Abstract
Sonodynamic therapy (SDT) is an emerging approach that involves a combination of low-intensity ultrasound and specialized chemical agents known as sonosensitizers. Ultrasound can penetrate deeply into tissues and can be focused into a small region of a tumor to activate a sonosensitizer which offers the possibility of non-invasively eradicating solid tumors in a site-directed manner. In this article, we critically reviewed the currently accepted mechanisms of sonodynamic action and summarized the classification of sonosensitizers. At the same time, the breath of evidence from SDT-based studies suggests that SDT is promising for cancer treatment.
Keywords: Sonodynamic therapy, Sonosensitizer, Cancer, Ultrasound, Reactive oxygen species
Introduction
As we all know that cancer has become the first killer of human health, and anticancer therapy has attracted more and more researchers' attention all over the world. At current stage, four main methods including surgery, chemotherapy, radiotherapy, and immunotherapy are clinically applied in cancer treatments. However, every kind of treatment has its own limitations which make rigorous challenges for cancer therapy. Surgery treatment has difficulty on clearing cancer cells entirely and can not cure the metastasized tumor. Chemotherapy and radiotherapy can effectively kill cancer cells to some extend, but they will damage normal tissues simultaneously. In addition, cancer cells will exert tolerance during the long-period of chemotherapy and radiotherapy, which is considered to be the main obstacle on cancer therapy. Immunotherapy, the burgeoning cancer treatment, is an effective treatment on cancers but it costs too much and is possible to cause cytokine storm. Therefore, finding an effective, secure, and low-cost treatment becomes extremely urgent.
Sonodynamic therapy (SDT) has been developed as a novel promising noninvasive approach derived from photodynamic therapy (PDT). In 1989, Yumita et al. 1 found several hematoporphyrin (HP) derivatives (HPDs) used in PDT also induced significant cell damage when they are activated with ultrasound. It has since been demonstrated that several newly-generated HPDs have potential to be used as sonosensitizers for tumor treatment in combination with ultrasound 2- 4, which is referred to SDT 5. The major difference between SDT and PDT is the energy source used to activate the sensitizers (ultrasound versus light). Due to the short penetration depth of light, PDT is not effective for the treatment of deep-seated tumors 6. However, the significant advantage of SDT over PDT is that ultrasound can be tightly focused with penetration in soft tissue up to several tens of centimeters 7. Therefore, SDT overcomes the major limitation of PDT. The sonodynamic efficiency of SDT mainly relies on the generation of reactive oxygen species (ROS) through the simultaneous combination of low intensity ultrasound, molecular oxygen and a sonosensitizer 8. SDT has been a promising novel treatment modality which yielded impressive anticancer effects on both in vitro and in vivo studies 9. In this article, we review the current investigations on SDT with a particular focus on its function mechanisms and application in cancer treatment.
Possible function mechanisms of SDT
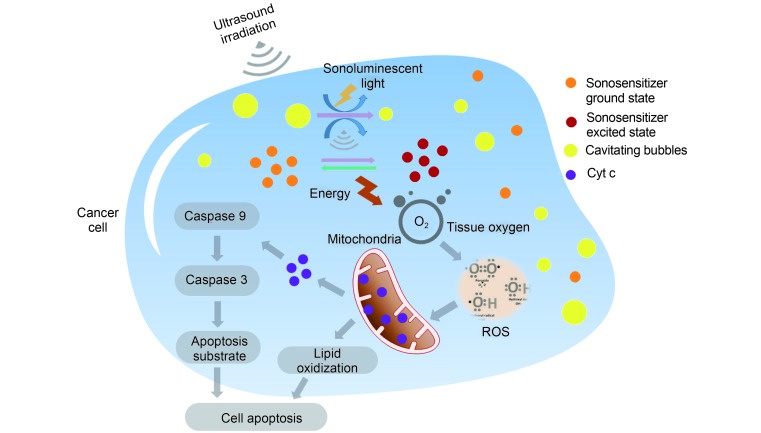
PDT is described as a dynamic interaction involving light, a photosensitive agent and oxygen. Upon activation, the photosensitizer is promoted from its ground state (S 0) to the first excited singlet state (S 1), from which point a chain of further electronic transitions can occur. This pathway involves an intersystem crossing from the sensitizers S 1 state to its longer-lived triplet state (T 1). In the absence of triplet state population, the excited photosensitizer returns to its ground state with the emission of light, known as fluorescence, and/or by means of radiationless transitions whereby energy is given to or taken up by another particle or system. Population of the triplet state is necessary to produce the sufficient quantity of ROS to initiate the cell death. In most cases, the key ROS of PDT is singlet oxygen ( 1O 2), which is the lowest excited electronic state of oxygen 10, 11. This mechanism seems to be applied to SDT, however, the generation of ROS during ultrasound treatment is not fully understood until now 12, 13. The interaction of ultrasound with aqueous environment results in a unique phenomenon known as acoustic cavitation 14, which can activate sensitizers to generate ROS 13. Based on the acoustic cavitation, sonoluminescence, a process whereby light is generated upon irradiation of a solution with the ultrasound, is believed as another key mechanism to generate ROS 15. The possible mechanisms of SDT are shown in Figure 1 and briefly discussed as follows.
1.
Possible mechanisms of SDT. Ultrasound irradiation induces cavitation around the surface of cancer cells. The energy provided by the collapse of cavitating bubbles initiates the formation of sonoluminescent light in cancer cells. Thus, sonosensitizer is activated from its ground state into an excited state. As the activated sonosensitizer returns to the ground state, the released energy can be transferred to the circumambient oxygen to produce a large amount of ROS including oxygen ion, peroxide and singlet oxygen, which subsequently mediate the mitochondrial-dependent cell apoptosis through the damage of mitochondria membrane and the release of Cyt c.
Acoustic cavitation
SDT involves the synergistic interaction of ultrasound and some chemical compounds termed as sonosensitizers 16. The sonication parameters in SDT (usually 1.0–2.0 MHz at an intensity of 0.5–3.0 W/cm 2) have been selected to produce cavitation in a cell culture or tumor 17. Cavitation process involves the nucleation, growth, and implosive collapse of gas-filled bubbles under the appropriate ultrasound conditions. It may be essentially classified into stable and inertial cavitation 14. Bubbles of stable cavitation oscillate, creating a streaming of the surrounding liquid which results in a mixture of the surrounding media while the gas bubbles in inertial cavitation process grow to a near resonance size and expand to a maximum before collapse violently 18.
The extreme temperatures up to 10000 K and pressures of 81 MPa in the surrounding microenvironment resulted from the released energy by this implosion are viewed as a sonochemical reactor 13, 17. Under the function of this sonochemical reactor, a sonosensitizer attached to the surface of a tumor cell will be activated from its ground state into an excited state when it is exposed to the ultrasound, and as the activated sonosensitizer returns to the ground state, the released energy can be transferred to the circumambient oxygen to produce a large amount of ROS, which subsequently mediates cellular toxicity directly 19. Konno et al. 20 found that HP-treated cells were sensitive to the ultrasound at low intensities, although it was shown not to induce inertial cavitation. It is interesting to note that the ultrasound intensities employed in this study have given rise to sonoluminescence via stable cavitation. Yasuda et al. 21 have demonstrated that cavitation cloud would be useful for efficient generation of ROS using nonlinear propagation effect.
Sonoluminescence
Sonoluminescence is the emission of light from cavitating bubbles when ultrasound irradiation induces cavitation around the surface of tumor cells 22. Umemura et al. 23 had studied the emission of this light in saline solutions and proposed that light from sonoluminescence could activate sonosensitizers such as HP in a similar manner to PDT. Although these experiments carried out in vitro may not represent the results found in vivo, it is reasonable to assume that the emission spectrum of sonoluminescence in water should be similar to its spectrum within the tissues with a large of water content. Sazgarnia et al. 24 successfully confirmed sonoluminescence in gel-based phantoms using protoporphyrin IX (PpIX) coupled to gold nanoparticles after ultrasonic irradiation at a frequency of 1.1MHz. In their study, gold nanoparticles was applied as nucleation centers for cavitation and sonoluminescence signals were detected at 350–450 nm, 450–550 nm and 550–650 nm, respectively.
It has been suggested that SDT can induce the ROS production in the form of sonoluminescent light by inertial cavitation, a process of creating microbubbles in liquid environments such as cellular cytoplasm 13. When microbubbles implode, they give off substantial amount of energy, thus initiate the emission of sonoluminescent light, and subsequently leads to the production of ROS. The energy released from microbubble implosion can also severely damage malignant cells by hydrodynamic shear forces, e.g., destroying vital cytoskeletal structures of the cells 25. Cheng et al. 26 demonstrated that 5-aminolevulinic acid (5-ALA)-induced PpIX which are mainly located on the mitochondria, can induce THP-1 macrophage apoptosis by generating a large amount of ROS in mitochondria after the production of sonoluminescence.
Anticancer effect of SDT
From the above-mentioned advantages, it can be seen that SDT is an emerging approach that offers the possibility of non-invasively eradicating solid tumors in a site-directed manner. A high therapeutic efficacy of SDT in cancers is resulting from the comprehensive functions. Many investigations have confirmed that the production of a large amount of intracellular ROS induced by SDT can produce direct cytotoxicity in cancer cells. Recently, some investigations also showed that SDT possesses the modulation effects on cancer microenvironment, e.g., the suppression effect on tumor vasculature and the stimulation effect on the tumor immunity, which inhibit the growth of cancers. Here, we reviewed the currently accepted mechanisms by which ultrasound activates sensitizers to elicit therapeutic effects on cancers.
Cytotoxicity induced by SDT in cancer cells
Upon absorbing low energy ultrasound, sonosensitizers at tumor sites can covert oxygen to various highly reactive ROS, such as singlet oxygen, leading to the irreversible damages of cancer cells directly. In the study reported by Chen et al. 27, human lung adenocarcinoma cells SPCA-1 and mice bearing SPCA-1 tumor xenograft were exposed to the ultrasound in presence or absence of chlorin e6 (Ce6). The results showed that the accumulation concentration of Ce6 in tumor tissue was remarkably higher than that in normal muscle near tumor. In vivo, the ultrasound (0.4–1.6 W/cm 2) or Ce6 (10–40 mg/kg) alone exhibited no remarkable anti-tumor effects, but the combination of ultrasound (1.6 W/cm 2) with Ce6 hampered tumor growth significantly ( P<0.05). Flow cytometry analysis showed that Ce6-mediated sonodynamic effect was mainly through the process of cell necrosis induced by ROS.
High level of intracellular ROS triggered by SDT can damage the mitochondrial membrane through promoting lipid peroxidation, thus causing the depolarization of mitochondrial membrane potential and the increase of mitochondrial membrane permeability 28. Sun et al. 29 developed an experimental system to monitor the intracellular ROS and mitochondrial membrane potential (MMP) loss in real-time during ultrasonic irradiation in order to achieve the optimal parameters. THP-1 derived macrophages were incubated with 5-ALA and then sonicated at different intensities. When the intensity of ultrasound was 0.48 W/cm 2, the ROS elevation and MMP loss were observed in THP-1 derived macrophages during ultrasonic irradiation. More recent reports suggest the intracellular production of ROS is responsible for mediating cytotoxic effects in SDT 30.
The damages of mitochondrial membrane can further cause the release of cytochrome c (Cyt c) from the mitochondria to the cytoplasm and the subsequent activation of caspase-dependent apoptosis pathway. Su et al. 31 investigated the occurrence of apoptosis and autophagy in human leukemia K562 cells after treatment with PpIX-mediated SDT. The mitochondrial-dependent apoptosis was clearly observed through morphological observation and biochemical analysis. Besides that, SDT was shown to induce the autophagy in K562 cells. The same group also investigated the apoptosis behavior of U937 cells induced by HP monomethylether (HMME)-mediated SDT. Compared with the control, ultrasound-alone, and HMME-alone groups, the intracellular ROS production was greatly increased in the combined SDT group 32. The role of intracellular calcium overload in the apoptosis of C6 glioma cells with treatment of HMME-mediated SDT was investigated by Hao et al. 33. The results showed that the intracellular ROS production increased, the mitochondrial membrane potential decreased, and Cyt c was released from the mitochondria with treatment of SDT in the media supplemented with Ca 2+. Altogether, the intracellular ROS production was directly related to the increase of Ca 2+ concentration, and as a result the cell apoptosis was improved.
Modulation effects of SDT on cancer microenviroment
Cancer microenvironment, also called tumor microen- vironment, describes cellular environment in which the tumor exists, including surrounding blood vessels, immune cells, fibroblasts, bone marrow-derived inflammatory cells, lymphocytes, signaling molecules, and the extracellular matrix. The tumor and the surrounding microenvironment are closely related and interact constantly. Tumors can influence the microenvironment by releasing extracellular signals, promoting tumor angiogenesis and inducing peripheral immune tolerance, while the tumor microenvironment provide the support for the growth and metastasis of cancer cells 34. Recently, several investigations have showed that SDT can efficiently modulate the tumor microenvironment.
Gao et al. 35 investigated the effects of SDT on the tumor microenvironment, especially the tumor angiogenesis, which is required for invasive tumor growth and metastasis and constitutes an important point in the control of cancer progression. They found that the ROS production induced by SDT could significantly inhibit the proliferation, migration and invasion of endothelial cells, and the tube formation. Furthermore, in a tumor xenograft mouse model, SDT was found to remarkably suppress the tumor growth, intratumoral vascularity, and expression of vascular endothelial growth factor in tumor cells. An ultrastructural study showed the obvious damage and disruption of tumor microvasculature after STD. Some other studies also confirmed the suppression effect of SDT on the tumor angiogenesis 36, 37.
Similar to PDT, SDT can also modulate the tumor microenvironment to generate an immune response. The destruction of tumors induced by SDT may lead to the in situ immunity forming in the body when the immune cells are exposed to the tumor debris and immune stimulatory substances that are present in the tumor. In the study reported by Wang et al. 38, they investigated the transfor- mation of macrophages and dendritic cells (DCs) in the tumor microenvironment during 5-ALA-mediated SDT in mice transplanted with B16F10 melanomas. The results showed that the tumor growth was restrained and the macrophage and DC passivity was remarkably reversed in B16F10 melanoma-bearing mice by 5-ALA-mediated SDT. For example, CD68 expression level increased and CD163 expression level decreased, indicating that M2 macrophages were converted to the M1 phenotype in tumor microen- vironment; CD80 and CD86 expression levels both increased, suggesting that DCs in tumor microenvironment tend to mature after SDT treatment. Furthermore, the cytokines such as INF-γ, TNF-α and IL-10 also significantly increased at the same time.
Sonosensitizers
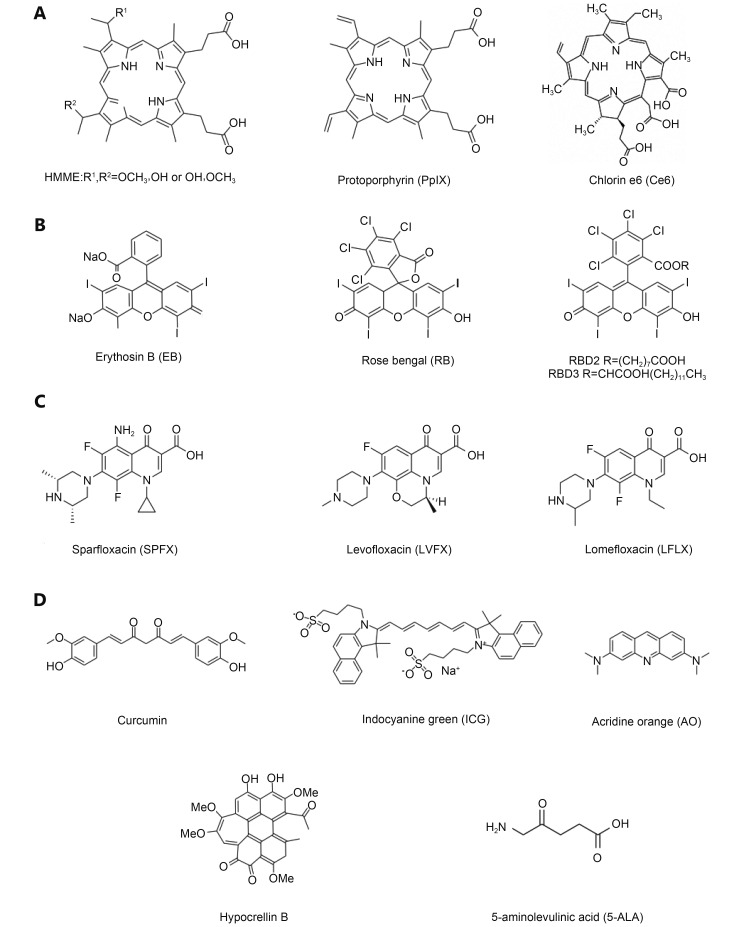
Sonosensitizer can maximize the effects of ultrasonic irradiation, thus is considered as another important element in SDT. Based on the known mechanisms of cell apoptosis or cell death induced by SDT, sonosensitizers have achieved rapid development in the past decades. Moreover, the selection of a suitable sonosensitizer in carrying out SDT on cancers has become an important issue in current investigations of SDT. Here, we introduced several kinds of sonosensitizers mainly including porphyrin-based sonosensitizers, xanthene-based sonosensitizers, non-steroidal anti-inflammatory drug-based sonosensitizers, and other sonosensitizers. Their chemical structures are shown in Figure 2 . These sonosensitizers have been extensively studied in some investigations of SDT in cancer treatment.
2.
Chemical structures of porphyrin-based sonosensitizers (A), xanthene-based sonosensitizers (B), non-steroidal anti-inflammatory drug-based sonosensitizers (C), and other sonosensitizers (D).
Porphyrin-based sonosensitizers
The widely used sonosensitizers at present are mainly the first generation photosensitizers such as photofrin, porphyrin and its derivatives. Figure 2A lists the several representative porphyrin-based sonosensitizers that have been studied extensively in the development of SDT. HMME is a HP-related photosensitizer and exhibits several advantages in cancer therapy, such as the high selectivity for tumor, the rapid removal from normal tissues and the relatively low toxicity. Previous reports showed that HMME combined with ultrasonic irradiation obviously enhanced the intracellular ROS production 39 and the expression of Bax, caspase-3 and caspase-9 proteins 40, and also displayed significant improvement on the suppression of cell viability and the induction of cell apoptosis 41. Recent studies on human leukemia U937 cells 32 and osteosarcoma 42 revealed that HMME combined with ultrasonic irradiation might be a promising approach for cancer therapy. PpIX is a novel HPD and displays stronger anticancer activities than HP under the same ultrasonic condition because that it is much easier to be taken up by cancer cells 43. However, HP and HPD have phototoxicity on the skin, thus markedly prevent their clinical applications. The second generation of sonosensitizers such as Ce6 is promised to overcome this disadvantage. Ce6 is a monomer compound which consists of single chemical structure. Previous studies showed that Ce6 can be selectively accumulated in tumor tissues and rapidly cleared from the normal tissues 44. Li et al. 45 observed that Ce6 mediated SDT can activate the mitochondrial-dependent apoptosis pathway in human chronic myelogenous leukemia K562 cells.
Xanthene-based sonosensitizers
The xanthene dyes ( Figure 2B ) such as erythrosin B (EB) and rose bengal (RB) have been used as sonosensitizers and exhibit very high sonodynamic efficiency under the ultrasound. The in vitro cytotoxicity of RB can be significantly enhanced by the ultrasonic irradiation, but its in vivo applications are limited by its low accumulation in tumor tissues, rapid sequestration in the liver and subsequent clearance 46. In recent years, chemical modification of RB has attracted considerable attention to overcome its disadvantage. In view that the amphiphilicity is favorable for accumulation of drugs in tumor, several amphiphilic RB derivatives have been synthesized. Sugita et al. 47 combined carboxylation (RBD2) and alkylation (RBD3) to synthesize amphiphilic RB derivatives, which exhibit more efficient in vivo anticancer effects than RB under the ultrasound due to their enhanced accumulation in tumors 48.
Non-steroidal anti-inflammatory-based sonosensitizers
Recently, several non-steroidal anti-inflammatory drugs have been found to have significant anticancer effects under the ultrasound. Quinolone compounds, a kind of clinically used anti-infection drugs with broad-spectrum, exhibit obvious photosensitivity. Because many sonosensitizers are derived from photosensitizers, quinolone compounds are deduced to have sonodynamic efficiency. For example, fluoroquinolone antibiotics were used as sonosensitizers to exert anti-tumor effects against sarcoma 180 cells in vitro ( Figure 2C ) 49. Besides, ciprofloxacin (CPFX), gatifloxacin (GFLX), lomefloxacin (LFLX), and sparfloxacin (SPFX) could promote the death of cancer cells under the ultrasound sonification at 2 W/cm 2 for 30 and 60 s. Non-steroidal anti-inflammatory drugs tenoxicam 50 and piroxicam 51 also exhibited remarkably enhanced cytotoxicities in sarcoma 180 cells after ultrasonic irradiation, which were partially contributed to the production of intracellular ROS.
Other sonosensitizers
With the development of SDT, seeking new sonosensitizers with higher sonodynamic efficiency and less toxicity has become an important issue for the further clinical application of SDT. Many small-molecular agents have been used as sonosensitizers and displayed relatively high sonodynamic efficiency against tumors both in vitro and in vivo. These small-molecular agents include curcumin, indocyanine green (ICG), acridine orange, hypocrellin B, and 5-ALA and so on ( Figure 2D ). Besides, the chemical modification of small-molecular sonosensitizers with hydrophilic polymers and the preparation of nanosensitizers, referring to the nanoparticles that possess the photo/sonodynamic activities in themselves, also have been explored on in cancer treatment.
Curcumin, an active ingredient of turmeric, shows natural anti-tumor effects with strong potency. Growing evidences reveal that the visible light can activate curcumin to have significantly enhanced toxicity in cancer cells, which demonstrates that curcumin is an herbal photosensitizer. Further investigations suggest that curcumin can be used as a novel sonosensitizer for clinical treatment on atheroscle- rosis 52. Some experimental results have demonstrated that curcumin exhibited significant sonodynamic effects on THP-1-derived macrophages and curcumin-based SDT could be a promising treatment method for atherosclerosis. Although the current researches do not use curcumin-mediated SDT to treat cancers directly, the obtained data suggest that curcumin is very likely to be used as a novel and efficient sonosensitizer in cancer therapy 53. ICG is a near infrared-absorbing dye and has been clinically applied in disease diagnosis. Many studies have used ICG as a photosensitizer in photothermal treatment and PDT 54 for cancer. A recent study showed that the combined treatment of ICG and ultrasonic irradiation decreased cell viability by 65% in mouse sarcoma cells. More interesting, ICG combined with light and ultrasound at the same time resulted in 90% of the cell to death and significantly inhibited the in vivo growth of sarcoma cells. From the obtained results, it could be deduced that ICG-mediated PDT and SDT combined treatment has great potential for cancer therapy 55. In a recent study, a water-soluble fluorochrome acridine orange was used as a sonosensitizer to treat S180 cells in vitro 56. After ultrasonic irradiation at potency of 2 W/cm 2 for 60 s, acridine orange exhibited significant inhibitory activity on the proliferation of S180 cells. It was interesting that sonodynamic efficiency of acridine orange was obviously suppressed by L-histidine and D-mannitol, the ROS scavengers. It suggested that the generation of ROS induced by acridine orange-mediated SDT might play an important role in its anticancer effects.
Hypocrellin B, as a phytochrome, is first discovered and in-depth studied by Chinese scientists. Hypocrellin B is extracted from a parasitic fungus called hypocrelline and has been used as a photosensitizer for PDT. Besides, its sonodynamic efficiency has been also evaluated in vitro. As reported by Wang et al. 57, hypocrellin B combined with ultrasound significantly promoted the death of nasopharyngeal carcinoma cells and HepG2 cells. It is worth to note that the increased ROS production was observed in HepG2 cells with treatment of hypocrellin B-mediated SDT. Moreover, hypocrellin B possesses many advantages over other sonosensitizers such as wide source, easy purification, low toxicity, and fast clearance. 5-ALA is a novel photo/sonosensitizer developed in recent years. As a precursor of PpIX, 5-ALA cannot produce photo/ sonodynamic activities directly, but its anabolic product PpIX exhibits relatively high photo/sonodynamic efficiency 58. Under normal circumstances, the amount of intracellular 5-ALA is very small. However, 5-ALA can be selectively uptaken by active cells and further transferred to PpIX when it is delivered into the body. A recent study showed that 5-ALA-mediated SDT displayed significant apoptotic effects in human tongue squamous carcinoma (SAS) cells and its effect mechanisms involved the generation of intracellular ROS, the enhancement of lipid peroxidation and the loss of mitochondrial membrane potential 59.
Chemical modification of sonosensitizers with polyethylene glycol (PEG) can improve their solubility and enhance their sonodynamic efficiency. Komori et al. 60 synthesized PEGylated LFLX derivatives by conjugating LFLX with methoxyl PEG and investigated their effects on sarcoma 180 cells under the ultrasound. The results demonstrated that the sonodynamic effects of PEGylated LFLXs were obviously stronger than those of LFLX. This suggests that the chemical structural optimization is favorable for improving the sonodynamic efficiency of sonosensitizers.
Recently, some nanoparticles have been found to possess the photo/sonodynamic activities in themselves, thus they are called as nanosensitizers. For example, TiO 2 nanoparticles can strongly absorb the ultraviolet light or untrasound and subsequently trigger the generation of ROS. Therefore, TiO 2 nanoparticles can be used as a novel sono/photosensitizer for SDT 61/PDT 62. Recent studies showed that SiO 2 nanoparticles possess the same properties as TiO 2 nanoparticles. Osminkina et al. 63 investigated the cytotoxicity and sonosensitivity of silicon nanoparticles (SiNPs) prepared from porous silicon nanowires (SiNWs). The combined treatment of SiNPs and ultrasound substantially inhibited the growth of cancer cells. These results open a new perspective for the usage of biocompatible porous SiNPs in cancer treatments.
Nanocarriers for tumor-targeted delivery of sonosensitizers
Although the above sonosensitizers shown in Figure 2 are preferentially distributed to solid tumors to some extent, it should be mentioned that the superfluous sonosensitizers can be uptaken by the normal tissues. It has been assuredly known as a major challenge in the clinical application of SDT because these sonosensitizers simultaneously possess photodynamic sensitivities, which can cause serious hypersensitivity when the patients are exposed to the light 64. The rapid development of nanocarrier technology in recent decades makes it possible to solve this problem. Many nanocarriers have been designed for tumor-targeted delivery of sonosensitizers via the enhanced permeation and retention (EPR) effect or other active targeting effects, e.g., the specific affinity of ligands or antibodies on the surface of nanosystems for the receptors over-expressed by tumor cells. Moreover, nanocarriers can be used as platform for different therapeutic agents, thus effectively combine different cancer treatment methods.
Sazgarnia et al. 65 prepared PpIX-conjugated gold nanopar- ticles and evaluated their sonodynamic efficiency on an animal model with colon cancer. The results showed that this nanoparticle system successfully delivered PpIX targeting to the tumor, significantly decreased its toxicity on the normal tissues and remarkably enhanced synergistic effects on the tumor by combing PpIX-mediated SDT with gold nanoparticle-mediated thermotherapy. Hu et al. 66 prepared stearic acid-grafted chitosan oligosaccharide micelles with loading of Ce6. These micelles improved the cellular internalization of Ce6, significantly enhanced its sonodynamic efficiency, and decreased its toxic and side effects compared to Ce6 liquores. In Nomikou's study 67, microbubble-sonosensitizer conjugates were prepared by covalently attaching rose bengal to lipid-shelled microbu- bbles. These microbubble-sonosensitizer conjugates triggered the increased intracellular ROS level in the presence of an acoustic filed, thus enhanced SDT-mediated cytotoxic effects on target cancer cells. Further investigations confirmed their in vivo antitumor effects using human xenograft tumor animal models.
In our investigation previously reported 68, HP was conjugated to PEG to synthesize HPP, which could form nanoparticles in aqueous media by self-assembly. HPP nanoparticles displayed strong capability for loading chemotherapeutic drug doxorubicin (DOX), thus efficiently combined PDT and chemotherapy to reverse drug resistance of human breast cancer MCF-7/ADR cells. In MCF-7/ADR tumor-bearing mice, this nanoparticle system exhibited excellent tumor-targeting property and successfully realized the tumor ablation. Besides, we also prepared HP and DOX co-loaded Pluronic F68 (HPDF) nanomicelles and evaluated their capability for combining SDT and chemotherapy to treat hepatocellular carcinoma (HCC) in our recent study ( Figure 3 ). The results have shown that HPDF nanomicelles combined with ultrasonic irradiation (1.0 MHz, 1.5 W/cm 2, 30 s) exhibited significant synergistic effects on the cytotoxicity, apoptosis, and cell-cycle arrest of HCC HepG2 cells. Furthermore, HPDF nanomicelles also exhibited excellent HCC-targeting delivery capability in HepG2 tumor-bearing nude mice and remarkably inhibited the tumor growth, angiogenesis, and collagen deposition after being combined with ultrasonic irradiation (1.0 MHz, 3 W/cm 2).
3.
HPDF nanomicelles exerted efficient anti-hepatoma effects both in vitro and in vivo. (A) The preparation route of HPDF nanomicelles. (B) The transmission electron microscopic image of HPDF nanomicelles. HPDF nonamicelles induced the cell apoptosis (C) and the cell cycle arrest (D) in hepatoma HepG2 cells. (E) The tissue distributions of HPDF nanomicelles in HepG2 tumor-bearing mice. (F) The comparison for tissue distributions of HPDF nanomicelles.
Combination of SDT with other cancer treatment methods
SDT shows great potential as a novel strategy for cancer treatment, but its single application cannot achieve tumor ablation completely. Therefore, SDT is very likely to be an adjunctive method for clinical cancer treatment. Currently, many investigations have shown that SDT combined with other treatment methods such as chemotherapy, PDT, hyperthermotherapy, etc, can achieve significant synergistic effects against the growth of tumors both in vitro and in vivo. Here, we reviewed the recent advances of SDT combined with other methods for cancer treatment.
SDT combined with chemotherapy
Chemotherapy is a very important method in clinical cancer treatment, whereas cancer drug resistance either natural or acquired greatly limits its wide applications in clinic. To enhance the sensitivity of cancers to chemotherapeutic drugs is always a focused issue in the research field of cancer treatment. First, the ultrasound can selectively improve the uptake of chemotherapeutic drugs in cancer cells, thus reduce their toxic and side effects on normal cells and tissues 9. Second, SDT can activate the mitochondria-caspase signaling pathway 69 and down-regulate the expression levels of ATP-binding cassette (ABC) transporters 70, which are favorable for enhancing the sensitivity of cancer cells to chemotherapeutic drugs. In addition, some nanosonosensitizers such as TiO 2 nanoparticles can be used for loading and targeted delivery of chemotherapeutic drugs in order to improve the therapeutic efficacy of chemotherapy on cancers 71. Taken together, the combined treatment of SDT with chemotherapy will likely achieve synergistic therapeutic effects on cancers. Here, we summarized some strategies recently used for combining SDT and chemotherapy to treat cancers.
Wang et al. 72 evaluated the efficacy of administration of DOX in combination with PpIX-assisted low-intensity ultrasound in K562/DOX cells. Under the optimal condition, the combination treatment significantly aggravated the death of multidrug-resistant leukemia k562/DOX cells by comparison with either monotherapy. Synergistic effects on DNA damage, generation of intracellular ROS and inhibition of P-glycoprotein (a classic ATP-binding cassette efflux transporter) were evidently detected. Gao et al. 73 found that DOX combined with Ce6-mediated SDT exhibited significant synergistic effects against the proliferation of human breast cancer MDA-MB-231 cells. Furthermore, these effects were schedule-dependent and became stronger when DOX was added after Ce6-mediated SDT. Osaki et al. 74 examined the therapeutic enhancement of bleomycin when combined with 5-ALA-based SDT on mouse mammary tumor cells. The results suggested that the mechanisms of tumor shrinkage induced by combination treatment of 5-ALA-based SDT and bleomycin involved not only the direct killing of cancer cells but also the vascular shutdown.
McEwan et al. 75 prepared an oxygen-loaded microbubble (O 2MB) platform for carrying the sensitizer Rose Bengal (O 2MB-RB) or the antimetabolite 5-fluorouracil (O 2MB-5FU) to targetedly treat pancreatic cancer by combining SDT and antimetabolite therapy. The results showed that the combined treatment of SDT and antimetabolite therapy significantly inhibited the proliferations of three different pancreatic cancer cell lines (BxPc-3, MIA PaCa-2 and PANC-1) and reduced the tumor growth in SCID mice bearing human xenograft BxPC-3 tumor compared to either therapy alone. Moreover, O 2MBs could effectively deliver O 2 to the tumor microenvironment, thus enhance the efficacy of therapies that depend on O 2. Altogether, the use of MBs to facilitate delivery of O 2 as well as the sensitizer/ antimetabolite, combined with the possibility to activate the sensitizer using externally applied ultrasound, provides a more targeted approach with improved efficacy and reduced side effects when compared with conventional systemic administration of antimetabolite drugs alone.
SDT combined with PDT (SPDT)
SPDT is an emerging approach in anticancer field using combination of SDT and PDT. The basis of this novel therapy is to administer a very small amount of sensitizer, which can be activated by both the ultrasound and the light simultaneously, thus produce mechanical, sonochemical and photochemical activities 76 on tumors.
As a non-invasive therapy method, PDT has been gradually applied for treatment of various cancers since the 1990s 77. However, the low penetration capability of laser light limits the wide applications of PDT 78. Inspired by PDT, Yumita et al. 1 developed a novel method named SDT using ultrasound to irritate sensitizers. Compared with PDT, the ultrasound that can easily penetrate deep tissue layers is used in SDT, thereby can make up the major limitation of PDT 9, 79. Liu et al. 80 evaluated the combined effects of SDT and PDT by using sinoporphyrin sodium (DVDMS), a newly identified sensitizer that shows great potential in both SDT and PDT, on breast cancer. In their study, DVDMS-mediated SPDT elicited much serious cytotoxicity compared with either SDT or PDT alone, and greatly suppressed the tumor growth in 4T1 xenograft mouse model. Moreover, DVDMS-mediated SPDT produced no obvious effects on body weight and major organs in tumor-bearing mice. The results suggested that by combination of SDT and PDT, the sensitizer DVDMS could produce enhanced therapeutic effects on tumors and reduced toxicity on normal tissues. Miyoshi et al. 81 used TiO 2 nanoparticles and 5-ALA respectively as sonosensitizer and photosensitizer for combination of SDT and PDT to treat cancer. Several combination forms were applied in an animal tumor model and oral-administrations of 0.2%-TiO 2 nanoparticles and 1 mM 5-ALA, respectively, followed by the ultrasonic and laser irradiations and achieved the strongest anti-tumor effects. The results suggest that SDT followed by PDT is more favorable for exerting synergistic effects on cancers.
SDT combined with hyperthermotherapy
In view that cancer cells can be inactivated by heating tumorous regions, hyperthermotherapy is currently believed as one of the promising approaches in cancer therapy. Both hyperthermotherapy and SDT have been studied alone and in combination with other modalities to treat cancers 82. Clearly, combination therapies have more success stories to tell than any of the modalities alone. Hyperthermia induced by ultrasound was investigated and found to be more advantageous because of its manageability in terms of focusing, control and sufficient tissue attenuation coefficient for deep tumor targets 83. Moreover, hyperthermia combined with non-thermal ultrasound also showed synergistic effects on cell killing 84. Kondo and Kano 84 treated mouse L-cells with a low-intensity ultrasound (1 MHz, 3.7 W/cm 2) and/or 44 °C hyperthermia. More significant synergistic effects on cell killing were observed in combination treatment of ultrasound and hyperthermia. Ju et al. 85 reported that 5-ALA-mediated SDT combined with hyperthermia could significantly inhibit the growth of human glioma both in vitro and in vivo, compared to SDT alone. Hyperthermia conspicuously enhanced the induction effects of 5-ALA-mediated SDT on cell apoptosis and ROS intracellular production. The anti-tumor effects of combination treatment were closely related to 5-ALA concentration, ultrasound exposure time and temperature. The results suggest that SDT in combination with hyperthermotherapy can be used as a potent strategy for cancer treatment.
Shock waves (SWs) mediated SDT
Despite the effectiveness that SDT has demonstrated in many experimental tumor models, there is a limited understanding of the mechanism of interaction between ultrasound and sonosensitizer in tumor tissues, even though inertial cavitation seems to play a crucial role. The most important parameters for inducing inertial cavitation are the ultrasound insonation technique used and peak ultrasound wave pressure. Therapeutic ultrasound usually produces non-thermal effects that are difficult to isolate from the thermal ones. In view of that, some research groups tried to use SWs to activate sonosensitizers, in order to minimize the thermal effect produced by ultrasound and enhance inertial cavitation 86, 87.
SWs are sharp discontinuities involving a sudden change in pressure and density which can induce in vivo bioeffects. A typical pressure waveform at the focus in water consists of a compressive wave with a peak positive pressure in the range of 30–150 MPa and a phase duration of 0.5–3 μs, followed by a tensile wave with a peak negative pressure that drops to 20 MPa and a duration of 2–20 μs, which is responsible for cavitation occurring. The low peak negative acoustic pressures of common therapeutic ultrasound are usually 0.2 MPa and produce stable cavitation 88, 89. SWs have been used in extracorporeal SW lithotripsy for many years where it non-invasively treats patients with stone diseases. The in vivo treatment of tumors by SWs alone has been shown to be ineffective in inhibiting tumor growth 88, whereas some evidence has been obtained to suggest that combining SWs and sonosensitizer results in sonodynamic efficiency on the inhibition of tumor growth 90- 92. For example, Foglietta et al. 93 investigated the anticancer effects of SDT based on SWs in a Mat B-III syngeneic rat breast cancer model. The SDT-treated group saw a significant decrease in tumor size after treatment with PpIX precursor 5-ALA and SWs. This occurred together with significant increase in apparent diffusion coefficients between pre- and post-treatment tumor magnetic resonance images and strong increase in necrotic and apoptotic histological features at 72 h after treatment. The results show that the anticancer effect of SDT was remarkably boosted by SWs, thus suggesting that SWs-mediated SDT can be developed as an innovative and valid approach for cancer treatment.
Conclusions
A significant body of data has demonstrated therapeutic effectiveness of SDT in cancer treatment. In view of the significant depth that ultrasound penetrates tissue, SDT provides an advantage over PDT, in which less penetrating light is employed. More work needs to be done before SDT is accepted as an adjuvant or replacement method for traditional cancer treatment. A greater understanding of mechanisms underlying the generation of ROS induced by SDT will surely enable the design of more effective sonosensitizers and help to reasonably control ultrasound dosimetry and therapeutic response. Some investigations have found that SDT can exert indirect inhibitory effects on cancer growth by modulating the tumor microenvironment, but its modulation mechanisms remain unknown. The strategy of combining SDT with chemotherapy, thermal therapy and PDT is gaining more legitimacy as many studies have validated their synergistic effects. Besides, nanocarriers display enormous advantages for tumor-targeted delivery of sonosensitizers and combination of SDT with other treatment methods. More data necessary to ascertain the actual anticancer activities of SDT will be acquired in the near future, thereby determining whether SDT warrants further study as a novel strategy for cancer therapy.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81573005 and 81371671).
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Yumita N, Nishigaki R, Umemura K, Umemura S. Hematoporphyrin as a sensitizer of cell-damaging effect of ultrasound. Jpn J Cancer Res. 1989;80:219–22. doi: 10.1111/j.1349-7006.1989.tb02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin ZH, Miyoshi N, Ishiguro K, Umemura S, Kawabata K, Yumita N, et al. Combination effect of photodynamic and sonodynamic therapy on experimental skin squamous cell carcinoma in C3H/HeN mice. J Dermatol. 2000;27:294–306. doi: 10.1111/j.1346-8138.2000.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 3.Yumita N, Umemura S. Sonodynamic therapy with photofrin Ⅱ on AH130 solid tumor. Pharmacokinetics, tissue distribution and sonodynamic antitumoral efficacy of photofrin Ⅱ. Cancer Chemother Pharmacol. 2003;51:174–8. doi: 10.1007/s00280-002-0523-6. [DOI] [PubMed] [Google Scholar]
- 4.Yumita N, Nishigaki R, Umemura S. Sonodynamically induced antitumor effect of photofrin Ⅱ on colon 26 carcinoma. J Cancer Res Clin Oncol. 2000;126:601–6. doi: 10.1007/PL00008471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milowska K, Gabryelak T. Enhancement of ultrasonically induced cell damage by phthalocyanines in vitro. Ultrasonics. 2008;48:724–30. doi: 10.1016/j.ultras.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Huang Z. A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat. 2005;4:283–93. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoogenboom M, Eikelenboom D, den Brok MH, Heerschap A, Fütterer JJ, Adema GJ. Mechanical high-intensity focused ultrasound destruction of soft tissue: working mechanisms and physiologic effects. Ultrasound Med Biol. 2015;41:1500–17. doi: 10.1016/j.ultrasmedbio.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Shibaguchi H, Tsuru H, Kuroki M, Kuroki M. Sonodynamic cancer therapy: a non-invasive and repeatable approach using low-intensity ultrasound with a sonosensitizer. Anticancer Res. 2011;31:2425–9. [PubMed] [Google Scholar]
- 9.Trendowski M. The promise of sonodynamic therapy. Cancer Metastasis Rev. 2014;33:143–60. doi: 10.1007/s10555-013-9461-5. [DOI] [PubMed] [Google Scholar]
- 10.Niedre M, Patterson MS, Wilson BC. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem Photobiol. 2002;75:382–91. doi: 10.1562/0031-8655(2002)075<0382:DNILDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.O'Connor AE, Gallagher WM, Byrne AT. Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol. 2009;85:1053–74. doi: 10.1111/j.1751-1097.2009.00585.x. [DOI] [PubMed] [Google Scholar]
- 12.Didenko YT, McNamara WB, 3rd, Suslick KS. Molecular emission from single-bubble sonoluminescence. Nature. 2000;407:877–9. doi: 10.1038/35038020. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal I, Sostaric JZ, Riesz P. Sonodynamic therapy——a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;11:349–63. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Kremkau FW, Kaufmann JS, Walker MM, Burch PG, Spurr CL. Ultrasonic enhancement of nitrogen mustard cytotoxicity in mouse leukemia. Cancer. 1976;37:1643–7. doi: 10.1002/1097-0142(197604)37:4<1643::aid-cncr2820370404>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Hu Z, Fan H, Lv G, Zhou Q, Yang B, Zheng J, et al. 5-aminolevulinic acid-mediated sonodynamic therapy induces anti-tumor effects in malignant melanoma via p53-miR-34a-SIRT1 axis. J Dermatol Sci. 2015;79:155–62. doi: 10.1016/j.jdermsci.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Kondo Y, Tokuda N, Furukawa K, Ando R, Uchikawa M, Zhang Q, et al. Efficient generation of useful monoclonal antibodies reactive with globotriaosylceramide using knockout mice lacking Gb3/CD77 synthase. Glycoconj J. 2011;28:371–84. doi: 10.1007/s10719-011-9335-4. [DOI] [PubMed] [Google Scholar]
- 17.Misik V, Riesz P. Free radical intermediates in sonodynamic therapy. Ann N Y Acad Sci. 2000;899:335–48. doi: 10.1111/j.1749-6632.2000.tb06198.x. [DOI] [PubMed] [Google Scholar]
- 18.Frulio N, Trillaud H, Deckers R, Lepreux S, Moonen C, Quesson B. Influence of ultrasound induced cavitation on magnetic resonance imaging contrast in the rat liver in the presence of macromolecular contrast agent. Invest Radiol. 2010;45:282–7. doi: 10.1097/RLI.0b013e3181dac2a7. [DOI] [PubMed] [Google Scholar]
- 19.McHale AP, Callan JF, Nomikou N, Fowley C, Callan B. Sonodynamic therapy: concept, mechanism and application to cancer treatment. Adv Exp Med Biol. 2016;880:429–50. doi: 10.1007/978-3-319-22536-4_22. [DOI] [PubMed] [Google Scholar]
- 20.Konno T, Watanabe J, Ishihara K. Enhanced solubility of paclitaxel using water-soluble and biocompatible 2-methacryloyloxyethyl phosphorylcholine polymers. J Biomed Mater Res A. 2003;65:209–14. doi: 10.1002/jbm.a.10481. [DOI] [PubMed] [Google Scholar]
- 21.Yasuda J, Yoshizawa S, Umemura S-i. Quantitative assessment of reactive oxygen species generation by cavitation incepted efficiently using nonlinear propagation effect. Recent Developments in Nonlinear Acoustics: International Symposium on Nonlinear Acoustics Including the International Sonic Boom Forum. 2015;1685:040003. [Google Scholar]
- 22.Yin H, Chang N, Xu S, Wan M. Sonoluminescence characterization of inertial cavitation inside a BSA phantom treated by pulsed HIFU. Ultrason Sonochem. 2016;32:158–64. doi: 10.1016/j.ultsonch.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Umemura S, Yumita N, Nishigaki R, Umemura K. Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn J Cancer Res. 1990;81:962–6. doi: 10.1111/j.1349-7006.1990.tb02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sazgarnia A, Shanei A, Eshghi H, Hassanzadeh-Khayyat M, Esmaily H, Shanei MM. Detection of sonoluminescence signals in a gel phantom in the presence of protoporphyrin IX conjugated to gold nanoparticles. Ultrasonics. 2013;53:29–35. doi: 10.1016/j.ultras.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Zhou X, Gao Y, Zheng B, Tang F, Huang J. Recent progress in development of new sonosensitizers for sonodynamic cancer therapy. Drug Discov Today. 2014;19:502–9. doi: 10.1016/j.drudis.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Cheng J, Sun X, Guo S, Cao W, Chen H, Jin Y, et al. Effects of 5-aminolevulinic acid-mediated sonodynamic therapy on macrophages. Int J Nanomedicine. 2013;8:669–76. doi: 10.2147/IJN.S39844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen B, Zheng R, Liu D, Li B, Lin J, Zhang W. The tumor affinity of chlorin e6 and its sonodynamic effects on non-small cell lung cancer. Ultrason Sonochem. 2013;20:667–73. doi: 10.1016/j.ultsonch.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 28.McEwan C, Owen J, Stride E, Fowley C, Nesbitt H, Cochrane D, et al. Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours. J Control Release. 2015;203:51–6. doi: 10.1016/j.jconrel.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Sun X, Xu H, Shen J, Guo S, Shi S, Dan J, et al. Real-time detection of intracellular reactive oxygen species and mitochondrial membrane potential in THP-1 macrophages during ultrasonic irradiation for optimal sonodynamic therapy. Ultrason Sonochem. 2015;22:7–14. doi: 10.1016/j.ultsonch.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Hachimine K, Shibaguchi H, Kuroki M, Yamada H, Kinugasa T, Nakae Y, et al. Sonodynamic therapy of cancer using a novel porphyrin derivative, DCPH-N -Na(I), which is devoid of photosensitivity. Cancer Sci. 2007;98:916–20. doi: 10.1111/j.1349-7006.2007.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su X, Wang P, Yang S, Zhang K, Liu Q, Wang X. Sonodynamic therapy induces the interplay between apoptosis and autophagy in K562 cells through ROS. Int J Biochem Cell Biol. 2015;60:82–92. doi: 10.1016/j.biocel.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 32.Su X, Wang P, Wang X, Cao B, Li L, Liu Q. Apoptosis of U937 cells induced by hematoporphyrin monomethyl ether-mediated sonodynamic action. Cancer Biother Radiopharm. 2013;28:207–17. doi: 10.1089/cbr.2012.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao D, Song Y, Che Z, Liu Q. Calcium overload and in vitro apoptosis of the C6 glioma cells mediated by sonodynamic therapy (hematoporphyrin monomethyl ether and ultrasound) Cell Biochem Biophys. 2014;70:1445–52. doi: 10.1007/s12013-014-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber CE, Kuo PC. The tumor microenvironment. Surg Oncol. 2012;21:172–7. doi: 10.1016/j.suronc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Gao Z, Zheng J, Yang B, Wang Z, Fan H, Lv Y. Sonodynamic therapy inhibits angiogenesis and tumor growth in a xenograft mouse model. Cancer Lett. 2013;335:93–99. doi: 10.1016/j.canlet.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Xiong W, Wang P, Hu J, Jia Y, Wu L, Chen X, et al. A new sensitizer DVDMS combined with multiple focused ultrasound treatments: an effective antitumor strategy. Sci Rep. 2015;5:17485. doi: 10.1038/srep17485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song D, Yue W, Li Z, Li J, Zhao J, Zhang N. Study of the mechanism of sonodynamic therapy in a rat glioma model. Onco Targets Ther. 2014;7:1801–10. doi: 10.2147/OTT.S52426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Hu Z, Wang X, Gu C, Gao Z, Cao W, Zheng J. 5-Aminolevulinic acid-mediated sonodynamic therapy reverses macrophage and dendritic cell passivity in murine melanoma xenografts. Ultrasound Med Biol. 2014;40:2125–33. doi: 10.1016/j.ultrasmedbio.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Li JH, Yue W, Huang Z, Chen ZQ, Zhan Q, Ren FB, et al. Calcium overload induces C6 rat glioma cell apoptosis in sonodynamic therapy. Int J Radiat Biol. 2011;87:1061–6. doi: 10.3109/09553002.2011.584938. [DOI] [PubMed] [Google Scholar]
- 40.Dai S, Hu S, Wu C. Apoptotic effect of sonodynamic therapy mediated by hematoporphyrin monomethyl ether on c6 glioma cells in vitro. Acta Neurochir (Wien) 2009;151:1655–61. doi: 10.1007/s00701-009-0456-5. [DOI] [PubMed] [Google Scholar]
- 41.Sun H, Ge W, Gao X, Wang S, Jiang S, Hu Y, et al. Apoptosis-promoting effects of hematoporphyrin monomethyl ether-sonodynamic therapy (HMME-SDT) on endometrial cancer. PloS one. 2015;10:e0137980. doi: 10.1371/journal.pone.0137980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian Z, Quan X, Leung AW, Xiang J, Xu C. Hematoporphyrin monomethyl ether enhances the killing of ultrasound on osteosarcoma cells involving intracellular reactive oxygen species and calcium ion elevation. Integr Cancer Ther. 2010;9:365–9. doi: 10.1177/1534735410379013. [DOI] [PubMed] [Google Scholar]
- 43.Zhu B, Liu Q, Wang Y, Wang X, Wang P, Zhang L, et al. Comparison of accumulation, subcellular location, and sonodynamic cytotoxicity between hematoporphyrin and protoporphyrin IX in L1210 cells. Chemotherapy. 2010;56:403–10. doi: 10.1159/000317743. [DOI] [PubMed] [Google Scholar]
- 44.Klyta M, Ostasiewicz P, Jurczyszyn K, Duś K, Latos-Grażyński L, Pacholska-Dudziak E, et al. Vacata- and divacataporphyrin: new photosensitizers for application in photodynamic therapy-an in vitro study. Lasers Surg Med. 2011;43:607–13. doi: 10.1002/lsm.21086. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Wang P, Wang X, Su X, Liu Q. Involvement of mitochondrial and reactive oxygen species in the sonodynamic toxicity of chlorin e6 in human leukemia K562 cells. Ultrasound Med Biol. 2014;40:990–1000. doi: 10.1016/j.ultrasmedbio.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 46.Nonaka M, Yamamoto M, Yoshino S, Umemura S, Sasaki K, Fukushima T. Sonodynamic therapy consisting of focused ultrasound and a photosensitizer causes a selective antitumor effect in a rat intracranial glioma model. Anticancer Res. 2009;29:943–50. [PubMed] [Google Scholar]
- 47.Sugita N, Iwase Y, Yumita N, Ikeda T, Umemura S. Sonodynamically induced cell damage using rose bengal derivative. Anticancer Res. 2010;30:3361–6. [PubMed] [Google Scholar]
- 48.Sugita N, Hosokawa M, Sunaga N, Yumiko I, Nagahiko Y, Toshihiko I, et al. Sonodynamically-induced cytotoxicity by rose bengal derivative and microbubbles in isolated sarcoma 180 cells. J Appl Phys. 2015;54:7–16. [Google Scholar]
- 49.Huang D, Okada K, Komori C, Itoi E, Suzuki T. Enhanced antitumor activity of ultrasonic irradiation in the presence of new quinolone antibiotics in vitro. Cancer Sci. 2004;95:845–9. doi: 10.1111/j.1349-7006.2004.tb02192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakusabe N, Okada K, Sato K, Kamada S, Yoshida Y, Suzuki T. Enhanced sonodynamic antitumor effect of ultrasound in the presence of nonsteroidal anti-inflammatory drugs. Jpn J Cancer Res. 1999;90:1146–51. doi: 10.1111/j.1349-7006.1999.tb00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okada K, Itoi E, Miyakoshi N, Nakajima M, Suzuki T, Nishida J. Enhanced antitumor effect of ultrasound in the presence of piroxicam in a mouse air pouch model. Jpn J Cancer Res. 2002;93:216–22. doi: 10.1111/j.1349-7006.2002.tb01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang F, Gao Q, Guo S, Cheng J, Sun X, Li Q, et al. The sonodynamic effect of curcumin on THP-1 cell-derived macrophages. Biomed Res Int. 2013;2013:737264. doi: 10.1155/2013/737264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng L, Sun X, Zhu X, Lv F, Zhong Z, Zhang F, et al. Apoptosis of THP-1 derived macrophages induced by sonodynamic therapy using a new sonosensitizer hydroxyl acetylated curcumin. PloS one. 2014;9:e93133. doi: 10.1371/journal.pone.0093133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim TI, Jeong KH, Shin MK. Verrucous epidermal nevus (ven) successfully treated with indocyanine green (ICG) photodynamic therapy (PDT) JAAD Case Rep. 2015;1:312–4. doi: 10.1016/j.jdcr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nomikou N, Sterrett C, Arthur C, McCaughan B, Callan JF, McHale AP. The effects of ultrasound and light on indocyanine-green-treated tumour cells and tissues. ChemMedChem. 2012;7:1465–71. doi: 10.1002/cmdc.201200233. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki N, Okada K, Chida S, Komori C, Shimada Y, Suzuki T. Antitumor effect of acridine orange under ultrasonic irradiation in vitro. Anticancer Res. 2007;27:4179–84. [PubMed] [Google Scholar]
- 57.Wang X, Leung AW, Jiang Y, Yu H, Li X, Xu C. Hypocrellin B-mediated sonodynamic action induces apoptosis of hepatocellular carcinoma cells. Ultrasonics. 2012;52:543–6. doi: 10.1016/j.ultras.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Lawrence JE, Steele CJ, Rovin RA, Belton RJ Jr, Winn RJ. Dexamethasone alone and in combination with desipramine, phenytoin, valproic acid or levetiracetam interferes with 5-ALA-mediated PpIX production and cellular retention in glioblastoma cells. J Neurooncol. 2016;127:15–21. doi: 10.1007/s11060-015-2012-x. [DOI] [PubMed] [Google Scholar]
- 59.Lv Y, Fang M, Zheng J, Yang B, Li H, Xiuzigao Z. Low-intensity ultrasound combined with 5-aminolevulinic acid administration in the treatment of human tongue squamous carcinoma. Cell Physiol Biochem. 2012;30:321–33. doi: 10.1159/000339067. [DOI] [PubMed] [Google Scholar]
- 60.Komori C, Okada K, Kawamura K, Suzuki N, Chida S, Suzuki T. Sonodynamic effects of lomefloxacin derivatives conjugated with methoxy polyethylene glycol on sarcoma 180 cells. Anticancer Res. 2009;29:243–8. [PubMed] [Google Scholar]
- 61.Ninomiya K, Fukuda A, Ogino C, Shimizu N. Targeted sonocatalytic cancer cell injury using avidin-conjugated titanium dioxide nanoparticles. Ultrason Sonochem. 2014;21:1624–8. doi: 10.1016/j.ultsonch.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 62.Ninomiya K, Noda K, Ogino C, Kuroda S, Shimizu N. Enhanced oh radical generation by dual-frequency ultrasound with TiO 2 nanoparticles: its application to targeted sonodynamic therapy . Ultrason Sonochem. 2014;21:289–94. doi: 10.1016/j.ultsonch.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 63.Osminkina LA, Sivakov VA, Mysov GA, Georgobiani VA, Natashina UA, Talkenberg F, et al. Nanoparticles prepared from porous silicon nanowires for bio-imaging and sonodynamic therapy. Nanoscale Res Lett. 2014;9:463. doi: 10.1186/1556-276X-9-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costley D, Mc Ewan C, Fowley C, McHale AP, Atchison J, Nomikou N, et al. Treating cancer with sonodynamic therapy: a review. Int J Hyperthermia. 2015;31:107–17. doi: 10.3109/02656736.2014.992484. [DOI] [PubMed] [Google Scholar]
- 65.Sazgarnia A, Shanei A, Meibodi NT, Eshghi H, Nassirli H. A novel nanosonosensitizer for sonodynamic therapy: In vivo study on a colon tumor model. J Ultrasound Med. 2011;30:1321–9. doi: 10.7863/jum.2011.30.10.1321. [DOI] [PubMed] [Google Scholar]
- 66.Hu FQ, Jiang XH, Huang X, Wu XL, Yuan H, Wei XH, et al. Enhanced cellular uptake of chlorine e6 mediated by stearic acid-grafted chitosan oligosaccharide micelles. J Drug Target. 2009;17:384–91. doi: 10.1080/10611860902894325. [DOI] [PubMed] [Google Scholar]
- 67.Nomikou N, Fowley C, Byrne NM, McCaughan B, McHale AP, Callan JF. Microbubble-sonosensitiser conjugates as therapeutics in sonodynamic therapy. Chem Commun (Camb) 2012;48:8332–34. doi: 10.1039/c2cc33913g. [DOI] [PubMed] [Google Scholar]
- 68.Ren Y, Wang R, Liu Y, Guo H, Zhou X, Yuan X, et al. A hematoporphyrin-based delivery system for drug resistance reversal and tumor ablation. Biomaterials. 2014;35:2462–70. doi: 10.1016/j.biomaterials.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Tang W, Liu Q, Zhang J, Cao B, Zhao P, Qin X. In vitro activation of mitochondria-caspase signaling pathway in sonodynamic therapy-induced apoptosis in sarcoma 180 cells. Ultrasonics. 2010;50:567–76. doi: 10.1016/j.ultras.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Xu ZY, Wang K, Li XQ, Chen S, Deng JM, Cheng Y, Wang ZG. The ABCG2 transporter is a key molecular determinant of the efficacy of sonodynamic therapy with photofrin in glioma stem-like cells. Ultrasonics. 2013;53:232–8. doi: 10.1016/j.ultras.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 71.Shen S, Wu L, Liu J, Xie M, Shen H, Qi X, et al. Core-shell structured Fe 3O 4@TiO 2-doxorubicin nanoparticles for targeted chemo-sonodynamic therapy of cancer . Int J Pharm. 2015;486:380–8. doi: 10.1016/j.ijpharm.2015.03.070. [DOI] [PubMed] [Google Scholar]
- 72.Wang X, Jia Y, Su X, Wang X, Zhang K, Feng X, et al. Combination of protoporphyrin IX-mediated sonodynamic treatment with doxorubicin synergistically induced apoptotic cell death of a multidrug-resistant leukemia K562/DOX cell line. Ultrasound Med Biol. 2015;41:2731–9. doi: 10.1016/j.ultrasmedbio.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Gao HJ, Zhang WM, Wang XH, Zheng RN. Adriamycin enhances the sonodynamic effect of chlorin e6 against the proliferation of human breast cancer mda-mb-231 cells in vitro. J South Medical University. 2010;30:2291–4. [PubMed] [Google Scholar]
- 74.Osaki T, Ono M, Uto Y, Ishizuka M, Tanaka T, Yamanaka N, et al. Sonodynamic therapy using 5-aminolevulinic acid enhances the efficacy of bleomycin. Ultrasonics. 2016;67:76–84. doi: 10.1016/j.ultras.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 75.McEwan C, Kamila S, Owen J, Nesbitt H, Callan B, Borden M, et al. Combined sonodyn amic and antimetab olite therapy for the improved treatment of pancreatic cancer using oxygen loaded microbubbles as a delivery vehicle. Biomaterials. 2016;80:20–32. doi: 10.1016/j.biomaterials.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 76.Sadanala KC, Chaturvedi PK, Seo YM, Kim JM, Jo YS, Lee YK, et al. Sono-photodynamic combination therapy: A review on sensitizers. Anticancer Res. 2014;34:4657–64. [PubMed] [Google Scholar]
- 77.Davids LM, Kleemann B. Combating melanoma: The use of photodynamic therapy as a novel, adjuvant therapeutic tool. Cancer Treat Rev. 2011;37:465–75. doi: 10.1016/j.ctrv.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 78.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–7. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 79.Wood AK, Sehgal CM. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med Biol. 2015;41:905–28. doi: 10.1016/j.ultrasmedbio.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu Y, Wang P, Liu Q, Wang X. Sinoporphyrin sodium triggered sono-photodynamic effects on breast cancer both in vitro and in vivo. Ultrason Sonochem. 2016;31:437–48. doi: 10.1016/j.ultsonch.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 81.Miyoshi N, Kundu SK, Tuziuti T, Yasui K, Shimada I, Ito Y. Combination of sonodynamic and photodynamic therapy against cancer would be effective through using a regulated size of nanoparticles. Nanosci Nanoeng. 2016;4:1–11. doi: 10.13189/nn.2016.040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hornback NB. Historical aspects of hyperthermia in cancer therapy. Radiol Clin North Am. 1989;27:481–8. [PubMed] [Google Scholar]
- 83.Lizzi FL, Ostromogilsky M. Analytical modelling of ultrasonically induced tissue heating. Ultrasound Med Biol. 1987;13:607–18. doi: 10.1016/0301-5629(87)90058-5. [DOI] [PubMed] [Google Scholar]
- 84.Kondo T, Kano E. Enhancement of hyperthermic cell killing by non-thermal effect of ultrasound. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;51:157–66. doi: 10.1080/09553008714550591. [DOI] [PubMed] [Google Scholar]
- 85.Ju D, Yamaguchi F, Zhan G, Higuchi T, Asakura T, Morita A, et al. Hyperthermotherapy enhances antitumor effect of 5-aminolevulinic acid-mediated sonodynamic therapy with activation of caspase-dependent apoptotic pathway in human glioma. Tumour Biol. 2016;37:10415–26. doi: 10.1007/s13277-016-4931-3. [DOI] [PubMed] [Google Scholar]
- 86.Maruyama M, Asano T, Uematsu T, Nakagohri T, Hasegawa M, Miyauchi H, et al. Enhancement of the antitumor effect by combined use of high-energy shock waves and ATX-70. Jpn J Cancer Res. 1995;86:800–1. doi: 10.1111/j.1349-7006.1995.tb03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maruyama M, Asano T, Nakagohri T, Uematsu T, Hasegawa M, Miyauchi H, et al. Application of high energy shock waves to cancer treatment in combination with cisplatin and ATX-70. Anticancer Res. 1999;19:1989–93. [PubMed] [Google Scholar]
- 88.Lukes P, Zeman J, Horak V, Hoffer P, Pouckova P, Holubova M, et al. In vivo effects of focused shock waves on tumor tissue visualized by fluorescence staining techniques. Bioelectrochemistry. 2015;103:103–10. doi: 10.1016/j.bioelechem.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 89.Lukes P, Sunka P, Hoffer P, Stelmashuk V, Pouckova P, Zadinova M, et al. Focused tandem shock waves in water and their potential application in cancer treatment. Shock Waves. 2014;24:51–7. [Google Scholar]
- 90.Catalano MG, Costantino L, Fortunati N, Bosco O, Pugliese M, Boccuzzi G, et al. High energy shock waves activate 5'-aminolevulinic acid and increase permeability to paclitaxel: antitumor effects of a new combined treatment on anaplastic thyroid cancer cells. Thyroid. 2007;17:91–9. doi: 10.1089/thy.2006.0142. [DOI] [PubMed] [Google Scholar]
- 91.Serpe L, Canaparo R, Berta L, Bargoni A, Zara GP, Frairia R. High energy shock waves and 5-aminolevulinic for sonodynamic therapy: effects in a syngeneic model of colon cancer. Technol Cancer Res Treat. 2011;10:85–93. doi: 10.7785/tcrt.2012.500182. [DOI] [PubMed] [Google Scholar]
- 92.Canaparo R, Varchi G, Ballestri M, Foglietta F, Sotgiu G, Guerrini A, et al. Polymeric nanoparticles enhance the sonodynamic activity of meso-tetrakis (4-sulfonatophenyl) porphyrin in an in vitro neuroblastoma model. Int J Nanomedicine. 2013;8:4247–63. doi: 10.2147/IJN.S51070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Foglietta F, Canaparo R, Francovich A, Arena F, Civera S, Cravotto G, et al. Sonodynamic treatment as an innovative bimodal anticancer approach: shock wave-mediated tumor growth inhibition in a syngeneic breast cancer model. Discov Med. 2015;20:197–205. [PubMed] [Google Scholar]