Abstract
Sleep deprivation (SD) disrupts dopamine (DA) signaling and impairs attention. However, the interpretation of these concomitant effects requires a better understanding of dopamine’s role in attention processing. Here we test the hypotheses that D2/D3 receptors (D2/D3R) in dorsal and ventral striatum would distinctly regulate the activation of attention regions and that, by decreasing D2/D3, SD would disrupt these associations. We measured striatal D2/D3R using positron emission tomography with [11C]raclopride and brain activation to a visual attention (VA) task using 4-Tesla functional magnetic resonance imaging. Fourteen healthy men were studied during rested wakefulness and also during SD. Increased D2/D3R in striatum (caudate, putamen and ventral striatum) were linearly associated with higher thalamic activation. Subjects with higher D2/D3R in caudate relative to ventral striatum had higher activation in superior parietal cortex and ventral precuneus, and those with higher D2/D3R in putamen relative to ventral striatum had higher activation in anterior cingulate. SD impaired the association between striatal D2/D3R and VA-induced thalamic activation, which is essential for alertness. Findings suggest a robust DAergic modulation of cortical activation during the VA task, such that D2/D3R in dorsal striatum counterbalanced the stimulatory influence of D2/D3R in ventral striatum, which was not significantly disrupted by SD. In contrast, SD disrupted thalamic activation, which did not show counterbalanced DAergic modulation but a positive association with D2/D3R in both dorsal and ventral striatum. The counterbalanced dorsal versus ventral striatal DAergic modulation of VA activation mirrors similar findings during sensorimotor processing (Tomasi et al., 2015) suggesting a bidirectional influence in signaling between the dorsal caudate and putamen and the ventral striatum.
Introduction
Attention allows us to focus on one aspect of information (that is, the moving ball) while ignoring irrelevant information (that is, other moving objects in the scene), an ability severely compromised by sleep deprivation (SD).1 Attention engages a distributed network of brain regions for focusing on specific stimuli or the surroundings, and for resolving conflict between multiple cues.2 Several neurotransmitters are implicated in the modulation of these attention components, including cholinergic, noradrenergic and dopaminergic systems.3, 4 During the last decade, there has been an increased interest on the role of dopamine (DA) in the modulation of attention5 as stimulant medications enhance DA signaling in the human brain6, 7, 8 and improve attention under excessive sleepiness.9, 10
Previous studies have shown that SD decreases striatal D2/D3R availability, impairs performance and alters brain activation during attention tasks.11, 12, 13, 14, 15, 16, 17 Specifically, SD has been shown to impair performance to attention demanding cognitive tasks and to reduce arousal and alertness.18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 Concomitant with these behavioral changes, SD increases functional magnetic resonance imaging (fMRI) signals in the thalamus, which is essential for alertness,30 while reducing fMRI signals in superior parietal (SPC) and prefrontal (PFC) cortices during a visual attention (VA) task.30, 31
The role of DA in the regulation of thalamic and PFC activity is well established.32, 33 For instance, D2/D3 receptors (D2/D3R) in the ventral striatum (VS) have been associated with fMRI activation of the medial PFC during visual attention to rewards,34 and D2/D3R in the dorsal striatum have been associated with neural processing in the PFC during inhibitory control35 and executive functioning.36, 37 However, the role of DA in the regulation of the SPC has not been investigated. Thus, while SD-related changes in the PFC and thalamic activation30 may have reflected the decreases in DA function during SD,11, 13 the association between the decreases in DA function and the changes in brain activation during SD are still largely unknown.
We recently showed that a balance between dorsal caudate versus VS in D2/D3R mediated the modulation of brain activation to a cognitive task.38 Thus, we predicted that fMRI signals during an attention task would show distinct linear associations with the dorsal and ventral striatal regions such that higher D2/D3R availability in the dorsal versus VS regions would be associated with greater cortical activation, and that SD would disrupt these associations.
Hence, in this work, we test the linear association between D2/D3R in the dorsal and ventral striatum and VA activation in thalamus, SPC and PFC, which are the three critical components of the attention networks.2 We measured D2/D3R using positron emission tomography (PET) and VA activation with 4-Tesla fMRI in 14 healthy men. Subjects were scanned with PET and fMRI twice, after one night of normal sleep (that is, under rested wakefulness (RW)) and also after one night of SD. We hypothesized that cortical activation responses would reflect the relative availability of D2/D3R in the dorsal (caudate, putamen) versus ventral striatum, whereas thalamic responses that are necessary for alertness30 would show an association with both dorsal and ventral striatum. We further predicted that SD would disrupt the modulation of striatal signaling in the indirect striatocortical pathway by virtue of the downregulation of striatal D2/D3 receptors that follows SD, which we have shown is associated with a concomitant impairment in cognitive performance.11
Materials and methods
Subjects
Fourteen healthy, non-smoking, right-handed men (age 32±8 years, education: 16±2 years) participated in the study. At α=0.05 and 80% power, this sample size allowed us to detect large effects (r=0.6) of SD on the association between D2/D3R and fMRI activation. The subjects were included if they were able to understand and give informed consent, and were 18 to 50 years old. They were screened carefully with a detailed medical history as well as physical and neurological examinations. The subjects were excluded if they had (1) urine positive for psychotropic drugs; (2) present or past history of dependence on alcohol or other drugs of abuse; (3) present or past history of neurological or psychiatric disorders (including sleep disorders); (4) cardiovascular disease or diabetes; (5) history of head trauma with loss of consciousness for more than 30 min; (6) medical conditions that may alter the brain function; (7) used psychoactive medications in the past month (that is, opiate analgesics, stimulants, sedatives); (8) used prescription (non-psychiatric) medication(s); or (9) contraindications to MRI environment (metallic implants/claustrophobia). The study participants signed a written consent approved by the Institutional Review Board at Brookhaven National Laboratory before the study. The subjects were asked to keep a diary of the number of hours slept per night for the 2-week duration of the study and this corresponded to an average of 7±1 h per night (range, 5–8 h).
SD and RW sessions
All the subjects were kept overnight at the Brookhaven National Laboratory campus before their scheduled sessions (Figure 1a) to ensure that that they had a good night rest for the RW session (6.7±0.9 h of sleep; range 5–8.5 h) or they did not sleep during the night for the SD session (supervised by a team member). For the SD session, the total time of sleep deprivation, computed from the subject’s wake up time on the check-in day until the end of fMRI session, was 30–35 h. The SD and RW sessions were scheduled 2 weeks apart. The subjects did not have food after midnight and no caffeinated beverages were permitted during the study. PET and MRI acquisition were done sequentially on the same day, either after RW or SD. On the RW day, the subjects were awakened at 0700 h and brought to the imaging suite. A nurse remained with the subjects to ensure they stayed awake throughout the study. The PET sessions (RW and SD) took place between 1100 h and 1400 h and the MRI sessions (RW and SD) took place between 1500 h and 1700 h. Half the studies started with the RW session; the remaining studies started with the SD session to control for practice effects on brain activation.39
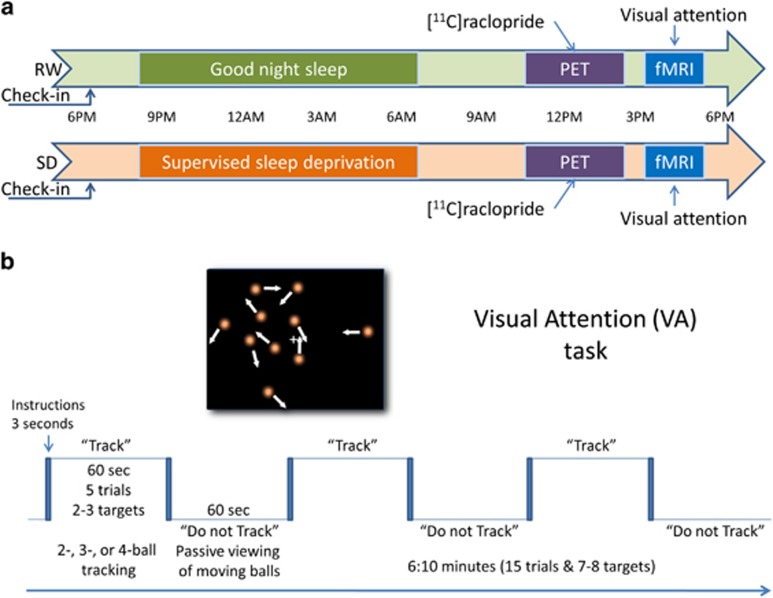
Figure 1.
Study design. (a) Fourteen healthy, non-smoking, right-handed men were kept overnight onsite before their scheduled imaging sessions to ensure that that they had a good night rest (rested wakefulness (RW) session) or they did not sleep during the night (sleep deprivation (SD) session). All the subjects underwent [11C]raclopride positron emission tomography (PET) to assess D2/D3R in the striatum and 4-Tesla blood-oxygenation-level-dependent functional magnetic resonance imaging (BOLD-fMRI) to map brain activation to a visual attention (VA) task during RW and during SD. (b) The parametric VA task had a blocked design in which subjects either tracked 2, 3 or 4 balls out of 10 moving balls (task epochs) or viewed them passively (rest epochs).
PET imaging
A Siemens HR+ tomograph with 4.5 mm isotropic resolution was used to collect dynamic PET images in three-dimensional mode. Twenty emission scans were obtained from the time of injection up to 54 min immediately after injection of [11C]raclopride (4–8 mCi; specific activity 0.5–1.5 Ci μm−1). Arterial sampling was used to quantify total carbon-11 and unchanged [11C]raclopride in plasma. The distribution volume (DV) was computed for each imaging voxel using a graphical analysis technique for reversible systems.40 These images were then spatially normalized to the stereotactic space of the Montreal Neurological Institute using a 12-parameter affine transformation. A custom Montreal Neurological Institute template, which was previously developed using DV images acquired with [11C]raclopride and the same PET scanning sequence41 was used for the spatial normalization of the DV images. The intensity of the DV images was normalized to that in the cerebellum (left and right regions of interest) to quantify the non-displaceable binding potential (BPND) in each voxel. BPND images were spatially smoothed (8-mm isotropic Gaussian kernel) using the statistical parametric mapping package SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK).
Anatomical region of interest analyses
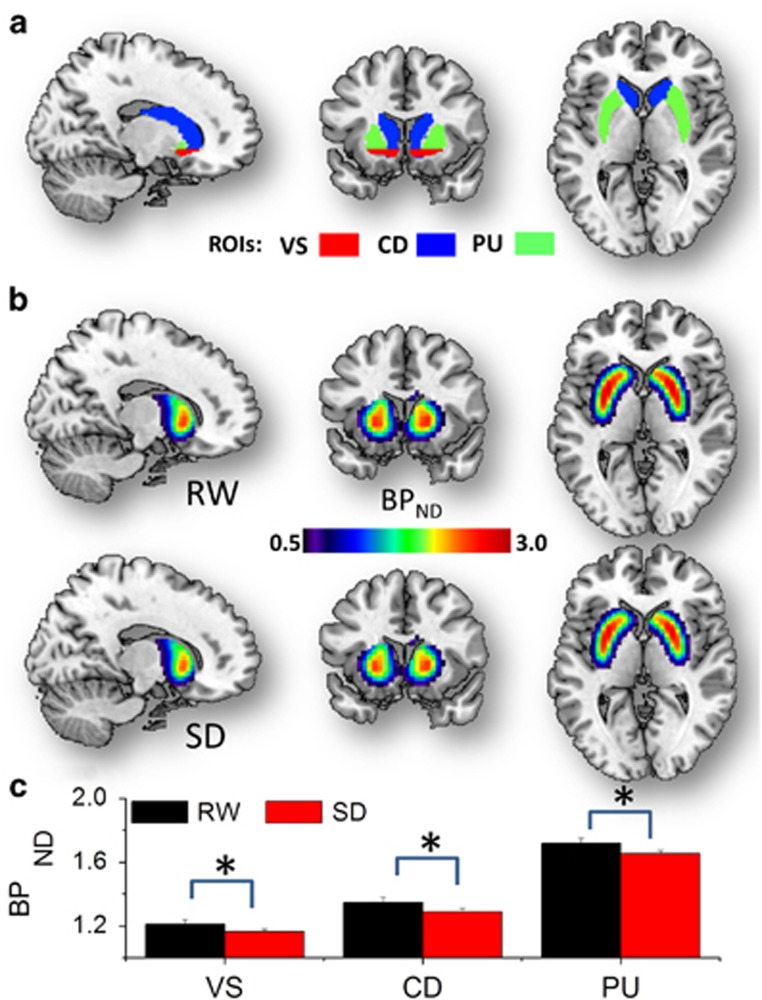
In-house software written in IDL (Exelis Visual Information Solutions, Boulder, CO, USA) and the Automated Anatomical Labeling (AAL) atlas42 were used to define three bilateral anatomical regions of interest (ROIs): putamen (PU), caudate (CD) and VS (Figure 1a). The CD ROI included all voxels in dorsal caudate, as defined in the AAL atlas planes −6 mm<z<14 mm. The VS ROI encompassed the inferior planes of pre-commissural caudate and putamen (6 mm<y⩽27 mm; −11 mm<z⩽−6 mm) in the AAL atlas. Average BPND values were computed for each subject independently for these ROIs. We chose to report the average BPND values in the whole anatomy of the striatal regions to minimize human errors or potential confounds resulting from the utilization of arbitrary thresholds.
VA paradigm
After the PET session, the subjects underwent fMRI with a VA task that was described previously.39, 43, 44, 45, 46 This fMRI task was used previously to assess visual attention activation in healthy controls,39, 44, 47, 48, 49 human immunodeficiency virus patients,45, 50, 51, 52 marijuana53 and cocaine46, 54 abusers as well as to assess the effects of functional connectivity,54, 55 sleep deprivation,30 dopamine transporters56 and stimulants57 on VA activation. The ball-tracking task activates attention-related brain regions (prefrontal, parietal, and occipital cortices, thalamus, and cerebellum). The blocked VA task had 3 difficulty levels (2-, 3, and 4-ball tracking). Each of the three fMRI runs lasted 6 min and was composed by three ‘TRACK’ epochs interleaved with three ‘DO NOT TRACK’ epochs. ‘TRACK’ epochs interleave five tracking and five respond periods (Figure 1b). In these epochs, a target set of balls (2, 3 or 4 out of 10 balls) is briefly highlighted. Then all the balls start to move. The subjects’ task is to fixate on the center cross and track the target balls as they move randomly (simulated Brownian motion) across the display with instantaneous angular speed of 3° per second. At the end of tracking periods, the balls stop moving and a new set of balls is highlighted; the subjects’ are instructed to press a button if the highlighted balls are the target set. After a 0.5-s delay, the original target balls are re-highlighted to re-focus the subjects’ attention on the target balls. ‘DO NOT TRACK’ epochs are composed of five consecutive ‘resting’ periods. In these epochs, all the 10 balls move and stop in the same manner as during ‘TRACK’ epochs; however, no balls are highlighted, and subjects are instructed to not track the balls and view them passively. The subjects performed a brief training session (~10 min) of a shortened version of the paradigm outside of the scanner to ensure that they understood and were able to perform the tasks. There were three fMRI runs (two-, three- and four-ball tracking). Each one of these runs had 231 image volumes (4 dummy volumes, 7 fixation cross baseline volumes, 112 passive-viewing volumes and 112 ball-tracking volumes).
Different versions of the two-, three- and four-ball-tracking tasks were used in each session (SD and RW). The stimuli were created using Matlab (MathWorks, Natick, MA, USA) and presented to the subjects on MRI-compatible goggles (Resonance Technology, Northridge, CA, USA) connected to a personal computer. The display software was synchronized with the MRI acquisition using a trigger pulse. All button press events were recorded to determine RT and performance accuracy during fMRI.
MRI data acquisition
The blood-oxygenation-level-dependent (BOLD) contrast was used to assess fMRI activation in a 4-Tesla whole-body Varian/Siemens MRI scanner. A T2*-weighted single-shot gradient-echo planar imaging sequence (TE/TR=20/1600 ms, 4 mm slice thickness, 1 mm gap, 35 coronal slices, 3.1 mm in-plane resolution, 64 × 64 matrix size, 90°-flip angle, 231 time points, bandwidth: 200.00 kHz) covering the whole brain was used for this purpose. Padding was used to minimize motion. Task performance and subject motion were determined immediately after each fMRI trial.58 Anatomical images were collected using T2-weighted hyperecho (TE/TR=42/10 000 ms, echo train length=16, 256 × 256 matrix size, 30 coronal slices, 0.86 × 0.86 mm in-plane resolution, 5 mm thickness, 1 mm gap, 2-min scan time) and T1-weighted three-dimensional MDEFT (TE/TR=7/15ms, 0.94 × 0.94 × 1 mm spatial resolution, axial orientation, 256 readout and 192 × 96 phase-encoding steps, 16-min scan time) sequences. These structural MRI scans were reviewed to rule out gross morphological abnormalities in the brain.
Data processing
The first four volumes in the time series were discarded to avoid non-equilibrium effects in the fMRI signal. Subsequent analyses were performed with SPM8. Spatial realignment was performed with a fourth degree B-spline function without weighting and without warping; head motion was less than 2-mm translations and 2° rotations for all scans. Spatial normalization to the stereotactic space of the Montreal Neurological Institute was performed using a 12-parameter affine transformation with medium regularization, 16-nonlinear iterations, 3 × 3 × 3 mm3 voxel size and the standard SPM8 EPI template. Spatial smoothing was carried out using an 8-mm (full width at half maximum) Gaussian kernel. A general linear model59 was used to calculate the BOLD contrasts for each VA load condition (two, three and four balls), session (RW and SD) and subject. The blocked analysis was based on a box-car design defined by the onsets of the ‘TRACK’ epochs, convolved with the canonical hemodynamic response function, as a low-pass filter, and a high-pass filter (256 s time cutoff).
Statistical analyses
Simple (SLR) and multiple (MLR) linear regression analyses were used to assess the association between the fMRI signals in the brain and the D2/D3R measures across subjects, using VA load and session as covariates in SPM8. Five SLR models were used with regressors that reflected the absolute BPND values extracted from CD (SLR1), PU (SLR2) and VS (SLR3), as well as the relative BPND measures CD/VS (SLR4) and PU/VS (SLR5). Two different MLR models were used to study the combined influence of receptors in VS and in CD (MLR1), as well as that of receptors in PU and VS (MLR2). Specifically, the fMRI responses at a given voxel, S(x, y, z), were modeled using the affine transformation:
where i and j are CD and VS, or PU and VS, the scalar maps α (x, y, z) are the slopes that quantify the efficiency of the linear association between D2/D3R and brain activation and ε is the intercept of the MLR. Independent MLR analyses were carried for RW and SD as well as for the combined RW and SD sample. For all analyses, statistical significance was set as PFWE<0.05, corrected for multiple comparisons in the whole brain with the random field theory and a family-wise error correction at the cluster level. A cluster-forming threshold P<0.001 (two-sided) and a minimum cluster size of 100 voxels were used for this purpose.
Results
Behavior
The fMRI and behavioral data in this work were previously reported in a study that documented SD-related decreases in VA performance and fMRI activation differences between RW and SD.30 Briefly, subjects reported higher sleepiness before the SD session than before the RW session (RW: 3.8±0.5; s.d.: 8.8±0.4; P<0.0001, paired t-test). Increased sleepiness correlated linearly with performance accuracy during the fMRI tasks (R=0.59; P=0.025). Performance accuracy during fMRI decreased with increased task difficulty (from two balls to four balls; P<0.0001; two-way ANOVA) and was lower during the SD session than during the RW session (P=0.02). Reaction time (RT) during the fMRI did not differ significantly across tasks or sessions. There were no statistically significant load × session interaction effects on subject’s performance (accuracy or RT). In the present study, we studied the association between brain activation during the VA task and D2/D3R measures in the dorsal and ventral striatum.
D2/D3R
The average BPND values, which were computed without BPND thresholds over the anatomical volumes of CD, PU and VS (see the 'Methods' section), were lower for SD than for RW for all striatal ROIs (VS: 1.21±0.03 (RW) and 1.16±0.02 (s.d.); CD: 1.35±0.03 (RW) and 1.29±0.02 (s.d.); PU: 1.72±0.03 (RW) and 1.65±0.02 (s.d.); mean±s.e.; P<0.05, two-sided paired t-test, df=13; Figure 2). The BPND ROI measures showed high correlations across subjects and were higher during RW than during s.d. (P<0.05). The differences in the ‘relative’ BPND measures between RW and s.d. were not significant (CD/VS: 1.11±0.01 (RW) and 1.11±0.01 (s.d.); PU/VS: 1.42±0.01 (RW) and 1.42±0.01 (s.d.); P>0.2, two-sided paired t-test, df=13).
Figure 2.
D2/D3R binding. (a) Average non-displaceable binding potential (BPND) values reflecting D2/D3R levels were computed in three bilateral anatomical striatal regions of interest (ROIs): ventral striatum (VS), dorsal caudate (CD) and putamen (PU), superimposed on three orthogonal views of the human brain. (b) Average BPND maps across subjects for the sleep deprivation (SD) and rested wakefulness (RW) conditions, highlighting the high availability of D2/D3R in the striatum. (c) Bar plot quantifying the average BPND measures in the ROIs for RW and SD and highlighting the significantly lower availability of D2/D3R for SD than for RW (*P<0.05, two-sided). Sample size: 14 healthy, non-smoking, right-handed men. Error bars are s.e.m.
D2/D3R and brain activation
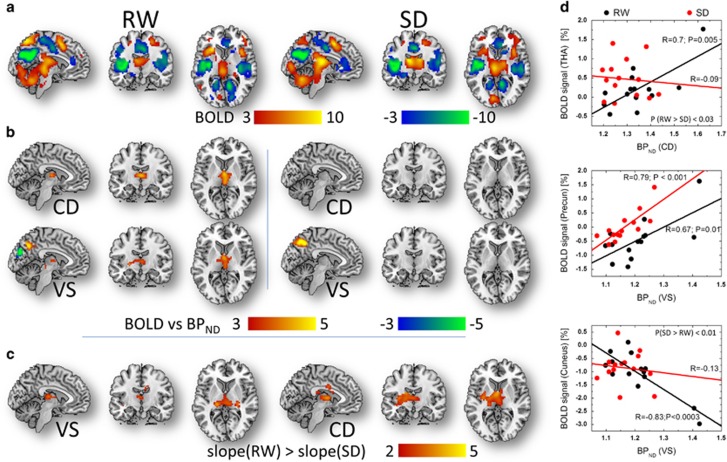
The SLR analysis revealed that fMRI signals in the thalamus increased linearly with D2/D3R across subjects during RW but not during SD, independently for CD, VS and PU (PFWE<0.003; Figure 3b and Table 1). The slopes of the linear associations between fMRI signals in the anterior thalamus and D2/D3R in the CD, and between fMRI signals in the posterior thalamus and D2/D3R in the VS were significantly steeper for RW than for SD (PFWE<0.02; Figure 3c and Table 1). During RW, higher availability of D2/D3R in the VS were associated with increased activation in precuneus and increased deactivation in cuneus; during SD only the fMRI signals in precuneus showed a linear association with D2/D3R in VS (PFWE<0.001; Figure 3b and Table 1). Figure 3d exemplifies the linear associations between D2/D3R measures in the striatum and fMRI signals in the thalamus, precuneus and cuneus, independently for RW and for SD.
Figure 3.
Visual attention activation versus dopamine (DA) receptors. Statistical significance (t-score) maps of brain activation responses for (a) rested wakefulness (RW) and for sleep deprivation (SD) conditions superimposed on three orthogonal views of the human brain (PFWE<0.0001) and (b) simple linear regression (SLR) slopes demonstrating the linear association across subjects between brain activation responses and D2/D3R separately for caudate (CD) and ventral striatum (VS; PFWE<0.001). (c) For VS and CD, the SLR slopes in the thalamus were significantly steeper for RW than for SD (PFWE<0.02). (d) Scatter plots showing the linear associations between D2/D3R measures in caudate (CD) and ventral striatum (VS), and the blood-oxygen-level dependent (BOLD) signals in thalamus, precuneus and cuneus, independently for the rested wakefulness (RW) and sleep deprivation (CD) conditions. Sample size: 14 healthy, non-smoking, right-handed men. FWE, family-wise error.
Table 1. Statistical significance for the linear associations between striatal D2/D3R measures and brain activation responses (BOLD) during the visual attention (VA) task under sleep deprivation (SD) and rested wakefulness (RW) conditions.
| Region | MNI coordinates (mm) | Brain activation | Session |
D
2
/D
3
R-BOLD SLR
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
Cluster level
|
Voxel level
|
|||||||||
| Name | BA/nucleus | x | y | z | VA, T | VA load, T | SD>RW, T | PFWE-corr. | k | PFWE-corr. | T | ||
| Caudate (CD) | |||||||||||||
| Thalamus | Anterior | 0 | −6 | 6 | 5.7 | NS | NS | RW | 0.001 | 220 | <0.0005 | 4.5 | |
| Middle Occipital | 19 | −27 | −84 | 24 | −4.1 | −1.7 | NS | SD | 0.023 | 109 | 0.006 | −4.5 | |
| Ventral striatum (VS) | |||||||||||||
| Precuneus | 7 | 3 | −63 | 39 | −7.0 | NS | NS | RW | 0.001 | 222 | <0.0005 | 5.3 | |
| Thalamus | Anterior | 0 | −3 | 6 | 4.0 | NS | NS | RW | 0.003 | 179 | 0.001 | 4.2 | |
| Cuneus | 18 | 6 | −81 | 27 | −12.0 | −1.7 | 2.0 | RW | 0.03 | 101 | 0.008 | −6.4 | |
| Precuneus | 7 | 6 | −54 | 45 | NS | NS | 2.2 | SD | 0.001 | 217 | <0.0005 | 5.8 | |
| Globus pallidus (GP) | |||||||||||||
| Thalamus | Ventral posterior | 24 | −15 | 0 | NS | NS | NS | RW | 0 | 389 | <0.0005 | 4.7 | |
| Precuneus | 7 | 0 | −63 | 36 | −12.3 | NS | NS | RW | 0.004 | 170 | 0.001 | 4.7 | |
| Cuneus | 18 | 6 | −81 | 27 | −12.0 | −1.7 | 2.0 | RW | 0.031 | 101 | 0.008 | −5.6 | |
| Middle Occipital | 19 | −27 | −78 | 33 | −9.0 | −2.4 | NS | RW | 0.015 | 125 | 0.004 | −5.6 | |
| Middle Occipital | 39 | 42 | −78 | 18 | 3.0 | NS | NS | RW | 0 | 283 | <0.0005 | −4.9 | |
| Putamen (PU) | |||||||||||||
| Thalamus | Ventral posterior | 24 | −12 | 0 | NS | NS | 1.7 | RW | 0 | 355 | <0.0005 | 4.5 | |
| Middle Occipital | 19 | −27 | −78 | 33 | −9.0 | −2.4 | NS | RW | 0.002 | 194 | 0.001 | −6.7 | |
| Middle Occipital | 39 | 42 | −78 | 18 | 3.0 | NS | NS | RW | 0 | 340 | <0.0005 | −5.3 | |
| Lingual | 37 | 24 | −51 | −9 | −3.9 | NS | NS | RW | 0.005 | 164 | 0.001 | −4.6 | |
| CD | |||||||||||||
| Thalamus | Pulvinar | 18 | −24 | 15 | 8.3 | NS | 2.8 | RW>SD | 0.02 | 430 | 0.001 | 5.0 | |
| VS | |||||||||||||
| Thalamus | Pulvinar | 18 | −24 | 18 | 7.1 | NS | 2.5 | RW>SD | 0.002 | 665 | <0.0005 | 5.5 | |
Abbreviations: BOLD, blood-oxygen-level dependent; FWE-corr., family-wise error corrected; NS, not significant; SLR, simple linear regression.
Sample size: 14 healthy non-smoking men.
Balanced influence of D2/D3R in dorsal versus ventral striatum on fMRI signals
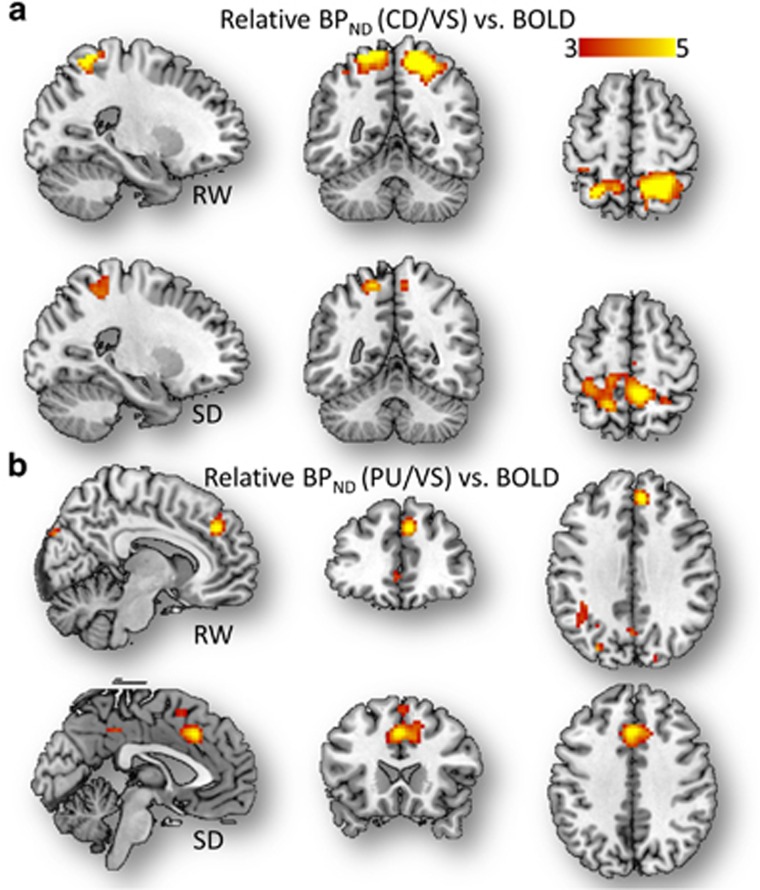
The SLR analysis also revealed significant linear associations between the ‘relative’ CD-to-VS ratio of D2/D3R measures and the fMRI signals in SPC (positive slope), regions that showed prominent brain activation to the VA task during RW but attenuated activation during SD (Table 2), and in precuneus (negative slope), a region that showed significant fMRI deactivation (negative BOLD signals) during the VA tasks, independently for RW and for SD (PFWE<0.03, cluster corrected for multiple comparisons in the whole brain; Figure 4 and Table 2).
Table 2. Statistical significance for the linear associations between relative striatal D2/D3R measures and brain activation responses (BOLD) during the visual attention (VA) task under sleep deprivation (SD) and rested wakefulness (RW) conditions.
| Region | MNI coordinates (mm) | Brain activation | Session |
Relative D
2
/D
3
R-BOLD SLR
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
Cluster level
|
Voxel level
|
|||||||||
| Name | BA | x | y | z | VA, T | VA load, T | SD>RW, T | PFWE-corr. | k | PFWE-corr. | T | ||
| Caudate-to-ventral striatum ratio (CD/VS) | |||||||||||||
| Superior parietal | 7 | 27 | −57 | 63 | 14.9 | NS | −3.1 | RW | 0.003 | 186 | 0.001 | 7.3 | |
| Superior parietal | 5 | −18 | −51 | 66 | 4.5 | NS | −2.4 | RW | <0.0005 | 382 | <0.0005 | 6.5 | |
| Precuneus | 7 | 3 | −66 | 39 | −9.6 | NS | −1.9 | RW | 0.028 | 103 | 0.007 | −4.4 | |
| Precuneus | 5 | −6 | −42 | 60 | −6.4 | 2.3 | NS | SD | <0.0005 | 514 | <0.0005 | 5.7 | |
| Precuneus | 7 | 9 | −69 | 33 | −14.0 | 1.7 | NS | SD | 0.007 | 148 | 0.002 | 5.4 | |
| Globus pallidus-to-ventral striatum ratio (GP/VS) | |||||||||||||
| Supramarginal | 40 | −57 | −39 | 27 | −5.5 | −1.8 | NS | RW | 0.005 | 160 | 0.001 | 4.8 | |
| Cingulum | 32 | 0 | 21 | 42 | 11.9 | 3.5 | NS | SD | 0.004 | 172 | 0.001 | −5.8 | |
| Putamen-to-ventral striatum ratio (PU/VS) | |||||||||||||
| Lingual | 18 | −15 | −87 | −6 | −2.4 | NS | NS | RW | <0.0005 | 349 | <0.0005 | 5.8 | |
| Calcarine | 17 | 15 | −60 | 15 | −14.1 | −3.1 | 3.0 | RW | <0.0005 | 287 | <0.0005 | 5.5 | |
| Cingulum | 24 | 0 | 24 | 39 | 7.8 | 1.7 | NS | SD | 0.006 | 155 | 0.002 | −5.5 | |
Abbreviations: BOLD, blood-oxygen-level dependent; FWE-corr., family-wise error corrected; NS, not significant; SLR, simple linear regression.
Sample size: 14 healthy non-smoking men.
Figure 4.
Parietal activation versus relative D2/D3R in dorsal to ventral striatum. (a and b) Statistical significance (t-score) maps for simple linear regression (SLR) slopes demonstrating the linear association across subjects between brain activation responses and the caudate (CD) to ventral striatum (VS) (a) and putamen (PU) to VS (b) ratios of D2/D3R measures for rested wakefulness (RW) and for sleep deprivation (SD), superimposed on three orthogonal views of the human brain. Sample size: 14 healthy, non-smoking, right-handed men. Significance threshold: PFWE<0.002, cluster corrected for multiple comparisons in the whole brain. BOLD, blood-oxygen-level dependent; FWE, family-wise error.
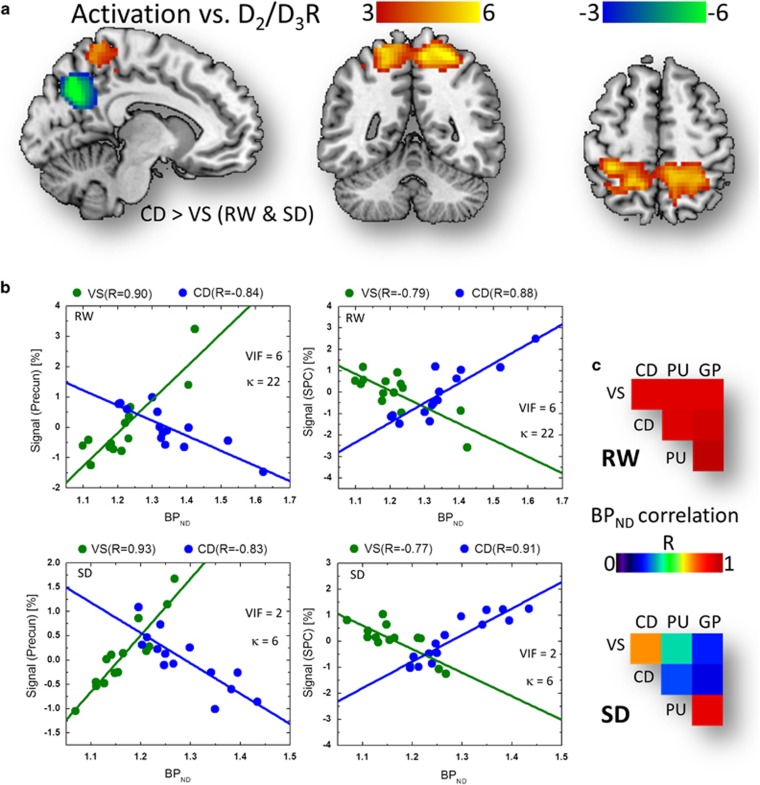
The MLR analysis showed a bilinear association between brain activation responses in parietal cortex and D2/D3R in VS and in CD (Figure 5a). Specifically, in precuneus, the fMRI responses predicted by D2/D3R in VS showed a positive correlation with BPNDVS, whereas those predicted by D2/D3R in CD showed a negative correlation with BPNDCD (PFWE<0.0005, cluster corrected for multiple comparisons in the whole brain; RW and SD conjunction contrast), and the MLR slope was significantly steeper for VS than for CD (αVS>αCD, PFWE<0.0005; Figure 5b). Conversely, the predicted responses in SPC showed negative correlation with BPNDVS and positive correlation with BPNDCD (PFWE<0.0005), and the MLR slope was significantly steeper for CD than for VS (αCD>αVS, PFWE<0.002; Figure 5b). Although the SLR association between the relative CD-to-VS D2/D3R measures and the fMRI signals accounted for less than 22% of the variance in the fMRI data, the MLR association accounted for more than 52% of the variance in the fMRI signal in SPC and precuneus. However, because the BPNDCD and BPNDVS regressors exhibited high correlation (R=0.91 for RW and 0.71 for SD; Figure 5c), we evaluated the risk of multicollinearity in the MLR model using the variance inflation factor, VIF=1/(1−R2), and the condition number, κ=|λmax/λmin|, a standard measure reflecting the ratio between the maximum and minimum eigenvalues, λ, of the correlation matrix computed from BPNDVS and BPNDCD. Depending on κ and VIF, the significance of the multicollinearity problem is usually classified as low (κ<30, VIF<10) or high (κ>30, VIF>10).60, 61 In the present work, the risk of multicollinearity for the BPNDCD and BPNDVS regressors did not exceed these thresholds for any of the sessions and was lower for SD (κ=6 and VIF=2) than for RW (κ=28 and VIF=6).
Figure 5.
Balanced dopaminergic (DAergic) effects on parietal activation. (a) Statistical significance (t-score) maps for multiple linear regression (MLR) slopes demonstrating the linear associations across subjects between average non-displaceable binding potential (BPND) measures in caudate (CD) and ventral striatum (VS) and brain activation responses in the superior parietal cortex (SPC; red-yellow pattern) and precuneus (blue-green pattern) during visual attention for rested wakefulness (RW) and for sleep deprivation (SD; conjunction analysis), superimposed on three orthogonal views of the human brain. Significance threshold: PFWE<0.002, cluster corrected for multiple comparisons in the whole brain. (b) Scatter plots showing the linear associations between the predicted signals (BPNDVS and BPNDCD; see the 'Methods' section) in SPC and precuneus and the corresponding BPND measures in CD and VS. (c) BPND correlation matrix showing the Pearson correlation factors (R; computed across subjects) between average D2/D3R measures in VS, CD, putamen (PU) and globus pallidus (GP), for RW and for SD conditions. Sample size: 14 healthy, non-smoking, right-handed men. FWE, family-wise error; κ, condition number; VIF, variance inflation factor.
The fMRI responses in SMA, a PFC region that was increasingly activated by parametric VA load increases (BOLD signal=0.52±0.07% load effect=0.16%±0.10% mean±90% confidence interval; Table 2) and in ACC increased in proportion to the ‘relative’ PU-to-VS ratio (PU/VS) of BPND measures. Visual cortex deactivation was enhanced by VA load increases and attenuated by SD, and decreased in proportion to the relative PU-to-VS ratio of BPND measures during RW (PFWE<0.005; Figure 4 and Table 2). Similarly during SD, ACC activation showed a negative association with the PU-to-VS ratio of BPND measures (Table 1; PFWE<0.001).
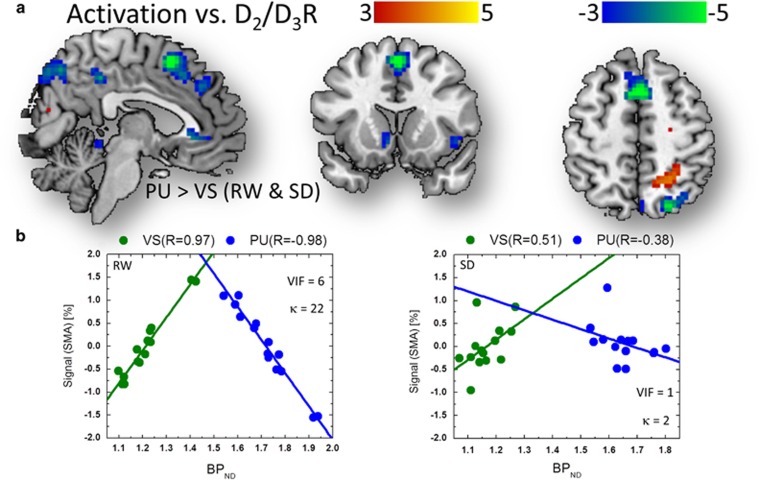
The MLR analysis confirmed the bilinear association between brain activation responses and D2/D3R in VS and PU during RW and SD (Figure 6a). Specifically, in SMA, the fMRI responses predicted by D2/D3R in VS showed a positive linear association with BPNDVS, whereas those predicted by D2/D3R in PU showed a negative linear association with BPNDPU (PFWE<0.03; RW and SD conjunction contrast), and the MLR slope was significantly steeper for VS than for PU (αVS>αPU, PFWE<0.005; Figure 6b). In cuneus, the fMRI responses predicted by D2/D3R in PU showed a positive correlation with BPNDPU, whereas those predicted by D2/D3R in VS showed a negative correlation with BPNDVS (PFWE<0.001; RW and SD conjunction contrast), and the MLR slope was significantly steeper for PU than for VS (αPU>αVS, PFWE<0.005). The SLR association accounted for 38% of the variance in the fMRI data in SMA during RW (27% during SD). The MLR association accounted for 52% of the variance in the fMRI signal in SMA during RW (27% during SD). The risk of multicollinearity for the BPNDPU and BPNDVS regressors was lower for SD (κ=2 and VIF=1) than for RW (κ=22 and VIF=6).
Figure 6.
Balanced dopaminergic (DAergic) effects on prefrontal activation. (a) Statistical significance (t-score) maps for multiple regression analysis (MLR) slopes demonstrating the negative linear associations across subjects between average non-displaceable binding potential (BPND) measures in putamen (PU) and ventral striatum (VS) and brain activation responses in the supplementary motor area (SMA; blue-green pattern) during visual attention for rested wakefulness (RW) and for sleep deprivation (SD; conjunction analysis), superimposed on three orthogonal views of the human brain. Significance threshold: PFWE<0.005, cluster corrected for multiple comparisons in the whole brain. (b) Scatter plots showing the linear associations between the predicted signals (BPNDVS and BPNDPU; see the 'Methods' section) in SMA and the corresponding BPND measures in PU and VS.
Sleep-deprivation effects: behavior vs brain activation
Across all ball-tracking conditions, SD-related decreases in performance accuracy were linearly associated with SD-related decreases in VA activation in the PFC (BA=24; R=0.52; P<0.0004; linear regression, df=41).
Discussion
Here we demonstrate a distinct involvement of D2/D3R in the different striatal regions in the fMRI activation of brain regions involved in the alerting, orienting and executive components of attention2 during the VA task. We found that D2/D3R in dorsal striatum counterbalance D2/D3R in ventral striatum in the modulation of activation responses to a VA task, which corroborates our previous findings using a sensorimotor reaction time task.38 We also found that the SD-related reduction in the availability of D2/D3R in the striatum was associated with (1) decreased strength in the linear association between thalamic activation and D2/D3R in CD, PU and VS during SD and (2) a robust bilinear association between the activation of frontal and parietal regions and D2/D3R in dorsal relative to ventral striatal regions that attenuated the effects of SD. This study also documents a counterbalanced association between caudate versus VS D2/D3R in the deactivation of the default-mode network during VA.
Thalamus
The thalamus, the gateway to the cortex,62 is essential for alerting attention2 and for arousal63 and has an important role in the regulation of sleep and wakefulness.64 Here we show for the first time the role of D2/D3R-mediated dopamine signaling in the activation of the thalamus. Specifically, thalamic activation increased in proportion to D2/D3R in the striatum during the RW condition but not during the SD condition, when D2/D3R availability was significantly reduced and thalamic activation was higher than for the RW condition. As the thalamus mediates the interaction between attention and arousal in humans63 and is involved in the alerting component of attention,2, 65, 66 the increased thalamic activation14, 15, 16, 17, 30, 67 likely reflects an adaptation to compensate for reduced DAergic signaling due to lower D2/D3R during SD. Previous studies have documented associations between striatal D2/D3R and cortical fMRI responses to emotion, visual attention, decision-making and inhibitory control tasks.34, 35, 68, 69, 70 These studies, however, did not report an association between D2/D3R and fMRI signals in the thalamus. Dopamine is a neuromodulator that changes the efficacy of other neurotransmitters as a function of ongoing neuronal activity.71 The effect of DA on neuronal firing is believed to improve signal to noise for the detection of task-specific neuronal activation in electrophysiological studies.72, 73 Thus, by decreasing non-task-related activity, DA stimulation increases efficiency and results in lower activation of task-specific regions.72 Therefore, the higher thalamic activation for SD than for RW is consistent with decreased efficiency due to lower DAergic signaling during SD. Alternatively it could also reflect an increased modulation by noradrenergic signaling as SD also disrupt noradrenergic activity.74
SPC
The SPC is essential for orienting attention2, 75 and projects to multiple cortical and subcortical areas (including thalamus) and is engaged in cognitive operations such as selective attention and top-down control of attention.31, 76, 77, 78, 79, 80, 81, 82, 83, 84 Here we show that the fMRI signals in SPC increased in proportion to the relative availability of D2/D3R in CD to that in VS such that the higher the CD-to-VS ratio of D2/D3R, the higher the activation in SPC. The SPC, which is consistently activated by the VA task,39, 43, 44, 46, 48, 85 showed lower fMRI activation during SD than during RW.30 However, significant differences between RW and SD in the linear association of SPC activation and striatal D2/D3R were not found. Thus, the lower cortical activation for SD than for RW commonly reported in neuroimaging studies14, 15, 16, 17, 30, 67, 86, 87, 88, 89, 90 likely reflects effects of SD on other neurotransmitter systems (that is, cholinergic or noradrenergic).
The MLR findings suggest that D2/D3R in CD and VS have distinct roles in the modulation of SPC responses during VA. Indeed, the association between D2/D3R and fMRI signals in SPC was significantly stronger when two regressors (BPNDVS and BPNDCD; R2=0.52) were used in the MLR model, compared with one regressor (BPNDVS/BPNDCD; R2=0.22). This finding supports the existence of a balanced D2/D3R modulation of cortical activation responses from CD and VS, which is consistent with our recent findings using a sensorimotor reaction time task in a different sample of healthy subjects.38 The reproducibility of the MLR findings across the RW and SD conditions strongly supports the existence of a balanced D2/D3R modulation between CD and VS for the SPC activation to a VA task that is robust to the SD challenge.
SMA and ACC
The ACC and PFC have been implicated in the executive component of attention2, 75 and are involved in target detection and awareness.91 We found an association between the relative availability of D2/D3R in the striatum and the fMRI signals in ACC and SMA, such that increased D2/D3R in VS proportionally increased the fMRI signal in ACC/SMA and increased D2/D3R in PU proportionally decreased it. These findings are consistent with the well-established role of DA on executive function in the human brain,92 including its role in response control.93 DA modulation in ACC is important for executive function,94, 95 and DA modulation in SMA is important for response inhibition and response initiation.93, 96, 97 Though most studies on the DAergic modulation of executive function identify the CD as the striatal region that mediates this effect,98, 99, 100 others implicate the PU.101, 102, 103 Our findings suggest that during the VA task, DA modulates executive attention through counterbalanced D2/D3R signaling from PU and VS. Interestingly, fMRI activation in SMA and ACC and its association with D2/D3R did not differ for SD and RW, providing support for a robust and balanced DAergic modulation of executive attention.
Precuneus
The fMRI signals in the ventral anterior precuneus showed linear association with the ‘relative’ availability of D2/D3R in CD and VS such that the higher the CD-to-VS ratio of D2/D3R, the greater the deactivation in precuneus, both during RW and during SD. The MLR findings suggest that D2/D3R in CD and VS mediate a balanced modulation of deactivation in precuneus, which is reproducible across sessions and robust to the SD challenge. This is consistent with the role of DA in the modulation of the precuneus,56, 104 a major hub in the default-mode network105, 106 that deactivates during the VA task.47 Note that a recent study on functional subdivisions of the precuneus revealed that ventral anterior precuneus, but not the dorsal precuneus, is connected to the default-mode network.107 This major association area has reciprocal connections with superior and inferior parietal, prefrontal, and occipital cortices as well as subcortical regions,108 including the thalamus.109 The precuneus, is also involved in alertness110 and activates during spatial43, 47, 111 and orienting79, 112 attention. Because DA innervation in the parietal cortex is scarce,113, 114 the association between D2/D3R documented here suggests indirect DA modulation through thalamo–cortical pathways rather than a direct modulation. The enhanced deactivation of the precuneus in subjects with higher CD-to-VS ratio of D2/D3R could reflect regulation of CD in orienting attention by facilitating attention processing while inhibiting the posterior default-mode network.
We have shown that SD decreases the specific binding of [11C]raclopride (measured as reduced D2/D3 receptor availability in striatum), which we initially interpreted to reflect increased competition for binding secondary to an increase in DA release during SD.11 However, a follow-up study showed that the changes in DA triggered by the stimulant drug methylphenidate were not affected by SD, which was a finding not consistent with SD increasing DA release.13 Moreover this was supported by microdialysis experiments in which we showed that SD did not increase DA release.13 This led us to conclude that the decreases in [11C]raclopride’s specific binding reflected a downregulation of D2/D3 receptors in striatum by SD. Though the mechanisms underlying the D2/D3 receptor downregulation by SD are unclear, we speculated that increases in adenosine following SD mediate the internalization of D2/D3 receptors.115, 116 Indeed, we subsequently showed that caffeine, which is an adenosine antagonist led to an increase in D2/D3 receptors in striatum, presumably by interfering with adenosine-mediated internalization of D2/D3 receptors.117 Regardless of the mechanism, what our current findings are showing is that despite the overall reductions in striatal D2/D3 receptors with SD the activation/deactivation in ACC, SMA, SPC and precuneus to VA is buffered by the counterbalanced modulation of D2/D3 receptor signaling in the dorsal relative to the VS through the indirect striatocortical pathway.
Limitations
The multicollinearity of the D2/D3R regressors limits the generalizability of our approach. As the multicollinearity problem increases, the regression model estimates become unstable and their standard errors might get inflated. As multicollinearity is considered a potential concern only if VIF>10 or κ>30,60, 61 the MLR model for the RW condition (VIF=6 and κ=28) was deemed viable. Furthermore, similar MLR patterns were observed for the SD condition that had significantly lower multicollinearity risk (VIF<2 and κ<6) than the RW condition, demonstrating the reproducibility of the MLR findings. Also we ascribe a modulatory role to D2/D3R on the activation responses to the VA task on the basis of finding significant associations, but future studies that vary the levels of DA signaling are needed to confirm this. We cannot assess the influence of NA (noradrenaline) on VA activation. It is known that the DAergic circuits interact with NAergic circuits118 and that wakefulness-promoting medications such as modafinil may enhance arousal in humans by activation of the NAergic locus coeruleus.119 Thus, the SD-related activation changes may reflect NA changes to sustain arousal during SD.
In conclusion, our study documents a significant involvement of DA signaling through striatal D2/D3R in the orchestration of visual attention. SD disrupted DA’s regulation of the thalamus but not that of the SPC and PFC. Our findings also corroborate a balanced involvement of D2/D3R signaling in dorsal striatum (CD and PU) versus that in VS for the regulation of brain activation in regions involved in the VA task.
Acknowledgments
We thank Lisa Muench, Colleen Shea, Joanna Fowler and Youwen Xu for radiopharmaceutical preparation and quality control; Millard Jayne, Yana Studentsova, Frank Telang, Pauline Carter and Barbara Hubbard for subject care and protocol oversight; Karen Apelskog for protocol coordination; Michael Schueller for cyclotron operations; Dave Alexoff and Jean Logan for pharmacokinetic modeling and Ruiliang Wang for MRI data acquisition. We also thank the subjects who volunteered to participate in this study. The research was supported by the National Institute of Health’s Intramural Research Program (National Institute on Alcohol Abuse and Alcoholism; Y1AA-3009) using infrastructure of Brookhaven National Laboratory under Contract DE-AC02-98CH10886.
Footnotes
The authors declare no conflict of interest.
References
- Lim J, Dinges D. Sleep deprivation and vigilant attention. Ann N Y Acad Sci 2008; 1129: 305–322. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss B, Fossella J, Flombaum J, Posner M. The activation of attentional networks. Neuroimage 2005; 26: 471–479. [DOI] [PubMed] [Google Scholar]
- Posner M, Rothbart M, Sheese B, Voelker P. Control networks and neuromodulators of early development. Dev Psychol 2012; 48: 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J, AC N, Frith C. The noradrenergic alpha2 agonist clonidine modulates behavioural and neuroanatomical correlates of human attentional orienting and alerting. Cereb Cortex 2001; 11: 73–84. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol 2002; 67: 53–83. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Fowler J, Logan J, Gerasimov M, Maynard L et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001; 21: RC121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L, Houle S, Kapur S, Busto U. Oral D-amphetamine causes prolonged displacement of [11C]raclopride as measured by PET. Synapse 2004; 51: 27–31. [DOI] [PubMed] [Google Scholar]
- Volkow N, Fowler J, Logan J, Alexoff D, Zhu W, Telang F et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA 2009; 301: 1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken B, Salinsky M, Elsas S. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin Neurophysiol 2006; 119: 1885–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden C, Housden C, Aggarwal R, Sahakian B, Darzi A. Effect of pharmacological enhancement on the cognitive and clinical psychomotor performance of sleep-deprived doctors: a randomized controlled trial. Ann Surg 2012; 255: 222–227. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Telang F, Fowler J, Logan J, Wong C et al. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci 2008; 28: 8454–8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers U, Veltman D, van Tol M, Kloet R, Boellaard R, Lammertsma A et al. Neurophysiological effects of sleep deprivation in healthy adults, a pilot study. PLoS One 2015; 10: e0116906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Tomasi D, Wang G, Telang F, Fowler J, Logan J et al. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J Neurosci 2012; 32: 6711–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M, Tan J, Zheng H, Parimal S, Weissman D, Zagorodnov V et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci 2008; 28: 5519–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A, Rakitin B, Basner R, Gazes Y, Steffener J, Stern Y. fMRI activation during failures to respond key to understanding performance changes with sleep deprivation. Behav Brain Res 2011; 218: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Huettel S, Chuah L, Payne J, Chee M. Sleep deprivation biases the neural mechanisms underlying economic preferences. J Neurosci 2011; 31: 3712–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M, Chuah L, Venkatraman V, Chan W, Philip P, Dinges D. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: correlations of fronto-parietal activation with performance. Neuroimage 2006; 31: 419–428. [DOI] [PubMed] [Google Scholar]
- Wesensten N, Belenky G, Kautz M, Thorne D, Reichardt R, Balkin T. Maintaining alertness and performance during sleep deprivation: modafinil versus caffeine. Psychopharmacology (Berl) 2002; 159: 238–247. [DOI] [PubMed] [Google Scholar]
- Wesensten N, Killgore W, Balkin T. Performance and alertness effects of caffeine, dextroamphetamine, and modafinil during sleep deprivation. J Sleep Res 2005; 14: 255–266. [DOI] [PubMed] [Google Scholar]
- Pilcher J, Huffcutt A. Effects of sleep deprivation on performance: a meta-analysis. Sleep 1996; 19: 318–326. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne J, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults—a model for healthy aging? Sleep 2000; 23: 1067–1073. [PubMed] [Google Scholar]
- Harrison Y, Horne J. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl 2000; 6: 236–249. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne J. Sleep loss and temporal memory. Q J Exp Psychol A 2000; 53: 271–279. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Söderström M, Karlsson A, Lekander M, Akerstedt T, Lindroth N et al. Less effective executive functioning after one night's sleep deprivation. J Sleep Res 2005; 14: 1–6. [DOI] [PubMed] [Google Scholar]
- Hsieh S, Cheng I, Tsai L. Immediate error correction process following sleep deprivation. J Sleep Res 2007; 16: 137–147. [DOI] [PubMed] [Google Scholar]
- Tsai L, Young H, Hsieh S, Lee C. Impairment of error monitoring following sleep deprivation. Sleep 2005; 28: 707–713. [DOI] [PubMed] [Google Scholar]
- Jennings J, Monk T, van der Molen M. Sleep deprivation influences some but not all processes of supervisory attention. Psychol Sci 2003; 14: 473–479. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Hitzemann R, Fowler J, Pappas N, Lowrimore P et al. Depression of thalamic metabolism by lorazepam is associated with sleepiness. Neuropsychopharmacology 1995; 12: 123–132. [DOI] [PubMed] [Google Scholar]
- Fiset P, Paus T, Daloze T, Plourde G, Meuret P, Bonhomme V et al. Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study. J Neurosci 1999; 19: 5506–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang R, Telang F, Boronikolas V, Jayne M, Wang G et al. Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex 2009; 19: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Geng J, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol 2004; 14: 212–217. [DOI] [PubMed] [Google Scholar]
- Graham GD, Howseman AM, Rothman DL, Lantos G, Fayad PB, Brass LM et al. Proton magnetic resonance spectroscopy of metabolites after cerebral infarction in humans. Stroke 1991; 22: 143. [Google Scholar]
- Posner M, Rothbart M. Toward a physical basis of attention and self regulation. Phys Life Rev 2009; 6: 103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio S, Romero M, Romero F, Wong C, Alia-Klein N, Tomasi D et al. Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse 2010; 64: 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani D, Lee B, Robertson C, Tabibnia G, Morgan A, De Shetler N et al. Striatal dopamine D2/D3 receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci 2012; 32: 7316–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 1993; 14: 169–177. [DOI] [PubMed] [Google Scholar]
- Volkow N, Gur R, Wang G, Fowler J, Moberg P, Ding Y et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 1998; 155: 344–349. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Wang G, Volkow N. Balanced modulation of striatal activation from D2/D3 receptors in caudate and ventral striatum: disruption in cannabis abusers. Hum Brain Mapp 2015; 36: 3154–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Practice-induced changes of brain function during visual attention: a parametric fMRI study at 4 Tesla. Neuroimage 2004; 23: 1414–1421. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler J, Volkow N, Wolf A, Dewey S, Schlyer D et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 1990; 10: 740–747. [DOI] [PubMed] [Google Scholar]
- Wang G, Smith L, Volkow N, Telang F, Logan J, Tomasi D et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry 2011; 17: 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RBH. Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol 1998; 80: 2657–2670. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Peters RJ, Koch C, Braun J, Chang L, Ernst T. Brain areas specific for attentional load in a motion tracking task. J Cogn Neurosci 2001; 13: 1048–1058. [DOI] [PubMed] [Google Scholar]
- Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E et al. Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol 2004; 56: 259–272. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein R, Telang F, Maloney T, Alia-Klein N, Caparelli E et al. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psych Res Neuroimaging 2007; 155: 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli E, Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum Brain Mapp 2006; 27: 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli E, Ernst T. Different activation patterns for working memory load and visual attention load. Brain Res 2007; 1132: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli E, Ernst T. Sex differences in sensory gating of the thalamus during auditory interference of visual attention tasks. Neurosci 2008; 151: 1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo-Dukelow M et al. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann Neurol 2009; 65: 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Nakama H, Stokes B, Ernst T. Antiretroviral treatment is associated with increased attentional load-dependent brain activation in HIV patients. J Neuroimmune Pharmacol 2008; 3: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Holt J, Yakupov R, Jiang C, Ernst T. Lower cognitive reserve in the aging human immunodeficiency virus-infected brain. Neurobiol Aging 2013; 34: 1240–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain 2006; 129: 1096–1112. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow N, Wang R, Carrillo J, Maloney T, Alia-Klein N et al. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One 2010; 5: e10815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang R, Wang G, Volkow N. Functional connectivity and brain activation: a synergistic approach. Cereb Cortex 2013; 24: 2619–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N, Wang R, Telang F, Wang G, Chang L et al. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One 2009; 4: e6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N, Wang G, Wang R, Telang F, Caparelli E et al. Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage 2011; 54: 3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T. k-Space based summary motion detection for functional magnetic resonance imaging. Neuroimage 2003; 20: 1411–1418. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Franckowiak RSJ. Statistical parametric maps in functional imaging: a general approach. Hum Brain Map 1995; 2: 189–210. [Google Scholar]
- O’Brien R. A caution regarding rules of thumb for variance inflation factors. Qual Quant 2007; 41: 673–690. [Google Scholar]
- Freud R, Littell R. SAS System for Regression, 3rd Edn. SAS Institute and John Wiley and Sons: Cary, NC, USA, 2003. [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz R. Guarding the gateway to cortex with attention in visual thalamus. Nature 2008; 456: 391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas C, Rees G, Howseman A, Josephs O, Turner R, Frith C. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci 1998; 18: 8979–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux M, Chen J, Lonjers P, Bazhenov M, Timofeev I. The impact of cortical deafferentation on the neocortical slow oscillation. J Neurosci 2014; 34: 5689–5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian B, Lehrer D, Shi B, Narayanan T, Strohmeyer P, Buchsbaum M et al. Measuring dopamine neuromodulation in the thalamus: using [F-18]fallypride PET to study dopamine release during a spatial attention task. Neuroimage 2006; 31: 139–152. [DOI] [PubMed] [Google Scholar]
- Vandewalle G, Balteau E, Phillips C, Degueldre C, Moreau V, Sterpenich V et al. Daytime light exposure dynamically enhances brain responses. Curr Biol 2006; 16: 1616–1621. [DOI] [PubMed] [Google Scholar]
- Ma N, Dinges D, Basner M, Rao H. How acute total sleep loss affects the attending brain: a meta-analysis of neuroimaging studies. Sleep 2015; 38: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast T, Siessmeier T, Wrase J, Braus D, Smolka M, Buchholz H et al. Ratio of dopamine synthesis capacity to D2 receptor availability in ventral striatum correlates with central processing of affective stimuli. Eur J Nucl Med Mol Imaging 2008; 35: 1147–1158. [DOI] [PubMed] [Google Scholar]
- Volkow N, Tomasi D, Wang G, Telang F, Fowler J, Wang R et al. Hyperstimulation of striatal D2 receptors with sleep deprivation: implications for cognitive impairment. Neuroimage 2009; 45: 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Ghahremani D, Morales A, Robertson C, Ishibashi K, Morgan A et al. Risk-taking behavior: dopamine d2/d3 receptors, feedback, and frontolimbic activity. Cereb Cortex 2015; 25: 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin E, Rebec G. Dopaminergic modulation of glutamate-induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol 1996; 75: 142–153. [DOI] [PubMed] [Google Scholar]
- Volkow N, Fowler J, Wang G, Telang F, Logan J, Wong C et al. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS One 2008; 3: e2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E, Thorpe S, Boytim M, Szabo I, Perrett D. Responses of striatal neurons in the behaving monkey. 3. Effects of iontophoretically applied dopamine on normal responsiveness. Neurosci 1984; 12: 1201–1212. [DOI] [PubMed] [Google Scholar]
- Mallick B, Singh A. REM sleep loss increases brain excitability: role of noradrenaline and its mechanism of action. Sleep Med Rev 2011; 15: 165–178. [DOI] [PubMed] [Google Scholar]
- Posner M, Walker J, Friedrich F, Rafal R. Effects of parietal injury on covert orienting of attention. J Neurosci 1984; 4: 1863–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman G. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 2002; 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe J, Wylie G, Javitt D, Robertson I et al. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Res Cogn Brain Res 2004; 20: 132–143. [DOI] [PubMed] [Google Scholar]
- Lawrence N, Ross T, Hoffmann R, Garavan H, Stein E. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci 2003; 15: 1028–1038. [DOI] [PubMed] [Google Scholar]
- Le T, Pardo J, Hu X. 4 T-fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. J Neurophysiol 1998; 79: 1535–1548. [DOI] [PubMed] [Google Scholar]
- de Fockert J, Rees G, Frith C, Lavie N. The role of working memory in visual selective attention. Science 2001; 291: 1803–1806. [DOI] [PubMed] [Google Scholar]
- Leonards U, Sunaert S, Van Hecke P, Orban G. Attention mechanisms in visual search—an fMRI study. J Cogn Neurosci 2000; 12(Suppl 2): 61–75. [DOI] [PubMed] [Google Scholar]
- Adler C, Sax K, Holland S, Schmithorst V, Rosenberg L, Strakowski S. Changes in neuronal activation with increasing attention demand in healthy volunteers: an fMRI study. Synapse 2001; 42: 266–272. [DOI] [PubMed] [Google Scholar]
- Buchel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ. The functional anatomy of attention to visual motion: a functional MRI study. Brain 1998; 121: 1281–1294. [DOI] [PubMed] [Google Scholar]
- Arrington C, Carr T, Mayer A, Rao S. Neural mechanisms of visual attention: object-based selection of a region in space. J Cogn Neurosci 2000; 12(Suppl 2): 106–117. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Caparelli EC, Chang L, Ernst T. fMRI-acoustic noise alters brain activation during working memory tasks. Neuroimage 2005; 27: 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M, Choo W. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci 2004; 24: 4560–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M, Chuah Y. Functional neuroimaging and behavioral correlates of capacity decline in visual short-term memory after sleep deprivation. Proc Natl Acad Sci USA 2007; 104: 9487–9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond S, Brown G, Gillin J, Stricker J, Wong E, Buxton R. Altered brain response to verbal learning following sleep deprivation. Nature 2000; 403: 655–657. [DOI] [PubMed] [Google Scholar]
- Drummond S, Brown G, Stricker J, Buxton R, Wong E, Gillin J. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport 1999; 10: 3745–3748. [DOI] [PubMed] [Google Scholar]
- Chuah Y, Venkatraman V, Dinges D, Chee M. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci 2006; 26: 7156–7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S, Posner M. The attention system of the human brain: 20 years after. Annu Rev Neurosci 2012; 35: 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Ko J, Strafella A. Striatal dopamine release during performance of executive functions: a [(11)C] raclopride PET study. Neuroimage 2006; 33: 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins T. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 2013; 108: 44–79. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cognit Sci 2000; 4: 215–222. [DOI] [PubMed] [Google Scholar]
- MacDonald Ar, Cohen J, Stenger V, Carter C. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000; 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Li C, Huang C, Constable R, Sinha R. Imaging response inhibition in a stop-signal task: neural correlates independent of signal monitoring and post-response processing. J Neurosci 2006; 26: 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky S, Simmonds D. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci 2008; 20: 751–761. [DOI] [PubMed] [Google Scholar]
- Rinne J, Portin R, Ruottinen H, Nurmi E, Bergman J, Haaparanta M et al. Cognitive impairment and the brain dopaminergic system in Parkinson disease: [18 F]fluorodopa positron emission tomographic study. Arch Neurol 2000; 57: 470–475. [DOI] [PubMed] [Google Scholar]
- Marié R, Barré L, Dupuy B, Viader F, Defer G, Baron J. Relationships between striatal dopamine denervation and frontal executive tests in Parkinson's disease. Neurosci Lett 1999; 260: 77–80. [DOI] [PubMed] [Google Scholar]
- Jokinen P, Brück A, Aalto S, Forsback S, Parkkola R, Rinne J. Impaired cognitive performance in Parkinson's disease is related to caudate dopaminergic hypofunction and hippocampal atrophy. Parkinsonism Relat Disord 2009; 15: 88–93. [DOI] [PubMed] [Google Scholar]
- Müller U, Wächter T, Barthel H, Reuter M, von Cramon D. Striatal [123I]beta-CIT SPECT and prefrontal cognitive functions in Parkinson's disease. J Neural Transm 2000; 107: 303–319. [DOI] [PubMed] [Google Scholar]
- Cropley V, Fujita M, Bara-Jimenez W, Brown A, Zhang X, Sangare J et al. Pre- and post-synaptic dopamine imaging and its relation with frontostriatal cognitive function in Parkinson disease: PET studies with [11C]NNC 112 and [18 F]FDOPA. Psychiatry Res 2008; 163: 171–182. [DOI] [PubMed] [Google Scholar]
- Siepel F, Brønnick K, Booij J, Ravina B, Lebedev A, Pereira J et al. Cognitive executive impairment and dopaminergic deficits in de novo Parkinson's disease. Mov Disord 2014; 29: 1802–1808. [DOI] [PubMed] [Google Scholar]
- Braskie M, Landau S, Wilcox C, Taylor S, O'Neil J, Baker S et al. Correlations of striatal dopamine synthesis with default network deactivations during working memory in younger adults. Hum Brain Mapp 2011; 32: 947–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N. Functional connectivity density mapping. Proc Natl Acad Sci USA 2010; 107: 9885–9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow N. Association between functional connectivity hubs and brain networks. Cereb Cortex 2011; 21: 2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li C. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage 2012; 59: 3548–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A, Trimble M. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006; 129: 564–583. [DOI] [PubMed] [Google Scholar]
- Fernández-Espejo D, Soddu A, Cruse D, Palacios E, Junque C, Vanhaudenhuyse A et al. A role for the default mode network in the bases of disorders of consciousness. Ann Neurol 2012; 72: 335–343. [DOI] [PubMed] [Google Scholar]
- Cavanna A. The precuneus and consciousness. CNS Spectr 2007; 12: 545–552. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Sawamoto N et al. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage 1999; 10: 193–199. [DOI] [PubMed] [Google Scholar]
- Simon O, Mangin J, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron 2002; 33: 475–487. [DOI] [PubMed] [Google Scholar]
- Berger B, Trottier S, Verney C, Gaspar P, Alvarez C. Regional and laminar distribution of the dopamine and serotonin innervation in the macaque cerebral cortex: a radioautographic study. J Comp Neurol 1988; 273: 99–119. [DOI] [PubMed] [Google Scholar]
- Herrera-Marschitz M, Goiny M, Utsumi H, Ungerstedt U. Mesencephalic dopamine innervation of the frontoparietal (sensorimotor) cortex of the rat: a microdialysis study. Neurosci Lett 1989; 97: 266–270. [DOI] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A et al. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem 2002; 277: 18091–18097. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela D, Romero-Fernandez W, Tarakanov A, Ciruela F, Agnati L, Fuxe K. On the existence of a possible A2A-D2-β-Arrestin2 complex: A2A agonist modulation of D2 agonist-induced β-arrestin2 recruitment. J Mol Biol 2011; 406: 687–699. [DOI] [PubMed] [Google Scholar]
- Volkow N, Wang G, Logan J, Alexoff D, Fowler J, Thanos P et al. Caffeine increases striatal dopamine D2/D3 receptor availability in the human brain. Transl Psychiatry 2015; 5: e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao H, Li C. Resting-state functional connectivity of the locus coeruleus in humans: in comparison with the ventral tegmental area/substantia nigra pars compacta and the effects of age. Cereb Cortex 2015; doi: 10.1093/cercor/bhv172; e-pub ahead of print. [DOI] [PMC free article] [PubMed]
- Hou R, Freeman C, Langley R, Szabadi E, Bradshaw C. Does modafinil activate the locus coeruleus in man? Comparison of modafinil and clonidine on arousal and autonomic functions in human volunteers. Psychopharmacology (Berl) 2005; 181: 537–549. [DOI] [PubMed] [Google Scholar]