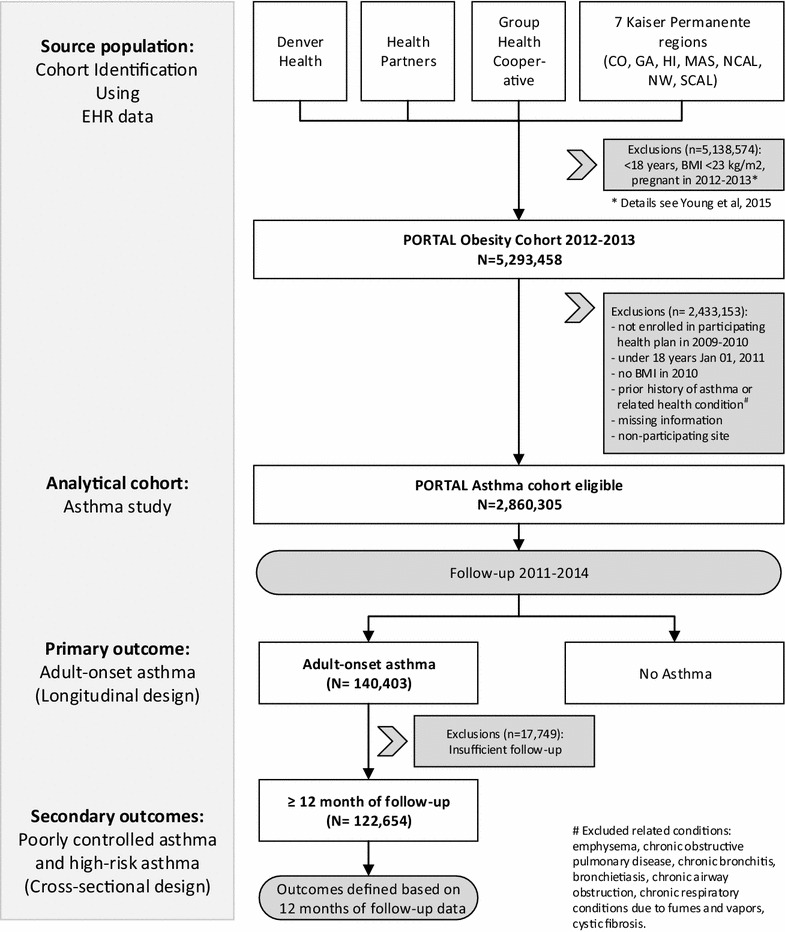
Study flow chart modified from Young et al. [8]. BMI body mass index; EHR electronic health records; PORTAL patients outcomes research to advance learning
Table 1.
Exclusion and censoring criteria
| Medical condition | International classification of disease, 9th modification ICD-9 diagnosis code |
|---|---|
| Emphysema | 492.x |
| Chronic obstructive pulmonary disease including chronic bronchitis | 490.x–491.x |
| Bronchiectasis | 494.x |
| Chronic airway obstruction | 496.x |
| Chronic respiratory conditions due to fumes and vapors | 506.4 |
| Cystic fibrosis | 277.0 |
| Prior history of asthma | 493.x |
Weight and height
We used weight and height measurements extracted from outpatient clinic visits before but closest to the index date (at maximum 1 year prior to the index date) to calculate baseline BMI [8]. Weight is routinely measured as part of obtaining vital signs during outpatient clinic visits at PORTAL sites. Height is typically assessed less often, as it is considered to be more static. BMI is calculated within the EHR. A height <4 ft or ≥8 ft or a weight <50 lbs or ≥1000 lbs were considered implausible and removed from the data. Similarly, a calculated BMI <5 kg/m2 or ≥90 kg/m2 was excluded. A total of 6954 (0.1 %) individuals were excluded from the cohort because they had no biologically plausible weight, height, or BMI values.
We categorized individuals based on their baseline BMI as normal weight (BMI <25 kg/m2), overweight (25.0–29.9 kg/m2), obese class 1 (30.0–34.9 kg/m2), obese class 2 (35.0–39.9 kg/m2), obese class 3 (40.0–49.9 kg/m2), and obese class 4 (≥50 kg/m2) [11, 12]. We classified Asians in the same manner for the main analysis. Because the baseline BMI used for this study was in 2011 not 2012/2013 as for the PORTAL overweight/obesity cohort, the normal weight category includes of individuals with a BMI <23 kg/m2 before 2012/2013. For a secondary analysis, we classified Asians as normal weight (BMI <23 kg/m2), overweight (23.0–24.9 kg/m2), obese class 1 (25.0–29.9 kg/m2), obese class 2 (30.0–34.9 kg/m2), obese class 3 (35.0–39.9 kg/m2), and obese class 4 (≥40 kg/m2) [13].
Race and ethnicity
Based on the self-reported identification from administrative records, individuals were categorized as Asian, Black or African American, Hispanic, Native Hawaiian or other Pacific Islander, American Indian/Alaskan Native (AIAN), non-Hispanic White, or other/unknown [14]. Hispanic ethnicity took priority over any racial category.
Socioeconomic status
As indicator of socioeconomic status, two measures were used: neighborhood education and insurance through government health care assistance programs.
Neighborhood education was estimated using geospatial entity object codes (geocodes) that linked addresses to 2010 United States census data at the block group level. The probability of high school graduation or less within a block group was used and low neighborhood education defined as the probability of a high school education or less above the population median. Individuals with missing neighborhood education (n = 82,833) were assigned the mean probability of high school graduation for their region.
Information on insurance through government health care assistance programs for individuals with low income and limited resources such as Medicaid (yes/no) was extracted from insurance plan administrative information. Individuals with missing insurance information (n = 853) were assigned to no government health care assistance programs.
Oral contraceptive use
Because female sex hormones are a risk factor for asthma [3] and are associated with obesity, woman were identified as using oral contraceptives if oral contraceptives were dispensed at least once at any point between 2009 and the end of follow-up for the primary study outcome.
Definition of study outcomes
The primary study outcome was adult-onset asthma defined as (1) one or more hospitalization or emergency department visit with any diagnosis of asthma [International Classification of Disease (ICD), 9th modification ICD-9 diagnosis code 493.x], (2) two or more ambulatory visits with diagnosis of asthma, or (3) at least one visit with a diagnosis of asthma in combination with at least one dispensing of asthma-related medication in the year following the diagnosis [15, 16]. The outcome date was defined as the first date for which a patient met 1 of the above criteria.
The secondary study outcomes were poorly controlled asthma with or without high AMR as indicator for difficulties to achieve asthma control and high-risk asthma. For these secondary study outcomes, the cohort was limited to individuals with adult-onset asthma, at least 12 months of follow-up after the first diagnosis, and medication dispense information available (n = 140,403).
The secondary outcomes were defined based on asthma medications and asthma-related utilization as follows:
Asthma medication dispenses were extracted from the EHR. Medications classified as controllers included inhaled corticosteroids (flunisolide, mometasone, triamcinolone, ciclesonide, fluticasone, budesonide, beclomethasone), combined inhaled corticosteroids/long-acting β-agonists (fluticasone/salmeterol, mometasone/formoterol, budesonide/formoterol), methylxanthines (aminophylline, dyphylline, theophylline), mast cell stabilizer (cromolyn), and leukotriene receptor antagonists (montelukast, zafirlukast, zileuton) [17]. Medications classified as controllers did not include oral corticosteroids. Reliever (rescue) medications included inhaled short-acting β-agonists (albuterol, levalbuterol, metaproterenol) [17]. For persons with asthma and at least 1 year of follow-up, the number and type of medications dispensed during the 1-year period following the incidence date of asthma diagnosis were examined for characterization of asthma severity and control.
An asthma medication ratio was calculated according to the specifications of the National Committee for quality assurance [17]. The asthma medication ratio (AMR) is defined as the number of controller medication fills divided by the sum of the number of controller and rescue medication fills. Both medications were weighted for the number of doses per container to account for differences between products [17, 18]. The AMR generally is calculated over a 1-year period and ranges from 0 to 1 where 1 is ideal (i.e., all controller and no rescue medications). We categorized the AMR as “high” (>=0.5) or “low” (<0.5), which previously has been shown to be associated with patient outcomes [16, 19, 20].
The amount of dispensing of rescue medication over 1 year is an indicator of long-term asthma control [16, 20]. Asthma control was defined based on the number of rescue medication canisters dispensed in the first 12 months after diagnosis and categorized as “poorly controlled” (>=6 canisters) or “adequately controlled” (<6 canisters) [18]. Poorly controlled asthma can be related to factors such as higher asthma severity, inadequate controller prescribing, poor medication adherence, environmental exposures or unaddressed comorbidities [21].
To distinguish between poorly controlled with low or high AMR as indicator for difficulties to achieve asthma control, we divided patients with poorly controlled asthma into those with low (<0.5) and high AMR (>=0.5) [18, 19].
High-risk asthma was defined as having 1 or more asthma-related emergency department visits and/or 1 or more oral steroid dispensing within 7 days of an asthma-related ambulatory visit during the 1-year follow up [18].
Cohort follow-up
For the primary study outcome, adult-onset asthma, individuals were passively followed through June 30, 2014 using information from the EHR. The follow-up time was calculated from January 1, 2011 until the first occurrence of one of the following events: diagnosis of adult-onset asthma, diagnosis of other chronic pulmonary diseases, the end of health care coverage, death, or the end of follow-up on December 31, 2014.
Statistical analysis
Demographic and clinical characteristics were summarized as absolute numbers and proportions and reported by weight status. Chi squared tests were used to assess differences in proportions of demographic and clinical characteristics. These characteristics were examined and compared for each site to ensure there were no systematic site-level differences.
The association between baseline weight status and asthma risk was evaluated using hazard ratios (HR) estimated from Cox proportional hazard models with person-time of follow-up as the time scale adjusted for or stratified by age, sex, race/ethnicity, insurance through government health care assistance programs, neighborhood education level, oral contraceptive use, and site. We examined the association of BMI and asthma risk within strata of potential effect modifiers. Tests of linear trends across weight category were conducted by considering weight category as a continuous variable in the multivariate model. To investigate whether the association between asthma risk and BMI category was modified by other potential risk factors for asthma, we performed tests for multiplicative interaction using likelihood-ratio tests. All HRs are displayed with 95 % confidence intervals (CI); reported P values for trends and interactions are based on 2-sided tests.
Among adults with adult-onset asthma and at least 12 months of follow-up (Fig. 1), associations among weight status and asthma-specific outcomes were assessed. We also examined how associations were modified by sex, race, and other factors. Due to smaller cell sizes, Asian, Pacific Islander, and AIAN races were combined into one category for these secondary analyses. Odds ratios (OR) for the association between weight status and poorly controlled asthma with low or high AMR, and high-risk asthma were estimated using logistic regression adjusted for age, sex, race/ethnicity, insurance, neighborhood education level, oral contraceptive use and site. Sensitivity analyses were performed excluding persons with missing neighborhood education (n = 82,833) and insurance (n = 853) information as well as removing education and insurance as covariates with essentially unaltered results. All statistical analyses were conducted using SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA).
Results
The PORTAL cohort is ethnically and racially diverse with 49.4 % non-Hispanic Whites, 23.0 % Hispanics, 11.4 % Blacks and 10.5 % Asians (Table 2). With increasing weight class, individuals were more likely to be in the age group between 40–60 years of age, female, non-White, a recipient of care through a government insurance program for individuals with low income and limited resources such as Medicaid, and a resident of a neighborhood with higher educational attainment.
Table 2.
Distribution of baseline demographic characteristics of PORTAL patients (n = 2,860,305)
| Total population | BMI (kg/m2) | ||||||
|---|---|---|---|---|---|---|---|
| <25 | 25.0–29.9 | 30.0–34.9 | 35.0–39.9 | 40.0–49.9 | ≥50 | ||
| N (%) | |||||||
| Age (years) | |||||||
| 18–39 | 700,548 (24.5 %) | 187,257 (31.2 %) | 260,014 (22.5 %) | 141,290 (21.5 %) | 64,996 (23.7 %) | 39,778 (26.6 %) | 7213 (30.1 %) |
| 40–65 | 1,507,052 (52.7 %) | 269,106 (44.9 %) | 605,983 (52.4 %) | 369,320 (56.3 %) | 159,459 (58.1 %) | 88,592 (59.3 %) | 14,592 (60.9 %) |
| 65+ | 652,705 (22.8 %) | 143,604 (23.9 %) | 290,840 (25.1 %) | 145,162 (22.1 %) | 50,046 (18.2 %) | 20,906 (14.0 %) | 2147 (9.0 %) |
| Sex | |||||||
| Male | 1,307,645 (45.7 %) | 223,739 (37.3 %) | 587,681 (50.8 %) | 323,679 (49.4 %) | 114,094 (41.6 %) | 50,906 (34.1 %) | 7546 (31.5 %) |
| Female | 1,552,660 (54.3 %) | 376,228 (62.7 %) | 569,156 (49.2 %) | 332,093 (50.6 %) | 160,407 (58.4 %) | 98,370 (65.9 %) | 16,406 (68.5 %) |
| Race/ethnicity | |||||||
| Non-Hispanic White | 1,413,998 (49.4 %) | 305,329 (50.9 %) | 575,541 (49.8 %) | 318,138 (48.5 %) | 132,652 (48.3 %) | 71,183 (47.7 %) | 11,155 (46.6 %) |
| Hispanic | 656,642 (23.0 %) | 105,589 (17.6 %) | 263,032 (22.7 %) | 173,604 (26.5 %) | 72,069 (26.3 %) | 36,972 (24.8 %) | 5376 (22.4 %) |
| Black | 327,148 (11.4 %) | 46,157 (7.7 %) | 113,792 (9.8 %) | 87,652 (13.4 %) | 44,754 (16.3 %) | 29,181 (19.5 %) | 5612 (23.4 %) |
| Asian | 301,722 (10.5 %) | 108,670 (18.1 %) | 138,957 (12.0 %) | 40,504 (6.2 %) | 9845 (3.6 %) | 3422 (2.3 %) | 324 (1.4 %) |
| Pacific Islander | 37,719 (1.3 %) | 7335 (1.2 %) | 13,976 (1.2 %) | 8660 (1.3 %) | 4321 (1.6 %) | 2821 (1.9 %) | 606 (2.5 %) |
| AIAN | 14,552 (0.5 %) | 2493 (0.4 %) | 5189 (0.4 %) | 3675 (0.6 %) | 1850 (0.7 %) | 1115 (0.7 %) | 230 (1.0 %) |
| Other/unknown | 108,524 (3.8 %) | 24,394 (4.0 %) | 46,350 (4.0 %) | 23,539 (3.6 %) | 9010 (3.3 %) | 4582 (3.1 %) | 649 (2.7 %) |
| Health care assistance | |||||||
| No | 2,809,762 (98.2 %) | 590,175 (98.4 %) | 1,139,813 (98.5 %) | 643,938 (98.2 %) | 268,196 (97.7 %) | 144,760 (97.0 %) | 22,880 (95.5 %) |
| Yes | 50,543 (1.8 %) | 9792 (1.6 %) | 17,024 (1.5 %) | 11,834 (1.8 %) | 6305 (2.3 %) | 4516 (3.0 %) | 1072 (4.5 %) |
| Neighborhood education | |||||||
| Low | 1,387,326 (48.5 %) | 328,707 (54.8 %) | 582,814 (50.4 %) | 295,934 (45.1 %) | 114,239 (41.6 %) | 57,322 (38.4 %) | 8310 (34.7 %) |
| High | 1,472,979 (51.5 %) | 271,260 (45.2 %) | 574,023 (49.6 %) | 359,838 (54.9 %) | 160,262 (58.4 %) | 91,954 (61.6 %) | 15,642 (65.3 %) |
| Oral contraceptives (women only) | |||||||
| Yes | 467,551 (16.3 %) | 133,053 (22.2 %) | 168,619 (14.6 %) | 91,033 (13.9 %) | 43,140 (15.7 %) | 26,925 (18.0 %) | 4781 (20.0 %) |
| No | 2,392,754 (83.7 %) | 466,914 (77.8 %) | 988,218 (85.4 %) | 564,739 (86.1 %) | 231,361 (84.3 %) | 122,351 (82.0 %) | 19,171 (80.0 %) |
Chi squared tests were used to assess differences in proportions of demographic and clinical characteristics across different weight classes. P < 0.001 for all demographic and clinical characteristics shown
AIAN American Indian/American Native; BMI body mass index
During 11,753,217 person-years of follow-up (median follow-up time 4.5 years), we identified 140,403 individuals who met the study definition of adult-onset asthma. In multivariate-adjusted models, a new diagnosis of asthma was more common among women, younger individuals, members of selected racial groups, and persons with lower socioeconomic status (SES) (Fig. 2).
Fig. 2.
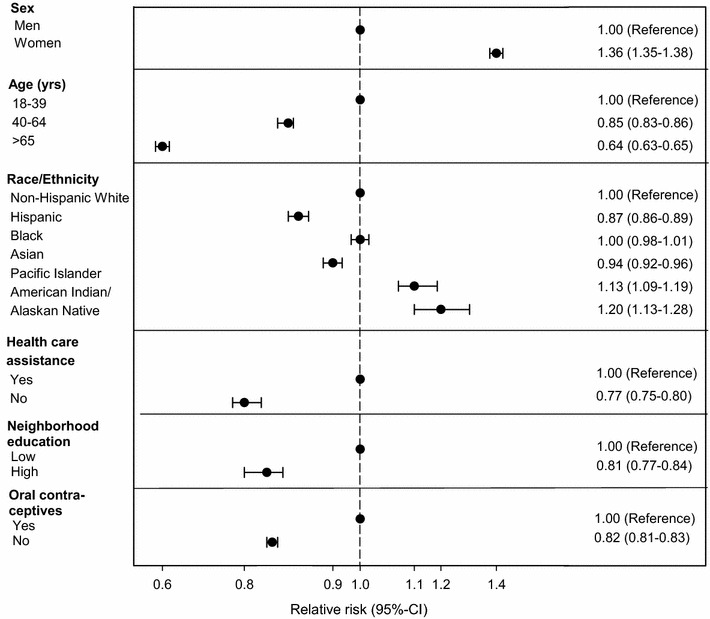
Risk for asthma by age, sex, race and sociodemographic characteristics
The risk for adult-onset asthma was strongly and positively associated with BMI categories. In multivariate-adjusted models, the hazard of developing adult-onset asthma progressively increased with higher BMI categories, from a 12 % increase among persons with a BMI of 25.0–29.9 kg/m2 (HR 1.12, 95 % CI 1.10, 1.14) to an almost 250 % increase among persons with a BMI ≥50 kg/m2 (HR 2.49, 95 % CI 2.38, 2.60) (Table 3).
Table 3.
Adjusted HR of asthma according to weight class by race, age, and sex
| BMI (kg/m2) | P for trend | P for interaction | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases | <25 | 25.0–29.9 | 30.0–34.9 | 35.0–39.9 | 40.0–49.9 | ≥50 | ||||||||
| Ref | HR | 95 % CI | HR | 95 % CI | HR | 95 % CI | HR | 95 % CI | HR | 95 % CI | ||||
| All ages | 140,403 | 1.00 | 1.12 | 1.10, 1.14 | 1.37 | 1.35, 1.40 | 1.64 | 1.61, 1.68 | 1.97 | 1.92, 2.01 | 2.49 | 2.38, 2.60 | <0.0001 | – |
| Age (years) | ||||||||||||||
| 18–39 | 41,812 | 1.00 | 1.03 | 1.00, 1.06 | 1.18 | 1.15, 1.22 | 1.34 | 1.30, 1.39 | 1.57 | 1.51, 1.64 | 1.89 | 1.75, 2.04 | <0.0001 | <0.001 |
| 40–65 | 74,755 | 1.00 | 1.18 | 1.15, 1.20 | 1.46 | 1.42, 1.49 | 1.77 | 1.72, 1.82 | 2.17 | 2.11, 2.24 | 2.79 | 2.64, 2.94 | <0.0001 | |
| 65+ | 23,836 | 1.00 | 1.25 | 1.21, 1.30 | 1.65 | 1.59, 1.72 | 2.11 | 2.01, 2.21 | 2.47 | 2.32, 2.63 | 3.52 | 3.05, 4.06 | <0.0001 | |
| Sex | ||||||||||||||
| Male | 51,018 | 1.00 | 1.07 | 1.04, 1.10 | 1.26 | 1.23, 1.30 | 1.50 | 1.45, 1.56 | 1.78 | 1.70, 1.85 | 2.19 | 2.01, 2.39 | <0.0001 | <0.001 |
| Female | 89,385 | 1.00 | 1.14 | 1.12, 1.16 | 1.43 | 1.40, 1.46 | 1.71 | 1.67, 1.75 | 2.05 | 2.00, 2.10 | 2.59 | 2.47, 2.72 | <0.0001 | |
| Race/ethnicity | ||||||||||||||
| Non-Hispanic White | 73,049 | 1.00 | 1.13 | 1.10, 1.15 | 1.38 | 1.35, 1.41 | 1.65 | 1.60, 1.69 | 1.99 | 1.93, 2.05 | 2.55 | 2.40, 2.70 | <0.0001 | <0.001 |
| Hispanic | 28,386 | 1.00 | 1.07 | 1.03, 1.11 | 1.29 | 1.24, 1.34 | 1.59 | 1.53, 1.67 | 1.90 | 1.81, 2.00 | 2.29 | 2.08, 2.53 | <0.0001 | |
| Black | 16,855 | 1.00 | 1.01 | 0.96, 1.06 | 1.22 | 1.16, 1.28 | 1.41 | 1.33, 1.49 | 1.69 | 1.59, 1.79 | 2.04 | 1.85, 2.24 | <0.0001 | |
| Asian | 14,623 | 1.00 | 1.23 | 1.19, 1.28 | 1.62 | 1.54, 1.70 | 2.08 | 1.93, 2.25 | 2.34 | 2.09, 2.62 | 4.45 | 3.39, 5.83 | <0.0001 | |
| Pacific Islander | 2332 | 1.00 | 1.00 | 0.88, 1.13 | 1.21 | 1.07, 1.38 | 1.39 | 1.20, 1.61 | 1.44 | 1.22, 1.69 | 2.07 | 1.60, 2.68 | <0.0001 | |
| American Indian/American native | 994 | 1.00 | 1.13 | 0.92, 1.38 | 1.32 | 1.07, 1.63 | 1.58 | 1.25, 1.99 | 2.14 | 1.68, 2.72 | 2.61 | 1.78, 3.82 | <0.0001 | |
| Other/unknown | 4164 | 1.00 | 1.08 | 1.00, 1.18 | 1.32 | 1.20, 1.45 | 1.56 | 1.39, 1.75 | 1.95 | 1.71, 2.23 | 2.42 | 1.82, 3.21 | <0.0001 | |
| Men only | ||||||||||||||
| Age (years) | ||||||||||||||
| 18–39 | 15,196 | 1.00 | 0.98 | 0.94, 1.02 | 1.10 | 1.05, 1.15 | 1.20 | 1.13, 1.28 | 1.39 | 1.29, 1.50 | 1.66 | 1.43, 1.93 | <0.0001 | <0.001 |
| 40–65 | 26,659 | 1.00 | 1.13 | 1.08, 1.17 | 1.35 | 1.29, 1.41 | 1.63 | 1.55, 1.72 | 1.96 | 1.84, 2.08 | 2.48 | 2.22, 2.78 | <0.0001 | |
| 65+ | 9163 | 1.00 | 1.20 | 1.13, 1.28 | 1.50 | 1.41, 1.61 | 1.96 | 1.80, 2.13 | 2.46 | 2.19, 2.77 | 3.29 | 2.38, 4.55 | <0.0001 | |
| Race/ethnicity | ||||||||||||||
| Non-Hispanic White | 27,157 | 1.00 | 1.09 | 1.05, 1.13 | 1.28 | 1.23, 1.33 | 1.54 | 1.46, 1.61 | 1.85 | 1.75, 1.96 | 2.17 | 1.92, 2.45 | <0.0001 | <0.001 |
| Hispanic | 9889 | 1.00 | 0.97 | 0.90, 1.03 | 1.13 | 1.06, 1.21 | 1.39 | 1.28, 1.50 | 1.61 | 1.46, 1.77 | 1.84 | 1.51, 2.23 | <0.0001 | |
| Black | 5091 | 1.00 | 0.95 | 0.87, 1.03 | 1.11 | 1.01, 1.21 | 1.16 | 1.04, 1.29 | 1.39 | 1.23, 1.57 | 1.68 | 1.35, 2.09 | <0.0001 | |
| Asian | 5731 | 1.00 | 1.20 | 1.13, 1.28 | 1.56 | 1.44, 1.69 | 2.14 | 1.89, 2.42 | 2.18 | 1.79, 2.66 | 4.41 | 2.81, 6.95 | <0.0001 | |
| Pacific Islander | 916 | 1.00 | 0.99 | 0.80, 1.22 | 1.12 | 0.90, 1.40 | 1.20 | 0.93, 1.55 | 1.25 | 0.94, 1.66 | 2.08 | 1.41, 3.08 | <0.0001 | |
| AIAN | 304 | 1.00 | 0.83 | 0.58, 1.18 | 1.00 | 0.69, 1.45 | 1.30 | 0.85, 1.97 | 1.62 | 1.00, 2.61 | 2.55 | 1.19, 5.45 | <0.0001 | |
| Other/unknown | 1930 | 1.00 | 1.04 | 0.92, 1.18 | 1.19 | 1.03, 1.37 | 1.38 | 1.15, 1.66 | 1.74 | 1.39, 2.18 | 3.55 | 2.36, 5.34 | <0.0001 | |
| Women only | ||||||||||||||
| Age (years) | ||||||||||||||
| 18–39 | 26,616 | 1.00 | 1.05 | 1.02, 1.09 | 1.23 | 1.19, 1.27 | 1.42 | 1.36, 1.48 | 1.66 | 1.58, 1.74 | 1.99 | 1.83, 2.18 | <0.0001 | <0.001 |
| 40–65 | 48,096 | 1.00 | 1.19 | 1.16, 1.22 | 1.51 | 1.47, 1.55 | 1.82 | 1.77, 1.89 | 2.25 | 2.17, 2.33 | 2.88 | 2.71, 3.07 | <0.0001 | |
| 65+ | 14,673 | 1.00 | 1.27 | 1.22, 1.34 | 1.74 | 1.66, 1.83 | 2.18 | 2.05, 2.32 | 2.48 | 2.30, 2.67 | 3.60 | 3.06, 4.22 | <0.0001 | |
| Race/ethnicity | ||||||||||||||
| Non-Hispanic White | 45,892 | 1.00 | 1.14 | 1.11, 1.17 | 1.44 | 1.40, 1.48 | 1.70 | 1.65, 1.76 | 2.05 | 1.97, 2.12 | 2.68 | 2.51, 2.87 | <0.0001 | <0.001 |
| Hispanic | 18,497 | 1.00 | 1.11 | 1.06, 1.16 | 1.36 | 1.30, 1.42 | 1.68 | 1.59, 1.77 | 2.01 | 1.89, 2.13 | 2.46 | 2.20, 2.76 | <0.0001 | |
| Black | 11,764 | 1.00 | 1.04 | 0.98, 1.11 | 1.29 | 1.21, 1.37 | 1.53 | 1.43, 1.64 | 1.82 | 1.69, 1.95 | 2.18 | 1.96, 2.43 | <0.0001 | |
| Asian | 8892 | 1.00 | 1.26 | 1.20, 1.32 | 1.65 | 1.55, 1.76 | 2.05 | 1.86, 2.25 | 2.42 | 2.10, 2.78 | 4.44 | 3.17, 6.23 | <0.0001 | |
| Pacific Islander | 1416 | 1.00 | 0.99 | 0.85, 1.16 | 1.26 | 1.07, 1.49 | 1.51 | 1.25, 1.81 | 1.55 | 1.26, 1.90 | 2.04 | 1.44, 2.88 | <0.0001 | |
| AIAN | 690 | 1.00 | 1.29 | 1.01, 1.64 | 1.48 | 1.15, 1.91 | 1.71 | 1.29, 2.26 | 2.37 | 1.78, 3.14 | 2.73 | 1.75, 4.26 | <0.0001 | |
| Other/unknown | 2234 | 1.00 | 1.10 | 0.99, 1.24 | 1.43 | 1.26, 1.62 | 1.69 | 1.46, 1.97 | 2.09 | 1.77, 2.47 | 1.86 | 1.26, 2.77 | <0.0001 | |
| Oral contraceptives (women only) | ||||||||||||||
| Yes | 32,399 | 1.00 | 1.09 | 1.06, 1.12 | 1.34 | 1.30, 1.38 | 1.54 | 1.48,1.60 | 1.80 | 1.72,1.88 | 2.06 | 1.89, 2.24 | <0.0001 | <0.001 |
| No | 56,986 | 1.00 | 1.19 | 1.16, 1.21 | 1.51 | 1.47, 1.55 | 1.84 | 1.78,1.89 | 2.23 | 2.16, 2.30 | 2.97 | 2.80, 3.15 | <0.0001 | |
Hazard ratios were estimated from Cox proportional hazard models adjusted for age (continuous), sex (male, female), race/ethnicity (non-Hispanic White, Hispanic, Black, Asian, Pacific Islander, AIAN, other or unknown), insurance through government health care assistance programs (yes/no), neighborhood education level (continuous probability of education below high school), oral contraceptive use (yes/no), and site (Denver Health, Group Health Cooperative, HealthPartners and seven Kaiser Permanente regions). Tests of linear trend across weight category were conducted by considering weight category as a continuous variable in the multivariate model
CI confidence intervals; HR hazard ratios; AIAN American Indian/Alaskan Native
To evaluate whether the association between BMI and adult-onset asthma was modified by age, sex and race, we tested for interaction effects and repeated our analyses within subgroups defined by those variables (Table 3). The association of BMI to asthma incidence appeared to be stronger among women than among men, and in older (40–65 and 65+ year) compared with younger individuals (18–39 year; P value for interaction <0.001).
The association of BMI and asthma incidence also appeared to be stronger among Asians and weaker among Blacks than among other races/ethnicities (P value for interaction < 0.001, Fig. 3). When applying Asian-specific BMI thresholds to compare the risk for asthma across the different weight classes, the association of obesity to asthma was comparable between Asians and non-Hispanic Whites (Fig. 3).
Fig. 3.
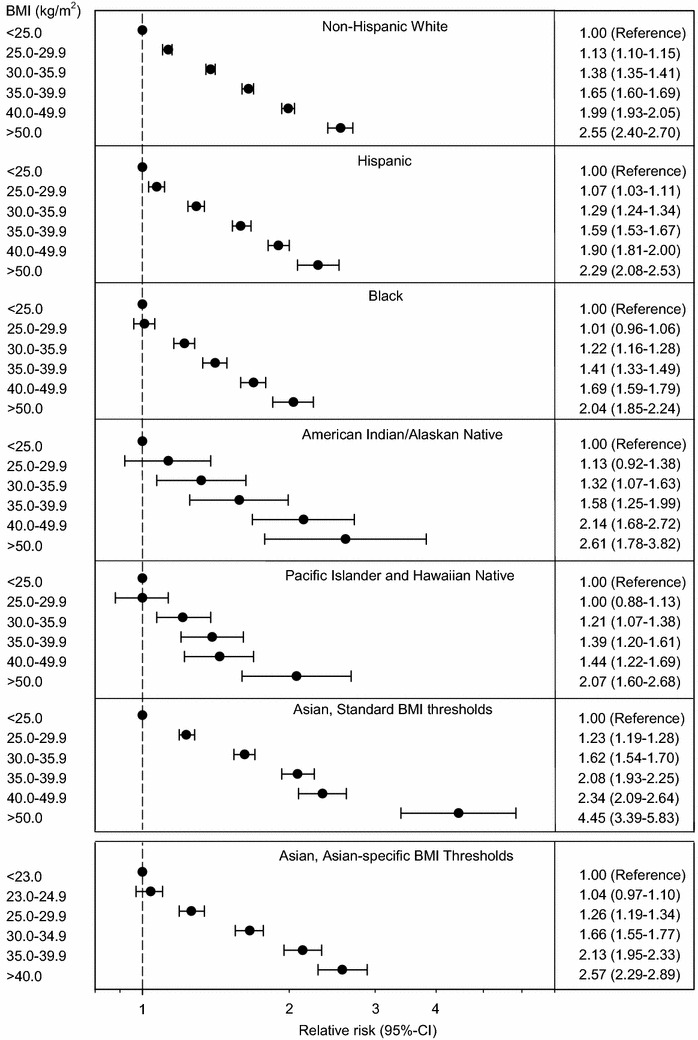
Risk for asthma across the different weight class
For women, the association of BMI to adult-onset asthma was stronger among oral contraceptives non-users than among users (P value for interaction < 0.001). For example, the HR was 2.97 (95 % CI 2.80, 3.15) for non-users and 2.06 (95 % CI 1.89, 2.24) for users with a BMI ≥50 kg/m2 when compared with their normal weight counterparts (Table 3).
The association of body weight to adult-onset asthma in all subgroups defined by age and race was roughly comparable among men and women after further stratifying by sex (Table 3).
In individuals with adult-onset asthma and at least 1 year of follow up, we examined the association of body weight class and asthma outcomes. Poorly controlled asthma (3083 cases) including poorly controlled asthma with high AMR (758 cases), and high-risk asthma (17,204 cases) were more prevalent at higher BMI categories (Table 4). The OR for poorly controlled asthma for individuals with a BMI <25 kg/m2, 25.0–29.9 kg/m2, 30.0–34.9 kg/m2, 40.0–49.9 kg/m2, and ≥50 kg/m2 were 1.00, 0.97, 1.03, 1.13, 1.13 and 1.46 (95 % CI 1.13, 1.88), respectively. The OR for poorly controlled asthma with high AMR for individuals with a BMI <25 kg/m2, 25.0–29.9 kg/m2, 30.0–34.9 kg/m2, 40.0–49.9 kg/m2, and ≥50 kg/m2 were 1.00, 1.12, 1.21, 1.26, 1.25 and 2.08 (95 % CI 1.31, 3.31), respectively. The OR for high-risk asthma were 1.00, 1.03, 1.17, 1.30, 1.37, and 1.63 (95 % CI 1.45, 1.84), respectively.
Table 4.
Adjusted odds ratio of poorly controlled asthma with or without high AMR and high-risk asthma
| BMI (kg/m2) | P for trend | P for interaction | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases | <25 | 25.0–29.9 | 30.0–34.9 | 35.0–39.9 | 40.0–49.9 | ≥50 | ||||||||
| Ref | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | ||||
| Poorly controlled asthma (any AMR) | ||||||||||||||
| All ages | 3083 | 1.00 | 0.97 | 0.87, 1.08 | 1.03 | 0.91, 1.15 | 1.13 | 0.99, 1.29 | 1.13 | 0.97, 1.31 | 1.46 | 1.13, 1.88 | 0.001 | – |
| Age (years) | ||||||||||||||
| 18–39 | 721 | 1.00 | 1.17 | 0.96, 1.43 | 1.15 | 0.92, 1.44 | 1.30 | 0.99, 1.70 | 1.17 | 0.85, 1.59 | 0.85 | 0.43, 1.68 | 0.268 | <0.001 |
| 40–65 | 1842 | 1.00 | 0.91 | 0.78, 1.05 | 1.00 | 0.86, 1.17 | 1.04 | 0.87, 1.24 | 1.13 | 0.93, 1.37 | 1.42 | 1.04, 1.94 | 0.005 | |
| 65+ | 520 | 1.00 | 0.85 | 0.65, 1.10 | 0.87 | 0.66, 1.15 | 1.17 | 0.85, 1.61 | 0.87 | 0.57, 1.35 | 3.24 | 1.75, 6.01 | 0.076 | |
| Sex | ||||||||||||||
| Male | 1367 | 1.00 | 1.03 | 0.87, 1.22 | 1.12 | 0.93, 1.34 | 1.27 | 1.03, 1.58 | 1.26 | 0.98, 1.63 | 1.48 | 0.92, 2.38 | 0.002 | 0.695 |
| Female | 1716 | 1.00 | 0.94 | 0.82, 1.08 | 0.97 | 0.84, 1.13 | 1.06 | 0.89, 1.26 | 1.07 | 0.88, 1.28 | 1.44 | 1.07, 1.95 | 0.039 | |
| Race/ethnicity | ||||||||||||||
| Non-Hispanic White | 1845 | 1.00 | 1.02 | 0.89, 1.17 | 0.94 | 0.81, 1.09 | 1.05 | 0.89, 1.25 | 1.10 | 0.90, 1.33 | 1.27 | 0.90, 1.79 | 0.264 | 0.055 |
| Hispanic | 459 | 1.00 | 0.86 | 0.63, 1.16 | 1.05 | 0.77, 1.44 | 1.16 | 0.82, 1.64 | 1.18 | 0.80, 1.76 | 1.73 | 0.90, 3.33 | 0.017 | |
| Black | 375 | 1.00 | 0.64 | 0.44, 0.92 | 0.94 | 0.66, 1.33 | 1.00 | 0.69, 1.47 | 0.92 | 0.61, 1.38 | 1.38 | 0.77, 2.48 | 0.057 | |
| Asian/PI/AIANa | 293 | 1.00 | 1.05 | 0.77, 1.42 | 1.45 | 1.03, 2.04 | 1.41 | 0.89, 2.25 | 1.02 | 0.50, 2.06 | 2.86 | 1.20, 6.82 | 0.018 | |
| Other/unknown | 111 | 1.00 | 1.14 | 0.65, 1.98 | 1.23 | 0.67, 2.27 | 1.52 | 0.75, 3.07 | 1.31 | 0.56, 3.07 | – | – | 0.477 | |
| Poorly controlled asthma with high AMRb | ||||||||||||||
| All ages | 758 | 1.00 | 1.12 | 0.90, 1.41 | 1.21 | 0.95, 1.53 | 1.26 | 0.95, 1.65 | 1.25 | 0.92, 1.71 | 2.08 | 1.31, 3.31 | 0.009 | – |
| Age (years) | ||||||||||||||
| 18–39 | 124 | 1.00 | 1.39 | 0.84, 2.28 | 1.65 | 0.97, 2.81 | 1.55 | 0.81, 2.99 | 0.84 | 0.34, 2.08 | 1.24 | 0.29, 5.31 | 0.572 | 0.075 |
| 40–65 | 508 | 1.00 | 1.14 | 0.85, 1.52 | 1.21 | 0.89, 1.64 | 1.18 | 0.83, 1.68 | 1.48 | 1.03, 2.14 | 2.32 | 1.36, 3.95 | 0.005 | |
| 65+ | 126 | 1.00 | 0.86 | 0.52, 1.44 | 0.89 | 0.51, 1.56 | 1.28 | 0.69, 2.39 | 0.52 | 0.18, 1.53 | 2.03 | 0.47, 8.82 | 0.812 | |
| Sex | ||||||||||||||
| Male | 314 | 1.00 | 1.33 | 0.92, 1.94 | 1.23 | 0.82, 1.85 | 1.53 | 0.96, 2.43 | 1.52 | 0.88, 2.64 | 2.48 | 1.03, 5.97 | 0.054 | 0.502 |
| Female | 444 | 1.00 | 1.00 | 0.75, 1.33 | 1.22 | 0.91, 1.64 | 1.12 | 0.79, 1.58 | 1.13 | 0.77, 1.64 | 1.87 | 1.08, 3.24 | 0.076 | |
| Race/ethnicity | ||||||||||||||
| Non-Hispanic White | 461 | 1.00 | 1.31 | 0.98, 1.75 | 1.17 | 0.86, 1.60 | 1.12 | 0.78, 1.62 | 1.39 | 0.94, 2.04 | 2.15 | 1.19, 3.87 | 0.141 | 0.357 |
| Hispanic | 106 | 1.00 | 0.81 | 0.42, 1.57 | 1.19 | 0.62, 2.28 | 1.20 | 0.58, 2.49 | 1.27 | 0.57, 2.87 | 1.33 | 0.30, 5.96 | 0.171 | |
| Black | 82 | 1.00 | 0.75 | 0.33, 1.68 | 1.06 | 0.49, 2.31 | 1.24 | 0.55, 2.81 | 0.78 | 0.31, 2.00 | 1.20 | 0.32, 4.53 | 0.615 | |
| Asian/PI/AIANa | 80 | 1.00 | 0.92 | 0.52, 1.62 | 1.26 | 0.66, 2.42 | 1.68 | 0.73, 3.84 | 0.46 | 0.06, 3.48 | 6.22 | 1.76, 21.94 | 0.098 | |
| Other/unknown | 29 | 1.00 | 1.14 | 0.35, 3.71 | 1.96 | 0.59, 6.47 | 2.48 | 0.65, 9.51 | 0.85 | 0.09, 7.74 | – | – | 0.395 | |
| High-risk asthma | ||||||||||||||
| All ages | 17,204 | 1.00 | 1.03 | 0.98, 1.08 | 1.17 | 1.11, 1.24 | 1.30 | 1.23, 1.38 | 1.37 | 1.29, 1.47 | 1.63 | 1.45, 1.84 | <0.0001 | – |
| Age (years) | ||||||||||||||
| 18–39 | 4998 | 1.00 | 0.93 | 0.86, 1.01 | 1.04 | 0.95, 1.14 | 1.13 | 1.01, 1.26 | 1.35 | 1.20, 1.51 | 1.60 | 1.30, 1.97 | <0.0001 | <0.001 |
| 40–65 | 8986 | 1.00 | 1.11 | 1.03, 1.20 | 1.30 | 1.21, 1.41 | 1.48 | 1.35, 1.61 | 1.50 | 1.37, 1.65 | 1.68 | 1.44, 1.97 | <0.0001 | |
| 65+ | 3220 | 1.00 | 1.06 | 0.95, 1.19 | 1.16 | 1.02, 1.31 | 1.25 | 1.08, 1.45 | 1.17 | 0.97, 1.40 | 1.84 | 1.25, 2.70 | <0.0001 | |
| Sex | ||||||||||||||
| Male | 5681 | 1.00 | 0.94 | 0.86, 1.02 | 1.10 | 1.01, 1.21 | 1.24 | 1.11, 1.38 | 1.28 | 1.12, 1.46 | 1.69 | 1.32, 2.16 | <0.0001 | <0.001 |
| Female | 11,523 | 1.00 | 1.08 | 1.01, 1.14 | 1.20 | 1.13, 1.28 | 1.33 | 1.24, 1.43 | 1.41 | 1.31, 1.53 | 1.64 | 1.43, 1.87 | <0.0001 | |
| Race/ethnicity | ||||||||||||||
| Non-Hispanic White | 8776 | 1.00 | 1.04 | 0.97, 1.11 | 1.18 | 1.10, 1.27 | 1.34 | 1.23, 1.45 | 1.37 | 1.25, 1.51 | 1.69 | 1.43, 2.00 | <0.0001 | <0.001 |
| Hispanic | 3666 | 1.00 | 0.90 | 0.81, 1.01 | 1.03 | 0.92, 1.16 | 1.18 | 1.04, 1.35 | 1.21 | 1.04, 1.40 | 1.20 | 0.90, 1.59 | <0.0001 | |
| Black | 2412 | 1.00 | 0.88 | 0.76, 1.03 | 1.08 | 0.93, 1.26 | 1.04 | 0.88, 1.23 | 1.20 | 1.00, 1.42 | 1.48 | 1.14, 1.92 | <0.0001 | |
| Asian/PI/AIANa | 2030 | 1.00 | 1.31 | 1.16, 1.48 | 1.45 | 1.25, 1.67 | 1.56 | 1.29, 1.89 | 1.91 | 1.50, 2.42 | 2.32 | 1.52, 3.56 | <0.0001 | |
| Other/unknown | 320 | 1.00 | 0.74 | 0.54, 1.02 | 0.79 | 0.55, 1.13 | 1.27 | 0.85, 1.89 | 1.27 | 0.80, 2.01 | 1.26 | 0.48, 3.31 | 0.063 | |
ORs were estimated from logistic regression models adjusted for age (continuous), sex (male, female), race/ethnicity (non-Hispanic White, Hispanic, Black, Asian/PI/AIAN, or other/unknown), insurance through government health care assistance programs (yes/no), neighborhood education level (continuous probability of education below high school), oral contraceptive use (yes/no), and site (Denver Health, Group Health Cooperative, HealthPartners and seven Kaiser Permanente regions). Tests of linear trend across weight category were conducted by considering weight category as a continuous variable in the multivariate model
AIAN American Indian/American Native; CI confidence interval; OR odds ratio; PI Pacific Islander
aDue to sample size limitations, these groups were combined for the outcomes related to asthma control
bSubgroup of poorly controlled asthma
The magnitude of the association between BMI and poorly controlled asthma (P value for interaction < 0.001) and high-risk asthma (P value for interaction < 0.001) was stronger among younger compared with older individuals, while the magnitude of the association between BMI and poorly controlled asthma with high AMR was comparable across all age groups (P value for interaction = 0.075). The magnitude of the association between BMI and high-risk asthma (P value for interaction < 0.001) was stronger among Asians, Pacific Islanders and AIAN than among other races, while the magnitude of the association between BMI and poorly controlled asthma (P value for interaction = 0.055), especially for poorly controlled asthma with high AMR (P value for interaction = 0.357) was comparable across all racial/ethnic groups.
Discussion
Obesity has been previously recognized as a risk factor for adult-onset asthma. We demonstrated that excessive body weight was associated with a 40 % higher risk of asthma for individuals affected by obesity with a BMI of 30.0–34.9 kg/m2 and almost 250 % higher risk for individuals with a BMI of over 50 kg/m2 compared to individuals with a normal weight BMI <25 kg/m2. We also found that the magnitude of this association may be altered by age, sex, and race. Women, older adults, and individuals of Asian origin carried a higher obesity-related risk to develop asthma during adulthood than other population groups. Furthermore, among individuals with asthma, greater BMI was associated with higher odds for poorly controlled and high-risk asthma across all racial and ethnic groups. In addition, the obesity-related risk for poorly controlled and high-risk asthma increased with age, and was marginally to significantly higher among individuals of Asian origin.
Asthma is a heterogeneous disease with many phenotypes, and age at disease onset is an important factor in separating the phenotypes. Patients with childhood-onset asthma are typically atopic with a relatively good prognosis [22]. In contrast, patients with adult-onset asthma are most often non-atopic females without a family history of asthma or allergies, have a less favorable prognosis, and are more likely to develop persistent airflow limitation [3, 23, 24]. Asthma in patients affected by obesity, especially in women, has been identified as a distinct phenotype characterized by frequent symptoms, frequent exacerbations and high health care utilization [24]. The results from our study contribute to the evidence that asthma is not only more likely to develop in individuals affected by obesity, but also that existing asthma is complicated by obesity, with some demographic subgroups experiencing worse asthma control than others. With more than one-third of U. S. adults considered obese, clinical care approaches, asthma risk stratification algorithms and population management strategies may benefit from taking into account that obesity can alter asthma outcomes for some population groups.
Several cross-sectional and prospective studies, as well as systematic reviews and meta-analyses, provide compelling evidence that obesity increases the risk of asthma in children and adults [4, 25–27]. However, only a few studies were able to distinguish between child- and adult-onset of asthma and provide estimates for the magnitude of the association of obesity class 2 and higher [4]. According to a meta-analysis based on prospective studies, the odds of developing asthma for individuals with obesity compared to those with normal weight were twice as high (OR 1.92, 95 % CI 1.43–2.59) [4]. Cross-sectional data from the National Health and Nutrition Examination Survey (NHANES), 2001–2004, showed an OR of 1.80 (95 % CI 1.40–2.30) for a BMI of 30.0–34.9 kg/m2, and 2.40 (95 % CI 1.60–3.70) for a BMI >40.0 kg/m2 compared to their normal weight counterparts [28]. In the Black Women’s Health Study, the incidence rate ratios ranged from 1.26 (95 % CI 1.05–1.51) for women with overweight to 2.85 (95 % CI 2.19–3.72) for women with a BMI ≥40 kg/m2 when compared to women with a BMI of 20–24.9 kg/m2 [29]. Compared to other studies [4, 28, 29], our HRs are slightly lower, but this can be partially explained by the higher BMI of our normal weight control group (23.0–24.9 as opposed to 19.0–24.9).
Several mechanisms for the contribution of obesity to asthma risk have been implicated [3, 30]. Obesity is associated with increased levels of adipokines, which may affect the airway directly, rather than through increased airway inflammation [31]. Additionally, mechanical factors such as reduced functional residual capacity that can occur with more severe obesity may result in expiratory flow limitation and airway closure [32].
Sex has been previously shown to alter the association between obesity and asthma. In a meta-analyses, the odds for asthma among women with overweight and obesity were almost 70 % higher (OR 1.68, 95 % CI 1.45, 1.94) than that found for men with overweight and obesity (1.46, 95 % CI 1.05, 2.02) compared to their counterparts with normal weight [4]. Extending these findings, we observed an asthma risk increase between 43 and 260 % for women and between 26 and 220 % for men with obesity class 1 to class 4 compared to their counterparts with normal weight. While both sexes in our study showed a strong risk increase with higher BMI, the association was significantly altered by sex, showing a higher obesity-related burden for asthma in women. The obesity-related risk for asthma was also modified by age, showing an increased risk with higher age in both, men and women. A potential role of hormonal factors as explanation of sex differences is supported by the effect modification of oral contraceptive use among women. The obesity-related risk for asthma was more pronounced in women who did not use oral contraceptives (OR 2.97, 95 % CI 2.80, 3.15) than in those taking oral contraceptives (OR 2.06, 95 % CI 1.89, 2.24). Results from other studies indicated that oral contraceptive use lowered the risk for asthma in girls during puberty [33] and decreased the risk for asthma and asthma exacerbations in Scottish women [34].
One previous study investigated the effect modification of race/ethnicity on the association between BMI and risk of asthma [35]. Results from that study suggested that obesity is a strong risk factor for asthma among Blacks (OR for BMI ≥30 kg/m2 = 2.90, 95 % CI 1.20, 7.00) and Hispanic men (OR for BMI ≥30 kg/m2 = 2.70, 95 % CI 1.10, 6.30) but not in non-Hispanic White men (OR for BMI ≥30 kg/m2 = 1.00, 95 % CI 0.60, 1.70) compared to their counterparts with normal weight of the same race/ethnicity. However, the statistical power was limited due to a relatively small sample size for minority men [35]. These findings are not consistent with our results. We noted a comparable obesity–related burden across most races/ethnicities in men and women except for individuals of Asian origin and Blacks. While Asians appeared to have a slightly lower overall risk for asthma compared to Non-Hispanic Whites in the present study, they exhibited a greater increase in asthma risk with increasing BMI than other races/ethnicities. However, when applying Asian-specific BMI thresholds to define overweight and obesity, the relation of obesity to asthma risk was comparable with most other races. In Blacks, the increase in asthma risk associated with higher BMI appeared to be smaller than in other races.
While asthma is a common comorbidity among persons with obesity, our study suggests that obesity not only increases the risk for asthma, but also adversely affects asthma severity and control. Increasing obesity was associated with less favorable asthma outcomes across all racial and ethnic groups. But similar to the risk of developing adult-onset asthma, the risk associated with obesity class 4 for poorly controlled and high risk asthma was greater in adults who were elderly, and was marginally to significantly greater among individuals of Asian origin when using standard BMI thresholds. In contrast, increasing obesity was associated with poorly controlled asthma with high AMR across all subgroups.
Several biological and non-biological mechanisms have been suggested to explain a relationship between obesity and poor asthma outcomes, including reduced corticosteroid responsiveness in obese individuals [36–38], the influence of immunomodulatory adipokines [39, 40], deleterious effects on pulmonary mechanics [39], low vitamin D levels [41, 42], and obesity-related comorbidities such as gastro-esophageal reflux disease (GERD) and depression [40, 43]. However, a recent study suggested that obesity was associated with poor asthma control even after adjusting for GERD, depression, and corticosteroid use [15]. No studies could be identified that investigated the modification of association between obesity and asthma outcomes by race and ethnicity, age, and sex.
We noted several potential limitations. Due to the inclusion criteria for the PORTAL obesity cohort, individuals were required to have a BMI of at least 23.0 kg/m2 in 2012 or 2013. Thus, our study population may not reflect normal weight individuals ranging from 19.0–22.9 kg/m2 who are included in most other studies. However, the difference in the range of normal weight will lead to an underestimation of the risk associated with high BMI. Furthermore, this is unlikely to affect the interactions that were the focus of the present study. A unique strength of our study is that we examined the risk for asthma among individuals with severe obesity and examined relevant subgroups stratified by sex, age, and race. Although we were not able to account for changes in the BMI during follow-up, this is unlikely to influence the results, considering the relatively short follow-up and the time of exposure to obesity.
Our definition of adult-onset may have resulted in misclassification of recrudescent pediatric-onset asthma as adult-onset asthma, especially in younger adults. However, the misclassification is unlikely to be differential across weight classes and, therefore, should not affect the nature or the magnitude of the results presented here.
Several studies have suggested that asthma, especially adult-onset asthma, may be overdiagnosed in individuals affected by obesity [44, 45]. Individuals with asthma and obesity have a lower lung function and more comorbidities compared to individuals with asthma who are normal weight; if making urgent visits for respiratory symptoms, individuals affected by obesity are more likely to receive a misdiagnosis of asthma [46]. For the present study, a potential overdiagnosis of asthma in individuals affected by obesity when seeking urgent care would result in an overestimation of the association between obesity and asthma, especially the association between obesity and high-risk asthma. We can also not exclude that differential utilization of health care service such as higher utilization among women or lower utilization among Hispanics or Blacks may have contributed to differential chance of diagnosis with asthma.
Moreover, the relatively short follow-up times may mask some effect modification that may be apparent over longer periods of time. Generally, a short follow-up is likely to bias results towards the null. Future studies with a longer follow-up period are required to identify those effect modifications.
A small proportion of the study population was classified as other or unknown race/ethnicity. Incomplete assessment of race/ethnicity is usually caused by shorter enrollment [14, 47] and unlikely to be differential across different weight classes and, therefore, unlikely to affect the results. In addition, risk estimates of individuals classified as other or unknown were roughly comparable to the largest represented racial groups, i.e., non-Hispanic Whites, Hispanic, and Blacks, and unlikely to mask a significant effect modification in a small racial subgroup with differential classification as other or unknown racial origin.
Conclusions
We found that the associations between obesity and asthma and asthma control were significantly altered by age, sex, and race. Among individuals with asthma, increasing obesity was associated with poorer asthma outcomes, which was more pronounced in adults who were older. Understanding the role of obesity in the development of adult-onset asthma will help to improve asthma treatment algorithms, design patient-centered outcome studies to better understand patient barriers to asthma control, and to develop targeted interventions.
Authors’ contributions
All authors substantially contributed to conception and design, to the acquisition of data, and the analysis and interpretation of data. CK and MKG drafted the article and it was critically reviewed for important intellectual content by all other authors. All authors read and approved the final manuscript.
Acknowledgements
David Arterburn, MD, MPH (Group Health Cooperative), Gregory Nichols, Ph.D. (Kaiser Permanente North-West) and Dennis Tolsma, MPH, (Kaiser Permanente Georgia) provided data to contribute to this study.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
The datasets generated during and/or analyzed during the current study are not publicly available due privacy concerns but are available from the corresponding author on reasonable request.
Declarations
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of Kaiser Permanente Southern California (KPSC) as the lead site. The requirement for informed consent was waived. The IRBs at the other sites reviewed the protocol and subsequently ceded review to the KPSC IRB.
Funding
This study used the infrastructure developed by the PORTAL (Patient Outcomes Research to Advance Learning) Network, a consortium of four integrated delivery systems (Kaiser Permanente, Group Health Cooperative, HealthPartners, and Denver Health) and their affiliated research centers, with funding support from a contract awarded by the Patient-Centered Outcomes Research Institute (PCORI) (Grant No. CDRN-1306-04681).
Abbreviations
- AIAN
American Indian/Alaskan Native
- AMR
asthma medication ratio
- BMI
body mass index
- CI
confidence intervals
- EHR
electronic health record
- GERD
gastro-esophageal reflux disease
- HR
hazard ratios
- ICD
International Classification of Disease
- IRB
Institutional Review Board
- KPSC
Kaiser Permanente Southern California
- NHANES
National Health and Nutrition Examination Survey
- PORTAL
patients outcomes research to advance learning
- OR
odds ratio
- SES
socioeconomic status
- WHO
World Health Organization
Contributor Information
Corinna Koebnick, Email: Corinna.Koebnick@kp.org.
Heidi Fischer, Email: Heidi.Fischer@kp.org.
Matthew F. Daley, Email: Matthew.F.Daley@kp.org
Assiamira Ferrara, Email: Assiamira.Ferrara@kp.org.
Michael A. Horberg, Email: Michael.Horberg@kp.org
Beth Waitzfelder, Email: Beth.E.Waitzfelder@kp.org.
Deborah Rohm Young, Email: Deborah.R.Young@kp.org.
Michael K. Gould, Email: Michael.K.Gouold@kp.org
References
- 1.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011;(32):1–14. [PubMed]
- 3.de Nijs SB, Venekamp LN, Bel EH. Adult-onset asthma: is it really different? Eur Respir Rev. 2013;22:44–52. doi: 10.1183/09059180.00007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lugogo NL, Kraft M, Dixon AE. Does obesity produce a distinct asthma phenotype? J Appl Physiol. 1985;2010(108):729–734. doi: 10.1152/japplphysiol.00845.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor B, Mannino D, Brown C, Crocker D, Twum-Baah N, Holguin F. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008;63:14–20. doi: 10.1136/thx.2007.082784. [DOI] [PubMed] [Google Scholar]
- 7.McGlynn EA, Lieu TA, Durham ML, Bauck A, Laws R, Go AS, et al. Developing a data infrastructure for a learning health system: the PORTAL network. J Am Med Inform Assoc. 2014;21:596–601. doi: 10.1136/amiajnl-2014-002746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young DR, Waitzfelder BA, Arterburn D, Nichols GA, Ferrara A, Koebnick C, et al. The patient outcomes research to advance learning (PORTAL) network adult overweight and obesity cohort: development and description. JMIR Res Protoc. 2016;5:e87. doi: 10.2196/resprot.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. doi: 10.7812/TPP/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sukumaran L, McCarthy NL, Li R, Weintraub ES, Jacobsen SJ, Hambidge SJ, et al. Demographic characteristics of members of the vaccine safety datalink (VSD): a comparison with the United States population. Vaccine. 2015;33:4446–4450. doi: 10.1016/j.vaccine.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sturm R. Increases in morbid obesity in the USA: 2000–2005. Public Health. 2007;121:492–496. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Technical report series 894: obesity: preventing and managing the global epidemic. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 13.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 14.Derose SF, Contreras R, Coleman KJ, Koebnick C, Jacobsen SJ. Race and ethnicity data quality and imputation using U.S. Census data in an integrated health system: the Kaiser Permanente Southern California experience. Med Care Res Rev. 2013;70:330–345. doi: 10.1177/1077558712466293. [DOI] [PubMed] [Google Scholar]
- 15.Schatz M, Zeiger RS, Yang SJ, Chen W, Sajjan S, Allen-Ramey F, et al. Prospective study on the relationship of obesity to asthma impairment and risk. J Allergy Clin Immunol Pract. 2015;3:560–565. doi: 10.1016/j.jaip.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Schatz M, Zeiger RS, Vollmer WM, Mosen D, Apter AJ, Stibolt TB, et al. Validation of a beta-agonist long-term asthma control scale derived from computerized pharmacy data. J Allergy Clin Immunol. 2006;117:995–1000. doi: 10.1016/j.jaci.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Quality Assurance. Measures HEDIS 2015. http://www.ncqa.org. 2015. Accessed Sept 10.
- 18.Schatz M, Zeiger RS, Yang SJ, Chen W, Crawford WW, Sajjan SG, et al. Relationship of asthma control to asthma exacerbations using surrogate markers within a managed care database. Am J Manag Care. 2010;16:327–333. [PubMed] [Google Scholar]
- 19.Schatz M, Zeiger RS, Vollmer WM, Mosen D, Mendoza G, Apter AJ, et al. The controller-to-total asthma medication ratio is associated with patient-centered as well as utilization outcomes. Chest. 2006;130:43–50. doi: 10.1378/chest.130.1.43. [DOI] [PubMed] [Google Scholar]
- 20.Schatz M, Nakahiro R, Crawford W, Mendoza G, Mosen D, Stibolt TB. Asthma quality-of-care markers using administrative data. Chest. 2005;128:1968–1973. doi: 10.1378/chest.128.4.1968. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler ME. Managing asthma in primary care: putting new guideline recommendations into context. Mayo Clin Proc. 2009;84:707–717. doi: 10.4065/84.8.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paaso EM, Jaakkola MS, Rantala AK, Hugg TT, Jaakkola JJ. Allergic diseases and asthma in the family predict the persistence and onset-age of asthma: a prospective cohort study. Respir Res. 2014;15:152. doi: 10.1186/s12931-014-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amelink M, de Nijs SB, de Groot JC, van Tilburg PM, van Spiegel PI, Krouwels FH, et al. Three phenotypes of adult-onset asthma. Allergy. 2013;68:674–680. doi: 10.1111/all.12136. [DOI] [PubMed] [Google Scholar]
- 24.Ilmarinen P, Tuomisto LE, Kankaanranta H. Phenotypes, risk factors, and mechanisms of adult-onset asthma. Mediators Inflamm. 2015;2015:514868. doi: 10.1155/2015/514868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Arch Dis Child. 2006;91:334–339. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YC, Dong GH, Lin KC, Lee YL. Gender difference of childhood overweight and obesity in predicting the risk of incident asthma: a systematic review and meta-analysis. Obes Rev. 2013;14:222–231. doi: 10.1111/j.1467-789X.2012.01055.x. [DOI] [PubMed] [Google Scholar]
- 27.Juel CT, Ali Z, Nilas L, Ulrik CS. Asthma and obesity: does weight loss improve asthma control? a systematic review. J Asthma Allergy. 2012;5:21–26. doi: 10.2147/JAA.S32232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McHugh MK, Symanski E, Pompeii LA, Delclos GL. Prevalence of asthma among adult females and males in the United States: results from the National Health and Nutrition Examination Survey (NHANES), 2001–2004. J Asthma. 2009;46:759–766. doi: 10.1080/02770900903067895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coogan PF, Palmer JR, O’Connor GT, Rosenberg L. Body mass index and asthma incidence in the Black Women’s Health Study. J Allergy Clin Immunol. 2009;123:89–95. doi: 10.1016/j.jaci.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–1093. doi: 10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, et al. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012;186:598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 1985;2010(108):206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 33.Wei J, Gerlich J, Genuneit J, Nowak D, Vogelberg C, von Mutius E, et al. Hormonal factors and incident asthma and allergic rhinitis during puberty in girls. Ann Allergy Asthma Immunol. 2015;115:21–27. doi: 10.1016/j.anai.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 34.Nwaru BI, Sheikh A. Hormonal contraceptives and asthma in women of reproductive age: analysis of data from serial national Scottish Health Surveys. J R Soc Med. 2015;108:358–371. doi: 10.1177/0141076815588320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S, Camargo CA., Jr Sex-race differences in the relationship between obesity and asthma: the behavioral risk factor surveillance system, 2000. Ann Epidemiol. 2003;13:666–673. doi: 10.1016/S1047-2797(03)00054-1. [DOI] [PubMed] [Google Scholar]
- 36.Anderson WJ, Lipworth BJ. Does body mass index influence responsiveness to inhaled corticosteroids in persistent asthma? Ann Allergy Asthma Immunol. 2012;108:237–242. doi: 10.1016/j.anai.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 38.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178:682–687. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 40.Gibson PG. Obesity and asthma. Ann Am Thorac Soc. 2013;10(Suppl):S138–S142. doi: 10.1513/AnnalsATS.201302-038AW. [DOI] [PubMed] [Google Scholar]
- 41.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol. 2010;126:52–58. doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris SS, Dawson-Hughes B. Reduced sun exposure does not explain the inverse association of 25-hydroxyvitamin D with percent body fat in older adults. J Clin Endocrinol Metab. 2007;92:3155–3157. doi: 10.1210/jc.2007-0722. [DOI] [PubMed] [Google Scholar]
- 43.Boudreau M, Bacon SL, Ouellet K, Jacob A, Lavoie KL. Mediator effect of depressive symptoms on the association between BMI and asthma control in adults. Chest. 2014;146:348–354. doi: 10.1378/chest.13-1796. [DOI] [PubMed] [Google Scholar]
- 44.Aaron SD, Vandemheen KL, Boulet LP, McIvor RA, Fitzgerald JM, Hernandez P, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121–1131. doi: 10.1503/cmaj.081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Huisstede A, Castro Cabezas M, van de Geijn GJ, Mannaerts GH, Njo TL, Taube C, et al. Underdiagnosis and overdiagnosis of asthma in the morbidly obese. Respir Med. 2013;107:1356–1364. doi: 10.1016/j.rmed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Pakhale S, Doucette S, Vandemheen K, Boulet LP, McIvor RA, Fitzgerald JM, et al. A comparison of obese and nonobese people with asthma: exploring an asthma-obesity interaction. Chest. 2010;137:1316–1323. doi: 10.1378/chest.09-2491. [DOI] [PubMed] [Google Scholar]
- 47.Smith N, Iyer RL, Langer-Gould A, Getahun DT, Strickland D, Jacobsen SJ, et al. Health plan administrative records versus birth certificate records: quality of race and ethnicity information in children. BMC Health Serv Res. 2010;10:316. doi: 10.1186/1472-6963-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]


