Abstract
CCAAT/enhancer binding protein β (C/EBPβ) is a widely expressed transcription factor whose activity is regulated by oncogenic Ha-RasV12 signaling. C/EBPβ is essential for the development of mouse skin tumors containing Ras mutations and can cooperate with RasV12 to transform NIH 3T3 cells. Here we have investigated Ras-induced phosphorylation of C/EBPβ in fibroblasts and report a novel proline-directed phosphoacceptor site at Ser64 within the transactivation domain. Ser64 phosphorylation was induced by activated Ras and Raf but was not blocked by chemical inhibitors of MEK1/2, phosphatidylinositol 3-kinase, JNK, or p38 mitogen-activated protein kinases. Ser64 was efficiently phosphorylated in vitro by the cyclin-dependent kinases Cdk2 and Cdc2. Thr189, previously identified as an ERK1/2 phosphorylation site that regulates C/EBPβ activity, was also a substrate for Cdk phosphorylation. Ser64 and Thr189 phosphorylation was low in serum-starved (G0) cells but was strongly increased in mid-G1 cells and in cells arrested in S or M phase. In addition, phosphorylation on both sites was blocked by treating cells with the Cdk inhibitor roscovitine. In contrast to wild-type C/EBPβ, which enhances transformation of NIH 3T3 cells, mutants bearing alanine substitutions at Ser64 and/or Thr189 inhibited RasV12-induced focus formation. Our findings support a role for C/EBPβ as a nuclear effector of Ras signaling and transformation, and they indicate that cell cycle-dependent phosphorylation of C/EBPβ on Ser64 and Thr189 is required to promote Ras-induced transformation of NIH 3T3 cells.
Ras proteins function as key relays for many signal transduction pathways that transmit extracellular cues into the cell (1, 17, 23). A significant proportion of mammalian tumors contain mutant Ras genes (e.g., H-RasV12) encoding constitutively active Ras proteins that are uncoupled from upstream activating signals. Activated Ras provides an unabated proliferative stimulus in the cell which, in combination with other oncogenic mutations, causes neoplastic transformation and cancer (4). Oncogenic Ras activates several downstream pathways, including the classical serum-stimulated signal transduction cascade Ras-Raf-MEK-ERK, that impinge on nuclear transcription factors as well as cytoplasmic targets (30). Although biological responses to Ras vary with the cell context, in tumor cells oncogenic Ras activates a program of gene expression that is permissive for passage through the cell cycle. The downstream effectors that interpret Ras-dependent signals are complex and remain to be fully elucidated (14, 23). However, several transcription factors, including members of the AP-1, Ets (Sap1, Elk-1), Myc, and NF-κB protein families (10, 13, 20, 33), are known targets of Ras signaling in normal and transformed cells.
The transcription factor CCAAT/enhancer binding protein β (C/EBPβ) is another nuclear effector of Ras signaling. The transcriptional activity of C/EBPβ can be stimulated by coexpression of oncogenic Ras through a mechanism that depends at least partly on phosphorylation of Thr235 (in human C/EBPβ, also known as NF-IL-6) (26). The Thr235 residue, which is conserved in the rat (Thr189) and mouse (Thr188), is a substrate for ERK1/2 (12, 26). Mutation of the ERK site impairs RasV12-mediated activation of the human, avian, and rodent C/EBPβ proteins in reporter assays (18, 26, 39). Furthermore, the ERK site is essential for Ras-induced transcriptional cooperativity between C/EBPβ and serum response factor as well as for the physical association of these two proteins (11).
Analysis of C/EBPβ-null mice using the mouse skin model of multistage carcinogenesis has revealed a critical role for C/EBPβ in Ras-mediated tumorigenesis. Wild-type mice subjected to a 7,12-dimethylbenz[a]antracene/12-O-tetradecanoylphorbol 13-acetate skin carcinogenesis protocol develop papillomas that are dependent on H-Ras or Ki-Ras mutations (3, 38). In contrast, C/EBPβ−/− mice display a complete absence of skin tumors (39). C/EBPβ deficiency also reduces the number and size of skin tumors in mice expressing a v-H-Ras transgene, providing further evidence that C/EBPβ participates in Ras-mediated tumorigenesis. It has been proposed that C/EBPβ has antiapoptotic functions during skin tumorigenesis, since carcinogen-treated C/EBPβ−/− mice display a ∼17-fold increase in epidermal apoptotic cells compared to wild-type animals. Thus, C/EBPβ may be engaged in a prosurvival pathway that suppresses programmed cell death in early stages of skin tumorigenesis. Moreover, low levels of ectopic C/EBPβ expression have been found to augment RasV12-induced focus formation in NIH 3T3 cells. Collectively, these results indicate an important role for C/EBPβ in Ras-mediated transformation of keratinocytes and fibroblasts. The involvement of C/EBPβ in human cancers has been further supported by a recent study showing that C/EBPβ expression is highly correlated with cyclin D1-dependent tumors and that C/EBPβ physically associates with cyclin D1 and may mediate cyclin D1-dependent activation of a set of target genes that are important for cell proliferation and tumorigenesis (19).
To further understand the regulation of C/EBPβ activity by oncogenic Ras signaling and its role in neoplastic transformation, we have investigated changes in C/EBPβ phosphorylation that are induced by RasV12. We report the identification of a novel C/EBPβ phosphoacceptor, Ser64, located in the N-terminal transactivation domain (TAD). Several lines of evidence indicate that Ser64 and Thr189 are substrates for the cyclin-dependent kinases (Cdk's) Cdk2 and Cdc2, and possibly for related kinases. Alanine substitutions at Ser64 or Thr189 impaired the ability of C/EBPβ to stimulate Ras-induced focus formation in NIH 3T3 cells and instead caused C/EBPβ to inhibit transformation. These findings suggest that phosphorylation of Ser64 and Thr189 by Cdks during specific stages of the cell cycle is essential for C/EBPβ to facilitate oncogenic transformation by H-Ras.
MATERIALS AND METHODS
Cells and reagents.
L cells (ATCC CCL-1; murine fibroblasts) and NIH 3T3 cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics. Kinase inhibitors (PD98059, U0126, LY294002, SB203580, SP600125, and roscovitine) were purchased from Calbiochem. Anisomycin and nocodazole were obtained from Calbiochem, and hydroxyurea was purchased from Sigma-Aldrich.
Plasmid constructs.
pcDNA3.1-C/EBPβ vectors were generated by subcloning C/EBPβ inserts (EcoRI-HindIII fragments) from pMEX constructs (37) into pcDNA3.1 (Invitrogen). Serine 64 or Thr189 was replaced with alanine by site-directed mutagenesis using the QuikChange mutagenesis kit (Stratagene). The alanine substitutions were verified by sequencing the entire C/EBPβ gene (LMT Sequencing Facility, Frederick, Md.). The H-RasV12 expression vector was kindly provided by C. Der, and a constitutively active Raf-1 vector was obtained from J. Muller and D. Morrison.
Cell labeling and immunoprecipitation.
L cells were seeded at a density of 8 × 105 per 10-cm plate and transfected (Fugene6) the following day with 7.5 μg of pcDNA3.1-C/EBPβ, with or without 2.5 μg of pcDNA3-H-RasV12. One day later, the plates were washed with phosphate-buffered saline (PBS) and serum starved for 4 h. Cells were washed in serum-free, phosphate-free medium, incubated in the same medium for 20 min, and then incubated overnight in fresh serum- and phosphate-free medium containing 0.4 mCi of 32Pi (Amersham-Pharmacia)/ml. After 10 h, the cells were lysed in radioimmunoprecipitation assay (RIPA) buffer, and C/EBPβ was immunoprecipitated from the lysates by incubation for 3 h with 10 μl of antiserum and protein A-Sepharose beads. Washed immunoprecipitates were eluted in protein sample buffer, separated on precast sodium dodecyl sulfate (SDS)-12% polyacrylamide gels (Novex), and transferred to Immobilon membranes (Millipore). After exposure to X-ray film (X-Omat AR; Kodak), C/EBPβ bands were excised for tryptic phosphopeptide analysis.
Tryptic digestion and phosphopeptide analysis.
Immobilon strips were cut into small pieces and washed sequentially with distilled H2O and 50 mM NH4HCO3 (pH 7.92), then blocked for 1 h in 50 mM NH4HCO3 containing 1% Triton X-100. Membranes were digested overnight at 37°C by using 10 μg of sequencing grade trypsin (Roche) in the presence of 0.1% SDS. Supernatants containing released tryptic fragments were removed, and total 32P was determined by scintillation counting. The tryptic digests were adjusted to pH 2 with 20% trifluoroacetic acid and were subjected to reversed-phase high-performance liquid chromatography (HPLC) on a Waters 3.9- by 300-mm C18 column. Chromatography was performed using a gradient of 0 to 30% acetonitrile in 0.05% trifluoroacetic acid over 90 min at a flow rate of 1 ml/min. One-milliliter fractions were collected and counted for 32P in a Beckman 6500 scintillation counter (25). 32P-containing peptides were coupled to Sequalon disks and subjected to solid-phase Edman degradation using a model 420 Applied Biosystems sequencer. Cycle fractions were spotted onto Whatman no. 1 paper, and radioactivity was quantitated by using a phosphorimager.
Antibodies.
The phospho-C/EBPβ (Ser64) antiserum was prepared by synthesizing a 12-mer phosphopeptide consisting of an N-terminal Cys (for coupling) and the sequence Glu-Arg-Ala-Ile-Asp-Phe-Ser(PO4)-Pro-Tyr-Leu-Glu, coupling it to keyhole limpet hemocyanin (KLH), and immunizing rabbits by standard methods. The resulting antiserum showed reactivity against both unmodified and phosphorylated C/EBPβ and subsequently was depleted of reactivity against nonphosphorylated C/EBPβ by passage over an affinity column containing the unmodified peptide, as follows. The peptide Cys-Glu-Arg-Ala-Ile-Asp-Phe-Ser-Pro-Tyr-Leu-Glu was suspended in Sulfolink sample preparation buffer (Pierce Sulfolink kit) and solubilized by dropwise addition of dimethyl sulfoxide. Three milligrams of the unmodified peptide was coupled through the amino-terminal Cys residue to 2 ml of Sulfolink coupling gel (Pierce) by using conditions recommended by the manufacturer. Crude antiserum (5 ml) was applied to a 3-ml protein A-Sepharose column (Amersham-Pharmacia) in 0.1 M Tris (pH 8.0), washed extensively, and eluted with 6 ml of 0.1 M glycine (pH 3.0). The eluted immunoglobulin G (IgG) fraction was neutralized by the addition of Tris (pH 8.0) and dialyzed against 1× Tris-buffered saline (TBS). The IgG fraction was applied three times serially to the unmodified peptide column, and the flowthrough fraction was collected and used as the phospho-C/EBPβ antiserum. Bovine serum albumin (BSA) was added to a final concentration of 2 mg/ml, and the preparation was filtered through a 0.22-μm-pore-size Millipore membrane and stored at 4°C. To prepare phospho-C/EBPβ (Thr189) antiserum, a 12-mer phosphopeptide, Cys-Ser-Ser-Ser-Ser-Pro-Pro-Gly-Thr(PO4)-Pro-Ser-Pro-Ala, was synthesized, coupled to KLH as described above, and prepared for immunization by standard protocols. The resulting antiserum showed exclusive reactivity against phospho-Thr189-modified C/EBPβ. For the experiment of Fig. 7, a commercially available antibody recognizing phospho-Thr189 (rat) was used (“phospho-Thr235”; Cell Signaling Technology). C/EBPβ (C-19) and phospho-c-Jun (KM-1) antibodies were obtained from Santa Cruz Biotechnology, Inc. The affinity-depleted phospho-C/EBPβ (Ser64) antiserum was used in Western blots at a dilution of 1:600, the phospho-C/EBPβ (Thr189) antiserum was used at 1:2,000, the commercial phospho-C/EBPβ (Thr235) antibody was used at 1:1,000, and the horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Promega) was used at 1:20,000. Blots were developed with Supersignal West Pico reagent (Pierce).
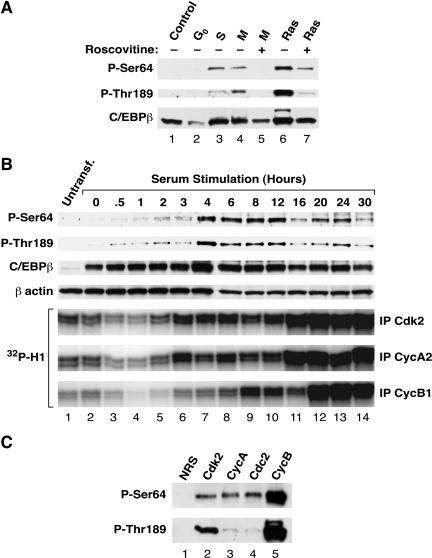
FIG. 7.
Cell cycle-dependent phosphorylation of C/EBPβ. (A) L cells transfected with C/EBPβ were blocked at the indicated phases of the cell cycle by serum withdrawal (G0) or treatment with hydroxyurea (S phase) or nocodazole (M phase). Lysates (50 μg per lane) were separated by SDS-12% PAGE and transferred to Immobilon membranes. Duplicate panels were analyzed with antibodies against P-Ser64 (upper panel), P-Thr189 (middle panel), or unmodified C/EBPβ (lower panel). The effect of blocking Cdk1 and Cdk2 activity was tested by using the inhibitor roscovitine (lanes 5 and 7). (B) L cells were transfected with 5 μg of pcDNA3.1-C/EBPβ. After 24 h, the cells were placed in serum-free DMEM for 48 h; the cells were then stimulated to proliferate by switching them to growth medium containing 10% fetal bovine serum. Extracts were prepared by lysis in RIPA buffer at 0 h (starved cells) and at the indicated times after serum stimulation. Cell lysates (25 μg) were analyzed for C/EBPβ phosphorylation by Western blotting using phosphospecific antibodies. (C) Extracts from L cells arrested in mitotsis with nocodazole were immunoprecipitated with antisera against Cdk2, cyclin A, Cdc2, or cyclin B, or with normal rabbit serum (NRS). Precipitates were washed with kinase buffer, and 1 μg of purified C/EBPβ was added together with 100 μM ATP. After 30 min at room temperature, kinase reactions were terminated by addition of sample buffer, and products were separated on a 12% gel. Phosphorylation at Ser64 and Thr189 was determined by Western blotting with phosphospecific antibodies.
Preparation of cell lysates and Western blotting.
L cells (8 × 105 per 10-cm plate) were transfected with 7.5 μg of the pcDNA3.1-C/EBPβ expression plasmid with or without 2.5 μg of H-RasV12 or Raf-1 vectors, by using 2 μl of Fugene reagent (Roche) per μg of DNA. The total amount of plasmid (10 μg) was equalized in all experiments by including appropriate amounts of pcDNA3.1 DNA. After 24 h, cells were washed with PBS and cultured for approximately 16 h in serum-free medium prior to lysis. Chemical inhibitors were included upon serum withdrawal and were maintained in the medium for 16 h. Whole-cell lysates were prepared by using RIPA buffer supplemented with protease and phosphatase inhibitors. Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes by standard methods. Membranes were blocked in TBS supplemented with 0.1% Tween 20 and 5% BSA, which was maintained in all subsequent antibody and washing steps.
In vitro phosphorylation of C/EBPβ by purified Cdks.
A 0.5-μg sample of purified glutathione S-transferase (GST)-Cdk2 or GST-Cdk3 was incubated with 16 ng of GST-Cak1p for 2.5 h in the presence of 375 μM (for Western blots) or 50 μM (for radiolabeling) ATP. GST-cyclin A174-432 (0.77 μg) was added, and incubation continued for 1.5 h at room temperature. For Western blotting experiments (Fig. 5 and 6C), 2 μg of purified C/EBPβ (wild type or S64A) was added and incubated for approximately 3 h at room temperature. For radiolabeling, 1.5 μCi of [γ-32P]ATP and 2 μg of wild-type or mutant C/EBPβ were added, and reaction mixtures were incubated for 30 min at room temperature, followed by addition of 5 μl of 5× SDS sample buffer. Active cyclin D1-Cdk4 or -Cdk6 complexes (0.5 μg) were used as described previously (15).
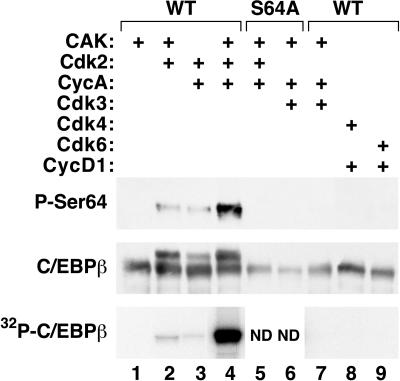
FIG. 5.
Ser64 is phosphorylated by Cdk2 in vitro. Wild-type or mutant C/EBPβ proteins were phosphorylated by active Cdk-cyclin complexes. Samples were then subjected to Western blot analysis using antibodies against the S64-phosphorylated form of C/EBPβ (upper panel) or total C/EBPβ (middle panel). Kinase reactions were also performed in the presence of [γ-32P]ATP, and the products were analyzed by SDS-PAGE and autoradiography (lower panel). ND, not determined.
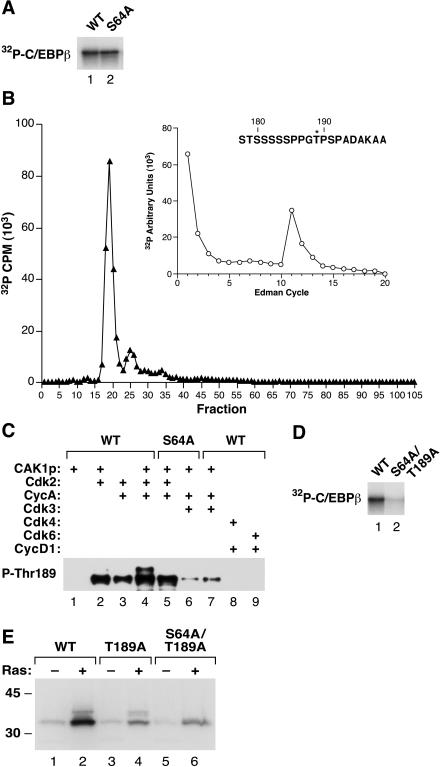
FIG. 6.
Thr189 and Ser64 are Cdk2 phosphoacceptors. (A) Purified wild-type C/EBPβ and S64A were phosphorylated in vitro by Cdk2 in the presence of [γ-32P]ATP. The products were resolved by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and visualized by autoradiography. (B) Labeled S64A protein was released from the membrane by digestion with chymotrypsin, and the products were separated by reverse-phase HPLC. Inset shows Edman sequencing of the peak at fraction 19, with radiolabel released at cycle 11; this assignment corresponds to Thr189. (C) Cdk2 phosphorylates Thr189. The Cdk reactions shown in Fig. 5 were analyzed by immunoblotting with an anti-P-Thr189 antibody. (D) Wild-type C/EBPβ and S64A/T189A proteins were labeled with Cdk2 and [γ-32P]ATP as described for panel A. (E) Wild-type, T189A, and S64A/T189A C/EBPβ were expressed in L cells in the absence or presence of RasV12, and the cells were labeled with 32Pi. C/EBPβ immunoprecipitates were prepared and analyzed by SDS-PAGE and autoradiography.
Cell cycle blockade and Western blotting for C/EBPβ, phospho-Ser64, and phospho-Thr189.
L cells were seeded at 8 × 105 per 10-cm dish and transfected with 7.5 μg of a C/EBPβ expression plasmid with or without 2.5 μg of a RasV12 expression plasmid. For G1 arrest, cells were held in serum-free medium for 36 h. For other phase-specific arrests, cells were treated for 36 h with 2 μM hydroxyurea (S phase) or 150 ng of nocodazole/ml (M phase). Where indicated, 500 nM roscovitine was added for the final 24 h of treatment. All dishes were harvested by lysis in RIPA buffer (250 μl per dish), followed by a clearing spin. Extracts were separated on SDS-12% PAGE gels, transferred to Immobilon membranes, and immunoblotted with the appropriate antisera.
Cell cycle time course.
L cells were plated 12 h prior to transfection (2 × 105 per 10-cm dish). Each dish was transfected with 5 μg of pcDNA3.1-C/EBPβ by using Fugene6 (Roche) according to the manufacturer's recommendations. Twenty-four hours later, the cells were switched to serum-free DMEM for 48 h. The cells were then stimulated to reenter the cell cycle by replacing the starvation medium with fresh medium containing 10% fetal bovine serum. Cells were harvested by lysis in RIPA buffer at various time points after serum stimulation, and C/EBPβ phosphorylation was analyzed as described above. Measurements of kinase activity for Cdk2, cyclin A2, and cyclin B1 were performed as described elsewhere (5).
Immunoprecipitation and in vitro kinase assays.
For each immunoprecipitation, one 15-cm dish was seeded with 2 × 106 L cells. After 16 h the cells were treated with 150 ng of nocodazole/ml for 36 h. Plates were rinsed with PBS and lysed in 1 ml of PBS containing 0.2% Triton X-100, 10% glycerol, 5 mM EDTA, 25 mM β-glycerophosphate, 25 mM p-nitrophenylphosphate, and 10 mM NaF and supplemented with phenylmethylsulfonyl fluoride (PMSF), dithiothreitol (DTT), leupeptin, aprotinin, pepstatin, sodium pyrophosphate, and sodium vanadate. The lysate was frozen on dry ice, thawed on wet ice, and cleared by centrifugation at 4°C for 15 min in a microcentrifuge. Each supernatant received 5 μl of polyclonal antisera against Cdk2, Cdc2, cyclin A, or cyclin B or normal rabbit serum and 100 μl of a 10% slurry of protein A beads in PBS. Immunoprecipitates were formed by mixing for 4 h at 4°C. The beads were washed twice with 1 ml of lysis buffer and twice with 1 ml of kinase reaction buffer (20 mM morpholinepropanesulfonic acid [MOPS] [pH 7.2], 25 mM β-glycerophosphate, 1 mM EGTA, 1 mM sodium vanadate, 1 mM DTT, 12.5 mM MgCl2, 0.2 mM PMSF, supplemented with aprotinin and antipain). As a positive control, 1.5 μg of histone H1 (Roche) was used as a substrate and labeled in the presence of 20 μCi of [γ-32P]ATP (3,000 Ci/mmol). C/EBPβ (1 μg) purified from a bacterial expression system and dialyzed against PBS was used as the substrate in all other reactions, which included 100 μM cold ATP. Reactions proceeded for 30 min at room temperature and were terminated by the addition of SDS-PAGE sample buffer. Samples were boiled for 3 min, separated on SDS-12% PAGE gels, transferred to Immobilon membranes, and probed with the appropriate antisera.
Electrophoretic mobility shift assays.
RIPA whole-cell lysates were prepared from transfected L cells rinsed with cold PBS containing vanadate and PMSF. Cells were scraped into 1 ml of the same buffer, pelleted, and lysed in 400 μl of RIPA buffer supplemented with 0.2 mM PMSF, 0.1 mM vanadate, 20 mM NaF, 0.1 mM pyrophosphate, and 5 ng (each) of leupeptin, pepstatin, and aprotinin/ml. Lysates were cleared by centrifugation for 10 min at 4°C. Protein concentrations were determined by a Bradford assay (Pierce). DNA-binding reaction mixtures contained 5 μg of protein extract, 20 mM HEPES (pH 7.5), 5% Ficoll, 1 mM EDTA, 200 mM NaCl, 1 mM DTT, 0.01% NP-40, 400 ng of BSA/ml, and 40 ng of poly(dI-dC)/ml. Protein extracts were diluted into 26-μl binding reaction mixtures (the RIPA buffer volume never exceeded 10% of the total, and the final detergent concentration was held constant in all reactions) and incubated for 20 min at room temperature, and the complexes were analyzed by electrophoresis using 5% acrylamide-Tris-borate-EDTA gels. Where indicated, 1 μl of an anti-C/EBPβ C-terminal antiserum (Santa Cruz C-19) was included in the binding reaction.
NIH 3T3 cell focus assays.
NIH 3T3 cells were maintained in DMEM plus 10% fetal bovine serum and were plated at a density of 5 × 105 per 60-mm dish. The following day, cells were transfected with calcium phosphate precipitates as described previously (7, 39). Transformed foci were scored after 2 weeks (7).
RESULTS
Persistent Ha-RasV12 signaling stimulates C/EBPβ phosphorylation in fibroblasts.
To investigate the effect of oncogenic Ras signaling on C/EBPβ phosphorylation, we expressed C/EBPβ in L cell fibroblasts with or without RasV12, starved the cells for serum, and labeled them with 32Pi. Labeled C/EBPβ was immunoprecipitated from cell lysates, separated by SDS-PAGE, transferred to Immobilon membranes, and subjected to autoradiography (Fig. 1A). A comparison of immunoprecipitates from cells transfected with C/EBPβ alone (lane 1) or with RasV12 (lane 2) showed that C/EBPβ is a phosphoprotein and that RasV12 increases C/EBPβ phosphorylation severalfold, in accordance with previous observations (26). Phospho-amino acid analysis of labeled C/EBPβ isolated from Ras-expressing cells showed that C/EBPβ contains phosphoserine and phosphothreonine but not phosphotyrosine (data not shown). Oncogenic Ras also induced the appearance of a C/EBPβ species with reduced electrophoretic mobility (lane 2), suggesting that this could be a hyperphosphorylated form of the protein.
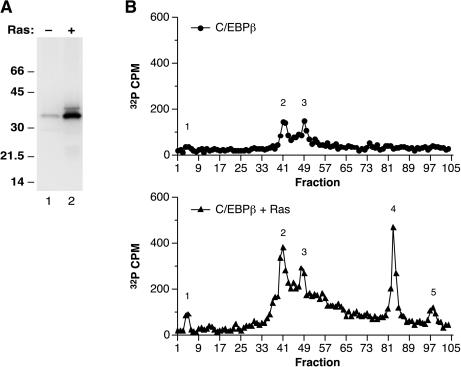
FIG. 1.
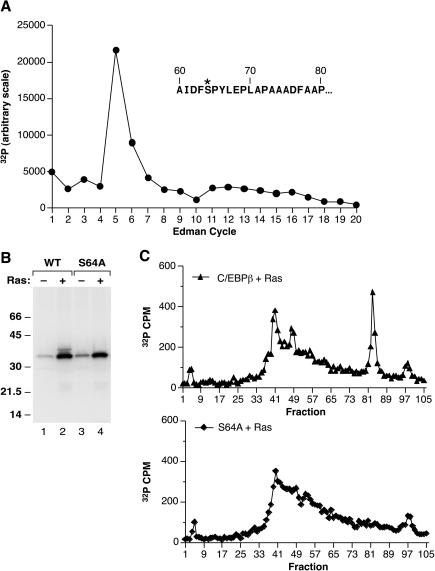
C/EBPβ phosphorylation in L cells is stimulated by coexpression of RasV12. (A) L cells were transfected with a vector encoding the p35 form of rat C/EBPβ (9, 37) alone (−) or with a RasV12 expression vector (+) and were labeled for 10 h in serum-free medium containing 32Pi. C/EBPβ immunoprecipitates were prepared, resolved by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and visualized by autoradiography. (B) Labeled C/EBPβ proteins from panel A were released from the membrane by trypsin digestion, and the products were separated by reverse-phase HPLC. Phosphopeptide peaks are numbered 1 to 5.
Ras induces C/EBPβ phosphorylation on Ser64.
To identify the sites of phosphorylation on C/EBPβ, we generated phosphopeptide profiles of in vivo radiolabeled protein using trypsin digestion and reverse-phase HPLC. As shown in Fig. 1B, RasV12 increased the incorporation of phosphate into tryptic peaks 1 to 3, which were detected at low levels even in the absence of Ras; in addition, two new peaks (peaks 4 and 5) were induced by Ras. When the prominent phosphopeptide corresponding to peak 4 (approximately fraction 80) was subjected to Edman degradation, 32P was released at cycle 5 of the sequencing reaction (Fig. 2A). Inspection of the predicted tryptic products showed three candidate peptides that contain Ser or Thr located 5 residues from their N termini. Two of these are small peptides derived from the leucine zipper region that are predicted to elute early in the HPLC gradient and, therefore, probably do not correspond to peak 4. The third candidate is a 42-amino-acid peptide (residues 60 to 101) expected to elute much later in the gradient. Based on these considerations, Ser64 was the most likely phosphoacceptor site for peak 4.
FIG. 2.
RasV12 induces phosphorylation on serine 64 of C/EBPβ. (A) HPLC fractions encompassing RasV12-induced peak 4 (Fig. 1) were pooled and subjected to Edman degradation. The label released at each sequencing cycle was quantitated by phosphorimaging. The N-terminal sequence of a candidate tryptic peptide (amino acids 60 to 101) containing serine at position 5 (labeled with an asterisk) is shown in the inset. (B) Wild-type and mutant (S64A) C/EBPβ proteins were expressed in L cells without (−) or with (+) RasV12, labeled with 32P, and immunoprecipitated as described for Fig. 1. (C) Samples from panel B (wild-type C/EBPβ + Ras and S64A C/EBPβ + Ras) were subjected to tryptic digestion and reverse-phase HPLC.
To confirm that C/EBPβ is phosphorylated on Ser64, we changed this residue to Ala, expressed the S64A mutant protein in L cells with or without RasV12, and labeled the cells with 32P (Fig. 2B). S64A did not produce the slower-migrating species observed for wild-type C/EBPβ (compare lanes 2 and 4), demonstrating that phosphorylation of Ser64 is responsible for the Ras-induced mobility shift. Furthermore, tryptic phosphopeptide analysis showed that peak 4 was completely absent from the S64A mutant protein (Fig. 2C). These results define Ser64 as a novel Ras-induced phosphoacceptor site in C/EBPβ.
Characterization of the signaling pathway regulating Ser64 phosphorylation.
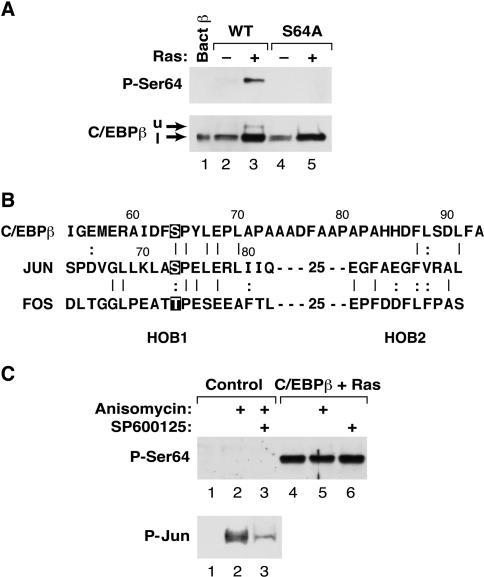
To facilitate further analysis of Ser64 phosphorylation, we raised an antiserum against a synthetic peptide containing phospho-Ser64. The immune serum was depleted of reactivity against the nonphosphorylated epitope by serial passage over a column containing the unmodified peptide. As shown in Fig. 3A, the affinity-depleted phospho-C/EBPβ (Ser64) antiserum did not react with bacterially expressed C/EBPβ in a Western blot (lane 1) but did recognize the upper band induced by RasV12 in transfected cells (lane 3). The antiserum also exhibited no reactivity against the S64A mutant (lanes 4 and 5). In contrast, a conventional C/EBPβ antibody (lower panel) recognized all C/EBPβ species. Thus, the phospho-C/EBPβ antiserum displays specificity for the phospho-Ser64 form of C/EBPβ.
FIG. 3.
Analysis of Ser64 phosphorylation using a phosphospecific antibody. (A) Characterization of an anti-P-Ser64 C/EBPβ antiserum. Lysates were prepared from L cells expressing wild-type or S64A C/EBPβ, with or without RasV12, and analyzed by Western blotting using either anti-P-Ser64 C/EBPβ (upper panel) or a conventional C/EBPβ antiserum (lower panel). The upper band (u) recognized by the anti-P-Ser64 antibody and the lower band (l) recognized predominantly by the conventional antibody are indicated by arrows. (B) Sequence alignment of the TAD regions from C/EBPβ, c-Jun, and c-Fos. Ser64 in C/EBPβ, the JNK site in c-Jun (Ser73), and the analogous Thr phosphoacceptor in c-Fos are highlighted. HOB1 and HOB2, homology boxes 1 and 2. (C) Ser64 is not a target of JNKs. L cells were mock transfected (control) or transfected with C/EBPβ and RasV12; then they were either left untreated or treated for 30 min with anisomycin (to activate JNKs) and/or the JNK inhibitor SP600125. Lysates were prepared and analyzed by Western blotting using anti-P-Ser64 (upper panel) or anti P-Jun (lower panel) antibodies.
Ser64 is located within a conserved segment of the TAD (Fig. 3B) that is shared by all C/EBP family members (28, 37). This region of C/EBPβ was previously noted for its similarity to homology box 1 (HOB1), a sequence showing modest homology to the TADs of Fos, Jun, and C/EBP family members (34, 37). HOB1 of Jun encompasses a Jun N-terminal kinase (JNK) phosphoacceptor site at Ser73, and phosphorylation of this site by JNKs potently stimulates transactivation (reviewed in reference 8). Since an alignment of the HOB1 regions of C/EBPβ, Jun, and Fos shows that Ser64 is analogous to Ser73 in c-Jun and is a proline-directed kinase site (Fig. 3B), we examined whether Ser64 might be a target for JNKs. L cells were transfected with C/EBPβ and Ras and treated either with anisomycin to activate JNKs or with the JNK inhibitor SP600125, followed by analysis of Ser64 phosphorylation by Western blotting (Fig. 3C). Anisomycin did not increase Ras-induced phosphorylation of Ser64 (compare lanes 4 and 5), and SP600125 failed to inhibit this modification (compare lanes 4 and 6). In contrast, endogenous JNK activity was induced by anisomycin, and JNK activation was substantially blocked by SP600125 (as determined by analysis of c-Jun phosphorylation [Fig. 3C, lower panel]), demonstrating the effectiveness of these agents. Thus, C/EBPβ Ser64 does not appear to be a substrate for JNKs.
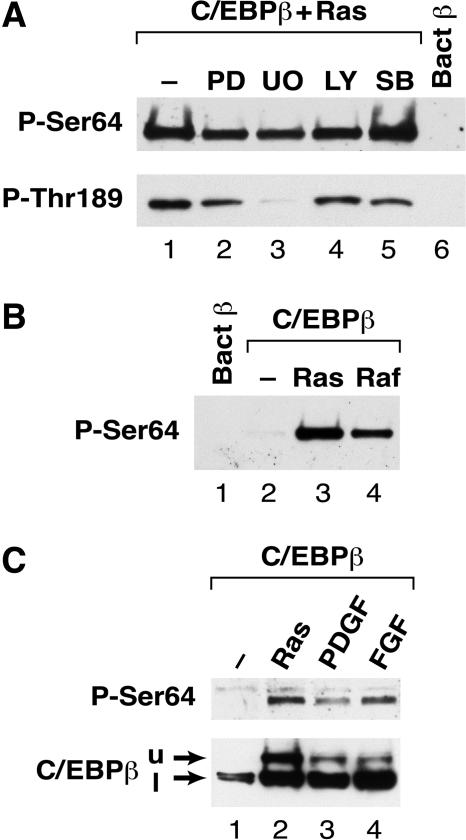
We next investigated whether Ser64 phosphorylation could be blocked by inhibitors of several kinases that are downstream effectors of Ras signaling. L cells transfected with C/EBPβ and RasV12 were treated with inhibitors of MEK1/2 (PD98059 and U0126), phosphoinositol (PI) 3-kinase (LY294002), and p38 mitogen-activated protein (MAP) kinases (SB203580), and extracts were analyzed for Ser64 phosphorylation (Fig. 4A). None of these agents significantly blocked Ser64 phosphorylation. Consistent with the results of the kinase inhibitors, expression of dominant-negative forms of ERK1, ERK2, JNK1, and JNK2 were also ineffective at blocking Ras-induced phosphorylation of Ser64 (data not shown). We also analyzed the samples for phosphorylation on Thr189, which was previously identified as an ERK1/2 substrate (26). To monitor Thr189 phosphorylation, we used a C/EBPβ antibody specific for phospho-Thr189 (see Materials and Methods). Western blotting showed that Thr189 becomes phosphorylated in response to expression of oncogenic Ras (Fig. 4A, lower panel, and data not shown). C/EBPβ reactivity with the phospho-Thr189 antibody was partially inhibited by treating the cells with PD98059 (lane 2) and nearly completely blocked by U0126 (lanes 3 and 4) but not by LY294002 or SB203580. Thus, U0126 efficiently inhibits Ras-induced phosphorylation on Thr189 but not on Ser64. These results suggest that the kinase responsible for modification of Ser64 is largely independent of the MEK/ERK cascade and is not activated by pathways involving PI 3-kinase or p38 stress kinases.
FIG. 4.
Analysis of the signaling pathway regulating Ser64 phosphorylation. (A) L cells were transfected with vectors for C/EBPβ and RasV12 (lanes 1 to 6) and then treated for 16 h in serum-free medium containing no inhibitor (lane 1) or the following inhibitors: 20 μM PD98059 (lane 2), 5 μM U0126 (lane 3), 50 μM LY294002 (lane 4), and 10 μM SB203580 (lane 5). Whole-cell lysates were prepared and analyzed by Western blotting using anti-P-Ser64 (upper panel) or anti- P-Thr189 (lower panel) antibodies. Bacterial C/EBPβ (lane 6) was included as a negative control. (B) Activated Raf-1 induces Ser64 phosphorylation. Cells were transfected with C/EBPβ alone (lane 2) or with RasV12 (lane 3) or constitutively active Raf-1 (lane 4). Cell extracts were analyzed by Western blotting using the anti-P-Ser64 antibody. Bacterial C/EBPβ was included as a control (lane 1). (C) PDGF and bFGF induce phosphorylation on Ser64. L cells were transfected with C/EBPβ, starved for serum overnight, and then either left untreated (lane 1) or stimulated for 4.5 h with 50 ng of PDGF/ml (lane 3) or 50 ng of bFGF/ml (lane 4). C/EBPβ coexpressed with RasV12 was included as a control (lane 2). Extracts were prepared and analyzed by Western blotting using anti-P-Ser64 (upper panel) and an antibody recognizing total C/EBPβ (lower panel). The upper (u) and lower (l) C/EBPβ species are indicated.
To determine if the signaling pathway leading to Ser64 phosphorylation involves the Raf-1 kinase, we expressed C/EBPβ together with constitutively active (CA) Raf-1 in L cells and analyzed C/EBPβ phosphorylation on Ser64 (Fig. 4B). Ser64 phosphorylation was induced by CA-Raf (lane 4) to a level comparable to that observed for RasV12 (lane 3). Thus, constitutive Raf-1 signaling can efficiently activate Ser64 phosphorylation. We also investigated whether Ser64 becomes modified in response to growth factors that signal through Ras (32, 35). Exposure of C/EBPβ-transfected cells to 50 ng of platelet-derived growth factor (PDGF) or basic fibroblast growth factor (bFGF)/ml for 4.5 h induced Ser64 phosphorylation (Fig. 4C, lanes 3 and 4, respectively). Thus, intracellular signals initiated by activation of receptor tyrosine kinases can stimulate C/EBPβ modification on Ser64.
Ser64 is phosphorylated in vitro by Cdks.
The preceding experiments indicate that Ser64 phosphorylation in cells is not mediated by ERK1/2, JNK1/2, or p38 MAP kinases, despite the fact that Ser64 is a proline-directed phosphoacceptor site that could be a target for MAP kinase-related kinases. Since Cdk's constitute another major class of proline-directed kinases, we examined the ability of several purified Cdks (Cdk2, -3, -4, and -6) to phosphorylate bacterially expressed C/EBPβ in vitro. Each kinase was tested in combination with its appropriate cyclin (cyclin A for Cdk2 and Cdk3, cyclin D1 for Cdk4 and Cdk6), and the products were examined by Western blotting for the presence of phospho-Ser64 (Fig. 5). Recombinant Cdk2 showed significant kinase activity toward Ser64 (lanes 2 to 4), with the strongest phosphorylation observed when both cyclin A and Cdk-activating kinase (CAK) were included in the reaction (lane 4). Western blot analysis using a conventional C/EBPβ antibody (middle panel) showed a significant amount of the slow-migrating C/EBPβ band in reactions containing Cdk2, which also suggests phosphorylation on Ser64. As expected, the S64A mutant protein did not undergo this Cdk2-induced mobility shift (lane 5). None of the other Cdk's had detectable Ser64 kinase activity (lanes 7 to 9), even though each Cdk was active, as judged by its ability to phosphorylate histone H1 or Rb (data not shown). Analysis of C/EBPβ phosphorylation by labeling with [γ-32P]ATP gave similar results (lower panel).
We next asked if Cdk2 phosphorylates C/EBPβ exclusively on Ser64 or whether other sites are also modified. Wild-type C/EBPβ and the S64A mutant were incubated with Cdk2, cyclin A, and CAK in the presence of [γ-32P]ATP, and the products were analyzed by SDS-PAGE and autoradiography (Fig. 6A). Both proteins were efficiently labeled by Cdk2, indicating that another site(s) in addition to Ser64 is a Cdk2 target. Chymotryptic digestion of the 32P-labeled S64A protein revealed a major labeled peak eluting at HPLC fraction 19 (Fig. 6B). Edman sequencing of this peptide showed label released at cycle 11 (Fig. 6B), and phospho-amino acid analysis revealed that the peptide contains phospho-Thr (data not shown). These data unambiguously identify Thr189 as a second Cdk2 phosphoacceptor in C/EBPβ. This assignment was subsequently confirmed by performing phospho-Thr189 Western blot analysis of C/EBPβ products from Cdk kinase assays (Fig. 6C). Wild-type C/EBPβ phosphorylated by Cdk2 displayed strong reactivity with the phospho-Thr189 antibody (lanes 2 to 4), as did the S64A mutant protein (lane 5). Cdk3 also weakly phosphorylated Thr189 (lanes 6 to 7), while Cdk4 and -6 were ineffective (lanes 8 to 9). Lastly, we generated a mutant lacking both sites (S64A/T189A) and examined phosphorylation of this protein with purified Cdk2 and [γ-32P]ATP (Fig. 6D). The S64A T189A mutant was labeled much less efficiently than wild-type C/EBPβ, indicating that Ser64 and Thr189 are the primary targets of Cdk2 phosphorylation in vitro.
32Pi labeling of wild-type, T189A, and S64A/T189A proteins expressed in L cells showed that 32P incorporation is reduced, but not eliminated, in the mutant proteins and is still enhanced by coexpression of RasV12 (Fig. 6E). Thus, there are other phosphorylation sites in addition to Ser64 and Thr189 that respond to RasV12 signaling. The identity of this site (or sites) is currently under investigation in our laboratory.
Cell cycle-dependent phosphorylation of Ser64 and Thr189.
To investigate whether Cdks phosphorylate C/EBPβ in vivo, we expressed C/EBPβ in L cells and arrested the cells at various stages of the cell cycle by serum withdrawal (G0) or treatment with hydroxyurea (S phase) or nocodazole (M phase). Analysis of cell extracts showed minimal phosphorylation of Ser64 and Thr189 in asynchronous cells (Fig. 7A, lane 1) and G0 cells (lane 2). In contrast, cells treated with hydroxyurea (lane 3) or nocodazole (lane 4) exhibited much higher levels of phosphorylation on both sites. Phosphorylation at these two sites in M-phase cells was blocked by treating the cells with the Cdk inhibitor roscovitine (lane 5); RasV12-induced phosphorylation was also substantially inhibited by the drug (compare lanes 6 and 7). These data suggest that C/EBPβ is phosphorylated by Cdks or Cdk-related kinases in proliferating cells.
We further examined cell cycle-dependent phosphorylation of Ser64 and Thr189 in synchronized cultures of proliferating cells. L cells were transfected with C/EBPβ, arrested in G0 by serum withdrawal, and stimulated to reenter the cell cycle by serum addition. C/EBPβ phosphorylation was analyzed at various times after serum treatment (Fig. 7B). Ser64 phosphorylation was low in starved cells and remained low for several hours after serum stimulation, but increased significantly at 4 h and was maintained at this level until 16 h, when it diminished. A second peak of Ser64 phosphorylation appeared at 20 to 24 h. The biphasic pattern of induction was observed in an independent experiment (data not shown) and is thus a reproducible effect. Phospho-Thr189 was induced with similar kinetics, suggesting that phosphorylation of Ser64 and Thr189 in vivo is coordinately regulated and that these sites may be substrates for the same kinase(s). To determine whether the induction of Ser64/Thr189 phosphorylation correlates with Cdk activation in late-G1- and S-phase cells, we analyzed Cdk2 and cyclin A2- and cyclin B1-associated kinase activities in the extracts by immunoprecipitation and in vitro phosphorylation of histone H1 (Fig. 7B, lower panels). Cdk2 activity showed a modest increase at 3 h but was enhanced significantly at the 16- to 30-h time points; this pattern was mirrored by cyclin A2 kinase activity. Cyclin B1 activity rose at 8 to 12 h, diminished at 16 h, and then underwent a large increase during the 20- to 30-h interval. Thus, high cyclin B1-associated kinase activity coincides with the second phase of C/EBPβ phosphorylation.
Immune complex kinase assays were used to test whether Cdks isolated from cells show kinase activity toward C/EBPβ (Fig. 7C). Cdk immunoprecipitates prepared from nocodazole-arrested (M-phase) cell extracts utilizing antibodies against Cdk2, Cdc2, or their associated cyclins (cyclin A2 and cyclin B1, respectively) phosphorylated Ser64, with cyclin B complexes being especially active (lane 5). Similar results were obtained for Thr189 phosphorylation (Fig. 7C, lower panel), although in this case the Cdk2 and cyclin B immunoprecipitates (lanes2 and 5) were more active than cyclin A or Cdc2 immunoprecipitates (lanes 3 and 4). These results demonstrate that both Cdk2 and Cdc2 are able to phosphorylate Ser64 and Thr189 and may be physiologically relevant C/EBPβ kinases.
Ser64 and Thr189 are required for C/EBPβ to augment RasV12 transformation of NIH 3T3 cells.
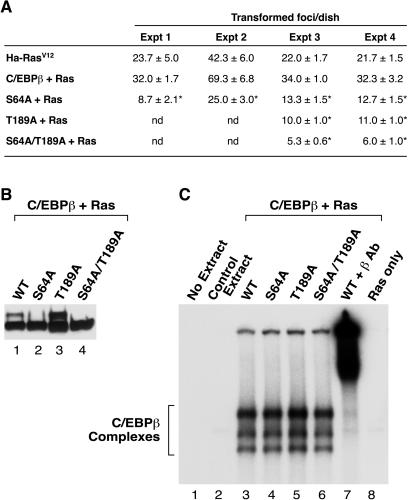
In a previous study we observed that low levels of C/EBPβ expression enhance transformation of NIH 3T3 cells by activated Ras or Raf, whereas dominant-negative C/EBPβ blocks transformation (39). To investigate whether Ser64 phosphorylation is required for the pro-oncogenic activity of C/EBPβ, we transfected the S64A mutant, as well as the T189A and S64A/T189A mutants, together with RasV12 into NIH 3T3 cells and assayed focus formation (Fig. 8A). Expression of wild-type C/EBPβ increased the number of Ras-transformed foci by ∼50%, in accordance with previous observations (39). In contrast, the S64A mutant decreased focus formation by approximately twofold, while the T189A mutant had a similar inhibitory effect (see also reference 39). The S64A/T189A protein was more inhibitory, diminishing focus formation three- to fourfold. Thus, the mutant proteins behave like dominant-negative C/EBPβ in blocking Ras transformation, and the two mutations act additively to inhibit transformation. The expression of each C/EBPβ protein in NIH 3T3 cells was equivalent (Fig. 8B), as were their DNA-binding activities (Fig. 8C), showing that the mutant phenotypes are not due to differences in expression or DNA binding. We conclude that phosphorylation of Ser64 and Thr189 is critical for C/EBPβ to enhance RasV12-induced transformation of NIH 3T3 cells.
FIG. 8.
Mutation of Ser64 and/or Thr189 impairs the ability of C/EBPβ to cooperate with RasV12 in NIH 3T3 focus assays. (A) NIH 3T3 cells were transfected with 10 ng each of the C/EBPβ and RasV12 expression vectors and scored 2 weeks later for the presence of transformed foci, as described previously (7). Transfections were performed in triplicate, and the data were averaged (± standard deviations); results from four independent experiments are shown. Transfection of C/EBPβ alone produced no transformed foci. *, P < 0.01 relative to Ha-RasV12 (by Student's t test). (B) NIH 3T3 cells were transfected with expression vectors for RasV12 and the indicated C/EBPβ protein. The cells were serum starved for 16 h, RIPA buffer lysates were prepared, and 25 μg of each extract was analyzed for C/EBPβ expression by Western blotting. (C) Extracts from panel B were analyzed by an electrophoretic mobility shift assay using equivalent amounts of total protein in binding reactions containing a radiolabeled C/EBP binding site probe. Extracts from mock-transfected cells (lane 2) and RasV12-transfected cells (lane 8) were included as controls. A C/EBPβ antibody supershift (lane 7) shows that all of the observed DNA-binding species contain C/EBPβ.
DISCUSSION
Phosphorylation of C/EBPβ by cell cycle-dependent kinases.
We have identified Ser64 as a novel Ras-induced site of phosphorylation in C/EBPβ. Ser64 is located in a segment of the C/EBPβ TAD region that displays weak homology to Fos and Jun. Our results indicate that Ser64 is phosphorylated by Cdk2 and Cdc2 (Cdk1) and possibly also by an unidentified kinase that becomes activated in mid-G1 phase. These conclusions are based on the following observations: (i) recombinant and cell-derived Cdk2 and Cdc2 proteins can phosphorylate Ser64 in vitro; (ii) Ser64 phosphorylation in dividing cells is cell cycle regulated and can be blocked by the Cdk inhibitor roscovitine; and (iii) the initial peak of Ser64 phosphorylation observed after cells exit G0 and enter the cell cycle does not appear to correlate with Cdk2 or Cdc2 activity.
Phosphorylation of Ser64 in vitro was specific to Cdk2 and Cdc2, as we did not observe modification of this site by purified Cdk4 and Cdk6 (Fig. 5) or by several other kinases, including Cdk7, ERK2, BMK1/ERK5, p38 MAP kinases, Rsk2, and GSK3 (data not shown). C/EBPβ phosphorylation by Cdk2 occurred exclusively on Ser64 and Thr189, despite the presence of three additional Ser/Thr-Pro sequences in the protein. Whereas Ser64 appears to be uniquely phosphorylated by Cdk's, Thr189 can be modified in vitro by Cdk2 and Cdc2 and also by purified ERKs (12, 26; our unpublished data). Hence, this site may be targeted by multiple kinases. Phosphorylation of both Thr189 and Ser64 was sensitive to the Cdk inhibitor roscovitine. Since roscovitine does not inhibit ERK1/2 (2), Thr189 may be phosphorylated primarily by Cdks, rather than ERKs, in vivo. In any case, our findings provide the first evidence that Thr189 is a Cdk substrate.
It was somewhat unexpected to find that Ser64 is a target of Cdk's, since we initially identified this residue as a Ras-induced phosphoacceptor site. However, the reduced phosphorylation of Ser64 in serum-starved cells is consistent with their arrest in G0 or early G1, when Cdk's are inactive. In RasV12-expressing serum-starved cells, the lack of external mitogenic signals is overcome by constitutive, high-level Ras signaling that continuously drives the cell cycle by activating cyclin D1 expression via the MEK-ERK and PI 3-kinase pathways (22). The idea that Ser64 modification is induced by cell cycle entry and is not directly targeted by a Ras signal transduction pathway is also supported by the kinetics of phosphorylation levels after serum stimulation, which do not increase until 4 h postinduction (Fig. 7). This is considerably later than would be expected if phosphorylation were catalyzed by a signal-dependent kinase such as ERK or Rsk. Nonetheless, there could also be a more direct effect of MEK/ERK signaling on Cdk activation, since it has been reported that ERK1/2 is required for nuclear localization of Cdk2-cyclin E complexes (16) as well as for the functional activation of nuclear Cdk2-cyclin E complexes to regulate progression into S phase (21). This cross talk between Cdks and ERK1/2 may account for the partial inhibition of Ser64 and Thr189 phosphorylation by MEK inhibitors (Fig. 4A). Clearly, further studies are required to fully elucidate the cellular kinases and upstream pathways responsible for modifying Ser64 and Thr189 in response to Ras and other cellular signals.
Cdk2 was originally thought to be an essential kinase that is required for the G1/S transition (with cyclin E) and for passage through S phase (with cyclin A). This view has now been challenged by the finding that abolition of Cdk2 expression in human tumor cell lines has little effect on their ability to proceed through the cell cycle (36), as well as by two studies showing that Cdk2 knockout mice are viable and that cells derived from these animals proliferate in culture (5, 29). Thus, either Cdk2 does not provide an essential cell cycle function or, more likely, cells express a compensating kinase. We have observed that Ser64 phosphorylation occurs in Cdk2-deficient mouse embryonic fibroblasts, including during G1 phase (T. Sebastian, E. Aleem, P. Kaldis, and P. F. Johnson, unpublished data), demonstrating that Ser64 is not modified exclusively by Cdk2. Some Ser64 phosphorylation in the mutant cells can be accounted for by Cdc2 (Cdk1) activity, but this should occur only during S and G2/M phases. Thus, there may be another Cdk2-like kinase that phosphorylates Ser64 during mid- and late-G1 phase. This is also suggested by the fact that phospho-Ser64 in synchronized L cells is induced prior to significant activation of Cdk2 (Fig. 7B). It is interesting to consider the possibility that this unidentified C/EBPβ kinase is identical to the activity that compensates for the loss of Cdk2 in Cdk2-null cells.
One potential candidate for the putative G1 Ser64 kinase is Cdk8. A recent study showed that an inhibitory mediator complex harboring Cdk8 associates with C/EBPβ and that activation of C/EBPβ by oncogenic Ras signaling induces exchange of the mediator complex for an active form that lacks Cdk8 (24). The interaction of a Cdk8-containing mediator complex with C/EBPβ raises the possibility that Cdk8 might be involved in phosphorylating Ser64 and/or Thr189. One known function of Cdk8 (as well as Cdk7 and Cdk9) is to phosphorylate the C-terminal domain (CTD) of the large subunit of RNA polymerase II (31). In yeast, Cdk8/cyclin C also phosphorylates the activation domain of the Ste12 transcription factor (27). However, little is known about Cdk8 substrates in mammalian cells. Whether Cdk8 is in fact a Ras-inducible C/EBPβ kinase requires further investigation.
Functional consequences of Ser64 phosphorylation.
Despite its location in the TAD, we did not observe any effect of mutating Ser64 on Ras-stimulated transcriptional activity of C/EBPβ in reporter assays (data not shown). However, Ser64 clearly plays an important role in the ability of C/EBPβ to augment transformation by Ha-RasV12 in NIH 3T3 cells, as does Thr189 (39) (Fig. 8). Thus, modification of both Thr189 and Ser64 is critical for C/EBPβ to enhance neoplastic transformation by RasV12. The mechanism by which C/EBPβ promotes Ras-induced transformation of NIH 3T3 cells remains to be elucidated. However, one plausible model is that C/EBPβ regulates transcription of a critical target gene(s) whose product stimulates oncogenic transformation, and Ser64 phosphorylation is required for C/EBPβ to efficiently activate this gene. This possibility is supported by the observation that a transcriptionally inert form of C/EBPβ lacking the TAD inhibits Ras transformation (39). Thus, phosphorylation of the TAD on Ser64 could promote the association (or disassociation) of an auxiliary protein that facilitates transcription of the target gene(s). In fact, the S64A mutation was found to significantly diminish C/EBPβ-dependent activation of proinflammatory cytokine genes in lipopolysaccharide-stimulated pre-B cells (C. J. Spooner, J. D. Shuman, P. F. Johnson, and R. C. Schwartz, unpublished data). Thus, in some contexts Ser64 modification is critical for transcription of C/EBPβ target genes.
Presently, no protein is known to interact with the Ser64 region of C/EBPβ, although the HOB1/TAD module is conserved within the C/EBP family, and this sequence activates transcription when fused to the GAL4 DNA-binding domain (18, 37). Interestingly, serine occurs at the analogous residue within the HOB1 region of each C/EBP family member (37), yet C/EBPβ is unique in containing Ser-Pro at this position. It is possible that C/EBPα, -β, -δ, and -ɛ are all regulated by phosphorylation of this conserved serine but that each protein is modified by a distinct kinase. In this regard we note that among the C/EBP proteins, only C/EBPβ is able to augment Ras-induced focus formation in NIH 3T3 cells (39).
A prosurvival role for C/EBPβ was reported by Buck and colleagues (6), who found that murine C/EBPβ is phosphorylated on Thr217 by RSK in hepatic stellate cells exposed to CCl4 and that phosphorylation of this site creates an “XVXD box” which binds and inhibits initiator caspases. They observed that C/EBPβ is essential for survival of these stressed cells, leading to the proposal that C/EBPβ blocks apoptosis by direct inhibition of caspases and that its transcriptional activity is dispensable for suppression of apoptosis. However, this nontranscriptional function for C/EBPβ probably does not account for its pro-oncogenic role in NIH 3T3 cells, which requires the TAD (39) and is abolished by mutation of Ser64 or Thr189. Our results favor a transcriptional mechanism in which Ras-induced phosphorylation of C/EBPβ on Ser64 and Thr189 is required to activate a critical target gene(s) that promotes the survival and/or proliferation of transformed cells.
A study by Lamb et al. (19) has implicated C/EBPβ in cellular transformation by cyclin D1 and suggested that C/EBPβ mediates the ability of cyclin D1 to activate transcription of target genes whose expression is correlated with specific cancers. Cyclin D1 and C/EBPβ also interact physically, leading to the proposal that a cyclin D1-C/EBPβ complex activates transcription of cyclin D1-regulated genes (19). Therefore, we considered that C/EBPβ phosphorylation might regulate its binding to cyclin D1 during specific phases of the cell cycle. However, preliminary results from coimmunoprecipitation experiments indicate that mutation of Ser64 and/or Thr189 does not significantly affect the formation of C/EBPβ-cyclin D1 complexes (J. Wessells and P. F. Johnson, unpublished data), arguing against this model. Nonetheless, it remains possible that phosphorylation of Ser64 and/or Thr189 alters C/EBPβ-dependent regulation of cyclin D1 target genes and that disrupted transcription of these genes by the S64A or T189A mutants accounts for their inhibitory effects on Ras transformation.
In summary, we show for the first time that C/EBPβ is phosphorylated by Cdks on Ser64 and Thr189 in a cell cycle-dependent manner. These two modifications are critical for C/EBPβ to facilitate oncogenic transformation of immortalized fibroblasts expressing Ha-RasV12. Our findings thus provide new insights into the posttranslational regulation of C/EBPβ by Ras signaling and the role of C/EBPβ in oncogenic transformation.
Acknowledgments
We thank Tad Gusczczynski for assistance with the phosphopeptide mapping experiments, Channing Der for the H-RasV12 vector and NIH 3T3 cells, Jurgen Muller and Deborah Morrison for Raf-1 vectors, and members of the Johnson laboratory for advice and discussion.
This research was supported in part by a grant from the National Cancer Institute (CA46637-RCS).
REFERENCES
- 1.Ayllon, V., and A. Rebollo. 2000. Ras-induced cellular events. Mol. Membr. Biol. 17:65-73. [DOI] [PubMed] [Google Scholar]
- 2.Bain, J., H. McLauchlan, M. Elliott, and P. Cohen. 2003. The specificities of protein kinase inhibitors: an update. Biochem. J. 371:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balmain, A., and K. Brown. 1988. Oncogene activation in chemical carcinogenesis. Adv. Cancer Res. 51:147-182. [DOI] [PubMed] [Google Scholar]
- 4.Barbacid, M. 1987. ras genes. Annu. Rev. Biochem. 56:779-827. [DOI] [PubMed] [Google Scholar]
- 5.Berthet, C., E. Aleem, V. Coppola, L. Tessarollo, and P. Kaldis. 2003. Cdk2 knockout mice are viable. Curr. Biol. 13:1775-1785. [DOI] [PubMed] [Google Scholar]
- 6.Buck, M., V. Poli, T. Hunter, and M. Chojkier. 2001. C/EBPβ phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol. Cell 8:807-816. [DOI] [PubMed] [Google Scholar]
- 7.Clark, G. J., A. D. Cox, S. M. Graham, and C. J. Der. 1995. Biological assays for Ras transformation. Methods Enzymol. 255:395-412. [DOI] [PubMed] [Google Scholar]
- 8.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 9.Descombes, P., and U. Schibler. 1991. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67:569-579. [DOI] [PubMed] [Google Scholar]
- 10.Finco, T. S., J. K. Westwick, J. L. Norris, A. A. Beg, C. J. Der, and A. S. Baldwin, Jr. 1997. Oncogenic Ha-Ras-induced signaling activates NF-κB transcriptional activity, which is required for cellular transformation. J. Biol. Chem. 272:24113-24116. [DOI] [PubMed] [Google Scholar]
- 11.Hanlon, M., and L. Sealy. 1999. Ras regulates the association of serum response factor and CCAAT/enhancer-binding protein beta. J. Biol. Chem. 274:14224-14228. [DOI] [PubMed] [Google Scholar]
- 12.Hanlon, M., T. W. Sturgill, and L. Sealy. 2001. ERK2- and p90(Rsk2)-dependent pathways regulate the CCAAT/enhancer-binding protein-beta interaction with serum response factor. J. Biol. Chem. 276:38449-38456. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, R., B. Spiegelman, D. Hanahan, and R. Wisdom. 1996. Cellular transformation and malignancy induced by ras require c-jun. Mol. Cell. Biol. 16:4504-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joneson, T., and D. Bar-Sagi. 1997. Ras effectors and their role in mitogenesis and oncogenesis. J. Mol. Med. 75:587-593. [DOI] [PubMed] [Google Scholar]
- 15.Kaldis, P., A. A. Russo, H. S. Chou, N. P. Pavletich, and M. J. Solomon. 1998. Human and yeast cdk-activating kinases (CAKs) display distinct substrate specificities. Mol. Biol. Cell 9:2545-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keenan, S. M., C. Bellone, and J. J. Baldassare. 2001. Cyclin-dependent kinase 2 nucleocytoplasmic translocation is regulated by extracellular regulated kinase. J. Biol. Chem. 276:22404-22409. [DOI] [PubMed] [Google Scholar]
- 17.Kolch, W. 2000. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351:289-305. [PMC free article] [PubMed] [Google Scholar]
- 18.Kowenz-Leutz, E., G. Twamley, S. Ansieau, and A. Leutz. 1994. Novel mechanism of C/EBP β (NF-M) transcriptional control: activation through derepression. Genes Dev. 8:2781-2791. [DOI] [PubMed] [Google Scholar]
- 19.Lamb, J., S. Ramaswamy, H. L. Ford, B. Contreras, R. V. Martinez, F. S. Kittrell, C. A. Zahnow, N. Patterson, T. R. Golub, and M. E. Ewen. 2003. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell 114:323-334. [DOI] [PubMed] [Google Scholar]
- 20.Langer, S. J., D. M. Bortner, M. F. Roussel, C. J. Sherr, and M. C. Ostrowski. 1992. Mitogenic signaling by colony-stimulating factor 1 and ras is suppressed by the ets-2 DNA-binding domain and restored by myc overexpression. Mol. Cell. Biol. 12:5355-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lents, N. H., S. M. Keenan, C. Bellone, and J. J. Baldassare. 2002. Stimulation of the Raf/MEK/ERK cascade is necessary and sufficient for activation and Thr-160 phosphorylation of a nuclear-targeted CDK2. J. Biol. Chem. 277:47469-47475. [DOI] [PubMed] [Google Scholar]
- 22.Marshall, C. 1999. How do small GTPase signal transduction pathways regulate cell cycle entry? Curr. Opin. Cell Biol. 11:732-736. [DOI] [PubMed] [Google Scholar]
- 23.Marshall, C. J. 1996. Ras effectors. Curr. Opin. Cell Biol. 8:197-204. [DOI] [PubMed] [Google Scholar]
- 24.Mo, X., E. Kowenz-Leutz, H. Xu, and A. Leutz. 2004. Ras induces mediator complex exchange on C/EBPβ. Mol. Cell 13:241-250. [DOI] [PubMed] [Google Scholar]
- 25.Morrison, D. K., G. Heidecker, U. R. Rapp, and T. D. Copeland. 1993. Identification of the major phosphorylation sites of the Raf-1 kinase. J. Biol. Chem. 268:17309-17316. [PubMed] [Google Scholar]
- 26.Nakajima, T., S. Kinoshita, T. Sasagawa, K. Sasaki, M. Naruto, T. Kishimoto, and S. Akira. 1993. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc. Natl. Acad. Sci. USA 90:2207-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson, C., S. Goto, K. Lund, W. Hung, and I. Sadowski. 2003. Srb10/Cdk8 regulates yeast filamentous growth by phosphorylating the transcription factor Ste12. Nature 421:187-190. [DOI] [PubMed] [Google Scholar]
- 28.Nerlov, C., and E. B. Ziff. 1994. Three levels of functional interaction determine the activity of CCAAT/enhancer binding protein-alpha on the serum albumin promoter. Genes Dev. 8:350-362. [DOI] [PubMed] [Google Scholar]
- 29.Ortega, S., I. Prieto, J. Odajima, A. Martin, P. Dubus, R. Sotillo, J. L. Barbero, M. Malumbres, and M. Barbacid. 2003. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 35:25-31. [DOI] [PubMed] [Google Scholar]
- 30.Pearson, G., F. Robinson, T. Beers Gibson, B. E. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 31.Riedl, T., and J. M. Egly. 2000. Phosphorylation in transcription: the CTD and more. Gene Expr. 9:3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlessinger, J., and D. Bar-Sagi. 1994. Activation of Ras and other signaling pathways by receptor tyrosine kinases. Cold Spring Harbor Symp. Quant. Biol. 59:173-179. [DOI] [PubMed] [Google Scholar]
- 33.Sklar, M. D., E. Thompson, M. J. Welsh, M. Liebert, J. Harney, H. B. Grossman, M. Smith, and E. V. Prochownik. 1991. Depletion of c-myc with specific antisense sequences reverses the transformed phenotype in ras oncogene-transformed NIH 3T3 cells. Mol. Cell. Biol. 11:3699-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutherland, J. A., A. Cook, A. J. Bannister, and T. Kouzarides. 1992. Conserved motifs in fos and jun define a new class of activation domain. Genes Dev. 6:1810-1819. [DOI] [PubMed] [Google Scholar]
- 35.Takehara, K. 2000. Growth regulation of skin fibroblasts. J. Dermatol. Sci. 24(Suppl. 1):S70-S77. [DOI] [PubMed] [Google Scholar]
- 36.Tetsu, O., and F. McCormick. 2003. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell 3:233-245. [DOI] [PubMed] [Google Scholar]
- 37.Williams, S. C., M. Baer, A. J. Dillner, and P. F. Johnson. 1995. CRP2 (C/EBPβ) contains a bipartite regulatory domain that controls transcriptional activation, DNA binding and cell specificity. EMBO J. 14:3170-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuspa, S. H. 1994. The pathogenesis of squamous cell cancer: lessons learned from studies of skin carcinogenesis. Cancer Res. 54:1178-1189. [PubMed] [Google Scholar]
- 39.Zhu, S., K. Yoon, E. Sterneck, P. F. Johnson, and R. C. Smart. 2002. CCAAT/enhancer binding protein-beta is a mediator of keratinocyte survival and skin tumorigenesis involving oncogenic Ras signaling. Proc. Natl. Acad. Sci. USA 99:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]