Abstract
Background
Amebic liver abscess is a rare disease in high-income countries. Recurrence of amebic liver abscess is even rarer with only a few previous reports. Here we present a patient who developed three subsequent amebic liver abscesses over a sixteen-year period.
Case presentation
A Caucasian male developed recurrent amebic liver abscesses, when aged 23, 27 and 39 years. Only on the first occasion did this coincide with a recent visit to the tropics. The patient received adequate treatment during each episode. Possible explanations are persistent asymptomatic carrier state, cysts passage in his family, re-infection or chance.
Conclusion
We describe the unusual case of a healthy male who developed recurrent amebic liver abscesses over a long period despite adequate treatment. Possible pathophysiological explanations are explored.
Keywords: Entamoeba histolytica, Amebiasis, Carrier state environment, Immune response, Relapse, Treatment
Background
Intestinal amebiasis and amebic liver abscess, caused by the protozoan Entamoeba histolytica, are rarely reported from high-income countries. It is mostly encountered as an imported disease from regions with poor sanitation levels. Infection occurs through ingestion of E. histolytica cysts in contaminated food or water. Most infections remain asymptomatic while approximately 10 % of patients develop invasive disease and less than 1 % of the infections result in liver abscess [1]. Recurrence of amebic liver abscess after appropriate treatment with the combination of a nitroimidazole derivate with a luminal agent is rare with only a few previous reports [2–4]. The majority of those recurrences could be explained by failure to administer an adequate luminal agent [5–8]. We report an extraordinary case of a Caucasian (Dutch) male patient, who developed amebic liver abscesses three times over a period of 16 years, despite adequate combination treatment on each occasion.
Case presentation
A previously healthy 23-year old, Caucasian man was admitted for the first time 19 years ago (1997), at the age of 23 with a 4-week history of upper right abdominal pain, loose stools and fever. Three months previously he had made a 5-week trip through Indonesia, Thailand and Malaysia. Laboratory testing revealed elevated inflammatory parameters and a slightly increased gamma-glutamyltransferase (Table 1). Ultrasound of the abdomen demonstrated a vaguely defined area in the right liver lobe compatible with an abscess and Entamoeba spp. cysts were detected in a stool sample, supporting the diagnosis of an amebic liver abscess. On admission no specific antibodies against E. histolytica were detectable using an ELISA method. The liver abscess was not aspirated. The patient was treated with metronidazole followed by diloxanide furoate with good clinical response. Serology for E. histolytica was positive one month later. Six months later, stool examination by microscopy for cysts was negative.
Table 1.
Clinical characteristics of the 3 episodes of amebic liver abscesses
| 1st episode (1997) | 2nd episode (2001) | 3rd episode (2013) | |
|---|---|---|---|
| Age (years) | 23 | 27 | 39 |
| Symptoms | 4 weeks fever, sweating, upper right abdominal pain | 5 days spiking fever, malaise | 5 days spiking fever, sweating, upper abdominal pain |
| Physical examination | Temperature 38.4, upper right abdominal tenderness | No abnormalities | temperature 37.1, upper abdominal tenderness |
| Laboratory tests (reference range) | |||
| Hemoglobin mmol/L (8.5–11.0) | 7,2 | 8,3 | 8,7 |
| Leucocytes × 109/L (4.0–10.0) | 11 | 19,7 | 24,4 |
| C-reactive protein mg/L (0–5) | ND | 276 | 339b |
| Erythrocyte sedimentation rate mm/hr (<15) | 94 | 63 | 74 |
| Bilirubin total µmol/L (0–17) | 8 | 29 | 15 |
| Aspartate aminotransferase U/L (0–35) | 23 | 19 | 28b |
| Alanine aminotransferase U/L (0–45) | 69 | 46 | 23 |
| Alkaline phosphatase U/L (0–390) | ND | 107 | 99 |
| Lactate dehydrogenase U/L (0–248) | 244 | 288 | 160 |
| Gamma-glutamyltransferase U/L (0–55) | 190 | 58 | 63b |
| Creatinine µmol/L (64–104) | ND | 101 | 86 |
| Radiology | Liver abscess in the right lobe, size 6 × 5 cm (ultrasound) | Abscess high in the liver, size 10 cm (CT) | Liver abscess in the right lobe, size 4.9 × 4.5 cm with satellite abscesses (CT)b |
| Additional tests | |||
| Stool microscopy | Entamoeba spp. Cysts | Negative | Entamoeba spp. cysts and trophozoites |
| Stool PCR E. histolytica | ND | ND | positive |
| Serology for amebiasis | <1:40, 1 month later 1:320 | 1:320 | 1:640 and 1:640a |
| Abscess fluid | ND | Culture negative, no amebic trophozoites | ND |
| Treatment | Metronidazole 750 mg tid 7 days | Metronidazole 750 mg tid 10 days | Metronidazole 750 mg tid 10 days |
| Diloxanide furoate 500 mg tid 10 days | Diloxanide furoate 500 mg tid 10 days | Paromomycin 500 mg tid 10 days | |
| Aspiration and drainage liver abscess | |||
CT computed tomography, PCR polymerase chain reaction, ND not determined
aResults from samples collected June 9, 2013 and September 4, 2013, respectively (cut off <1:40)
bOne week after presentation during the 3rd episode the patient was readmitted and C-reactive protein decreased to 46 mg/L, aspartate aminotransferase increased to and 42 U/L and gamma-glutamyltransferase increased to 129 U/L, the size of the abscess increased to 5 × 7 cm
cState of the art treatment on all occasions: nitroimidazole derivate followed by a luminal agent
Four years later (2001) the patient was admitted with a 10 cm large abscess high in the liver with a 5-day history of spiking fever and malaise. Since 1997 he had travelled once to Nepal and Bangladesh in 1999. The patient was again diagnosed with an amebic liver abscess, which was supported by positive serology results. Treatment was initiated with metronidazole. However, fever persisted and after 5 days percutaneous drainage of the abscess was performed. In the aspirate no trophozoites of E. histolytica were found and the bacterial cultures remained negative. The recovery hereupon was uneventful and a 10-day course of metronidazole was followed by diloxanide furoate for 10 days. Ultrasound of the liver demonstrated complete resolution of the abscess 6 months later.
Finally, three years ago—with a twelve year interval (2013)—the patient was admitted for the third time with a 5-day history of spiking fever and upper abdominal pain. Two years earlier, in 2011, the patient had been in Thailand for one week and had made short trips to China (in 2006, 2009 and 2012). Laboratory examination revealed increased levels of inflammation markers and computed tomography (CT) showed an abscess of 5 cm in the right liver lobe with small satellite abscesses. For the third time the diagnosis of amebic liver abscess was made, based on positive serology results and the detection of E. histolytica in a stool sample by polymerase chain reaction (PCR). Treatment was started with metronidazole. Initially the patient responded well and was discharged on day 5. After 10 days of metronidazole, paromomycin was started. One week after discharge, the patient was readmitted with persistent low-grade fever and abdominal pain. C-reactive protein concentration had decreased (46 mg/L), but slightly increased levels of aspartate aminotransferase (42 U/L) and gamma-glutamyltransferase (129 U/L) were noted. A new CT-scan revealed a subtle increase in the size of the abscess with subcapsular extension (Fig. 1). Percutaneous drainage was deferred because the clinical condition improved. The patient subsequently made an uncomplicated recovery and has remained in excellent condition to date. There were no indications of immune deficiencies. Normal amounts of T- and B-cells, immunoglobulins and complement factors were found (Table 2). In addition a human immunodeficiency virus (HIV) test was negative.
Fig. 1.
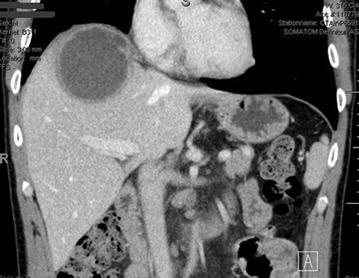
Upper abdominal computed tomography scan revealing an abscess of 7 cm in the right liver lobe with a small margin of liver tissue to the right hemidiaphragm and subcapsular extension
Table 2.
Laboratory evaluation of the immune system of the patient
| Variable | Reference range adults | Results |
|---|---|---|
| B-cells (×109/L) | 0.10–0.40 | 0.27 |
| T-cells (×109/L) | 0.70–1.90 | 1.50 |
| CD4 (×109/L) | 0.40–1.30 | 0.90 |
| CD8 (×109/L) | 0.20–0.70 | 0.56 |
| NK-cells (×109/L) | 0.10–0.40 | 0.29 |
| IgG (g/L) | 7.00–16.00 | 11.10 |
| IgG1 (g/L) | 4.90–11.40 | 9.25 |
| IgG2 (g/L) | 1.50–6.40 | 2.60 |
| IgG3 (g/L) | 0.20–1.10 | 0.51 |
| IgG4 (g/L) | 0.08–1.40 | 0.55 |
| IgA (g/L) | 0.76–3.91 | 2.33 |
| IgM (g/L) | 0.45–2.30 | 1.46 |
| C3 (g/L) | 0.90–1.80 | 1.11 |
| C4 (g/L) | 0.10–0.40 | 0.20 |
| C1Q (g/L) | 0.05–0.25 | 0.24 |
| M-protein | negative |
Stool samples of the patient’s wife, who accompanied him from the first amebic abscess episode onwards, and their 2 children were investigated for E. histolytica. The wife tested positive for E. histolytica by PCR examination of the stool, but E. histolytica serology was negative. She was treated with paromomycin 500 mg tid for 10 days.
Discussion
Our patient suffered from recurrent amebic liver abscesses on three separate occasions over a period of 16 years. Each time the diagnosis of amebic liver abscess was based on the clinical presentation, ultrasound or CT imaging and positive E. histolytica serology. The first and third episode Entamoeba spp. cysts were detected in stool samples. The abscess was aspirated only in the second episode; no trophozoites of E. histolytica were detected. The material was obtained 5 days after initiation of therapy, which probably accounts for the negative result of microscopy. The diagnosis of amebic liver abscess was further supported in each episode by clinical response after appropriate treatment.
Only a small proportion of E. histolytica-infected individuals develop invasive disease, whereas the majority of individuals carry the parasite within the gut without clinical symptoms [1]. Previous studies have indicated that both cell-mediated immunity and macrophage-mediated effector mechanisms are involved in host resistance to E. histolytica infection [9]. Neutrophils activated by interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α), or lipopolysaccharides (LPS) have amebicidal activity in vitro by releasing reactive oxygen species. Macrophages are also amebicidal after stimulation with IFN-γ or TNF-α. Important for the host defence against E. histolytica is a proper functioning of natural killer T cells (NKT cells) for the production of sufficient IFN-γ and nitric oxide, which induces the amebicidal activity of macrophages [9–11]. Moran et al. suggested that defects in production of reactive oxygen species are a risk factor for recurrence of amebic liver abscess [12]. There is also a protective role for lectin signalling and specific human leukocyte antigen (HLA) II alleles. A study of children in Bangladesh suggested a potential protective association with the HLA class II allele DQB1*0601 and the heterozygous haplotype DQB1*0601/DRB1*1501 for invasive amebiasis [13].
There is no evidence of immune deficiencies in our patient, as the absolute number of NKT cells and other immune cells was within the normal range with an otherwise uneventful medical history.
In contrast to amebic colitis, amebic liver abscess occurs more often in adult males. It is 10 times as common in men as in women and is a rare disease in children [1, 14]. Lotter et al. investigated in a mouse model for amebic liver abscess the role of female and male sexual hormones. Removal of testosterone by orchiectomy reduced the size of abscesses in male mice, while substitution of testosterone in female mice increased the size of amebic liver abscesses. This may be attributable to the inhibitory effect of testosterone on the IFN-γ production by NKT cells [15].
There are several possible explanations for the recurrence of amebic liver abscesses in this patient. The patient had travelled frequently to Asia, and therefore he may have simply been re-infected during his travels. Alternatively, recurrent disease may have occurred due to transmission of cysts between household members. Transmission of E. histolytica within households has been reported earlier [16]. The patient’s wife tested positive for E. histolytica cysts in the stool when investigated after her husband’s third episode. After 2002 she had travelled annually to China (Beijing) and had accompanied her husband during travel to Thailand and Nepal. A repeated infection from household members could have been supported by genotyping the strain(s) by means of PCR-fingerprinting [17]. Unfortunately, no material from past episodes was available to test this hypothesis. Persistent asymptomatic carrier state appears to be highly unlikely since the patient was treated appropriately for persisting intraluminal cysts on all occasions. In addition, the intervals would be exceptionally long for recurrences resulting from inadequate treatment which usually occur within months, not years. On the other hand, Blessmann et al. showed that diloxanide furoate was less effective than paromomycin in eradicating E. histolytica in asymptomatic cyst carriers [18]. Some form of immune dysfunction or genetic susceptibility could also have played a role, but this possibility seems unlikely. Obviously all speculation on the mechanism(s) involved remains hypothetical and this remarkable patient history may ultimately simply have been a matter of chance.
Of note in this case is the decision during the third episode to continue conservative treatment without drainage. In a recent Cochrane review there was no benefit for drainage in addition to metronidazole in case of uncomplicated amebic liver abscess [19]. Aspiration should be considered for patients with either a large left-lobe abscesses, a lack of clinical response within 5 days and in case of uncertainty about the diagnosis [1]. Our patient had a right lobe abscess of 7 cm—with a suspicion of subcapsular expansion - but with a clear margin to the right pleural cavity (Fig. 1). His clinical condition improved during metronidazole treatment, illustrated by the disappearance of the spiking fever and declining C-reactive protein levels (339–46 in 2 weeks).
Conclusion
We present the unusual case of a healthy Dutch male with recurrent amebic liver abscesses on three separate occasions over a period of 16 years despite adequate treatment. While the exact nature of the underlying pathophysiological mechanism(s) will remain obscure, we explored some of the possibilities such as cysts from an asymptomatic close contact or an as yet unidentified immune deficiency in the host. Our observation serves as a welcome reminder of the unpredictable course of amebiasis even in the absence of recent travel to tropical regions.
Authors’ contributions
DC- conceived the case report, wrote the initial manuscript and performed literature review. PW was responsible for the patient’s management episode 3, contributed to the acquisition of data and made critical revision of the manuscript. LV was responsible for the patient’s management episode 1, contributed to the acquisition of data and made critical revision of the manuscript. JH contributed to the acquisition of data and made critical revision of the manuscript. PG made critical revision of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Marian Humphrey is thanked for English grammar correction of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images.
Abbreviations
- CT
computed tomography
- PCR
polymerase chain reaction
- HIV
human immunodeficiency virus
- IFN-γ
interferon-γ
- TNF-α
tumour necrosis factor-α
- LPS
lipopolysaccharides
- NKT cells
natural killer T cells
- HLA
human leukocyte antigen
Contributor Information
D. Creemers-Schild, Email: dinaschild@hotmail.com
P. J. J. van Genderen, Email: p.van.genderen@havenziekenhuis.nl
L. G. Visser, Email: l.g.visser@lumc.nl
J. J. van Hellemond, Phone: +31622279138, Email: j.vanhellemond@erasmusmc.nl
P. J. Wismans, Email: p.wismans@havenziekenhuis.nl
References
- 1.Stanley SL., Jr Amoebiasis. Lancet. 2003;361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- 2.Bauer AG, Schalm SW, Stuiver PC. Failure of conventional treatment to prevent relapse of hepatic amoebiasis. Neth J Med. 1981;24:6–9. [PubMed] [Google Scholar]
- 3.Ramiro M, Moran P, Olvera H, Curiel O, Gonzalez E, Ramos F, et al. Reincidence of amebic liver abscess: a case report. Arch Med Res. 2000;31:S1–S3. doi: 10.1016/S0188-4409(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 4.Hwang EW, Cheung L, Mojtahed A, Cartwright CA. Relapse of intestinal and hepatic amebiasis after treatment. Dig Dis Sci. 2011;56:677–680. doi: 10.1007/s10620-010-1492-y. [DOI] [PubMed] [Google Scholar]
- 5.Singal DK, Mittal A, Prakash A. Recurrent amebic liver abscess. Indian J Gastroenterol. 2012;31:271–273. doi: 10.1007/s12664-012-0210-4. [DOI] [PubMed] [Google Scholar]
- 6.Nespola B, Betz V, Brunet J, Gagnard JC, Krummel Y, Hansmann Y, et al. First Case of amebic liver abscess 22 years after the first occurrence. Parasite. 2015;22:20. doi: 10.1051/parasite/2015020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyon C, Greve E, Hag B, Cuilleron M, Jospe R, Nourrisson C, et al. Amebic liver abscess and late recurrence with no travel in an endemic area. Med Sante Trop. 2013;23:344–346. doi: 10.1684/mst.2013.0189. [DOI] [PubMed] [Google Scholar]
- 8.Dooley CP, O’Morain CA. Recurrence of hepatic amebiasis after successful treatment with metronidazole. J Clin Gastroenterol. 1988;10:339–342. doi: 10.1097/00004836-198806000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Moonah SN, Jiang NM, Petri WA., Jr Host immune response to intestinal amebiasis. PLoS Pathog. 2013;9:e1003489. doi: 10.1371/journal.ppat.1003489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotter H, Gonzalez-Roldan N, Lindner B, Winau F, Isibasi A, Moreno-Lafont M, et al. Natural killer T cells activated by a lipopeptidophosphoglycan from Entamoebe histolytica are critically important to control amebic liver abscess. PLoS Pathog. 2009;5:e1000434. doi: 10.1371/journal.ppat.1000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seydel KB, Smith SJ, Stanley SL., Jr Innate immunity to amebic liver abscess is dependent on gamma interferon and nitric oxide in a murine model of disease. Infect Immun. 2000;68:400–402. doi: 10.1128/IAI.68.1.400-402.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moran P, Rico G, Ramiro M, Olvera H, Ramos F, Gonzalez E, et al. Defective production of reactive oxygen intermediates (ROI) in a patient with recurrent amebic liver abscess. Am J Trop Med Hyg. 2002;67:632–635. doi: 10.4269/ajtmh.2002.67.632. [DOI] [PubMed] [Google Scholar]
- 13.Duggal P, Haque R, Roy S, Mondal D, Sack RB, Farr BM, et al. Influence of human leukocyte antigen class II alleles on susceptibility to Entamoebe histolytica infection in Bangladeshi children. J Infect Dis. 2004;189:520–526. doi: 10.1086/381272. [DOI] [PubMed] [Google Scholar]
- 14.Acuna-Soto R, Maguire JH, Wirth DF. Gender distribution in asymptomatic and invasive amebiasis. Am J Gastroenterol. 2000;95:1277–1283. doi: 10.1111/j.1572-0241.2000.01525.x. [DOI] [PubMed] [Google Scholar]
- 15.Lotter H, Helk E, Bernin H, Jacobs T, Prehn C, Adamski J, et al. Testosterone increases susceptibility to amebic liver abscess in mice and mediates inhibition of IFNγ secretion in natural killer T cells. PLoS ONE. 2013;8:e55694. doi: 10.1371/journal.pone.0055694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vreden SG, Visser LG, Verweij JJ, Blotkamp J, Stuiver PC, Aguirre A, et al. Outbreak of amebiasis in a family in The Netherlands. Clin Infect Dis. 2000;31:1101–1104. doi: 10.1086/318153. [DOI] [PubMed] [Google Scholar]
- 17.Ali IK, Zaki M, Clark CG. Use of PCR amplification of tRNA gene-linked short tandem repeats for genotyping Entamoeba histolytica. J Clin Microbiol. 2005;43:5842–5847. doi: 10.1128/JCM.43.12.5842-5847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blessmann J, Tannich E. Treatment of asymptomatic intestinal Entamoeba histolytica infection. N Engl J Med. 2002;347:1384. doi: 10.1056/NEJM200210243471722. [DOI] [PubMed] [Google Scholar]
- 19.Chavez-Tapia NC, Hernandez-Calleros J, Tellez-Avila FI, Torre A, Uribe M. Image-guided percutaneous procedure plus metronidazole versus metronidazole alone for uncomplicated amoebic liver abscess. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD004886.pub2. [DOI] [PubMed] [Google Scholar]


