Abstract
Implantation and the establishment of pregnancy in mammals involves an intricate interplay of hormones, cytokines, growth factors, proteins, lipids, ions and the extracellular matrix between the uterine epithelium, stroma, immune cells and the conceptus trophectoderm. The divergent nature of implantation in the mouse, human and pig provides not only an interesting contrast in the establishment of pregnancy and early embryonic development but also intriguing similarities with regard to early endometrial-conceptus signaling. An interesting pro-inflammatory cytokine expressed in a number of mammalian species during the period of implantation is interleukin-1β (IL1B). The presence of IL1B might be involved with immunotolerance at the maternal-placental interface and has been proposed as one of the mediators in placental viviparity. The production of IL1B and other proinflammatory cytokines might play a role in establishing pregnancy through modulation of the nuclear factor kappa-B (NFKB) system in a number of species. A model for the regulation of cellular progesterone receptor expression and NFKB activation for endometrial receptivity and conceptus attachment is continuing to evolve and is discussed in the present review.
Keywords: Endometrium, Conceptus, Pregnancy, Interleukin-1β, Nuclear factor kappa-B
Introduction
Implantation and the establishment of pregnancy in mammals is one of the most fascinating phenomena of reproductive biology. The intricate interplay of hormones, cytokines, growth factors, proteins, lipids, ions and the extracellular matrix (ECM) between the uterine epithelium, stroma, immune cells and the conceptus trophectoderm that establishes pregnancy is complex and distinct among species (Moffett and Loke 2006; Wang and Dey 2006; Bazer et al. 2010). The period of in vivo development of early cleaving embryos to blastocysts is less variable and can be mimicked outside the oviduct and uterus in vitro under proper culture conditions. Following hatching from the zona pellucida, the blastocyst is exposed to direct contact with the maternal uterine luminal (LE) and glandular (GE) epithelium and a milieu of endometrial secretions. The developing conceptus now initiates one of the most critical periods that is required for the maintenance of the pregnancy and implantation and that precedes placental transformation for nutrient exchange with the maternal system throughout pregnancy.
The opportunity for invasive implantation in rodents, primates and humans (the trophoblast erodes the uterine LE and basal matrix allowing migration into the underlying stroma and ECM of the lamina propria) or the non-invasive placental attachment to the uterine endometrial surface (trophoblast attachment to the uterine LE with no or limited erosion of the uterine surface epithelium) typical of large domestic farm species (cow, sheep, goat, horse and pig) is dependent upon the opening of the uterine “window of receptivity” (Dey et al. 2004; Fazleabas et al. 2004; Fazleabas 2007; Slayden and Keater 2007). A physical barrier to receptivity for uterine adhesion in a number of species, including the human, is the expression of high-molecular-weight mucin O-linked glycoproteins such as MUC1 by the uterine epithelia (Burghardt et al. 1997; Meseguer et al. 1998; Aplin 1999). In general, MUC1 on the surface of the uterine epithelia represents a non-adhesive state for blastocyst attachment in mice (Braga and Gendler 1993), baboons (Hild-Petito et al. 1996; Julian et al. 2005), sheep (Johnson et al. 2001) and pigs (Bowen et al. 1996) and is removed prior to the time of conceptus attachment. Removal of MUC1 permits conceptus attachment to the uterine LE through the expression of integrins and numerous possible adhesive molecules on the conceptus and uterine surface, as described in previous reviews (Burghardt et al. 2002; Carson 2002; Aplin and Kimber 2004; Spencer et al. 2004; Farach-Carson and Carson 2007; Singh and Aplin 2009; van Mourik et al. 2009).
The opening of the window of receptivity is regulated primarily by the pattern of ovarian estrogen and progesterone release and the expression of endometrial steroid receptors established during a normal estrous cycle of domestic farm species or menstrual cycle in humans. Although ovarian estrogen from the developing ovulatory follicle is critical for priming the endometrium, progesterone and the localization of its cellular receptor play an essential role in establishing the proper uterine environment for uterine attachment and early development of the conceptus (Spencer and Bazer 2002). Changes in the uterine LE and GE needed for opening the implantation window are controlled by the rapid and sustained increase in plasma progesterone following ovulation (Spencer and Bazer 2002; Spencer et al. 2004; Geisert et al. 2006). The role of progesterone in opening the window for implantation during early pregnancy is associated with cell-specific changes in the expression of the endometrial progesterone receptor (PGR). Progesterone is the ligand for two receptor isoforms, PGRA and PGRB, which are members of the nuclear receptor superfamily of transcription factors (Mulac-Jericevic and Conneely 2004). Endometrial expression of both receptors is involved with endometrial function but selective knockout studies in mice have indicated that PGRA is the essential form of the receptor for implantation and development (Fernandez-Valdivia et al. 2005). Epithelial PGR has been demonstrated to be a key regulator of uterine epithelial-stromal crosstalk crucial for uterine development and function in mice (Franco et al. 2011). A clear spatiotemporal association exists between the decline or removal of PGR from the endometrial LE and GE (stroma maintain PGR) and receptivity for conceptus implantation (Spencer et al. 2004; Geisert et al. 2006). The length of endometrial exposure to progesterone regulates the timing of PGR down-regulation in LE and GE of large domestic farm species. Administration of exogenous progesterone immediately following ovulation shortens the estrous cycle of sheep (Ottobre et al. 1980) and cattle (Garrett et al. 1988a), whereas the estrous cycle is lengthened in ewes treated with a PGR antagonist (Morgan et al. 1993). Treatment of pregnant pigs with a PGR antagonist following ovulation delays PGR down-regulation in the uterine LE and GE and alters the timing of uterine gene expression and conceptus survival (Mathew et al. 2011). Administration of progesterone following ovulation has been used to accelerate early conceptus growth in both sheep (Lawson and Cahill 1983; Satterfield et al. 2006) and cattle (Garrett et al. 1988b; Mann et al. 2006; Carter et al. 2008) and advance uterine receptivity for the transfer of asynchronous older embryos in cattle (Geisert et al. 1991). Advancement in uterine development with progesterone is consistent with accelerated down-regulation of epithelial PGR and accelerated release of uterine growth factors for the developing sheep conceptus (Satterfield et al. 2009). The down-regulation of PGR in endometrial epithelia (Fig. 1) is a conserved event among mammals associated with the opening of the implantation window in the mouse (Tan et al. 1999), pig (Geisert et al. 1994; Mathew et al. 2011), sheep (Spencer and Bazer 1995), cattle (Meikle et al. 2001), horse (Hart et al. 2005), baboon (Fazleabas et al. 1999) and human (Lessey et al. 1988, 1996; Critchley and Saunders 2009). A suitable uterine environment for the peri-implantation and activation of implantation is established through the loss of PGR from the epithelial cells but the maintenance of PGR in the stromal cell layer stimulates the expression and secretion of progestamedins such as fibroblast growth factor 10 and hepatocyte growth factor (Spencer and Bazer 2002; Cunha et al. 2004), which activate multiple uterine genes involved with growth, morphogenesis, the synthesis of enzymes and enzyme inhibitors, the ECM and cell adhesion prior to trophoblast attachment to the uterine LE (Geisert et al. 2006; van Mourik et al. 2009; Bazer et al. 2009, 2010). Establishment of a receptive endometrium to conceptus attachment is thus regulated through progesterone induction of epithelial PGR loss allowing finely synchronized alterations in the ECM of the LE (exposing attachment factors) and balanced secretion of numerous cytokines, prostaglandins, growth factors, enzymes and their inhibitors (Spencer et al. 2004; Wang and Dey 2006; Bazer et al. 2010).
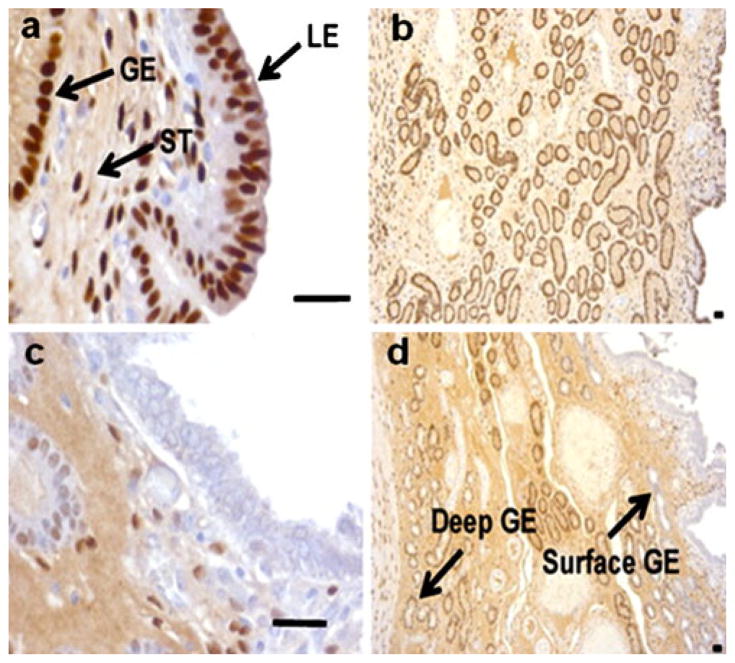
Fig. 1.
Immunohistochemistry for progesterone receptor B (PGRB) in the endometrium on days 8 (a, b) and 12 (c, d) of pregnancy in the pig. Note the loss of PGRB in the luminal epithelium (LE) and surface glandular epithelium (GE) on day 12 when the pig conceptuses would initiate attachment to the uterine surface (ST stroma). Bars 50 μm (from Mathew et al. 2011)
Although the rodent, human, pig and other domestic farm species provide contrasting models of implantation (invasive vs noninvasive) and placentation (hemochorial vs epitheliochorial), similarities exist in the uterine responses to estrogen (E2) required for the establishment of pregnancy in the mouse and pig. Rodents have an invasive type of implantation and thus, have served as valuable models regarding uterine-conceptus interactions necessary for implantation in the human; however, distinct species differences do exist. Following initial priming with E2, endometrial receptivity for implantation of the human blastocyst requires only continuous progesterone stimulation rather than ovarian E2 to induce uterine responsiveness as occurs in rodents (Simon et al. 2003; Wang and Dey 2006). As in humans, placental attachment to the uterine surface in the pig is regulated by progesterone. Although pig conceptuses are highly invasive when placed outside the luminal uterine environment (Samuel and Perry 1972), the pig has a diffuse central-type implantation leading to an epitheliochorial type of placentation because of endometrial secretion of multiple protease inhibitors that block invasion through the LE (Fazleabas et al. 1983; Geisert and Yelich 1997). Attachment of the trophoblast to the LE is preceded by conceptus secretion of E2 to signal the establishment of the pregnancy (Geisert et al. 2006). The divergent nature of implantation in the mouse, human and pig provides not only an interesting contrast in the establishment of pregnancy and early embryonic development but also intriguing similarities with early endometrial-conceptus signaling; this will be the focus for the remainder of the review.
Conceptus signaling and development
Early development of porcine conceptuses is unique compared with that of other large domestic farm species (Bazer et al. 2009, 2010). Porcine conceptuses undergo rapid trophoblast differentiation and expansion between days 11 to 12 of gestation (Geisert et al. 1982a). Conceptuses develop from a 1- to 2-mm sphere into a 9- to 10-mm ovoid shape between days 10 to 12 and then a rapid transition occurs to tubular and filamentous forms by elongation at 30–40 mm/h to >100 mm in length in less than 1–2 h (Geisert et al. 2006). The elongating conceptuses produce E2 that stimulates secretions from the uterine LE and GE; this is closely linked to the initiation of trophoblast attachment to the uterine LE (Burghardt et al. 1997; White et al. 2005; Bazer et al. 2010). Conceptus E2 secretion acts through epithelial estrogen receptor 1 (ESR1) and directly stimulates uterine gene expression, release of uterine secretions (Geisert et al. 1993) and changes in the LE and GE needed for trophoblast attachment such as alteration in integrin expression (Burghardt et al. 2002) and endometrial GE secretion of osteopontin (Garlow et al. 2002; White et al. 2005; Johnson et al. 2003, 2009). Estrogen release might also have an autocrine effect on conceptus development, as ESR2 is expressed in early pre- and post-elongated day 12 conceptuses and then decreases significantly after rapid trophoblast elongation (Ying et al. 2000; Kowalski et al. 2002). Initial trophoblast elongation is followed by a continuation of placental growth until the conceptus reaches over a meter in length by day 16 (Perry and Rowlands 1962).
Porcine conceptus elongation is rapidly followed by attachment to the endometrial LE from days 13 to 18 of pregnancy (Burghardt et al. 1997). Conceptus E2 release has been proposed as the pregnancy recognition signal that maintains the function of the corpora lutea (CL) by preventing development of the endometrial luteolytic mechanism (Bazer et al. 1984). Expression of ESR1 protein peaks in the endometrial LE and GE on day 12 of pregnancy but remains in the epithelia from days 13 to 18 of pregnancy, although at lower abundance (Geisert et al. 1993; Sukjumlong et al. 2003). In the pig, the acute (days 11 to 12) and chronic (days 15 to 20) synthesis and release of E2 by the developing conceptuses provide the signal to maintain the CL throughout gestation (Geisert et al. 1987). The two phases of conceptus E2 secretion alter the movement of endometrial prostaglandin F2α (PGF2α) release into the uterine lumen rather than toward the uterine vasculature as occurs during the estrous cycle (Bazer et al. 1984). During early pregnancy, a large accumulation of both PGF2α and prostaglandin E (PGE) occurs in the uterine lumen (Geisert et al. 1982b). Elongating conceptuses induce an acute release of calcium and continued secretion of uterine proteins into the uterine lumen (Geisert et al. 1982b) and stimulate a local hyperemia surrounding the elongated conceptus (Keys et al. 1986). Early trophoblast expansion regulates and limits the final placental surface area in the epitheliochorial type of placentation in the pig. Uterine and conceptus factors involved with inducing rapid trophoblast elongation are of critical importance. Both embryonic and maternal endometrial syntheses of cytokines and growth factors are essential for providing the synergistic environment for the endocrinological and immunological signals needed for the establishment of pregnancy (Geisert and Yelich 1997; Spencer and Bazer 2004; Bazer et al. 2010), similar to that in other mammalian species (Wang and Dey 2006; Chaouat et al. 2007; Lea and Sandra 2007; Guzeloglu-Kayisli et al. 2009; Banerjee and Fazleabas 2010).
The rapid elongation of pig conceptuses on day 12 involves the expression of many conceptus genes (Ross et al. 2003a; Blomberg and Zuelke 2004; Degrelle et al. 2009; Blomberg et al. 2008; le Blomberg et al. 2010) and the presentation of adhesion factors on the endometrial LE to allow continuous adhesive attachment of the placenta throughout gestation (Bowen et al. 1996; Jaeger et al. 2001; Burghardt et al. 2002; Johnson et al. 2009). A number of endometrial growth factors and cytokines that are possible mediators of attachment and implantation at the conceptus-uterine interface of the mouse and human have been described (Carson et al. 2000; Wang and Dey 2006; Banerjee and Fazleabas 2010). In addition, induction of uterine PG synthesis via prostaglandin-endoperoxide synthase-2 (PTGS2) plays an essential role in the modulation of angiogenesis, cell proliferation and differentiation (Matsumoto et al. 2002). Development and attachment of the pig conceptus involves spatiotemporal expression of a similar cascade of paracrine and autocrine cytokines as described for mouse and human implantation. Uterine and/ or conceptus expression of insulin-like growth factors 1 and 2 (IGF1, IGF2; Green et al. 1995), IGF-binding proteins (Lee et al. 1998; Ashworth et al. 2005), epidermal growth factor (EGF; Vaughan et al. 1992; Kennedy et al. 1994), heparin-binding EGF (Kim et al. 1995), transforming growth factors (Massuto et al. 2010a, 2010b), leukemia inhibitory factor (LIF; Anegon et al. 1994; Modric et al. 2000), fibroblast growth factor-7 (Ka et al. 2000, 2007), interleukin-1β (IL1B; Tuo et al. 1996; Ross et al. 2003b), IL6 (Anegon et al. 1994; Modric et al. 2000), conceptus interferons (IFNτ and IFNΔ; Cross and Roberts 1989; Lefevre and Boulay 1993) and PTGS2 (Wilson et al. 2002; Ashworth et al. 2006) have been studied during the critical period of conceptus attachment in the pig.
IL1B role in implantation
An intriguing pro-inflammatory cytokine expressed in a number of mammalian species during the period of implantation is IL1B (Simon et al. 1994a, 1997a; Takacs and Kauma 1996; Kruessel et al. 1997; Schäfer-Somi et al. 2008). The presence of IL1B might play a role in immunotolerance at the maternal-placental interface and has been proposed as one of the mediators in placental viviparity (Paulesu et al. 2008). IL1, previously termed leukocyte endogenous mediator, was first identified as a mediator of the acute-phase inflammatory response. The ability of IL1B to invoke inflammation is dependent on the expression of members of the IL1 system, which belongs to the IL1B/TLR superfamily. The IL1 system consists in two agonists (IL1A and IL1B), two receptors, i.e., IL1R1 (functional) and IL1R2 (pseudo-receptor), converting enzymes, receptor accessory proteins and multiple isoforms of receptor antagonists (Mantovani et al. 1998). Early studies indicated an essential role of IL1B in implantation as repeated injections of IL1 receptor antagonist into pregnant mice prior to implantation caused implantation failure (Simon et al. 1994b, 1998). However, mice with a deficiency in either IL1B (Zheng et al. 1995) or caspase 1 (CASP1), which is involved with cleaving the pro-IL1B to release the bioactive form from cells (Li et al. 1995), are fertile. In addition, IL1R1 knockout mice (Abbondanzo et al. 1996) have only a slightly reduced litter size and IL1 receptor antagonist knockout mice only have growth retardation after weaning (Horai 2005). Although the essential need of IL1B expression at implantation has not been clearly resolved, its expression and stimulation of other factors involved with implantation have been established.
Habitual abortion in women is associated with a decrease in expression of IL1B and IL6 (von Wolff et al. 2000) suggesting a role for IL1B in the maintenance of pregnancy. Preimplantation human embryos express IL1B (Barañao et al. 1997) and IL1R1 is present in the uterine endometrium (Takacs and Kauma 1996). Human embryos selectively up-regulate β3 integrin in endometrial epithelial cells during implantation; this is induced at least partially through IL1B (Simon et al. 1997b). IL1B has been demonstrated to induce endometrial expression of LIF (Sawai et al. 1997; Gonzalez et al. 2004), PTGS2 (Huang et al. 1998; Tamura et al. 2002) and leptin (Dimitriadis et al. 2005), to inhibit stromal cell differentiation (Frank et al. 1995) and to stimulate the release of placental metalloproteinase 9 (Librach et al. 1994), IL6 (Kauma et al. 1994), colony-stimulating factor-1 (Kauma 1993) and chorionic gonadotropin (Yagel et al. 1989; Seki et al. 1997) in the human.
IL1 system during implantation in the primate
In the human and primate, implantation has been characterized as an inflammatory type response. A number of cytokines have been identified at the implantation site and many of these molecules are of embryonic origin (Tabibzadeh and Sun 1992; Tabibzadeh 1994). IL1 was identified as one such paracrine factor that modulates the communication between the maternal endometrium and embryo (Simon et al. 1994a, 1994b; Krussel et al. 2003; Paulesu et al. 2008). IL1 is a key regulator of the inflammatory response and is currently recognized as a cytokine capable of a wide spectrum of effects on numerous cell types (Bankers-Fulbright et al. 1996). The two forms of IL1 agonists (IL1A and IL1B) bind to the same IL1R1 and, therefore, also show similar, if not identical, biological activities.
In human endometrium, both ligands have a ubiquitous presence in epithelium, stroma and endothelial cells (Tabibzadeh 1994). Purified cytotrophoblasts in culture release IL1B in the manner that parallels their invasive potential (Librach et al. 1994). The cytotrophoblasts isolated from first trimester placentae produce approximately four times more IL1B than those isolated from second and third trimester placentae (Librach et al. 1994). Successful implantation after in vitro fertilization has been correlated to high concentrations of both IL1A and IL1B in the culture medium of human embryos (Karagouni et al. 1998). However, the role of IL1A in the human or non-human primate endometrium has not been as clearly established as IL1B (Simon et al. 1994b; Krussel et al. 2003). IL1 is produced by macrophages, stromal cells and trophoblast cells (Kauma 2000). The expression of IL1R1 throughout the human menstrual cycle follows a triphasic pattern, both in epithelial and in stromal cells. Protein expression is low in the proliferative phase, moderate during the peri-ovulatory and the implantation phases and intense at the end of the cycle (Bigonnesse et al. 2001). In early human implantation sites, immunostaining for IL1R1 has been reported for syncytiotrophoblast and hyperplastic endometrial glands in the maternal decidua, with only weak staining in stromal cells (Hu et al. 1992; Simon et al. 1994a). The expression of the IL1 system in single blastomeres of preimplantation human embryos has also been documented and the selective release of IL1 from human embryo is observed only when embryos are co-cultured with human endometrial epithelial cells (Krussel et al. 1998; De los Santos et al. 1996). The third member of the IL1 ligand family is the natural IL-1 receptor antagonist (IL1RN). IL1RN can block the binding of IL1A and IL1B to its receptor and its binding to the receptor does not result in signal transduction (Bankers-Fulbright et al. 1996). As has been reported, IL1RN prevents embryonic implantation through a direct effect on the transformation of the epithelial plasma membrane at the time of implantation as a result of the down-regulation of integrins α4, αv and β3 (Simon and Dominguez 2004). Although these data suggest an important role for the IL1 system during implantation (Krussel et al. 2003), as previously discussed, the targeted deletion of IL1R1I and IL1 do not prevent the ability of these animals to reproduce (Abbondanzo et al. 1996; Zheng et al. 1995).
To determine whether IL1 plays a physiological role in vivo, Strakova et al. (2005) investigated the synergistic effect of human chorionic gonadotrophin (hCG) and IL1B on endometrial function by using a baboon model of simulated pregnancy. Infusion both of hCG and of hCG plus IL1B induced marked differences in the distribution of α-smooth muscle actin, proliferation marker Ki67, decidualization marker IGFBP1 and PTGS1. The most marked effect of IL1B was the induction of IGFBP1 protein in stromal cells close to the apical surface, whereas PTGS1 was down-regulated in the glandular epithelium. Protein arrays of uterine flushings showed significant suppression of death receptors, Fas and tumor necrosis factor (TNF) receptor 1, in the groups treated with hCG alone or with hCG plus IL1B, suggesting an inhibition of apoptosis. Subsequent studies have confirmed that hCG inhibits endometrial apoptosis, both in vivo and in vitro (Lovely et al. 2005; Jasinska et al. 2006). Additionally, cytotoxic T lymphocyte antigen-4, matrix metalloproteinase-3 (MMP-3) and IL4 were suppressed in treated animals compared with controls (Strakova et al. 2005). However, no differences were observed in the cytokine profile between baboons treated with hCG alone or with hCG plus IL1B. The study of Strakova et al. (2005) confirmed that, in preparation for pregnancy, functional changes modulated by hCG and IL1B lead to the inhibition of apoptosis and the development of an immunotolerant environment. Furthermore, the results suggest distinct functions associated with IL1B. The presence of IL1B specifically regulates decidualization (as evidenced by IGFBP1 expression) and PTGS1 protein regulation, which represent functional changes associated with the presence of the conceptus during early pregnancy and is in agreement with in vitro studies on both baboon and human stromal fibroblasts (Strakova et al. 2000; Tarantino et al. 1992; Kim et al. 1999a, 1999b).
During implantation, trophoblast migration and invasion is modulated by several factors, including IGFBP1, which is the major secretory product of the primate decidua (Irving and Lala 1995; Hamilton et al. 1998; Rutanen et al. 1988). Decidualization is a major change that occurs in the endometrium after conception and involves the differentiation of stromal fibroblasts into decidual cells (Kearns and Lala 1983; Kliman 2000; Brosens and Gellersen 2010). The decidualization response is characterized by local edema, influx of immune cells and the transformation of stromal fibroblasts into secretory decidual cells (Gellersen et al. 2007) and defects in this process lead to multiple reproductive pathologies associated with pregnancy (Karpovich et al. 2005; Klemmt et al. 2006; Brosens and Gellersen 2010). However, the exact molecular mechanisms regulating this complex process of stromal cell transformation are still unknown.
Since IL1 has been identified as one modulator of the communication between human maternal endometrium and the embryo (Simon and Dominguez 2004), investigations have indicated the possible involvement of IL1B in decidualization. IL1B induces PTGS2 expression, PGE2 synthesis and in the presence of steroid hormones, IGFBP1 expression in human and baboon stromal fibroblasts (Strakova et al. 2000). Dissociation of filamentous actin is essential for IGFBP1 expression during decidualization (Kim et al. 1999a, 1999b). Forces of tension generated within the cytoskeleton by actin and myosin determine the organization of the cytoskeleton (Elson 1988). Cytoskeletal changes can also be induced from the outside of the cells by disruption of their ECM, which serves as external scaffold for the cells (Huang and Ingber 1999). Degradation of ECM by MMPs in stromal cells can result in intracellular cytoskeleton changes. IL1B induces mRNA expression and synthesis of proMMP-3 protein in baboon stromal fibroblasts (Strakova et al. 2000) in agreement with results from a previous study of human stromal fibroblasts (Rawdanowicz et al. 1994). Results also indicate that IL1B stimulates the phosphorylation of extracellular-signal-regulated kinase (ERK) and p38 mitogen-activated kinases in a time-dependent manner (Strakova et al. 2000). The results with specific inhibitors suggest that ERK and p38 pathways are involved in the IL1B-induced proMMP-3 synthesis (Strakova et al. 2003). Recent data on the role of IL1B in the decidualization process point to a central role in the remodeling of the cytoskeleton, a process that is critical for the inhibition of apoptosis and the initiation of differentiation (Kim et al. 1999a, 1999b; Jasinska et al. 2006). Destabilization of the cytoskeleton by inhibitors of myosin light chain kinase or myosin II ATPase accelerates the decidualization induced by cAMP but inhibits the decidualization induced by IL1B (Ihnatovych et al. 2007). Changes in actin dynamics, specifically the stabilization of filamentous actin, negatively impact decidualization and prevent the translocation of the actin-binding protein cofilin to the nucleus, an essential response to permit stromal cell differentiation (Ihnatovych et al. 2009). Furthermore, cofilin-mediated actin reorganization in uterine epithelial cells might also be important in preparation for blastocyst implantation, since dysregulation of this reorganization is evident in baboons with endometriosis and might lead to the decreased fertility associated with this disease (Braundmeier and Fazleabas 2009; Morris et al. 2011). Paracrine stimulation by IL1B secreted by decidualized stromal cells enhances trophoblast migration and impaired IL1B leads to fetal death further emphasizing the importance of this cytokine in the establishment of pregnancy (Ashley et al. 2010; Gonzalez et al. 2011).
In summary, the transformation of stromal cells into fully differentiated decidual cells is a complex process. Results of both in vivo and in vitro studies suggest that IL1B contributes to this process by reorganizing the actin cytoskeleton and indirectly increasing intracellular cAMP concentrations by up-regulating PTGS2 and PGE2. Inhibition of this process, either by disrupting or stabilizing the cytoskeleton, results in impaired decidulization and induction of apoptosis, both of which lead to impaired fertility.
IL1 system during implantation in the pig
Porcine conceptus IL1B gene expression and release of IL1B protein into the uterine lumen is temporally and spatially associated with rapid trophoblast elongation between days 11 and 12 of pregnancy (Ross et al. 2003a, 2003b). IL1B, an inducer of phospholipase A2 (Kol et al. 2002), might regulate the release of arachidonic acid from the phospholipid bilayer allowing an increase in membrane fluidity necessary for remodeling of the trophectoderm during elongation and its conversion to prostaglandins, which are needed for placental attachment during the establishment of pregnancy. Filamentous (day 12) porcine conceptuses express PTGS2 mRNA (Wilson et al. 2002), which is temporally associated with IL1B expression. Therefore, conceptus IL1B secretion possibly has an autocrine effect by inducing the rapid morphological transformation of the conceptuses on day 12 of pregnancy. Indeed, conceptus IL1B mRNA expression is rapidly increased during trophoblast elongation but decreases over 2000-fold immediately following the completion of the elongation process (Ross et al. 2003b). Because pro-IL1B lacks a signal sequence, its activation and secretion require cleavage by an intracellular cysteine protease (Fantuzzi and Dinarello 1999). IL1B-converting enzyme, also known as caspase-1 (CASP1), transforms IL1B to its biologically active form. Conceptus expression of CASP1 occurs coincidentally with IL1B secretion on days 12 and 13 of pregnancy (G.L. Morgan, J.W. Ross and R.D. Geisert, unpublished). Analyses of genome sequences and expressed sequence tags (EST) of the domestic pig (Sus scrofa domestica) indicate that two forms of IL1B are expressed in the pig. The close proximity of both of the genes on chromosome 3 suggests a gene duplication event resulting in a porcine embryonic form of IL1B. The classical IL1B is expressed in macrophages and endometrial tissue, whereas the embryonic form of IL1B has only been detected in the early elongating porcine conceptus prior to attachment to the uterine LE (C.K. Tuggle, Iowa State University, personal communication). The two predicted protein sequences are 86% identical and are least homologous near the N-terminus, as CASP1 cleaves this portion of the peptide resulting in a functional protein (D.J. Mathew, M.C. Lucy and R.D. Geisert, unpublished). Interestingly, in the case of embryonic IL1B, a proline is inserted just two amino acids away from the predicted CASP1 cleavage site. Porcine embryonic IL1B is secreted only within a brief window associated with conceptus elongation on day 12 of pregnancy. The specific roles of conceptus secretion of IL1B in trophoblast elongation and uterine receptivity have not been determined.
The pig conceptus isoform of IL1B signaling could be critical not only for trophoblast elongation in the pig but also for the initiation of events essential to implantation and placentation for the establishment and maintenance of pregnancy. The pig endometrium expresses IL1R1, accessory protein and antagonist, which are involved in stimulating a pro-inflammatory response (Ross et al. 2003b). Secretion of IL1B and E2 by pig conceptuses might establish immunological interactions between the endometrium and conceptus during placental attachment and maintenance of the CL function. Endometrial expression of PTGES1 and PGE2 are increased by IL1B in vitro (Franczak et al. 2010); this might play a role in blocking luteolysis and provide immunotolerance during pregnancy.
Many of the biological events during conceptus implantation in the pig resemble an acute phase pro-inflammatory response (Geisert et al. 2003). IL1B secreted by the conceptus has been implicated in inducing the inflammatory response during implantation in other species (Martin et al. 2002; Simon et al. 1995). The expression of IL1B increases prior to the initiation of blastocyst implantation in the mouse (Takacs and Kauma 1996; Kruessel et al. 1997) and might be an initiator of conceptus-uterine cross-talk during pregnancy in the human (Lindhard et al. 2002). IL1B induces PTGS2 gene expression in human endometrial stromal cells (Huang et al. 1998). Activation of PTGS2 expression in the endometrium and conceptus might be regulated through NFKB activation stimulated by IL1B (Kniss et al. 2001). An increase occurs in endometrial IL1R1 and IL1RAP gene expression on day 12 of pregnancy in the pig (Ross et al. 2003b). Interestingly, IL1R1 and IL1R2 are also molecular markers for uterine receptivity in mice (Reese et al. 2001). Conceptus secretion of IL1B induces a pregnancy-specific increase of salivary lipocalin (SAL1) from the GE only during the period of porcine conceptus elongation on day 12 (Seo et al. 2011). Lipocalins belong to a family of extracellular proteins that bind hydrophobic molecules and act as lipid transporters. Uterine secretion of SAL1 possibly serves as a scavenger for toxic products during the period of rapid conceptus elongation, which involves alterations in lipid movement. IL1B-stimulated endometrial GE expression of Sal1 and E2-induced stanniocalcin expressed in the LE (Song et al. 2009) are markers of implantation in the pig.
Implantation and the NFKB pathway
The NFKB family of transcription factors regulates the tissue immune function and inflammatory and acute phase responses (Ghosh and Hayden 2008; Hayden and Ghosh 2011). NFKB is regulated through receptor activation by a variety of stimuli such as the bacterial endotoxin lipopolysaccharide, oxidative stress, radiation exposure and specific cytokines, particularly, IL1B and TNFA. NFKB is a cytoplasmic heterodimer composed of various Rel family proteins (Ghosh et al. 1998). The heterodimer of p50 and p65 is the most abundant form of NFKB in eukaryotes. NFKB is sequestered in an inactive form within the cytoplasm through binding by inhibitors of NFKB, known as IκBs (Ali and Mann 2004). IL1B and TNFA receptor activation stimulates the classical pathway for the phosphorylation of two serines in IκB by IκB kinase composed of two catalytic units, IKKα and IKKβ and the regulatory subunit IKKγ. Phosphorylation of IκB results in its release from the NFKB complex and degradation by polyubiquitination in the 26S proteasome (Ghosh et al. 1998). Release of IκB allows the nuclear translocation of NFKB (p65:p50 dimer) for binding to specific κB-sites in the promoter region of target genes to activate transcription. Activation of nuclear κB sites also stimulates IκB to bind NFKB and translocate as an inactive complex back to the cytoplasm (negative feedback loop). Activation of NFKB targets a variety of genes, including those for cell adhesion molecules, cytokines, growth factors, anti-apoptotic factors and immunoreceptors. Genes containing κB sites that are transcriptionally regulated by NFKB include those for many cytokines (TNFα, IL1, IL2, IL6, IL12, LIF and GMCSF), chemokines (IL8 and RANTES) and enzymes such as PTGS2 (Ali and Mann 2004).
IL1B possibly invokes an endometrial NFKB-stimulated inflammatory response through the induction of PTGS2 gene expression and prostaglandin secretion, which is a required maternal signaling component for the establishment of a successful pregnancy in a number of species. Establishment of pregnancy in mice has been shown to involve the NFKB system. Not only does the activation of NFKB during the implantation window in mice occur (Nakamura et al. 2004b) but it has also been shown to be required for uterine receptivity through its affects on LIF expression, as LIF administration restores normal implantation during pregnancy when NFKB function is compromised (Nakamura et al. 2004a). Activation of NFKB in the human endometrium has been shown to regulate IL6 and LIF production (Laird et al. 2000) and is associated with implantation (Page et al. 2002; King et al. 2010). Interestingly, peak LIF secretion by the pig uterus occurs on day 12 of the estrous cycle (Anegon et al. 1994), as does the up-regulation of uterine PTGS2 (Ashworth et al. 2006), an NFKB-regulated gene (Kniss et al. 2001). Endometrial gene expression of p65 and p50 increases between days 5 to 12 of the estrous cycle and pregnancy in the pig suggesting that NFKB is involved in regulating uterine function (Ross et al. 2010). Endometrial NFKB translocation to the nucleus (activation) during pregnancy (Fig. 2) is specifically localized only to uterine LE adjacent to the overlying IL1B-secreting elongating pig conceptuses (Mathew et al. 2011).
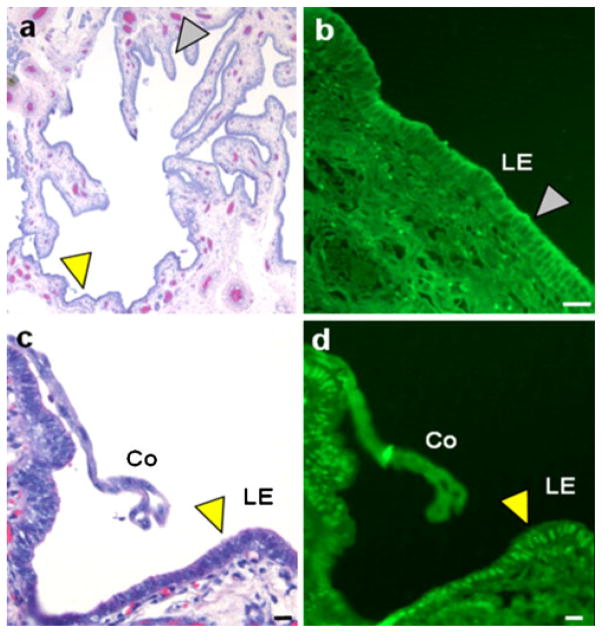
Fig. 2.
Image of a hematoxylin-stained cross-section of uterine lumen from a day-12 pregnant pig (a) indicating the location of a conceptus attaching to the uterine luminal epithelium (c, d). b Immunofluorescence for NFKB is localized in the cytoplasm of the luminal epithelium (LE) distal to the conceptus (white arrowhead in a, b). c Enlarged image of a hematoxylin-stained conceptus (Co) attaching to the uterine luminal epithelium on day 12 of pregnancy (yellow arrowhead in a, c). d Immunofluorescence for NFKB has migrated to the nuclei of the LE (yellow arrowhead) near the elongating conceptus indicating that NFKB activation is locally induced by the conceptuses. Bars 50 μm (from Mathew et al. 2011)
Endometrial activation of NFKB provides a unique model to explain the coordinate down-regulation of epithelial PGR and concomitant induction of many cytokines involved with early conceptus development and implantation. The available evidence indicates that an interaction between NFKB and PGR can occur (Kalkhoven et al. 1996) which might explain the timing of endometrial cytokine and PTGS2 expression being under uterine control. Progesterone exerts an inhibitory effect on pro-inflammatory chemokine and PTGS2 synthesis by blocking the NFKB pathway (McKay and Cidlowski 1999), whereas loss of endometrial progesterone stimulation is associated with enhanced in vivo chemokine release in women (Jones et al. 1997). Knockout mice lacking PGR have a strong uterine inflammatory response (Lydon et al. 1995). Mutual repression between PGR and NFKB activation has been reported (Kalkhoven et al. 1996; McKay and Cidlowski 1998) and this mechanism might control the timing of cytokine release for the establishment and maintenance of pregnancy. Progesterone has been proposed to inhibit NFKB activation through its receptor by directly interfering with NFKB binding to its consensus DNA response element, inhibiting transcription of ligands and receptors that activate NFKB, or increasing expression of binding proteins that inhibit NFKB activity (Davies et al. 2004). Davies et al. (2004) have indicated that liganded PGR inhibits NFKB activation. Therefore, the presence of PGR in cells might inhibit NFKB activation until either (1) the withdrawal of progesterone as would be the case during the end of the estrous cycle or parturition, or (2) PGR is down-regulated in progesterone-sensitive epithelial cells.
Conceptus expression of IL1B would be consistent with the continued activation of NFKB, whereas the synchronous E2 secretion by pig conceptuses would provide a negative effect to prevent a full inflammatory reaction that would be detrimental to conceptus survival. In the pig, conceptus secretion of E2 mirrors the increase and decrease of uterine luminal IL1B between days 12 and 15 of pregnancy (Ross et al. 2003b). The pig endometrial expression of ESR1 during conceptus trophoblast elongation (Geisert et al. 1993; Sukjumlong et al. 2003) might regulate the uterine inflammatory response during the conceptus release of IL1B. Negative reciprocal cross-talk between the activation of ESR and NFKB has been described in other tissues (Evans et al. 2001; Quaedackers et al. 2007). Thus, conceptus E2 secretion might play an important role in modulating the inflammatory response induced by the implanting conceptus. However, recent information has indicated that E2 and IL1B have an additive stimulation on PGE2 production through the up-regulation of prostaglandin E synthetase in human endometrial cells (King et al. 2010). This is consistent with the increase in PGE synthesis and secretion induced by IL1B in the pig endometrium (Franczak et al. 2010).
In the pig, conceptus IL1B gene expression and secretion declines dramatically by day 15 of pregnancy together with a decrease in E2 production (Ross et al. 2003b). However, a second sustained phase of estrogen secretion by conceptuses occurs that is necessary to maintain CL function beyond 28 days of gestation (Geisert et al. 1987). The second sustained increase of conceptus estrogen secretion is temporally associated with a pregnancy-specific increase in endometrial CASP1 mRNA on days 15 and 18 of gestation (Ashworth et al. 2010). The increase in endometrial CASP1 is not associated with the endometrial release of IL1B for which the uterine luminal content is decreased on days 15 and 18 of pregnancy, suggesting the involvement of an alternate substrate. Pro-IL18, which has structural similarities to pro-IL1B and is involved with the modulation of the immune system through the induction of interferon-γ, is another substrate for CASP1 (Fantuzzi and Dinarello 1999). Although similar to IL1B, IL18 binds to a unique IL18 receptor and therefore does not function through the NFKB pathway (Lee et al. 2004). IL18 is expressed by the human endometrial epithelia and is suggested to regulate maternal-embryo interplay during the establishment of pregnancy (Yoshino et al. 2001). Porcine endometrial IL18 mRNA expression increases from day 10 to 15 of the estrous cycle with mRNA expression increasing by 10-fold on day 18 of pregnancy (Ashworth et al. 2010). However, the uterine luminal content of IL18 increases on days 15 and 18 of pregnancy in contrast to no change during the estrous cycle because of the increase in CASP1 induced by the developing conceptuses. The conceptus factor that stimulates the increase in CASP1 in the uterine epithelium is unknown. The increased endometrial expression of CASP1 and the release of IL18 into the uterine lumen might induce the secretion of interferon-γ by conceptuses (Cencic and La Bonnardiere 2002) to modulate the maternal immune system through STAT1 at the interface between trophectoderm and uterine LE (Joyce et al. 2007). The loss of IL1B stimulation and switch to endometrial IL18 production during placental attachment in the pig would decrease the potential pro-inflammatory stimulation of the conceptuses following trophoblast elongation. Repression of the NFKB activation following attachment might be important for controlling cytokine and immune function following implantation (Hadfield et al. 2011).
Concluding remarks
A model for the regulation of cellular PGR expression and NFKB activation for endometrial receptivity and conceptus attachment is continuing to evolve. Clearly, the loss of the PGR from the uterine LE initiates the period of conceptus implantation and early development following blastocyst hatching from the zona pellucida. The down-regulation of the uterine epithelial PGR during the estrous cycle permits the activation of the NFKB system, possibly through Toll-like receptors (Ross et al. 2010). The loss of epithelial PGR shifts progesterone stimulation of uterine function to the stroma, which retains PGR (Bazer et al. 2010). Stromal progestamedins and other regulatory factors stimulate gene activation of multiple cytokines, growth factors and prostaglandin for conceptus growth. Removal of MUC1 from the LE surface permits adhesion between the conceptus trophoblast and uterine LE surface receptors. The NFKB system is activated during the period of implantation in the pig (Mathew et al. 2011), mouse (Nakamura et al. 2004b) and human (Page et al. 2002). Production of IL1B can localize the activation of NFKB to enhance the LE expression of LIF together with leptin in the human to induce implantation (Gonzalez et al. 2004). Moreover, ILlB increases LE expression of integrin β3 in the human (Simon et al. 1997b); this will aid conceptus attachment to the uterine surface, activation of PTGS2 for implantation and secretion of cytokines, growth factors and proteases for conceptus development and uterine remodeling (van Mourik et al. 2009). Estrogen might serve to control the pro-inflammatory response induced by IL1B activation of NFKB. Once the initial stimulation of the pro-inflammatory response of IL1B on the endometrium has occurred, maternal activation of IL18 takes place through an increase in endometrial CASP1 activity. Both the pig and mouse have an active IL18 system following conceptus attachment and initiation of implantation (Croy et al. 2003; Ashworth et al. 2010). Endometrial secretion of IL18 regulates the immune response via stimulating interferon-γ through either uterine immune cells (Laird et al. 2006) or, in the case of the pig, conceptus trophectoderm (Joyce et al. 2007). The current model obviously cannot mirror the process of implantation in all mammalian species but does present similarities in the interplay between the conceptus-uterine-immune systems for the establishment of pregnancy.
Acknowledgments
The work in our laboratories is supported by National Research Initiative Competitive Grant no. 2007-35203-17836 from the USDA Cooperative State Research, Education, and Extension Service (R.D.G. and M.C.L.) and RO 1 grant NIH HD 42280 (A.T.F.).
Contributor Information
Rodney Geisert, Email: geisertr@missouri.edu, Division of Animal Sciences, University of Missouri, Columbia, Mo., USA.
Asgerally Fazleabas, Department of Obstetrics and Gynecology, and Reproductive Biology, Michigan State University, East Lansing, Mich., USA.
Mathew Lucy, Division of Animal Sciences, University of Missouri, Columbia, Mo., USA.
Daniel Mathew, Division of Animal Sciences, University of Missouri, Columbia, Mo., USA.
References
- Abbondanzo SJ, Cullinan EB, McIntyre K, Labow MA, Stewart CL. Reproduction in mice lacking a functional type 1 IL-1 receptor. Endocrinology. 1996;137:3598–3601. doi: 10.1210/endo.137.8.8754793. [DOI] [PubMed] [Google Scholar]
- Ali S, Mann DA. Signal transduction via the NF-κB pathway: targeted treatment modality for infection, inflammation and repair. Cell Biochem Funct. 2004;22:67–79. doi: 10.1002/cbf.1082. [DOI] [PubMed] [Google Scholar]
- Anegon I, Cuturi MC, Godard A, Moreau M, Terqui M, Martinat-Botte F, Soulillou JP. Presence of leukaemia inhibitory factor and interleukin 6 in porcine uterine secretions prior to conceptus attachment. Cytokine. 1994;6:493–499. doi: 10.1016/1043-4666(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Aplin JD. MUC1 glycosylation in endometrium: possible roles of the apical glycocalyx at implantation. Hum Reprod. 1999;14:17–25. doi: 10.1093/humrep/14.suppl_2.17. [DOI] [PubMed] [Google Scholar]
- Aplin JD, Kimber SJ. Trophoblast-uterine interactions at implantation. Reprod Biol Endocrinol. 2004;2:48. doi: 10.1186/1477-7827-2-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley RL, Henkes LE, Bouma GJ, Pru JK, Hansen TR. Deletion of the Isg15 gene results in up-regulation of decidual cell survival genes and down-regulation of adhesion genes: implication for regulation by IL-1beta. Endocrinology. 2010;151:4527–4536. doi: 10.1210/en.2010-0166. [DOI] [PubMed] [Google Scholar]
- Ashworth MD, Ross JW, Allen DT, Stein DR, Spicer LJ, Geisert RD. Endocrine disruption of uterine insulin-like growth factor (IGF) expression in the pregnant gilt. Reproduction. 2005;130:545–551. doi: 10.1530/rep.1.00821. [DOI] [PubMed] [Google Scholar]
- Ashworth MD, Ross JW, Hu J, White FJ, Stein DR, Desilva U, Johnson GA, Spencer TE, Geisert RD. Expression of porcine endometrial prostaglandin synthase during the estrous cycle and early pregnancy, and following endocrine disruption of pregnancy. Biol Reprod. 2006;74:1007–1015. doi: 10.1095/biolreprod.105.046557. [DOI] [PubMed] [Google Scholar]
- Ashworth MD, Ross JW, Stein DR, White FJ, Desilva UW, Geisert RD. Endometrial caspase 1 and interleukin-18 expression during the estrous cycle and peri-implantation period of porcine pregnancy and response to early exogenous estrogen administration. Reprod Biol Endocrinol. 2010;8:33. doi: 10.1186/1477-7827-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P, Fazleabas AT. Endometrial responses to embryonic signals in the primate. Int J Dev Biol. 2010;54:295–302. doi: 10.1387/ijdb.082829pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankers-Fulbright JL, Kalli KR, McKean DJ. Interleukin-1 signal transduction. Life Sci. 1996;59:61–83. doi: 10.1016/0024-3205(96)00135-x. [DOI] [PubMed] [Google Scholar]
- Barañao RI, Piazza A, Rumi LS, Polak de Fried E. Determination of IL-1 and IL-6 levels in human embryo culture-conditioned media. Am J Reprod Immunol. 1997;37:191–194. doi: 10.1111/j.1600-0897.1997.tb00212.x. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Marengo SR, Geisert RD, Thatcher WW. Exocrine versus endocrine secretion of prostaglandin F in the control of pregnancy in swine. Anim Reprod Sci. 1984;7:115–132. [Google Scholar]
- Bazer FW, Spencer TE, Johnson GA, Burghardt RC, Wu G. Comparative aspects of implantation. Reproduction. 2009;138:195–209. doi: 10.1530/REP-09-0158. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Wu G, Spencer TE, Johnson GA, Burghardt RC, Bayless K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod. 2010;16:135–152. doi: 10.1093/molehr/gap095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigonnesse F, Labelle Y, Akoum A. Triphasic expression of interleukin-1 receptor type I in human endometrium throughout the menstrual cycle of fertile women and women with unexplained infertility. Fertil Steril. 2001;75:79–87. doi: 10.1016/s0015-0282(00)01634-4. [DOI] [PubMed] [Google Scholar]
- Blomberg LA, Zuelke KA. Serial analysis of gene expression (SAGE) during porcine embryo development. Reprod Fertil Dev. 2004;16:87–92. doi: 10.10371/RD03081. [DOI] [PubMed] [Google Scholar]
- Blomberg L, Hashizume K, Viebahn C. Blastocyst elongation, trophoblastic differentiation, and embryonic pattern formation. Reproduction. 2008;135:181–195. doi: 10.1530/REP-07-0355. [DOI] [PubMed] [Google Scholar]
- Blomberg A, le Schreier L, Li RW. Characteristics of peri-implantation porcine concepti population and maternal milieu influence the transcriptome profile. Mol Reprod Dev. 2010;77:978–989. doi: 10.1002/mrd.21253. [DOI] [PubMed] [Google Scholar]
- Bowen JA, Bazer FW, Burghardt RC. Spatial and temporal analysis of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vivo. Biol Reprod. 1996;55:1098–1106. doi: 10.1095/biolreprod55.5.1098. [DOI] [PubMed] [Google Scholar]
- Braga VMM, Gendler SJ. Modulation of Muc-1 mucin expression in the mouse uterus during the estrus cycle, early pregnancy and placentation. J Cell Sci. 1993;105:397–405. doi: 10.1242/jcs.105.2.397. [DOI] [PubMed] [Google Scholar]
- Braundmeier AG, Fazleabas AT. The non-human primate model of endometriosis: research and implications for fecundity. Mol Hum Reprod. 2009;15:577–586. doi: 10.1093/molehr/gap057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosens JJ, Gellersen B. Something new about early pregnancy: decidual biosensoring and natural embryo selection. Ultrasound Obstet Gynecol. 2010;36:1–5. doi: 10.1002/uog.7714. [DOI] [PubMed] [Google Scholar]
- Burghardt RC, Bowen JA, Newton GR, Bazer FW. Extracellular matrix and the implantation cascade in pigs. J Reprod Fertil Suppl. 1997;52:151–164. [PubMed] [Google Scholar]
- Burghardt RC, Johnson GA, Jaeger LA, Ka H, Garlow JE, Spencer TE, Bazer FW. Integrins and extracellular matrix proteins at the maternal-fetal interface in domestic animals. Cells Tissues Organs. 2002;172:202–217. doi: 10.1159/000066969. [DOI] [PubMed] [Google Scholar]
- Carson DD. The glycobiology of implantation. Front Biosci. 2002;7:d1535–d1544. doi: 10.2741/A858. [DOI] [PubMed] [Google Scholar]
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- Carter F, Forde N, Duffy P, Wade M, Fair T, Crowe MA, Evans AC, Kenny DA, Roche JF, Lonergan P. Effect of increasing progesterone concentration from day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reprod Fertil Dev. 2008;20:368–375. doi: 10.1071/rd07204. [DOI] [PubMed] [Google Scholar]
- Cencic A, La Bonnardiere C. Trophoblastic interferon-gamma: current knowledge and possible role(s) in early pig pregnancy. Vet Res. 2002;33:139–157. doi: 10.1051/vetres:2002003. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Dubanchet S, Ledée N. Cytokines: important for implantation? J Assist Reprod Genet. 2007;24:491–505. doi: 10.1007/s10815-007-9142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HO, Saunders PT. Hormone receptor dynamics in a receptive human endometrium. Reprod Sci. 2009;16:191–199. doi: 10.1177/1933719108331121. [DOI] [PubMed] [Google Scholar]
- Cross JC, Roberts RM. Porcine conceptuses secrete an interferon during the preattachment period of early pregnancy. Biol Reprod. 1989;40:1109–1118. doi: 10.1095/biolreprod40.5.1109. [DOI] [PubMed] [Google Scholar]
- Croy BA, He H, Esadeg S, Wei Q, McCartney D, Zhang J, Borzychowski A, Ashkar AA, Black GP, Evans SS, Chantakru S, Heuvel M, van den Paffaro VA, Jr, Yamada AT. Uterine natural killer cells: insights into their cellular and molecular biology from mouse modelling. Reproduction. 2003;126:149–160. doi: 10.1530/rep.0.1260149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- Davies S, Dai D, Feldman I, Pickett G, Leslie KK. Identification of a novel mechanism of NF-κB inactivation by progesterone through progesterone receptors in Hec50co poorly differentiated endometrial cancer cells: induction of A20 and ABIN-2. Gynecol Oncol. 2004;94:463–470. doi: 10.1016/j.ygyno.2004.05.028. [DOI] [PubMed] [Google Scholar]
- De los Santos MJ, Mercader A, Francés A, Portolés E, Remohí J, Pellicer A, Simon C. Role of endometrial factors in regulating secretion of components of the immunoreactive human embryonic interleukin-1 system during embryonic development. Biol Reprod. 1996;54:563–574. doi: 10.1095/biolreprod54.3.563. [DOI] [PubMed] [Google Scholar]
- Degrelle SA, Le Ann B, Garrett WM, Li RW, Talbot NC. Comparative proteomic and regulatory network analyses of the elongating pig conceptus. Proteomics. 2009;9:2678–2694. doi: 10.1002/pmic.200800776. [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25:341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod. 2005;11:613–630. doi: 10.1093/humupd/dmi023. [DOI] [PubMed] [Google Scholar]
- Elson EL. Cellular mechanics as an indicator of cytoskeletal structure and function. Annu Rev Biophys Biophys Chem. 1988;17:397–430. doi: 10.1146/annurev.bb.17.060188.002145. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Eckert A, Lai K, Adelman SJ, Harnish DC. Reciprocal antagonism between estrogen receptor and NF-kappaB activity in vivo. Circ Res. 2001;89:823–830. doi: 10.1161/hh2101.098543. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1β: two cytokine substrates for ICE (caspase-1) J Clin Immun. 1999;19:1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- Farach-Carson MC, Carson DD. Perlecan—a multifunctional extracellular proteoglycan scaffold. Glycobiology. 2007;17:897–905. doi: 10.1093/glycob/cwm043. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT. Physiology and pathology of implantation in the human and nonhuman primate. Semin Reprod Med. 2007;25:405–409. doi: 10.1055/s-2007-991037. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Geisert RD, Bazer FW, Roberts RM. Relationship between release of plasminogen activator and estrogen by blastocysts and secretion of plasmin inhibitor by uterine endometrium in the pregnant pig. Biol Reprod. 1983;29:225–238. doi: 10.1095/biolreprod29.1.225. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Kim JJ, Srinivasan S, Donnelly KM, Brudney A, Jaffe RC. Implantation in the baboon: endometrial responses. Semin Reprod Endocrinol. 1999;17:257–265. doi: 10.1055/s-2007-1016233. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Kim JJ, Strakova Z. Implantation: embryonic signals and the modulation of the uterine environment—a review. Placenta. 2004;25:S26–S31. doi: 10.1016/j.placenta.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valdivia R, Mukherjee A, Mulac-Jericevic B, Conneely OM, DeMayo FJ, Amato P, Lydon JP. Revealing progesterone’s role in uterine and mammary gland biology: insights from the mouse. Semin Reprod Med. 2005;23:22–37. doi: 10.1055/s-2005-864031. [DOI] [PubMed] [Google Scholar]
- Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, Jeong JW, Spencer TE, Behringer RR, Lydon JP, Demayo FJ. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J. 2011 doi: 10.1096/fj.11-193334. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franczak A, Zmijewska A, Kurowicka B, Wojciechowicz B, Kotwica G. Interleukin 1β-induced synthesis and secretion of prostaglandin E2 in the porcine uterus during various periods of pregnancy and the estrous cycle. J Physiol Pharmacol. 2010;61:733–742. [PubMed] [Google Scholar]
- Frank GR, Brar AK, Jikihara H, Cedars MI, Handwerger S. Interleukin-1 beta and the endometrium: an inhibitor of stromal cell differentiation and possible autoregulator of decidualization in humans. Biol Reprod. 1995;52:184–191. doi: 10.1095/biolreprod52.1.184. [DOI] [PubMed] [Google Scholar]
- Garlow JE, Ka H, Johnson GA, Burghardt RC, Jaeger LA, Bazer FW. Analysis of osteopontin at the maternal–placental interface in pigs. Biol Reprod. 2002;66:718–725. doi: 10.1095/biolreprod66.3.718. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Geisert RD, Zavy MT, Gries LK, Wettemann RP, Buchanan DS. Effect of exogenous progesterone on prostaglandin F2 alpha release and the interestrous interval in the bovine. Prostaglandins. 1988a;36:85–96. doi: 10.1016/0090-6980(88)90104-9. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Geisert RD, Zavy MT, Morgan GL. Evidence for maternal regulation of early conceptus growth and development in beef cattle. J Reprod Fertil. 1988b;84:437–446. doi: 10.1530/jrf.0.0840437. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Yelich JV. Regulation of conceptus development and attachment in pigs. J Reprod Fertil Suppl. 1997;52:133–149. [PubMed] [Google Scholar]
- Geisert RD, Brookbank JW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig. II. Cellular remodeling of the porcine blastocysts during elongation on day 12 of pregnancy. Biol Reprod. 1982a;27:941–955. doi: 10.1095/biolreprod27.4.941. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Renegar RH, Thatcher WW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig. I. Interrelationships between preimplantation development of the pig blastocyst and uterine endometrial secretions. Biol Reprod. 1982b;27:925–939. doi: 10.1095/biolreprod27.4.925. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Biggers BG, Wettemann RP, Zavy MT. Length of pseudopregnancy and pattern of uterine protein release as influenced by time and duration of oestrogen administration in the pig. J Reprod Fert. 1987;79:163–172. doi: 10.1530/jrf.0.0790163. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Fox TC, Morgan GL, Wells ME, Wettemann RP, Zavy MT. Survival of bovine embryos transferred to progesterone-treated asynchronous recipients. J Reprod Fertil. 1991;92:475–482. doi: 10.1530/jrf.0.0920475. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Brenner RM, Moffatt JR, Harney JP, Yellin T, Bazer FW. Changes in estrogen receptor protein, mRNA expression and localization in the endometrium of cyclic and pregnant gilts. Reprod Fert Dev. 1993;5:247–260. doi: 10.1071/rd9930247. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Pratt T, Bazer FW, Mayes JS, Watson GH. Immunocytochemical localization and changes in endometrial progestin receptor protein during the porcine oestrous cycle and early pregnancy. Reprod Fert Dev. 1994;6:749–760. doi: 10.1071/rd9940749. [DOI] [PubMed] [Google Scholar]
- Geisert RD, Ashworth MD, Malayer JR. Endometrial expression of inter-α-trypsin inhibitor heavy chains in cyclic and pregnant gilts. Reproduction. 2003;126:621–627. [PubMed] [Google Scholar]
- Geisert RD, Ross JW, Ashworth MD, White FJ, Johnson GA, DeSilva Y. Maternal recognition of pregnancy signal or endocrine disruptor: the two faces of oestrogen during establishment of pregnancy in the pig. Soc Reprod Fertil Suppl. 2006;62:131–145. [PubMed] [Google Scholar]
- Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Ann Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Neufeld J, Reimann K, Wittmann S, Samalecos A, Wolf A, Bamberger AM, Gellersen B. Expansion of human trophoblastic spheroids is promoted by decidualized endometrial stromal cells and enhanced by heparin-binding epidermal growth factor-like growth factor and interleukin-1 beta. Mol Hum Reprod. 2011;17:421–433. doi: 10.1093/molehr/gar015. [DOI] [PubMed] [Google Scholar]
- Gonzalez RR, Rueda BR, Ramos MP, Littell RD, Glasser S, Leavis PC. Leptin-induced increase in leukemia inhibitory factor and its receptor by human endometrium is partially mediated by interleukin 1 receptor signaling. Endocrinology. 2004;145:3850–3857. doi: 10.1210/en.2004-0383. [DOI] [PubMed] [Google Scholar]
- Green ML, Simmen RCM, Simmen FA. Developmental regulation of steroidogenic enzyme gene expression in the preiimplantation porcine conceptus: a paracrine role for insulin-like growth factor-I. Endocrinology. 1995;136:3961–3970. doi: 10.1210/endo.136.9.7649105. [DOI] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Kayisli UA, Taylor HS. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin Reprod Med. 2009;27:62–79. doi: 10.1055/s-0028-1108011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfield KA, McCracken SA, Ashton AW, Nguyen TG, Morris JM. Regulated suppression of NF-κB throughout pregnancy maintains a favourable cytokine environment necessary for pregnancy success. J Reprod Immunol. 2011;89:1–9. doi: 10.1016/j.jri.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Hamilton GS, Lysiak JJ, Han VK, Lala PK. Autocrine-paracrine regulation of human trophoblast invasiveness by insulin-like growth factor (IGF)-II and IGF-binding protein (IGFBP)-1. Exp Cell Res. 1998;244:147–156. doi: 10.1006/excr.1998.4195. [DOI] [PubMed] [Google Scholar]
- Hart LS, Carling SJ, Joyce MM, Johnson GA, Vanderwall DK, Ott TL. Temporal and spatial associations of oestrogen receptor alpha and progesterone receptor in the endometrium of cyclic and early pregnant mares. Reproduction. 2005;130:241–250. doi: 10.1530/rep.1.00596. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. NF-κB in immunobiology. Cell Res. 2011;21:223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hild-Petito S, Fazleabas AT, Julian J, Carson DD. Mucin (Muc-1) expression is differentially regulated in uterine luminal and glandular epithelia of the baboon (Papio anubis) Biol Reprod. 1996;54:939–947. doi: 10.1095/biolreprod54.5.939. [DOI] [PubMed] [Google Scholar]
- Horai R. IL-1 receptor antagonist knockout mice. Nihon Rinsho. 2005;63(Suppl 1):55–58. [PubMed] [Google Scholar]
- Hu XL, Yang Y, Hunt JS. Differential distribution of interleukin-1 alpha and interleukin-1 beta proteins in human placentas. J Reprod Immunol. 1992;22:257–268. doi: 10.1016/0165-0378(92)90047-8. [DOI] [PubMed] [Google Scholar]
- Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1:E131–E138. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- Huang JC, Liu DY, Yadollah S, Wu KV, Dawood MY. Interleukin-1β induces cyclooxygenase-2 gene expression in cultured endometrial stromal cells. J Clin Endocrinol Metab. 1998;83:538–541. doi: 10.1210/jcem.83.2.4533. [DOI] [PubMed] [Google Scholar]
- Ihnatovych I, Hu W, Martin JL, Fazleabas AT, Lanerolle P, de Strakova Z. Increased phosphorylation of myosin light chain prevents in vitro decidualization. Endocrinology. 2007;148:3176–3184. doi: 10.1210/en.2006-1673. [DOI] [PubMed] [Google Scholar]
- Ihnatovych I, Livak M, Reed J, Lanerolle P, de Strakova Z. Manipulating actin dynamics affects human in vitro decidualization. Biol Reprod. 2009;81:222–230. doi: 10.1095/biolreprod.108.074666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving JA, Lala PK. Functional role of cell surface integrins on human trophoblast cell migration: regulation by TGF-beta, IGF-II, and IGFBP-1. Exp Cell Res. 1995;217:419–427. doi: 10.1006/excr.1995.1105. [DOI] [PubMed] [Google Scholar]
- Jaeger LA, Johnson GA, KAH, Garlow JG, Burghardt RC, Spencer TE, Bazer FW. Functional analysis of autocrine and paracrine signaling at the uterine-conceptus interface in pigs. Reprod Suppl. 2001;58:191–207. [PubMed] [Google Scholar]
- Jasinska A, Strakova Z, Szmidt M, Fazleabas AT. Human chorionic gonadotropin and decidualization in vitro inhibits cytochalasin-D-induced apoptosis in cultured endometrial stromal fibroblasts. Endocrinology. 2006;147:4112–4121. doi: 10.1210/en.2005-1577. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Bazer FW, Jaeger LA, Ka H, Garlow JE, Pfarrer C, Spencer TE, Burghardt RC. Muc-1, integrin, and osteopontin expression during the implantation cascade in sheep. Biol Reprod. 2001;65:820–828. doi: 10.1095/biolreprod65.3.820. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Burghardt RC, Bazer FW, Spencer TE. Minire-view. Osteopontin: roles in implantation and placentation. Biol Reprod. 2003;69:1458–1471. doi: 10.1095/biolreprod.103.020651. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Bazer FW, Burghardt RC, Spencer TE, Wu G, Bayless KJ. Conceptus–uterus interactions in pigs: endometrial gene expression in response to estrogens and interferons from conceptuses. Reprod Fertil Suppl. 2009;66:321–332. [PubMed] [Google Scholar]
- Jones RL, Kelly RW, Critchley HO. Chemokine and cyclooxygenase-2 expression in human endometrium coincides with leukocyte accumulation. Hum Reprod. 1997;12:1300–1306. doi: 10.1093/humrep/12.6.1300. [DOI] [PubMed] [Google Scholar]
- Joyce MM, Burghardt RC, Geisert RD, Burghardt JR, Hooper RN, Ross JW, Ashworth MD, Johnson GA. Pig conceptuses secrete estrogen and interferons to differentially regulate uterine STAT1 in a temporal and cell type-specific manner. Endocrinology. 2007;148:4420–4431. doi: 10.1210/en.2007-0505. [DOI] [PubMed] [Google Scholar]
- Julian J, Enders AC, Fazleabas AT, Carson DD. Compartmental distinctions in uterine Muc-1 expression during early pregnancy in cynomolgous macaque (Macaca fascicularis) and baboon (Papio anubis) Hum Reprod. 2005;20:1493–1503. doi: 10.1093/humrep/deh801. [DOI] [PubMed] [Google Scholar]
- Ka H, Spencer TE, Johnson GA, Bazer FW. Keratinocyte growth factor: expression by endometrial epithelia of the porcine uterus. Biol Reprod. 2000;62:1772–1778. doi: 10.1095/biolreprod62.6.1772. [DOI] [PubMed] [Google Scholar]
- Ka H, Al-Ramadan S, Erikson DW, Johnson GA, Burghardt RC, Spencer TE, Jaeger LA, Bazer FW. Regulation of expression of fibroblast growth factor 7 in the pig uterus by progesterone and estradiol. Biol Reprod. 2007;77:172–180. doi: 10.1095/biolreprod.106.056309. [DOI] [PubMed] [Google Scholar]
- Kalkhoven E, Wissink S, Saag PT, van der Burg B. Negative interaction between the RelA (p65) subunit of NF-κB and the progesterone receptor. J Biol Chem. 1996;271:6217–6224. doi: 10.1074/jbc.271.11.6217. [DOI] [PubMed] [Google Scholar]
- Karagouni EE, Chryssikopoulos A, Mantzavinos T, Kanakas N, Dotsika EN. Interleukin-1beta and interleukin-1alpha may affect the implantation rate of patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 1998;70:553–559. doi: 10.1016/s0015-0282(98)00243-x. [DOI] [PubMed] [Google Scholar]
- Karpovich N, Klemmt P, Hwang JH, McVeigh JE, Heath JK, Barlow DH, Mardon HJ. The production of interleukin-11 and decidualization are compromised in endometrial stromal cells derived from patients with infertility. J Clin Endocrinol Metab. 2005;90:1607–1612. doi: 10.1210/jc.2004-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauma SW. Interleukin-1 beta stimulates colony-stimulating factor-1 production in human term placenta. J Clin Endocrinol Metab. 1993;76:701–703. doi: 10.1210/jcem.76.3.8445029. [DOI] [PubMed] [Google Scholar]
- Kauma SW. Cytokines in implantation. J Reprod Fertil Suppl. 2000;55:31–42. [PubMed] [Google Scholar]
- Kauma SW, Turner TT, Harty JR. Interleukin-1 beta stimulates interleukin-6 production in placental villous core mesenchymal cells. Endocrinology. 1994;134:457–460. doi: 10.1210/endo.134.1.8275959. [DOI] [PubMed] [Google Scholar]
- Kearns M, Lala PK. Life history of decidual cells: a review. Am J Reprod Immunol. 1983;3:78–82. doi: 10.1111/j.1600-0897.1983.tb00219.x. [DOI] [PubMed] [Google Scholar]
- Kennedy TG, Brown KD, Vaughan TJ. Expression of the genes for the epidermal growth factor receptor and its ligands in porcine oviduct and endometrium. Biol Reprod. 1994;50:751–756. doi: 10.1095/biolreprod50.4.751. [DOI] [PubMed] [Google Scholar]
- Keys JL, King GJ, Kennedy TG. Increased uterine vascular permeability at the time of embryonic attachment in the pig. Biol Reprod. 1986;34:405–411. doi: 10.1095/biolreprod34.2.405. [DOI] [PubMed] [Google Scholar]
- Kim GY, Besner GE, Steffen CL, McCarthy DW, Downing MT, Luquette MH, Abad MS, Brigstock DR. Purification of heparin-binding epidermal growth factor-like growth factor from pig uterine luminal flushings, and its production by endometrial tissues. Biol Reprod. 1995;52:561–571. doi: 10.1095/biolreprod52.3.561. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jaffe RC, Fazleabas AT. Insulin-like growth factor binding protein-1 expression in baboon endometrial stromal cells: regulation by filamentous actin and requirement for de novo protein synthesis. Endocrinology. 1999a;140:997–1004. doi: 10.1210/endo.140.2.6474. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Wang J, Bambra C, Das SK, Dey SK, Fazleabas AT. Expression of cyclooxygenase-1 and -2 in the baboon endometrium during the menstrual cycle and pregnancy. Endocrinology. 1999b;140:2672–2678. doi: 10.1210/endo.140.6.6716. [DOI] [PubMed] [Google Scholar]
- King AE, Collins F, Klonisch T, Sallenave JM, Critchley HO, Saunders PT. An additive interaction between the NFkappaB and estrogen receptor signalling pathways in human endometrial epithelial cells. Hum Reprod. 2010;25:510–518. doi: 10.1093/humrep/dep421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85:564–572. doi: 10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliman HJ. Uteroplacental blood flow. The story of decidualization, menstruation, and trophoblast invasion. Am J Pathol. 2000;157:1759–1768. doi: 10.1016/S0002-9440(10)64813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniss DA, Rovin B, Fertel RH, Zimmerman PD. Blockade NF-kB activation prohibits TNF-α-induced cyclooygenase-2 gene expression in ED27 trophoblast-like cells. Placenta. 2001;22:80–89. doi: 10.1053/plac.2000.0591. [DOI] [PubMed] [Google Scholar]
- Kol S, Kehat I, Adashi EY. Ovarian interleukin-1-induced gene expression: privileged genes threshold theory. Med Hypotheses. 2002;58:6–8. doi: 10.1054/mehy.2001.1389. [DOI] [PubMed] [Google Scholar]
- Kowalski AA, Graddy LG, Vale-Cruz DS, Choi I, Katzenellenbogen BS, Simmen FA, Simmen RC. Molecular cloning of porcine estrogen receptor-β complementary DNAs and developmental expression in periimplantation embryos. Biol Reprod. 2002;66:760–769. doi: 10.1095/biolreprod66.3.760. [DOI] [PubMed] [Google Scholar]
- Kruessel JS, Huang HY, Wen Y, Kloodt AR, Bielfeld P, Polan ML. Different pattern of interleukin-1β-(IL-1β), interleukin-1 receptor antagonist-(IL-1ra) and interleukin-1 receptor type I-(IL-1R tI) mRNA-expression in single preimplantation mouse embryos at various developmental stages. J Reprod Immunol. 1997;33:103–120. doi: 10.1016/s0165-0378(97)00030-2. [DOI] [PubMed] [Google Scholar]
- Krüssel JS, Simon C, Rubio MC, Pape AR, Wen Y, Huang HY, Bielfeld P, Polan ML. Expression of interleukin-1 system mRNA in single blastomeres from human preimplantation embryos. Hum Reprod. 1998;13:2206–2211. doi: 10.1093/humrep/13.8.2206. [DOI] [PubMed] [Google Scholar]
- Krüssel JS, Bielfeld P, Polan ML, Simon C. Regulation of embryonic implantation. Eur J Obstet Gynecol Reprod Biol. 2003;110(Suppl 1):S2–S9. doi: 10.1016/s0301-2115(03)00167-2. [DOI] [PubMed] [Google Scholar]
- Laird SM, Tuckerman EM, Cork BA, Li TC. Expression of nuclear factor kappa B in human endometrium; role in the control of interleukin 6 and leukaemia inhibitory factor production. Mol Hum Reprod. 2000;6:34–40. doi: 10.1093/molehr/6.1.34. [DOI] [PubMed] [Google Scholar]
- Laird SM, Tuckerman EM, Li TC. Cytokine expression in the endometrium of women with implantation failure and recurrent miscarriage. Reprod Biomed Online. 2006;13:13–23. doi: 10.1016/s1472-6483(10)62011-1. [DOI] [PubMed] [Google Scholar]
- Lawson RA, Cahill LP. Modification of the embryo-maternal relationship in ewes by progesterone treatment early in the oestrous cycle. J Reprod Fertil. 1983;67:473–475. doi: 10.1530/jrf.0.0670473. [DOI] [PubMed] [Google Scholar]
- Lea RG, Sandra O. Immunoendocrine aspects of endometrial function and implantation. Reproduction. 2007;134:389–404. doi: 10.1530/REP-07-0167. [DOI] [PubMed] [Google Scholar]
- Lee CY, Green ML, Simmen RCM, Simmen FA. Proteolysis of insulin-like growth factor-binding proteins (IGFBPs) within the pig uterine lumen associated with peri-implantation conceptus development. J Reprod Fert. 1998;112:369–377. doi: 10.1530/jrf.0.1120369. [DOI] [PubMed] [Google Scholar]
- Lee J-K, Kim S-H, Lewis EC, Azam T, Reznikov LL, Dinarello CA. Differences in signaling pathways by IL-1β and IL-18. Proc Nat Acad Sci USA. 2004;101:8815–8820. doi: 10.1073/pnas.0402800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre F, Boulay V. A novel and atypical type one interferon gene expressed by trophoblast during early pregnancy. J Biol Chem. 1993;268:19760–19768. [PubMed] [Google Scholar]
- Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCary KS., Jr Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67:334–340. doi: 10.1210/jcem-67-2-334. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Yeh I, Castelbaum AJ, Fritz MA, Ilesanmi AO, Korzeniowski P, Sun J, Chwalisz K. Endometrial progesterone receptors and markers of uterine receptivity in the window of implantation. Fertil Steril. 1996;65:477–483. [PubMed] [Google Scholar]
- Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Librach CL, Feigenbaum SL, Bass KE, Cui TY, Verastas N, Sadovsky Y, Quigley JP, French DL, Fisher SJ. Interleukin-1 beta regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J Biol Chem. 1994;269:17125–17131. [PubMed] [Google Scholar]
- Lindhard A, Bentin-Ley U, Ravn V, Islin H, Hviid T, Rex S, Bangsboll S, Sorensen S. Biochemical evaluation of endometrial function at the time of implantation. Fertil Steril. 2002;78:221–233. doi: 10.1016/s0015-0282(02)03240-5. [DOI] [PubMed] [Google Scholar]
- Lovely LP, Fazleabas AT, Fritz MA, McAdams DG, Lessey BA. Prevention of endometrial apoptosis: randomized prospective comparison of human chorionic gonadotropin versus progesterone treatment in the luteal phase. J Clin Endocrinol Metab. 2005;90:2351–2356. doi: 10.1210/jc.2004-2130. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Mann GE, Fray MD, Lamming GE. Effects of time of progesterone supplementation on embryo development and interferon-tau production in the cow. Vet J. 2006;171:500–503. doi: 10.1016/j.tvjl.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Muzio M, Ghessi P, Colotta C, Introna M. Regulation of inhibitory pathways of the interleukin-1 system. Ann N Y Acad Sci. 1998;840:338–351. doi: 10.1111/j.1749-6632.1998.tb09573.x. [DOI] [PubMed] [Google Scholar]
- Martin J, Dominquez F, Avila S, Castrillo JL, Remohi J, Pellicer A, Simon C. Human endometrial receptivity: gene regulation. J Reprod Immunol. 2002;55:131–139. doi: 10.1016/s0165-0378(01)00140-1. [DOI] [PubMed] [Google Scholar]
- Massuto DA, Hooper RN, Kneese EC, Johnson GA, Ing NH, Weeks BR, Jaeger LA. Intrauterine infusion of latency-associated peptide (LAP) during early porcine pregnancy affects conceptus elongation and placental size. Biol Reprod. 2010a;82:534–542. doi: 10.1095/biolreprod.109.081893. [DOI] [PubMed] [Google Scholar]
- Massuto DA, Kneese EC, Johnson GA, Burghardt RC, Hooper RN, Ing NH, Jaeger LA. Transforming growth factor beta (TGFB) signaling is activated during porcine implantation: proposed role for latency-associated peptide interactions with integrins at the conceptus-maternal interface. Reproduction. 2010b;139:465–478. doi: 10.1530/REP-09-0447. [DOI] [PubMed] [Google Scholar]
- Mathew DJ, Sellner EM, Green JC, Okamura CS, Anderson LL, Lucy MC, Geisert RD. Uterine progesterone receptor expression, conceptus development, and ovarian function in pigs treated with RU 486 during early pregnancy. Biol Reprod. 2011;84:130–139. doi: 10.1095/biolreprod.110.086843. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ma W-G, Daikoku T, Zhao X, Paria BC, Das SK, Trzaskos JM, Dey SK. Cyclooxygenase-2 differentially directs uterine angiogenesis during implantation in mice. J Biol Chem. 2002;277:29260–29267. doi: 10.1074/jbc.M203996200. [DOI] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA. Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: mechanisms of mutual antagonism. Mol Endocrinol. 1998;12:45–56. doi: 10.1210/mend.12.1.0044. [DOI] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- Meikle A, Sahlin L, Ferraris A, Masironi B, Blanc JE, Rodriguez-Irazoqui M, Rodriguez-Pinon M, Kindahl H, Forsberg M. Endometrial mRNA expression of oestrogen receptor alpha, progesterone receptor and insulin-like growth factor-I (IGF-I) throughout the bovine oestrous cycle. Anim Reprod Sci. 2001;68:45–56. doi: 10.1016/s0378-4320(01)00143-9. [DOI] [PubMed] [Google Scholar]
- Meseguer M, Pellicer A, Simon C. MUC1 and endometrial receptivity. Mol Hum Reprod. 1998;4:1089–1098. doi: 10.1093/molehr/4.12.1089. [DOI] [PubMed] [Google Scholar]
- Modric T, Kowalski AA, Green ML, Simmen RCM, Simmen FA. Pregnancy-dependent expression of leukaemia inhibitory factor (LIF), LIF receptor-β and interleukin 6 (IL-6) messenger ribonucleic acids in the porcine female reproductive tract. Placenta. 2000;21:345–353. doi: 10.1053/plac.1999.0493. [DOI] [PubMed] [Google Scholar]
- Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- Morgan GL, Geisert RD, McCann JP, Bazer FW, Ott TL, Mirando MA, Stewart M. Failure of luteolysis and extension of the interoestrous interval in sheep treated with the progesterone antagonist mifepristone (RU 486) J Reprod Fertil. 1993;98:451–457. doi: 10.1530/jrf.0.0980451. [DOI] [PubMed] [Google Scholar]
- Morris K, Ihnatovych I, Ionetz E, Reed J, Braundmeier A, Strakova Z. Cofilin and slingshot localization in the epithelium of uterine endometrium changes during the menstrual cycle and in endometriosis. Reprod Sci. 2011;18:1014–1024. doi: 10.1177/1933719111401663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourik MS, van Macklon NS, Heijnen CJ. Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J Leukoc Biol. 2009;85:4–19. doi: 10.1189/jlb.0708395. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128:139–146. doi: 10.1530/rep.1.00189. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kimura T, Ogita K, Nakamura T, Takemura M, Shimoya K, Koyama S, Tsujie T, Koyama M, Murata Y. NF-kappaB activation at implantation window of the mouse uterus. Am J Reprod Immunol. 2004a;51:16–21. doi: 10.1046/j.8755-8920.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kimura T, Ogita K, Koyama S, Tsujie T, Tsutsui T, Shimoya K, Koyama M, Kaneda Y, Murata Y. Alteration of the timing of implantation by in vivo gene transfer: delay of implantation by suppression of nuclear factor kappaB activity and partial rescue by leukemia inhibitory factor. Biochem Biophys Res Commun. 2004b;321:886–892. doi: 10.1016/j.bbrc.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Ottobre JS, Lewis GS, Thayne WV, Inskeep EK. Mechanism by which progesterone shortens the estrous cycle of the ewe. Biol Reprod. 1980;23:1046–1053. doi: 10.1095/biolreprod23.5.1046. [DOI] [PubMed] [Google Scholar]
- Page M, Tuckerman EM, Li TC, Laird SM. Expression of nuclear factor kappa B components in human endometrium. J Reprod Immunol. 2002;54:1–13. doi: 10.1016/s0165-0378(01)00122-x. [DOI] [PubMed] [Google Scholar]
- Paulesu L, Jantra S, Ietta F, Brizzi R, Bigliardi E. Interleukin-1 in reproductive strategies. Evol Dev. 2008;10:778–788. doi: 10.1111/j.1525-142X.2008.00292.x. [DOI] [PubMed] [Google Scholar]
- Perry JS, Rowlands IW. Early pregnancy in the pig. J Reprod Fert. 1962;4:175–188. [Google Scholar]
- Quaedackers ME, Brink CE, van den Saag PT, van der Tertoolen LG. Direct interaction between estrogen receptor alpha and NF-kappaB in the nucleus of living cells. Mol Cell Endocrinol. 2007;273:42–50. doi: 10.1016/j.mce.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Rawdanowicz TJ, Hampton AL, Nagase H, Woolley DE, Salamonsen LA. Matrix metalloproteinase production by cultured human endometrial stromal cells: identification of interstitial collagenase, gelatinase-A, gelatinase-B, and stromelysin-1 and their differential regulation by interleukin-1 alpha and tumor necrosis factor-alpha. J Clin Endocrinol Metab. 1994;79:530–536. doi: 10.1210/jcem.79.2.8045973. [DOI] [PubMed] [Google Scholar]
- Reese J, Das SK, Paria BC, Lim H, Song H, Matsumoto H, Knudtson KL, DuBois RN, Dey SK. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J Biol Chem. 2001;47:44137–44145. doi: 10.1074/jbc.M107563200. [DOI] [PubMed] [Google Scholar]
- Ross JW, Ashworth MD, Hurst AG, Malayer JR, Geisert RD. Analysis and characterization of differential gene expression during rapid trophoblastic elongation in the pig using suppression subtractive hybridization. Reprod Biol Endocrinol. 2003a;1:23. doi: 10.1186/1477-7827-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JW, Malayer JR, Ritchey JW, Geisert RD. Involvement of the interleukin-1β system in porcine trophoblastic elongation and at the fetal-maternal interface during peri-implantation development. Biol Reprod. 2003b;69:1251–1259. doi: 10.1095/biolreprod.103.015842. [DOI] [PubMed] [Google Scholar]
- Ross JW, Ashworth MD, Mathew D, Reagan P, Ritchey JW, Hayashi K, Spencer TE, Lucy M, Geisert RD. Activation of the transcription factor, nuclear factor kappa-B, during the estrous cycle and early pregnancy in the pig. Reprod Biol Endocrinol. 2010;8:39. doi: 10.1186/1477-7827-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutanen EM, Pekonen F, Mäkinen T. Soluble 34K binding protein inhibits the binding of insulin-like growth factor I to its cell receptors in human secretory phase endometrium: evidence for autocrine/paracrine regulation of growth factor action. J Clin Endocrinol Metab. 1988;66:173–180. doi: 10.1210/jcem-66-1-173. [DOI] [PubMed] [Google Scholar]
- Samuel CA, Perry JS. The ultrastructure of pig trophoblast transplanted to an ectopic site in the uterine wall. J Anat. 1972;113:139–149. [PMC free article] [PubMed] [Google Scholar]
- Satterfield MC, Bazer FW, Spencer TE. Progesterone regulation of preimplantation conceptus growth and galectin 15 (LGALS15) in the ovine uterus. Biol Reprod. 2006;75:289–296. doi: 10.1095/biolreprod.106.052944. [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Song G, Kochan KJ, Riggs PK, Simmons RM, Elsik CG, Adelson DL, Bazer FW, Zhou H, Spencer TE. Discovery of candidate genes and pathways in the endometrium regulating ovine blastocyst growth and conceptus elongation. Physiol Genomics. 2009;39:85–99. doi: 10.1152/physiolgenomics.00001.2009. [DOI] [PubMed] [Google Scholar]
- Sawai K, Matsuzaki N, Okada T, Shimoya K, Koyama M, Azuma C, Saji F, Murata Y. Human decidual cell biosynthesis of leukemia inhibitory factor: regulation by decidual cytokines and steroid hormones. Biol Reprod. 1997;56:1274–1280. doi: 10.1095/biolreprod56.5.1274. [DOI] [PubMed] [Google Scholar]
- Schäfer-Somi S, Beceriklisoy HB, Budik S, Kanca H, Aksoy OA, Polat B, Cetin Y, Ay SS, Aslan S. Expression of genes in the canine pre-implantation uterus and embryo: implications for an active role of the embryo before and during invasion. Reprod Domest Anim. 2008;43:656–663. doi: 10.1111/j.1439-0531.2007.00966.x. [DOI] [PubMed] [Google Scholar]
- Seki H, Zosmer A, Elder MG, Sullivan MH. The regulation of progesterone and hCG production from placental cells by interleukin-1beta. Biochim Biophys Acta. 1997;1336:342–348. doi: 10.1016/s0304-4165(97)00042-1. [DOI] [PubMed] [Google Scholar]
- Seo H, Kim M, Choi Y, Ka H. Salivary lipocalin is uniquely expressed in the uterine endometrial glands at the time of conceptus implantation and induced by interleukin 1beta in pigs. Biol Reprod. 2011;84:279–287. doi: 10.1095/biolreprod.110.086934. [DOI] [PubMed] [Google Scholar]
- Simon C, Dominguez F. Embryonic-endometrial interactions at implantation in humans. Gynecol Obstet Invest. 2004;57:28–30. [PubMed] [Google Scholar]
- Simon C, Frances A, Piquette G, Hendrickson M, Milki A, Polan ML. Interleukin-1 system in the materno-trophoblast unit in human implantation: immunohistochemical evidence for autocrine/paracrine function. J Clin Endocrinol Metab. 1994a;78:847–854. doi: 10.1210/jcem.78.4.8157710. [DOI] [PubMed] [Google Scholar]
- Simon C, Frances A, Piquette GN, Danasouri I, el Zurawski G, Dang W, Polan ML. Embryonic implantation in mice is blocked by interleukin-1 receptor antagonist. Endocrinology. 1994b;134:521–528. doi: 10.1210/endo.134.2.8299552. [DOI] [PubMed] [Google Scholar]
- Simon C, Pellicer A, Polan ML. Interleukin-1 system crosstalk between embryo and endometrium in implantation. Hum Reprod. 1995;10:43–54. doi: 10.1093/humrep/10.suppl_2.43. [DOI] [PubMed] [Google Scholar]
- Simon C, Mercader A, Gimeno MJ, Pellicer A. The interleukin-1 system and human implantation. Am J Reprod Immunol. 1997a;37:64–72. doi: 10.1111/j.1600-0897.1997.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Simon C, Gimeno MJ, Mercader A, O’Connor JE, Remohí J, Polan ML, Pellicer A. Embryonic regulation of integrins beta 3, alpha 4, and alpha 1 in human endometrial epithelial cells in vitro. J Clin Endocrinol Metab. 1997b;82:2607–2616. doi: 10.1210/jcem.82.8.4153. [DOI] [PubMed] [Google Scholar]
- Simon C, Valbuena D, Krüssel J, Bernal A, Murphy CR, Shaw T, Pellicer A, Polan ML. Interleukin-1 receptor antagonist prevents embryonic implantation by a direct effect on the endometrial epithelium. Fertil Steril. 1998;70:896–906. doi: 10.1016/s0015-0282(98)00275-1. [DOI] [PubMed] [Google Scholar]
- Simon C, Domínguez F, Valbuena D, Pellicer A. The role of estrogen in uterine receptivity and blastocyst implantation. Trends Endocrinol Metab. 2003;14:197–199. doi: 10.1016/s1043-2760(03)00084-5. [DOI] [PubMed] [Google Scholar]
- Singh H, Aplin JD. Adhesion molecules in endometrial epithelium: tissue integrity and embryo implantation. J Anat. 2009;215:3–13. doi: 10.1111/j.1469-7580.2008.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayden OD, Keater CS. Role of progesterone in nonhuman primate implantation. Sem Reprod Med. 2007;25:418–430. doi: 10.1055/s-2007-991039. [DOI] [PubMed] [Google Scholar]
- Song G, Dunlap KA, Kim J, Bailey DW, Spencer TE, Burghardt RC, Wagner GF, Johnson GA, Bazer FW. Stanniocalcin 1 is a luminal epithelial marker for implantation in pigs regulated by progesterone and estradiol. Endocrinology. 2009;150:936–945. doi: 10.1210/en.2008-1026. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW. Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod. 1995;53:1527–1545. doi: 10.1095/biolreprod53.6.1527. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci. 2002;7:d1879–d1898. doi: 10.2741/spencer. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW. Uterine and placental factors regulating conceptus growth in domestic animals. J Anim Sci. 2004;82(E-Suppl):E4–E13. doi: 10.2527/2004.8213_supplE4x. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Johnson GA, Burghardt RC, Bazer FW. Progesterone and placental hormone actions on the uterus: insights from domestic animals. Biol Reprod. 2004;71:2–10. doi: 10.1095/biolreprod.103.024133. [DOI] [PubMed] [Google Scholar]
- Strakova Z, Srisuparp S, Fazleabas AT. Interleukin-1beta induces the expression of insulin-like growth factor binding protein-1 during decidualization in the primate. Endocrinology. 2000;141:4664–4670. doi: 10.1210/endo.141.12.7810. [DOI] [PubMed] [Google Scholar]
- Strakova Z, Szmidt M, Srisuparp S, Fazleabas AT. Inhibition of matrix metalloproteinases prevents the synthesis of insulin-like growth factor binding protein-1 during decidualization in the baboon. Endocrinology. 2003;144:5339–5346. doi: 10.1210/en.2003-0471. [DOI] [PubMed] [Google Scholar]
- Strakova Z, Mavrogianis P, Meng X, Hastings JM, Jackson KS, Cameo P, Brudney A, Knight O, Fazleabas AT. In vivo infusion of interleukin-1beta and chorionic gonadotropin induces endometrial changes that mimic early pregnancy events in the baboon. Endocrinology. 2005;146:4097–4104. doi: 10.1210/en.2005-0380. [DOI] [PubMed] [Google Scholar]
- Sukjumlong S, Kaeoket K, Dalin A-M, Persson E. Immunohistochemical studies on oestrogen receptor alpha (ERα) and the proliferative marker Ki-67 in the sow uterus at different stages of the oestrous cycle. Reprod Domest Anim. 2003;38:5–12. doi: 10.1046/j.1439-0531.2003.00383.x. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. Cytokines and the hypothalamic-pituitary-ovarian-endometrial axis. Hum Reprod. 1994;9:947–967. doi: 10.1093/oxfordjournals.humrep.a138621. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S, Sun XZ. Cytokine expression in human endometrium throughout the menstrual cycle. Hum Reprod. 1992;7:1214–1221. doi: 10.1093/oxfordjournals.humrep.a137829. [DOI] [PubMed] [Google Scholar]
- Takacs R, Kauma S. The expression of interleukin-1α, interleukin-1β, and interleukin-1 receptor type I mRNA during preimplantation mouse development. J Reprod Immunol. 1996;32:27–35. doi: 10.1016/s0165-0378(96)00987-4. [DOI] [PubMed] [Google Scholar]
- Tamura M, Sebastian S, Yang S, Gurates B, Fang Z, Bulun SE. Interleukin-1beta elevates cyclooxygenase-2 protein level and enzyme activity via increasing its mRNA stability in human endometrial stromal cells: an effect mediated by extracellularly regulated kinases 1 and 2. J Clin Endocrinol Metab. 2002;87:3263–3273. doi: 10.1210/jcem.87.7.8594. [DOI] [PubMed] [Google Scholar]
- Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–5321. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino S, Verhage HG, Fazleabas AT. Regulation of insulin-like growth factor-binding proteins in the baboon (Papio anubis) uterus during early pregnancy. Endocrinology. 1992;130:2354–2362. doi: 10.1210/endo.130.4.1372242. [DOI] [PubMed] [Google Scholar]
- Tuo W, Harney JP, Bazer FW. Developmentally regulated expression of interleukin-1β by peri-implantation conceptuses in swine. J Reprod Immunol. 1996;31:185–198. doi: 10.1016/0165-0378(96)00975-8. [DOI] [PubMed] [Google Scholar]
- Vaughan TJ, James PS, Pascall JC, Brown KD. Expression of the genes for TGFα, EGF and EGF receptor during early pig development. Development. 1992;116:663–669. doi: 10.1242/dev.116.3.663. [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- White FJ, Ross JW, Joyce MM, Geisert RD, Burghardt RC, Johnson GA. Steroid regulation of cell specific secreted phosphoprotein 1 (osteopontin) expression in the pregnant porcine uterus. Biol Reprod. 2005;73:1294–1301. doi: 10.1095/biolreprod.105.045153. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fahrenkrug SC, Smith TPL, Rohrer GA, Ford SP. Differential expression of cyclooxygenase-2 around the time of elongation in the pig conceptus. Anim Reprod Sci. 2002;71:229–237. doi: 10.1016/s0378-4320(02)00029-5. [DOI] [PubMed] [Google Scholar]
- Wolff M, von Thaler CJ, Strowitzki T, Broome J, Stolz W, Tabibzadeh S. Regulated expression of cytokines in human endometrium throughout the menstrual cycle: dysregulation in habitual abortion. Mol Hum Reprod. 2000;6:627–634. doi: 10.1093/molehr/6.7.627. [DOI] [PubMed] [Google Scholar]
- Yagel S, Lala PK, Powell WA, Casper RF. Interleukin-1 stimulates human chorionic gonadotropin secretion by first trimester human trophoblast. J Clin Endocrinol Metab. 1989;68:992–995. doi: 10.1210/jcem-68-5-992. [DOI] [PubMed] [Google Scholar]
- Ying CW, Hsu W-L, Hong W-F, Cheng WTK, Yang Y-C. Estrogen receptor is expressed in pig embryos during perimplantation development. Mol Reprod Dev. 2000;55:83–88. doi: 10.1002/(SICI)1098-2795(200001)55:1<83::AID-MRD11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Koga K, Tsutsumi O, Yano T, Fujii T, Kugu K, Momoeda M, Fujiwara T, Tomita K, Taketani Y. Evidence for the expression of interleukin (IL)-18, IL-18 receptor and IL-18 binding protein in the human endometrium. Mol Hum Reprod. 2001;7:649–654. doi: 10.1093/molehr/7.7.649. [DOI] [PubMed] [Google Scholar]
- Zheng H, Fletcher D, Kozak W, Jiang M, Hofmann KJ, Conn CA, Soszynski D, Grabiec C, Trumbauer ME, Shaw A, et al. Resistance to fever induction and impaired acute-phase response in interleukin-1 beta-deficient mice. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]