Abstract
Emerging evidence suggests that the γ subunit composition of an individual G protein contributes to the specificity of the hundreds of known receptor signaling pathways. Among the twelve γ subtypes, γ3 is abundantly and widely expressed in the brain. To identify specific functions and associations for γ3, a gene-targeting approach was used to produce mice lacking the Gng3 gene (Gng3−/−). Confirming the efficacy and specificity of gene targeting, Gng3−/− mice show no detectable expression of the Gng3 gene, but expression of the divergently transcribed Bscl2 gene is not affected. Suggesting unique roles for γ3 in the brain, Gng3−/− mice display increased susceptibility to seizures, reduced body weights, and decreased adiposity compared to their wild-type littermates. Predicting possible associations for γ3, these phenotypic changes are associated with significant reductions in β2 and αi3 subunit levels in certain regions of the brain. The finding that the Gng3−/− mice and the previously reported Gng7−/− mice display distinct phenotypes and different αβγ subunit associations supports the notion that even closely related γ subtypes, such as γ3 and γ7, perform unique functions in the context of the organism.
The G protein βγ dimer performs numerous roles in the signal transduction process, from membrane targeting of the α subunit (12), to recognition of receptors (23), to activation of effectors (7), to modulation of various proteins affecting the signal intensity or duration (24). In fact, there is potential for a large number of different βγ dimers arising from combinatorial association of the 5 β and 12 γ subtypes. The current challenge is to identify which of these βγ dimers actually exist in vivo and to establish their roles in particular signaling pathways and biological processes. Although transfection and reconstitution provide valuable information on the potential interactions of the β and γ subtypes, these strategies fall short of identifying their actual associations and functions in the context of the organism. By contrast, a gene deletion approach represents a powerful method for determining this information by analyzing the resulting phenotype in knockout mice.
The γ3 subtype has characteristic features that led us to believe that mice with a targeted disruption of Gng3 would display a distinctive phenotype. Gng3 is predominantly expressed in the brain (1, 5, 17), where its expression increases during postnatal development (1, 34). Within the brain, γ3 is widely expressed in neurons rather than glial cells (2, 22, 33). In vitro RNA suppression studies predict a specific function for γ3 in coupling various receptors to changes in calcium channel activity (9, 25, 26). These in vitro studies also suggested that γ3 functions in several different heterotrimer combinations: αo2β1γ3 (9), α13β1γ3 (26), and αq/11β1/3γ3 (25).
In the present study, we show that Gng3−/− mice exhibit changes in seizure susceptibility and body weight on both mixed and C57BL/6J genetic backgrounds. Although the receptor signaling pathway(s) responsible for this complex phenotype is not yet identified, we show that loss of the γ3 subunit produces concurrent reductions in levels of the β2, β1, and αi3 subunits in certain brain regions and that a deficiency of the γ3 subunit does not impair regulation of adenylyl cyclase activity. In a previous study, Schwindinger et al. generated mice lacking the Gng7 gene, which encodes the γ7 subtype, and identified a unique function for γ7 together with αolf in stimulation of adenylyl cyclase activity in the striatum (39). Comparison of Gng3−/− mice and Gng7−/− mice reveals different phenotypes, different heterotrimeric partners, and different signal transduction pathways, demonstrating the unique functions of these two G protein γ subtypes in vivo.
MATERIALS AND METHODS
Production of mice.
Targeted embryonic stem cells, chimeric mice, and F1 heterozygotes with a floxed Gng3 allele (Gng3+/fl) were produced at Lexicon Genetics, Inc. (The Woodlands, Tex.). To provide the potential for conditional inactivation of Gng3 in future studies, the targeting vector included a 1.9-kb cassette containing a loxP site and a PGK-neoR selectable marker in the first intron (52 bp upstream of the initiation codon), and a second, 59-bp loxP cassette in the 3′ untranslated region (immediately downstream of the termination codon). Gng3+/fl mice were bred to mice carrying the Cre recombinase driven by the EIIa adenovirus promoter, FVB/N-Tgn(EIIa-Cre)C5379Lmgd (Jackson Laboratory, Bar Harbor, Maine), to produce mice bearing the deleted Gng3 allele (Gng3−). Gng3+/− mice were then backcrossed to C57BL/6J mice (Jackson Laboratory) for 5 generations. Gng3+/− mice were intercrossed to produce the mice used in these studies, either before (i.e., N0 backcross) or after (i.e., N5 backcross) 5 backcrosses to C57BL/6J mice.
Animal care and approval.
Mice were segregated by sex and group housed in ventilated racks (Thoren Caging Systems, Inc., Hazelton, Pa.). Mice were given access to water and Mouse Diet 9F (Purina Mills, LLC, St. Louis, Mo.) ad libitum. Environmental factors included temperature and humidity control and a 12-h light-dark cycle. Mice were maintained as virus antibody free and parasite free. Animal research protocols were approved by the Geisinger Clinic institutional animal care and use committee.
Genotyping.
Southern blot analysis was performed on genomic tail DNA digested with HindIII. The probe used for this analysis was a 1-kb fragment 3′ of the modified Gng3 allele. Alternatively, PCR analysis was performed using primers (Invitrogen, Rockville, Md.) shown in Table 1. Briefly, primers flanking the 3′ loxP site (JR282 and JR286) were used to competitively amplify the Gng3 and Gng3fl alleles, while a third primer, upstream of the 5′ loxP site (JR416), was included to simultaneously amplify the Gng3− allele. The bacteriophage P1 Cre transgene was amplified with JR353 and JR354.
TABLE 1.
Primers used in this study
| Name | Descriptiona | Sequence |
|---|---|---|
| JR282 | Gng3 3′ UTR antisense | GAG TAG AAG GTG CTT GGA GT |
| JR289 | Gng3 exon 1 sense | ACG CAA GAT GGT GGA ACA GC |
| JR416 | Gng3 5′ sense | TCT GGG CAG AAC TTA AGC TG |
| JR353 | Cre sense | GTT CGC AAG AAC CTG ATG GAC A |
| JR354 | Cre antisense | CTA GAG CCT GTT TTG CAC GTT C |
| JR175 | Eef1a2 sense | GGA ATG GTG ACA ACA TGC TG |
| JR174 | Eef1a2 antisense | CGT TGA AGC CTA CAT TGT CC |
| JR443 (a) | Bscl2 antisense | TAG TGG AAG TGC ACA GGG CTG |
| JR444 | Bscl2 common sense | GAC CCA CCG TTC TTC AGA GCT |
| JR445 (b) | Bscl2 exon 1 sense | CCT GAC ACA GCA CTT AGC ACC |
| JR446 (c) | Bscl2 alt exon 1 sense | ACG TTG TCC TCT GAG GCT CTG |
| Gapds | Gapd sense | TGA AGG TCG GTG TGA ACG GAT TTG GC |
| Gapdas | Gapd antisense | CAT GTA GGC CAT GAG GTC ACC AC |
UTR, untranslated region; alt, alternate.
Expression of the Gng3 and Bscl2 genes.
PCR amplification of cDNA in the murine multiple tissue cDNA panel (BD Biosciences, Palo Alto, Calif.) was performed according to the manufacturer's instructions. RNA was isolated from whole brain or adipose tissues by using TRIzol reagent (Invitrogen). First-strand cDNA was prepared from 2 μg of RNA by using Moloney murine leukemia virus reverse transcriptase (Promega), and this was used as a template to amplify the Gng3, Bscl2, or elongation factor transcripts with the primers shown in Table 1.
Audiogenic seizures.
Audiogenic seizures were induced in 6- to 12-week-old mice (21). Mice were placed in a clear plastic breeding box (27 by 27 by 20 cm) with a filter paper lid and were allowed to explore for 1 min. Five metal keys on a ring were shaken above the box for 10 s to produce a sound of 85 to 95 dB near the bottom of the box, as measured with a sound level meter (Tandy Corp., Fort Worth, Tex.). Observation was continued for at least 1 min after cessation of the sound. All sessions were videotaped and then graded by two observers who were unaware of the genotypes of the mice.
Immunoblot analysis.
To examine the expression of G protein subunits in the brain, Western blot analysis was performed on cholate-solubilized membranes prepared from the cortex, hippocampus, cerebellum, striatum, or whole brain, as described previously (39). Antisera for Ras (BD Biosciences) and for the rat Na+/K+-ATPase β subunit (Research Diagnostics, Inc., Flanders, N.J.) were used at a 1:2,000 dilution. Antisera for αi1, αi3, and αq/11 (Calbiochem, La Jolla, Calif.) were used at a 1:1,000 dilution. Antisera for αi1/i3, αi2, αo (1:500), β1 (1:500), β2 (1:500), γ2, γ3, γ5 (1:100), and γ7 have been described previously (5, 13, 47) and were used at a 1:200 dilution except as indicated. Antisera for αs (a generous gift from Catherine Berlot) were used at a 1:500 dilution, antisera for αolf (a generous gift from Denis Hervé) were used at 1:2,000, and antisera for α12 or α13 (generous gifts from N. Dhanasekaran) were used at 1:1,000. His-tagged G protein subunits (CytoSignal Research Products, Irvine, Calif.) were used as standards for quantitative immunoblotting.
GTPγS binding assay.
Agonist-stimulated [35S]GTPγS autoradiography was performed as previously described (32, 43) with slight modifications. Following CO2 euthanasia, brains were removed and frozen in isopentane at −35°C. Coronal sections (thickness, 20 μm) at the levels of the striatum and hippocampus were cut on a cryostat at −20°C and mounted on gelatin-coated slides. Slides were equilibrated in 50 mM Tris-HCl-3 mM MgCl2-0.2 mM EGTA-100 mM NaCl (pH 7.4) (assay buffer) at 25°C for 10 min and were then preincubated in assay buffer containing 2 mM GDP and adenosine deaminase (9.5 mU/ml) at 25°C for 15 min. Assays were conducted by incubating slides in assay buffer with 2 mM GDP, adenosine deaminase (9.5 mU/ml), and 0.04 nM [35S]GTPγS (250 Ci/mmol; New England Nuclear Corp., Boston, Mass.) with (stimulated) or without (basal) agonist at 25°C for 2 h. Agonists included [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO) (Drug Supply Program, National Institute on Drug Abuse), WIN 55,212-2, and (−)-N6-(2-phenylisopropyl)adenosine (PIA) (Sigma Chemical Co., St. Louis, Mo.) at a concentration (10 μM) that has been shown to produce maximal, antagonist-reversible stimulation (32, 43). Slides were rinsed twice in 50 mM Tris-HCl, pH 7.4, at 4°C for 2 min each time and then in H2O at 4°C for 30 s. Slides were dried overnight and exposed to Biomax MR film (Eastman Kodak Co., Rochester, N.Y.) for 24 h in the presence of 14C-labeled microscales. Films were digitized with a Sony XC-77 video camera and analyzed by using the NIH IMAGE program for Macintosh computers. Net agonist-stimulated [35S]GTPγS binding was calculated by subtracting basal binding from agonist-stimulated binding. Data are reported as means ± standard errors for triplicate sections of brains from six mice per group. Statistical comparison between wild-type and knockout mice was performed by analysis of variance followed by post hoc analysis using the two-tailed nonpaired Student t test.
Adenylyl cyclase assay.
Membranes were prepared as previously described (39) from cerebellums of Gng3−/− and Gng3+/+ mice. Adenylyl cyclase activity was determined by incubating membranes (20 μg of protein) at 30°C for 10 min in a solution containing 0.1 ml of an agonist-specific buffer, 0.5 μCi of [α-32P]ATP, 0.1 mM ATP, 0.05 mM GTP, and an ATP regenerating system consisting of 5 mM creatine phosphate and 50 U of creatine phosphokinase/ml. For the A1 adenosine agonist, cyclopentyl adenosine (10 to 1,000 nM), the buffer consisted of 50 mM HEPES (pH 7.4), 1 mM EGTA, 5 mM MgCl2, 0.01 mM rolipram, and 1 U of adenosine deaminase/ml (30). For the cannabinoid agonist anandamide (1 to 10 μM), the buffer consisted of 50 mM Tris · HCl (pH 7.4), 2 mM MgSO4, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), and 0.03 mM cAMP (6). Reactions were terminated by addition of 0.1 ml of 2% sodium dodecyl sulfate, 40 mM ATP, 1.4 mM cAMP, and 0.06 μCi of [2,8-3H]cAMP and heating to 95°C for 3 min. The resulting [32P]cAMP and the added [3H]cAMP were isolated by Dowex and alumina chromatography (19) and quantified in an LS-6500 scintillation counter (Beckman) using ScinitiSafe Plus 50% LSC-Cocktail (Fisher).
RESULTS
Targeted disruption of the Gng3 gene.
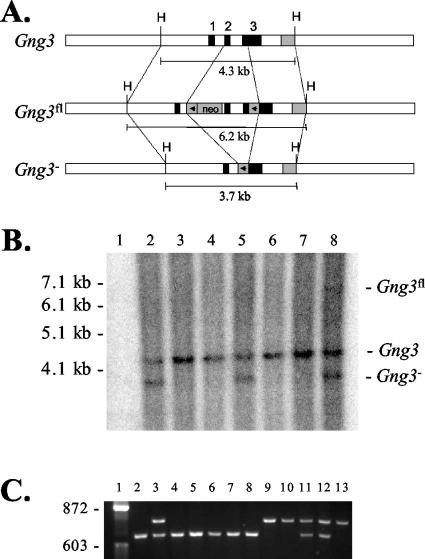
Mice with a targeted deletion of the Gng3 gene were produced in two steps (Fig. 1A). First, mice with a floxed Gng3 allele (Gng3fl) were created by using a targeting vector in which the complete coding region of the gene was flanked by loxP sites. This provides the potential for generation of inducible or tissue-specific knockouts in future studies. Second, mice with a deleted Gng3 allele (Gng3−) were produced by crossing Gng3fl mice with mice expressing the Cre transgene under the control of the EIIa adenovirus promoter. As shown by Southern blot analysis, this resulted in the excision of the complete coding region of the Gng3 gene (Fig. 1B). To produce the mice used in subsequent experiments, mice carrying the Gng3− allele were either intercrossed, to obtain mice on a mixed genetic background, or backcrossed for 5 generations, to produce mice on a C57BL/6J genetic background. PCR analysis was used to identify mice carrying the Gng3− allele (Fig. 1C). Heterozygous crosses produced litters of the expected size (6.2 ± 3.3 pups) in the expected 1:2:1 ratio (112 Gng3+/+ pups, 225 Gng3+/− pups, and 119 Gng3−/− pups), indicating that disruption of Gng3 does not affect survival to weaning. In sequential matings of one homozygous pair, Gng3−/− mice were fertile and weaned litters of apparently normal size (9.3 ± 1.2 pups).
FIG. 1.
(A) Targeting strategy. (Top) Wild-type allele (Gng3) with exons 1 to 3 (solid boxes) and the 3′ probe (shaded box). (Center) Targeted allele (Gng3fl) with a loxP-neoR cassette in intron 1 (arrowhead and “neo”) and a loxP cassette in the 3′ untranslated sequence (arrowhead). (Bottom) Deleted allele (Gng3−) with one remaining loxP site (arrowhead) following Cre-mediated excision of the coding region of Gng3. Expected sizes of HindIII (H) fragments are indicated. (B) Southern blot of tail biopsy DNA digested with HindIII and detected with the 3′ probe. Lane 1, molecular weight ladder; lanes 2 to 8, progeny of a Gng3+/fl × TgN(EIIa-Cre) cross, with all lanes showing the 4.3-kb wild-type Gng3 allele and lanes 2, 5, and 8 also showing the 3.7-kb Gng3− allele. A small amount of residual Gng3fl allele can be seen in lane 8. (C) PCR products obtained with primers JR282, JR286, and JR416 of tail biopsy DNA from progeny of a Gng3+/− × Gng3+/− cross. Lane 1, φX HaeIII marker; lanes 2 and 4 to 8, wild-type mice with a single 629-bp band derived from primers JR282 and JR286; lanes 9, 10, and 13, Gng3−/− mice with a single 755-bp band derived from primers JR282 and JR416; lanes 3, 11, and 12, heterozygous Gng3+/− mice with both bands.
Abolished expression of the Gng3 gene.
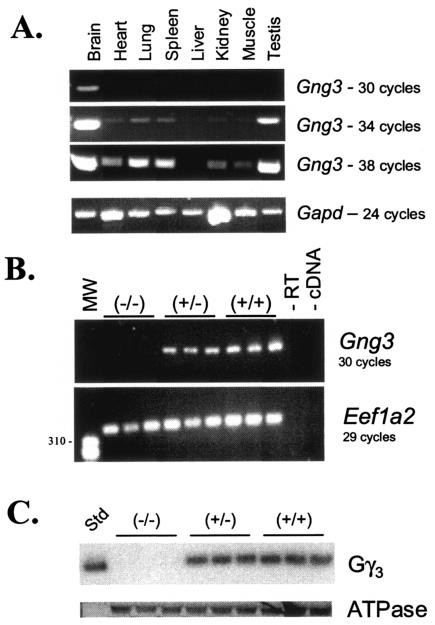
To confirm the effectiveness of the targeted gene deletion, expression of Gng3 was examined in the brain, where the gene was most highly expressed (Fig. 2A). Reverse transcription-PCR (RT-PCR) analysis demonstrated that γ3 mRNA was present at reduced levels in brains from Gng3+/− mice and was absent in brains from Gng3−/− mice (Fig. 2B). Likewise, Western blot analysis with γ3-specific antisera (20) showed that the γ3 protein was reduced by 35% ± 5% in cholate-solubilized membrane extracts of brains from Gng3+/− mice and was not detected in brains from Gng3−/− mice (Fig. 2C).
FIG. 2.
(A) RT-PCR products obtained after 30, 34, or 38 cycles of amplification with primers JR282 and JR286, showing relative expression of Gng3 in various tissues (top three panels), or products from 24 cycles with primers for glyceraldehyde-3-phosphate dehydrogenase (Gapd), confirming approximately equal amounts of cDNA in all reactions (bottom panel). (B) (Top) RT-PCR products obtained after 30 cycles of amplification with primers JR282 and JR286 for Gng3, from cDNA prepared from whole brains of three wild-type, three Gng3+/−, and three Gng3−/− mice, without added reverse transcriptase (−RT), or without added cDNA (−cDNA). (Bottom) RT-PCR products obtained after 29 cycles of amplification with primers JR174 and JR175 for elongation factor 1 α2 (Eef1a2), from identical aliquots of the cDNAs. (C) Western blot analysis of proteins prepared from Sf9 cells expressing γ3 (Std) or brains of three Gng3−/−, three Gng3+/−, and three wild-type mice. Top panel shows that γ3 is reduced in membranes from Gng3+/− mice and absent in membranes from Gng3−/− mice. Bottom panel shows that sodium/potassium ATPase β-subunit levels are equal in all lanes.
Preserved expression of the Bscl2 gene.
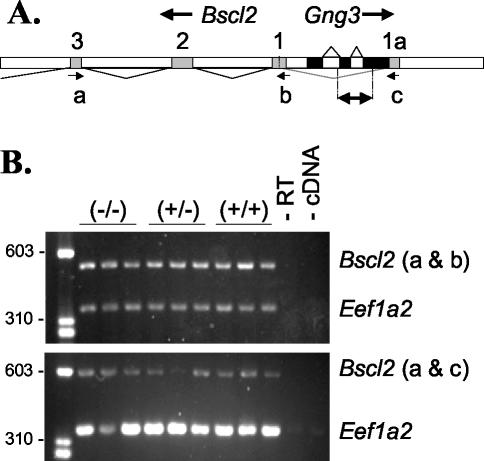
The Gng3 gene is located head-to-head with the Bscl2 gene, previously known as Gng3lg (10). Because of this arrangement, these two genes may share promoter elements. Moreover, our analysis of expressed sequence tags (e.g., GenBank accession no. CB566482 and BI855301) reveals the existence of an alternate first exon that places the Gng3 gene within the alternate first intron of the Bscl2 gene (Fig. 3A). To rule out the possibility that mice carrying the Gng3− allele may show altered expression of the Bscl2 gene, RT-PCR analysis was performed on total RNA obtained from brains and adipose tissue. This analysis showed that both Bscl2 mRNA transcripts were expressed at comparable levels in brains from Gng3+/+, Gng3+/−, and Gng3−/− mice (Fig. 3B). Likewise, Bscl2 mRNA transcripts were detected at similar levels in white or brown adipose tissue from Gng3+/+ and Gng3−/− mice (data not shown). Taken together, these results show that Gng3 deletion produces loss of γ3 mRNA and protein (Fig. 2) without affecting the expression of the closely linked Bscl2 gene.
FIG. 3.
(A) Organization of the Bscl2 and Gng3 genes, showing the first few exons (exons 1 to 3) of Bscl2 (shaded boxes), including the alternate exon 1 (1a), and the three exons of Gng3 (solid boxes). The region replaced in the targeted allele, Gng3−, is indicated by the double arrow. Arrows mark positions of primers used for amplification of Bscl2: the antisense primer JR443 (a), the exon 1-specific sense primer JR445 (b), and the alternate exon 1-specific sense primer JR446 (c). (B) Duplex RT-PCR of brain cDNA from three Gng3−/− (−/−), three Gng3+/− (+/−), and three wild-type (+/+) mice, with primers for exon 1 of Bscl2 (upper panel), the alternate exon 1 of Bscl2 (lower panel), and elongation factor Eef1a2 (both panels), showing approximately equal levels of either Bscl2 transcript across all genotypes.
Increased seizure susceptibility of Gng3−/− mice.
Gng3−/− mice exhibit increased susceptibility to seizures, consistent with the normally high expression of Gng3 in the brain. This was first observed on a mixed genetic background (i.e., N0 backcross) when animal workers noticed that Gng3−/− mice suffered from recurrent seizures upon cage changing. Overall, 24% of Gng3−/− mice were observed to have recurrent handling-induced seizures, with the earliest seizure occurring at the age of 2 months. In comparison, 8% of wild-type littermates were observed to have seizures, with the earliest occurring at 14 months. These seizures were characterized by vocalizations, excessive salivation, wild running, explosive jumping, tonic-clonic convulsions, and tonic hindlimb extension. Moreover, the occurrence of these seizures was associated with a reduced life span. Approximately 40% of the Gng3−/− mice died between the ages of 2 months and 1 year compared to 10% of wild-type littermates over the same observation period. Because they showed no prior clinical signs, we suspect that the Gng3−/− mice on a mixed genetic background were dying as a result of their recurrent seizures.
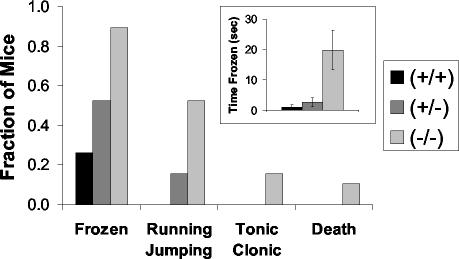
Intriguingly, none of the Gng3−/− mice on the C57BL/6J background (i.e., N5 backcross) were observed to have seizures upon cage changing. Moreover, the absence of these seizures was associated with an increased life span. Nevertheless, the Gng3−/− mice on the C57BL/6J background still showed an increased frequency and severity of seizures induced by other means (Fig. 4). In response to a loud noise, 90% of Gng3−/− mice exhibited mild seizure activity that was characterized by freezing for a mean time of 19.8 ± 6.5 s. In addition, 50% of Gng3−/− mice displayed moderate seizure activity that was characterized by wild running and explosive jumping; 15% of Gng3−/− mice displayed severe seizure activity that was characterized by tonic-clonic convulsions with extension; and 10% of Gng3−/− mice died. In contrast, only 25% of their wild-type littermates displayed even the mildest form of seizure activity that was characterized by freezing for a mean time of 1.8 ± 0.8 s. Gng3+/− mice had an intermediate frequency and severity of audiogenic seizures. Taken together, these results indicate that Gng3−/− mice on different genetic backgrounds show increased susceptibility to seizures induced by various means, suggesting a role for γ3 in the regulation of neuronal excitability.
FIG. 4.
Audiogenic seizures induced by a 90-dB noise with a duration of 10 s for mice on a C57BL/6J genetic background (i.e., N5 backcross). The fraction of mice displaying a seizure of the indicated severity is shown by genotype for Gng3−/−, Gng3+/−, and wild-type mice (19 mice in each group). Note that Gng3−/− mice were more likely to demonstrate any seizure-like activity and more often progressed to more severe seizures. (Inset) Time frozen in response to auditory stimulus by genotype. Note that Gng3−/− mice remained frozen for significantly longer than their wild-type littermates.
Reduced weight and decreased adiposity of Gng3−/− mice.
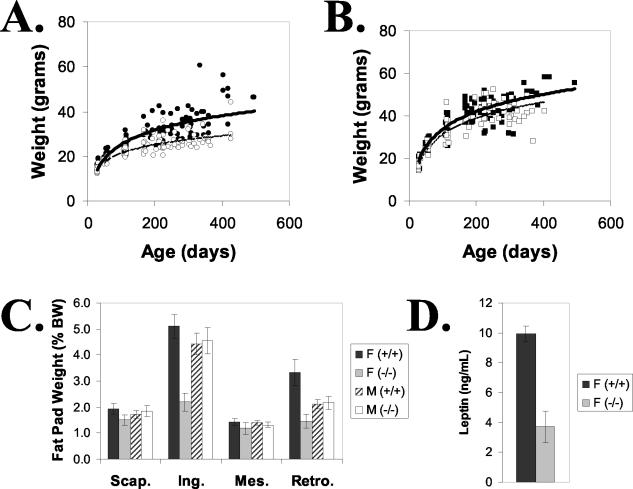
Gng3−/− mice also displayed reduced body weights. This was first observed on a mixed genetic background and was later confirmed on a C57BL/6J background (Fig. 5A and B). Although indistinguishable at weaning, the Gng3−/− mice on the C57BL/6J background failed to gain weight at the same rate as wild-type littermates. This difference was most pronounced among female Gng3−/− mice, which weighed approximately 25% less than their wild-type siblings by the age of 1 year. Female Gng3−/− mice also showed a striking reduction in inguinal and retroperitoneal fat pad masses (Fig. 5C). This effect was associated with a dramatic decrease in serum leptin levels among female Gng3−/− mice (Fig. 5D). Consistent with the smaller difference in body weight, male Gng3−/− mice showed no significant reduction in fat pad masses (Fig. 5B and C). Taken together, these results indicate that Gng3−/− mice on different genetic backgrounds exhibit reduced body weights, suggesting an additional role for γ3 in the regulation of appetite and/or metabolism.
FIG. 5.
Lean phenotype of Gng3−/− mice on C57BL/6J genetic background (i.e., N5 backcross). (A) Older, female Gng3−/− mice (open circles) weighed less than wild-type littermates (filled circles). (B) Weight differences for male Gng3−/− mice (open squares) and wild-type littermates (filled squares) were not as pronounced. Weights were obtained at 3 to 9 irregular intervals, between the ages of 4 and 70 weeks, for 14 to 18 mice in each group. (C) Fat pad weights expressed as a percentage of body weight (% BW). Female Gng3−/− mice had significantly reduced inguinal (Ing.) and retroperitoneal (Retro.) fat pad weights, but interscapular (Scap.) and mesenteric (Mes.) fat pad weights were unchanged. Fat pad weights of male Gng3−/− mice were not different from those of wild-type littermates. Data are means ± standard errors for five to seven mice in each group. (D) Serum leptin levels were significantly reduced in female Gng3−/− mice. Data are means ± standard errors for five or six mice in each group.
Possible basis for altered phenotype of Gng3−/− mice.
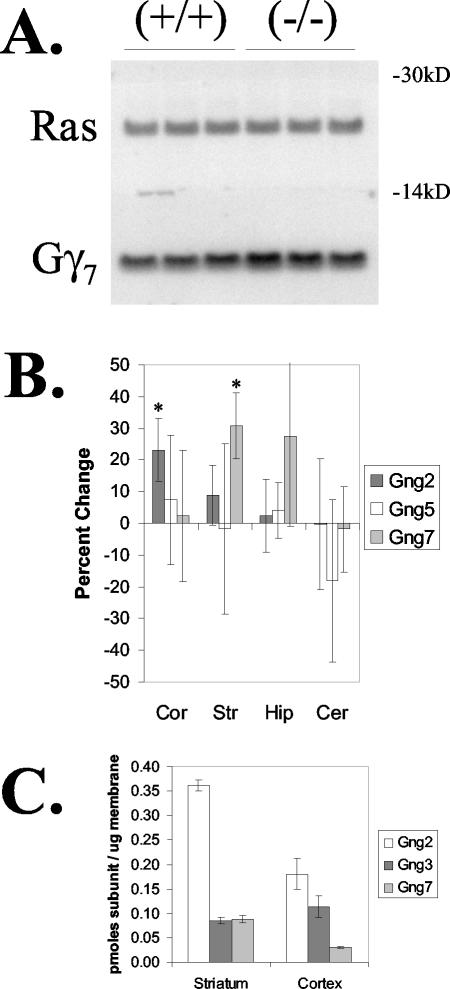
The existence and disparate nature of the phenotypes in Gng3−/− mice indicate a requirement for γ3 in different brain regions or distinct signaling pathways affecting neuronal excitability and body weight regulation that cannot be compensated for by related subtypes. Of the known γ subtypes, γ2, γ3, and γ7 are highly expressed in the brain, where they show partly overlapping patterns of expression in the cortex, striatum, hippocampus, and cerebellum (2). To determine whether loss of γ3 produces compensatory changes in the expression of other γ subtypes, we performed immunoblot analysis to compare the levels of various γ subtypes in several brain regions from Gng3+/+ and Gng3−/− mice (Fig. 6A). The nature and extent of the observed changes were found to differ between brain regions (Fig. 6B). In this regard, γ2 levels were increased slightly, by 23% ± 10%, in the cortices of Gng3−/− mice, while γ7 levels were increased modestly, by 31% ± 10%, in the striatum, and the level of γ5 was not altered in any of the brain regions examined from these animals. To provide a physiological context for the observed changes, we next performed quantitative immunoblot analysis to determine the concentrations of γ2, γ3, and γ7 in these regions (Fig. 6C). In wild-type mice, γ3 and γ7 are expressed at comparable levels in the striatum, while γ2 and γ3 are detected at roughly similar levels in the cortex. Thus, the relatively small increases in γ7 subunit levels in the striatum and γ2 subunit levels in the cortex would not be expected to fully compensate for the loss of γ3 subunit in Gng3−/− mice.
FIG. 6.
Expression of other γ subtypes. (A) Representative immunoblot of cholate-extractable membrane protein (20 μg) from striata of three wild-type (+/+) and three Gng3−/− (−/−) mice. The top portion of the blot was incubated with a Ras antiserum to correct for variations in protein loading. The bottom portion was incubated with a γ7 antiserum. Note the increased level of γ7 in the striata of Gng3−/− mice. (B) Quantification of immunoblots for γ2 (Gng2), γ5 (Gng5), and γ7 (Gng7) in the cortex (Cor), striatum (Str), hippocampus (Hip), and cerebellum (Cer). Relative levels in Gng3−/− mice are shown as the percent change from levels in wild-type mice. Data are means ± standard deviations for three mice in each group. *, P < 0.05 for comparison of Gng3−/− to Gng+/+ mice by Student's t test. (C) Concentrations of γ2, γ3, and γ7 in the cortex and striatum, showing approximately equimolar amounts of γ2 and γ3 in the cortex and of γ3 and γ7 in the striatum. Data are expressed as picomoles of γ subunit per microgram of membrane cholate extract and are means ± standard errors for three or four wild-type mice.
Possible G protein subunit associations revealed by Gng3−/− mice.
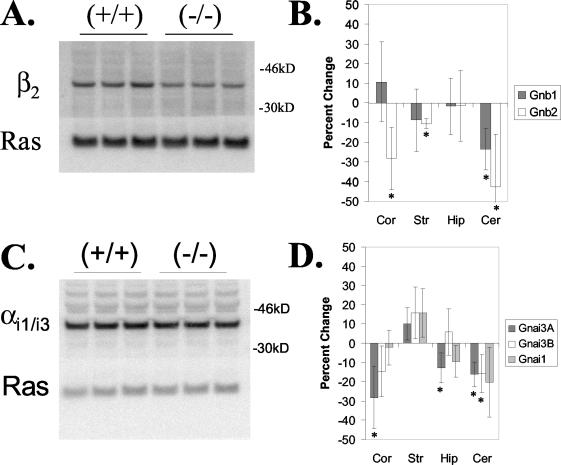
Under physiological conditions, G proteins function as heterotrimers whose specific αβγ subunit combinations determine their particular roles in receptor signaling pathways (37). Increasing evidence suggests that the composition of the γ subunit may play a critical role in the assembly of specific αβγ subunit combinations. In this regard, it was shown previously that loss of γ7 coordinately suppresses the levels of β1 (47) or αolf (39), suggesting a functional association between them. To determine whether loss of γ3 produces a similar effect, we performed immunoblot analysis to compare the levels of various α and β subunits in several brain regions from Gng3+/+ and Gng3−/− mice. The type and extent of suppression was found to differ between brain regions (Fig. 7). In this regard, the level of β1 was reduced by 24% ± 11% in the cerebellum, while levels of β2 were reduced by 11% ± 2%, 28% ± 16%, and 42% ± 27% in the striatum, cortex, and cerebellum, respectively (Fig. 7B). No significant decreases in the levels of αi1, αi2, αq/11, αo, αs, αolf, α12, or α13 were observed in the cerebellums, cortices, hippocampi, or striata of Gng3−/− mice (data not shown). However, small decreases of 13% ± 8% to 28% ± 16% in the levels of αi3 were seen in cortices, hippocampi, and cerebellums of Gng3−/− mice, with two different antisera cross-reacting with αi1 and αi3 showing a decrease, but an antiserum specific for αi1 showing no difference (Fig. 7D).
FIG. 7.
Expression of α and β subunits in Gng3−/− brain regions. (A) Representative immunoblot of β2 in cerebellum from wild type (+/+) and Gng3−/− (−/−) mice; the bottom portion was detected with Ras antisera to control for variations in protein loading. (B) Quantification of immunoblots for β1 (Gnb1) and β2 (Gnb2) in the cortex (Cor), striatum (Str), hippocampus (Hip), and cerebellum (Cer). Data are expressed as the percent change from the wild-type level in Gng3−/− mice and are means ± standard deviations for three mice in each group. *, P < 0.05 for comparison of Gng3−/− to Gng+/+ mice by Student's t test. (C) Representative immunoblot of αi3 in the cerebellum for wild-type (+/+) and Gng3−/− (−/−) mice. The top portion was detected with our αi1/i3 antisera; the bottom portion was detected with Ras antisera to control for variations in protein loading. (D) Quantification of immunoblots for αi1 (Gnai1) and αi3 in four brain regions. Two different antisera for αi3, our αi1/i3 antisera (Gnai3A) and Calbiochem αi3 antisera (Gnai3B), both cross-reacted with αi1 to varying degrees (data not shown). Note that levels of αi1/i3 were reduced in the cortex, hippocampus, and cerebellum, but levels of αi1 were unchanged in these regions, suggesting that levels of αi3 are reduced in these regions.
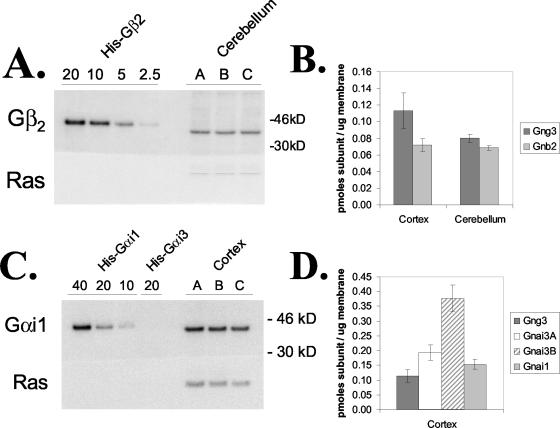
To provide physiological meaning for the observed changes, we next performed quantitative immunoblot analysis on several brain regions from wild-type mice (Fig. 8). The level of β2 was found to be approximately equal to that of γ3 in the cortices and cerebellums of wild-type mice (Fig. 8B). Thus, the 30 and 40% reductions in the levels of β2 in the cortices and cerebellums of Gng3−/− mice suggest that γ3 forms dimers with more than one β subunit in these brain regions of wild-type mice and that loss of γ3 is accompanied by loss of a β2γ3 dimer in these brain regions of Gng3−/− mice. The interpretation of the relative reductions in αi3 levels in several brain regions is complicated by the lack of specific antisera. Two antisera, which cross-react with αi3 and αi1 to various degrees, gave different expression levels for αi3 in the cortex (Fig. 8D), suggesting that the level of αi3 was either equal to or 3 times that of γ3. If the high-end estimate is correct, the 30% reduction in αi3 levels observed in the cortex could be accounted for by the loss of a unique αi3βγ3 heterotrimer.
FIG. 8.
Quantitative immunoblots of G protein subunits. (A) Representative immunoblot showing a standard curve of the His-tagged β2 subunit (20, 10, 5, or 2.5 ng/lane) and 20 μg of cholate extracts of cortical membranes from three wild-type mice (mice A, B, and C). The top portion of the blot was incubated with an antiserum for β2; the bottom portion was incubated with a Ras antiserum to confirm equal loading of the membrane cholate extract. (B) Concentrations of γ3 and β2 in the cortex and cerebellum, showing approximately equimolar amounts of both subunits. Data are expressed as picomoles of subunit per microgram of membrane cholate extract and are means ± standard deviations for three mice in each group. (C) Representative immunoblot showing a standard curve of the His-tagged αi1 subunit (40, 20, and 10 ng/lane); the His-tagged αi3 subunit (20 ng/lane), showing no cross-reactivity with αi1 antisera; and 20 μg of cholate extracts of cortical membranes from three wild-type mice (mice A, B, and C). The top portion of the blot was incubated with an antiserum for αi1, and the bottom portion was incubated with a Ras antiserum. (D) Concentrations of γ3, αi1, and αi3 in the cortex. Note that both available αi3 antisera cross-reacted with αi1 (data not shown) and that the two antisera gave different results, indicating that the concentration of αi3 was either equal to or 3 times that of γ3.
Screening of possible receptor signaling pathways affected in Gng3−/− mice.
The results presented above predict the existence of a specific Gi heterotrimer composed of αi3βγ3 subunits, whose assembly is limited in certain brain regions of Gng3−/− mice. This raises the possibility that one or more receptor signaling pathways whose actions are dependent on this specific Gi heterotrimer may be disrupted in these animals. To test this possibility, we used a novel technique to compare receptor activation of G proteins in brain sections by in vitro autoradiography of [35S]GTPγS binding. Our preliminary screen focused on the mu opioid, A1 adenosine, and CB1 cannabinoid receptors, since there was some evidence in the literature to suggest their coupling to Gi proteins and their involvement in signaling pathways affecting neuronal excitability and/or body weight regulation. The agonists DAMGO, PIA, and WIN 55,212-2 were used to visualize mu opioid-, A1 adenosine-, and CB1 cannabinoid receptor-stimulated [35S]GTPγS binding, respectively. Visual comparison of autoradiograms from wild-type and Gng3−/− mice did not reveal significant differences in basal or agonist-stimulated activity in any region. This observation was confirmed by densitometric analysis. Basal [35S]GTPγS binding did not differ between wild-type and Gng3−/− mice in the caudate-putamen (232 ± 9 versus 219 ± 7 nCi/g), anterior cingulate cortex (186 ± 8 versus 176 ± 6 nCi/g), hippocampus (202 ± 25 versus 187 ± 5 nCi/g), or hypothalamus (313 ± 23 versus 315 ± 11 nCi/g). Similarly, DAMGO-, WIN 55,212-2-, and PIA-stimulated [35S]GTPγS binding did not differ between wild-type and knockout mice in any region examined (Table 2). Thus, deletion of γ3 did not appear to affect mu opioid-, A1 adenosine-, or CB1 cannabinoid receptor-mediated signaling, at least in the brain regions examined.
TABLE 2.
Densitometric analysis of agonist-stimulated [35S]GTPγS autoradiography in brain regionsa
| Region | [35S]GTPγS binding (mean nCi/g ± SEM)b stimulated by the following agonist in the indicated micec: |
|||||
|---|---|---|---|---|---|---|
| WIN 55,212-2 |
DAMGO |
PIA |
||||
| WT | KO | WT | KO | WT | KO | |
| Cingulate cortex | 447 ± 7 | 438 ± 14 | 152 ± 24 | 116 ± 13 | 495 ± 33 | 508 ± 16 |
| Caudate-putamen | 414 ± 12 | 405 ± 12 | 266 ± 26 | 254 ± 14 | 363 ± 33 | 381 ± 8 |
| Hippocampus | 413 ± 24 | 433 ± 18 | ND | ND | 522 ± 20 | 506 ± 19 |
| Hypothalamus | 242 ± 20 | 232 ± 9 | 193 ± 15 | 196 ± 6 | ND | ND |
Brain sections were incubated with [35S]GTPγS, GDP, and maximally effective concentrations of the indicated agonists as described in Materials and Methods.
ND, not determined.
WT, wild type; KO, knockout.
Screening of possible effectors in Gng3−/− mice.
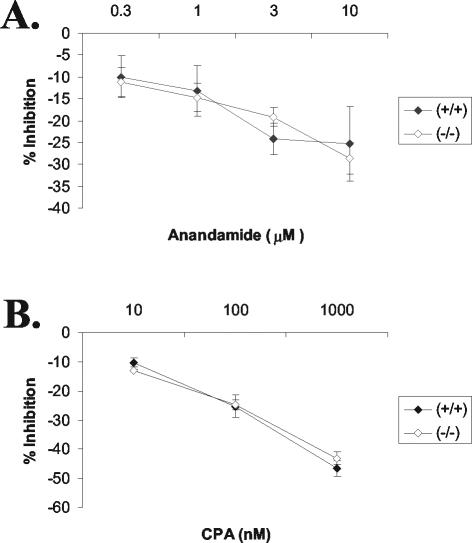
Given the observed reduction in αi3 levels in several brain regions of Gng3−/− mice, we tested the possibility that inhibition of adenylyl cyclase by one or more G protein-coupled receptors might be disrupted (Fig. 9). As a preliminary screen, we showed that the endogenous cannabinoid receptor agonist anandamide inhibited basal adenylyl cyclase activity equally well in cerebellar membranes from Gng3+/+ and Gng3−/− mice (Fig. 9A), while the A1 adenosine receptor agonist cyclopentyladenosine inhibited forskolin-stimulated adenylyl cyclase activity equally well in cerebellar membranes from these two lines of mice (Fig. 9B). Thus, deletion of γ3 did not appear to affect adenylyl cyclase signaling, at least for the receptors and brain regions examined.
FIG. 9.
Adenylyl cyclase assays. (A) Inhibition of adenylyl cyclase by a cannabinoid agonist, 0.3 to 10 μM anandamide, in cerebellar membranes, expressed as percent inhibition of GTP (50 μM)-stimulated activity (119 ± 12 pmol/mg of protein/min for Gng3+/+ mice or 125 ± 16 pmol/mg of protein/min for Gng3−/− mice). Data are means ± standard errors for six mice in each group. (B) Inhibition adenylyl cyclase by an A1 adenosine agonist, 10 to 1,000 nM cyclopentyladenosine (CPA), in cerebellar membranes, expressed as percent inhibition of forskolin (0.3 μM)-stimulated activity (136 ± 20 pmol/mg of protein/min for Gng3+/+ mice or 141 ± 13 pmol/mg of protein/min for Gng3−/− mice). Data are means ± standard errors for three mice in each group.
DISCUSSION
Gng3−/− mice exhibit a complex phenotype that includes neurological and metabolic abnormalities. The complexity of this phenotype suggests that γ3 may function in multiple brain regions and/or signaling pathways. In future studies, the ability to limit inactivation of the Gng3 gene to specific brain regions will allow further dissection of the functions of this gene in different neuronal populations.
Seizure-related phenotype.
Mice lacking Gng3 on a mixed genetic background (i.e., FVB/N, C57BL/6, and 129 strains) experience an increased frequency of spontaneous seizures and increased mortality rates compared to their wild-type littermate controls. An association with increased rates of death has been described for other knockout mice with spontaneous seizures. For instance, mice with a deficiency of a serotonin receptor, Htr2c, demonstrate spontaneous seizures that occasionally progress to respiratory arrest and death, with approximately 25% of mice dying by the age of 13 weeks and 50% of mice dying during 25 weeks of observation (46). Mice with a deficiency of the GABAB1 receptor, Gabbr1, exhibited recurrent spontaneous seizures typified by wild running and limb tonus and clonus, which frequently progressed to death, with a mean life expectancy of only 21 days (36).
Intriguingly, both the spontaneous seizure and mortality phenotypes of Gng3−/− mice are dependent on the genetic background. After crossing onto the C57BL/6J background for 5 generations, these seizure- and mortality-related changes are no longer observed. A similar decline in spontaneous seizures and sudden death was observed when serotonin 5-HT2C receptor-null mice were backcrossed to C57BL/6 mice (18). Notably, these results are consistent with a recent study showing that seizure susceptibility is strain dependent, with the C57BL/6 strain exhibiting a higher electroconvulsive threshold than the FVB/NJ and 129S3 strains (14). To confirm the presence of a modifying factor in the C57BL/6 genome that attenuates the seizure phenotype of Gng3−/− mice, we are currently in the process of backcrossing the Gng3−/− mice to more seizure-sensitive strains.
Although they no longer suffer spontaneous seizures, mice lacking Gng3 on the C57BL/6J genetic background still exhibit increased seizure susceptibility. A number of mouse models involving disruption of G protein-coupled receptors, their agonists, or intracellular effectors display increased susceptibility to seizures (31). Mice with a deficiency of somatostatin (4), the D2 dopamine receptor (3), or the neuropeptide Y5 receptor (28) all display increased susceptibility to kainic acid-induced seizures. The Frings mouse displays audiogenic seizures (44). Mice with a deficiency of neuropeptide Y (11), a serotonin receptor (46), the GABAB1 receptor (36), or the GIRK2 potassium channel (42) all exhibit spontaneous or handling-induced seizures. These mouse models suggest potential signaling pathways through which disruption of Gng3 might increase seizure susceptibility.
Weight-related phenotype.
Female mice lacking Gng3 show reduced body weights, decreased adiposity, and low leptin levels compared to their wild-type littermate controls. Importantly, the phenotype was not dependent on genetic background. The basis for this lean phenotype is currently under investigation. One possibility that was discounted was derangement of Bscl2 expression as a result of targeting of the Gng3 gene. The human BSCL2 gene has been linked to a form of congenital lipodystrophy (27). However, analysis of Gng3−/− mice revealed no disruption of Bscl2 expression in the brain, white fat, or brown fat. Therefore, the lean phenotype was due to loss of Gng3 expression. Possibilities under consideration include a change in food consumption and/or metabolic rate. A number of mouse models involving disruption of G protein-coupled receptors or their agonists affect body weight and adiposity. Deficiency of the melanin-concentrating hormone (MCH) prohormone, Pmch (41), or its receptor, Mch1r (29), produces a lean phenotype in mice as a result of increased metabolic rate. Mch1r is coupled to inhibition of adenylyl cyclase (35) and stimulation of calcium channels (16). Deficiency of Npy2r, Npy4r, or a combination of both receptors produces a lean mouse (38). Npy2r is coupled to inhibition of adenylyl cyclase and inhibition of calcium channels through a pertussin toxin-sensitive G protein (40), most likely αi3 and unknown βγ subunits (15). Particular interest revolves around these signaling pathways, since neuropeptide Y affects seizure susceptibility (11). Deficiency of the CB1 cannabinoid receptor, Cnr1, produces mice with a lean phenotype due in part to decreased caloric intake (8). Cnr1 is coupled to inhibition of presynaptic calcium channels that regulate neurotransmitter release (45). Again, special interest is focused on this signaling pathway, since Cnr1−/− mice showed a mortality phenotype similar to that of our Gng3−/− mice, with ∼25% of the mice dying by the age of 6 months (48).
Lack of functional compensation by other γ subtypes.
The presence of a phenotype in the Gng3−/− mice suggests that at least some of the functions of γ3 are unique and cannot be replaced by other related family members. The inability of other subunits to substitute for γ3 in the context of the organism could be due to a distinctive structural feature or a unique expression pattern of this gene. To begin to distinguish between these possibilities, we examined the effect of loss of γ3 on other γ subtypes. We showed that loss of γ3 results in a specific increase in the level of γ2 in the cortex and the level of γ7 in the striatum. These results suggest that modest adaptations in γ subunit expression may occur in a region-specific fashion in Gng3−/− mice. Such adaptations may reflect a compensatory change to replace the lost γ3 in the same signaling pathway. However, based on the relative levels of these γ subtypes within these brain regions, the relatively small changes in γ2 and γ7 would not be sufficient to functionally compensate for the much larger loss of γ3. Alternatively, such changes may reflect a secondary alteration in another signaling pathway or cell type. For example, in situ hybridization studies indicate that γ3 and γ7 are expressed in different neuronal populations within the striatum (2). Assuming that this pattern holds true in Gng3−/− mice, it suggests that these two γ subtypes normally function in distinct signaling pathways in different cell types, with the changes observed in Gng3−/− mice revealing a novel, functional interaction between the two.
Heterotrimeric associations of γ3.
To gain insight into possible associations of γ3, we have examined the effect of loss of γ3 on expression of β1 and β2 in different regions of the brain. Notably, there were significant reductions in the levels of β2 in the cortex, striatum, and cerebellum and in the level of β1 in the cerebellum. In vitro studies have previously demonstrated that the γ subunit enhances the stability of the β subunit; treatment of HEK293 cells with a ribozyme directed against the γ7 subunit results in a dramatic reduction in the half-life of the β1 subunit (47). We also examined the effect of loss of γ3 on levels of the various α subtypes in different regions of the brain. Intriguingly, we found a small, but specific, reduction in the levels of αi3 in the cortex, hippocampus, and cerebellum. Further studies will be needed to determine if γ3 associates with additional α subtypes in these brain regions.
Signal transduction pathways requiring γ3.
We have begun to look for possible receptor signaling pathways that are responsible for the observed phenotype of Gng3−/− mice. For this purpose, we employed a novel autoradiographic technique that measures receptor-mediated activation of G proteins in brain slices. Focusing on a small subset of receptors and brain regions that could be responsible for the observed phenotype, we saw no differences in mu opioid-, A1 adenosine-, and CB1 cannabinoid receptor-stimulated GTPγS binding in selected brain regions between wild-type and Gng3−/− mice. Obviously, we need to examine a much larger number of receptors and brain regions. Moreover, by using maximally effective concentrations of the receptor agonist, we may have missed a modest decrease in the potency (i.e., increase in the 50% effective concentration). Nonetheless, there does not appear to be a widespread disruption of receptor-mediated activation of G proteins in the regions examined to date.
Because immunoblot data revealed a small but significant reduction in the αi3 subunit levels, we also compared inhibition of adenylyl cyclase activity by the CB1 cannabinoid and A1 adenosine receptors. No differences in adenylyl cyclase activity were observed between wild-type and knockout mice, but other possible effectors remain to be examined.
Contrast to Gng7−/− mice.
Initial characterization of Gng3−/− mice reveals a complex phenotype that includes increased seizure susceptibility, decreased body weight, and reduced fat stores. This phenotype was associated with a modest decrease in the level of αi3 and no disruption of adenylyl cyclase signaling in several brain regions of Gng3−/− mice. Importantly, the phenotype of Gng3−/− mice contrasts sharply with that of Gng7−/− mice. Gng7−/− mice had no observable seizure activity and had normal body weight. However, they displayed an increased acoustic startle response and a trend toward decreased exploratory locomotor activity. This phenotype was associated with a decreased level of αolf and disruption of D1 dopamine receptor-stimulated adenylyl cyclase signaling in the striatum (39). Because γ3 and γ7 are structurally similar and are predominantly expressed in the brain, the finding that mice lacking one or the other subtype display distinctive phenotypes provides conclusive proof that they perform nonredundant roles in separate signaling pathways or biological processes. Collectively, these results support the notion that the γ subunit composition contributes to the specificity of signaling pathways in the context of the organism.
Acknowledgments
We are indebted to the outstanding technicians in our animal care facility: Cynthia J. Rhone, Gail L. Gregory, and Shannon Wescott. We are grateful to Sigrid Wattler and Michael C. Nehls at Lexicon Genetics, Inc., for the construction of the Gng3+/fl mice. We thank Nicole Schechter for technical assistance with the GTPγS binding.
This work was supported by NIH grants GM39867 (awarded to J.D.R.), DA-10770 and DA-05274 (awarded to D.E.S.), and DA-14277 (awarded to L.J.S.-S.).
REFERENCES
- 1.Asano, T., R. Morishita, K. Ohashi, M. Nagahama, T. Miyake, and K. Kato. 1995. Localization of various forms of the γ subunit of G protein in neural and nonneural tissues. J. Neurochem. 64:1267-1273. [DOI] [PubMed] [Google Scholar]
- 2.Betty, M., S. W. Harnish, K. J. Rhodes, and M. I. Cockett. 1998. Distribution of heterotrimeric G-protein β and γ subunits in the rat brain. Neuroscience 85:475-486. [DOI] [PubMed] [Google Scholar]
- 3.Bozzi, Y., D. Vallone, and E. Borrelli. 2000. Neuroprotective role of dopamine against hippocampal cell death. J. Neurosci. 20:8643-8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckmaster, P. S., V. Otero-Corchon, M. Rubinstein, and M. J. Low. 2002. Heightened seizure severity in somatostatin knockout mice. Epilepsy Res. 48:43-56. [DOI] [PubMed] [Google Scholar]
- 5.Cali, J. J., E. A. Balcueva, I. Rybalkin, and J. D. Robishaw. 1992. Selective tissue distribution of G protein γ subunits, including a new form of the γ subunits identified by cDNA cloning. J. Biol. Chem. 267:24023-24027. [PubMed] [Google Scholar]
- 6.Childers, S. R., T. Sexton, and M. B. Roy. 1994. Effects of anandamide on cannabinoid receptors in rat brain membranes. Biochem. Pharmacol. 47:711-715. [DOI] [PubMed] [Google Scholar]
- 7.Clapham, D. E., and E. J. Neer. 1997. G protein βγ subunits. Annu. Rev. Pharmacol. Toxicol. 37:167-203. [DOI] [PubMed] [Google Scholar]
- 8.Cota, D., G. Marsicano, M. Tschop, Y. Grubler, C. Flachskamm, M. Schubert, D. Auer, A. Yassouridis, C. Thone-Reineke, S. Ortmann, F. Tomassoni, C. Cervino, E. Nisoli, A. C. Linthorst, R. Pasquali, B. Lutz, G. K. Stalla, and U. Pagotto. 2003. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Investig. 112:423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degtiar, V. E., B. Wittig, G. Schultz, and F. Kalkbrenner. 1996. A specific Go heterotrimer couples somatostatin receptors to voltage-gated calcium channels in RINm5F cells. FEBS Lett. 380:137-141. [DOI] [PubMed] [Google Scholar]
- 10.Downes, G. B., N. G. Copeland, N. A. Jenkins, and N. Gautam. 1998. Structure and mapping of the G protein γ3 subunit gene and a divergently transcribed novel gene, Gng3lg. Genomics 53:220-230. [DOI] [PubMed] [Google Scholar]
- 11.Erickson, J. C., K. E. Clegg, and R. D. Palmiter. 1996. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature 381:415-421. [DOI] [PubMed] [Google Scholar]
- 12.Evanko, D. S., M. M. Thiyagarajan, D. P. Siderovski, and P. B. Wedegaertner. 2001. Gβγ isoforms selectively rescue plasma membrane localization and palmitoylation of mutant Gαs and Gαq. J. Biol. Chem. 276:23945-23953. [DOI] [PubMed] [Google Scholar]
- 13.Foster, K. A., P. J. McDermott, and J. D. Robishaw. 1990. Expression of G proteins in rat cardiac myocytes: effect of KCl depolarization. Am. J. Physiol. 259:H432-H441. [DOI] [PubMed] [Google Scholar]
- 14.Frankel, W. N., L. Taylor, B. Beyer, B. L. Tempel, and H. S. White. 2001. Electroconvulsive thresholds of inbred mouse strains. Genomics 74:306-312. [DOI] [PubMed] [Google Scholar]
- 15.Freitag, C., A. B. Svendsen, N. Feldthus, K. Lossl, and S. P. Sheikh. 1995. Coupling of the human Y2 receptor for neuropeptide Y and peptide YY to guanine nucleotide inhibitory proteins in permeabilized SMS-KAN cells. J. Neurochem. 64:643-650. [DOI] [PubMed] [Google Scholar]
- 16.Gao, X.-B., and A. N. van den Pol. 2002. Melanin-concentrating hormone depresses L-, N-, and P/Q-type voltage-dependent calcium channels in rat lateral hypothalamic neurons. J. Physiol. 542:273-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautam, N., J. Northup, H. Tamir, and M. I. Simon. 1990. G protein diversity is increased by associations with a variety of γ subunits. Proc. Natl. Acad. Sci. USA 87:7973-7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heisler, L. K., H.-M. Chu, and L. H. Tecott. 1998. Epilepsy and obesity in serotonin 5-HT2C receptor mutant mice. Ann. N. Y. Acad. Sci. 861:74-78. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, R. A., R. Alvarez, and Y. Salomon. 1994. Determination of adenylyl cyclase catalytic activity using single and double column procedures. Methods Enzymol. 238:31-56. [DOI] [PubMed] [Google Scholar]
- 20.Iñiguez-Lluhi, J. A., M. I. Simon, J. D. Robishaw, and A. G. Gilman. 1992. G protein βγ subunits synthesized in Sf9 cells. J. Biol. Chem. 267:23409-23417. [PubMed] [Google Scholar]
- 21.Kawai, H., M. L. Allende, R. Wada, M. Kono, K. Sango, C. Deng, T. Miyakawa, J. N. Crawley, N. Werth, U. Bierfreund, K. Sandhoff, and R. L. Proia. 2001. Mice expressing only monosialoganglioside GM3 exhibit lethal audiogenic seizures. J. Biol. Chem. 276:6885-6888. [DOI] [PubMed] [Google Scholar]
- 22.Liang, J. J., M. Cockett, and X. Z. Khawaja. 1998. Immunohistochemical localization of G protein β1, β2, β3, β4, β5, and γ3 subunits in the adult rat brain. J. Neurochem. 71:345-355. [PubMed] [Google Scholar]
- 23.Lim, W. K., C. S. Myung, J. C. Garrison, and R. R. Neubig. 2001. Receptor-G protein γ specificity: γ11 shows unique potency for A1 adenosine and 5-HT1A receptors. Biochemistry 40:10532-10541. [DOI] [PubMed] [Google Scholar]
- 24.Lodowski, D. T., J. A. Pitcher, W. D. Capel, R. J. Lefkowitz, and J. J. Tesmer. 2003. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gβγ. Science 300:1256-1262. [DOI] [PubMed] [Google Scholar]
- 25.Macrez-Leprêtre, N., F. Kalkbrenner, G. Schultz, and J. Mironneau. 1997. Distinct functions of Gq and G11 proteins in coupling α1-adrenoreceptors to Ca2+ release and Ca2+ entry in rat portal vein myocytes. J. Biol. Chem. 272:5261-5268. [DOI] [PubMed] [Google Scholar]
- 26.Macrez-Leprêtre, N., F. Kalkbrenner, J.-L. Morel, G. Schultz, and J. Mironneau. 1997. G protein heterotrimer Gα13β1γ3 couples the angiotensin AT1A receptor to increases in cytoplasmic Ca2+ in rat portal vein myocytes. J. Biol. Chem. 272:10095-10102. [DOI] [PubMed] [Google Scholar]
- 27.Magré, J., M. Delépine, E. Khallouf, T. Gedde-Dahl, Jr., L. Van Maldergem, E. Sobel, J. Papp, M. Meier, A. Mégarbané, A. Bachy, A. Verloes, F. H. d'Abronzo, E. Seemanova, R. Assan, N. Baudic, C. Bourut, P. Czernichow, F. Huet, F. Grigorescu, M. de Kerdanet, D. Lacombe, P. Labrune, M. Lanza, H. Loret, F. Matsuda, J. Navarro, A. Nivelon-Chevalier, M. Polak, J. J. Robert, P. Tric, N. Tubiana-Rufi, C. Vigouroux, J. Weissenbach, S. Savasta, J. A. Maassen, O. Trygstad, P. Bogalho, P. Freitas, J. L. Medina, F. Bonnicci, B. I. Joffe, G. Loyson, V. R. Panz, F. J. Raal, S. O'Rahilly, T. Stephenson, C. R. Kahn, M. Lathrop, and J. Capeau. 2001. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat. Genet. 28:365-370. [DOI] [PubMed] [Google Scholar]
- 28.Marsh, D. J., S. C. Baraban, G. Hollopeter, and R. D. Palmiter. 1999. Role of the Y5 neuropeptide Y receptor in limbic seizures. Proc. Natl. Acad. Sci. USA 96:13518-13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh, D. J., D. T. Weingarth, D. E. Novi, H. Y. Chen, M. E. Trumbauer, A. S. Chen, X. M. Guan, M. M. Jiang, Y. Feng, R. E. Camacho, Z. Shen, E. G. Frazier, H. Yu, J. M. Metzger, S. J. Kuca, L. P. Shearman, S. Gopal-Truter, D. J. MacNeil, A. M. Strack, D. E. MacIntyre, L. H. Van der Ploeg, and S. Qian. 2002. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc. Natl. Acad. Sci. USA 99:3240-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazzoni, M. R., S. Taddei, L. Giusti, P. Rovero, C. Galoppini, A. D'Ursi, S. Albrizio, A. Triolo, E. Novellino, G. Greco, A. Lucacchini, and H. E. Hamm. 2000. A Gαs carboxyl-terminal peptide prevents Gs activation by the A2A adenosine receptor. Mol. Pharmacol. 58:226-236. [DOI] [PubMed] [Google Scholar]
- 31.Meisler, M. H., J. Kearney, R. Ottman, and A. Escayg. 2001. Identification of epilepsy genes in human and mouse. Annu. Rev. Genet. 35:567-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore, R. J., R. Xiao, L. J. Sim-Selley, and S. R. Childers. 2000. Agonist-stimulated [35S]GTPγS binding in brain: modulation by endogenous adenosine. Neuropharmacology 39:282-289. [DOI] [PubMed] [Google Scholar]
- 33.Morishita, R., S. Saga, N. Kawamura, Y. Hashizume, T. Inagaki, K. Kato, and T. Asano. 1997. Differential localization of the γ3 and γ12 subunits of G proteins in the mammalian brain. J. Neurochem. 68:820-827. [DOI] [PubMed] [Google Scholar]
- 34.Morishita, R., H. Shinohara, H. Ueda, K. Kato, and T. Asano. 1999. High expression of the γ5 isoform of G protein in neuroepithelial cells and its replacement of the γ2 isoform during neuronal differentiation in the rat brain. J. Neurochem. 73:2369-2374. [DOI] [PubMed] [Google Scholar]
- 35.Pissios, P., D. J. Trombly, I. Tzameli, and E. Maratos-Flier. 2003. Melanin-concentrating hormone receptor 1 activates extracellular signal-regulated kinase and synergizes with Gs-coupled pathways. Endocrinology 144:3514-3523. [DOI] [PubMed] [Google Scholar]
- 36.Prosser, H. M., C. H. Gill, W. D. Hirst, E. Grau, M. Robbins, A. Calver, E. M. Soffin, C. E. Farmer, C. Lanneau, J. Gray, E. Schenck, B. S. Warmerdam, C. Clapham, C. Reavill, D. C. Rogers, T. Stean, N. Upton, K. Humphreys, A. Randall, M. Geppert, C. H. Davies, and M. N. Pangalos. 2001. Epileptogenesis and enhanced prepulse inhibition in GABAB1-deficient mice. Mol. Cell. Neurosci. 17:1059-1070. [DOI] [PubMed] [Google Scholar]
- 37.Robishaw, J. D., and C. H. Berlot. 2004. Translating G protein subunit diversity into functional specificity. Curr. Opin. Cell Biol. 16:1-4. [DOI] [PubMed] [Google Scholar]
- 38.Sainsbury, A., P. A. Baldock, C. Schwarzer, N. Ueno, R. F. Enriquez, M. Couzens, A. Inui, H. Herzog, and E. M. Gardiner. 2003. Synergistic effects of Y2 and Y4 receptors on adiposity and bone mass revealed in double knockout mice. Mol. Cell. Biol. 23:5225-5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwindinger, W. F., K. S. Betz, K. E. Giger, A. Sabol, S. K. Bronson, and J. D. Robishaw. 2003. Loss of G protein γ7 alters behavior and reduces striatal αolf level and cAMP production. J. Biol. Chem. 278:6575-6579. [DOI] [PubMed] [Google Scholar]
- 40.Shigeri, Y., and M. Fujimoto. 1994. Y2 receptors for neuropeptide Y are coupled to three intracellular signal transduction pathways in a human neuroblastoma cell line. J. Biol. Chem. 269:8842-8848. [PubMed] [Google Scholar]
- 41.Shimada, M., N. A. Tritos, B. B. Lowell, J. S. Flier, and E. Maratos-Flier. 1998. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 396:670-674. [DOI] [PubMed] [Google Scholar]
- 42.Signorini, S., Y. J. Liao, S. A. Duncan, L. Y. Jan, and M. Stoffel. 1997. Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc. Natl. Acad. Sci. USA 94:923-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sim, L. J., D. E. Selley, and S. R. Childers. 1995. In vitro autoradiography of receptor-activated G-proteins in rat brain by agonist-stimulated guanylyl 5′-[γ-[35S]thio]-triphosphate binding. Proc. Natl. Acad. Sci. USA 92:7242-7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skradski, S. L., A. M. Clark, H. Jiang, H. S. White, Y. H. Fu, and L. J. Ptacek. 2001. A novel gene causing a Mendelian audiogenic mouse epilepsy. Neuron 31:537-544. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan, J. M. 1999. Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J. Neurophysiol. 82:1286-1294. [DOI] [PubMed] [Google Scholar]
- 46.Tecott, L. H., L. M. Sun, S. F. Akana, A. M. Strack, D. H. Lowenstein, M. F. Dallman, and D. Julius. 1995. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 374:542-546. [DOI] [PubMed] [Google Scholar]
- 47.Wang, Q., B. K. Mullah, and J. D. Robishaw. 1999. Ribozyme approach identifies a functional association between the G protein β1γ7 subunits in the β-adrenergic receptor signaling pathway. J. Biol. Chem. 274:17365-17371. [DOI] [PubMed] [Google Scholar]
- 48.Zimmer, A., A. M. Zimmer, A. G. Hohmann, M. Herkenham, and T. I. Bonner. 1999. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc. Natl. Acad. Sci. USA 96:5780-5785. [DOI] [PMC free article] [PubMed] [Google Scholar]