Abstract
Smad4/DPC4, a common signal transducer in transforming growth factor beta (TGF-β) signaling, is frequently inactivated in human cancer. Although the ubiquitin-proteasome pathway has been established as one mechanism of inactivating Smad4 in cancer, the specific ubiquitin E3 ligase for ubiquitination-mediated proteolysis of Smad4 cancer mutants remains unclear. In this report, we identified the SCFSkp2 complex as candidate Smad4-interacting proteins in an antibody array-based screen and further elucidated the functions of SCFSkp2 in mediating the metabolic instability of cancer-derived Smad4 mutants. We found that Skp2, the F-box component of SCFSkp2, physically interacted with Smad4 at the physiological levels. Several cancer-derived unstable mutants exhibited significantly increased binding to Skp2, which led to their increased ubiquitination and accelerated proteolysis. These results suggest an important role for the SCFSkp2 complex in switching cancer mutants of Smad4 to undergo polyubiquitination-dependent degradation.
Loss of transforming growth factor beta (TGF-β) antiproliferative actions is a major hallmark in human cancer (8, 32, 44). TGF-β receptors and the intracellular signal transducers called Smads play a central role in the TGF-β signaling pathway (for the most recent reviews, see references 10 and 43). In response to TGF-β, receptor-regulated Smads (R-Smads) become phosphorylated and form a heterotrimeric complex with the common partner Smad4. The Smad complex is then transported into the nucleus, where it often cooperates with other transcriptional factors to regulate the transcription of TGF-β target genes (9, 31). Emergent evidence has demonstrated that alterations in Smad, particularly the common mediator Smad4, occur frequently in human diseases, including cancers (5, 8, 32, 44). For example, deletion and inactivating mutation of the human Smad4 gene occur in more than 50% of pancreatic cancer cases and in many other cancers (42). Thus, elucidation of the mechanism and functions of Smad4 regulation will provide insight into the role of Smad4 (as well as other Smads) in TGF-β physiology and cancer development.
The ubiquitin-proteasome pathway controls the turnover of many oncoproteins and tumor suppressors, thus playing a major role in cancer development (1, 2, 7, 11, 14). This pathway also regulates the stability of Smads. Recent studies have shown that R-Smads are posttranslationally modified by ubiquitin and can be irreversibly removed from the cell by the proteasome-mediated degradation system (25, 29, 53, 54). Ubiquitination, the covalent attachment of ubiquitin to proteins, predominantly serves to target proteins for degradation by 26S proteasomes (7). Conjugation of ubiquitin is accomplished by an enzymatic cascade involving E1, E2, and E3 enzymes. Ubiquitin E3 ligases play the most significant role in substrate specificity. For example, the HECT-class E3 ligases Smurf1 and Smurf2 as well as their Drosophila homolog, dSmurf, selectively target specific Smads for degradation (24, 25, 37, 53, 54). Smurf2-mediated degradation of Smad2 is a potential mechanism by which cancer cells may escape from TGF-β control. This notion is supported by our observations that Smurf2 inhibits TGF-β signaling (25) and also by the fact that Smurf2 is overexpressed in certain tumors (15). Other major E3 ligase groups, such as the Skp1-Cμl-F-box (SCF) complex, may also be involved in the regulation of Smad proteins. Indeed, a recent study reported the regulation of Smad3 stability by means of the ROC1-SCFFbw1a complex (16).
Our knowledge on the regulation of Smad4 by the ubiquitin-proteasome pathway, unlike that of R-Smads, is rather limited despite the key role of Smad4 in TGF-β signaling. Smad4 forms a heterotrimeric complex with R-Smads in response to TGF-β signals, but its activity is not regulated by TGF-β per se. Smad4 activity may be regulated by stability, as demonstrated by reports showing that the ubiquitin-proteasome pathway rapidly degrades cancer-derived mutants of Smad4 (33, 51). More recently it has been found that Ras and Jab1 promote the proteolytic degradation of Smad4 (39, 48). Recently, our laboratory and others have revealed that Smad4 is modified by SUMO-1, which increases the stability of Smad4 (23, 26, 27). While these are interesting observations, the molecular pathway for the degradation of Smad4 remains enigmatic. Furthermore, no connection has been established between specific ubiquitin E3 ligases and the rapid degradation of cancer-derived Smad4 mutants.
SCF ubiquitin E3 ligases are composed of Skp1, Cul-1, a Roc/Rbx RING finger protein, and a modular F-box protein. The F-box protein functions as a targeting subunit to recognize its substrate and thus determines the specificity in ubiquitination. Skp2 is the F-box component of the SCF ubiquitin E3 ligase that specifically recognizes and binds to its substrate (e.g., p27). Interestingly, Skp2 may function as an oncogene in cancer development (3, 17, 19, 45, 52). Here we report that Skp2 controls Smad4 degradation through the ubiquitination-proteasome pathway. Furthermore, we found that Smad4 mutants that naturally occur in cancer exhibited increased binding to Skp2 and that their degradation was consequently accelerated. Our findings identify a novel switching mechanism for mutation-induced SCFSkp2-mediated degradation of cancer-derived Smad4 mutants.
MATERIALS AND METHODS
Expression plasmids.
Mammalian expression plasmids for epitope (hemagglutinin [HA], Flag, or His)-tagged Smad4 have been described previously (26, 27). HA-ubiquitin was a gift from Bert O'Malley (35). Smad4 K507A and the cancer-derived G65V and L43S mutants were provided by Aristidis Moustakas (34). The Smad4 T276A mutant was a gift from Herbert Lin (38). Skp2 cDNA, beta-transducin repeat-containing protein (βTRCP) cDNA, and constructs for making recombinant SCFSkp2 proteins were provided by Wade Harper (20, 40). Skp2ΔF constructs were from Lars-Gunnar Larsson (47) and Williams Tansey (22).
Antibody array-based screen.
Exponentially growing 293T cells in one 15-cm dish were transfected with the expression plasmid for Flag-Smad4. Two days after transfection, cells were harvested in 5 ml of Flag lysis buffer (13). Cell extracts were added to a membrane filter arrayed with 400 antibodies (Hypromatrix), which was preblocked in a buffer containing 5% nonfat milk. After overnight incubation at 4°C, the presence of the antibody-antigen-Smad4 complex was detected by horseradish peroxidase-conjugated anti-Flag antibody, followed by chemiluminescence.
Ni-NTA precipitation, immunoprecipitation, and Western blotting.
For Ni-nitrilotriacetic acid (NTA) precipitation, HeLa cells were transfected with expression plasmids for His-tagged Smad4 and HA-tagged ubiquitin and Skp2 as specified in Fig. 5A. Forty-eight hours after transfection, cells were harvested and sonicated in guanidinium lysis buffer (6 M guanidinium HCl, 0.1 M NaPO4, 0.01 M Tris-Cl, pH 8). His-tagged Smad4 was then immobilized on Ni-NTA beads (Qiagen) and, after extensive washing, eluted in elution buffer (200 mM imidazole, 5% sodium dodecyl sulfate [SDS], 0.15 M Tris [pH 6.7], 30% glycerol, 0.72 M β-mercaptoethanol). Eluted proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE), transferred onto nitrocellulose (Schleicher and Schuell), and detected with an anti-His antibody (Santa Cruz Biotech) or anti-HA antibody (Roche) for Smad4-ubiquitin conjugation. Antibody-bound proteins were visualized by horseradish peroxidase-conjugated secondary antibody followed by chemiluminescence (Pierce).
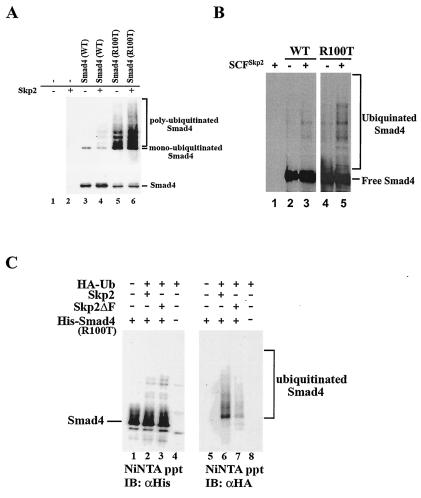
FIG. 5.
Skp2 promotes polyubiquitination of R100T. (A) Skp2 promotes polyubiquitination of the R100T mutant in vivo. HeLa cells were transfected with His-tagged wild-type Smad4 or Smad4 R100T and HA-ubiquitin. His-Smad4 was pulled down from cell lysates with nickel-NTA-agarose beads (Ni-NTA ppt) and then subjected to SDS-PAGE and Western blotting analyses with anti-HA antibodies to detect the pool of ubiquitinated Smad4 (upper). Levels of Smad4 in cells were detected with anti-His antibody (bottom). (B) Skp2 induces polyubiquitination of the R100T mutant in vitro. Ubiquitination of recombinant Smad4 or R100T was carried out as described in Materials and Methods. (C) The F box is critical for Smad4 ubiquitination. In vivo ubiquitination of R100T was done as described for panel A. Skp2ΔF has a deletion of the F box.
For Smad4-Skp2 interactions with immunoprecipitation (Fig. 2A), 293T cells were transfected with expression plasmids for differentially tagged Skp2 and Smad4 variants. Cells were harvested in Flag lysis buffer 48 h after transfection (13). To detect Skp2-bound Smad4, the cell lysates were subjected to immunoprecipitation with an appropriate antibody, as indicated in Fig. 2A and 2B, to retrieve the Smad4-Skp2 complex. Immunoprecipitation products were then subjected to Western blotting, as similarly described (12).
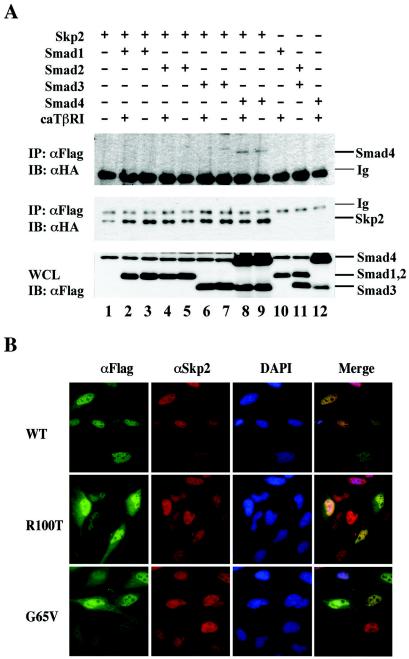
FIG. 2.
Skp2 specifically binds to Smad4. (A) Skp2 interacts with Smad4 but not other Smads. 293T cells were transfected with Flag-tagged Skp2 and HA-tagged Smads. Cell lysates were immunoprecipitated (IP) with anti-Flag antibody (M2; Sigma), followed by immunoblotting (IB) with an anti-HA antibody to detect Skp2-bound Smad (upper panel) and the level of immunoprecipitated Skp2 (middle panel). Whole-cell lysates (WCL) were also directly immunoblotted with anti-HA antibody to demonstrate expression of transfected Smads (lower panel). (B) Skp2 and Smad4 colocalize in the nucleus. HeLa cells were transfected with Flag-Smad4 (wild type, R100T, or G65V). Forty-eight hours after transfection, cells were fixed, blocked, and immunostained with appropriate antibodies, as indicated in Materials and Methods. WT, wild-type Smad4. 4′,6′-Diamidino-2-phenylindole (DAPI) stained DNA in the nucleus.
For endogenous Smad4-Skp2 interactions (Fig. 1B), 293T or HeLa cells were lysed in Flag buffer and immunoprecipitation was carried out with polyclonal antibodies for Skp2 (Zymed) or Smad4 (Santa Cruz Biotech) or a control antibody. The immunocomplex was blotted with anti-Smad4 monoclonal antibody B8 (Santa Cruz Biotech).
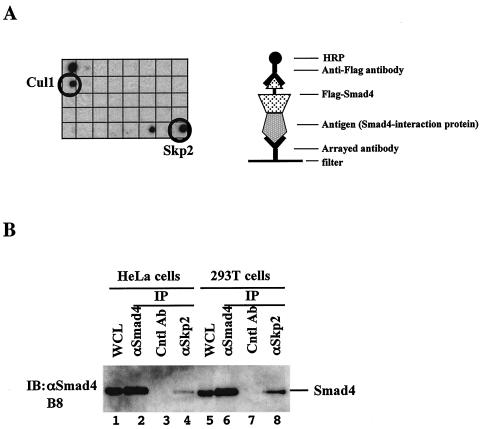
FIG. 1.
Smad4 interacts with Skp2 under physiological conditions. (A) Identification of Skp2 and Cul1 as Smad4-interacting proteins in an antibody array-based screen. A nitrocellulose membrane filter containing antibodies against 400 signal transduction proteins were blotted with extracts from 293T cells transfected with Flag-tagged Smad4. Assembly of a multiprotein complex involving the arrayed antibody, its antigen (potential Smad4-binding protein), and Smad4 was recognized by immunoblotting with a horseradish peroxidase-conjugated anti-Flag antibody and visualized by chemiluminescence. Only 1/10th of the filter with Cul1 and Skp2 antibodies is shown. (B) Endogenous interaction between Smad4 and Skp2. Exponentially growing HeLa cells and 293T cells were subjected to immunoprecipitation (IP) with polyclonal antibodies against Smad4 or Skp2 or a control antibody. Skp2-bound Smad4 was detected by anti-Smad4 (B8) Western blotting (IB). The levels of Smad4 in the immunoprecipitate and whole-cell lysate (WCL) are also shown.
Pulse-chase experiment.
HeLa cells were transfected with His-tagged Smad4 (wild type or G65V or R100T mutant) and Skp2; 48 h later, cells were pulsed for 30 min with 400 μCi of [35S]methionine-cysteine per ml and then chased in unlabeled medium supplemented with nonradioactive methionine-cysteine for various time periods, as indicated in Fig. 6D and E. Cell lysates were harvested and subjected to Ni-NTA precipitation. The precipitated Smad4 proteins were analyzed on SDS-PAGE and visualized by autoradiography.
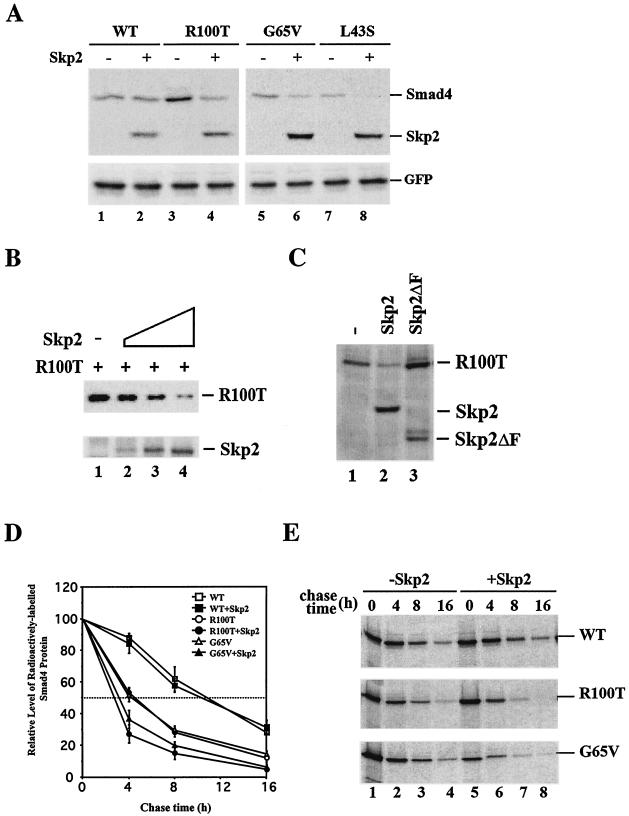
FIG. 6.
Skp2 promotes proteolysis of cancer-derived Smad4 mutants. (A) Skp2 reduces the steady-state level of cancer-derived Smad4 mutants. Flag-Skp2 and Flag-Smad4 (wild type or mutants) were transfected into HeLa cells. Cell lysates were subjected to anti-Flag Western blotting. GFP was included as a control for internal expression and equal loading. (B) Skp2 reduces the steady-state level of R100T. 293T cells were transfected with Flag-Smad4 R100T and an increasing dose of Skp2. Cell lysates were subjected to anti-Flag (for R100T) and anti-Skp2 Western blotting. (C) Skp2 mediates Smad4 degradation dependent of the F box. 293T cells were transfected with Flag-Skp2 (Skp2 or F box deletion mutant) and Flag-Smad4 (wild type and mutants). Cell lysates were subjected to anti-Flag Western blotting. (D and E) Skp2 reduces the half-lives of cancer-derived Smad4 mutants. The pulse-chase conditions are described in Materials and Methods. Three experiments were carried out. A representative is shown in panel E.
GST in vitro binding and pulldown assays.
The in vitro binding assay was carried as previously described (28). Briefly, 35S-labeled Smad4 proteins produced in vitro with a TNT kit (Promega), were incubated with 1 μg of glutathione S-transferase (GST) or GST-Skp2, as indicated in Fig. 2B and 3D. GST-Skp2-bound Smad4 variants were retrieved with glutathione-Sepharose beads at 4°C for 1 h. After extensive washing of GST fusions and binding proteins in 25 mM Tris-HCl (pH 8.0)-300 mM NaCl-1% Triton X-100, Skp2-bound Smad4 was separated by SDS-PAGE and visualized by autoradiography.
FIG. 3.
Skp2 exhibits stronger binding to cancer-derived Smad4 mutants. (A) Skp2 strongly interacts with R100T. 293T cells were transfected with Flag-tagged Skp2 and His-tagged Smad4 wild-type or R100T mutant. Immunoprecipitation-Western analysis was conducted as described for Fig. 2A. Ig, antibody heavy chain. WCL, whole-cell lysate. (B) βTRCP also binds to R100T. Transfection with Flag-Smad4 (wild type or R100T), HA-Skp2, and HA-βTRCP in 293T cells and subsequent immunoprecipitation-Western analysis were done as for panel A. (C) Skp2 strongly interacts with two other cancer-derived mutants. Immunoprecipitation-Western analysis was conducted as described for Fig. 2A except that anti-HA and anti-Flag antibodies were used for immunoprecipitation and immunoblotting, respectively. (D) Skp2 binds to Smad4 cancer mutants directly in vitro. The in vitro binding assay was done as described in Materials and Methods. WT, wild-type Smad4; Myc, positive control; GFP, negative control.
In vitro ubiquitination.
In vitro ubiquitination assays were performed as previously described (50). Recombinant baculoviruses expressing GST-Skp1, Cul1, Roc1, and Flag-Skp2 were kindly provided by Wade Harper. Baculoviruses were amplified in Sf9 cells, and coinfections were carried out in Hi-Five cells (Invitrogen). The SCF complex was purified by GST affinity chromatography and stored in QA buffer at −80°C (50). A typical ubiquitination reaction was carried out in a final volume of 30 μl containing 250 ng of E1 (Boston Biochemical), 3 μg of 6His-cdc34, 5 μg of SCFSkp2 complex, 1 μg of ubiquitin, 1.5 μg of methylated ubiquitin (Boston Biochem), 1 μM ubiquitin aldehyde, 2 μl MG132, 2.5 μl of 10× ER (50), and 1 mM dithiothreitol. The reaction mixture was incubated at 30°C for 2 h. Anti-Smad4 Western blot analysis was used to evaluate the results.
In vitro kinase assay.
The in vitro kinase assay was performed according to the manufacturer's instructions (Upstate Biotech). One microgram of either purified Smad4 protein (wild type or R100T) or GST-fused Smad4 protein on glutathione-Sepharose beads was incubated with 40 to 100 ng of recombinant active kinase proteins (Upstate), 100 μM unlabeled ATP in the absence or presence of 5 μCi of [γ-32P]ATP (Amersham) for 20 min at 30°C. The samples were subjected to SDS-PAGE, and phosphorylation of Smad4 protein was detected by antiphosphoserine Western blotting (Zymed) or autoradiography.
RNA interference.
The Skp2 short interfering RNA (siRNA) (GCAAAGGGAGTGACAAA) was from Dharmacon Research. The siRNA was transfected into AsPC1 cells with Lipofectamine 2000 (Invitrogen), as described before (27), and 48 h after siRNA transfection, cells were harvested for anti-Smad4 or anti-Skp2 Western blotting analysis.
Immunofluorescence.
HeLa cells transfected as specified in the text and figure legends (Fig. 2B) were grown on coverslips, fixed with cold methanol, and blocked with 2% bovine serum albumin in phosphate-buffered saline, pH 7. Cells were then stained with anti-Flag polyclonal (Sigma) or anti-Skp2 monoclonal (Zymed) antibodies, followed by fluorescein isothiocyanate-conjugated anti-rabbit or Texas Red-anti-mouse immunoglobulin antibody, and examined with a Zeiss Axioplan II microscope.
RESULTS
Smad4 and Skp2 interact under physiological conditions.
To search for novel regulators of Smad4, we carried out a protein interaction screen with an antibody-based array method. The array antibodies recognize specific antigens and also coprecipitate proteins (e.g., Smad4) that are associated with the antigen in the total cell lysate. To increase the abundance of Smad4, and thus its chance of binding to potential interacting proteins, we transfected a Flag-tagged Smad4 cDNA into 293T cells. Total cell lysates from transfected cells were incubated with a commercially available membrane filter spotted with antibodies against 400 different signal-transducing proteins. Immunodetection with anti-Smad4 recognizes Smad4 that binds to the specific antigen-antibody complex on the array. Of the 400 antibodies (against signal transducers and transcription factors) screened, approximately 20 candidate proteins were identified as proteins that potentially interact with Smad4. Skp2 and Cul1 were among these candidate Smad4-interacting proteins (Fig. 1A). Skp2 and Cul1 are components of the SCFSkp2 E3 ligase complex, suggesting their potential role in the regulation of Smad4 stability.
Skp2 is the substrate-binding unit of SCFSkp2 E3 ligase.
We next evaluated the ability of endogenous Smad4 to interact with endogenous Skp2. Proteins extracted from HeLa and 293T cells were immunoprecipitated with either anti-Skp2 or a control polyclonal antibody. Immunoprecipitated proteins were analyzed by Western blotting with an anti-Smad4 monoclonal antibody. As shown in Fig. 1B, endogenous Smad4 was present in the anti-Skp2-immunoprecipitated complex in both the HeLa and 293T cell extracts. Furthermore, as a control, unrelated antibody immunoglobulin G did not pull down Smad4 (Fig. 1B). Thus, Smad4 interacts with Skp2 at physiological levels.
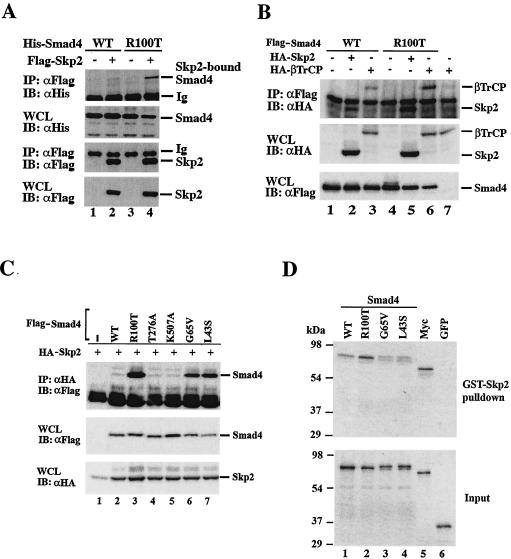
To verify that Skp2 is a true SMAD4-interacting protein, we performed a coimmunoprecipitation experiment. Mammalian expression plasmids for Flag-Skp2 and HA-labeled Smads 1 to 4 were transfected into 293T cells. Formation of the Skp2-Smad4 immunocomplex was detected by immunoprecipitation with anti-Flag antibody, followed by an anti-HA Western blot. In contrast, the R-Smads tested, Smad1, Smad2, and Smad3, could not interact with Skp2 (Fig. 2A). Furthermore, the interaction appeared to be independent of TGF-β receptor activation (Fig. 2A). These results demonstrate the specific association between Smad4 and Skp2.
Smad4 is a nucleocytoplasmic shuttling protein. We next investigated the subcellular location of the Skp2-Smad4 interaction. For this purpose, we carried out an immunofluorescence assay to determine whether the Smad4 and Skp2 proteins colocalize in cells. HeLa cells were transfected with Skp2 and Smad4 (wild type or cancer-derived R100T and G65V mutants). As shown in Fig. 2B, transfected Smad4 (wild type) was present in the nucleus, while two cancer-derived Smad4 mutants, R100T and G65V, were found in both the nucleus and the cytoplasm. The mutants carry an Arg-to-Thr mutation at amino acid 100 (R100T) or a Gly-to-Val mutation at amino acid 65 (G65V). Since the mutations cause rapid degradation of the mutant proteins in a proteasome-dependent manner (51), the transfected cells were treated with the proteasomal inhibitor MG132 to visualize the mutants in the cells. After leptomycin B treatment, the cells expressing these mutants stained only in the nucleus, suggesting that these mutants could undergo nuclear import (data not shown). Importantly, staining of Smad4 proteins (wild type or mutant) overlapped that of Skp2, which is a nuclear protein. These results further support the notion that Skp2 may serve as a ubiquitin E3 ligase for colocalized Smad4 proteins.
Unstable cancer-derived mutants of Smad4 display stronger binding to Skp2.
While some mutations inactivate the signaling activity of Smad4 protein, others, such as the R100T mutation, render it unstable. Since we had shown that Skp2 bound to Smad4, we sought to test whether this mutant had higher affinity to Skp2. Interestingly, we found that in 293T cells, anti-Flag(Skp2) immunoprecipitation could pull down not only Flag-tagged Skp2 (Fig. 3A, bottom, lanes 2 and 4), but also His-tagged Smad4 (Fig. 3A, upper, lanes 2 and 4). The level of immunoprecipitated R100T mutant (lane 4) was much higher than that of wild-type Smad4 (lane 2), which was barely detectable. A longer exposure of the blot clearly confirmed the interaction of wild-type Smad4 with Skp2 (data not shown). Thus, the unstable nature of the R100T mutant correlates well with its strong binding to Skp2, suggesting that SCFSKP2 serves as a ubiquitin E3 ligase for the Smad4 cancer mutant.
We also tested whether βTRCP, another Smad4-binding F-box protein (49), interacted with the R100T mutant. The βTRCP-R100T interaction was assessed by immunoprecipitation-coupled Western blotting analysis. The results in Fig. 3B clearly showed that, like Skp2, βTRCP also exhibited stronger binding to R100T mutant than did wild-type Smad4. In addition, overexpression of βTRCP also resulted in rapid degradation of Smad4 mutants (data not shown). The data suggest that Skp2 and βTRCP may both act as E3 ligases for degradation of Smad4 cancer mutants. However, our current study focused on the functions of Skp2.
To explore the possibility that increased binding to Skp2 is a common attribute of other unstable mutants of Smad4 derived from cancer cells, we tested the ability of two other mutants to interact with Skp2 in a coimmunoprecipitation assay. Smad4 G65V has a single Gly→Val substitution at amino acid 65 that was first identified in colorectal cancer (30), while L43S possesses a Leu→Ser mutation at amino acid 43 and is present in pancreatic cancer (21). Both G65V and L43S have a shorter half-life than wild-type Smad4 (33). Interestingly, the data in Fig. 3C indicate that both mutants exhibited a strong interaction with Skp2 which was comparable to the R100T-Skp2 interaction. In sharp contrast, synthetic mutants T276A and K507A had a very weak interaction with Skp2 (Fig. 3C). This suggests that increased binding to Skp2 might be responsible for the rapid turnover of the cancer-derived Smad4 mutants under study.
To establish whether Smad4 interacts with Skp2 directly, we carried out GST pulldown binding assays. The results in Fig. 3D clearly demonstrated that GST-Skp2 immobilized on glutathione-agarose beads could retrieve radioactively labeled in vitro-translated Smad4 but not green fluorescent protein (GFP) (a negative control). The level of Skp2-bound R100T was somewhat higher (about twice) than that of Skp2-bound wild-type Smad4, but not to the extent that we observed in vivo (Fig. 3A and C). Furthermore, Skp2 appeared to interact with the G65V and L43S mutants as with the wild type. These results suggest that the increased binding of Skp2 to Smad4 mutants in vivo may be primarily attributed to differential posttranslational modifications or subcellular localization of mutated Smad4 molecules.
Both F-box and LRR motifs mediate Skp2 interaction with Smad4.
Skp2 has a Skp1-binding F-box, three atypical leucine-rich repeats (LRRs), and the C-terminal seven typical LRRs. Skp2 binds to the substrate through its C-terminal seven LRRs (40). To determine whether this also holds true for Smad4 binding, we constructed a series of Skp2 constructs that harbored deletions of different domains. We next determined the ability of these deletion mutants to interact with R100T.
It was anticipated that use of R100T, which binds to Skp2 more strongly than wild-type Smad4 (Fig. 3A and C), should facilitate easy detection of the Smad4-Skp2 interaction. As expected, seven LRRs (as in amino acids 207 to 390 or 207 to 424) were sufficient to bind to R100T (Fig. 4A, lanes 5 and 3). Surprisingly, we found that deletion of seven LRRs did not abolish the capacity of Skp2 to interact with R100T (Fig. 4A, lane 2). Like seven LRRs, the F-box was not essential for binding to R100T (lane 6). Furthermore, the Skp2 mutant (amino acids 1 to 142) with removal of both the three LRRs and seven LRRs, still retained the ability to interact with R100T (Fig. 4A, lane 10). Further deletion of the F-box from amino acids 1 to 142 abolished binding to R100T, as seen in the Skp2 mutant (amino acids 1 to 96) (Fig. 4A, lane 15). The Smad4-binding capacity of the Skp2 deletion mutant (amino acids 1 to 142) but not the Skp2 deletion mutant (amino acids 1 to 96) was also reproduced in an in vitro binding assay (data not shown). These results suggest that besides seven LRRs, the usual substrate-binding motif, other regions of Skp2 are also in contact with Smad4 protein (Fig. 4C).
FIG.4.
Smad4 MH1 domain mediates its interaction with multiple regions of Skp2. (A) Mapping of Smad4-interacting domain on Skp2. 293T cells were transfected with Flag-tagged Smad4(R100T) and HA-tagged Skp2 deletion constructs. Skp2-bound Smad4 was detected with anti-HA immunoprecipitation coupled with anti-Flag immunoblotting. The levels of transfected proteins in whole-cell lysates (WCL) are shown (bottom panels). (B) Mapping of Skp2-interacting domain on Smad4. 35S-labeled Smad4 deletion mutants were generated with the TNT kit (Promega) and then incubated with immobilized GST-Skp2 protein. Smad4 fragments bound to Skp2 (left) and their inputs (right) are shown. (C) Schematic diagrams of Skp2 and Smad4 constructs. Deletion mutants of Skp2 and Smad4 are shown with color-coded domains. For Skp2, F indicates the F box and LRR indicates leucine-rich repeats. For Smad4, the MH1, linker, and MH2 domains are shown. The Skp2-Smad4 interaction is summarized on the right.
To map the Smad4 domain that interacts with Skp2, we performed a GST in vitro binding experiment with deletion mutants of Smad4. The interaction of these mutants with Skp2 is shown in Fig. 4B. Smad4N(1-185) and Smad4NL(1-323) bound to Skp2 (lanes 3 and 7). In contrast, deletion of the MH1 or N domain (as in the L, C1, and C2 mutants) abolished the ability to interact with Skp2 (lanes 4 to 6). Therefore, Smad4 interacted specifically through its MH1 domain with Skp2 (Fig. 4C).
SCFSkp2 induces ubiquitination of Smad4 cancer mutants.
Based on our observations that Skp2 displayed a stronger binding to Smad4 mutants (Fig. 3A and C), we further investigated whether Skp2 induced increased ubiquitination of these Smad4 mutants with R100T as an example. We transiently transfected HeLa cells with 6×His-Smad4, Flag-tagged Skp2, and HA-tagged ubiquitin (wild type). His-Smad4 was precipitated with Ni-NTA-agarose from cell lysates under denaturing conditions (to remove nonspecific Smad4-binding proteins), followed by anti-HA Western blotting. In agreement with an earlier report (27, 33, 51), R100T exhibited a high level of polyubiquitination in comparison to wild-type Smad4 (Fig. 5A, lanes 3 and 5). While the level of wild-type Smad4 ubiquitination increased moderately in cells overexpressing Skp2 (Fig. 5A, lane 4), coexpression of Skp2 induced a marked increase in polyubiquitination of R100T (Fig. 5A, lane 6).
To establish whether wild-type Smad4 or R100T is a direct substrate of SCFSkp2, in vitro assays were performed to determine whether SCFSkp2 mediates polyubiquitination of recombinant Smad4. Cell-free SCFSkp2complexes were assayed for the ability to attach polyubiquitin chains to recombinant wild-type Smad4 or the R100T mutant. As shown in Fig. 5B, the SCFSkp2 complex were capable of polyubiquitinating both wild-type Smad4 and R100T, suggesting that Smad4 and its mutant are a direct ubiquitination target of SCFSkp2 (lanes 3 and 5). The level of polyubiquitination of R100T was higher than that of wild-type Smad4. Furthermore, we compared the effect of a Skp2 F-box deletion mutant with that of wild-type Skp2. Skp2ΔF (the F-box deletion mutant) was unable to promote R100T polyubiquitination as efficiently as wild-type Skp2, emphasizing the importance of Skp2 F-box integrity in R100T ubiquitination (Fig. 5C, lane 7).
Skp2 promotes the degradation of Smad4 cancer mutants.
To confirm a relationship between SCFSkp2-mediated polyubiquitination and Smad4 degradation, we investigated whether SCFSkp2 could induce faster degradation of the cancer mutants. Expression plasmids for wild-type Smad4 and three Smad4 mutants were cotransfected with or without Skp2 into HeLa cells. Steady-state levels of Smad4 variants were assessed by Western blotting. Since Smad4 mutants are unstable proteins, larger amounts of their cDNAs were used for transfection. As illustrated in Fig. 6A, coexpression of Skp2 significantly reduced the levels of all three cancer mutants, whereas it had no effect on the steady-state level of wild-type Smad4. Furthermore, Skp2 promoted the degradation of R100T (Fig. 6B) and the other mutants (data not shown) in a dose-dependent manner.
In addition, the ability of Skp2 to degrade R100T was dependent on F-box integrity, since Skp2ΔF (the F-box deletion mutant) was unable to reduce the level of R100T protein (Fig. 6C). This observation is consistent with the data in Fig. 5C, showing that Skp2ΔF is unable to promote R100T polyubiquitination as efficiently as wild-type Skp2, emphasizing the importance of Skp2 F-box integrity in R100T degradation.
We next determined whether the diminished steady-state levels of the Smad4 mutants are correlated with their increased turnover. We examined the effect of Skp2 on the metabolic stability of Smad4, R100T, and G65V in a pulse-chase experiment. In the absence of Skp2, the half-life of wild-type Smad4 was around 10 h, whereas the R100T and G65V mutants each had a half-life of only 4 h (Fig. 6D and E), in agreement with previous studies (27, 33, 51). Notably, introduction of exogenous Skp2 shortened the half-lives of R100T and G65V to 2.5 and 3 h, respectively (Fig. 6D and E). This indicates that Skp2 expression reduced the half-lives of the cancer mutants by 25 to 35%, whereas it had no effect on the half-life of wild-type Smad4 (Fig. 6D and E).
Skp2 mediates proteasome-dependent proteolysis of endogenous R100T protein in AsPC1 cells.
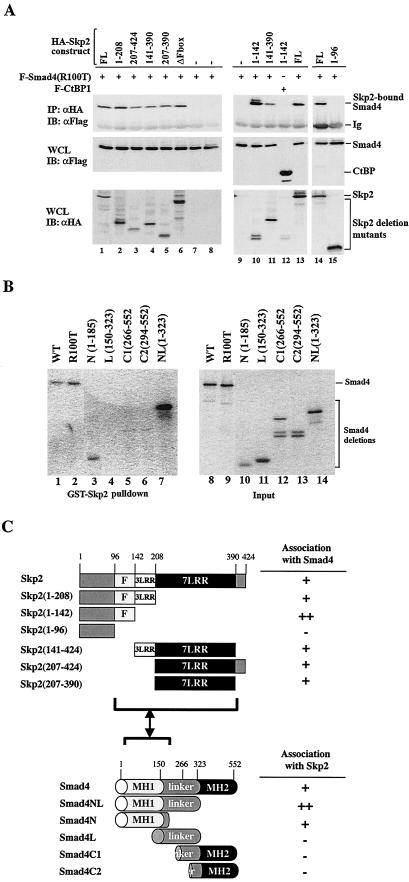
After establishing the role of Skp2 as the E3 ligase for Smad4 cancer mutants in transfection studies, we sought to determine whether depletion of endogenous Skp2 restored the level of Smad4 mutants in cells. To this end, an siRNA for Skp2 was transfected into the pancreatic cancer cell line AsPC1, which naturally carries the R100T mutation in Smad4. As shown in Fig. 7A, siSkp2 significantly reduced the expression of Skp2 by up to 80% (Fig. 7A, bottom). Furthermore, siSkp2 profoundly increased the level of Smad4 (Fig. 7A, top), which correlated inversely with the level of Skp2 protein. In sharp contrast, an irrelevant siRNA against Drosophila Smurf (siLack) had little effect on the Smad4 level (Fig. 7A, top).
FIG. 7.
Inverse correlation between endogenous Skp2 and R100T proteins. (A) Depletion of Skp2 increases the level of endogenous R100T. Pancreatic cancer AsPC1 cells, which carry a natural R100T mutation in Smad4, were transfected with siRNA against Skp2 (siSkp2) or the irrelevant siLack against Drosophila Smurf. The expression levels of Smad4 and Skp2 were assessed by Western blotting with the antibodies indicated. (B) The level of endogenous R100T decreases as the level of Skp2 increases in S phase. AsPC1 cells were serum starved for 24 h to reach a synchronized G0 phase and then stimulated with 20% fetal bovine serum for the indicated time. Cell extracts were subjected to Western blotting analysis with anti-Smad4 or anti-Skp2 antibodies. The percentage of cell cycle phases is shown.
Since Skp2 is an S-phase protein and causes R100T degradation (Fig. 6 and 7A), we next tested whether the steady-state level of the R100T mutant protein fluctuated during the cell cycle. We first synchronized AsPC1 cells by serum starvation and then determined the cell cycle phase by fluorescence-activated cell sorting analysis. As expected, the level of Skp2 protein increased dramatically in the S phase, which corresponds to 12 to 20 h after serum stimulation. Accordingly, the level of the R100T mutant decreased as that of Skp2 increased (Fig. 7B, lanes 4 to 6). Taken together, the results in Fig. 7 clearly demonstrate an inverse correlation between Skp2 and R100T in AsPC1 cells, further suggesting that Skp2 controls the turnover of Smad4 mutants in cancer cells.
JNK/p38 kinase induces serine phosphorylation and proteasomal degradation of R100T protein.
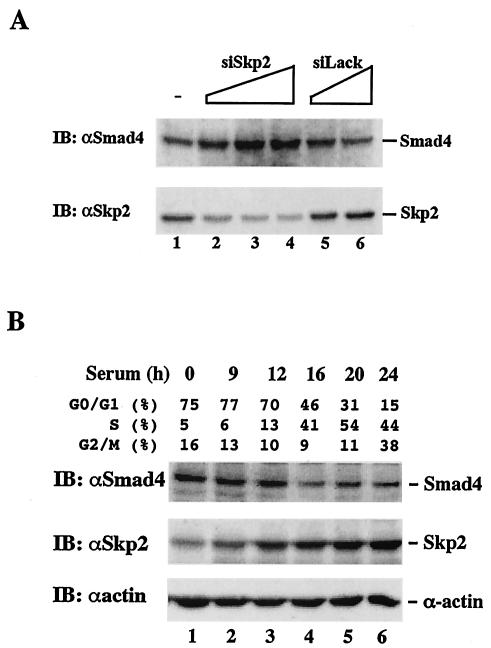
As SCF binds to most substrates in a phosphorylation-dependent manner (18), we wanted to test whether phosphorylation of Smad4 cancer mutants is a prerequisite for their proteasomal degradation. A previous study implicates the involvement of mitogen-activated protein kinase pathways in Ras-mediated Smad4 degradation (39). Thus, we examined whether inhibitors of ERK, Jun N-terminal kinase (JNK), or p38 kinase had any effect on blocking R100T degradation in AsPC1 cells. Consistent with the role of Skp2 in the proteasomal degradation of R100T, proteasomal inhibitor MG132 markedly increased the Smad4 level in AsPC1 cells (Fig. 8A, lane 2). As also shown in Fig. 8A, we found that the inhibition of ERK, JNK, and p38 kinase had different effects. Addition of SB203580 (a specific p38 kinase inhibitor) and JNK inhibitor II significantly increased the accumulation of Smad4 proteins to a level similar to that observed after MG132 addition. In contrast, Smad4 remained scarcely detectable in the presence of the specific MEK1 inhibitor U0126. Interestingly, R100T was also stabilized in the absence of high serum (Fig. 8A, lane 6), likely due to the lack of JNK and p38 kinase activities under serum-free conditions.
FIG. 8.
Proteasome-dependent proteolysis of endogenous R100T protein requires JNK- and p38-mediated phosphorylation of R100T. (A) Inhibition of p38 and JNK kinases and serum starvation markedly increase the level of endogenous R100T. AsPC1 cells were grown in regular medium supplemented with 10 or 0.2% fetal bovine serum and treated with vehicle (dimethyl sulfoxide) or the indicated chemical inhibitor. The expression level of Smad4 is shown. MG132, proteasomal inhibitor; U0126, MEK1 inhibitor; SB203580, p38 kinase inhibitor. (B) JNK/p38 kinase inhibitors reduce Smad4-Skp2 interaction. 293T cell transfection and immunoprecipitation-Western analysis were carried out as described for Fig. 3. SB, p38 kinase inhibitor SB203580. JNKI, JNK inhibitor II. (C) R100T is phosphorylated in vivo. 293T cells were transfected with His-tagged Smad or Skp2 constructs, and Ni-NTA precipitation (ppt) and Western blotting were carried out as described for Fig. 5A and in Materials and Methods except that an antiphosphoserine antibody (αPS; Zymed) was used to detect phospho-Smad4 (P-Smad4). (D) JNK and p38 kinase inhibitors inhibit R100T that is phosphorylated in vivo. Phosphoserine Western blotting was done as described for panel C. (E and F) Recombinant JNK phosphorylates Smad4 in vitro. (E) Bacterially expressed recombinant Smad4 was incubated with recombinant JNK (Upstate) in the presence or absence of JNK inhibitor II. The reaction was then subjected to Western blotting with phosphoserine antibody. (F) In vitro phosphorylation was carried out as in panel E except in the presence of radioactive [32P-γ]ATP. Phosphorylated Smad4 was visualized by autoradiography.
To provide further evidence for the role of the JNK and p38 kinases in promoting the degradation of Smad4 mutants, we determined whether the JNK and p38 kinase activities were important for Smad4-Skp2 interaction. 293T cells were first transfected with HA-Skp2 and Flag-tagged Smad4 (wild type or R100T) and treated with JNK and p38 kinase inhibitors, and an immunoprecipitation-Western assay was then performed to detect the presence of Smad4-associated Skp2. The results in Fig. 8B showed that the JNK and p38 inhibitors reduced the binding of R100T to Skp2, although the p38 inhibitor was less potent. The data suggest that JNK-mediated phosphorylation of the mutant precedes its Skp2 binding.
We next determined whether the R100T mutant was preferentially phosphorylated in cells. As shown Fig. 8C, the R100T mutant was strongly phosphorylated on serine residues (lane 2), whereas the phosphorylation of wild-type Smad4 or Smad3 was not detectable (lane 1 and 3). We noticed that the phosphoserine antibody (Zymed) recognized multiple retarded bands, indicating the presence of multiple serine phosphorylation sites on R100T as well as the G65V and L43S mutants (Fig. 8C and D). In addition, JNK and p38 kinase inhibitors reduced the level of R100T phosphorylation (Fig. 8D, lane 7 and 8). Furthermore, JNK and p38 kinases (data not shown) could directly phosphorylate the R100T mutant in an in vitro assay (Fig. 8E and F). Conversely, addition of the JNK inhibitor abolished the JNK-dependent phosphorylation of the recombinant R100T protein in antiphosphoserine Western blot analysis (Fig. 8E) and the in vitro 32P-labeled kinase assay (Fig. 8F).
Taken together, these results suggest an important role of the JNK and p38 kinases in the regulation of stability of Smad4 mutants in cancer cells. Mutants, particularly those carrying mutations in the MH1 domain, were preferentially phosphorylated by JNK or p38 kinase, likely on serine residues. JNK- and p38-mediated phosphorylation leads to increased binding of the mutants to the SCF E3 complex and ultimately causes rapid destruction of these mutants in cancer cells.
DISCUSSION
Smad proteins play a key role in cell growth arrest in response to TGF-β. Aberrations in TGF-β signaling, from dysfunction of receptors to Smad inactivation, can lead to deregulated cell proliferation or extracellular matrix deposition. Emergent evidence demonstrates that alterations in the common TGF-β effecter Smad4 occur frequently in human cancers (41). Thus, elucidating the mechanism of Smad4 regulation and its possible functions will provide insight into the role of Smad4 in tumorigenesis. Previous studies have shown that a number of Smad4 missense mutations found in cancer cause their accelerated proteolysis (27, 33, 51). Still, the E3 ligase that targets Smad4 cancer mutants for rapid destruction has never been identified. In this study, we ascertained a physiological association of Smad4 with the ubiquitin E3 ligase complex SCFSkp2, and we provide strong evidence for the role of SCFSkp2 in mediating rapid turnover of Smad4 cancer mutants.
SCFSkp2 is a ubiquitin E3 ligase for Smad4 mutants in cancer.
Identification of SCFSkp2 as an E3 ligase for Smad4 cancer mutants is consistent with the function of UbcH3/Cdc34, the E2 enzyme associated with Rbx1 in the SCF complex, in the degradation of Smad4 cancer mutants. A dominant negative mutant of UbcH3 inhibits ubiquitination and subsequent proteasomal degradation of Smad4 cancer mutants (33). In addition, our findings that Skp2 promotes ubiquitin-dependent degradation of Smad4 cancer mutants is consistent with both the oncogenic roles of Skp2 (3, 17, 19, 45, 52) and the tumor suppressor functions of Smad4 (41).
Skp2 controls the turnover of several key cell cycle regulatory proteins, including the tumor suppressor proteins retinoblastoma-related p130 and the cyclin-dependent kinase inhibitor p27Kip1 (4, 6, 46). Skp2 is the F-box component of the SCFSkp2 complex, which consists of Rbx1, Cul1, and Skp1, and it is the subunit that directly binds to substrates through its LRR motif. It has previously been shown that the level of Skp2 is inversely correlated with that of p27Kip1 in oral, colorectal, breast, and prostate cancers (3, 17, 19, 45, 52). Upregulation of Skp2 expression can thus contribute to tumorigenesis by initiating an increased turnover of growth inhibitors.
Our present work points to a novel mechanism for degrading the tumor suppressor Smad4. Unlike p27, which is rarely mutated in cancer, Smad4 is deleted or mutated in more than half of pancreatic cancers and in about one third of colon cancers with microsatellite instability (42). While some mutations inactivate the transcriptional capacity of Smad4, certain missense mutations in cancer cells, i.e., R100T, G65V, and L43S, probably introduce conformational changes to Smad4 which make it more susceptible to degradation. Our work suggests that these conformational changes have uniquely caused an increased affinity to the E3 ligase SCFSkp2. Without an increase in the Skp2 level, the increased binding to SCFSkp2 suffices to wipe out these mutants from cancer cells.
The same or a similar mechanism may operate for βTRCP-mediated regulation of the stability of Smad4 mutants. Like Skp2, βTRCP is also an F-box component of an SCF class E3 ligase and targets cell cycle regulators such as cdc25A, β-catenin, and IκBα for ubiquitin-dependent degradation (20; reviewed in references 7, 11, 14, and 18). After the original submission of our manuscript, Wan et al. reported that βTRCP promotes the turnover of wild-type Smad4 protein and inhibits basal TGF-β signaling (48). Interestingly, here we found that βTRCP also strongly binds to the cancer-derived mutants of Smad4 (Fig. 3B) and contributes to their rapid proteolysis in cells (data not shown), suggesting the existence of multiple ubiquitin E3 ligases for Smad4 mutants in cancer cells. Nonetheless, preferential binding and subsequent destruction of cancer-derived Smad4 mutants by SCFSkp2 and/or SCFβTRCP provides a growth advantage to cancer cells, since some of the mutants, such as G65V, are still able to partially transduce TGF-β signals when present (34).
Interplay among posttranslational modifications of Smad4: ubiquitination, phosphorylation. and sumoylation.
Substrate binding by F-box proteins is often facilitated by phosphorylation of the substrate (18). Prior phosphorylation is obviously not a requirement for Skp2 binding to normal Smad4 protein, as the highly purified recombinant Smad4 and Skp2 are capable of binding to each other in a cell-free system (data not shown). A few other Skp2 substrates, e.g., c-Myc and E2F1, bind to Skp2 independently of phosphorylation (22, 47). Conversely, our study demonstrates that Smad4 cancer mutants are preferably modified in cells and consequently possess higher affinity to Skp2. We observed that these mutants sometime migrate as a doublet, indicating possible phosphorylation.
Stabilization of endogenous R100T by p38 and JNK inhibitors further supports the notion that p38 and JNK kinases may induce phosphorylation of Smad4 mutants (Fig. 7B). Indeed, an antiphosphoserine antibody (Zymed) recognized the upper bands of R100T. These phosphorylated species of R100T disappeared after cells were treated with a JNK inhibitor (Fig. 8D). In a cell-free assay, JNK also phosphorylated recombinant Smad4, which could be completely inhibited by a JNK inhibitor (Fig. 8 and F). Furthermore, these inhibitors also blocked the degradation of transfected cancer mutants in the presence of Skp2 or Ras (data not shown). Oncogenic Ras has been reported to promote Smad4 degradation, likely through the MEK/ERK pathway, in transformed rat intestinal epithelial cells (39). Although we were unable to detect proteolysis of wild-type Smad4 by either Skp2 or Ras in HeLa or 293T cells, Skp2-mediated degradation of Smad4 cancer mutants appears to occur on serine residues through the p38 and/or JNK pathway (Fig. 8). A recent work reports the ERK-mediated phosphorylation of Smad4 at T277, but this phosphorylation appears to play a positive role in Smad4 signaling (38) and thus is unlikely to contribute to Smad4 degradation. Further identification of the phosphorylated serine residues will provide more insight into the proteasomal degradation of Smad4 mutants in cancer.
Recent studies have demonstrated the importance of sumoylation in regulating TGF-β signaling (23, 26, 27). While Smad4 is a sumoylated protein (23, 26, 27), the R100T mutant could not be modified by SUMO-1 (27) but had an increased level of ubiquitination (27, 33, 51). Our preliminary study further extends the inverse correlation between sumoylation and ubiquitination to other cancer mutants, i.e., G65V and L43S (data not shown). While sumoylation occurs in the nucleus and also enhances nuclear retention of wild-type Smad4 (27), the locations where the mutants become ubiquitinated and degraded remain unknown. It has previously been reported that mutants such as R100T and L43S are predominantly localized in the cytoplasm even in the presence of TGF-β, and G65V is also mostly cytoplasmic, although it undergoes partial nuclear import induced by TGF-β (34).
Since Skp2 is primarily localized in the nucleus, how does Skp2 ubiquitinate and degrade these mutants? Our current study demonstrates that MG132 treatment not only causes increases in the total levels of these mutants in cells but also significantly promotes their nuclear localization (Fig. 2B). Leptomycin B, an inhibitor of CRM1-dependent nuclear export, completely rendered these mutants in the nucleus (data not shown). These findings suggest that the cancer mutants have the ability to undergo nuclear localization (Fig. 2B). In the nucleus, where Skp2 is present, the mutants, which are not modified by SUMO (27), may be susceptible to Skp2-mediated ubiquitination and degradation (see our working model in Fig. 9). In agreement with this notion, Skp2 and Smad4 mutants colocalized in the nucleus in the presence of MG132 (Fig. 2B). However, our study has not ruled out the possibility that the Skp2-Smad4 complex may form in the nucleus but that the complex is rapidly exported to the cytoplasm, where Smad4 degradation takes place.
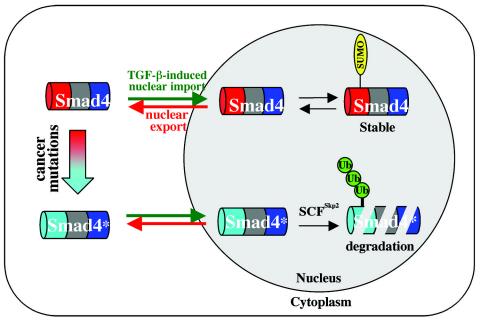
FIG. 9.
Working model for Smad4 regulation by ubiquitination and sumoylation. Smad4 undergoes nuclear import and export. Wild-type Smad4 can be sumoylated in the nucleus, which further enhances its nuclear retention. Cancer-derived mutants of Smad4 (Smad4*), especially those with mutations in the MH1 domain, show increased affinity for SCFSkp2 E3 ligase. SCFSkp2 induces polyubiquitination and mediates proteasome-dependent proteolysis of these mutants.
We have provided compelling evidence for an oncogenic function of the SCFSkp2 complex in the destruction of Smad4 mutants. Still, since Skp2 interacts with normal Smad4 under physiological conditions, one question remains: what is the effect of this interaction on normal TGF-β signaling? Our data indicate that wild-type Smad4 is not significantly affected by Skp2-mediated events. We observed only a mild increase in ubiquitination in vivo and no Skp2-induced reduction in wild-type Smad4 half-life. Thus, the function of Skp2 is somewhat different from that of βTRCP in the regulation of the basal level of wild-type Smad4. While βTRCP promotes the turnover of wild-type Smad4 protein and inhibits basal TGF-β signaling (48), Skp2 does not inhibit the TGF-β-mediated Smad4-dependent induction of reporter gene activity such as the SBE-luc response (data not shown). On the contrary, Skp2 unexpectedly stimulates and siSkp2 suppresses the TGF-β-induced SBE-luc reporter activity (data not shown).
We speculate that this positive effect may be explained by the degradation of an inhibitor of TGF-β signaling. It is also possible that Skp2 participates in the mono-ubiquitination of Smad4. Smad4 has been reported to be monoubiquitinated at Lys-507, which plays a positive role in Smad oligomerization and subsequent signaling (33). An E3 ligase has not yet been identified for Smad4 monoubiquitination. In addition, since Skp2 has been reported to act as a coactivator for Myc protein, Skp2 might potentiate Smad4 transcriptional activity by recruiting the proteasome's nonproteolytic functions to the Smad-DNA complex (22, 47). Nonetheless, the role of Skp2 in normal TGF-β signaling requires further investigation.
Acknowledgments
We thank Bert O'Malley, Aristidis Moustakas, Williams Tansey, Lars-Gunnar Larsson, Herbert Lin, and Wade Harper for many invaluable reagents and Xu Cao for personal communications.
This work was supported by grants from the American Cancer Society (X. Lin and X.-H.F) and National Institutes of Health (X.-H.F., X. Liu, and F.C.B.). X.-H.F. is a Leukemia and Lymphoma Society Scholar.
REFERENCES
- 1.Adams, J. 2003. The proteasome: structure, function, and role in the cell. Cancer Treat. Rev. 29(Suppl. 1):3-9. [DOI] [PubMed] [Google Scholar]
- 2.Bashir, T., and M. Pagano. 2003. Aberrant ubiquitin-mediated proteolysis of cell cycle regulatory proteins and oncogenesis. Adv. Cancer Res. 88:101-144. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Izhak, O., S. Lahav-Baratz, S. Meretyk, S. Ben-Eliezer, E. Sabo, M. Dirnfeld, S. Cohen, and A. Ciechanover. 2003. Inverse relationship between Skp2 ubiquitin ligase and the cyclin dependent kinase inhibitor p27Kip1 in prostate cancer. J. Urol. 170:241-245. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya, S., J. Garriga, J. Calbo, T. Yong, D. S. Haines, and X. Grana. 2003. SKP2 associates with p130 and accelerates p130 ubiquitylation and degradation in human cells. Oncogene 22:2443-2451. [DOI] [PubMed] [Google Scholar]
- 5.Blobe, G. C., W. P. Schiemann, and H. F. Lodish. 2000. Role of transforming growth factor beta in human disease. N. Engl. J. Med. 342:1350-1358. [DOI] [PubMed] [Google Scholar]
- 6.Carrano, A. C., E. Eytan, A. Hershko, and M. Pagano. 1999. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1:193-199. [DOI] [PubMed] [Google Scholar]
- 7.Ciechanover, A., A. Orian, and A. L. Schwartz. 2000. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays 22:442-451. [DOI] [PubMed] [Google Scholar]
- 8.Derynck, R., R. J. Akhurst, and A. Balmain. 2001. TGF-beta signaling in tumor suppression and cancer progression. Nat. Genet. 29:117-129. [DOI] [PubMed] [Google Scholar]
- 9.Derynck, R., Y. Zhang, and X.-H. Feng. 1998. Smads: transcriptional activators of TGF-beta responses. Cell 95:737-740. [DOI] [PubMed] [Google Scholar]
- 10.Derynck, R., and Y. E. Zhang. 2003. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425:577-584. [DOI] [PubMed] [Google Scholar]
- 11.Doherty, F. J., S. Dawson, and R. J. Mayer. 2002. The ubiquitin-proteasome pathway of intracellular proteolysis. Essays Biochem. 38:51-63. [DOI] [PubMed] [Google Scholar]
- 12.Feng, X.-H., Y. Zhang, R.-Y. Wu, and R. Derynck. 1998. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for Smad3 in TGF-β-induced transcriptional activation. Genes Dev. 12:2153-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng, X. H., E. H. Filvaroff, and R. Derynck. 1995. Transforming growth factor-beta (TGF-beta)-induced down-regulation of cyclin A expression requires a functional TGF-beta receptor complex. Characterization of chimeric and truncated type I and type II receptors. J. Biol. Chem. 270:24237-24245. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs, S. Y. 2002. The role of ubiquitin-proteasome pathway in oncogenic signaling. Cancer Biol. Ther. 1:337-341. [PubMed] [Google Scholar]
- 15.Fukuchi, M., Y. Fukai, N. Masuda, T. Miyazaki, M. Nakajima, M. Sohda, R. Manda, K. Tsukada, H. Kato, and H. Kuwano. 2002. High-level expression of the Smad ubiquitin ligase Smurf2 correlates with poor prognosis in patients with esophageal squamous cell carcinoma. Cancer Res. 62:7162-7165. [PubMed] [Google Scholar]
- 16.Fukuchi, M., T. Imamura, T. Chiba, T. Ebisawa, M. Kawabata, K. Tanaka, and K. Miyazono. 2001. Ligand-dependent degradation of smad3 by a ubiquitin ligase complex of roc1 and associated proteins. Mol. Biol. Cell 12:1431-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gstaiger, M., R. Jordan, M. Lim, C. Catzavelos, J. Mestan, J. Slingerland, and W. Krek. 2001. Skp2 is oncogenic and overexpressed in human cancers. Proc. Natl. Acad. Sci. USA 98:5043-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harper, J. W. 2002. A phosphorylation-driven ubiquitination switch for cell-cycle control. Trends Cell Biol. 12:104-107. [DOI] [PubMed] [Google Scholar]
- 19.Hershko, D., G. Bornstein, O. Ben-Izhak, A. Carrano, M. Pagano, M. M. Krausz, and A. Hershko. 2001. Inverse relation between levels of p27(Kip1) and of its ubiquitin ligase subunit Skp2 in colorectal carcinomas. Cancer 91:1745-1751. [DOI] [PubMed] [Google Scholar]
- 20.Jin, J., T. Shirogane, L. Xu, G. Nalepa, J. Qin, S. J. Elledge, and J. W. Harper. 2003. SCFbeta-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 17:3062-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, K., H. Kirkpatrick, A. Comer, F. M. Hoffmann, and A. Laughon. 1999. Interaction of Smad complexes with tripartite DNA-binding sites. J. Biol. Chem. 274:20709-20716. [DOI] [PubMed] [Google Scholar]
- 22.Kim, S. Y., A. Herbst, K. A. Tworkowski, S. E. Salghetti, and W. P. Tansey. 2003. Skp2 regulates Myc protein stability and activity. Mol. Cell 11:1177-1188. [DOI] [PubMed] [Google Scholar]
- 23.Lee, P. S., C. Chang, D. Liu, and R. Derynck. 2003. Sumoylation of Smad4, the common Smad mediator of transforming growth factor-beta family signaling. J. Biol. Chem. 278:27853-27863. [DOI] [PubMed] [Google Scholar]
- 24.Liang, Y. Y., X. Lin, M. Liang, F. C. Brunicardi, P. ten Dijke, Z. Chen, K. W. Choi, and X. H. Feng. 2003. dSmurf selectively degrades decapentaplegic-activated MAD, and its overexpression disrupts imaginal disc development. J. Biol. Chem. 278:26307-26310. [DOI] [PubMed] [Google Scholar]
- 25.Lin, X., M. Liang, and X.-H. Feng. 2000. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in TGF-beta signaling. J. Biol. Chem. 275:36818-36822. [DOI] [PubMed] [Google Scholar]
- 26.Lin, X., M. Liang, Y.-Y. Liang, F. C. Brunicardi, F. Melchior, and X.-H. Feng. 2003. Activation of TGF-β signaling by SUMO modification of tumor suppressor Smad4/DPC4. J. Biol. Chem. 278:18714-18719. [DOI] [PubMed] [Google Scholar]
- 27.Lin, X., M. Liang, Y. Y. Liang, F. C. Brunicardi, and X. H. Feng. 2003. SUMO-1/Ubc9 promotes nuclear accumulation and metabolic stability of tumor suppressor Smad4. J. Biol. Chem. 278:31043-31048. [DOI] [PubMed] [Google Scholar]
- 28.Lin, X., B. Sun, M. Liang, Y.-Y. Liang, A. Gast, J. Hildebrand, F. C. Brunicardi, F. Melchior, and X.-H. Feng. 2003. Opposed regulation of CtBP corepressor function by SUMOylation and PDZ binding. Mol. Cell 11:1389-1396. [DOI] [PubMed] [Google Scholar]
- 29.Lo, R. S., and J. Massagué. 1999. Ubiquitin-dependent degradation of TGF-beta-activated Smad2. Nat. Cell Biol. 1:472-478. [DOI] [PubMed] [Google Scholar]
- 30.MacGrogan, D., M. Pegram, D. Slamon, and R. Bookstein. 1997. Comparative mutational analysis of DPC4 (Smad4) in prostatic and colorectal carcinomas. Oncogene 15:1111-1114. [DOI] [PubMed] [Google Scholar]
- 31.Massagué, J., and D. Wotton. 2000. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 19:1745-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyazono, K., H. Suzuki, and T. Imamura. 2003. Regulation of TGF-beta signaling and its roles in progression of tumors. Cancer Sci. 94:230-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moren, A., U. Hellman, Y. Inada, T. Imamura, C. H. Heldin, and A. Moustakas. 2003. Differential ubiquitination defines the functional status of the tumor suppressor Smad4. J. Biol. Chem. 278:33571-33582. [DOI] [PubMed] [Google Scholar]
- 34.Moren, A., S. Itoh, A. Moustakas, P. Dijke, and C. H. Heldin. 2000. Functional consequences of tumorigenic missense mutations in the amino-terminal domain of Smad4. Oncogene 19:4396-4404. [DOI] [PubMed] [Google Scholar]
- 35.Nawaz, Z., D. M. Lonard, A. P. Dennis, C. L. Smith, and B. W. O'Malley. 1999. Proteasome-dependent degradation of the human estrogen receptor. Proc. Natl. Acad. Sci. USA 96:1858-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierreux, C. E., F. J. Nicolas, and C. S. Hill. 2000. Transforming growth factor beta-independent shuttling of Smad4 between the cytoplasm and nucleus. Mol. Cell. Biol. 20:9041-9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Podos, S. D., K. K. Hanson, Y. C. Wang, and E. L. Ferguson. 2001. The DSmurf ubiquitin-protein ligase restricts BMP signaling spatially and temporally during Drosophila embryogenesis. Dev. Cell. 1:567-578. [DOI] [PubMed] [Google Scholar]
- 38.Roelen, B. A., O. S. Cohen, M. K. Raychowdhury, D. N. Chadee, Y. Zhang, J. M. Kyriakis, A. A. Alessandrini, and H. Y. Lin. 2003. Phosphorylation of threonine 276 in Smad4 is involved in transforming growth factor-beta-induced nuclear accumulation. Am. J. Physiol. Cell Physiol. 285:C823-C830. [DOI] [PubMed] [Google Scholar]
- 39.Saha, D., P. K. Datta, and R. D. Beauchamp. 2001. Oncogenic ras represses transforming growth factor-beta /Smad signaling by degrading tumor suppressor Smad4. J. Biol. Chem. 276:29531-29537. [DOI] [PubMed] [Google Scholar]
- 40.Schulman, B. A., A. C. Carrano, P. D. Jeffrey, Z. Bowen, E. R. Kinnucan, M. S. Finnin, S. J. Elledge, J. W. Harper, M. Pagano, and N. P. Pavletich. 2000. Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature 408:381-386. [DOI] [PubMed] [Google Scholar]
- 41.Schutte, M. 1999. DPC4/SMAD4 gene alterations in human cancer, and their functional implications. Ann. Oncol. 10(Suppl. 4):56-59. [PubMed] [Google Scholar]
- 42.Schutte, M., R. H. Hiruban, L. Hedrick, K. Cho, G. M. Nadasdy, C. L. Weinstein, G. S. Bova, W. B. Isaacs, P. Cairns, H. Nawroz, D. Sidransky, R. A. Casero, P. S. Meltzer, S. A. Hahn, and S. E. Kern. 1996. DPC4 gene in various tumor types. Cancer Res. 56:2527-2530. [PubMed] [Google Scholar]
- 43.Shi, Y., and J. Massagué. 2003. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113:685-700. [DOI] [PubMed] [Google Scholar]
- 44.Siegel, P. M., and J. Massagué. 2003. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev Cancer 3:807-821. [DOI] [PubMed] [Google Scholar]
- 45.Signoretti, S., L. Di Marcotullio, A. Richardson, S. Ramaswamy, B. Isaac, M. Rue, F. Monti, M. Loda, and M. Pagano. 2002. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J. Clin. Investig. 110:633-641. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Tsvetkov, L. M., K. H. Yeh, S. J. Lee, H. Sun, and H. Zhang. 1999. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr. Biol. 9:661-664. [DOI] [PubMed] [Google Scholar]
- 47.von der Lehr, N., S. Johansson, S. Wu, F. Bahram, A. Castell, C. Cetinkaya, P. Hydbring, I. Weidung, K. Nakayama, K. I. Nakayama, O. Soderberg, T. K. Kerppola, and L. G. Larsson. 2003. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol. Cell 11:1189-1200. [DOI] [PubMed] [Google Scholar]
- 48.Wan, M., X. Cao, Y. Wu, S. Bai, L. Wu, X. Shi, N. Wang, and X. Cao. 2002. Jab1 antagonizes TGF-beta signaling by inducing Smad4 degradation. EMBO Rep. 3:171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wan, M., Y. Tang, E. Tytler, C. Lu, B. Jin, S. Vickers, L. Yang, X. Shi, and X. Cao. 2004. Smad4 protein stability is regulated by ubiquitin ligase SCF beta-TrCP1. J. Biol. Chem. 279:14484-14487. [DOI] [PubMed] [Google Scholar]
- 50.Wang, W., D. Ungermannova, L. Chen, and X. Liu. 2003. A negatively charged amino acid in Skp2 is required for Skp2-Cks1 interaction and ubiquitination of p27Kip1. J. Biol. Chem. 278:32390-32396. [DOI] [PubMed] [Google Scholar]
- 51.Xu, J., and L. Attisano. 2000. Mutations in the tumor suppressors Smad2 and Smad4 inactivate transforming growth factor beta signaling by targeting Smads to the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA 97:4820-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang, G., G. Ayala, A. De Marzo, W. Tian, A. Frolov, T. M. Wheeler, T. C. Thompson, and J. W. Harper. 2002. Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin. Cancer Res. 8:3419-3426. [PubMed] [Google Scholar]
- 53.Zhang, Y., C. Chang, D. J. Gehling, A. Hemmati-Brivanlou, and R. Derynck. 2001. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc. Natl. Acad. Sci. USA 98:974-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu, H., P. Kavsak, S. Abdollah, J. L. Wrana, and G. H. Thomsen. 1999. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 400:687-693. [DOI] [PubMed] [Google Scholar]