Abstract
Background:
The generation of bioartificial bone tissues may help to overcome the problems related to donor site morbidity and size limitations.
Materials and Methods:
In this paper, hydroxyapatite (HA) powder was made out of bovine bone by thermal analysis at 900°C and first, and then, porous HA (50 weight percentage) was produced by polyurethane sponge replication method. In order to improve the scaffold mechanical properties, they have been coated with poly hydroxybutyrate. In terms of phase studies, morphology, and specifying agent groups, the specific characterization devices such as X-ray diffraction and Fourier transform infrared, were employed. To compare the behavior of cellular scaffolds, they were divided into four groups of scaffolds. The osteoblast cells were cultured. To perform phase studies, analysis of Methylthiazole tetrazolium (MTT) and Trypan blue were carried out for the viability and attachment on the surface of the scaffold, and the specification of Scanning electron microscopy was employed for the morphology of the cells.
Results:
The results of MTT analysis performed on four groups of scaffolds have shown that Titanium oxide (Tio2) had no effect on cell growth alone and HA was the main factor of growth and cell osteoblast adhesion on the scaffold. Moreover, the results showed that the use of coating with poly-3-hydroxybutyrate saved the factors and placed the osteoblasts within the pore. Since the main part of bone consists of HA, the TiO2 accelerates the formation of apatite crystals at the scaffold surface which is the evidence for bone tissue regeneration.
Conclusions:
It is likely that the relation between HA and TiO2 leads to an increase in osteoblast adhesion and growth of cells on the scaffold surface.
Keywords: Osteoblast, poly- hydroxybutyrate, scaffold, tissue Engineering, titania
INTRODUCTION
Tissue Engineering is a young field of research. Initially, it was defined as “… an interdisciplinary field that applies the principles of engineering and the life sciences to the development of biological substitutes that restore, maintain, or improve tissue function.”[1] Among the human body organs, bone enjoys higher potential to reproduce; therefore, it accounts for a good sample in tissue engineering.[2]
Bone is a dynamic, highly vascularized tissue with the unique capacity to heal and remodel without leaving a scar.[3,4] It provides mechanical stability for the skeleton. This stability is required for load-bearing, locomotion and protection of internal organs. Furthermore, bone serves as a mineral reservoir and has the capacity to rapidly mobilize mineral stores if needed for homeostasis of the calcium blood level. The diversity of functional requirements of bone tissue is reflected by its complex architecture. In the adult skeleton bone tissue, it is either arranged in a trabecular pattern (cancellous bone) or in a compact pattern (cortical bone).[5] Cortical bone is almost solid with <10% porosity and is ubiquitously present in long, short, and flat bones. In contrast, cancellous bone is organized in a porous sponge-like pattern. This type of bone harbors a large part of the bone marrow and is essentially present in the metaphysis of long bones, i.e. the iliac crest and the vertebral bodies.[5]
Although bone tissue is populated by a variety of different cells, its functional integrity is maintained by three different cell types: osteoblasts, osteocytes and osteoclasts.[6,7] These tissue-specific cells are embedded in a highly complex matrix that consists of a mineralized (hydroxylapatite) and a nonmineralized component.[8,9] The nonmineralized, organic part containing collagens, glycoproteins, proteoglycans and sialoproteins plays an essential role in the control of growth and differentiation of osteoblasts, osteocytes, and osteoclasts as well as in bone remodeling.[10,11,12]
Bone regeneration is a highly efficient and a tightly regulated process that involves all the above-mentioned components of the bone tissue. Bone regeneration is the result of a continuous interplay between growth factors and cytokines for both initiation and regulation of the remodeling process.[13,14,15] The majority of fractures heal well under standard conservative or surgical therapy. However, extended bone defects following trauma or cancer resection or nonunions of fractures may require more sophisticated treatments. In these cases, bone grafting procedures, segmental bone transport, distraction osteogenesis or biomaterials are applied for reconstruction.[16,17,18,19]
Polyhydroxyalkanoates have been considered as a biopolymer family that will extend significantly the range of biomaterials suitable for tissue engineering.[20,21] Its mechanical properties have been shown to improve over polyhydroxybutyrate (PHB) that is also available in a large scale.[22,23] Synthetic and natural hydroxyapatites (HAs) have the same chemical composition and crystallographic properties as a bone joint.[24,25] Their biocompatibility and osteoconductive behavior are suitable for bone implants.[26] Studies showed that incorporation of HA into biomaterials would enhance mechanical performance or osteoblast responses.[27,28] Today, numerous studies have been conducted to improve the mechanical properties of HA.[29] One-way to overcome this limitation HA, is its use it as a background in a composite material, and as a biocompatible reinforcement material. In recent years, researchers have performed a lot of researches on composite HA-zirconia, HA- alumina, HA, and bioactive glass.[30,31,32] It has recently been reported that HA- titania can be a good combination of mechanical properties for bioactivity.[33] In addition, titania can increase the adhesion and growth of bone cells.[34] Due to the weaknesses observed in procedures, including low strength of the bioactive glass powder, heterogeneous polymer coating on the surface of the scaffold, relatively poor biocompatibility, and HA natural nanocrystals, poly-3-hydroxybutyrate (P3HB) coating was used on the surface of the scaffold by Foroughi et al. to improve the mechanical properties and fracture toughness of the coating.[35] In this study, the mechanical strength of the scaffold with a polymeric coating on the HA scaffold increased. However it was not good enough and needed reinforcement materials to have an effect on other desirable properties such as bioactivity of the scaffolds and production of higher porosity. After examining the physical and mechanical properties and the nature of titanium dioxide(TiO2), we decided to use this material to improve the mechanical properties and bioactivity of the scaffold.
As there was no report on the formation of scaffold by HA/Tio2 nanocomposite coated with P3HB solution and its comparison of cell viability and attachment on HA/Tio2 and HA without coating and HA coated with P3HB has been performed. This study mainly focuses on a scaffold design for cell evaluation of the seeded scaffolds. To optimize the seeding efficiency and to observe the cell proliferation into the inner structure, we developed a special design of the scaffold. The objective of the design was the maximization of the surface and, the facilitation of the seeding process to enhance cell adhesion and good supply of the scaffold interior with medium. The cultivation of cells seeded onto the scaffolds was carried out under static and dynamic conditions. Cell adhesion on the designed structure was also analyzed. In addition, cell growth into the bulk material and cell morphology was examined. Therefore, in this study, HA nanocrystal powder has been made out of bovine bone by thermal deposition method first, and then the porous scaffold has been constructed by polymer replication method. In order to improve the level of mechanical properties, they were coated by immersing it in P3HB polymer leading to the formation of higher compressive strength with more appropriate porous level scaffold. Therefore, this property can be considered as the distinguishing aspect of this type of scaffold. The use of P3HB polymer results in higher strenght, higher mechanical properties, and true porosity which are of high importance for the construction of scaffold making it more appropriate to be used in bone tissue engineering.
MATERIALS AND METHODS
Materials
HA Scaffolds were manufactured using a bovine bone. Commercial Polyurethane sponge (80 cpi) (Meat Co. Ltd. China) with an average pore size of 300 -700 μm was purchased. Powder of Tio2 with purity of 99.96% was purchased (Germany's Merck product with product code 1,00808,1000). Double-distilled water was prepared to build slurry ceramic. Solvent ammonium poly-methacrylate (APMP) as disperse (Mw = 10,000-160,000) (DARVAN C-N, R-T. Vanderbilt company, Inc., Norwalk USA) and powder of Carboxymethyl cellulose (CMC) as a bonding and increase the viscosity of the slurry was purchased. P3HB was purchased from Sigma- Aldrich Company. Chloroform (CHCl3) formula and purity of 5/99 were purchased from Spanish Oscar Lao company to prepare a polymer solution. Ethanol was obtained from Merck to be used in calculating porosity purity 9/99%.
Methods
Procurement of ceramic powder hydroxyapatite
After preparing bovine bone, first, it was boiled for 2 hours until its flesh and fats dissociated. Then, the bones were kept at the temperature of 60°C for 24 hours until its humidity disappeared. To prevent materials from being blackened with soot during the heating process, the bones were cut into small pieces (10 mm × 10 mm × 10 mm) with a saw and, then heated to 400°C for 3 hours in the air to analyze its organic compositions. The substance of this procedure was black ash of bone, which is used to produce HA. Next, to produce HA powder, the black ash experienced thermal operations for 2 hours in 900°C. One of the most important levels of constructing porous scaffold is the procurement of stable slurry by means of proper additives. In this paper, natural HA powder with a grain size of about 85nm was utilized for field's substance of scaffold.[35]
Fabrication of ceramic slurry
HA powder with 50 weight percentage was dissolved bit by bit in double-distilled water in order to prevent it from being a cold. After homogenizing the solution because of the need for right mechanical strength, it is advised that the amount of solid material which nestles on polymer sponge, should be large. Therefore, APMA is used for augmentation of weight percentage of solid substance from 1 weight percentage. Then, grout was stirred at the proper speed rate (300rpm) for 30 minutes until a homogenous solution was achieved. CMC powder (1 weight percentage) as a binder was gradually added to the solution in order to increase grout flowing. Then, the solution was stirred in 60°C until it turned into absolute homogeneity. The scaffold was used as a control sample. Hence, the slurry to produce 50 weight percentage HA composite scaffolds with 3 weight percentage TiO2 with lubricant and glue were prepared.
Preparation of porous hydroxyapatite scaffold
Commercial Polyurethane sponge(80cpi) (Meat Co., Ltd., China) with an average pore size of 300 -700 μm and (1 cm × 1 cm × 1cm) pieces were infiltrated with a controlled amount of slurry to achieve porous materials. The sponge was immersed into a ceramic slurry and squeezed to exhaust the contained air. The slurry was absorbed into the sponge while the body restores by elasticity. When the slurry filled the sponge space totally, the impregnated sponge was taken out and squeezed equally to remove excessive slurry. The gained composite porous body was dried for 24 hours naturally. In air heat treatment in four stages including (1) Putting samples in the temperature of 600°C with warming rate 5°C/min for 1 hour to complete burning of polyurethane sponge. (2) Increasing temperature from 600°C to 1200°C with warming rate 5°C/min. (3) Keeping it at 1200°C for 2 hours to sinter ceramic scaffold. (4) Decreasing temperature with cooling rate 5°C/min.
Scaffold Coated with poly-3-hydroxybutyrate
In this study, P3HB powder (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 10 ml CHCl3 (CHCl3, Sigma-Aldrich, USA) by 6 weight percentage for 6 hours in an oil bath at the temperature of 60°C, for the Polymer solution for coating ceramic scaffold to be achieved. Since CHCl3 is volatile, it is necessary to have a fridge device to produce this type of solution. Afterward, HA/Tio2 and HA scaffolds were immersed in a Polymer solution for 30 s and 60 s, and then to achieve consistency in every point of the coated scaffold and to extract extra Polymer solution, the samples were wrapped up in aluminum foil and were put in a centrifuge with 500 rpm for 30 sec. Finally, the samples were placed in a vacuum oven at room temperature for 24 hours.
Physical Characterization
X-ray diffraction of hydroxyapatite powder
In order to discuss structural changes and to study phases for HA nanocrystals and scaffolds, X-ray diffraction method (XRD, Philips X'Pert) was applied. A CuKα ray was used for the analysis. The scan rate was set to 1°/min, imposed voltage equates 30 KV and 30 mA current and diffraction angle (2-θ) ranged from 10° to 60°at a rate of 0.4°/min.
Fourier transforms infrared spectrometer
IR was used to characterize HA nanocrystals and scaffolds after sintering. A 2 -mg dried sample was carefully mixed with 300 mg dry KBr and pressed into a pellet using a macro- KBr die kit. The solid pellet was placed in a magnetic holder. The system was purged with dry air for 1 h to remove water vapor from the sample compartment. Fourier transform infrared (FTIR) spectroscopy (FT-IR: 6300, JASCO, Japan) has been used for studying functional groups and specifically, the degree of 2-hydroxylation of HA. Spectral analyses were performed using standard Nicolet and Microcal Origin (FTIR: 6300, JASCO, Japan) software. FT-IR spectra were taken of both as received and sintered HA nanocrystals and scaffolds.
Scanning electron microscopy
Scanning electron microscopy (SEM, Philips XL-30, the Netherlands) was used to study the HA nanocrystals and scaffold. Samples were coated with gold under an argon atmosphere.
Polyurethane sponge thermal gravimetric analysis
Thermal gravimetric analysis (TGA) was used to measure thermal stability and composition of the material. This measure showed the reduction of weight to the temperature changes. This study was used to reduce the weight of polyurethane sponge to temperature devices as TGA (TG/DTA, TGA 401, Sanatara, co.).
Calculating the porosity of scaffolds
A scaffold of weight (W) was immersed in a graduated cylinder containing a known volume (V1) of ethanol. The cylinder was placed in a vacuum to force the ethanol into the pores of the scaffold until no air bubble emerged from the scaffold. The total volume of the ethanol and scaffold was then recorded as V2. The volume difference (V2 - V1) was the volume of the skeleton of the scaffold. The scaffold was removed from the ethanol and the residual ethanol volume was measured as V3:
The apparent density of the scaffold, ρ, was evaluated as (1):[36]

The porosity of the open pores in the scaffold, ρ, was evaluated as (2):

Cell culture
Sterilization of scaffold
There are four groups which are used for cell culture studies; HA/Tio2 /P3HB, HA/PHB, HA/Tio2, and HA. For sterilization of each group, the samples were placed in a 9 cm plate. Scaffolds were sterilized in 75% ethanol for 3h under ultraviolet radiation for 1h, and then immersed in DMEM (Dubecco's Modified Eagle Medium, Invitrogen, California, USA) overnight.
Cell seeding
The human osteoblast cell lines Saos-II were purchased from the Pasteur Institute of Iran (IPI) (Tehran, Iran). Saos-II cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10% fetal Bovine serum (FBS), and 100 mg/ml streptomycin (Jinke Biotech). Cells were incubated at 37°C in a 5% CO2 incubator. Cells were harvested by trypsinization followed by addition of fresh culture medium to create a new single cell suspension. When the cells reached the plateau phase of growth, they were harvested by trypsinization, followed by addition of fresh culture medium to create a new single cell suspension with desired seeding cell number per 100 mg. Inoculation was performed in polystyrene 24-well flat-bottom culture plates. Scaffolds were placed in the center of the wells added with 1 ml cell suspension to allow full attachment of cells to scaffolds. Cultivation was conducted for 3 days. Culture media was changed every 4 days.[37]
Cell Viability
For attachment study, Saos-II was allowed to attach for 8 and 24 h, respectively. At each specified seeding time, the viability of the attached cells was quantified by methylthiazole tetrazolium (MTT) assay. Each sample was rinsed with phosphate buffer saline (PBS; Sigma Aldrich, USA) to remove unattached cells prior to MTT assay. Since no studies related to the expression of attachment proteins or the strength of the attached cells were carried out. This evaluation only served as the qualitative measure of the cell attachment study. For proliferation study, the cells were first allowed to attach on the specimens for 24 h. The proliferation of the cells on each specimen was determined after varying culture period 3 and 7 days, respectively. The viability of the cells was, again, quantified by MTT assay.[38]
Quantification of viable cells (methylthiazole tetrazolium assay)
The MTT assay is based on the reduction of the yellow tetrazolium salt to purple formazan crystals by dehydrogenase enzymes secreted from the mitochondria of metabolically active cells. The amount of the purple formazan crystals formed is proportional to the number of viable cells. First, each sample was incubated at 37°C for 1 h with 250 (10) μl/well of MTT solution at 0.5 (5) mg/ml without phenol red for a 24-well (or 96-well) scaffold. After incubation, MTT solution was removed. A buffer solution containing dimethyl sulfoxide (DMSO; Carlo Erba, Italy) of about 900 (100) μl/well and glycine buffer (pH = 10) of about 125 (5) μl/well was added to the wells to dissolve the formazan crystals. After 10 min of rotary agitation, the solutions were then transferred into a cuvette and placed in a Thermospectronic Genesis 10 UV- visible spectrophotometer, from which the absorbance at 540 nm representing the number of viable cells was measured.[39,40]
Cell attachment
The human osteoblast cell lines Saos-II were purchased from the IPI (Tehran, Iran). Saos-II cells were cultured in RPMI 1640 supplemented with 10% FBS, and 100 mg/ml streptomycin (Jinke Biotech). Cells were incubated at 37°C in a 5% CO2 incubator. Cells were harvested by trypsinization followed by addition of fresh culture medium to create a new single cell suspension. When the cells reached the plateau phase of growth, they were harvested by trypsinization, followed by addition of fresh culture medium to create a new single cell suspension with desired seeding cell number per 100 mg. Inoculation was performed in polystyrene24-well flat-bottom culture plates. Scaffolds were placed in the center of the wells added with 1 ml cell suspension to allow full attachment of cells to scaffolds. Cultivation was conducted for 3, 7 days. Culture media was changed every 4 days.
Trypan blue
Osteoblast cells from line of Saos-II were used for the assays. Briefly, osteoblast cells were released from nearly confluent cultures with trypsin (Sigma), washed with a solution containing trypsin inhibitor (Sigma). After 30 min, nonbound cells were washed away, and attached cells were fixed, stained, and counted.
Scanning electron microscopy examination
It has been tested in accordance with the standards ISO 10993-5 and carried directly. To study the adhesion and cell morphology, cell suspension containing 20,000 cells with UV light was seed on the surface of sterile scaffold. The specimens were washed twice by PBS and immersed in PBS containing 3% glutaraldehyde (pH = 7.4) for 4h. They were then dehydrated in increasing concentrations of ethanol (from 30%, 50%, 70%, 90%, and 95% to 100%), followed by lyophilization. They were then mounted on aluminum stumps, coated with gold in a sputtering device for 1.5 min at 15 mA and examined under a SEM (KYKY-2800, Tehran University, Tehran, Iran).[41,42]
Statistical analysis
Statistically significant differences were determined with a two-tailed student's t-test between four groups and a one-way ANOVA was used to compare the means of different data sets, and statistical significance was accepted at a 0.05 confidence level. The results are reported as a mean ± MTT and Trypan blue. P < 0.05 was considered statistically significant.
RESULTS
X-ray Diffraction of Hydroxyapatite Powder
Figure 1 is XRD pattern of the HA powder. According to standard JCPDS: 9-432 peaks 211, 112, 300 and 202 at a temperature of 900°C defines the natural HA powder by heating the bone because of its similarity to bone apatite used for medical purposes. Importantly, the difference in the broadening of the diffraction patterns reflects differences in average crystalline grain size.
Figure 1.
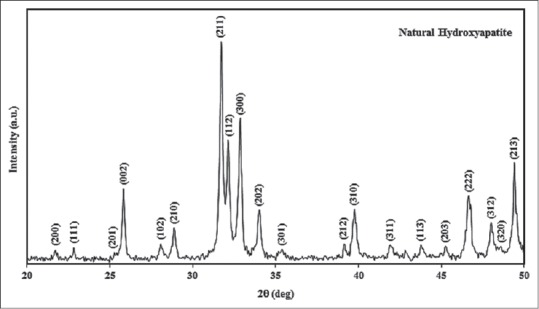
X-ray diffraction pattern of the powder hydroxyapatite (according to standard of JCPDS: 9-432)
Fourier transform infrared of hydroxyapatite powder
In order to study the presence of agent groups nanocrystals HA and also to study how these groups affected each other when they were compounding, the practice of FTIR test (FTIR: 6300, JASCO, Japan) was possible over pure nanocrystals HA. Findings of the test have been illustrated in Figure 2. Absorption spectrum related to HA in Figure 2 clearly showed peaks which belong to apatite phase. Observed peaks in the range of 570- 632/cm have to do with the bending vibration of PO4-3 bonds, and absorption at 1020/cm resulted from the stretching vibration of PO4-3 bonds. The OH- group of HA led to a wide peak in 3570/cm. Observed peaks in 1049/cm have to do with bending vibration of PO4-3 bonds and absorption at 1020/cm resulted from stretching vibration of PO4-3 bonds. A peak intensity is seen in the range of 1049 to reflect the stretch and also it represents a phosphate group attached to the carbon that is C-O-H. The OH- groups lead to make the link between their particles.
Figure 2.
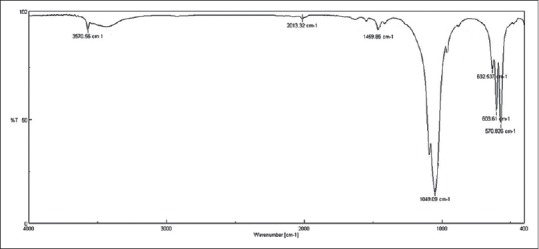
Fourier transform infrared absorption spectrum hydroxyapatite powder
According to Figure 3, FTIR spectroscopy is a technique which is used to obtain collects high spectral resolution data over a wide spectral range. This test is more appropriate for polymer samples. Pure HA sample without nTiO2 in the range of 1049/cm to peak intensity can be seen that represents the OH- groups attached to the carbon C-OH and also represents a phosphate group in bending motions. A single small peak is in1091/cm which represents the OH- attached to adjacent atoms that creating bonds between particles. In 3570/cm, a relatively sharp peak in the OH- groups which are the same molecular or structural to water. For pureTiO2 and in 1000/cm, there is not found any peak and this means that the OH- groups of the bending motions of the phosphate group does not exist. Addition of TiO2 to HA was caused a change in the peaks of the graph. A peak at around 3537/cm to 3432/cm was observed which according to the above explanation of the structural OH- groups, with increasing TiO2, a stronger hydrogen bond will be created and it is concluding the more strength of the composite. Around the 1124/cm, a small single peak is observable which is related to the carbonyl group. A sharp peak in the region of 3537/cm was detected which indicating stronger hydrogen bonds between molecules of HA and TiO2. For HA/TiO2 /P3HB composite, similar results were obtained.
Figure 3.
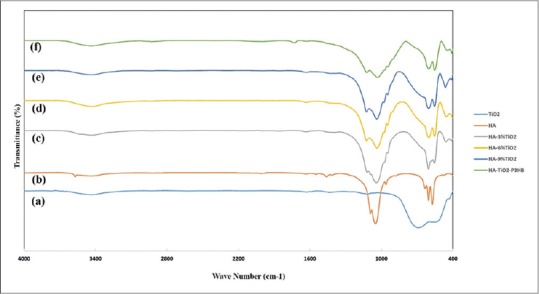
Fourier transform infrared pattern of scaffolds
Study Morphology of scaffolds
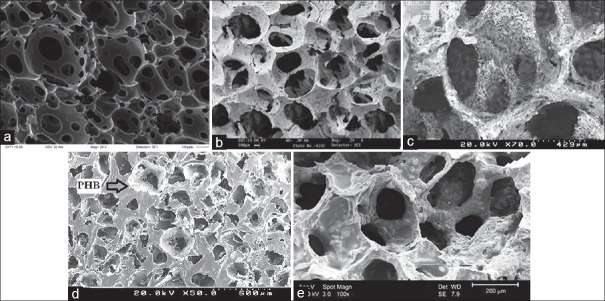
In order to investigate the microstructure of the four groups scaffolds, SEM was used. Figure 4a was taken before polyurethane sponge impregnated the ceramic slurry. According to Figure 4a, polyurethane sponge has a three dimensional structure with open porosity. Figure 4a displays electron micrographs of polyurethane with open pores in the range of 300-700 μm before immersion in a ceramic slurry. Figure 4b shows HA porous scaffold. Figure 4c illustrates micropores formed from nanocomposite HA/Tio2. Figure 4d micropores formed from nanocomposite HA/Tio2 coated with P3HB. Figure 4e indicates HA porous scaffold coated with P3HB coating [Figure 4].
Figure 4.
Scanning electron microscopy of scaffolds (a) polyurethane sponge (b) hydroxyapatite (c) hydroxyapatite/titanium dioxide (d) hydroxyapatite/titanium dioxide coated poly-3-hydroxybutyrate (e) hydroxyapatite coated poly-3-hydroxybutyrate
Study Thermal Gravimetric Analysis
In this study, thermal analysis done and is show complete burning temperature of polymer sponge specifies. Start analyzing temperature ambient temperature, in the presence of argon gas until the temperature of 600°C with speed 10°C/min were performed [Figure 5].
Figure 5.
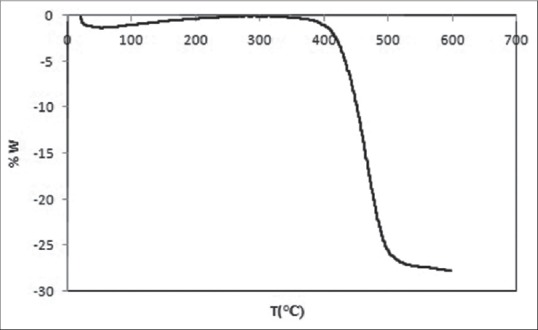
Thermal gravimetric analysis graph of polyurethane sponge and drawing temperature 200–300°C
Polymer sponge decomposition is done in two stages:
Approximately 200 starting and 350 continues, this temperature range is not very noticeable
The bulk polymer at temperatures between 600°C and 400°C has logged out; hence, taking the slow heating rate at this stage to prevent damage caused by gas emissions resulting from the thermal decomposition of the polymer structure is essential. Therefore, to avoid thermal shock and cracking, during the heat treatment, temperature with rate 5°C/min had climbed up to 600°C and then sponge for full burning, the temperature was kept for 1 h.
Calculation the porosity of scaffolds
For comparison and better selection of the appropriate scaffold porosity, Table 1 was prepared. This table can select the most suitable scaffold for cell culture.
Table 1.
Porosity of nanocomposite scaffolds for 50 weight percentage hydroxyapatite with 3 weight percentage titanium dioxide, 6 weight percentage poly-3-hydroxybutyrate
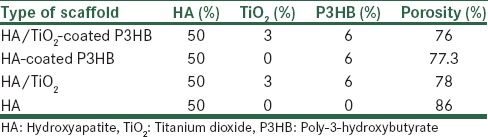
As you see in Table 1, HA 50 weight percentage scaffold has proper a porosity percentage but has little strength. HA 50 weight percentage coated P3HB 6 weight percentage has less porosity percentage than HA without coated P3HB but its strength is high. HA 50 weight percentage, TiO2 3 weight percentage without coated with P3HB weight percentagehas little porosity percentage, because titanium oxide was an agent to porosity was closed. HA 50 weight percentage, Tio2 3 weight percentage coated P3HB 6 weight percentagehas a good porosity percentage and strength. Therefore, with regard to results obtained from porosity, the best scaffold for cell culture is HA 50 weight percentage, Tio2 3 weight percentage and 6 weight percentage P3HB that you can see in Table 1. It is important to note that scaffold of HA 50 weight percentage with 6 weight percentage P3HB can be a scaffold because of its porosity percentage. Table 1 shows the data of porosity, 50 weight percentage HA, and Tio2 3 weight percentage coated 6 weight percentage. P3HB scaffold has been selected and shown with highlight.
Study cell viability
After 3, 7 days of cell culture in four scaffolds, the results showed that by increasing cultivation, the proliferation of osteoblasts on the scaffold has increased on group A. As shown in Figures 6a and b in group A, the cell density is much more than the cell density in the other group and there is a significant difference (ρ < 0.05) between these four groups.
Figure 6.
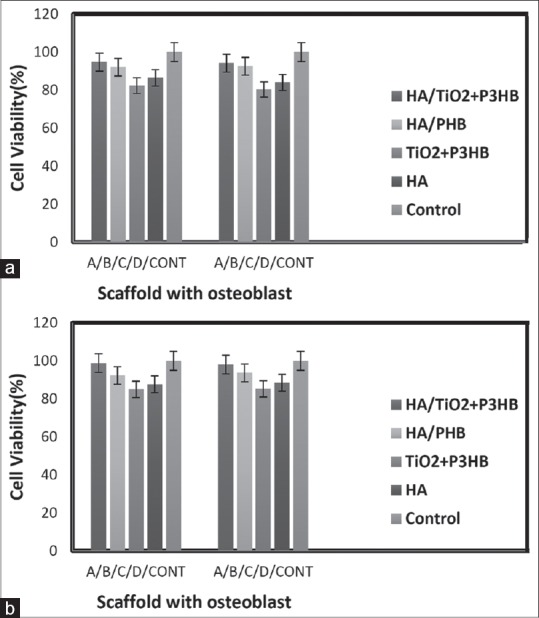
Cell proliferation assay using methylthiazole tetrazolium assay of three days growth of osteoblast on scaffolds made of hydroxyapatite/titanium dioxide + poly-3-hydroxybutyrate (a), hydroxyapatite/polyhydroxybutyrate (b), titanium dioxide + poly-3-hydroxybutyrate (c), hydroxyapatite (d) and control. (a) After 3 days and (b) after 7 days cell culture on Scaffolds) (n = 5, mean ± standard deviation, P < 0.05)
Studying cell attachment
Cell line Saos-II seeded on HA/TiO2 coating P3HB, respectively, showed better after 3 and 7 days adhesion than that of any other films [Figure 7]. HA/P3HB may be too smooth for cellular adhesion, while HA/TiO2 coating P3HB scaffold was too hydrophobic for cell attachment. These results further demonstrated that the introduction of A groups in HA/TiO2 coating P3HB scaffold was a positive factor for cell growth.
Figure 7.
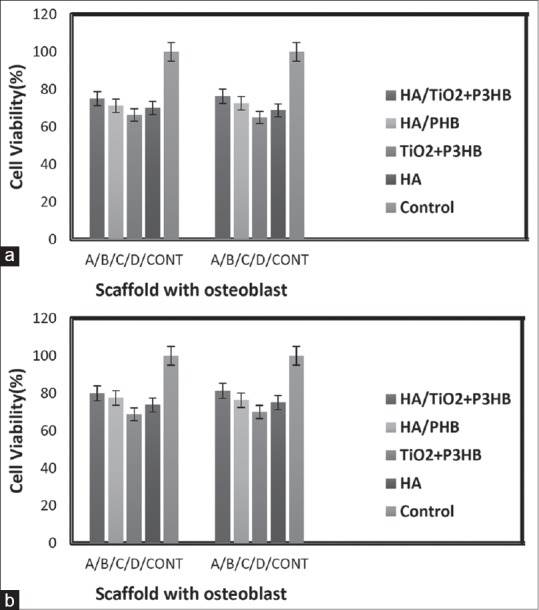
Study of cell attachment on the hydroxyapatite/titanium dioxide + poly-3-hydroxybutyrate, hydroxyapatite/polyhydroxybutyrate, titanium dioxide + poly-3-hydroxybutyrate, hydroxyapatite, and control, respectively. About 2 × 104 cells/well of Saos-II was seeded on each scaffold (n = 5). Saos-II cells adhered on the plate as 100% control. (a) After 3 days and (b) after 7 days cell culture on Scaffolds)
Studying surface morphology and cell growth behavior on biomaterials using scanning electron microscopy
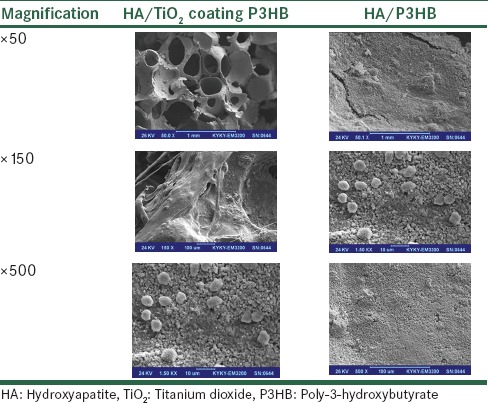
Table 2 shows selected SEM images of Saos-II that were cultured on HA/Tio2 coating P3HB and HA/P3HB scaffolds substrate for 24 h at two different magnifications (×50, ×150, and × 500; scale bar = 1mm, 10 and 100μm, respectively). For the low magnification images (×150), a good number of cells were attached on the HA/Tio2 coating P3HB surface. In addition, with increasing culture period, the number of cells on any given substrate was found to increase. The high-magnification images (×500) clearly showed that the attached cells exhibited the spindle shape, typical of active like fibroblastic cells, with an evidence of filopodia. Though not shown, some of the attached cells with round shape were also observed after 24 h in culture, but the cells appeared to be more elongated with increasing culture period. Compared to HA/Tio2 coating P3HB and HA/P3HB scaffolds, HA/Tio2 coating P3HB scaffold showed a larger porosity. The larger porosity should be better for cell growth. Significant differences were observed between cultures on various tested scaffolds. It seems that the growth pattern, cell aggregations and extracellular cell matrix production as well as the rates of colonization, could be observed to attach to these embedded scaffolds.
Table 2.
Selected scanning electron microscopy images of Saos-II that were cultured on hydroxyapatite/titanium dioxide coating poly-3-hydroxybutyrate and hydroxyapatite/oly-3-hydroxybutyrate scaffolds substrate for 24 h at two magnifications (×50, ×150, and×500; scale bar=1 mm, 10 and 100 μm, respectively)
DISCUSSION
In bone tissue engineering, usually a cellular mixture including biodegradable composite scaffold comprising bioactive elements and growth factors for recovery and reconstruction of natural tissue structure is used. The selection of appropriate biomaterials for the construction of scaffold is the foremost step in bone tissue engineering. The favorable scaffold is deemed as a pattern for tissue reconstruction, and plays a key role in the formation of the final engineered structure of tissue and its final performance. The biomaterials used in scaffold should support adhesion and cellular growth and proliferation both in vitro and in vivo.[43,44] They should possess several properties such as biocompatibility, biodegradability, and bioactivity. Despite the fact that TiO2 (Titania) and poly hydroxyl butyrate possess these requirements, lack of appropriate bioactivity in bone tissue engineering has caused restriction of their use in bone reconstruction. In spite of low mechanical properties, hydroxyl-apatite is a bioactive material with osteoconductivity potential that is suitable for bone tissue engineering.[45] Based on the conducted studies, construction of scaffold by means of immersion of sponge in ceramic slurry leads to creating scaffolds at high and uniform porosity percentages. Similarly, application of TiO2 causes us to have mechanical and physical properties, which are followed by a coarse surface for the growth of osteoblast cells and formation of cellular outgrowths. Rising quantity of porosity leads to reducing compressional strength and possible destruction in applications under loading in which using polymeric coating P3HP causes a noticeable increase in mechanical strength thus preventing the blockage of porosities. While HA/TiO2 includes positive effects on the growth of osteoblast cells, only application of HA due to its low mechanical properties, TiO2 because of low mechanical properties, and the related composite were used along with P3HB coating following lack of preparation of suitable and adequate conditions for the growth of osteoblast cells. The behavior of osteoblast cells on composite scaffold HA/TiO2 with a polymeric coating of poly hydroxyl butyrate compared to scaffold with scaffold HA with and without coating was explored in this study.
With respect to the given investigations on the composite scaffold, it was identified that use of polymeric coating did not lead to a noticeable reduction in porosities, and highly porous and 3-D homogeneous microstructure with suitable porosity size has been provided for adhesion, proliferation, internal growth, and migration of osteoblast cells. Based on the conducted studies, the average porosity size was 200μm in composite scaffolds, which were greatly suitable for adhesion, internal growth, and proliferation of osteoblast cells. The porosity rate of composite scaffold HA/TiO2 with and without polymeric coating is 76% and 78%, respectively, where this partial reduction is due to the presence of polymeric coating. This behavior may be expressed for HA coating (77.3%) and without coating (86%). Based on studies carried out by Foroughi et al., similar results were acquired on composite scaffold HA with and without polymeric coating P3HB as well so that application of polymeric coating on the surface of scaffold HA has not led to noticeable reduction in percentage of porosities and as a result strength was increased in scaffolds. This factor causes cellular migration and interconnectivity to take place with osteoblast cells culture.[46,47]
The studies of cell culture showed that composite scaffold HA/TiO2 with P3HB coating possessed better rate of adhesion and cellular growth compared to other groups and it was due to the presence of TiO2 as a strengthening phase and rising coarseness of surface. On the other hand, due to piezoelectric property, biocompatibility, and rising strength in porosities, the presence of P3HB has caused osteoblast cells to be easily embedded into porosities and to have better growth than groups without coating. Based on the conducted studies through MTT assay, one can express that the presence of a polymeric coating on the surface of scaffolds has caused improvement in cellular behavior compared to scaffolds without coating. Based on studies conducted by Saadat et al., they reported similar results in which the use of nanocomposite scaffold HA with P3HB coating causes rising activity and improvement in bioactivity of osteoblast cells.[48] Likewise, studies conducted by Zhang et al. also indicated that the presence of HA, and P3HB causes an increase in osteoconductivity and osteoinductivity.[49]
Finally, whereas nanocomposite scaffold HA/TiO2 has been used with coating P3HB for the first time and it has shown suitable results from growth and adhesion of osteoblast cells, it may be implied that this scaffold may be assumed as an appropriate candidate for designing bone tissues.
CONCLUSIONS
After more than 100 years of bone substitutes and almost 20 years of tissue engineering, there is still no satisfying therapeutic alternative to autogenous bone grafting for the treatment of large bony defects. However, in this research, nanocomposite scaffolds with 50 weight percentage of HA and Tio2 have been formed with the help of Polyurethane sponge with close porosity to natural bone, and afterward due to scaffold fragility, the scaffolds have been coated with P3HB to get a higher compressive strength scaffold, and then Cell lines Saos-II from osteoblast seed on four types of scaffold. The best Proliferation on Ma and Langer nanocomposite scaffolds with 50 weight percentage of HA and Tio2 coated P3HB were compared with the likes of HA coated PHB, HA/Tio2 without coated P3HB and HA without a coat. Findings of the study are as follows:
Highly porous by using Polyurethane sponge replication method with coated P3HB are mechanically strong and possess architectures suitable for osteoblast seeding and growth
HA/Tio2 composite coating with P3HB scaffold has advantages over its blend with HA both in mechanical properties and in promoting osteoblast growth although HA is bioactive and osteoconductive. The porous surface of HA/Tio2 composite coating P3HB could be an important factor for osteoblast proliferation
The HA/Tio2 composite coated P3HB scaffolds have been shown to have a higher osteoblast survival rate, more uniform cell distribution and growth. It could be useful for substitution of bone defect in vivo
These results indicate that the nanocomposite HA/Tio2 coating P3HB bone graft substitutes have the adequate morphology properties, pore size, and interconnectivity favored for tissue engineering
The in vitro results from this study showed that the cytotoxicity of the Tio2 bone graft substitutes was significantly lower compared to the commercial bone graft substitutes already used
The rigid surface by blend of HA/Tio2 has been the best condition for the proliferation of osteoblast
The HA/Tio2 composite coated with P3HB scaffold has good biocompatibility with bone osteoblast cells, and these cells could attach and proliferate on the surface
Eventually, the formation of cellular processes and extracellular matrix constructs that can provide adequate conditions for bone regeneration in vivo comparable to those of autogenous bone grafts will be developed
The HA/Tio2 composite coated with P3HB scaffold has better cell viability and cell attachment than HAP/Tio2 composite without coated with P3HB scaffold.
The HA/Tio2 composite coated with P3HB scaffold composites developed in this work are fully biocompatible materials that may be useful in tissue engineering applications such as bone implant scaffolds. When fabricating bone implants, it is desirable to make scaffolds with high porosity and large pore sizes that allow the migration, adhesion, and proliferation of cells such as osteoblasts to promote bone in growth.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
I would like to express my deep gratitude to Dr. Salehi, a faculty member of the University of Isfahan, and a faculty member of Medical Science University for their service and generous support to conduct this research.
REFERENCES
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Hollinger JO, Einhorn TA, Doll B, Sfeir C, editors. Bone tissue engineering. CRC press. 2004 Oct 14; [Google Scholar]
- 3.Buckwalter JA, Glimcher MJ, Cooper RR, Recker R. Bone biology. II: Formation, form, modeling, remodeling, and regulation of cell function. Instr Course Lect. 1996;45:387–99. [PubMed] [Google Scholar]
- 4.Buckwalter JA, Glimcher MJ, Cooper RR, Recker R. Bone biology. I: Structure, blood supply, cells, matrix, and mineralization. Instr Course Lect. 1996;45:371–86. [PubMed] [Google Scholar]
- 5.Ackerman LV, Spjut HJ, Abell MR. Bones and Joints (Monographs in Pathology) Baltimore: Williams and Wilkins; 1976. [Google Scholar]
- 6.Aubin JE. Bone stem cells. J Cell Biochem Suppl. 1998;30(31):73–82. [PubMed] [Google Scholar]
- 7.Owen M. The origin of bone cells. Int Rev Cytol. 1970;28:213–38. doi: 10.1016/s0074-7696(08)62544-9. [DOI] [PubMed] [Google Scholar]
- 8.Heinegård D, Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989;3:2042–51. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- 9.Robey PG, Fedarko NS, Hefferan TE, Bianco P, Vetter UK, Grzesik W, et al. Structure and molecular regulation of bone matrix proteins. J Bone Miner Res. 1993;8(Suppl 2):S483–7. doi: 10.1002/jbmr.5650081310. [DOI] [PubMed] [Google Scholar]
- 10.Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1:E131–8. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- 11.Lian JB, Stein GS. Development of the osteoblast phenotype: Molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop J. 1995;15:118–40. [PMC free article] [PubMed] [Google Scholar]
- 12.Nomura S, Takano-Yamamoto T. Molecular events caused by mechanical stress in bone. Matrix Biol. 2000;19:91–6. doi: 10.1016/s0945-053x(00)00050-0. [DOI] [PubMed] [Google Scholar]
- 13.Reddi AH. Initiation of fracture repair by bone morphogenetic proteins. Clinical orthopaedics and related research. 1998 Oct 1;355:S66–72. doi: 10.1097/00003086-199810001-00008. [DOI] [PubMed] [Google Scholar]
- 14.Ducy P. Cbfa1: A molecular switch in osteoblast biology. Dev Dyn. 2000;219:461–71. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1074>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84-A:1032–44. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Perry CR. Bone repair techniques, bone graft, and bone graft substitutes. Clinical orthopaedics and related research. 1999 Mar 1;360:71–86. doi: 10.1097/00003086-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Gugenheim JJ., Jr The Ilizarov method. Orthopedic and soft tissue applications. Clin Plast Surg. 1998;25:567–78. [PubMed] [Google Scholar]
- 18.Motoki DS, Mulliken JB. The healing of bone and cartilage. Clin Plast Surg. 1990;17:527–44. [PubMed] [Google Scholar]
- 19.Polykandriotis E, Stangl R, Hennig HH, Lennerz JK, Frank WM, Loos MD, et al. The composite vastus medialis-patellar complex osseomuscular flap as a salvage procedure after complex trauma of the knee – An anatomical study and clinical application. Br J Plast Surg. 2005;58:646–51. doi: 10.1016/j.bjps.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Williams SF, Martin DP, Horowitz DM, Peoples OP. PHA applications: Addressing the price performance issue: I. Tissue engineering. Int J Biol Macromol. 1999;25:111–21. doi: 10.1016/s0141-8130(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 21.Kunze C, Freier T, Kramer S, Schmitz KP. Anti-inflammatory prodrugs as plasticizers for biodegradable implant materials based on poly (3-hydroxybutyrate) J Mater Sci Mater Med. 2002;13:1051–5. doi: 10.1023/a:1020392606225. [DOI] [PubMed] [Google Scholar]
- 22.Doi Y, Kitamura S, Abe H. Microbial synthesis and character-ization of poly (3-hydroxybutyrate co-3-hydroxyhexanoate) Macromolecules. 1995;28:4822–8. [Google Scholar]
- 23.Yang X, Zhao K, Chen GQ. Effect of surface treatment on the biocompatibility of microbial polyhydroxyalkanoates. Biomaterials. 2002;23:1391–7. doi: 10.1016/s0142-9612(01)00260-5. [DOI] [PubMed] [Google Scholar]
- 24.Dalby MJ, Di Silvio L, Harper EJ, Bonfield W. Increasing hydroxyapatite incorporation into poly (methylmethacrylate) cement increases osteoblast adhesion and response. Biomaterials. 2002;23:569–76. doi: 10.1016/s0142-9612(01)00139-9. [DOI] [PubMed] [Google Scholar]
- 25.Di Silvio L, Dalby MJ, Bonfield W. Osteoblast behaviour on HA/PE composite surfaces with different HA volumes. Biomaterials. 2002;23:101–7. doi: 10.1016/s0142-9612(01)00084-9. [DOI] [PubMed] [Google Scholar]
- 26.Ramires PA, Romito A, Cosentino F, Milella E. The influence of titania/hydroxyapatite composite coatings on in vitro osteoblasts behaviour. Biomaterials. 2001;22:1467–74. doi: 10.1016/s0142-9612(00)00269-6. [DOI] [PubMed] [Google Scholar]
- 27.Galego N, Rozsa C, Sanchez R, Fung J, Vazquez A, Tomas JS. Characterization and application of poly (beta-hydroxyalkano-ates) family as composite biomaterials. Polym Test. 2000;19:485–92. [Google Scholar]
- 28.Chen LJ, Wang M. Production and evaluation of biodegradable composites based on PHB-PHV copolymer. Biomaterials. 2002;23:2631–9. doi: 10.1016/s0142-9612(01)00394-5. [DOI] [PubMed] [Google Scholar]
- 29.Sankapal BR, Sartale S, Ennaoui A. Chemical and electrochemical synthesis of nanosized TiO2 anatase for large-area photon conversion. C R Chim. 2006;9:702–7. [Google Scholar]
- 30.Chiba A, Kimura S, Raghukandan K, Morizono Y. Effect of alumina addition on hydroxyapatite biocomposites fabricated by underwater-shock compaction. Materials Science and Engineering: A. 2003 Jun 15;350(1):179–83. [Google Scholar]
- 31.Kim S, Bang H, Song J, Park S. Effect of fluoride additive on the mechanical properties of hydroxyapatite/alumina composites. Ceram Int. 2009;35:1647–50. [Google Scholar]
- 32.Juang H, Hon M. Fabrication and mechanical properties of hydroxyapatite-alumina composites. Mater Sci Eng. 1994;2:77–81. [Google Scholar]
- 33.Ravarian R, Moztarzadeh F, Solati Hashjin M, Rabiee SM, Khoshakhlagh P, Tahriri M. Synthesis, characterization and bioactivity investigation of bioglass/hydroxyapatite composite. Ceram Int. 2010;36:291–7. [Google Scholar]
- 34.Peltola T, Pätsi M, Rahiala H, Kangasniemi I, Yli-Urpo A. Calcium phosphate induction by sol-gel-derived titania coatings on titanium substrates in vitro. J Biomed Mater Res. 1998;41:504–10. doi: 10.1002/(sici)1097-4636(19980905)41:3<504::aid-jbm22>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 35.Foroughi MR, Karbasi S, Ebrahimi-Kahrizsangi R. Physical and mechanical properties of a poly-3-hydroxybutyrate-coated nanocrystalline hydroxyapatite scaffold for bone tissue engineering. J Porous Mater. 2012;19:667–75. [Google Scholar]
- 36.Ramay HR, Zhang M. Preparation of porous hydroxyapatite scaffolds by combination of the gel-casting and polymer sponge methods. Biomaterials. 2003;24:3293–302. doi: 10.1016/s0142-9612(03)00171-6. [DOI] [PubMed] [Google Scholar]
- 37.Wang YW, Wu Q, Chen J, Chen GQ. Evaluation of three-dimensional scaffolds made of blends of hydroxyapatite and poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) for bone reconstruction. Biomaterials. 2005;26:899–904. doi: 10.1016/j.biomaterials.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 38.Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules. 2006;7:2249–58. doi: 10.1021/bm060317c. [DOI] [PubMed] [Google Scholar]
- 39.Evans MD, Steele JG. Polymer surface chemistry and a novel attachment mechanism in corneal epithelial cells. J Biomed Mater Res. 1998;40:621–30. doi: 10.1002/(sici)1097-4636(19980615)40:4<621::aid-jbm14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 40.Hu SG, Jou CH, Yang MC. Protein adsorption, fibroblast activity and antibacterial properties of poly (3-hydroxybutyric acid-co-3-hydroxyvaleric acid) grafted with chitosan and chitooligosaccharide after immobilized with hyaluronic acid. Biomaterials. 2003;24:2685–93. doi: 10.1016/s0142-9612(03)00079-6. [DOI] [PubMed] [Google Scholar]
- 41.Chen C, Zhou XS, Zhuang YG, Dong LS. Thermal behavior and intermolecular interactions in blends of poly (3-hydroxy-butyrate) and maleated poly(3-hydroxybutyrate) with chito-san. J Appl Polym Sci. 2004;10:35–47. [Google Scholar]
- 42.O’Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433–41. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 43.Indolfi L, Baker AB, Edelman ER. The role of scaffold microarchitecture in engineering endothelial cell immunomodulation. Biomaterials. 2012;33:7019–27. doi: 10.1016/j.biomaterials.2012.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma PX, Langer R. Fabrication of biodegradable polymer foams for cell transplantation and tissue engineering. Methods Mol Med. 1999;18:47–56. doi: 10.1385/0-89603-516-6:47. [DOI] [PubMed] [Google Scholar]
- 45.Vaccaro AR. The role of the osteoconductive scaffold in synthetic bone graft. Orthopedics. 2002;25(5 Suppl):s571–8. doi: 10.3928/0147-7447-20020502-05. [DOI] [PubMed] [Google Scholar]
- 46.Foroughi MR, Karbasi S, Kahrizsangi RE. Physical and mechanical properties of a poly-3-hydroxybutyrate-coated nanocrystalstaline hydroxyapatite scaffold for bone tissue engineering. J Porous Master. 2012;19:667–675. [Google Scholar]
- 47.Montazeri M, Karbasi S, Foroughi MR, Monshi A, Ebrahimi-Kahrizsangi R. Evaluation of mechanical property and bioactivity of nano-bioglass 45S5 scaffold coated with poly-3-hydroxybutyrate. J Mater Sci Mater Med. 2015;26:62. doi: 10.1007/s10856-014-5369-z. [DOI] [PubMed] [Google Scholar]
- 48.Saadat A, Behnamghader A, Karbasi S, Abedi D, Soleimani M, Shafiee A. Comparison of acellular and cellular bioactivity of poly 3-hydroxybutyrate/hydroxyapatite nanocomposite and poly 3-hydroxybutyrate scaffolds. Biotechnology and bioprocess engineering. 2013 Jun 1;18(3):587–93. [Google Scholar]
- 49.Zhang S, Prabhakaran MP, Qin X, Ramakrishna S. Poly-3-hydroxybutyrate-co-3-hydroxyvalerate containing scaffolds and their integration with osteoblasts as a model for bone tissue engineering. J Biomater Appl. 2015;29:1394–406. doi: 10.1177/0885328214568467. [DOI] [PubMed] [Google Scholar]