Abstract
Nuclear factor κB (NF-κB) and activator protein 1 (AP-1) transcription factors regulate many important biological and pathological processes. Activation of NF-κB is regulated by the inducible phosphorylation of NF-κB inhibitor IκB by IκB kinase. In contrast, Fos, a key component of AP-1, is primarily transcriptionally regulated by serum responsive factors (SRFs) and ternary complex factors (TCFs). Despite these different regulatory mechanisms, there is an intriguing possibility that NF-κB and AP-1 may modulate each other, thus expanding the scope of these two rapidly inducible transcription factors. To determine whether NF-κB activity is involved in the regulation of fos expression in response to various stimuli, we analyzed activity of AP-1 and expression of fos, fosB, fra-1, fra-2, jun, junB, and junD, as well as AP-1 downstream target gene VEGF, using MDAPanc-28 and MDAPanc-28/IκBαM pancreatic tumor cells and wild-type, IKK1−/−, and IKK2−/− murine embryonic fibroblast cells. Our results show that elk-1, a member of TCFs, is one of the NF-κB downstream target genes. Inhibition of NF-κB activity greatly decreased expression of elk-1. Consequently, the reduced level of activated Elk-1 protein by extracellular signal-regulated kinase impeded constitutive, serum-, and superoxide-inducible c-fos expression. Thus, our study revealed a distinct and essential role of NF-κB in participating in the regulation of elk-1, c-fos, and VEGF expression.
Nuclear factor κB (NF-κB) and activator protein 1 (AP-1) are key transcription factors that orchestrate expression of many genes involved in inflammation, embryonic development, lymphoid differentiation, oncogenesis, and apoptosis (48, 62). NF-κB and AP-1 activities are induced by a plethora of physiological and environmental stimuli (5, 51).
The activity of NF-κB is regulated by its interaction with the family of NF-κB inhibitors known as IκB, which results in the formation of inactive NF-κB-IκB complexes in the cytoplasm (3, 4, 60). In response to various stimuli, the IκB kinase complex (IKK) then phosphorylates the IκB bound to the NF-κB complexes as substrates (8, 36, 45, 47). The subsequent proteasome-mediated degradation of IκB exposes the nuclear localization signal (NLS) of NF-κB, which releases the NF-κB proteins to be translocated to the nucleus, where they regulate the transcription of specific genes (5, 48).
AP-1 is a group of basic leucine zipper (bZIP) transcription factors consisting of the Fos (c-Fos, FosB, Fra1, and Fra2) and Jun (c-Jun, JunB, and JunD) families (54, 62). The predominant forms of AP-1 in most cells are Fos/Jun heterodimers which have a high affinity for binding to an AP-1 site, whereas Jun/Jun homodimers bind to the AP-1 site with low affinity (54, 62). A number of studies have shown that serum and growth factors that induce AP-1 do so by activating the extracellular signal-regulated kinase (ERK) subgroup of mitogen-activated protein kinases (MAPKs) (9, 27, 55). These activated members of MAPKs translocate to the nucleus to phosphorylate and thereby transcriptionally activate a subfamily of ETS domain transcription factors known as ternary complex factors (TCFs) that bind to fos promoters (9, 27, 55, 65, 80). fos, fosB, and other members of the AP-1 family of transcription factors are mainly regulated at their transcription through serum responsive elements (SREs) in their promoters (57, 76). For example, the regulation of c-fos expression is controlled by Elk, a member of TCFs that associates with the serum response factor (SRF) (11, 28, 49). The elk-1 gene encodes two spliced variants: elk-1 and an alternatively spliced variant known as Δelk-1, which is missing the SRF interaction domain and part of the elk-1 DNA binding domain (61). The ΔElk-1protein cannot form an SRF-dependent ternary complex with SRE to activate fos transcription (61). However, a variety of experiments have shown that Elk-1 proteins play a central role in the response of cells to many extracellular signals and control the expression of genes involved in cell cycle progression, differentiation, and apoptosis (62, 75). The mechanism by which Elk-1 activates transcription in response to various stimuli has been extensively studied; however, less is known about the regulation of elk-1 gene expression itself.
Even though NF-κB and AP-1 transcription factors are regulated by different mechanisms, they appear to be activated simultaneously by the same multitude of stimuli (1, 19, 37, 43, 71, 78). A number of reports also showed that these transcription factors appear to be regulated by the same intracellular signal transduction cascades. Indeed, the activation of JNK by inflammatory cytokines or by stress is often accompanied by the nuclear translocation of NF-κB, and many genes require the concomitant activation of AP-1 and NF-κB, suggesting that these transcription factors work cooperatively (77). One clue to the interactions between the AP-1 and NF-κB activation pathways was the finding that activation of the MAPK pathway leads to the activation of JNK and the IκB kinase complexes (42, 43, 83). In addition, a scaffold protein was discovered that participates in the activation of the JNK pathway and the nuclear translocation of NF-κB (69). Further, the response to AP-1 is strikingly enhanced when NF-κB subunits are present and vice versa (66). The p65 subunit of NF-κB can act like an accessory protein for the SRF in transfection assays (20). NF-κB and C/EBPβ transcription factors are accessory proteins for the RhoA-linked regulation of the activity of the SRF (50). More recent findings have shown that NF-κB and AP-1 play a crucial role in the regulation of FasL in the Fas-mediated thymineless death of colon carcinoma cells (26). Moreover, the differential binding of AP-1 and NF-κB to the interleukin-8 (IL-8) promoter regulates the cell type-specific induction of H2O2- and tumor necrosis factor alpha (TNF-α)-mediated IL-8 gene expression (41). On the basis of these collective findings, one could speculate, therefore, an intriguing possibility that NF-κB and AP-1 may modulate the activity of each other, thus expanding the scope of these two rapidly inducible transcription factors. This interesting possibility implies that a cellular response to certain stimuli is induced cooperatively by a network of transcription factors that are activated concurrently. In this study, we delineated the signaling cascades responsible for NF-κB-dependent AP-1 regulation and VEGF expression by using cells lacking key components of the NF-κB activation pathway. We provided the first evidence that NF-κB plays an essential role in regulation of elk-1 expression, which in turn can be activated by ERK to induce the expression of c-fos and fosB in response to certain stimuli.
MATERIALS AND METHODS
Reagents.
Doxycycline was purchased from Sigma. A stock solution of 8-mg/ml doxycycline was prepared immediately before its use in phosphate-buffered saline (PBS) with a pH of 7.4. The expression vector encoding IKK2 (S177, 181E), the constitutively active form of IKK2, was described by Schmidt et al. (58). The expression vectors encoding mutant elk-1 (DN-Elk-1) and fos (ΔFos) were generated as described by Vanhoutte et al. (74) and Yen et al. (85), respectively. elk-1 promoter was cloned by PCR into a luciferase reporter gene as previously described (44). The wild-type and mutated κB binding motifs in elk-1 promoter were generated by site-directed mutagenesis performed with a double-stranded, site-directed mutagenesis kit from Stratagene. Primary antibodies for immunoblotting (IκBα, VEGF, and β-actin) and the electrophoretic mobility shift assay (EMSA) (RelA/p65, p50, Fos, and Elk-1) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.) Radioisotopes were purchased from Amersham Biosciences.
Cell culture.
The human pancreatic tumor cell line MDAPanc-28 was originally established by M. Frazier and D. B. Evans (The University of Texas M. D. Anderson Cancer Center) (21). Wild-type, IKK1−/−, and IKK2−/− murine embryonic fibroblast (MEF) cells were provided by Inder M. Verma (Salk Institute, La Jolla, Calif.) (46, 47). Cells were maintained in plastic flasks as adherent monolayers in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, sodium pyruvate, nonessential amino acids, and l-glutamine in 5% CO2 at 37°C. For doxycycline stimulation, a stock solution of doxycycline (Sigma, The Woodlands, Tex.) was prepared with PBS at 8 mg/ml (pH 7.4) and diluted in PBS to allow a 1% volume addition of doxycycline to the experimental wells. Control cells were treated with a 1% volume of PBS, which is indicated in the figures and figure legends as 0-μg/ml doxycycline.
Transfection and luciferase assays.
One microgram each of the wild-type κB, mutant κB, wild-type AP-1, mutant AP-1, wild-type Elk-1, and mutant Elk-1 reporter plasmids containing the firefly luciferase reporter gene (79) and the pRL-TK plasmid, containing the Renilla luciferase gene under the control of the herpes simplex virus thymidine kinase promoter as an internal control, was cotransfected into cells in triplicate by the lipotransfection method (FuGENE 6; Roche, Indianapolis, Ind.) according to the manufacturer's recommendation. The activities of both firefly and Renilla luciferases were determined 48 h after transfection with the dual luciferase reporter assay system (Promega, Madison, Wis.). The luciferase activities were normalized to the Renilla luciferase activity of the internal control.
EMSA.
EMSA was performed with nuclear extracts prepared from control and stimulated cells as previously described (17). Briefly, 10 μg of nuclear extract was incubated with 1 μg of poly(dI-dC) (Pharmacia, Piscataway, N.J.) in 10 μl of binding buffer (75 mM NaCl, 15 mM Tris-HCl, [pH 7.5], 1.5 mM EDTA, 1.5 mM dithiothreitol, 25% glycerol, 20-mg/ml bovine serum albumin) for 40 min at 4°C. 32P-labeled probes contained the following double-stranded oligonucleotides, which were synthesized by Sigma and generated by a kinase reaction with polynucleotide kinase and [γ-32P]ATP (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.) (underlined portions of the sequences represent specific binding sites for the transcription factors; boldface letters represent mutated sites): wild-type κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′), mutant κB (5′-AGTTGAGGCGACTTTCCCAGGC-3′), wild-type AP-1 (5′-CGCTTGATGACTCAGCCGGAA-3′), mutant AP-1 (5′-CGCTTGATGACTTGGCCGGAA-3′), Oct-1 (5′-TGTCGAATGCAAATCACTAGAA-3′), wild-type Elk-1 (5′-GGATGTCCATATTAGGACATCT-3′), mutant Elk-1 (5′-GGATGTCCATATTATTACATCT-3′), wild-type κB/Elk-1 (5′-GCTCTGTAGGGAAGGGCCCGTCCCC-3′), and mutant κB/Elk-1 (5′-GCTCTGTAGAAAAGGGCGGGTCCCC-3′). Equal loading of nuclear extracts was monitored by Oct-1 binding. For competition assays, a 50-fold molar excess of unlabeled oligonucleotides was added to the binding reaction mixture. For supershift assays, 2 μl of polyclonal antibodies to p65, p50, p52, c-Rel, Elk-1, and c-Fos (Santa Cruz Biotechnology, Inc.) in the presence or absence of their neutralizing peptides were preincubated for 30 min with the nuclear extracts before the probe was added. The probe was allowed to bind for 20 min at room temperature. Reaction mixtures were analyzed on 4% polyacrylamide gels containing 0.25× TBE (22.5 mM Tris, 22.5 mM borate and 500 μM EDTA, pH 8.0) buffer. The gel was then dried for 1 h at 80°C and exposed to Kodak film (Eastman Kodak Co., Rochester, N.Y.) at −80°C.
Northern blot analysis.
For Northern blot analysis, total RNA was extracted with Trizol reagent (Life Technologies, Inc., Gaithersburg, Md.) according to the manufacturer's recommendation. Fifteen micrograms of total RNA was separated electrophoretically on a 1% denaturing formaldehyde-agarose gel, transferred to a Hybond nylon filter (MSI, Westborough, Mass.) in 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 N sodium citrate), and UV cross-linked with a UV Stratalinker 1800 (Stratagene, La Jolla, Calif.). To obtain a cDNA probe for the Northern blot, reverse transcription-PCR was performed as follows. One microgram of total RNA made from MDAPanc-28 cells was reverse transcribed at 42°C for 1 h in the presence of 100 ng of Oligo (dT) 12-18 primer (Life Technologies, Inc.), 250 μM deoxynucleoside triphosphates (dNTPs; Promega), 1× incubation buffer of avian myeloblastosis virus (AMV) reverse transcriptase (Roche), 20 U of RNase inhibitor (Roche), and 25 U of avian myeloblastosis virus reverse transcriptase (Roche) in a final volume of 20 μl. One microliter of the reaction mixture was then subjected to 30 PCR cycles (denaturing at 94°C for 1 min, annealing at 56°C for 1 min, and polymerization at 72°C for 1 min) in the presence of 0.25 U of Taq DNA polymerase (Roche), 1× PCR buffer (Roche), 250 μM dNTPs (Promega), and 500 nM specific primers as follows: human Elk-1 (product size, 941 bp; 5′-TCCTTCTCCAGCATGGGCTC-3′ and 3′-TCAGCCTCGGGGTAGGTGAA-5′); and mouse Elk-1 (product size, 900 bp; 5′-TCCTTCTCCAGCATGGGCTC-3′ and 3′-TCAGCCTCGGGGTAGGTGAA-5′). PCR products were extracted and subsequently cloned in a pCR2.1-TOPO vector (Invitrogen, Carlsbad, Calif.) for sequence analysis. The sequences of these cDNA fragments agreed with those from the GenBank database. The cDNA probes (EcoRI-EcoRI) were labeled with [α-32P]dCTP with the Roche random labeling kit and subjected to hybridization. Equal loading of mRNA samples was monitored by hybridizing the same membrane filter with the cDNA probe for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as described previously (32).
RNase protection assay.
RNA was isolated from wild-type, IKK1−/−, and IKK2−/− MEF cells stimulated with either doxycycline or 20% serum for the indicated dose and time, using Trizol reagent. An RNase protection assay was performed with the Riboquant multiprobe protection assay system according to the manufacturer's recommendation.
Western blot analysis.
Cytoplasmic extracts were prepared as previously described (17). Soluble protein was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (10% polyacrylamide) and electrophoretically transferred onto polyvinylidene difluoride membrane (Osmonics, Westborough, Mass.). The membrane was blocked with 5% nonfat milk in PBS containing 0.2% Tween 20 and incubated with affinity-purified mouse antibody to IκBα, c-Fos, and β-actin (Santa Cruz Biotechnology, Inc.). The membranes were washed in PBS containing 0.2% Tween 20 and probed with horseradish peroxidase-coupled secondary goat anti-rabbit or anti-mouse immunoglobulin G antibodies (Amersham, Arlington Heights, Ill.). The proteins were detected with the Lumi-Light Western blotting substrate (Roche) according to the manufacturer's instructions.
ChIP assay.
The chromatin immunoprecipitation (ChIP) assay was performed with a chromatin immunoprecipitation assay kit (Upstate Biotechnology, Lake Placid, N.Y.) as recommended by the manufacturer. Briefly, after MDAPanc28/puro and MDAPanc28/IκBαM cells (2 × 106 each) were stimulated with 40-μg/ml doxycycline for 24 h, formaldehyde (1% final concentration) was directly added to the culture medium for cross-linked reactions at 37°C for 10 min. Cells were lysed in 200 μl of SDS lysis buffer with a protease-inhibitor mixture and sonicated to generate DNA fragments 200 to 1,000 bp long. After a preclearing step for 30 min at 4°C, the lysates were incubated with anti-p65 antibody (Santa Cruz) at 4°C for overnight with rotation. Immune complexes were precipitated with 60 μl of salmon sperm DNA-protein A agarose, eluted with elution buffer (1% SDS, 0.1 M NaHCO3) after washing, and incubated at 65°C for 6 h. After proteinase K (20 μg/ml) digestion at 50°C for 2 h, DNA was extracted in phenol, precipitated with ethanol, resuspended in 20 μl of Tris-EDTA (TE) buffer, and subjected to PCR, using for elk-1 primers 5′-CGTTCCATCATTTCCCCTTA-3′ and 5′-TGCGTTTCCCTACAGCTCAC-3′, which corresponded to 167 bp of the elk-1 promoter region, including the predicted NF-κB binding site.
RESULTS
Blocking constitutive NF-κB activation inhibits AP-1 activity and the overexpression of c-fos.
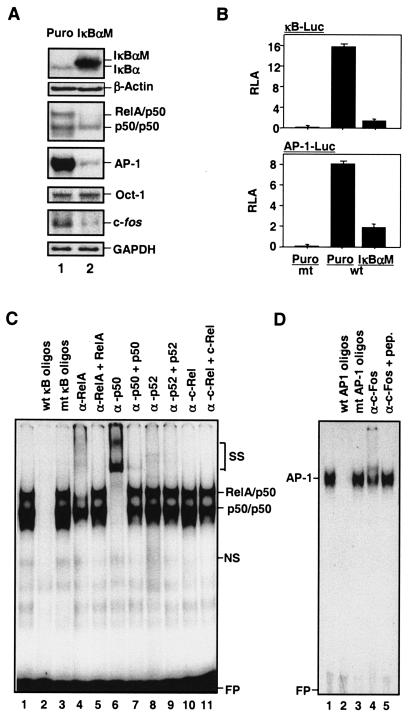
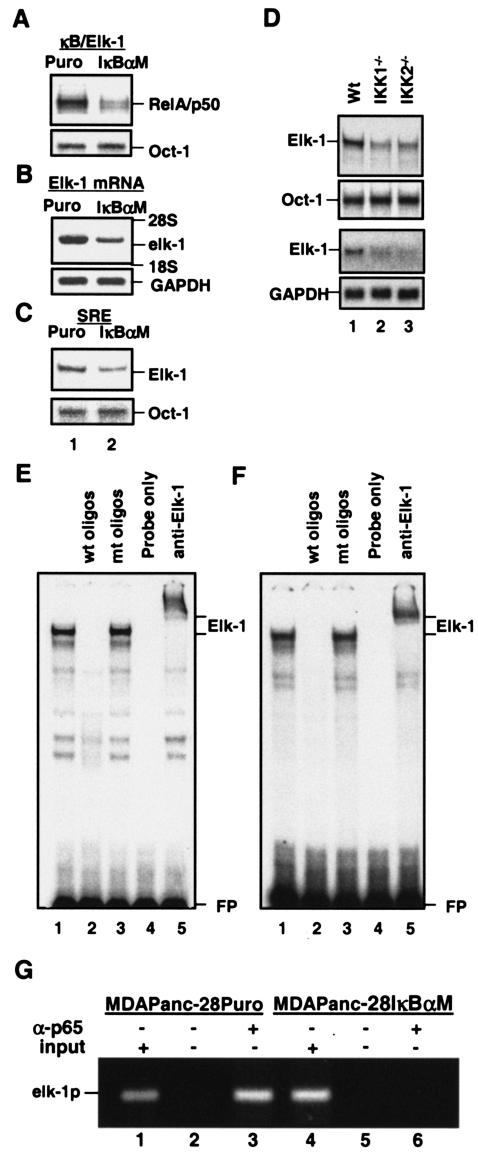
To determine whether constitutive NF-κB activity and AP-1 activity were coupled, NF-κB and AP-1 activities were analyzed in MDAPanc-28/IκBαM and MDAPanc-28/Puro cells established from pooled Flag-tagged IκBαM-infected and puromycin control retrovirus-infected MDAPanc-28 cells (22, 23). The expression of the Flag-IκBαM protein was confirmed by Western blot analysis (Fig. 1A). In MDAPanc-28/IκBαM cells, the constitutive DNA binding activity of RelA/p50 heterodimer was also completely inhibited, and the constitutive AP-1-DNA binding activity was concurrently downregulated significantly, whereas the Oct-1 DNA binding activity was not altered (Fig. 1A).
FIG. 1.
Suppression of constitutive NF-κB activity inhibits overexpression of c-fos and AP-1 activity. (A) The expression of IκBα and IκBαM in MDAPanc-28/Puro and MDAPanc-28/IκBαM cells was determined by Western blot analysis with the cytoplasmic protein extracts using β-actin as loading controls. The nuclear protein extracts from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells were subjected to EMSA with NF-κB, AP-1, and the Oct-1 probe as a control. Expression of c-fos in MDAPanc-28/Puro and MDAPanc-28/IκBαM cells was determined by Northern blot analysis with GAPDH as the loading control. (B) Luciferase reporter gene assays for NF-κB and AP-1 activities were performed with wild-type (wt) and mutant (mt) NF-κB and AP-1 luciferase reporter gene constructs transiently transfected into MDAPanc-28/Puro and MDAPanc-28/IκBαM cells. (C and D) Competition and supershift assays were performed with EMSA to determine the specificity of the constitutive RelA/p50 NF-κB and AP-1 DNA binding activities. Ten micrograms of nuclear extract from MDAPanc-28/Puro cells was incubated with a 50× excess of unlabeled wild-type NF-κB or AP-1 probe (C and D, lane 2), mutant NF-κB or AP-1 probe (C and D, lane 3), and anti-NF-κB and anti-Fos antibodies (α-), with and without control peptides, as indicated. FP, free probe.
To elucidate the mechanism underlying the inhibition of AP-1 activation by the IκBαM-mediated suppression of constitutive NF-κB activity, we first examined the transcriptional regulation of the c-fos gene, which encodes a major AP-1 component. Northern blot analysis showed that the overexpression of c-fos was significantly downregulated in response to IκBαM-mediated NF-κB inhibition (Fig. 1A). This finding was consistent with the significant inhibition of NF-κB- and AP-1-dependent reporter gene activities in the MDAPanc-28/IκBαM cells (Fig. 1B). Competition and supershift assays showed the presence of RelA and p50 in NF-κB DNA binding activity and c-Fos in AP-1 DNA binding activity (Fig. 1C and D). Taken together, these results suggest that constitutive NF-κB activity induces the overexpression of c-fos and activation of AP-1.
NF-κB activity is involved in ROS- and serum-induced AP-1 activation.
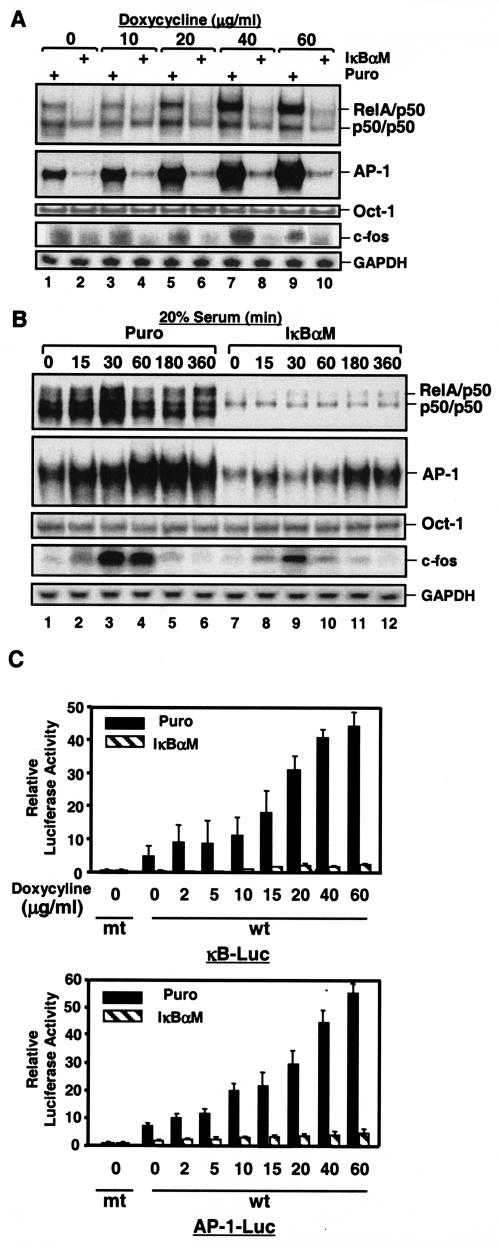
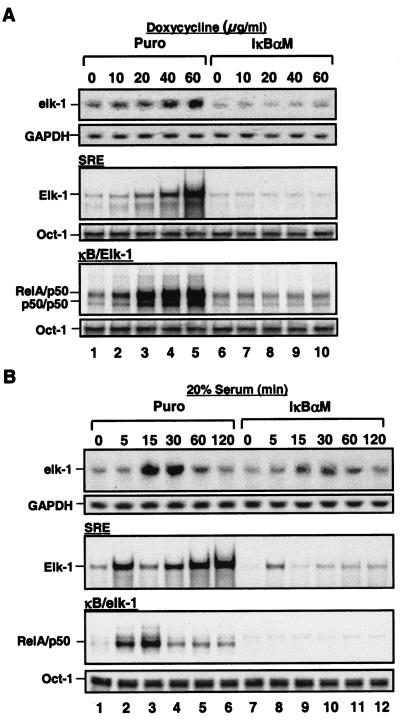
To further define the molecular mechanism by which the NF-κB signal pathway regulates AP-1 activity, we stimulated both MDAPanc-28/Puro and MDAPanc-28/IκBαM cells with serum and doxycycline, which strongly activates both NF-κB and AP-1 by inducing reactive oxygen species (ROS) (data not shown) (7, 39, 40). Doxycycline strongly activated both NF-κB and AP-1 in MDAPanc-28/Puro cells in a dose-dependent mode, but not in MDAPanc28/IκBαM cells (Fig. 2A). Consistent with the AP-1 activities in the MDAPacn-28/Puro cells, doxycycline induced c-fos expression in MDAPanc-28/Puro cells, but it had significantly less effect in MDAPanc-28/IκBαM cells (Fig. 2A). Furthermore, although serum activated both NF-κB and AP-1 in a time-dependent manner in MDAPanc-28/Puro cells, it did not do so in MDAPanc-28/IκBαM cells (Fig. 2B). Serum induction of c-fos expression was greatly reduced and delayed in MDAPanc-28/IκBαM cells (Fig. 2B). Moreover, doxycycline-induced NF-κB and AP-1 reporter gene activation seen in MDAPanc-28/Puro cells was almost completely absent in MDAPanc-28/IκBαM cells (Fig. 2C). These results further suggest that NF-κB activity is essential for doxycycline- and serum-induced c-fos expression and AP-1 activation.
FIG. 2.
Induction of AP-1 activity and fos expression by doxycycline-induced ROS requires NF-κB activation, and serum-induced AP-1 activity and fos expression are partially dependent on NF-κB activation. (A) EMSAs were performed to determine NF-κB and AP-1 activity by using the nuclear extracts isolated from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with increasing doses ofdoxycycline as indicated for 24 h. An Oct-1 probe was used as a control for quality and quantity of cell extracts. RNA was isolated from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with doxycycline for the indicated dosages and analyzed by Northern blotting with a human c-fos cDNA probe, and the same blot was rehybridized to a gapdh cDNA probe. (B) Inhibition of serum-induced activation of NF-κB and AP-1 was determined by using EMSAs with the nuclear extracts from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with 20% serum for the indicated times after 48 h of serum deprivation. An Oct-1 probe was used as a control for quality and quantity of cell extracts. RNA was isolated from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with 20% serum for the indicated time and analyzed by Northern blotting with a human c-fos cDNA probe, and the same blot was rehybridized to a gapdh cDNA probe. (C) IκBαM-mediated inhibition of the doxycycline-induced activation of NF-κB and AP-1 activities was determined with wild-type (wt) and mutant (mt) NF-κB and AP-1 luciferase reporter gene constructs transiently transfected into MDAPanc-28/Puro and MDAPanc-28/IκBαM cells.
Inducible AP-1 activation is inhibited in IKK1−/− and IKK2−/− MEFs.
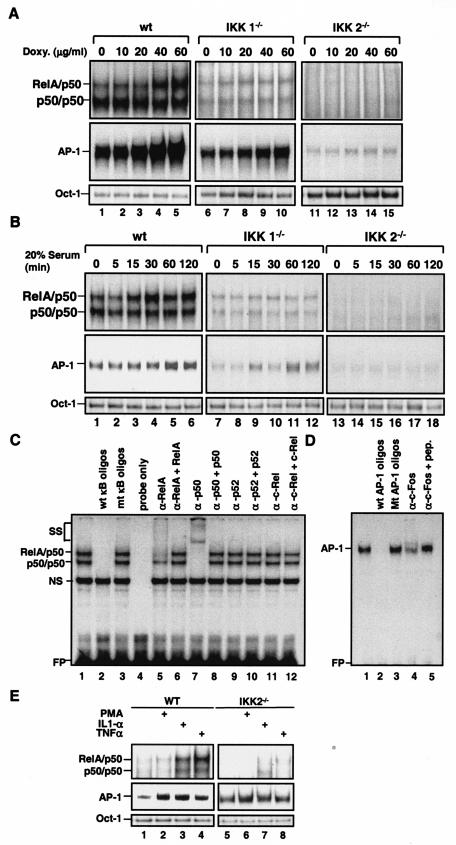
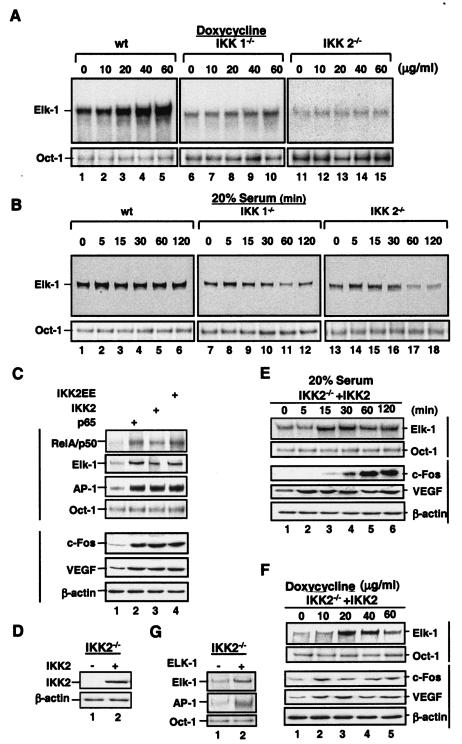
To further determine whether the induction of c-fos expression and AP-1 activity by doxycycline and serum is NF-κB dependent, doxycycline- and serum-activated AP-1 DNA binding was determined in wild-type, IKK1−/−, and IKK2−/− MEFs. EMSAs of these MEF cells showed that both NF-κB and AP-1 were activated by doxycycline and serum in wild-type MEFs, only partially in IKK1−/− cells, and almost not at all in IKK2−/− cells (Fig. 3A and B). The partial inhibition of serum-induced AP-1 activity in IKK1−/− MEF cells (Fig. 3B) was consistent with the limited impairment of serum-induced AP-1 activation in MDAPanc-28/IκBαM cells (Fig. 2B). Competition and supershift assays confirmed the presence of RelA and p50 in NF-κB DNA binding activity and c-Fos in AP-1-DNA binding activity (Fig. 3C and D). These results show that both doxycycline-induced AP-1 activation and serum-induced AP-1 activation were reduced or inhibited in IKK1−/− and IKK2−/− MEF cells, suggesting that NF-κB activity is involved in signaling steps in the regulation of AP-1 activation in response to serum and ROS. To determine whether the AP-1-regulatory function of NF-κB is limited to serum and doxycycline or also applies to other NF-κB inducers, wild-type and IKK2−/− MEF cells were stimulated with phorbol myristate acetate (PMA), IL-1α, and TNF-α for 30 min, and nuclear extracts were isolated for EMSA analysis. As shown in Fig. 3E, AP-1 DNA binding activity was induced by PMA, IL-1α, and TNF-α in wild-type but not in IKK2−/− cells. Taken together, these results suggest that NF-κB activation is one of the regulatory steps for AP-1 in response to several inducers such as serum, ROS, and cytokines.
FIG. 3.
Doxycycline- and serum-inducible NF-κB and AP-1 activities were inhibited in IKK1−/− and IKK2−/− MEF cells. Wild-type (wt), IKK1−/−, and IKK2−/− MEF cells were stimulated with doxycycline as indicated for 24 h (A), and wild-type, IKK1−/−, and IKK2−/− MEF cellswere stimulated with 20% serum for the indicated times after 48 h of serum starvation (B). Nuclear extracts were isolated and subjected to EMSA with NF-κB and AP-1 probes. (C and D) Competition and supershift assays were performed with EMSA to determine the specificity of the doxycycline-induced RelA/p50 NF-κB and AP-1 DNA binding activities. Ten micrograms of nuclear protein from doxycycline-stimulated wild-type MEF cells with a 50× excess of unlabeled wild-type NF-κB or AP-1 probe (C and D, lane 2) or mutant NF-κB or AP-1 probe (C and D, lane 3), along with anti-NF-κB and anti-Fos antibodies (α-), with and without control peptides, as indicated. FP, free probe. (E) Wild-type (WT) and IKK2−/− MEF cells were stimulated with PMA (50 μg/ml), IL-1α (10 ng/ml), and TNF-α (10 ng/ml) for 30 min, and EMSAs with NF-κB, AP-1, and Oct-1 probes were performed.
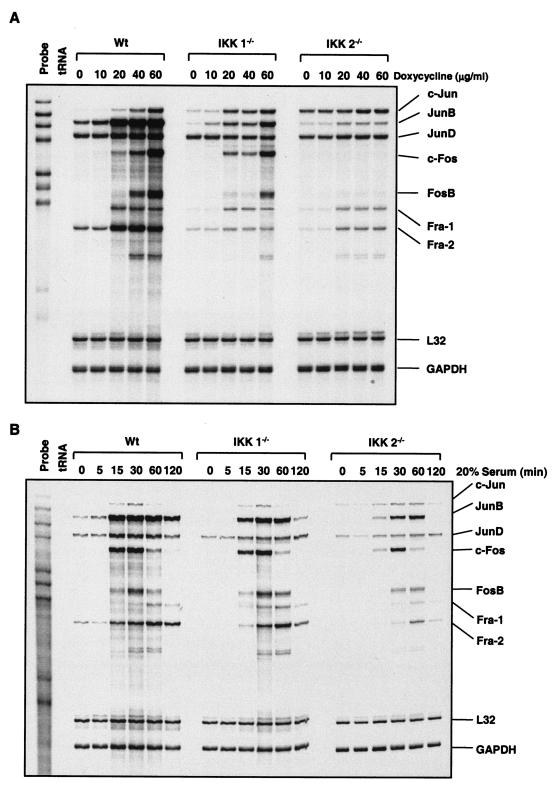
To determine which AP-1 component is expressed and induced by doxycycline and serum, RNase protection assays were performed with wild-type, IKK1−/−, and IKK2−/− MEF cells. Doxycycline stimulation induced the dose-dependent gene expression of c-jun, junB, c-fos, fosB, fra-1, and fra-2, but not junD, in wild-type MEF cells (Fig. 4A). However, the doxycycline-induced expression of c-jun, junB, c-fos, fosB, fra-1, and fra-2 was reduced and delayed in IKK1−/− cells, and expression of c-fos and fosB genes was not detected in IKK2−/− cells (Fig. 4A). c-jun expression was even elevated in IKK2−/− MEF cells (Fig. 4A). These results suggest that the regulation of the expression of c-jun, junB, fra-1, and fra-2 partially depends on IKK under doxycycline stimulation and that IKK2 activity is required for doxycycline to mediate c-fos and fosB expression.
FIG. 4.
Doxycycline- and serum-inducible expressions of the AP-1 family members were determined in wild-type (Wt), IKK1−/−, and IKK2−/− MEF cells. RNA was isolated from wild-type, IKK1−/−, and IKK2−/− MEF cells stimulated with either doxycycline or 20% serum for the indicated doses and times. RNase protection assays were carried out with the Riboquant multiprobe protection assay system to determine doxycycline (A)- and serum stimulation (B)-induced expression of AP-1 components in wild-type, IKK1−/−, and IKK2−/− MEF cells as indicated.
The induced expression of c-jun, junB, junD, c-fos, and fosB peaked from 15 to 30 min after serum stimulation, and the induction of fra-1 and fra-2 expression peaked at 60 min after serum stimulation in wild-type MEF cells (Fig. 4B). The serum induction of c-jun, junB, junD, c-fos, fosB, fra-1, and fra-2 gene expression decreased to some extent in IKK1−/− cells, consistent with serum-induced AP-1 DNA binding activity (Fig. 3B). In contrast, the serum-induced expression of c-jun, junB, junD, c-fos, fosB, fra-1, and fra-2 was considerably reduced and delayed in IKK2−/− MEF cells (Fig. 4B), consistent with the inhibition of AP-1 activation (Fig. 3B). Only the reduction and delay of c-fos and fosB induction by serum stimulation were observed in IKK2−/− cells, as opposed to the complete inhibition of fos and fosB induction by doxycycline stimulation (Fig. 4A). Serum-induced c-jun expression was not affected in IKK1−/− MEF cells and was even sustained in IKK2−/− MEF cells, consistent with findings of a previous study (68). These results suggest that, under serum stimulation, IKK2 but not IKK1 is involved in the regulation of the expression of AP-1 components. Taken together, these results suggest that NF-κB plays an important role in the regulation of the expression of c-fos and fosB in response to doxycycline stimulation but plays a less prominent role in the serum-regulated expression of c-fos and fosB.
NF-κB regulates elk-1 expression.
Because the SRE appears to be constitutively occupied in vivo, increased Elk-1 transcriptional activity is a more likely mechanism of c-fos induction (9, 25, 29, 35, 73). Therefore, we speculated that NF-κB-dependent elk-1 expression might play an important role in augmenting c-fos expression and in AP-1 induction in response to doxycycline stimulation. Our data (Fig. 1 to 4) suggest that the regulation of c-fos expression is a key step by which NF-κB regulates AP-1 activity. To determine whether NF-κB regulates elk-1 expression, we first analyzed NF-κB DNA binding activity to the κB-like motif found in the elk-1 promoter (κBelk-1) (44). We found that the constitutive DNA binding activity of the RelA/p50 heterodimer to the κBelk-1 motif was significantly reduced in MDAPanc-28/IκBαM cells (Fig. 5A). Consistent with this, expression of elk-1 mRNA and Elk-1 DNA binding activity to the SRE also decreased in MDAPanc-28/IκBαM cells (Fig. 5B and C). Similar decreases in Elk-1 DNA binding activity and the expression of elk-1 mRNA were observed in IKK1−/− and IKK2−/− MEF cells (Fig. 5D). Elk-1 activity in MDAPanc-28/Puro and in wild-type MEF cells was determined by competition and supershift assay (Fig. 5E and F). To further demonstrate that elk-1 is an NF-κB downstream target gene, we performed ChIP assays. The results showed that p65 (NF-κB) directly interacted with the promoter region of elk-l in MDAPanc-28/Puro cells, but this interaction was inhibited in MDAPanc-28/IκBαM cells (Fig. 5G). These results from NF-κB EMSA and ChIP assays suggest that NF-κB is one of the transcription factors that regulate elk-1 expression.
FIG. 5.
NF-κB activity regulates elk-1 expression. (A) EMSAs were performed with the nuclear extracts isolated from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells to determine the NF-κB binding activity to the NF-κB motif on the elk-1 promoter with an Oct-1 probe as a control. (B) elk-1 mRNA expression was determined by Northern blot analysis with RNA from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells with elk-1 cDNA and gapdh probe as a loading control. (C) Elk-1 DNA binding activity on SRE from the c-fos promoter was determined by EMSA with the nuclear extracts from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells (A) and an Oct-1 probe as a control. (D) EMSAs were performed with the nuclear extracts isolatedfrom wild-type (Wt), IKK1−/−, and IKK2−/− MEF cells to determine the Elk-1 DNA binding activity with the SRE probe. An Oct-1 probe was used as a control for loading. The expression of elk-1 was determined by Northern blot analysis with the RNA isolated from wild-type, IKK1−/−, and IKK2−/− MEF cells with mouse elk-1 cDNA and gapdh probes. (E and F) Elk-1 activity in MDAPanc-28/Puro (E) and wild-type MEF (F) cells was determined by competition and supershift assay with 10 μg of nuclear extract with a 50× excess of unlabeled wild-type and mutant Elk-1 probe (lanes 2 and 3) and anti-Elk-1 antibody as indicated. FP, free probe. (G) ChIP assays were performed with MDAPanc-28/Puro and MDAPanc-28/IκBαM cells with and without anti-p65 (NF-κB) antibody as indicated.
Induction of elk-1 expression by doxycycline and serum is dependent on NF-κB activity.
To determine whether the induction of elk-1 expression by doxycycline and serum is dependent on NF-κB activity, doxycycline- and serum-induced elk-1 expression and Elk-1 DNA binding activity were analyzed in MDAPanc-28/Puro and MDAPanc-28/IκBαM cells. NF-κB DNA binding activity to κBelk-1 was inhibited in MDAPanc-28/IκBαM cells, suggesting that the doxycycline-mediated induction of elk-1 expression and DNA binding activity was NF-κB dependent (Fig. 6A and B). The serum-induced DNA binding activity of NF-κB to the κB binding site in the elk-1 promoter was detected in MDAPanc-28/Puro cells but was completely inhibited in MDAPanc-28/IκBαM cells (Fig. 6B). The serum-induced expression of elk-1 and the DNA binding activity of Elk-1 were decreased in MDAPanc-28/IκBαM cells. The Elk-1 DNA binding activity induced by doxycycline and serum was inhibited in IKK1−/− and IKK2−/− MEF cells (Fig. 7A and B). Figure 7C shows that overexpression of a constitutively active IKK2 (S177, 181E), IKK2, and p65 (NF-κB) by transient transfection of wild-type MEF cells induced the DNA binding activity of RelA/p50 (NF-κB), Elk-1, and AP-1 and expression of c-fos and VEGF. IKK2 expression was reconstituted in IKK2−/− cells by establishing stable transfectants (Fig. 7D). The serum- and doxycycline-induced Elk-1 DNA binding activity and expression of c-fos and VEGF were restored in IKK2-reconstituted cells (Fig. 7E and F). Furthermore, overexpression of Elk-1 in IKK2−/− cells induced AP-1 DNA binding activity (Fig. 7G). Collectively, these results imply that the regulation of elk-1 and c-fos expression is NF-κB dependent.
FIG. 6.
NF-κB activity is essential for doxycycline- and serum-induced elk-1 expression. (A) Doxycycline- and (B) serum-induced NF-κB-dependent induction of elk-1 expression was determined by Northern blot analysis. RNA was isolated from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with doxycycline at the indicated doses and 20% serum for the indicated times and hybridized with a human elk-1 cDNA probe and rehybridized to a gapdh cDNA probe. (A) Doxycycline (A)- and serum (B)-induced NF-κB-dependent activation of Elk-1 was determined by EMSAs with NF-κB and SRE probes and the nuclear extracts isolated from MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with doxycycline as indicated for 24 h or with 20% serum for the indicated times after 48 h of serum withdrawal. An Oct-1 probe was used as a control for quality and quantity of cell extracts.
FIG. 7.
IKK is essential for doxycycline- and serum-induced elk-1 expression. Wild-type (wt), IKK1−/−, and IKK2−/− MEF cells were stimulated with doxycycline at the indicated doses for 24 h (A) or with 20% serum for the indicated times after 48 h of serum withdrawal (B). Nuclear extracts were isolated and subjected to EMSA with an SRE probe. (C) Nuclear and whole-cell extracts were isolated from the IKK2 (S177, 181E), IKK2, and p65 (NF-κB) transfectants and analyzed by EMSA with NF-κB, Elk-1, AP-1, and Oct-1 probes and Western blotting with anti-cFos, VEGF, and β-actin antibodies as indicated. (D) The protein extracts of the IKK2- or vector-transfected IKK2−/− clone were analyzed for the expression of transfected IKK2 in Western blotting with β-actin as loading controls. (E) IKK2-reconstituted cells from panel D were stimulated with 20% serum for the indicated times after 48 h of serum starvation. (F) IKK2-reconstituted cells from panel D were stimulated with doxycycline at the indicated doses for 24 h. Nuclear extracts from panels E and F were isolated and analyzed by EMSA with NF-κB, AP-1, and Oct-1 probes, and whole-cell extracts from both panels E and F were subjected to Western blot analysis with anti-c-Fos, -VEGF, and -β-actin antibodies as indicated. (G) The nuclear extracts from an IKK2−/− clone transfected with an expression plasmid encoding Elk-1 were analyzed by EMSA with SRE and AP-1 probes and with Oct-1 probe as a loading control.
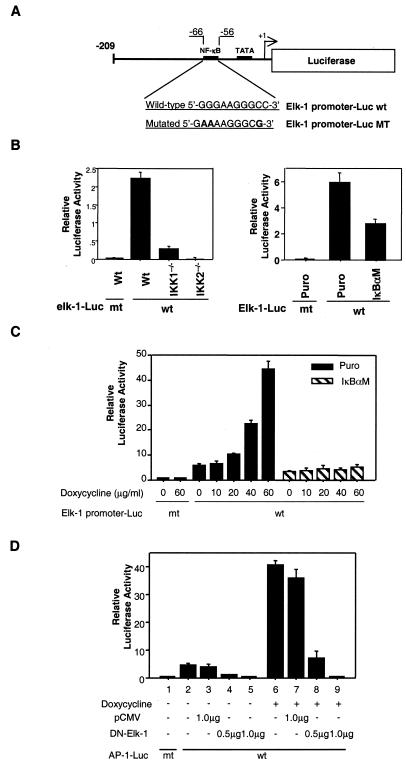
NF-κB participates the regulation of the elk-1 promoter.
To further investigate the regulation of elk-1 expression by NF-κB, elk-1 promoters with wild-type and mutated κB binding motifs were cloned by PCR into a luciferase reporter gene as previously described (Fig. 8A) (44). The luciferase reporter gene reporter gene activity of the elk-1 promoter was significantly decreased in MDAPanc-28/IκBαM cells and IKK1−/− and IKK2−/− MEF cells (Fig. 8B). Doxycycline-mediated elk-1 promoter reporter gene activity was induced in MDAPanc-28/Puro cells but almost completely inhibited in MDAPanc-28/IκBαM cells (Fig. 8C). These results suggest that elk-1 is one of the downstream target genes regulated by NF-κB in response to doxycycline stimulation. To determine the role of Elk-1 in doxycycline-mediated AP-1 activation, AP-1-dependent luciferase reporter gene activity was analyzed in MDAPanc-28/Puro cells transfected with or without a dominant-negative mutant Elk-1 (ΔElk-1) in the presence or absence of doxycycline. As shown in Fig. 8D, ΔElk-1 completely inhibited constitutive (lanes 4 and 5) and doxycycline-induced (lanes 8 and 9) activation of the AP-1 reporter gene, indicating that Elk-1 plays an essential role in both constitutive and doxycycline-inducible AP-1 activity.
FIG. 8.
NF-κB regulates the elk-1 promoter, and a dominant mutant Elk-1 inhibits AP-1 activity. (A) elk-1 promoter and elk-1 promoter luciferase reporter gene constructs with wild-type (wt) and mutant (MT) κBelk-1 sequences indicated. (B) Luciferase reporter gene assays for the basal activity of elk-1 promoter constructs with wild-type (Wt) κB and mutant (mt) κB binding sites were performed with wild-type, IKK1−/−, and IKK2−/− MEF cells as indicated. (C) Luciferase reporter gene assays for the inducible of elk-1 promoter activity were performed with MDAPanc-28/Puro and MDAPanc-28/IκBαM cells cotransfected with elk-1 promoter reporter gene constructs with wild-type and mutant κB binding sites and p-TK Renilla luciferase and then stimulated with doxycycline as indicated. (D) MDAPanc-28/Puro and MDAPanc-28/IκBαM cells were cotransfected either with the indicated amount of pCMV expression vector encoding the mutant Elk-1 or control pCMV vector, AP-1-luciferase reporter gene construct, and p-TK Renilla luciferase in the absence or presence of doxycycline stimulation. The transient transfections in panels B, C, and D were performed by the lipotransfection method (FuGENE 6; Roche). The activities of both firefly luciferase and Renilla luciferase were determined with the Promega dual-luciferase reporter assay system. The luciferase activities were normalized to the Renilla luciferase activity of the internal control. Data represent the mean ± standard error from three different experiments performed in triplicate.
NF-κB-dependent AP-1 activity regulates VEGF expression.
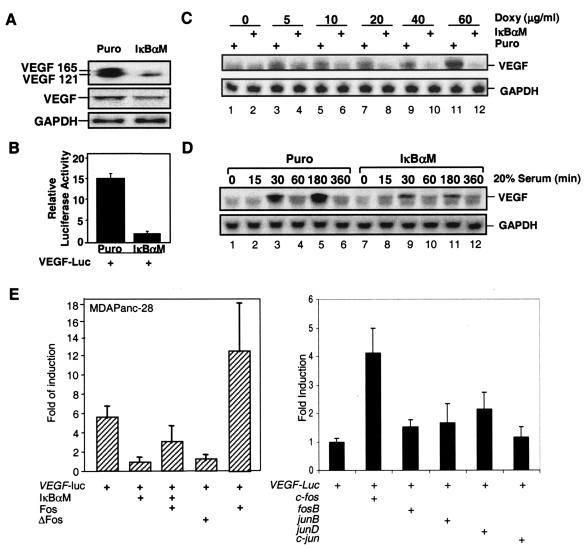
We and others have previously shown that the inhibition of NF-κB suppressed tumorigenesis and metastasis and downregulated VEGF expression in a number of cancer cell lines (6, 22, 23, 30, 38, 63). However, analysis of the VEGF regulation showed that several AP-1 and hypoxia-induced factor (HIF) elements, not the κB site present in the VEGF promoter and AP-1 and HIFs regulate VEGF expression (24, 56, 64, 70). We further analyzed NF-κB-mediated VEGF expression in Panc28/Puro and Panc28/IκBαM cells. The inhibition of constitutive NF-κB activity by IκBαM downregulated VEGF expression and VEGF promoter activity (Fig. 9A and B). IκBαM-mediated inhibition of NF-κB also completely suppressed doxycycline-induced VEGF expression (Fig. 9C). Downregulated serum-induced VEGF levels were also observed in MDAPanc-28/IκBαM cells (Fig. 9D). Interestingly, the transient induction of VEGF expression induced by serum occurred in early and late phases at 30 and 180 min. The early phase coincided with serum-induced transient c-fos expression, which peaked at 30 min, and the late phase overlapped with the appearance of delayed AP-1 activation, which occurred at 180 and 360 min (Fig. 2B). These results suggest that NF-κB-regulated AP-1 activity is connected to the regulation of VEGF expression.
FIG. 9.
NF-κB-mediated AP-1 activity regulates VEGF expression. (A) Western blot and Northern analyses of VEGF expression in MDAPanc-28/Puro and MDAPanc-28/IκBαM cells as indicated. (B) Luciferase reporter gene assays for the activity of a VEGF promoter construct were performed with MDAPanc-28/Puro and MDAPanc-28/IκBαM cells as indicated. (C and D) Northern blot analysis of VEGF expression in MDAPanc-28/Puro and MDAPanc-28/IκBαM cells stimulated with serum at various time intervals and doxycycline at different doses. (E) Luciferase reporter gene assays for the activity of a VEGF promoter construct were performed with the expression vectors encoding IκBαM, a dominant-negative Fos mutant (ΔFos), Fos, FosB, JunB, JunD, and c-Jun as indicated. The activities of both firefly luciferase and Renilla luciferase were determined by using the Promega dual-luciferase reporter assay system (Promega). The luciferase activities were normalized to the Renilla luciferase activity of the internal control. Data represent the mean ± standard error from three different experiments performed in triplicate.
To determine whether NF-κB-dependent AP-1 activity regulates the VEGF promoter, Panc-28/Puro cells were transfected with a VEGF promoter-luciferase reporter gene construct and various combinations of the expression vectors encoding IκBαM, dominant-negative Fos mutant (ΔFos), Fos, FosB, JunB, JunD, and c-Jun (Fig. 9E). These results showed that the VEGF promoter activity induced by the constitutive NF-κB activity in Panc28/Puro cells was inhibited by IκBαM and ΔFos, and IκBαM-mediated inhibition of VEGF promoter activity was overcome by cotransfection and overexpression of the c-fos gene (Fig. 9E). Furthermore, VEGF promoter-luciferase reporter gene assays showed that c-Fos is a more potent activator of VEGF promoter than other members of AP-1 family (Fig. 9E). These findings are consistent with the results shown in previous figures and suggest that NF-κB-regulated AP-1 activity induced VEGF expression.
DISCUSSION
Our results showed that the inhibition of NF-κB activity, achieved by stable expression of IκBαM in MDAPanc-28 cells or by MEF cells lacking IKK1 or IKK2, led to the inhibition of fos expression and AP-1 activity. We found that elk-1 was one of the NF-κB downstream genes. Inhibition of NF-κB reduced the level of Elk-1 protein to be activated by MAPK, thus impeding AP-1 activity and AP-1-dependent gene expression. We further found that the expression of VEGF, which possesses AP-1 binding motifs but not NF-κB in its promoter, was significantly downregulated in IκBαM transfectants (Fig. 9A and B). Previous studies also suggested that NF-κB regulates VEGF gene expression at the transcriptional level (22, 23, 31, 38, 63). Our results also showed that the constitutive and inducible NF-κB activities played key roles in upregulating the transcription of its downstream gene elk-1. The increased level of MAPK-activated Elk-1 further induced c-fos expression and AP-1 activity, which in turn increased VEGF gene expression. Thus, our findings provide a possible mechanism by which NF-κB regulates VEGF expression through Elk-1 and AP-1.
Transcription factors serve as integration centers of the different signaling cascades that control the expression of a given gene. The regulation of these transcription factors themselves is therefore of critical importance in determining the response to various physiological and environmental stimuli. Transcription factors can be regulated mainly by two means—concentration and activity—each of which can be modulated in diverse ways. For example, the activity of a transcription factor is often regulated by phosphorylation or dephosphorylation through multiple intracellular signal transduction pathways. The MAPKs are mediators of signal transduction from the cell surface to the nucleus, and NF-κB, Elk-1 and AP-1 are the targets of these MAPKs. For example, MAPK kinase kinase-3 (MEKK3), a mammalian serine/threonine kinase that directly activates JNKK1, a MAPK kinase that stimulates JNK1/2 and p38 MAPK, which in turn induces AP-1, has been shown to activate IKK1 and IKK2 in TNF-α-induced NF-κB activation (12, 18, 83, 86). In this way, two distinct transcription factors, NF-κB and AP-1, could be cooperatively regulated by MAPK signaling cascades, the activation of NF-κB could lead an increase in the transcription of elk-1 and c-fos, and the activation of Erk1/2, p38, and JNK could enhance the activity of Elk-1 and AP-1 transcription factors.
The coactivation of NF-κB and AP-1 can synergistically and effectively enhance the transcription of genes in response to a variety of stimuli. Many genes require the simultaneous activation of both transcription factors working cooperatively (10, 15, 84). For example, Stein et al. showed that the bZIP regions of c-Fos and c-Jun are capable of physically interacting with NF-κB/p65 through the Rel homology domain, which enhances DNA binding and biological function via both the κB and AP-1 response elements (66). This seems to explain one mechanism of cross talk between these distinct transcription factors. In our study, we initially found that the level of c-fos expression was significantly decreased in Panc-28/IκBαM cells, in which constitutive NF-κB activity was inhibited (Fig. 1A). Consistent with this, the transcription of junB, c-fos, fosB, and fra-2 genes was downregulated in MEF cells lacking IKK1 or IKK2 (Fig. 4A and B). These results led us to hypothesize that the transcriptional downregulation of AP-1 components, especially c-Fos, might play an important role in the regulation of NF-κB-dependent AP-1 downstream target genes. In our experiments, we used serum and doxycycline, which strongly induces both NF-κB and AP-1 activities through the generation of superoxide (unpublished data). Under doxycycline stimulation, most AP-1 components were induced at the transcriptional level in wild-type MEF cells, but less so in IKK1−/− cells, suggesting a general role for IKK1 in the regulation of AP-1 components stimulated by doxycycline (Fig. 4A). However, the inducible c-Fos and FosB were specifically inhibited, while the expression of other AP-1 components in IKK2−/− MEF cells was only weakly inhibited, suggesting that IKK2 is the key effector in the regulation of AP-1 by exerting specific control over the transcription of c-fos and fosB under doxycycline stimulation (Fig. 4A). However, under serum stimulation, c-fos and fosB expression in IKK2−/− cells was only weakly inhibited (Fig. 4B), suggesting the effect of IKK in the regulation of the specific AP-1 monomer is signal dependent. The inhibition of constitutive and inducible c-fos expression resulted in the downregulation of AP-1 activity and the expression of AP-1-regulated genes such as VEGF (Fig. 9).
To further clarify the mechanism by which NF-κB regulates the expression of c-fos, we determined the activity of Elk-1, a transcription factor that regulates c-fos transcription (28, 33, 72). We showed that the expression of elk-1 was transcriptionally regulated by NF-κB through an NF-κB-like binding motif in the elk-1 promoter and that the doxycycline-induced activation of the RelA/p65 heterodimer to this motif was significantly reduced in IκBαM-expressing cells (Fig. 5 and 6), which in turn inhibited the expression of elk-1, which decreased the level of Elk-1 protein that can be activated by MAPKs and the binding of Elk-1 to its binding motif in the c-Fos promoter (Fig. 8). In both IKK1- and IKK2-null cells, the expression level of Elk-1 was greatly reduced; however, it is unclear why only in IKK2−/− cells is the expression of c-fos and fosB predominantly inhibited. It is possible that IKK2 may be involved in regulation other members of the SRFs in addition to Elk-1.
Both Rac and p67phox are essential for the membrane-bound NADPH oxidase for the generation of superoxide (2, 16, 59). p67phox, which consists of two SH3 domains and has no catalytic activity, is a target of Rac (16). Overexpression of Rac induced the formation of ROS, and this may in part account for Rac-mediated NF-κB activation (67). Another target of Rac is POSH, which contains four SH3 domains (69). Overexpression of POSH induces both JNK and NF-κB activation (69). POSH interacts with JNK1 and -2, thus, appearing to act as a scaffold between Rac1 and the JNK pathway components and thereby perform a function not presently known for other scaffold proteins (81). Interestingly, the two C-terminal SH3 domains are sufficient to induce NF-κB translocation, but not JNK activation (67). It is possible that POSH may play a role in increasing JNK and NF-κB activities through the interaction of these four SH3 domains with different protein complexes. However, the mechanism by which POSH as a scaffold for multiprotein complexes enhances the specificity of either the JNK or NF-κB pathway is not clear.
In many cases, JNK and NF-κB are activated by same signaling pathways (12, 67, 82, 83), and the cross talk between these two pathways has been shown. Our findings show that NF-κB positively modulates the expression of c-Fos and AP-1 activity. Other findings showed that NF-κB could inhibit JNK signaling pathway (14, 52, 53, 68). For example, NF-κB activated Gadd45β, which in turn associated with MKK7 directly and blocked its further activation of JNK in response to TNF-α stimulation (14, 52). A recent report by Tang et al. suggested that in response to TNF-α, but not IL-1, X-chromosome-linked inhibitor of apoptosis (XIAP), an NF-κB downstream target gene, participated in the suppression of JNK signaling by NF-κB (68). Inactivation of JNK by NF-κB has been suggested to be a mechanism for the antiapoptotic effect of NF-κB upon TNF-α stimulation (14, 34, 68) because the JNK-dependent pathway has been shown to be required for TNF-α-induced apoptosis (13). There seem to be highly specific ways cross talk enables NF-κB activity to positively or negatively regulate JNK/AP-1 activities in response to different stimuli, such as TNF-α, serum, and superoxide. However, it is unclear how activation of both NF-κB and JNK pathways in cells achieves a response under different circumstances.
In summary, we have discovered a possible mechanism by which NF-κB regulates the expression of c-Fos and AP-1 activity through the controlling the expression level of Elk-1. When NF-κB and AP-1 are simultaneously activated by many inducers, the increased level of NF-κB downstream target gene elk-1 can be further activated by Erk1/2, p38, and JNK for greater induction of fos expression and AP-1 activation, which sequentially enhances the expression of AP-1 downstream target genes, thus allowing multiple regulatory steps and maximal target gene expression in response to a variety of stimuli.
Acknowledgments
We are grateful to Inder M. Verma for generously providing the wild-type, IKK1−/−, and IKK2−/− MEF cells. We thank David Galloway for editorial assistance.
This work was supported by grants from the National Cancer Institute (CA73675 and CA78778) and from the Lockton Fund for Pancreatic Cancer Research. G.M.S. is a recipient of a Fellowship of the Cancer League of Bern, Switzerland.
REFERENCES
- 1.Abate, C., L. Patel, F. J. Rauscher III, and T. Curran. 1990. Redox regulation of fos and jun DNA-binding activity in vitro. Science 249:1157-1161. [DOI] [PubMed] [Google Scholar]
- 2.Abo, A., E. Pick, A. Hall, N. Totty, C. G. Teahan, and A. W. Segal. 1991. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 353:668-670. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle, P. A., and D. Baltimore. 1988. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell 53:211-217. [DOI] [PubMed] [Google Scholar]
- 4.Baeuerle, P. A., and D. Baltimore. 1988. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science 242:540-546. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin, A. S., Jr. 1996. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 6.Bancroft, C. C., Z. Chen, G. Dong, J. B. Sunwoo, N. Yeh, C. Park, and C. Van Waes. 2001. Coexpression of proangiogenic factors IL-8 and VEGF by human head and neck squamous cell carcinoma involves coactivation by MEK-MAPK and IKK-NF-kappaB signal pathways. Clin. Cancer Res. 7:435-442. [PubMed] [Google Scholar]
- 7.Brar, S. S., T. P. Kennedy, A. R. Whorton, T. M. Murphy, P. Chitano, and J. R. Hoidal. 1999. Requirement for reactive oxygen species in serum-induced and platelet-derived growth factor-induced growth of airway smooth muscle. J. Biol. Chem. 274:20017-20026. [DOI] [PubMed] [Google Scholar]
- 8.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science 267:1485-1488. [DOI] [PubMed] [Google Scholar]
- 9.Cavigelli, M., F. Dolfi, F. X. Claret, and M. Karin. 1995. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. EMBO J. 14:5957-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins, T. S., L. F. Lee, and J. P. Ting. 2000. Paclitaxel up-regulates interleukin-8 synthesis in human lung carcinoma through an NF-kappaB- and AP-1-dependent mechanism. Cancer Immunol. Immunother. 49:78-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalton, S., and R. Treisman. 1992. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell 68:597-612. [DOI] [PubMed] [Google Scholar]
- 12.Deacon, K., and J. L. Blank. 1999. MEK kinase 3 directly activates MKK6 and MKK7, specific activators of the p38 and c-Jun NH2-terminal kinases. J. Biol. Chem. 274:16604-16610. [DOI] [PubMed] [Google Scholar]
- 13.Deng, Y., X. Ren, L. Yang, Y. Lin, and X. Wu. 2003. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell 115:61-70. [DOI] [PubMed] [Google Scholar]
- 14.De Smaele, E., F. Zazzeroni, S. Papa, D. U. Nguyen, R. Jin, J. Jones, R. Cong, and G. Franzoso. 2001. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature 414:308-313. [DOI] [PubMed] [Google Scholar]
- 15.Devary, Y., C. Rosette, J. A. DiDonato, and M. Karin. 1993. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science 261:1442-1445. [DOI] [PubMed] [Google Scholar]
- 16.Diekmann, D., A. Abo, C. Johnston, A. W. Segal, and A. Hall. 1994. Interaction of Rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science 265:531-533. [DOI] [PubMed] [Google Scholar]
- 17.Dong, Q. G., G. M. Sclabas, S. Fujioka, C. Schmidt, B. Peng, T. Wu, M. S. Tsao, D. B. Evans, J. L. Abbruzzese, T. J. McDonnell, and P. J. Chiao. 2002. The function of multiple IkappaB:NF-kappaB complexes in the resistance of cancer cells to Taxol-induced apoptosis. Oncogene 21:6510-6519. [DOI] [PubMed] [Google Scholar]
- 18.Ellinger-Ziegelbauer, H., K. Brown, K. Kelly, and U. Siebenlist. 1997. Direct activation of the stress-activated protein kinase (SAPK) and extracellular signal-regulated protein kinase (ERK) pathways by an inducible mitogen-activated protein kinase/ERK kinase kinase 3 (MEKK) derivative. J. Biol. Chem. 272:2668-2674. [DOI] [PubMed] [Google Scholar]
- 19.Fan, H., B. Sun, Q. Gu, A. Lafond-Walker, S. Cao, and L. C. Becker. 2002. Oxygen radicals trigger activation of NF-kappaB and AP-1 and upregulation of ICAM-1 in reperfused canine heart. Am. J. Physiol. Heart Circ. Physiol. 282:H1778-H1786. [DOI] [PubMed] [Google Scholar]
- 20.Franzoso, G., L. Carlson, K. Brown, M. B. Daucher, P. Bressler, and U. Siebenlist. 1996. Activation of the serum response factor by p65/NF-kappaB. EMBO J. 15:3403-3412. [PMC free article] [PubMed] [Google Scholar]
- 21.Frazier, M. L., E. Fernandez, R. de Llorens, N. M. Brown, S. Pathak, K. R. Cleary, J. L. Abbruzzese, K. Berry, M. Olive, A. Le Maistre, and D. B. Evans. 1996. Pancreatic adenocarcinoma cell line, MDAPanc-28, with features of both acinar and ductal cells. Int. J. Pancreatol. 19:31-38. [DOI] [PubMed] [Google Scholar]
- 22.Fujioka, S., G. M. Sclabas, C. Schmidt, W. A. Frederick, Q. G. Dong, J. L. Abbruzzese, D. B. Evans, C. Baker, and P. J. Chiao. 2003. Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin. Cancer Res. 9:346-354. [PubMed] [Google Scholar]
- 23.Fujioka, S., G. M. Sclabas, C. Schmidt, J. Niu, W. A. Frederick, Q. G. Dong, J. L. Abbruzzese, D. B. Evans, C. Baker, and P. J. Chiao. 2003. Inhibition of constitutive NF-kappaB activity by IkappaBalphaM suppresses tumorigenesis. Oncogene 22:1365-1370. [DOI] [PubMed] [Google Scholar]
- 24.Garrido, C., S. Saule, and D. Gospodarowicz. 1993. Transcriptional regulation of vascular endothelial growth factor gene expression in ovarian bovine granulosa cells. Growth Factors 8:109-117. [DOI] [PubMed] [Google Scholar]
- 25.Gille, H., M. Kortenjann, O. Thomae, C. Moomaw, C. Slaughter, M. H. Cobb, and P. E. Shaw. 1995. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 14:951-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harwood, F. G., S. Kasibhatla, I. Petak, R. Vernes, D. R. Green, and J. A. Houghton. 2000. Regulation of FasL by NF-kappaB and AP-1 in Fas-dependent thymineless death of human colon carcinoma cells. J. Biol. Chem. 275:10023-10029. [DOI] [PubMed] [Google Scholar]
- 27.Hipskind, R. A., D. Buscher, A. Nordheim, and M. Baccarini. 1994. Ras/MAP kinase-dependent and -independent signaling pathways target distinct ternary complex factors. Genes Dev. 8:1803-1816. [DOI] [PubMed] [Google Scholar]
- 28.Hipskind, R. A., V. N. Rao, C. G. Mueller, E. S. Reddy, and A. Nordheim. 1991. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature 354:531-534. [DOI] [PubMed] [Google Scholar]
- 29.Hodge, C., J. Liao, M. Stofega, K. Guan, C. Carter-Su, and J. Schwartz. 1998. Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2. J. Biol. Chem. 273:31327-31336. [DOI] [PubMed] [Google Scholar]
- 30.Huang, S., C. A. Pettaway, H. Uehara, C. D. Bucana, and I. J. Fidler. 2001. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 20:4188-4197. [DOI] [PubMed] [Google Scholar]
- 31.Huang, S., J. B. Robinson, A. Deguzman, C. D. Bucana, and I. J. Fidler. 2000. Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 60:5334-5339. [PubMed] [Google Scholar]
- 32.Hunt, K. K., J. B. Fleming, A. Abramian, L. Zhang, D. B. Evans, and P. J. Chiao. 1998. Overexpression of the tumor suppressor gene Smad4/DPC4 induces p21waf1 expression and growth inhibition in human carcinoma cells. Cancer Res. 58:5656-5661. [PubMed] [Google Scholar]
- 33.Janknecht, R., and A. Nordheim. 1992. Elk-1 protein domains required for direct and SRF-assisted DNA-binding. Nucleic Acids Res. 20:3317-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Javelaud, D., and F. Besancon. 2001. NF-kappa B activation results in rapid inactivation of JNK in TNF alpha-treated Ewing sarcoma cells: a mechanism for the anti-apoptotic effect of NF-kappa B. Oncogene 20:4365-4372. [DOI] [PubMed] [Google Scholar]
- 35.Karin, M. 1996. The regulation of AP-1 activity by mitogen-activated protein kinases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351:127-134. [DOI] [PubMed] [Google Scholar]
- 36.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 37.Karin, M., T. Takahashi, P. Kapahi, M. Delhase, Y. Chen, C. Makris, D. Rothwarf, V. Baud, G. Natoli, F. Guido, and N. Li. 2001. Oxidative stress and gene expression: the AP-1 and NF-kappaB connections. Biofactors 15:87-89. [DOI] [PubMed] [Google Scholar]
- 38.Kiriakidis, S., E. Andreakos, C. Monaco, B. Foxwell, M. Feldmann, and E. Paleolog. 2003. VEGF expression in human macrophages is NF-kappaB-dependent: studies using adenoviruses expressing the endogenous NF-kappaB inhibitor IkappaBalpha and a kinase-defective form of the IkappaB kinase 2. J. Cell Sci. 116:665-674. [DOI] [PubMed] [Google Scholar]
- 39.Kroon, A. M., B. H. Dontje, M. Holtrop, and C. Van den Bogert. 1984. The mitochondrial genetic system as a target for chemotherapy: tetracyclines as cytostatics. Cancer Lett. 25:33-40. [DOI] [PubMed] [Google Scholar]
- 40.Kroon, A. M., M. Holtrop, H. Fries, T. Melis, and C. van den Bogert. 1985. The effect of long-term inhibition of mitochondrial protein synthesis on the oxidation capacity of mitochondria for NADH-linked substrates. Biochem. Biophys. Res. Commun. 128:1190-1195. [DOI] [PubMed] [Google Scholar]
- 41.Lakshminarayanan, V., E. A. Drab-Weiss, and K. A. Roebuck. 1998. H2O2 and tumor necrosis factor-alpha induce differential binding of the redox-responsive transcription factors AP-1 and NF-kappaB to the interleukin-8 promoter in endothelial and epithelial cells. J. Biol. Chem. 273:32670-32678. [DOI] [PubMed] [Google Scholar]
- 42.Lee, F. S., J. Hagler, Z. J. Chen, and T. Maniatis. 1997. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell 88:213-222. [DOI] [PubMed] [Google Scholar]
- 43.Lee, S. W., S. I. Han, H. H. Kim, and Z. H. Lee. 2002. TAK1-dependent activation of AP-1 and c-Jun N-terminal kinase by receptor activator of NF-kappaB. J. Biochem. Mol. Biol. 35:371-376. [DOI] [PubMed] [Google Scholar]
- 44.Lehmann, U., P. Brocke, J. Dittmer, and A. Nordheim. 1999. Characterization of the human elk-1 promoter. Potential role of a downstream intronic sequence for elk-1 gene expression in monocytes. J. Biol. Chem. 274:1736-1744. [DOI] [PubMed] [Google Scholar]
- 45.Li, Q., G. Estepa, S. Memet, A. Israel, and I. M. Verma. 2000. Complete lack of NF-kappaB activity in IKK1 and IKK2 double-deficient mice: additional defect in neurulation. Genes Dev. 14:1729-1733. [PMC free article] [PubMed] [Google Scholar]
- 46.Li, Q., Q. Lu, J. Y. Hwang, D. Buscher, K. F. Lee, J. C. Izpisua-Belmonte, and I. M. Verma. 1999. IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 13:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li, Q., D. Van Antwerp, F. Mercurio, K. F. Lee, and I. M. Verma. 1999. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science 284:321-325. [DOI] [PubMed] [Google Scholar]
- 48.Li, Q., and I. M. Verma. 2002. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2:725-734. [DOI] [PubMed] [Google Scholar]
- 49.Marais, R., J. Wynne, and R. Treisman. 1993. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell 73:381-393. [DOI] [PubMed] [Google Scholar]
- 50.Montaner, S., R. Perona, L. Saniger, and J. C. Lacal. 1999. Activation of serum response factor by RhoA is mediated by the nuclear factor-kappaB and C/EBP transcription factors. J. Biol. Chem. 274:8506-8515. [DOI] [PubMed] [Google Scholar]
- 51.Pahl, H. L. 1999. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18:6853-6866. [DOI] [PubMed] [Google Scholar]
- 52.Papa, S., F. Zazzeroni, C. Bubici, S. Jayawardena, K. Alvarez, S. Matsuda, D. U. Nguyen, C. G. Pham, A. H. Nelsbach, T. Melis, E. D. Smaele, W. J. Tang, L. D'Adamio, and G. Franzoso. 2004. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat. Cell Biol. 6:146-153. [DOI] [PubMed] [Google Scholar]
- 53.Park, J. M., H. Brady, M. G. Ruocco, H. Sun, D. Williams, S. J. Lee, T. Kato, Jr., N. Richards, K. Chan, F. Mercurio, M. Karin, and S. A. Wasserman. 2004. Targeting of TAK1 by the NF-kappa B protein Relish regulates the JNK-mediated immune response in Drosophila. Genes Dev. 18:584-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ransone, L. J., and I. M. Verma. 1990. Nuclear proto-oncogenes fos and jun. Annu. Rev. Cell Biol. 6:539-557. [DOI] [PubMed] [Google Scholar]
- 55.Rao, V. N., and E. S. Reddy. 1994. elk-1 proteins interact with MAP kinases. Oncogene 9:1855-1860. [PubMed] [Google Scholar]
- 56.Salnikow, K., T. Kluz, M. Costa, D. Piquemal, Z. N. Demidenko, K. Xie, and M. V. Blagosklonny. 2002. The regulation of hypoxic genes by calcium involves c-Jun/AP-1, which cooperates with hypoxia-inducible factor 1 in response to hypoxia. Mol. Cell. Biol. 22:1734-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sassone-Corsi, P., and I. M. Verma. 1987. Modulation of c-fos gene transcription by negative and positive cellular factors. Nature 326:507-510. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt, C., B. Peng, Z. Li, G. M. Sclabas, S. Fujioka, J. Niu, M. Schmidt-Supprian, D. B. Evans, J. L. Abbruzzese, and P. J. Chiao. 2003. Mechanisms of proinflammatory cytokine-induced biphasic NF-kappaB activation. Mol. Cell 12:1287-1300. [DOI] [PubMed] [Google Scholar]
- 59.Segal, A. W., and A. Abo. 1993. The biochemical basis of the NADPH oxidase of phagocytes. Trends Biochem. Sci. 18:43-47. [DOI] [PubMed] [Google Scholar]
- 60.Sen, R., and D. Baltimore. 1986. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 47:921-928. [DOI] [PubMed] [Google Scholar]
- 61.Shao, N., Y. Chai, J. Q. Cui, N. Wang, K. Aysola, E. S. Reddy, and V. N. Rao. 1998. Induction of apoptosis by Elk-1 and deltaElk-1 proteins. Oncogene 17:527-532. [DOI] [PubMed] [Google Scholar]
- 62.Shaulian, E., and M. Karin. 2002. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4:E131-E136. [DOI] [PubMed] [Google Scholar]
- 63.Shibata, A., T. Nagaya, T. Imai, H. Funahashi, A. Nakao, and H. Seo. 2002. Inhibition of NF-kappaB activity decreases the VEGF mRNA expression in MDA-MB-231 breast cancer cells. Breast Cancer Res. Treat. 73:237-243. [DOI] [PubMed] [Google Scholar]
- 64.Shih, S. C., and K. P. Claffey. 2001. Role of AP-1 and HIF-1 transcription factors in TGF-beta activation of VEGF expression. Growth Factors 19:19-34. [DOI] [PubMed] [Google Scholar]
- 65.Shore, P., and A. D. Sharrocks. 1994. The transcription factors Elk-1 and serum response factor interact by direct protein-protein contacts mediated by a short region of Elk-1. Mol. Cell. Biol. 14:3283-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein, B., A. S. Baldwin, Jr., D. W. Ballard, W. C. Greene, P. Angel, and P. Herrlich. 1993. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 12:3879-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sulciner, D. J., K. Irani, Z.-X. Yu, V. J. Ferrans, P. Goldschmidt-Clermont, and T. Finkel. 1996. rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-κB activation. Mol. Cell. Biol. 16:7115-7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang, G., Y. Minemoto, B. Dibling, N. H. Purcell, Z. Li, M. Karin, and A. Lin. 2001. Inhibition of JNK activation through NF-kappaB target genes. Nature 414:313-317. [DOI] [PubMed] [Google Scholar]
- 69.Tapon, N., K. Nagata, N. Lamarche, and A. Hall. 1998. A new rac target POSH is an SH3-containing scaffold protein involved in the JNK and NF-kappaB signalling pathways. EMBO J. 17:1395-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tischer, E., R. Mitchell, T. Hartman, M. Silva, D. Gospodarowicz, J. C. Fiddes, and J. A. Abraham. 1991. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J. Biol. Chem. 266:11947-11954. [PubMed] [Google Scholar]
- 71.Toledano, M. B., and W. J. Leonard. 1991. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc. Natl. Acad. Sci. USA 88:4328-4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Treisman, R. 1987. Identification and purification of a polypeptide that binds to the c-fos serum response element. EMBO J. 6:2711-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Treisman, R., R. Marais, and J. Wynne. 1992. Spatial flexibility in ternary complexes between SRF and its accessory proteins. EMBO J. 11:4631-4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vanhoutte, P., J. L. Nissen, B. Brugg, B. D. Gaspera, M. J. Besson, R. A. Hipskind, and J. Caboche. 2001. Opposing roles of Elk-1 and its brain-specific isoform, short Elk-1, in nerve growth factor-induced PC12 differentiation. J. Biol. Chem. 276:5189-5196. [DOI] [PubMed] [Google Scholar]
- 75.Verger, A., and M. Duterque-Coquillaud. 2002. When Ets transcription factors meet their partners. Bioessays 24:362-370. [DOI] [PubMed] [Google Scholar]
- 76.Verma, I. M., and P. Sassone-Corsi. 1987. Proto-oncogene fos: complex but versatile regulation. Cell 51:513-514. [DOI] [PubMed] [Google Scholar]
- 77.Verma, I. M., J. K. Stevenson, E. M. Schwarz, D. Van Antwerp, and S. Miyamoto. 1995. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 9:2723-2735. [DOI] [PubMed] [Google Scholar]
- 78.von Knethen, A., D. Callsen, and B. Brune. 1999. NF-kappaB and AP-1 activation by nitric oxide attenuated apoptotic cell death in RAW 264.7 macrophages. Mol. Biol. Cell 10:361-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang, W., J. L. Abbruzzese, D. B. Evans, L. Larry, K. R. Cleary, and P. J. Chiao. 1999. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin. Cancer Res. 5:119-127. [PubMed] [Google Scholar]
- 80.Wang, Y., and R. Prywes. 2000. Activation of the c-fos enhancer by the erk MAP kinase pathway through two sequence elements: the c-fos AP-1 and p62TCF sites. Oncogene 19:1379-1385. [DOI] [PubMed] [Google Scholar]
- 81.Xu, Z., N. V. Kukekov, and L. A. Greene. 2003. POSH acts as a scaffold for a multiprotein complex that mediates JNK activation in apoptosis. EMBO J. 22:252-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yanagisawa, K., K. Tago, M. Hayakawa, M. Ohki, H. Iwahana, and S. Tominaga. 2003. A novel splice variant of mouse interleukin-1-receptor-associated kinase-1 (IRAK-1) activates nuclear factor-kappaB (NF-kappaB) and c-Jun N-terminal kinase (JNK). Biochem. J. 370:159-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang, J., Y. Lin, Z. Guo, J. Cheng, J. Huang, L. Deng, W. Liao, Z. Chen, Z. Liu, and B. Su. 2001. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat. Immunol. 2:620-624. [DOI] [PubMed] [Google Scholar]
- 84.Yasumoto, K., S. Okamoto, N. Mukaida, S. Murakami, M. Mai, and K. Matsushima. 1992. Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kB-like binding sites of the interleukin 8 gene. J. Biol. Chem. 267:22506-22511. [PubMed] [Google Scholar]
- 85.Yen, J., R. M. Wisdom, I. Tratner, and I. M. Verma. 1991. An alternative spliced form of FosB is a negative regulator of transcriptional activation and transformation by Fos proteins. Proc. Natl. Acad. Sci. USA 88:5077-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao, Q., and F. S. Lee. 1999. Mitogen-activated protein kinase/ERK kinase kinases 2 and 3 activate nuclear factor-kappaB through IkappaB kinase-alpha and IkappaB kinase-beta. J. Biol. Chem. 274:8355-8358. [DOI] [PubMed] [Google Scholar]