Abstract
Cognitive processing is highly dependent on the functional integrity of gamma-amino-butyric acid (GABA) interneurons in the brain. These cells regulate excitability and synaptic plasticity of principal neurons balancing the excitatory/inhibitory tone of cortical networks. Reduced function of parvalbumin (PV) interneurons and disruption of GABAergic synapses in the cortical circuitry result in desynchronized network activity associated with cognitive impairment across many psychiatric disorders, including schizophrenia. However, the mechanisms underlying these complex phenotypes are still poorly understood. Here we show that in animal models, genetic deletion of fibroblast growth factor 14 (Fgf14), a regulator of neuronal excitability and synaptic transmission, leads to loss of PV interneurons in the CA1 hippocampal region, a critical area for cognitive function. Strikingly, this cellular phenotype associates with decreased expression of glutamic acid decarboxylase 67 (GAD67) and vesicular GABA transporter (VGAT) and also coincides with disrupted CA1 inhibitory circuitry, reduced in vivo gamma frequency oscillations and impaired working memory. Bioinformatics analysis of schizophrenia transcriptomics revealed functional co-clustering of FGF14 and genes enriched within the GABAergic pathway along with correlatively decreased expression of FGF14, PVALB, GAD67 and VGAT in the disease context. These results indicate that Fgf14−/− mice recapitulate salient molecular, cellular, functional and behavioral features associated with human cognitive impairment, and FGF14 loss of function might be associated with the biology of complex brain disorders such as schizophrenia.
Introduction
Psychiatric diseases such as schizophrenia, depression and bipolar disorder are associated with cognitive deficits thought to arise from an imbalance between the excitatory and inhibitory (E/I) tone in cortical circuits.1, 2 Fast-spiking GABAergic parvalbumin (PV) interneurons have a fundamental role in maintaining E/I balance, controlling excitability and shaping the synaptic plasticity of principal neurons.3 Even though disrupted function of other types of inhibitory neurons has been associated with the etiology of brain disorders, changes in PV neurons are one of the most consistent associations. In animal models, reduced function of PV neurons results in desynchronized network activity, decreased gamma frequency oscillations and cognitive deficits, phenotypes that mimic clinical presentation and post-mortem tissue studies of virtually all human brain disorders associated with disrupted cognition.1, 4, 5, 6, 7, 8, 9, 10
In schizophrenia and bipolar patients, the number of PV interneurons and the expression level of molecular components of GABAergic synapses, such as glutamic acid decarboxylase 67 (GAD67) and vesicular gamma-amino-butyric acid (GABA) transporter (VGAT), are found decreased in post-mortem brains leading to the ‘GABA hypofunction hypothesis' as a potential etiology.1, 11, 12, 13, 14, 15, 16 Yet, the molecular understanding of how such a detrimental loss of the GABAergic system might lead to corrupted cortical networks manifesting in disease, remains poorly explored.
Recent large-scale genome-wide association studies identified FGF14 as locus of single-nucleotide polymorphisms and as such a potential disease-associated gene for schizophrenia, bipolar disease, depression, epilepsy and addictive behaviors,17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 corroborating initial reports of missense mutations in FGF14 as genetic links to the neurodegenerative disorder, spinocerebellar ataxia 27.28, 29 These results highlight the possibility of an as yet undiscovered and pivotal role for the FGF14 gene in psychiatric disorders.
Highly expressed in the central nervous system, FGF14 is an accessory protein of voltage-gated Na+ (Nav) channels at the axonal initial segment (AIS),30, 31, 32, 33, 34, 35, 36 a regulator of neuronal excitability,30, 37, 38, 39 a presynaptic organizer of glutamatergic synapses,40, 41 a scaffolding molecule for kinase signaling pathways33, 34, 42, 43 and a regulator of synaptic plasticity.41, 44, 45 At the circuitry and behavioral level, genetic deletion of FGF14 in Fgf14−/− mice results in presynaptic structural deficits of CA3–CA1 hippocampal synapses, decreased long-term potentiation, and cognitive deficits, aberrant responses to epileptic agents and decreased threshold for seizure induction.41, 44, 45 Furthermore, Fgf14−/− mice exhibit abnormal locomotor activity explained by a reduced response to dopamine receptor D2 agonists in the basal ganglia.45 Both phenotypes in the hippocampus and the basal ganglia could imply an impaired GABAergic tone supporting the emerging view of a tight correlation between the dopamine and the GABAergic systems.46, 47 Yet, evidence of FGF14 control of GABAergic inhibitory transmission is lacking.
Using a combination of confocal microscopy, patch-clamp electrophysiology, in vivo local field potential recordings and behavioral studies, we discovered that genetic deletion of Fgf14 in rodents leads to a reduced number of PV interneurons, decreased expression of GAD67 and VGAT, and reduced GABAergic transmission in CA1 pyramidal neurons associated with impaired gamma oscillations and working memory. Further, bioinformatics analysis of schizophrenia transcriptomics confirms FGF14 functional clustering with GABAergic synaptic signaling and identifies genetic covariance of FGF14, PVALB, GAD67 and VGAT in the disease, supporting Fgf14−/− mice as an attractive model to interrogate the biology of complex brain disorders associated with disrupted cognitive circuitry such as schizophrenia.
Materials and Methods
Animals
The primary Fgf14−/− colony was maintained at the animal facilities of the University of Texas Medical Branch and of the University of Torino following approved protocols. Genotypes were confirmed by in-house PCR analysis or by Charles River Laboratories International (Houston, TX, USA). Description of the animal husbandry is given in the Supplementary Material.
Immunofluorescence
Brain tissue derived from Fgf14−/− and Fgf14+/+ 4- to 5-month-old male littermates was processed for immunofluorescence staining using either 4% paraformaldehyde or liquid nitrogen vapor fixation followed by permeabilization, blocking, and primary and Alexa-conjugated secondary antibody staining as previously described48 and outlined in the Supplementary Material.
Data acquisition and image analysis
Multichannel confocal images were acquired using a Zeiss LSM-510 META (Carl Zeiss Microscopy, Jena, Germany) confocal microscope as previously described.49 Detailed information on image quantification can be found in the Supplementary Material.
Western blotting
Tissue homogenate preparation and western blotting were carried out as previously described50 and outlined in the Supplementary Material.
Whole-cell patch-clamp electrophysiology
Horizontal hippocampal slices were prepared from 4- to 5-month-old Fgf14−/− and Fgf14+/+ male littermates, and whole-cell patch-clamp recordings were performed at room temperature from CA1 pyramidal neurons using standard parameters previously described51, 52 and outlined in the Supplementary Material.
In vivo electroencephalogram
Extracellular field potentials were recorded in freely behaving mice using stainless steel electrodes stereotaxically implanted in the CA1 of the right hippocampus.53 Spectrograms were calculated using the software NeuroExplorer (Nex Technologies, Madison, AL, USA).
Behavioral tests
Tests were performed in Fgf14−/− and Fgf14+/+ 2- to 4-month-old males using the 4–8 version of the eight-arm radial maze.54 A detailed description of behavioral experiments and analysis are part of the Supplementary Material.
Human studies
As a computational gene co-expression search engine, SEEK (http://seek.princeton.edu/) was applied to identify a gene network co-expressed with FGF14. Functional annotation and pathway enrichment was performed with a built-in Gene Ontology and KEGG terms. Enriched data sets were downloaded from NCBI Gene Expression Omnibus, and the differential gene expression was analyzed by R Limma packages described in the Supplementary Material. We also applied GeneMANIA and STRING to the analysis of pathway enrichment and protein–protein interaction for the co-expressed genes of FGF14 in hippocampus. The method and result are detailed in the Supplementary Material.
Results
Anatomical abnormalities found in the CA1 hippocampal region of post-mortem brains from schizophrenia patients have been identified as an index of the disease severity and treatment responsiveness.55 These studies along with the reported association between hippocampal PV neurons with cognitive function in the normal brain and in schizophrenia, prompted us to begin our investigations in the CA1 hippocampal region.15, 16, 46, 47, 55 Thus, we first asked whether FGF14 was expressed in PV interneurons in the CA1 region. Confocal imaging confirmed that FGF14 is expressed at the AIS of cells across all CA1 sublayers (Figure 1a), including PV-positive neurons (Figure 1b). Therefore, we posited whether genetic ablation of Fgf14 might have physiological consequences for these inhibitory neurons.
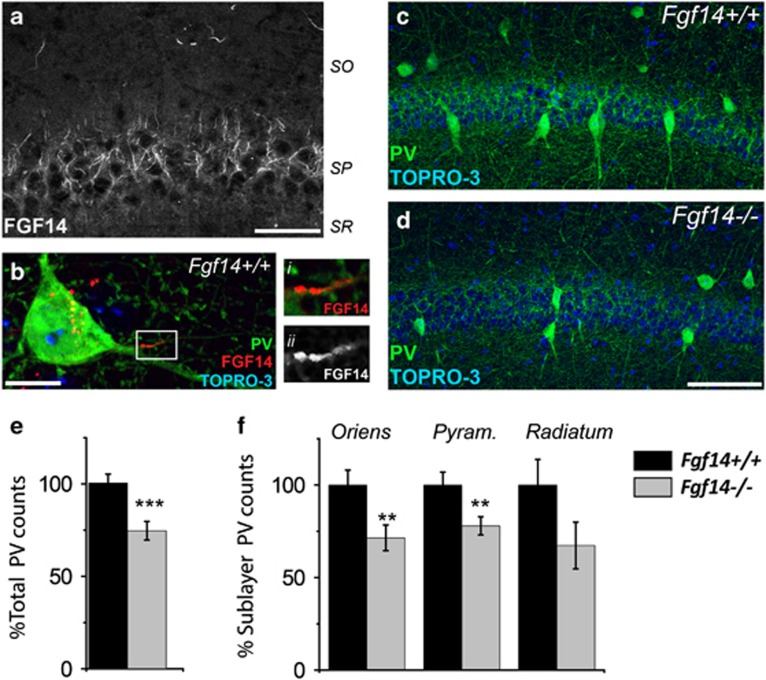
Figure 1.
Genetic deletion of Fgf14 results in structural changes in the CA1 parvalbumin (PV) interneurons. (a) FGF14 immunoreactivity is detectable at the axonal initial segment (AIS) of cells in CA1. (b) FGF14 (red) expressed in the soma and AIS of PV interneurons (green), i and ii represent zooms of the boxed area. (c, d) PV interneurons in the CA1 region of Fgf14+/+ and Fgf14−/− mice and respective higher resolution views of PV somas (i and ii). (e, f) Quantification of total PV interneurons in CA1 (380 cells in Fgf14+/+ and 282 in Fgf14−/−), and in specific subfields (oriens, pyramidalis and radiatum). Data represent mean±s.e.m., ***P<0.001; **P<0.02; *P<0.05 statistical differences were assessed by Student's t-test or non-parametric Mann–Whitney test. Scale bars, 40 μm (a); 10 μm (b); 100 μm (d).
To test this hypothesis, we first examined whether the overall number of PV interneurons in the CA1 area was affected by genetic loss of FGF14. Cell count quantification based on immunofluorescence staining revealed that the total number of PV-positive cells was significantly reduced in Fgf14−/− mice compared with wild-type controls (Figures 1c–e; 74.21%±5.02, 100%±4.89, P<0.001) with a most pronounced and significant reduction in the stratum oriens (SO; 71.42%±6.96, 100%±8.10, P<0.05) and in stratum pyramidalis (SP; 77.94%±4.96, 100%±7.06, P<0.05; Figure 1f). Notably, the expression level of PV per cell was not significantly different across groups (P=0.78, n=4 littermate mice; Supplementary Figures 1F–I); the neuropil composed of PV-positive neurites in either SP or SO was also unaffected by Fgf14 deletion (P=0.49 and 0.28; Supplementary Figures 1A–E). Furthermore, the total number of cells in the CA1 SP, which primarily includes pyramidal neurons, was also unchanged (P=0.24; Figures 1j–l). Thus, we concluded that deletion of Fgf14 leads to a cell- and subfield-specific reduction in the number of PV neurons in the CA1 hippocampal region.
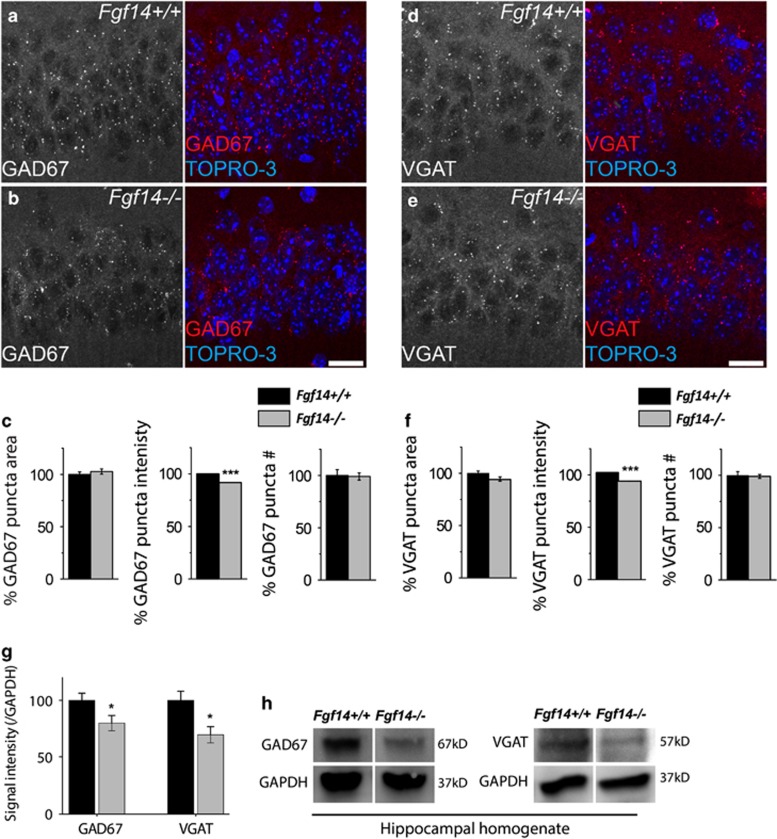
PV interneurons are the primary source of GABAergic synapses in the CA1 region. Reduction in GABA synthesis and its synaptic release machinery accompany loss of PV neurons in schizophrenia and other psychiatric disorders.4, 5, 6 Consistent with this, GAD67 mean fluorescence intensity in PV soma, the primary source of the enzyme pool, and in puncta across SP was significantly reduced in Fgf14−/− mice compared with littermate control (89.85%±3.08, 100%±3.96, P<0.05; and 91.86%±0.10, 100%±0.08, P<0.0001, respectively; Supplementary Figures 2A–D and Figures 2a–c). The area per puncta and total puncta number, though, were unchanged (P=0.10 and 0.88, respectively; Figure 2c). An additional marker and key regulatory protein at presynaptic GABAergic inputs is VGAT, the vesicular transporter that loads presynaptic vesicles with GABA, another marker associated with brain disorders.56, 57, 58 Along with GAD67, the mean fluorescence intensity of VGAT in PV soma and its mean content per puncta in SP were significantly decreased in Fgf14−/− mice compared with littermate controls (88.12%±3.06 vs 100%±3.18, P<0.01, n=4 littermate mice and 91.92%±0.14 vs 100%±0.135, P<0.001, n=3 littermate mice, respectively; Supplementary Figures 2E–H and Figures 2d–f), while puncta area and number were unchanged (P=0.66 and 0.79, n=3 mice; Figure 2f). Consistent with these results, Western blot analyses of hippocampal cell lysate revealed a significant reduction of GAD67 and VGAT protein content in Fgf14−/− animals compared with wild type (Figures 2g and h).
Figure 2.
The effect of Fgf14 genetic ablation on GABAergic presynaptic proteins in the hippocampal CA1 region. (a, b) GAD67 representative puncta in CA1 stratum pyramidalis (SP) at high magnification. (c) Quantification of GAD67 puncta area, puncta intensity and puncta number in the indicated genotypes. (d, e) Vesicular GABA transporter (VGAT) representative puncta in CA1 SP at high magnification. (f) Quantification of VGAT puncta area, puncta intensity and puncta number in the indicated genotypes. (g) Quantitative western blot analysis of GAD67 and VGAT from the hippocampus. (h) Immunoblot detection of GAD67 and VGAT in whole hippocampal homogenates from Fgf14−/−mice and Fgf14+/+ controls. Data represent mean±s.e.m., ***P<0.001; **P<0.02; *P<0.05 statistical differences were assessed by Student's t-test or non-parametric Mann–Whitney test. Scale bars, 20 μm (a, d). GABA, gamma-amino-butyric acid; GAD67, glutamic acid decarboxylase 67.
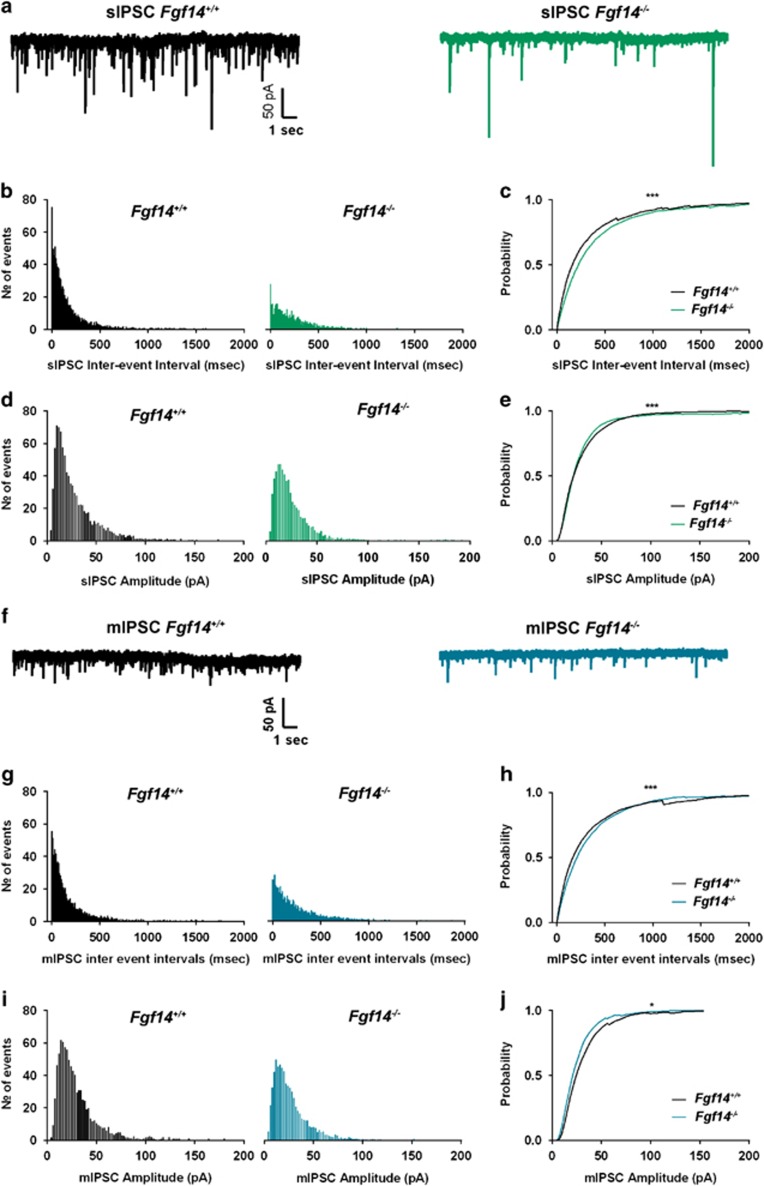
PV interneurons contribute greatly to the integrity of the GABAergic circuit in the CA1 region, posing the question of whether Fgf14 deletion might result in functional consequences for inhibitory transmission. Using patch-clamp electrophysiology, we recorded spontaneous and miniature inhibitory postsynaptic currents (sIPSCs and mIPSCs, respectively) from visually identified CA1 pyramidal neurons in Fgf14−/− and wild-type control mice (Figures 3a and f). We found that genetic deletion of Fgf14 led to a rightward shift in the probability distribution of sIPSC frequency (apparent loss in the higher frequency domain) accompanied by a reduction in the largest- and smallest-size synaptic event population (Figures 3c–e; P<0.001, Kolmogorov–Smirnov test). Spontaneous IPSCs are action potential-driven synaptic events and as such represent a compound readout of the firing status of presynaptic interneurons and their neurotransmitter release machinery. To examine GABA release mechanisms independently from interneuron spontaneous firing, we recorded mIPSCs in the presence of tetrodotoxin and found that, similarly to sIPSCs, the frequency distribution histograms showed lowered probability of short inter-event intervals in Fgf14−/− when compared with wild-type control mice (Figures 3f–h, P<0.001 with Kolmogorov–Smirnov test). This phenotype was paralleled by a loss of large- and small-amplitude mIPSCs in Fgf14−/− compared with wild-type control mice (Figures 3f, i and j). In both sIPSCs and mIPSCs, the averaged sIPSC frequency and amplitude between the two groups were not significantly different (Supplementary Figure 3) and no changes in rise and decay time were found across synaptic events (both sIPSCs and mIPSCs) of different genetic groups (Supplementary Figure 4). Thus, genetic deletion of Fgf14 leads to functional changes in the CA1 inhibitory circuitry that support the structural alterations demonstrated in Figures 1 and 2. Moreover, it suggests loss of interneuron firing (shift in sIPSC frequency) and pre- and postsynaptic modifications at GABA synapses (shift in frequency and amplitude distribution of mIPSCs, respectively).
Figure 3.
Genetic deletion of Fgf14 impairs GABAergic transmission in the CA1 region. Representative traces of whole-cell patch-clamp recordings showing effect of Fgf14 ablation on spontaneous inhibitory postsynaptic current (sIPSCs) (a) and miniature inhibitory postsynaptic current (mIPSCs) (f). (b) Inter-event-interval distribution of spontaneous GABAergic events in Fgf14+/+ (n=8 cells) and Fgf14−/−(n=10 cells) mice. (c) Inter-event-interval cumulative distribution plot for Fgf14+/+ and Fgf14−/−(sIPSCs; ***P<0.001, Kolmogorov–Smirnov test). (d) Amplitude distribution of spontaneous GABAergic events in Fgf14+/+ (n=8 cells) and Fgf14−/− (n=10 cells) mice. (e) Amplitude cumulative distribution plot for Fgf14+/+ and Fgf14−/− sIPSCs (***P<0.001; Kolmogorov–Smirnov test). (g) Inter-event-interval distribution of miniature GABAergic events in Fgf14+/+ (n=6 cells) and Fgf14−/− (n=7 cells) mice. (h) Inter-event-interval cumulative distribution plot for Fgf14+/+ and Fgf14−/− mIPSCs (***P<0.001 with Kolmogorov–Smirnov test). (i) Amplitude distribution of miniature GABAergic events in Fgf14+/+ (n=6 cells) and Fgf14−/− (n=7 cells) mice. (j) Amplitude cumulative distribution plot for Fgf14+/+ and Fgf14−/− sIPSCs (*P<0.05; Kolmogorov–Smirnov test).
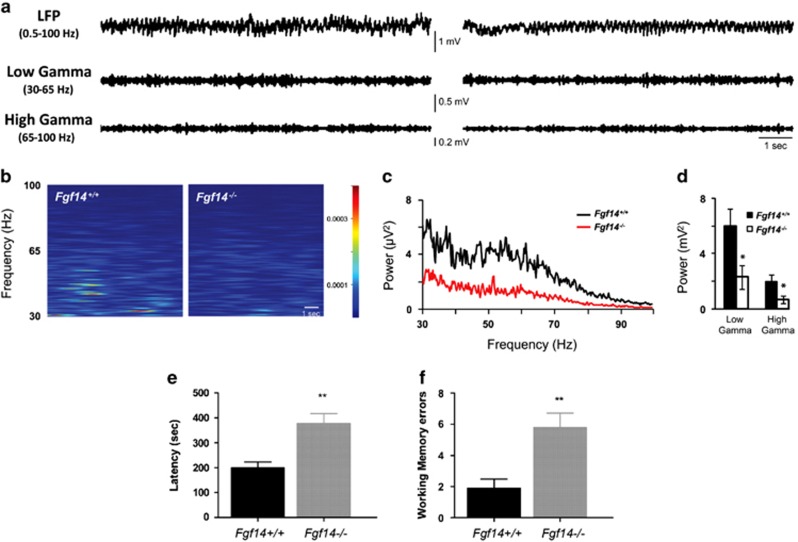
Reduced PV neuron function can desynchronize the CA1 network resulting in reduced gamma oscillations and impaired cognition. Thus, we postulated that gamma oscillations might be impaired upon ablation of Fgf14. To test this, gamma oscillations were recorded in the CA1 SR layer in Fgf14−/− and control mice by in vivo local field potential (Figure 4a). Spectral analysis within the 30–100 Hz range revealed that Fgf14−/− mice had a strong reduction in gamma oscillation power (2.94±0.11 μV2, n=7 compared with 7.92±0.17 μV2, n=7 in wild-type mice, P<0.05; Figures 4b and c) with both slow (low gamma, 30–65 Hz) and fast (high gamma, 65–100 Hz) gamma oscillations significantly impaired in Fgf14−/− mice compared with wild type (Fgf14−/−−/−, 2.29±0.85 and 0.65 ±0.25 μV2; wild-type control, 5.97±1.2 and 1.95±0.5 μV2; Figure 4d). Thus, consistent with disrupted GABAergic transmission, spectral analysis confirmed that FGF14 is required for the integrity of the cognitive circuitry. The combination of phenotypes observed in Fgf14−/− mice (Figures 1) has been associated with deficits in spatial working memory in animal models and patients afflicted with schizophrenia.1, 58, 59 Thus, we next evaluated the spatial working memory performance of Fgf14−/− mice using the eight-arm maze test. Analysis of the latency to perform the task showed that Fgf14−/− mice required a longer time to complete the task than wild type (n=20 wild type and n=19 Fgf14−/−, P<0.001; Figure 4e). Furthermore, revisiting errors (Figure 4f), which are directly linked to working memory performance, were more frequent in Fgf14−/− mice than in wild types (P<0.05), corroborating the cellular and functional phenotypes associated with genetic deletion of Fgf14.
Figure 4.
Genetic deletion of Fgf14 reduces gamma frequency and affects working memory. (a) Representative traces of 10-s in vivo electroencephalogram recordings in the CA1 region of hippocampus (local field potential; LFP) in Fgf14+/+ (left) and Fgf14−/− mice (right); filtered traces within low- and high-gamma band are also shown. (b) Spectrogram analysis of the above-mentioned traces in the gamma range (30–100 Hz). (c) Mean power spectral density of CA1 activity showing a marked decrease in gamma power in Fgf14−/− (n=7) with respect to Fgf14+/+ (n=7) mice, as revealed by power analysis within both low- and high gamma (d). (e) Fgf14−/− mice required a longer time to perform the eight-arm maze test (n=20 wild type and n=19 Fgf14−/−; P<0.001, t-test). (f) Analysis of working memory errors committed during the test day showed a significant difference between genotypes (P<0.05, t-test). Data are expressed as mean±s.e.m. *P<0.05.
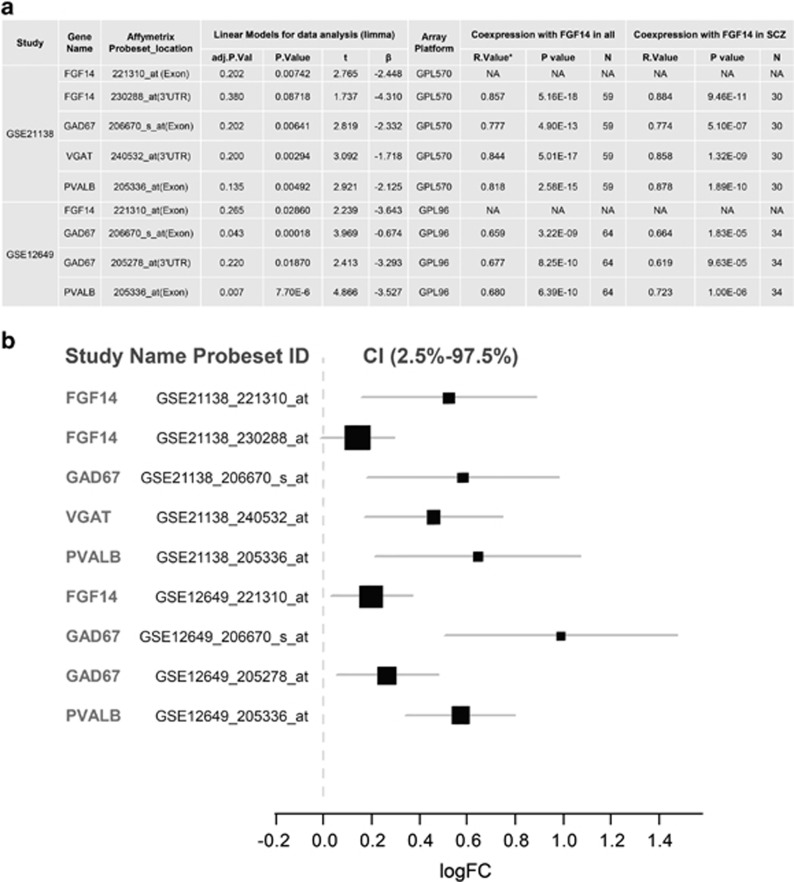
To provide translational value to our studies, we examined large transcriptomic data sets from schizophrenia post-mortem tissues deposited in the NCBI Gene Expression Omnibus, seeking genes whose expression might covary with that of FGF14. Through the SEEK-based gene co-expression search engine with built-in functional annotation and pathway enrichment by Gene Ontology terms (Supplementary Table 2) and by KEGG ortholog analysis, we found that FGF14 was enriched within the ‘GABAergic synapse' pathway (Supplementary Table 1) and its expression profile correlated with that of PVALB (P=0.004 in hippocampus; P=0.0059 in prefrontal cortex), GAD67 (P=0.0009 in hippocampus; P=0.0003 in prefrontal cortex) and VGAT (P=0.04 in hippocampus; P=0.069 in prefrontal cortex) (Supplementary Table 3) in tissue/disease-specific conditions including schizophrenia. Western blot analysis confirmed a significant reduction of GAD67 and VGAT protein expression in the hippocampus (Figures 2g and h) as well as in the prefrontal cortex (Supplementary Fig.5A, B) in Fgf14−/− mice compared with controls, providing further correlations between our mouse model and human studies. We subsequently analyzed expression of the FGF14, PVALB, GAD67 and VGAT genes and their correlations in two schizophrenia-enriched data sets and matched controls from the dorsal lateral prefrontal cortex.60, 61 Both data sets showed a significant decreased expression of FGF14, PVALB, GAD67 and VGAT (Figure 5a and Supplementary Figure 6), and a highly significant correlation between FGF14, PVALB, GAD67 and VGAT was found in all samples and in schizophrenia alone with the largest effect size in schizophrenia and controls for GAD67 (Figure 5b). The top enriched pathway such as ‘GABAergic synapse' was also confirmed by another pathway enrichment tool, GeneMANIA and the GABAergic gene cluster was identified by STRING as showed in Supplementary Table 4 and Supplementary Figure 8.
Figure 5.
Differential gene expression and correlation of FGF14, PVALB, VGAT and GAD67 in post-mortem control and schizophrenia samples. (a) The GSE21138 and GSE12649 data sets are both derived from previous studies60, 61 and deposited in NCBI Gene Expression Omnibus (GEO). Owing to significant deviation from the mean (>2 s.d.) in FGF14 gene expression (221310_at), five samples were removed from the GSE12649 data set. GPL96 represents Affymetrix Human Genome U133A Array; GPL570 represents Affymetrix Human Genome U133 Plus 2.0 Array. GPL96 had no probeset selected for VGAT (SLC32A1). The original P-value was adjusted by Benjamini and Hochberg (false discovery rate). The R-value represents a Pearson Correlation with significance at the 0.01 level (two-tailed). (b) Forest plot illustrates the effect size (logFC) of differential gene expression of FGF14, PVALB, VGAT and GAD67 between controls and schizophrenia patients in post-mortem dorsolateral prefrontal cortex (DLPFC) (BA46). Linear model and empirical Bayes method (Limma) was applied for assessing the differential gene expression of FGF14 and its co-expression genes, including PVALB, GAD67 and VGAT, in two independent data sets (GSE21138 and GSE12649) deposited in NCBI GEO. LogFC>0 suggest decreased gene expression in patients with schizophrenia. The forest plot was created by R rmeta package. VGAT, vesicular gamma-amino-butyric acid transporter.61, 62, 63, 64
Discussion
Here we provide new evidence for FGF14 in maintaining GABAergic activity in the CA1 hippocampal region, an area critical for cognitive function.65 Genetic deletion of Fgf14 leads to a decrease in the number of PV interneurons and in the expression level of the presynaptic GABAergic markers GAD67 and VGAT. These changes are associated with reduced inhibitory tone of pyramidal neurons, decreased gamma frequency oscillations and deficits in working memory. Bioinformatics analysis from human transcriptomics identified FGF14 as a component of GABAergic synaptic signaling and revealed a correlated decrease in FGF14, PVALB, GAD67 and VGAT gene expression in schizophrenia post-mortem tissues compared with matched controls. These results provide a new mechanistic role for FGF14, an emerging neuropsychiatric disease-associated gene17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 in the context of human brain disorders.
The identification of FGF14 immunoreactivity at the AIS of CA1 PV interneurons suggests this protein contributes to interneuron structural and functional diversity66 and as such might be part of the repertoire of signaling molecules dictating the cell cardinal and definitive specifications of PV neurons in the neural circuitry.1, 67 The cell type and sublayer-specific loss of PV neurons observed upon Fgf14 deletion confirms this hypothesis and supports the notion that FGF14 is indispensable for the development, maintenance and/or survival of PV interneurons in the CA1 region. Our post-mortem human tissue study identifies a significant correlation between the Fgf14 and PVALB genes, which might contribute to the loss of PV interneurons observed in Fgf14−/− mice.
We also found that the expression level of GAD67 and VGAT, two well-characterized disease-associated proteins critical for GABA synthesis and differentiation in addition to synaptogenesis of PV neurons,68, 69, 70, 71 are decreased in PV-positive somas and at inhibitory presynaptic terminals in Fgf14−/− animals. This phenotype is consistent with a diminished total pool of the two proteins, which might result from covariance of FGF14, GAD67 and VGAT at expression level as suggested by our bioinformatics analysis from schizophrenia samples.
Whether loss of PV neurons and deficits in GABAergic markers in Fgf14−/− brains occur through causative loops or are separate coincidental events remains to be determined. Evidence exists for two separate functions of FGF14 as a regulator of intrinsic excitability at the AIS30, 33, 34, 37, 38, 42, 43 and a presynaptic organizer.40, 41 Thus, phenotypes observed in Fgf14−/− hippocampi might arise from disruption of two independent functions of FGF14: one impairing intrinsic firing of PV neurons, causing cell death, arrested development and/or aberrant circuitry integration, and one disrupting the presynaptic GABA machinery (synthesis and loading) via downregulation of selective markers (that is, GAD67 and VGAT). Convergence of these disrupted functions might have fatal consequences for the final specifications and circuitry integration in PV neurons in the CA1 region. In an Fgf14 null condition, these uncompensated functions could be aggravated by concomitant loss of excitatory inputs, leading to a global remodeling of PV innervation fields,69 shifting cortical networks into a high-PV status with reduced plasticity.68
Given that the majority of GABAergic inputs in the CA1 area arise from PV interneurons, and that GAD67 and VGAT are essential for the synthesis and loading of GABA at presynaptic terminals, we posited that Fgf14 genetic deletion might impair hippocampal inhibitory transmission. Consistently, the distribution of sIPSC frequency (and amplitude) in Fgf14−/− CA1 pyramidal neurons was found populated by longer inter-event intervals compared with Fgf14+/+ suggesting that in Fgf14−/− mice the remaining inhibitory neurons might fire less than in control animals. sIPSCs are action potential-driven synaptic events and as such represent a compound readout of the firing status of presynaptic interneurons and the neurotransmitter release machinery. To examine GABA release mechanisms independent of interneuron spontaneous firing, we isolated mIPSCs. The distributions of frequency and amplitude of mIPSCs in Fgf14−/− mice were shifted with loss in short inter-event intervals and in smallest and largest amplitude events, respectively. These results highlight a combination of pre- and postsynaptic deficits in GABAergic transmission possibly induced by lower quantum content, probability of vesicle release or reduced number of vesicle, which might arise from structural and/or functional loss of a subset of inhibitory terminals. The lack in change of rise and decay time in the Fgf14−/− mice argues against significant changes in the mechanism of diffusion of GABA across the synaptic cleft, or in the composition of postsynaptic ionotropic GABA receptors. However, high-resolution structural analysis is required for confirmation.
In cortical areas loss in PV neuron function and changes in GABAergic activity can desynchronize the E/I network leading to reduced gamma oscillations, a phenotype associated with schizophrenia and other psychiatric disorders.1, 3, 72 In vivo electroencephalogram local field potential recordings in the CA1 region revealed that in Fgf14−/− animals the total, slow and fast, gamma band power was suppressed compared with control mice. These temporally segregated gamma oscillations reflect information carried by CA3 Schaffer collaterals and perforant path inputs, respectively.73, 74, 75 Thus, a reduction in both slow and fast gamma implies that both intra- and extra-hippocampal synaptic inputs might be compromised, possibly reflecting more widespread anomalies in Fgf14−/− brains, arising from the prefrontal cortex–thalamic–hippocampal loop.76 At the behavioral level, we show that Fgf14−/− animals exhibit impaired spatial working memory, complementing the array of deficits commonly found in psychiatric disorders associated with cognitive impairment.59
Human transcriptomics data confirmed functional clustering of FGF14 with GABAergic signaling and identified a highly correlated decrease of FGF14, PVALB, GAD67 and VGAT in schizophrenia post-mortem tissues, indicating possible genetic co-regulation of these genes. Thus, diminished expression of FGF14 in humans might be a risk factor for complex brain diseases associated with cognitive impairment such as schizophrenia. These findings extend the original studies linking the dominant negative FGF14F145S missense mutation to the inherited, rare disorder SCA27 to a much broader set of human brain diseases.
The range of phenotypes from molecular to behavioral observed in Fgf14−/− mice along with our corroborating human studies lay the groundwork for new mechanistic hypotheses on the biology and potential risk factors of cognitive impairment in schizophrenia and other complex brain disorders associated with E/I tone imbalance and disrupted development and plasticity of GABAergic signaling.77 These findings further strengthen the emerging role of the AIS and its molecular components in the biology of diseases such as schizophrenia, bipolar disorder and depression.42
Acknowledgments
We thank Dr Heather Lander for proof-reading the manuscript, Dr Neli Panova-Electronova and Elizabeth Crofton for technical support. This work was supported by the Saudi Arabia PhD scholarship M256406 (MAA), the Saudi Arabia PhD scholarship M256407 (TKA), the Italy PRIN Grant 20108WT59Y (FT), the NIH/NIEHS-T32ES007254 (TFJ), NSF-DMS 1320910 (DL) and the NIH Grant R01MH095995 (FL). HYM has received grant support from Activas, the American Foundation for Suicide Prevention, Auspex, Forum, Janssen, Mag T Therapeutics, Naurex, Reviva, Sumitomo Dainippon and Sunovion.
HYM has served as a consultant for Forum, Lundbeck, Reviva and Sunovion; he owns stocks in Acadia Pharmaceuticals, GlaxoSmithKline and SureGene. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 2012; 35: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen S, Liang S, Shehktman T, Kelsoe JR, Bipolar Genome S, Greenwood TA et al. Evidence for association of bipolar disorder to haplotypes in the 22q12.3 region near the genes stargazin, IFT27 and parvalbumin. Am J Med Genet B Neuropsychiatr Genet 2012; 159B: 941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011; 477: 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, Eggan SM, Lazarus MS, Huang ZJ, Volk DW, Lewis DA. Role of glutamic acid decarboxylase 67 in regulating cortical parvalbumin and GABA membrane transporter 1 expression: implications for schizophrenia. Neurobiol Dis 2013; 50: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Lazarus MS, Stoyak SR, Volk DW, Glausier JR, Huang ZJ et al. Cortical glutamic acid decarboxylase 67 deficiency results in lower cannabinoid 1 receptor messenger RNA expression: implications for schizophrenia. Biol Psychiatry 2012; 71: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry 2008; 13: 147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Morris HM. Cell and receptor type-specific alterations in markers of GABA neurotransmission in the prefrontal cortex of subjects with schizophrenia. Neurotox Res 2008; 14: 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 2005; 6: 312–324. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 2004; 174: 143–150. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Prefrontal cortical circuits in schizophrenia. Curr Top Behav Neurosci 2010; 4: 485–508. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP et al. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology 2011; 60: 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol 1994; 341: 95–116. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry 2002; 52: 708–715. [DOI] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EFStanley Neuropathology C. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry 2004; 9: 544. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res 2002; 55: 1–10. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry 2005; 57: 252–260. [DOI] [PubMed] [Google Scholar]
- Drgon T, Zhang PW, Johnson C, Walther D, Hess J, Nino M et al. Genome wide association for addiction: replicated results and comparisons of two analytic approaches. PLoS One 2010; 5: e8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungerius BJ, Hoogendoorn ML, Bakker SC, Van't Slot R, Bardoel AF, Ophoff RA et al. An association screen of myelin-related genes implicates the chromosome 22q11 PIK4CA gene in schizophrenia. Mol Psychiatry 2008; 13: 1060–1068. [DOI] [PubMed] [Google Scholar]
- Spencer JR, Darbyshire KM, Boucher AA, Kashem MA, Long LE, McGregor IS et al. Novel molecular changes induced by Nrg1 hypomorphism and Nrg1-cannabinoid interaction in adolescence: a hippocampal proteomic study in mice. Front Cell Neurosci 2013; 7: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HM, DeLong CJ, Bame M, Rajapakse I, Herron TJ, McInnis MG et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients. Transl Psychiatry 2014; 4: e375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Enoch MA, Srivastava V, Cummins-Oman JS, Ferrier C, Iarikova P et al. Genome-wide association identifies candidate genes that influence the human electroencephalogram. Proc Natl Acad Sci USA 2010; 107: 8695–8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson H, Shen Y, Avallone J, Sheidley BR, Pinsky R, Bergin AM et al. Copy number variation plays an important role in clinical epilepsy. Ann Neurol 2014; 75: 943–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X-L, Cheng X, Cai L, Tan G-H, Xu L, Feng X-Y et al. Conditional deletion of NRSF in forebrain neurons accelerates epileptogenesis in the kindling model. Cerebr Cortex 2011; 21: 2158–2165. [DOI] [PubMed] [Google Scholar]
- Liu Q-R, Drgon T, Johnson C, Walther D, Hess J, Uhl GR. Addiction molecular genetics: 639,401 SNP whole genome association identifies many “cell adhesion” genes. Am J Med Genet 2006; 141B: 918–925. [DOI] [PubMed] [Google Scholar]
- Hunter AM, Leuchter AF, Power RA, Muthen B, McGrath PJ, Lewis CM et al. A genome-wide association study of a sustained pattern of antidepressant response. J Psychiatr Res 2013; 47: 1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek EC, Bakker IM, Bevova MR, Bochdanovits Z, Rizzu P, Sondervan D et al. A fine-mapping study of 7 top scoring genes from a GWAS for major depressive disorder. PLoS One 2012; 7: e37384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 2011; 473: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusse E, de Koning I, Maat-Kievit A, Oostra BA, Heutink P, van Swieten JC. Spinocerebellar ataxia associated with a mutation in the fibroblast growth factor 14 gene (SCA27): A new phenotype. Mov Disord 2006; 21: 396–401. [DOI] [PubMed] [Google Scholar]
- Van Swieten JC, Brusse E, De Graaf BM, Krieger E, Van De Graaf R, De Koning I et al. A mutation in the fibroblast growth factor 14 gene is associated with autosomal dominant cerebellar ataxia. Am J Hum Genet 2003; 72: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Gerber BR, Lou JY, Kozel MA, Hartman H, Craig AM et al. The FGF14(F145S) mutation disrupts the interaction of FGF14 with voltage-gated Na+ channels and impairs neuronal excitability. J Neurosci 2007; 27: 12033–12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Lampert A, Kozel MA, Gerber BR, Rush AM, Nerbonne JM et al. FGF14 N-terminal splice variants differentially modulate Nav1.2 and Nav1.6-encoded sodium channels. Mol Cell Neurosci 2009; 42: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz R, Dover K, Laezza F, Shtraizent N, Huang X, Tchetchik D et al. Crystal structure of a fibroblast growth factor homologous factor (FHF) defines a conserved surface on FHFs for binding and modulation of voltage-gated sodium channels. J Biol Chem 2009; 284: 17883–17896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavkunov A, Panova N, Prasai A, Veselenak R, Bourne N, Stoilova-McPhie S et al. Bioluminescence methodology for the detection of protein-protein interactions within the voltage-gated sodium channel macromolecular complex. Assay Drug Dev Technol 2012; 10: 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavkunov AS, Wildburger NC, Nenov MN, James TF, Buzhdygan TP, Panova-Elektronova NI et al. The fibroblast growth factor 14: voltage-gated sodium channel complex is a new target of glycogen synthase kinase 3 (GSK3). J Biol Chem 2013; 288: 19370–19385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TF, Nenov MN, Wildburger NC, Lichti CF, Luisi J, Vergara F et al. The Na1.2 channel is regulated by GSK3. Biochim Biophys Acta 2015; 1850: 832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SR, Panova N, Stoilova-McPhie S, Laezza F. Protein-protein interactions based drug discovery against the voltage-gated sodium channel. Biophys J 2014; 106: 326a–326aa. [Google Scholar]
- Shakkottai VG, Xiao M, Xu L, Wong M, Nerbonne JM, Ornitz DM et al. FGF14 regulates the intrinsic excitability of cerebellar Purkinje neurons. Neurobiol Dis 2009; 33: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M, Schoorlemmer J, Williams A, Diwakar S, Wang Q, Huang X et al. Fibroblast growth factor homologous factors control neuronal excitability through modulation of voltage-gated sodium channels. Neuron 2007; 55: 449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch MK, Carrasquillo Y, Ransdell JL, Kanakamedala A, Ornitz DM, Nerbonne JM. Intracellular FGF14 (iFGF14) is required for spontaneous and evoked firing in cerebellar Purkinje neurons and for motor coordination and balance. J Neurosci 2015; 35: 6752–6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Pablo JL, Pitt GS. FGF14 regulates presynaptic Ca2+ channels and synaptic transmission. Cell Rep 2013; 4: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M, Xu L, Laezza F, Yamada K, Feng S, Ornitz DM. Impaired hippocampal synaptic transmission and plasticity in mice lacking fibroblast growth factor 14. Mol Cell Neurosci 2007; 34: 366–377. [DOI] [PubMed] [Google Scholar]
- Hsu WC, Nilsson CL, Laezza F. Role of the axonal initial segment in psychiatric disorders: function, dysfunction, and intervention. Front Psychiatry 2014; 5: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu WC, Nenov MN, Shavkunov A, Panova N, Zhan M, Laezza F. Identifying a kinase network regulating FGF14:Nav1.6 complex assembly using split-luciferase complementation. PLoS One 2015; 10: e0117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DF, Xiao M, Xu L, Yamada KA, Ornitz DM. Impaired spatial learning and defective theta burst induced LTP in mice lacking fibroblast growth factor 14. Neurobiol Dis 2007; 1: 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Bardgett ME, Wong M, Wozniak DF, Lou J, McNeil BD et al. Ataxia and paroxysmal dyskinesia in mice lacking axonally transported FGF14. Neuron 2002; 35: 25–38. [DOI] [PubMed] [Google Scholar]
- Shah A, Lodge DJ. A loss of hippocampal perineuronal nets produces deficits in dopamine system function: relevance to the positive symptoms of schizophrenia. Transl Psychiatry 2013; 3: e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boley AM, Perez SM, Lodge DJ. A fundamental role for hippocampal parvalbumin in the dopamine hyperfunction associated with schizophrenia. Schizophr Res 2014; 157: 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshammari MA, Alshammari TK, Laezza F. Improved methods for fluorescence microscopy detection of macromolecules at the axon initial segment. Front Cell Neurosci 2016; 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshammari MA, Alshammari TK, Nenov MN, Scala F, Laezza F. Fibroblast growth factor 14 modulates the neurogenesis of granule neurons in the adult dentate gyrus. Mol Neurobiol 2015. [DOI] [PMC free article] [PubMed]
- Tempia F, Hoxha E, Negro G, Alshammari MA, Alshammari T, Panova-Elektronova N et al. Parallel fiber to Purkinje cell synaptic impairment in a mouse model of spinocerebellar ataxia type 27. Front Cell Neurosci 2015; 9: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenov MN, Laezza F, Haidacher SJ, Zhao Y, Sadygov RG, Starkey JM et al. Cognitive enhancing treatment with a PPARgamma agonist normalizes dentate granule cell presynaptic function in Tg2576 APP mice. J Neurosci 2014; 34: 1028–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenov MN, Tempia FMP, Denner L, Dineley KT, Laezza F. Impaired firing properties of dentate granule neurons in an Alzheimer's disease animal model are rescued by PPARgamma agonism. J Neurophysiol 2014; 113: 1712–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambiaghi M, Cursi M, Magri L, Castoldi V, Comi G, Minicucci F et al. Behavioural and EEG effects of chronic rapamycin treatment in a mouse model of tuberous sclerosis complex. Neuropharmacology 2013; 67: 1–7. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M et al. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J Neurosci 2007; 27: 10765–10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierhut KC, Grassmann R, Kaufmann J, Steiner J, Bogerts B, Schiltz K. Hippocampal CA1 deformity is related to symptom severity and antipsychotic dosage in schizophrenia. Brain 2013; 136(Pt 3): 804–814. [DOI] [PubMed] [Google Scholar]
- Sawada K, Barr AM, Nakamura M, Arima K, Young CE, Dwork AJ et al. Hippocampal complexin proteins and cognitive dysfunction in schizophrenia. Arch Gen Psychiatry 2005; 62: 263–272. [DOI] [PubMed] [Google Scholar]
- Sawada K, Young CE, Barr AM, Longworth K, Takahashi S, Arango V et al. Altered immunoreactivity of complexin protein in prefrontal cortex in severe mental illness. Mol Psychiatry 2002; 7: 484–492. [DOI] [PubMed] [Google Scholar]
- Hoftman GD, Volk DW, Bazmi HH, Li S, Sampson AR, Lewis DA. Altered cortical expression of GABA-related genes in schizophrenia: illness progression vs developmental disturbance. Schizophr Bull 2015; 41: 180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, Baxter MG. Animal models of working memory: A review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neurosci Biobehav Rev 2013; 37: 2111–2124. [DOI] [PubMed] [Google Scholar]
- Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B et al. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res 2008; 1239: 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Bundo M, Kato T. Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol Genet 2005; 14: 241–253. [DOI] [PubMed] [Google Scholar]
- Jimenez D, Labate D, Kakadiaris IA, Papadakis M. Improved automatic centerline tracing for dendritic and axonal structures. Neuroinformatics 2014; 13: 227–244. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M et al. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J Neurosci 2007; 27: 10765–10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B et al. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res 2008; 1239: 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, Jay TM. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol 2013; 23: 1165–1181. [DOI] [PubMed] [Google Scholar]
- Chand AN, Galliano E, Chesters RA, Grubb MS. A distinct subtype of dopaminergic interneuron displays inverted structural plasticity at the axon initial segment. J Neurosci 2015; 35: 1573–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 2008; 321: 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 2013; 504: 272–276. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y et al. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron 2007; 54: 889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E et al. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci 2004; 24: 9598–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristo G, Wu C, Chattopadhyaya B, Ango F, Knott G, Welker E et al. Subcellular domain-restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat Neurosci 2004; 7: 1184–1186. [DOI] [PubMed] [Google Scholar]
- Craig MT, McBain CJ. Fast gamma oscillations are generated intrinsically in CA1 without the involvement of fast-spiking basket cells. J Neurosci 2015; 35: 3616–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron 2003; 37: 311–322. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Suh J, Takeuchi D, Tonegawa S. Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell 2014; 157: 845–857. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 2009; 462: 353–357. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry 2010; 68: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Watson SJ, Akil H. The fibroblast growth factor family: neuromodulation of affective behavior. Neuron 2012; 76: 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.