Abstract
Lipopolysaccharide (LPS) signaling is critical for the innate immune response to gram-negative bacteria. Here, evidence is presented for LPS stimulation of sphingosine kinase (SPK) in the RAW 264.7 murine macrophage cell line and rat primary hepatic macrophages (HMs). LPS treatment of RAW 264.7 cells resulted in a time- and dose-dependent activation of SPK and membrane translocation of SPK1. Further, LPS-induced SPK activation was blocked by SPK1-specific small interfering RNA (siRNA). Overexpression of Toll-like receptor 4 and MD2, the receptor and coreceptor of LPS, in HEK 293 cells activated SPK activity in the absence of LPS treatment. Inhibition of SPK by the pharmacological inhibitor N,N-dimethylsphingosine (DMS) or SPK1-specific siRNA blocked LPS stimulation of extracellular signal-regulated kinase 1/2 and p38 but enhanced LPS-induced c-Jun N-terminal kinase activation. The SPK inhibitor DMS and dominant-negative SPK1 also blocked LPS activation of Elk-1 and NF-κB reporters in RAW 264.7 cells. Inhibition of SPK sensitized RAW 264.7 cells and HMs to LPS-induced apoptosis. These data demonstrate the critical role of SPK1 in LPS signaling in macrophages and suggest that SPK1 is a potential therapeutic target to block hyperimmune responses induced by gram-negative bacteria.
As the first line of defense against infection by microbial pathogens, the innate immune system recognizes a limited number of conserved pathogen-associated molecular patterns (22). Among these, lipopolysaccharide (LPS), the major outer membrane component in gram-negative bacteria, is the principal constituent that is recognized by the innate immune system (57). LPS activates a variety of mammalian cell types, including monocytes and macrophages (56, 57). Despite its potent stimulation of the innate immune system, the underlying mechanism of LPS signaling is not fully understood. LPS binds to LPS-binding protein in the bloodstream, followed by binding of the LPS-binding protein-LPS complex to a cell surface glycoprotein, CD14, which facilitates the transfer of LPS from extracellular space to a membrane receptor complex (56, 62). It has been shown that the Toll-like receptor 4 (TLR4), a homologue of Drosophila Toll, is responsible for LPS-specific recognition (45). Mice lacking this receptor (TLR4−/−) are hyporesponsive to LPS (20). These and other studies demonstrate that TLR4 is required for LPS signaling. Furthermore, MD-2, a glycoprotein that binds the extracellular domain of TLR4, has been shown to enhance TLR4-dependent LPS response (52, 68). Mice lacking MD-2 (MD-2−/−) do not respond to LPS due to abnormal intracellular distribution of TLR4 (40). Thus, MD-2 is also indispensable for proper LPS signaling.
The intracellular signaling molecules involved in LPS signaling are not entirely known. Downstream of the receptor, MyD88, IL-1 receptor-associated kinase, tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6), and transforming growth factor β-activated kinase 1 (TAK1) have been reported as signaling intermediates proximal to LPS receptors (3, 34). Upon LPS stimulation, TAK1 phosphorylates IκB-kinase β to activate the NF-κB pathway (59). Phosphorylation of IκBs leads to their ubiquitination and degradation, thereby producing active NF-κB (8), which mediates LPS-induced expression of many inflammatory mediators (26). LPS also activates mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinase 1/2 (ERK1/2) (61), c-Jun N-terminal kinase (JNK) (18), and p38 (19). The mechanism of this activation is not entirely known. It has been suggested that LPS activates ERK1/2 through the Ras-Raf1-mitogen-activated kinase/ERK kinase pathway in human monocytes (17). However, in human alveolar macrophages, protein kinase C zeta (PKCζ) and mitogen-activated kinase/ERK kinase have been suggested to mediate LPS-induced ERK1/2 activation (37). Upon LPS treatment, TAK1 phosphorylates MAPK kinase 6, which activates p38 and JNK (59). MAPK activation is coupled to the production of certain inflammatory cytokines by phosphorylation and activation of the transcription factor Elk-1 (55). Blocking MAPK activation with pharmacological inhibitors reduced LPS induction of some inflammatory cytokines, including IL-1, IL-8, and TNF-α (7, 23, 51).
Since macrophage play an important role in immunity, the life span of activated macrophages is carefully regulated. The role of LPS in macrophage cell death has been reported. In the presence of gamma interferon, LPS can induce macrophage apoptosis (1). On the other hand, some studies have shown that LPS can protect monocytes and macrophages from apoptosis (16, 58).
Sphingosine-1-phosphate (S1P) is a unique signaling molecule in that it can act both as an extracellular ligand for the EDG family of G-protein-coupled receptors and as an intracellular second messenger (54). S1P is synthesized from sphingosine by sphingosine kinase (SPK), which regulates the intracellular level of S1P. In mammals, two types of SPK have been identified: sphingosine kinases 1 (SPK1) (28) and 2 (SPK2) (30). Many growth factors and cytokines have been shown to activate SPK, including fetal calf serum, platelet-derived growth factor (43), nerve growth factor (14), basic fibroblast growth factor (48), TNF-α (64), IL-1β (44), and vascular endothelial growth factor (VEGF) (53). Cross-linking of immunoglobulin receptors FcγRI and FcɛRI induces SPK activation and the accumulation of intracellular S1P (9, 35). Activation of G-protein-coupled receptors, such as the m2 and m3 muscarinic acetylcholine receptors, also induces SPK activation (36). PKC has been implicated in some receptor-mediated events leading to SPK activation (4, 12, 53). In many of the above signaling pathways, ERK1/2 activation relies on SPK activity (47, 64). In endothelial cells and tumor cells, we have found that SPK mediates VEGF-induced activation of Ras, Raf, and ERK1/2 by downregulating Ras-GTPase-activating protein activity (53, 63). The SPK signaling pathway also mediates transcription factor activation by various growth factors and cytokines. In human umbilical vein endothelial cells (HUVECs), SPK mediates NF-κB activation by TNF-α (64).
It is known that cell proliferation and survival are enhanced by S1P produced by SPK1 but not that produced by SPK2, whereas the S1P precursors ceramide and sphingosine are associated with apoptosis and cell growth arrest (11, 21, 31). The relative intracellular ratio between S1P and sphingosine is believed to determine the cell fate (54). Therefore, activation of SPK1 protects cells from apoptosis. For instance, TNF-α activates sphingosine kinase and protects HUVEC cells from apoptosis (65). In contrast, TNF-α induces apoptosis in HUVECs that are pretreated with an SPK inhibitor (66).
The TLR4 and interleukin 1 (IL-1) receptors share a similar cytoplasmic domain of approximately 200 amino acids (Toll/IL-1 receptor homologous region) (2). The reported role of IL-1 as an SPK stimulator (44) prompted us to determine whether LPS signaling might also be mediated through SPK activation. We conducted our study on RAW 264.7 murine macrophage cell line and rat primary hepatic macrophages (HMs) (or Kupffer cells). The latter type accounts for 70 to 80% of total macrophages (67). Our data show that SPK is activated by LPS and mediates LPS-induced cell survival in RAW 264.7 cells and HMs. Further, our data show that LPS treatment of RAW 264.7 cells does not induce apoptosis; however, LPS treatment induces apoptosis of RAW 264.7 cells that are pretreated with inhibitors of SPK. These data highlight the important role of SPK1 in LPS modulation of cell survival and production of inflammatory cytokines.
MATERIALS AND METHODS
Reagents.
Cell culture media were purchased from Invitrogen. LPS (from Escherichia coli 0127:B8), phorbol 12-myristate 13-acetate (PMA), and methylthiazoletetrazolium (MTT) were purchased from Sigma. N,N-dimethylsphingosine (DMS), sphingosine, S1P, and pertussis toxin (PTX) were purchased from CalBiochem. [γ-32P]ATP was purchased from Perkin-Elmer Life and Analytical Sciences. Human recombinant CD14 was purchased from R&D System.
Antibodies.
Antibodies to total and phosphorylated ERK1/2, JNK, and p38 were purchased from Cell Signaling Technology. The antibody to β-actin was purchased from Sigma. The antibody to caveolin was from BD Biosciences. Rabbit polyclonal antibody to mouse SPK1 was a generous gift from Yoshiko Banno (Gifu University School of Medicine, Gifu, Japan) and was described previously (39). Another rabbit polyclonal antibody to SPK1 was prepared by Proteintech Group. The antibody was raised against the synthetic peptide RSEELGRWDALVVMS conjugated to keyhole limpet hemocyanin. Rabbit polyclonal antibody to SPK2 was a generous gift from Shun-ichi Nakamura (Kobe University Graduate School of Medicine, Kobe, Japan) and described previously (21). All secondary antibodies used for Western blotting were purchased from Santa Cruz Biotechnology.
Cell cultures.
RAW 264.7 cell, a murine macrophage cell line, was obtained from Alan Epstein (University of Southern California) and maintained in RPMI 1640 with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin (Pen-Strep) at 37°C with 5% CO2. For serum starvation, RAW 264.7 cells were cultured in RPMI 1640 medium with 0.5% FBS and 1% Pen-Strep. Primary HMs, i.e., Kupffer cells, were isolated from male Wistar rats by the Nonparenchymal Liver Cell Core of the USC-UCLA Research Center for Alcoholic Liver and Pancreatic Diseases as described previously (67). HMs were maintained in Dulbecco's modified Eagle's medium (DMEM) high-glucose medium with 5% FBS and 1% Pen-Strep and used within 3 days of isolation. For serum starvation, HMs were cultured in DMEM high-glucose medium with 0.5% FBS and 1% Pen-Strep. HEK 293 parental cells (HEK), HEK 293 cells stably transfected with a TLR4 expression vector (HEK-TLR4), and HEK 293 cells stably transfected with both TLR4 and MD2 expression vectors (HEK-TLR4/MD2) were provided by Jesse Chow (Eisai Research Institute) (68). All three cell lines were also stably transfected with an NF-κB-dependent ELAM-1 luciferase reporter plasmid (pELAM-luc). HEK, HEK-TLR4, and HEK-TLR4/MD2 cells were maintained in DMEM high-glucose medium with 10% FBS and 1% Pen-Strep. For serum starvation, HEK, HEK-TLR4, and HEK-TLR4/MD2 cells were cultured in DMEM high-glucose media with 0.5% FBS and 1% Pen-Strep.
SPK assay.
RAW 264.7 cells were cultured to confluence in 12- or 6-well plates and serum starved overnight. Ten million HMs were seeded in 60-mm plates, cultured for 2 days, and then serum starved for 4 h. Cells were stimulated with various amounts of LPS. At the indicated times, cells were harvested and SPK activities in cell extracts were determined as described previously (53).
Assay of total cellular sphingosine and S1P levels.
Cells were cultured to confluence in 100-mm plates and serum starved with RPMI 1640 medium containing 0.5% FBS overnight. One set of cells was treated with 1 μg of LPS/ml for various times. Then, the cellular sphingosine and S1P levels were measured as described previously (53).
Western blot.
Cell lysates (about 20 μg of total protein) were separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE) and transferred to an Immun-Blot polyvinylidene difluoride membrane (Bio-Rad). The membrane was first blocked in 5% milk for 1 h and then incubated overnight with primary antibody (1:2,000) at 4°C. After three washes with TTBS (20 mM Tris, 500 mM NaCl, 0.1% Tween 20 [pH 7.5]), the membrane was incubated with alkaline phosphatase-conjugated secondary antibody (1:5,000 to 1:10,000 dilution) for 1 h at room temperature. The membrane was then extensively washed with TTBS and developed with Super-Signal West Femto maximum-sensitivity substrate (Pierce).
Cell fractionation.
RAW 264.7 cells were cultured to confluence in 100-mm dishes. Cells were serum starved overnight and then stimulated with 1 μg of LPS/ml for 1 h. Next, cell fractionation was conducted as previously described (24). All fractions were subjected to Western blot analysis.
Detection of MAPK activation and IκB degradation.
RAW 264.7 cells were cultured to confluence in six-well dishes. Five million HMs were seeded in each well of a six-well dish and cultured for 2 days. Cells were then stimulated with indicated concentration of LPS for the indicated times. Where indicated, cells were preincubated with the SPK inhibitor (DMS, 10 μM) for 30 min or with PTX (100 ng/ml) for 3 h. Cells were lysed in SDS-PAGE sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 10% glycerol, 50 mM dithiothreitol, 0.01% bromophenol blue). Cell lysates (about 20 μg of total protein) were separated by SDS-10% PAGE and subjected to Western blot analysis.
siRNA transfection.
Small interfering RNA (siRNA) for mouse SPK1 (5′-GGGCAAGGCUCUGCAGCUCdTdT-3′) and control sequence (5′-GGGCAAGGCCUUGCAGCUCdTdT-3′) were synthesized by the USC/Norris Cancer Center Microchemical Core Facility. Transfection was performed with oligofectamine (Invitrogen) according to the manufacturer's instructions. siRNA (175 nm) was used in each transfection. Twenty-four to forty-eight hours after transfection, cells were assayed for SPK and MAPK activities.
DNA transfection.
For transfections, RAW 264.7 cells were grown to 60% confluence in 12- or 6-well plates in RPMI 1640 medium containing 10% FBS and transiently transfected with various vectors, using TransFast reagent (Promega) according to the manufacturer's instructions.
Luciferase assay.
The murine SPK1 dominant-negative construct was a catalytically inactive mutant (G81D) and was described previously (53). A 3×NF-κB-luciferase reporter construct (Igκ-Luc) was a generous gift from Ebrahim Zandi (University of Southern California) and has been described previously (38). pEF-β-Gal, a β-galactosidase (β-Gal) expression vector, was a generous gift from Kalle Saksela (University of Tampere, Tampere, Finland). RAW 264.7 cells were transiently transfected with the PathDetect Elk-1 trans-reporting system (Stratagene) or Igκ-Luc, along with a pEF-β-Gal vector. About 2 days after transfection, cells were stimulated with LPS (10 ng/ml) for 10 to 12 h. Reporter luciferase activity was measured using the Luciferase Assay system (Promega). The data were normalized with β-Gal activity in each sample.
The HEK 293 parental cell line, HEK-TLR4, and HEK-TLR4/MD2 were stably transfected with an NF-κB-dependent ELAM-1 luciferase reporter plasmid (pELAM-luc) (68). Cells were seeded in 48-well plates, grown to confluence, and serum starved for overnight. Cells were stimulated with 100 ng of LPS/ml and 250 ng of recombinant human CD14/ml for 18 h. Reporter luciferase activity was measured using the Luciferase Assay system (Promega).
Cell viability assay.
Cell viability was measured using the MTT dye conversion assay. RAW 264.7 cells were seeded in 48-well plates at a density of 30,000 cells/well in 0.25 ml of culture medium for 2 days. Cells were pretreated with 5 μM DMS for 30 min and then stimulated with 10 ng of LPS/ml for 24 h. Then, 100 μl of 5-mg/ml MTT was added to each well and incubated for approximately 1 to 2 h. At the end of the incubation period, the medium was removed and formazan product was solubilized with acidic isopropanol (90% isopropanol, 0.5% SDS, 40 mM HCl). The absorbance was measured at a wavelength of 570 nm with reference at 650 nm in a 96-well enzyme-linked immunosorbent assay (ELISA) plate.
Cell death detection.
Apoptotic cell death was measured with a quantitative sandwich-ELISA assay detecting mono- and oligonucleosomes (Cell Death Detection ELISAPLUS kit, from Roche). RAW 264.7 cells were seeded in 48-well plates at a density of 30,000 cells/well in 0.25 ml of culture medium for 2 days. Cells were pretreated with 5 μM DMS for 30 min and then stimulated with 10 ng of LPS/ml for 24 h. For siRNA assay, RAW 264.7 cells were seeded in 24-well plates and transfected with 175 nM siRNA as described above. One day after transfection, the RAW 264.7 cells were treated with 10 ng of LPS/ml for 24 h. HMs were seeded in 24-well plates at a density of 1 million cells/well and cultured for 2 days. HMs were then pretreated with 6 μM DMS for 30 min and stimulated with 10 ng of LPS for 24 h. Cells were then lysed on the plate, and the ELISA was performed according to manufacturer's instruction. The absorbance at 405 nm minus that at 490 nm was measured.
Caspase-3 activity assay.
Caspase-3 activity was measured with a colorimetric assay kit (Sigma). RAW 264.7 cells were seeded in 100-mm dishes at a density of 3.5 × 106 cells/dish for 1 day. Cells were pretreated with 5 μM DMS for 30 min and then stimulated with 10 ng of LPS/ml for 20 h. Then cells were lysed at a concentration of 100 million cells/ml, and the cell lysates were incubated together with acetyl-Asp-Glu-Val-Asp p-nitroanilide at 37°C according to the manufacturer's instructions. The absorbance at 405 nm was measured.
RESULTS
LPS stimulates SPK activity.
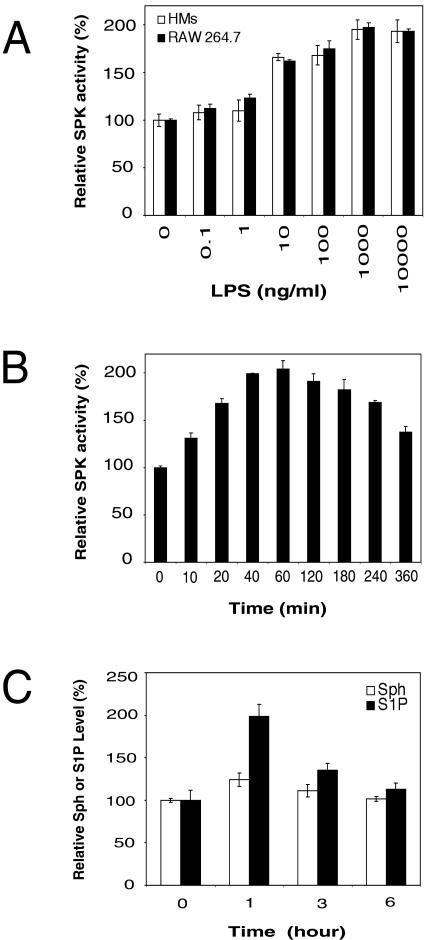
We first examined SPK activity in LPS-stimulated RAW 264.7 mouse macrophage cells and rat primary HMs. We found that LPS stimulated SPK activity in a dose-dependent manner (Fig. 1A). In these cells, SPK activity responded to LPS over a very broad concentration range (from 1 ng/ml to 10 μg/ml). In RAW 264.7 cells, LPS-induced SPK activation reached a maximum value (2.0-fold increase) at 40 to 60 min after LPS addition and decreased slightly thereafter (120 to 360 min) (Fig. 1B). The time course of this activation was longer compared to that observed with other SPK stimuli, such as TNF and VEGF, where SPK activity reaches its peak within 20 min of stimulation (53, 64).
FIG. 1.
LPS activates SPK in macrophages. (A) Dose-dependent activation of SPK by LPS. Serum-starved RAW 264.7 cells and HMs were stimulated with different doses of LPS for 40 min. Then, the SPK activity in cell lysate was assayed by measuring [γ-32P]ATP incorporation into sphingosine-1-phosphate. (For RAW 264.7 cells, relative SPK activity of 100% = 35 pmol/min/mg of protein. For HMs, relative SPK activity of 100% = 32 pmol/min/mg of protein). (B) Time course of SPK activation by LPS. RAW 264.7 cells were serum starved overnight and treated with 1 μg of LPS/ml for the indicated time period. Then, the SPK activity in cell lysate was assayed. (C) Analysis of total cellular sphingosine (Sph) and S1P levels in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells were serum starved overnight and treated with 1 μg of LPS/ml for the indicated time period, and then the relative levels of total cellular Sph and S1P were determined.
We measured the total cellular sphingosine and S1P levels in RAW 264.7 cells before and after LPS stimulation. These cells were serum starved overnight and then stimulated with LPS. Upon LPS treatment, the levels of sphingosine and S1P increased during the first hour (1.2-fold and 2.0-fold increases, respectively) and gradually decreased thereafter (at 3 and 6 h).
SPK1 is the major form of SPK activated by LPS.
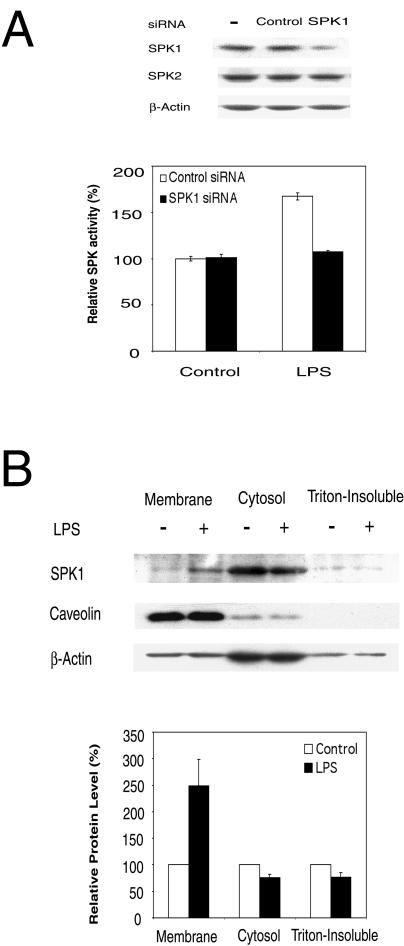
There are two isoforms of SPKs in mammalian cells, SPK1 and SPK2 (28, 30). Previous work in our lab suggested that SPK1 but not SPK2 is required for VEGF-stimulated Ras and ERK1/2 activation (53). Here, we used siRNA to downregulate the level of SPK1 and determine whether this affects LPS-induced SPK activation. In RAW 264.7 cells, the endogenous SPK1 level was reduced by 79% ± 7% following treatment with SPK1-specific siRNA (175 nM), whereas the expression levels of endogenous SPK2 and β-actin were not affected (Fig. 2A). SPK1-specific siRNA almost completely blocked LPS-induced SPK activation in RAW 264.7 cells (Fig. 2A). These results indicate that SPK1 is the major form of SPK that is activated by LPS.
FIG. 2.
SPK1 is the major form of SPKs activated by LPS. (A) SPK1-specific siRNA blocks LPS-induced SPK activation. The upper panel shows that mouse SPK1 siRNA specifically down-regulated the expression of SPK1 but not SPK2 in RAW 264.7 cells. RAW 264.7 cells were treated with 175 nM siRNA for 48 h, and then cell lysates were subjected to Western blot analysis. The lower panel shows that SPK1 siRNA inhibited the activation of SPK by LPS. RAW 264.7 cells were transfected with 175 nM SPK1 siRNA for 24 h, serum starved overnight, and then treated with 10 ng of LPS/ml for 40 min. The SPK assay was conducted. (B) LPS-induced membrane translocation of SPK1. RAW 264.7 cells were treated in the absence or presence of 1 μg of LPS/ml for 1 h. Cell lysates were then fractioned into cytosol, membrane, and triton-insoluble membrane extracts by ultracentrifugation. Equal amounts of protein (30 μg) from each fraction were subjected to Western blot analysis (top panel). The band densities on the Western blot were analyzed by a densitometer, and relative ratios between LPS-treated and control results were reported. The value for controls was arbitrarily set to 100% (the bottom panel).
LPS-induced translocation of SPK1 to the plasma membrane.
It has been reported that SPK1 translocates to the plasma membrane in response to PDGF or PMA stimulation (24, 49). In this study, we conducted a cell fractionation experiment with RAW 264.7 cells and determined the amount of SPK1 in each fraction by Western blotting. We found a 2.5-fold ± 0.5-fold increase of SPK1 in the membrane fraction in response to LPS treatment and a concomitant decrease in the cytosolic and triton-insoluble (cytoskeletal) fractions (Fig. 2B). Upon LPS stimulation, the levels of SPK1 in the cytosolic and triton-insoluble fractions were reduced by 24% ± 6% and 23% ± 8%, respectively. Our data suggest that both the cytosolic and cytoskeletal pools of SPK1 translocate to the plasma membrane in response to LPS.
Overexpression of both TLR4 and MD2 activates SPK.
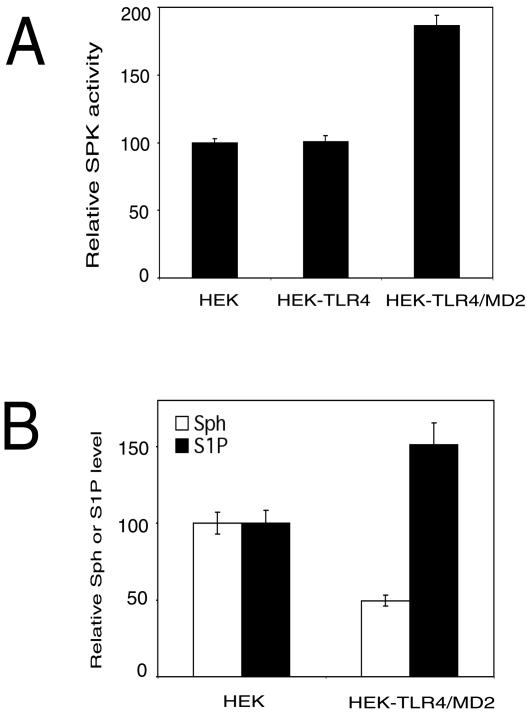
TLR4 has been recently identified as an LPS signaling receptor (45). However, in some cell lines, the TLR4-mediated response to LPS also requires the presence of the MD2 coreceptor (52). Here, we sought to determine whether TLR4 and MD2 are involved in LPS-induced SPK activation by using HEK 293 cells stably transfected with vectors expressing either TLR4 alone or both TLR4 and MD2. Overexpression of TLR4 alone did not activate SPK, whereas overexpression of both TLR4 and MD2 in HEK 293 cells increased SPK activity by 1.9-fold compared to that for the HEK 293 parental cell line (Fig. 3A). We also determined the levels of sphingosine and S1P in these cell lines. We found that HEK 293 cells overexpressing both TLR4 and MD2 had a lower level of sphingosine and a higher level of S1P than the HEK 293 parental cell line (Fig. 3B). Thus, overexpression of the LPS receptors (TLR4 and MD2) causes a ligand-independent activation of at least one of its downstream targets, namely, SPK. Similar results have been reported for HEK 293T cells, where overexpression of TLR4 and MD2 in induced a ligand-independent activation of NF-κB (52).
FIG. 3.
Overexpression of TLR4 and MD2 activates SPK. (A) The relative SPK activity in HEK 293 cells (HEK), HEK 293 stably transfected with TLR4 (HEK-TLR4), and HEK 293 stably transfected with both TLR4 and MD2 (HEK-TLR4/MD2). Cells were serum starved overnight and SPK activity in cell lysate was assayed. (For HEK 293 cells, relative SPK activity of 100% = 36 pmol/min/mg of protein). (B) Relative sphingosine and S1P levels in HEK 293 cells (HEK) and HEK 293 cells stably transfected with TLR4 and MD2 (HEK-TLR4/MD2). Cells were serum starved overnight, and relative levels of total cellular sphingosine (Sph) and S1P were determined.
SPK is involved in LPS activation of MAPK.
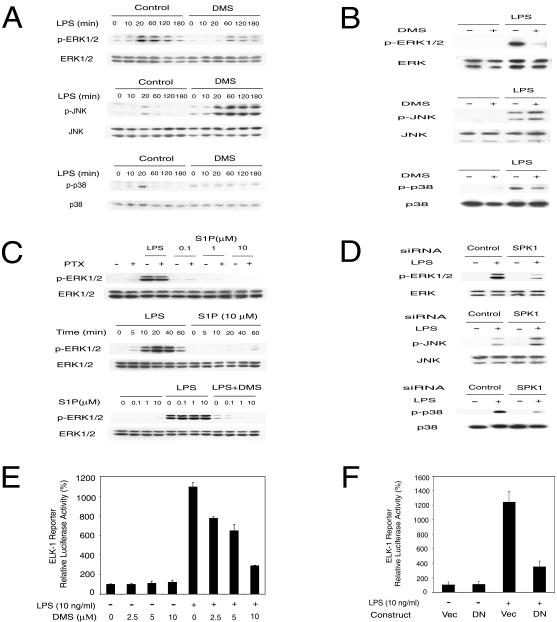
LPS is known to activate three major MAPKs: ERK1/2, JNK, and p38, which play an important role in LPS-induced cellular effects (18, 19, 61). We determined whether SPK activation is involved in these pathways by treating cells with a pharmacological inhibitor of SPK or with an SPK1-specific siRNA. First, we tested whether DMS, a competitive inhibitor of SPK (15), could inhibit LPS-stimulated ERK1/2, JNK, and p38 activation (phosphorylation) in RAW 264.7 cells and rat primary HMs. Phosphorylation of the MAPKs was detected by phospho-specific antibodies. In RAW 264.7 cells, LPS stimulated ERK1/2, JNK, and p38 phosphorylation in a time-dependent manner. ERK1/2 phosphorylation peaked at 20 min after LPS stimulation and decreased slowly over 3 h. Phosphorylation of JNK and p38 also peaked at 20 min and returned quickly to basal levels (Fig. 4A). When RAW 264.7 cells and HMs were pretreated with 10 μM DMS, LPS-induced ERK1/2 and p38 phosphorylation was blocked, whereas LPS-induced JNK phosphorylation was enhanced (Fig. 4A and B).
FIG. 4.
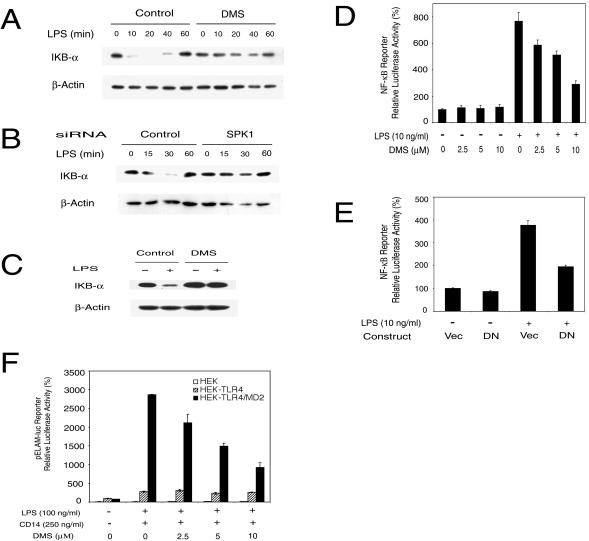
SPK mediates LPS-induced MAPK and Elk-1 activation. (A) MAPK activation by LPS was modulated by SPK inhibitor DMS in RAW 264.7 cells. The top panel shows that ERK1/2 activation by LPS was inhibited by DMS. The middle panel shows that JNK activation by LPS was enhanced by DMS. The bottom panel shows that p38 activation by LPS was inhibited by DMS. RAW 264.7 cells were pretreated with or without 10 μM DMS for 30 min and then stimulated with 10 ng of LPS/ml in the indicated time course. The phospho-MAPK and total MAPK in cell lysates were detected by Western blot. (B) MAPK activation by LPS was modulated by SPK inhibitor DMS in primary HMs. The top panel shows that ERK1/2 activation by LPS was inhibited by DMS. The middle panel shows that JNK activation by LPS was enhanced by DMS. The bottom panel shows that p38 activation by LPS was inhibited by DMS. HMs were pretreated with or without 10 μM DMS for 30 min and then stimulated with 10 ng of LPS/ml for 20 min. MAPK activation was assayed as described for panel A. (C) LPS activation of ERK1/2 is not through membrane S1P receptor. The upper panel shows that PTX did not block LPS-induced ERK1/2 activation. RAW 264.7 cells were pretreated with or without 100 ng of PTX/ml for 3 h and then stimulated with 10 ng of LPS/ml for 20 min or indicated concentrations of S1P for 10 min. The middle panel shows that extracellular S1P does not activate ERK1/2 in RAW 264.7 cells. RAW 264.7 cells were treated with 10 ng of LPS/ml or 10 μM S1P for the indicated time. The lower panel shows that extracellular S1P does not rescue the inhibition of LPS-induced ERK1/2 activation by DMS. RAW 264.7 cells were pretreated with or without 10 μM DMS for 30 min and then stimulated with 10 ng of LPS/ml and indicated concentrations of S1P for 20 min. ERK1/2 activation was assayed as described for panel A. (D) MAPK activation by LPS was modulated by SPK1 siRNA. The top panel shows that SPK1 siRNA blocked ERK1/2 activation by LPS. The middle panel shows that SPK1 siRNA enhanced JNK activation by LPS. The bottom panel shows that SPK1 siRNA blocked p38 activation by LPS. RAW 264.7 cells were treated with 175 nM siRNA for 24 h and then stimulated with LPS (10 ng/ml) for 20 min. MAPK activation was assayed as described for panel A. (E) Elk-1 activation by LPS was inhibited by the SPK inhibitor DMS. RAW 264.7 cells were transfected with the Elk-1 trans-reporting system (Stratagene) along with a pEF-β-Gal vector. Two days after transfection, cells were stimulated with LPS (10 ng/ml) in the presence of the indicated concentrations of DMS for 12 h. Reporter luciferase activity was measured using the luciferase assay system (Promega). Data were normalized with β-Gal activity in each sample. (F) DN-SPK1 inhibited LPS-induced Elk-1 activation. RAW 264.7 cells were transfected with the Elk-1 trans-reporting system, pEF-β-Gal and either a control vector or DN-SPK1. The reporter assay was conducted as described for panel E.
Next, we tested whether SPK1-specific siRNA would affect the LPS-induced activation of MAPKs. In RAW 264.7 cells treated with SPK1-specific siRNA, LPS-induced ERK1/2 and p38 phosphorylation was blocked and LPS-induced JNK phosphorylation was enhanced. In contrast, LPS-induced phosphorylation of all three MAPKs was unaffected in cells treated with control siRNA (Fig. 4D). Thus, our data with the pharmacological inhibitor DMS and SPK1-specific siRNA demonstrate that SPK is involved in the LPS regulation of MAPKs.
LPS signaling mediated by intracellular S1P.
Since S1P can act as an extracellular ligand for the EDG family of membrane receptors and lead to the activation of ERK1/2, we also tested whether LPS might activate ERK1/2 indirectly through the EDG family of receptors by stimulating S1P release from the cells. We have shown that PTX, which ADP ribosylates and inactivates Gi, G0 and Gt, blocks ERK1/2 activation in T24 cells induced by exogenous S1P but not VEGF (53). Pretreatment with PTX did not inhibit LPS-induced ERK1/2 activation in RAW 264.7 cells (Fig. 4C, top panel). The S1P1 receptor is known to signal solely through Gi (27). Thus, the failure of PTX to block LPS-induced ERK1/2 activation indicated that the S1P1 receptor was not involved in LPS signaling. However, other S1P membrane receptors might be involved in LPS signaling. Therefore, we tested whether exogenous S1P could activate ERK1/2 in RAW 264.7 cells. Addition of exogenous S1P to the culture medium failed to activate ERK1/2 in RAW 264.7 cells (Fig. 4C, middle panel). Although exogenous S1P did not activate ERK1/2, we cannot rule out the possibility that LPS may activate ERK1/2 via a synergy between extracellular S1P and other TLR4 signaling events. If this were true, extracellular S1P should be able to revert the DMS inhibition of LPS-induced ERK1/2 activation. To test this possibility, RAW 264.7 cells pretreated with DMS were stimulated with LPS and various concentrations of exogenous S1P. Exogenous S1P failed to reverse the DMS inhibition of LPS-induced ERK1/2 activation (Fig. 4C, bottom panel). Taken together, these results suggest that S1P acts as an intracellular secondary messenger rather than an extracellular ligand for EDG receptors in LPS signaling.
SPK mediates LPS activation of Elk-1.
MAPK pathways are coupled to the production of certain proinflammatory cytokines through phosphorylation and activation of Elk-1, resulting in increased levels of c-fos and enhanced formation of transcription factor AP-1 (55). Therefore, we examined the effect of the SPK inhibitor DMS and a plasmid expressing dominant-negative SPK1 (DN-SPK1) (53) on LPS-induced activation of an Elk-1 trans-reporting system (Stratagene). LPS-stimulated Elk-1 reporter activity was blocked by DMS in a dose-dependent manner (Fig. 4E). Cotransfection of a dominant-negative mutant of SPK1 (DN-SPK1) also inhibited the LPS-induced Elk-1 reporter activation (Fig. 4F). These data suggest that SPK is involved in LPS activation of Elk-1.
SPK is involved in LPS activation of NF-κB.
The transcription factor NF-κB is involved in the expression of many inflammatory mediators. Degradation of the inhibitory subunit (IκB) of the NF-κB and IκB complex leads to activation of NF-κB (26). We first examined the effect of the SPK inhibitor DMS and SPK1-specific siRNA on LPS-induced degradation of IκB-α. In RAW 264.7 cells, LPS-induced IκB-α degradation was blocked by DMS and SPK1-specific siRNA (Fig. 5A and B). Similarly, DMS also inhibited LPS-induced IκB-α degradation in rat primary HMs (Fig. 5C). Next, we examined the effect of the SPK inhibitor DMS and DN-SPK1 expression on LPS-induced activation of an NF-κB luciferase reporter gene. In RAW 264.7 cells, LPS-stimulated NF-κB reporter activity was blocked by DMS in a dose-dependent manner (Fig. 5D). Cotransfection with a DN-SPK1-expressing plasmid also inhibited LPS-stimulated NF-κB reporter activity (Fig. 5E). These data suggest that SPK is involved in LPS activation of NF-κB.
FIG. 5.
SPK mediates LPS-induced IκB-α degradation and NF-κB activation. (A) LPS-induced IκB-α degradation was inhibited by DMS in RAW 264.7 cells. RAW 264.7 cells were pretreated with or without 10 μM DMS for 30 min and then stimulated with 5 ng of LPS/ml for the indicated time course. IκB-α levels in cell lysates were detected by Western blotting. (B) LPS-induced IκB-α degradation was inhibited by SPK1 siRNA. RAW 264.7 cells were treated with 175 nM siRNA for 40 h and then stimulated with LPS (2 ng/ml) for the indicated time course. The IκB-α level was detected as described for panel A. (C) LPS-induced IκB-α degradation was inhibited by DMS in primary HMs. HMs were pretreated with or without 10 μM DMS for 30 min and then stimulated with 10 ng of LPS/ml for 20 min. IκB-α level was detected as described for panel A. (D) DMS inhibited NF-κB activation by LPS. RAW 264.7 cells were transiently transfected with Igκ-Luc along with a pEF-β-Gal vector. About 2 days after transfection, cells were stimulated with LPS (10 ng/ml) in the presence of indicated concentrations of DMS for 10 h. Reporter luciferase activity was measured using the Luciferase Assay system (Promega). Data were normalized with β-Gal activity in each sample. (E) DN-SPK1 inhibited LPS-induced NF-κB activation. RAW 264.7 cells were transfected with Igκ-Luc, pEF-β-Gal, and either a control vector or DN-SPK1. The reporter assay was conducted as described for panel D. (F) DMS only inhibited LPS-induced NF-κB activation in HEK 293 cells overexpressing both TLR4 and MD2. HEK 293 parental cells (HEK), HEK 293 cells overexpressing TLR4 (HEK-TLR4), and HEK 293 cells overexpressing both TLR4 and MD2 (HEK-TLR4/MD2) were plated on a 48-well plate, grown to confluence, and serum starved overnight. All three cell lines were also stably transfected with an NF-κB-dependent ELAM-1 luciferase reporter plasmid (pELAM-luc). Cells were stimulated with 100 ng of LPS/ml and 250 ng of recombinant human CD14/ml for 18 h. Reporter luciferase activity was measured using the Luciferase Assay system (Promega).
We also studied the effect of DMS on LPS-induced NF-κB activation in HEK 293 cells stably transfected with vectors expressing either TLR4 alone or both TLR4 and MD2. Previously, we found that overexpression of both TLR4 and MD2 but not TLR4 alone increased cellular SPK activity (Fig. 3). It has been reported that soluble CD14 does not induce activation of a NF-κB reporter but enhances LPS-induced NF-κB activation in HEK 293 cells overexpressing TLR4 (10). Therefore, we stimulated the cells with LPS plus soluble CD14. HEK 293 cells overexpressing either TLR4 alone or both TLR4 and MD2 transfected with an NF-κB luciferase reporter displayed similar levels of reporter activity as seen in the parental HEK 293 cells (Fig. 5F). LPS and soluble CD14 treatment of the cells overexpressing both TLR4 and MD2 resulted in a dramatic activation of the NF-κB reporter compared to results with LPS-treated HEK 293 parental cells or HEK 293 cells expressing only TLR4. Pretreatment of cells overexpressing both TLR4 and MD2 with DMS resulted in a dose-dependent inhibition of LPS-induced NF-κB reporter activity such that 10 μM DMS resulted in a 70% inhibition of NF-κB reporter activity (Fig. 5F). Because HEK 293 cells do not produce IL-1 and TNF (60), which can activate NF-κB, we suggest that LPS induction of the NF-κB reporter is a direct effect of signaling downstream of SPK rather than being due to secretion of these cytokines, which are also known to activate NF-κB in an SPK-dependent manner.
SPK protects LPS-activated macrophages from apoptosis.
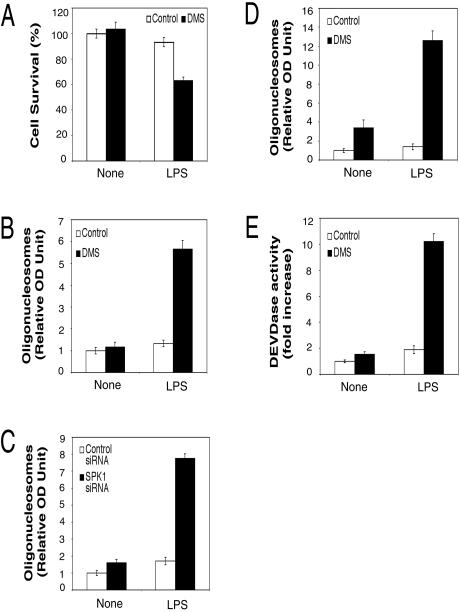
SPK1 activation changes the relative intracellular ratio of S1P and sphingosine and promotes cell growth and survival (54). In contrast, inhibition of SPK1 should favor apoptosis. It has been reported that DMS, a competitive inhibitor of SPK, sensitized HUVECs to caspase-3 activation and apoptosis (65). Here, we tested the effect of DMS and SPK1-specific siRNA on LPS-induced apoptosis using assays for cell viability, apoptotic cell death, and caspase-3 activity. Cell viability was measured using an MTT dye conversion assay. For RAW 264.7 cells, treatment with either LPS or DMS alone did not reduce cell survival significantly, whereas treatment with both LPS and DMS led to a significant decrease in cell survival (Fig. 6A). A reduced number of viable cells might be due to either apoptosis or cell growth arrest. To distinguish these possibilities, we examined apoptotic cell death with a quantitative sandwich-ELISA assay detecting mono- and oligonucleosomes (Cell Death Detection ELISAPLUS kit; Roche). In RAW 264.7 cells and rat primary HMs, treatment with either LPS or DMS alone did not induce significant cell death, whereas treatment with both LPS and DMS led to significant increase in these apoptotic markers and cell death (Fig. 6B and D). Consistent with these data, treatment with either LPS or SPK1-specific siRNA did not induce cell death, whereas treatment with both LPS and SPK1-specific siRNA induced significant apoptosis in RAW 264.7 cells (Fig. 6C). Since DMS sensitizes HUVECs to TNF-α-induced caspase-3 activation (65), we also examined the effect of DMS on LPS-induced caspase-3 activation. Caspase-3 activity was measured with a colorimetric assay kit (Sigma). In RAW 264.7 cells, treatment with either LPS or DMS did not induce significant activation of caspase-3, whereas treatment with both LPS and DMS led to a significant increase of caspase-3 activity (Fig. 6E). Taken together, these data suggest that LPS activates a balance of proapoptotic and antiapoptotic signals. However, when the prosurvival signal of SPK is inhibited by DMS or siRNA, the balance of signals is shifted in favor of the LPS-induced proapoptotic signals.
FIG. 6.
DMS and SPK1 siRNA sensitized LPS-activated macrophages to apoptosis. (A) DMS decreased cell survival in LPS-activated RAW 264.7 cells. RAW 264.7 cells were pretreated with 5 μM DMS for 30 min and then treated with 10 ng of LPS/ml for 24 h. Cell viability was measured by the MTT assay. (B) DMS sensitized LPS-activated RAW 264.7 cells to apoptosis. RAW 264.7 cells were pretreated with 5 μM DMS for 30 min and then treated with 10 ng of LPS/ml for 24 h. Apoptotic cell death was measured with a quantitative sandwich-ELISA assay detecting mono- and oligonucleosomes. The relative values for optical density at 405 nm minus that at 490 nm are reported. The value for untreated control was arbitrarily set to 1. (C) SPK1 siRNA sensitized LPS-activated RAW 264.7 cells to apoptosis. RAW 264.7 cells were transfected with control or SPK1 siRNA. One day after transfection, cells were pretreated with 5 μM DMS for 30 min and then treated with 10 ng of LPS/ml for 24 h. The apoptotic cell death was assayed as described for panel B. (D) DMS sensitized LPS-activated primary HMs to apoptosis. HMs were pretreated with 6 μM DMS for 30 min and then treated with 10 ng of LPS/ml for 24 h. The apoptotic cell death was assayed as described for panel B. (E) DMS enhanced LPS-induced caspase-3 activation. RAW 264.7 cells were pretreated with 5 μM DMS for 30 min and then treated with 10 ng of LPS/ml for 20 h. Then, the caspase-3 activities in cell lysates were assayed by measuring the hydrolysis of acetyl-Asp-Glu-Val-Asp p-nitroanilide. The relative values for optical density at 405 nm are reported.
DISCUSSION
The data presented in this paper demonstrate that LPS activates SPK in a RAW 264.7 murine macrophage cell line and rat primary HMs. In RAW 264.7 cells, the kinetics of activation is slow, starting as early as 10 min after treatment with LPS and peaking at 40 to 60 min after treatment. We note that the kinetics of MAPK regulation peaks around 20 min after LPS stimulation and this regulation is dependent on SPK (Fig. 4 and 5). We suggest that the level of SPK activity at 10 or 20 min (at 20 min, a 1.7-fold increase of SPK activity was observed) is sufficient for maximal regulation of MAPKs.
There are two forms of SPKs in mammalian cells: SPK1 and SPK2 (28, 30). Several lines of evidence suggest that SPK1 is the major form of SPK that responds to LPS. First, we have reported that SPK1, but not SPK2, is required for VEGF-stimulated Ras and MAPK activation (53). Second, we found that treatment with SPK1-specific siRNA abolished LPS-induced SPK activation in RAW 264.7 cells. Third, we found that SPK1 translocates to the plasma membrane in response to LPS stimulation. Fourth, Liu and colleagues recently reported that SPK2 is proapoptotic, in contrast to SPK1, which protects cells from apoptosis (31). Our observation that inhibition of SPK sensitizes cells to LPS-induced apoptosis therefore suggests that LPS signaling activates SPK1 to promote cell survival. Taken together, these data support the conclusion that LPS treatment stimulates SPK1 activity.
Our results showed that the intracellular sphingosine level was slightly increased after LPS stimulation. Activation of sphingomyelinase often mediates increases in cellular ceramide, which was converted to sphingosine by ceramidase (11). For example, TNF-α treatment results in activation of sphingomyelinase and increased levels of ceramide and sphingosine (13, 42). In human neutrophils, the sphingosine level increased slower than that of ceramide, suggesting that sphingosine was produced from the degradation of ceramide by ceramidase (42). In RAW 264.7 cells, the ceramide level increased rapidly after LPS treatment (33). In our studies, the slight increase in the sphingosine level could also result from ceramide degradation. As a result of SPK activation, we observed approximately a twofold increase of the S1P level. Although we observed that both sphingosine and S1P levels increased after LPS treatment, the relative ratio between sphingosine and S1P levels was changed. Furthermore, the local balance between the two sphingolipids near the LPS receptor may be even more pronounced in favor of S1P than was suggested by our measurements of total cellular sphingolipids. The balance between sphingosine and S1P decides the allergic responsiveness in mast cells (46). In addition, exogenous sphingosine blocks LPS-induced NF-κB activation and TNF expression in rabbit alveolar macrophages (32). Therefore, we suggest that the balance between sphingosine and S1P may also decide the responsiveness and the cell fate in LPS-activated macrophages.
TLR4 was recently identified as the LPS signaling receptor (45). However, in some cell lines, the TLR4-mediated response to LPS also requires the presence of the coreceptor MD2 (52). Here we tested whether both TLR4 and MD-2 were required for LPS-induced SPK activation. Overexpression of TLR4 alone was not enough to activate SPK, but overexpression of both TLR4 and MD2 led to a significant activation of SPK in the absence of LPS. Compared to the parental cell line, HEK 293 cells stably transfected with TLR4 and MD2 had a lower concentration of sphingosine but a higher level of S1P. This further indicates a higher basal SPK activity in cells overexpressing both TLR4 and MD-2. Our data suggested that both TLR4 and MD-2 are involved in the activation of SPK. Consistent with this finding, DMS inhibited the LPS-induced NF-κB reporter activation in HEK 293 cells overexpressing both TLR4 and MD2 but had no effect in HEK 293 parental cells or HEK 293 cells overexpressing only TLR4. However, in contrast to SPK activity, which is maximally activated by overexpression of TLR4 and MD2 in the absence of LPS, NF-κB reporter activity is dependent on LPS in cells overexpressing both TLR4 and MD2. This suggests that LPS induction of NF-κB reporter activity is dependent on both SPK-dependent and SPK-independent signals. While overexpression of TLR4 and MD2 is sufficient to activate SPK in the absence of LPS, other TLR4- and MD2-mediated events required for NF-κB activation are dependent on LPS but independent of SPK activation.
PKC has been implicated in some receptor-mediated events leading to SPK activation (4, 12, 53). We have reported that purified PKC can phosphorylate and activate recombinant mouse SPK1 in an ATP-dependent manner (53). Recently we found that the concentration of conventional PKC inhibitors required to inhibit SPK activation in RAW 264.7 cells (W. Wu, unpublished data) is higher than that required to inhibit signaling in T24 cells (53). This suggests that a different PKC isoform may be involved in LPS-induced SPK activation in T24 and RAW 264.7 cells. We found that a cell-permeable PKCζ inhibitor blocks LPS-induced activation of SPK in RAW 264.7 cells (W. Wu, unpublished data). PKCζ has been reported to mediate ERK1/2 activation by LPS in human alveolar macrophages (37). Furthermore, PKCζ-deficient mice displayed impaired TNF-α- and IL-1-induced activation of NF-κB (29). We are currently testing whether PKCζ is indeed involved in linking LPS to SPK activation.
The recruitment of SPK1 to the TNF receptor is mediated by a direct interaction with TRAF2 (66). TLR4 also recruits a TRAF family member, TRAF6 (34). We are currently exploring how SPK1 might be recruited to the activated TLR4. It will be interesting to determine whether SPK1 can interact directly with TRAF6 in a manner similar to the SPK1 interaction with TRAF2 in TNF signaling. Interestingly, the TRAF2-binding sequence in SPK1 (66) shares homology with reported consensus sequence for the TRAF6-binding motif (69). Furthermore, PKCζ has been reported to interact with dimerized TRAF6 (50), and we find that PKCζ may be required for LPS induction of SPK activity. Perhaps the recruitment of both PKCζ and SPK1 to activated TRAF6 aids in phosphorylation of SPK1 by PKC. We are currently testing this possibility.
SPK has been reported to be involved in ERK1/2 activation in several signaling pathways (47, 64). Not surprisingly, our studies with RAW 264.7 cells and rat primary HMs showed that LPS activation of ERK1/2 was blocked by treatment with the SPK inhibitor DMS or SPK1-specific siRNA. Since LPS also activates two other major MAPKs (p38 and JNK), we extended our study to examine the effect of DMS and SPK1-siRNA on these kinases. Both DMS and the SPK1-specific siRNA blocked LPS-induced p38 activation but enhanced LPS-induced JNK activation. These findings are consistent with those reported previously for S1P's role in TNF signaling (12). All three LPS-activated MAPKs have been linked to subsequent cytokine expression involving phosphorylation and activation of Elk-1 (55). In our studies, LPS-induced Elk-1 reporter activity was blocked by the SPK inhibitor DMS and expression of DN-SPK1.
The NF-κB/Rel family of transcription factors mediates LPS-induced expression of many proinflammatory mediators (26). TNF-α-induced NF-κB activation was blocked by the SPK inhibitor DMS and exogenous sphingosine (32, 64). Consistent with these findings, our studies with RAW 264.7 cells and rat primary HMs showed that both DMS and SPK1-specific siRNA blocked LPS-induced degradation of IκB-α, a process which is necessary for NF-κB activation. DMS and DN-SPK1 also inhibited LPS-induced activation of the NF-κB reporter in RAW 264.7 cells. LPS is known to stimulate the production and secretion of TNF, which in turn can activate NF-κB. Consequently, the inhibition of the NF-κB reporter by DMS and DN-SPK1 could reflect either a direct effect on LPS signaling or an inhibition of TNF signaling. We found that IκB-α degradation occurred as early as 10 min after LPS stimulation. Furthermore, we showed that DMS blocked LPS stimulation of an NF-κB reporter in HEK 293 cells overexpressing TLR4 and MD-2. Since HEK 293 cells do not express IL-1 and TNF (60), it is likely that the effect of DMS on LPS-induced NF-κB activation is a direct effect on LPS signaling, i.e., independent of cytokine production and secretion.
The relative levels of S1P and its precursors, sphingosine and ceramide, determine whether cells survive or undergo apoptosis (54). In RAW 264.7 cells, LPS increased the intracellular levels of ceramide (33), which can be converted to sphingosine. Both ceramide and sphingosine are proapoptotic. In contrast, the activation of SPK1 reduces the intracellular levels of ceramide and sphingosine by converting them to S1P and thereby promotes cell survival. Moreover, ERK, p38, and NF-κB are reported to protect LPS-stimulated macrophages or neutrophils from apoptosis (6, 25, 41), whereas JNK mediates LPS-induced apoptosis in macrophages (5). Our finding that LPS activation of SPK1 enhances ERK1/2, p38, and NF-κB activities but suppresses JNK activity further suggests that SPK may protect LPS-activated macrophages from apoptosis. Indeed, our studies with RAW 264.7 cells and rat primary HMs showed that treatment with DMS or SPK1-specific siRNA sensitized LPS-activated macrophages to apoptosis. These observations are consistent with those reported for TNF-α-treated endothelial cells, where inhibition of SPK sensitized cells to TNF-α-induced apoptosis (65). From these data, we conclude that SPK1 activation can protect LPS-activated macrophages from apoptosis. Further, our findings suggest that SPK1 is a potential therapeutic target in blocking hyperimmune responses induced by LPS.
Acknowledgments
We are grateful to Yoshiko Banno, Jesse Chow, Kalle Saksela, Alan Epstein, Hidekazu Tsukamoto, Shun-ichi Nakamura, and Ebrahim Zandi for providing reagents. We thank Ebrahim Zandi for critical reading of this manuscript.
This work was supported by NIH grant RO1-CA50261 (D.B.).
REFERENCES
- 1.Albina, J. E., S. Cui, R. B. Mateo, and J. S. Reichner. 1993. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J. Immunol. 150:5080-5085. [PubMed] [Google Scholar]
- 2.Anderson, K. V. 2000. Toll signaling pathways in the innate immune response. Curr. Opin. Immunol. 12:13-19. [DOI] [PubMed] [Google Scholar]
- 3.Bowie, A., and L. A. O'Neill. 2000. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J. Leukoc. Biol. 67:508-514. [DOI] [PubMed] [Google Scholar]
- 4.Buehrer, B. M., E. S. Bardes, and R. M. Bell. 1996. Protein kinase C-dependent regulation of human erythroleukemia (HEL) cell sphingosine kinase activity. Biochim. Biophys. Acta 1303:233-242. [DOI] [PubMed] [Google Scholar]
- 5.Castrillo, A., P. G. Través, P. Martín-Sanz, S. Parkinson, P. J. Parker, and L. Bosca. 2003. Potentiation of protein kinase C ζ activity by 15-deoxy-Δ12,14-prostaglandin J2 induces an imbalance between mitogen-activated protein kinases and NF-κB that promotes apoptosis in macrophages. Mol. Cell. Biol. 23:1196-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro-Alcaraz, S., V. Miskolci, B. Kalasapudi, D. Davidson, and I. Vancurova. 2002. NF-kappa B regulation in human neutrophils by nuclear I kappa B alpha: correlation to apoptosis. J. Immunol. 169:3947-3953. [DOI] [PubMed] [Google Scholar]
- 7.Chan, E. D., and D. W. Riches. 2001. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38(mapk) in a mouse macrophage cell line. Am. J. Physiol. Cell Physiol. 280:C441-C450. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Z., J. Hagler, V. J. Palombella, F. Melandri, D. Scherer, D. Ballard, and T. Maniatis. 1995. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 9:1586-1597. [DOI] [PubMed] [Google Scholar]
- 9.Choi, O. H., J. H. Kim, and J. P. Kinet. 1996. Calcium mobilization via sphingosine kinase in signalling by the Fc epsilon RI antigen receptor. Nature 380:634-636. [DOI] [PubMed] [Google Scholar]
- 10.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-100692. [DOI] [PubMed] [Google Scholar]
- 11.Cuvillier, O. 2002. Sphingosine in apoptosis signaling. Biochim. Biophys. Acta 1585:153-162. [DOI] [PubMed] [Google Scholar]
- 12.Cuvillier, O., G. Pirianov, B. Kleuser, P. G. Vanek, O. A. Coso, S. Gutkind, and S. Spiegel. 1996. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381:800-803. [DOI] [PubMed] [Google Scholar]
- 13.Dressler, K. A., S. Mathias, and R. N. Kolesnick. 1992. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science 255:1715-1718. [DOI] [PubMed] [Google Scholar]
- 14.Edsall, L. C., G. G. Pirianov, and S. Spiegel. 1997. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J. Neurosci. 17:6952-6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edsall, L. C., J. R. Van Brocklyn, O. Cuvillier, B. Kleuser, and S. Spiegel. 1998. N,N-dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry 37:12892-12898. [DOI] [PubMed] [Google Scholar]
- 16.Fahy, R. J., A. I. Doseff, and M. D. Wewers. 1999. Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J. Immunol. 163:1755-1762. [PubMed] [Google Scholar]
- 17.Guha, M., M. A. O'Connell, R. Pawlinski, A. Hollis, P. McGovern, S. F. Yan, D. Stern, and N. Mackman. 2001. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood 98:1429-1439. [DOI] [PubMed] [Google Scholar]
- 18.Hambleton, J., S. L. Weinstein, L. Lem, and A. L. DeFranco. 1996. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. USA 93:2774-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 20.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 21.Igarashi, N., T. Okada, S. Hayashi, T. Fujita, S. Jahangeer, and S. Nakamura. 2003. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J. Biol. Chem. 278:46832-46839. [DOI] [PubMed] [Google Scholar]
- 22.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis, B. W., T. H. Harris, N. Qureshi, and G. A. Splitter. 2002. Rough lipopolysaccharide from Brucella abortus and Escherichia coli differentially activates the same mitogen-activated protein kinase signaling pathways for tumor necrosis factor alpha in RAW 264.7 macrophage-like cells. Infect. Immun. 70:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson, K. R., K. P. Becker, M. M. Facchinetti, Y. A. Hannun, and L. M. Obeid. 2002. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane. Extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA). J. Biol. Chem. 277:35257-35262. [DOI] [PubMed] [Google Scholar]
- 25.Karahashi, H., K. Nagata, K. Ishii, and F. Amano. 2000. A selective inhibitor of p38 MAP kinase, SB202190, induced apoptotic cell death of a lipopolysaccharide-treated macrophage-like cell line, J774.1. Biochim. Biophys. Acta 1502:207-223. [DOI] [PubMed] [Google Scholar]
- 26.Karin, M., M. Delhase, and A. Rossi. 2000. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin. Immunol. 12:85-98. [DOI] [PubMed] [Google Scholar]
- 27.Kluk, M. J., and T. Hla. 2002. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim. Biophys. Acta 1582:72-80. [DOI] [PubMed] [Google Scholar]
- 28.Kohama, T., A. Olivera, L. Edsall, M. M. Nagiec, R. Dickson, and S. Spiegel. 1998. Molecular cloning and functional characterization of murine sphingosine kinase. J. Biol. Chem. 273:23722-23728. [DOI] [PubMed] [Google Scholar]
- 29.Leitges, M., L. Sanz, P. Martin, A. Duran, U. Braun, J. F. Garcia, F. Camacho, M. T. Diaz-Meco, P. D. Rennert, and J. Moscat. 2001. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol. Cell 8:771-780. [DOI] [PubMed] [Google Scholar]
- 30.Liu, H., M. Sugiura, V. E. Nava, L. C. Edsall, K. Kono, S. Poulton, S. Milstien, T. Kohama, and S. Spiegel. 2000. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 275:19513-19520. [DOI] [PubMed] [Google Scholar]
- 31.Liu, H., R. E. Toman, S. Goparaju, M. Maceyka, V. E. Nava, H. Sankala, S. G. Payne, M. Bektas, I. Ishii, J. Chun, S. Milstien, and S. Spiegel. 2003. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J. Biol. Chem. 278:40330-40336. [DOI] [PubMed]
- 32.Lo, C. J., M. Fu, F. R. Lo, and H. G. Cryer. 1999. Macrophage TNF mRNA expression induced by LPS is regulated by sphingomyelin metabolites. Shock 11:411-415. [PubMed] [Google Scholar]
- 33.MacKichan, M. L., and A. L. DeFranco. 1999. Role of ceramide in lipopolysaccharide (LPS)-induced signaling. LPS increases ceramide rather than acting as a structural homolog. J. Biol. Chem. 274:1767-1775. [DOI] [PubMed] [Google Scholar]
- 34.Martin, M. U., and H. Wesche. 2002. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim. Biophys. Acta 1592:265-280. [DOI] [PubMed] [Google Scholar]
- 35.Melendez, A., R. A. Floto, D. J. Gillooly, M. M. Harnett, and J. M. Allen. 1998. FcgammaRI coupling to phospholipase D initiates sphingosine kinase-mediated calcium mobilization and vesicular trafficking. J. Biol. Chem. 273:9393-9402. [DOI] [PubMed] [Google Scholar]
- 36.Meyer zu Heringdorf, D., H. Lass, R. Alemany, K. T. Laser, E. Neumann, C. Zhang, M. Schmidt, U. Rauen, K. H. Jakobs, and C. J. van Koppen. 1998. Sphingosine kinase-mediated Ca2+ signalling by G-protein-coupled receptors. EMBO J. 17:2830-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monick, M. M., A. B. Carter, D. M. Flaherty, M. W. Peterson, and G. W. Hunninghake. 2000. Protein kinase C zeta plays a central role in activation of the p42/44 mitogen-activated protein kinase by endotoxin in alveolar macrophages. J. Immunol. 165:4632-4639. [DOI] [PubMed] [Google Scholar]
- 38.Munoz, E., G. Courtois, P. Veschambre, P. Jalinot, and A. Israel. 1994. Tax induces nuclear translocation of NF-κB through dissociation of cytoplasmic complexes containing p105 or p100 but does not induce degradation of IκB alpha/MAD3. J. Virol. 68:8035-8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murate, T., Y. Banno, T. K. K., K. Watanabe, N. Mori, A. Wada, Y. Igarashi, A. Takagi, T. Kojima, H. Asano, Y. Akao, S. Yoshida, H. Saito, and Y. Nozawa. 2001. Cell type-specific localization of sphingosine kinase 1a in human tissues. J. Histochem. Cytochem. 49:845-855. [DOI] [PubMed] [Google Scholar]
- 40.Nagai, Y., S. Akashi, M. Nagafuku, M. Ogata, Y. Iwakura, S. Akira, T. Kitamura, A. Kosugi, M. Kimoto, and K. Miyake. 2002. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3:667-672. [DOI] [PubMed] [Google Scholar]
- 41.Nolan, B., A. Duffy, L. Paquin, M. De, H. Collette, C. M. Graziano, and P. Bankey. 1999. Mitogen-activated protein kinases signal inhibition of apoptosis in lipopolysaccharide-stimulated neutrophils. Surgery 126:406-412. [PubMed] [Google Scholar]
- 42.Ohta, H., Y. Yatomi, E. A. Sweeney, S. Hakomori, and Y. Igarashi. 1994. A possible role of sphingosine in induction of apoptosis by tumor necrosis factor-alpha in human neutrophils. FEBS Lett. 355:267-270. [DOI] [PubMed] [Google Scholar]
- 43.Olivera, A., and S. Spiegel. 1993. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature 365:557-560. [DOI] [PubMed] [Google Scholar]
- 44.Pitson, S. M., P. A. Moretti, J. R. Zebol, P. Xia, J. R. Gamble, M. A. Vadas, R. J. D'Andrea, and B. W. Wattenberg. 2000. Expression of a catalytically inactive sphingosine kinase mutant blocks agonist-induced sphingosine kinase activation. A dominant-negative sphingosine kinase. J. Biol. Chem. 275:33945-33950. [DOI] [PubMed] [Google Scholar]
- 45.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 46.Prieschl, E. E., R. Csonga, V. Novotny, G. E. Kikuchi, and T. Baumruker. 1999. The balance between sphingosine and sphingosine-1-phosphate is decisive for mast cell activation after Fc epsilon receptor I triggering. J. Exp. Med. 190:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rani, C. S., F. Wang, E. Fuior, A. Berger, J. Wu, T. W. Sturgill, D. Beitner-Johnson, D. LeRoith, L. Varticovski, and S. Spiegel. 1997. Divergence in signal transduction pathways of platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) receptors. Involvement of sphingosine 1-phosphate in PDGF but not EGF signaling. J. Biol. Chem. 272:10777-10783. [DOI] [PubMed] [Google Scholar]
- 48.Rius, R. A., L. C. Edsall, and S. Spiegel. 1997. Activation of sphingosine kinase in pheochromocytoma PC12 neuronal cells in response to trophic factors. FEBS Lett. 417:173-176. [DOI] [PubMed] [Google Scholar]
- 49.Rosenfeldt, H. M., J. P. Hobson, M. Maceyka, A. Olivera, V. E. Nava, S. Milstien, and S. Spiegel. 2001. EDG-1 links the PDGF receptor to Src and focal adhesion kinase activation leading to lamellipodia formation and cell migration. FASEB J. 15:2649-2659. [DOI] [PubMed] [Google Scholar]
- 50.Sanz, L., M. T. Diaz-Meco, H. Nakano, and J. Moscat. 2000. The atypical PKC-interacting protein p62 channels NF-kappaB activation by the IL-1-TRAF6 pathway. EMBO J. 19:1576-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scherle, P. A., E. A. Jones, M. F. Favata, A. J. Daulerio, M. B. Covington, S. A. Nurnberg, R. L. Magolda, and J. M. Trzaskos. 1998. Inhibition of MAP kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J. Immunol. 161:5681-5686. [PubMed] [Google Scholar]
- 52.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shu, X., W. Wu, R. D. Mosteller, and D. Broek. 2002. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of Ras and mitogen-activated protein kinases. Mol. Cell. Biol. 22:7758-7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spiegel, S., and S. Milstien. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4:397-407. [DOI] [PubMed] [Google Scholar]
- 55.Treisman, R. 1996. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 8:205-215. [DOI] [PubMed] [Google Scholar]
- 56.Triantafilou, M., and K. Triantafilou. 2002. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 23:301-304. [DOI] [PubMed] [Google Scholar]
- 57.Ulevitch, R. J., and P. S. Tobias. 1999. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 11:19-22. [DOI] [PubMed] [Google Scholar]
- 58.von Knethen, A., D. Callsen, and B. Brune. 1999. NF-kappaB and AP-1 activation by nitric oxide attenuated apoptotic cell death in RAW 264.7 macrophages. Mol. Biol. Cell 10:361-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, C., L. Deng, M. Hong, G. R. Akkaraju, J. Inoue, and Z. J. Chen. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412:346-351. [DOI] [PubMed] [Google Scholar]
- 60.Wang, Q., R. Dziarski, C. J. Kirschning, M. Muzio, and D. Gupta. 2001. Micrococci and peptidoglycan activate TLR2→MyD88→IRAK→TRAF→NIK→IKK→NF-κB signal transduction pathway that induces transcription of interleukin-8. Infect. Immun. 69:2270-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinstein, S. L., J. S. Sanghera, K. Lemke, A. L. DeFranco, and S. L. Pelech. 1992. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J. Biol. Chem. 267:14955-14962. [PubMed] [Google Scholar]
- 62.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]
- 63.Wu, W., X. Shu, H. Hovsepyan, R. D. Mosteller, and D. Broek. 2003. VEGF receptor expression and signaling in human bladder tumors. Oncogene 22:3361-3370. [DOI] [PubMed] [Google Scholar]
- 64.Xia, P., J. R. Gamble, K. A. Rye, L. Wang, C. S. Hii, P. Cockerill, Y. Khew-Goodall, A. G. Bert, P. J. Barter, and M. A. Vadas. 1998. Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proc. Natl. Acad. Sci. USA 95:14196-14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xia, P., L. Wang, J. R. Gamble, and M. A. Vadas. 1999. Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J. Biol. Chem. 274:34499-34505. [DOI] [PubMed] [Google Scholar]
- 66.Xia, P., L. Wang, P. A. Moretti, N. Albanese, F. Chai, S. M. Pitson, R. J. D'Andrea, J. R. Gamble, and M. A. Vadas. 2002. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J. Biol. Chem. 277:7996-8003. [DOI] [PubMed] [Google Scholar]
- 67.Xiong, S., H. She, H. Takeuchi, B. Han, J. F. Engelhardt, C. H. Barton, E. Zandi, C. Giulivi, and H. Tsukamoto. 2003. Signaling role of intracellular iron in NF-kappaB activation. J. Biol. Chem. 278:17646-17654. [DOI] [PubMed] [Google Scholar]
- 68.Yang, H., D. W. Young, F. Gusovsky, and J. C. Chow. 2000. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J. Biol. Chem. 275:20861-20866. [DOI] [PubMed] [Google Scholar]
- 69.Ye, H., J. R. Arron, B. Lamothe, M. Cirilli, T. Kobayashi, N. K. Shevde, D. Segal, O. K. Dzivenu, M. Vologodskaia, M. Yim, K. Du, S. Singh, J. W. Pike, B. G. Darnay, Y. Choi, and H. Wu. 2002. Distinct molecular mechanism for initiating TRAF6 signalling. Nature 418:443-447. [DOI] [PubMed] [Google Scholar]