Abstract
Major depressive disorder (MDD) is a serious mental disorder that negatively affects the quality of life of many individuals, and is a heavy economic burden to society. In recent years it was thought that depression is a ‘disconnection syndrome'. Disorganized brain activity and un-modulated emotion responses were considered the key neuropathologies underlying depression. In the present study, we investigated the alteration of whole brain network connectivity in 28 first-episode, drug-naive patients, using resting-state functional magnetic resonance imaging and a new analytical method called voxel-based eigenvector centrality mapping. We found that compared with normal controls, MDD patients had lower functional connectivity in the bilateral middle frontal gyrus, insula, hippocampus, amygdala and cerebellum, and higher functional connectivity in the medial prefrontal cortex. The functional connectivity strength at the right hippocampus (r=−0.413, P=0.032) and the right insula (r=−0.372, P=0.041) negatively correlated with the severity of the disease. We further examined coordination among these regions, and found that frontal–subcortical connection was reduced and insula–mPFC connection was increased. These results are consistent with previous hypotheses on the neural mechanism of MDD, and provide further evidence that emotion networks are already interrupted in early stages of depression.
Introduction
Major depressive disorder (MDD) is a serious psychiatric mood disorder, which typically presents as persistent dysthymia, anhedonia and occasional suicidal ideation and behaviors.1 The lifetime prevalence of MDD ranges from 1.52 to 16.2%3 across various studies. MDD exerts a heavy socioeconomic burden because of increased disability and suicide rates.4 The neuropathology underlying MDD is still unclear. Therefore, even at present, the diagnosis and treatment of depression can be challenging.
In recent years, it has been suggested that depression is not localized to a single brain area and that a network perspective is necessary to explain its complex etiology. Based on the fact that widespread brain areas were found abnormal in MDD patients,5, 6 constituting an ‘emotion network', more and more researchers have begun to believe that depression is a ‘disconnection syndrome'.7, 8 Alteration in important nodes of the emotion network may induce aberrant function of the whole network, causing depression. Specifically, it has been suggested that frontal–subcortical neural circuits play an important role in the pathogenesis of MDD.9 Interrupted coordination within and among large-scale functional networks were also implicated.10, 11 Both gray matter and white matter deficits in these populations might be the reason for disrupted network functions.12, 13, 14 Indeed, a network perspective supports the high heterogeneity of depression, and explains how different treatment methods might take effect.15
Although these hypotheses have found support from neuroimaging-based network studies, inconsistency still exists, possibly due to the different methods used in these studies. In the past, seed-based functional connectivity had been extensively used to explore the coordination between a specific region of interest (ROI) and the whole brain, or among several pre-specified ROIs. While it is useful to test a particular hypothesis, the selection of ROIs is quite subjective. As different researchers use different ROIs, it is difficult to make direct comparisons across studies.16, 17 In addition, the selected ROIs may not be truly representative of the designated region because of segmentation problems18 or template bias19 or high heterogeneity within them. Different ROI selection methods may greatly influence the result of network connectivity analysis.20 This problem also exists in ROI-based whole brain graph theory analysis.21 Despite the fact that some data-driven methods were used, such as independent component analysis, they usually explain sub-network connectivity10 or interaction among large-scale functional networks.22 Therefore, a simple voxel-based, bias-free method is desirable for future studies and clinical applications assessing brain network changes in MDD patients.
In the present study, we aimed to assess the brain network alterations in MDD patients, using resting-state functional magnetic resonance imaging and a new graph-based method called eigenvector centrality mapping (ECM). This method can objectively detect all the brain areas serving as communication hubs, which have greater connectivity with other parts of the brain. It can be performed in a voxel-wise manner and does not require manual selection of the seed regions, therefore it is free of researcher selection bias. We hypothesize that emotion-related networks may have already been interrupted in early MDD patients, and these alterations can be detected using ECM.
Materials and methods
Subjects
We recruited 28 MDD patients in the present study. Unipolar depression was diagnosed according to DSM-IV criteria by an experienced psychiatrist. Scores of the Hamilton Rating Scale for Depression (HRSD), Beck Depression Index (BDI) and the Mini-Mental State Examination (MMSE) were obtained from all subjects. Twenty-seven normal controls (NC) without any history of neurologic or psychiatric disorders, or brain trauma at any time of their lives, were also enrolled. Table 1 shows detailed characteristics of the two groups. All patients were first-episode, drug-naive patients with short disease durations. All the subjects signed written informed consent before taking part in the study. This research was approved by the Medical Ethic Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine.
Table 1. Demographic characteristics.
| Patients | Controls | P | |
|---|---|---|---|
| Age (years) | 30.9±9.5 | 27.5±6.4 | 0.124 |
| M/F | 11/17 | 13/14 | 0.591 |
| Duration (m) | 5.5±3.5 | — | — |
| HRSD | 29.1±4.9 | — | — |
| BDI | 32.9±8.3 | — | — |
Abbreviations: BDI, Beck depression index; F, female; HRSD, Hamilton rating scale for depression; M, male.
fMRI data acquisition
Scanning was carried out on a 3.0 T magnetic resonance scanner (Siemens 3.0T Trio Tim, Munich, Germany). Participants were fitted with soft earplugs, positioned comfortably in the coil, and instructed to relax and remain still. Head motion was minimized with foam pads. Participants were excluded if they had excessive head motion with >2 mm of translation or 2° of rotation, which was confirmed on a workstation immediately after the scanning. High-resolution three-dimensional T1-weighted images were acquired with a three-dimensional magnetization-prepared rapid acquisition gradient echo sequence with the following parameters: repetition time 8.5 ms, echo time 3.4 ms, flip angle 12°, 156 axial slices with 1 mm thickness, axial field of view 24 × 24 cm2 and matrix 256 × 256. Blood oxygen level-dependent images were acquired using an echo planar imaging sequence. The acquisition parameters were: time of repetition=2000 ms, time of echo=30 ms, flip angle=90°, 30 interleaved descending slices and voxel size=3.8 × 3.8 × 5.0 mm3. Several other sequences were also scanned and the total duration for each subject lasted about 40 min.
Preprocessing
Preprocessing was performed using the Data Processing Assistant for Resting-State fMRI (http://www.restfmri.net) and the Resting-State fMRI Data Analysis Toolkit (Rest, V1.8, http://www.restfmri.net), based on statistical parametric mapping-8 (SPM8, www.fil.ion.ucl.ac.uk/spm/) and Matlab R2010 (www.mathworks.com). The first 10 images were excluded from the analysis. The remaining images were corrected for slice timing with the middle slice used as a reference, realigned to remove head motion, normalized into the standard space, and resampled to a 3 × 3 × 3 mm3 voxel size. The resulting images were then smoothed using a 4-mm Gaussian kernel before proceeding to the next step.
Functional connectivity analysis
The human brain is organized as a complex network with small world properties.23 Therefore, graph-based analysis could provide valuable information for elucidating the brain's network structures. Eigenvector centrality is a particular type of graph-based method that identifies important nodes in the network. It does so by counting both the number and the quality of connections so that a node with few connections to some other high-ranking nodes may outrank one with a larger number of low-ranking connections.24 Google's ‘PageRank' algorithm25 is a variant of eigenvector centrality. Like its success in the web search engine, eigenvector centrality has also been proven to be valuable in analyzing human brain networks.26, 27
Here, the ECM of the pre-processed image data was performed using the fast ECM tool (https://code.google.com/p/bias/source/browse/matlab/fastECM), which yielded a voxel-wise measure of relevance to the functional brain network. Compared with the traditional ECM calculation method, the fast ECM tool is faster and computationally more efficient because it computes matrix-vector products without having to compute or store the connectivity matrix.28 Detailed analysis procedures can be seen in the study by Wink et al.28
By using ECM, we successfully identified several brain regions where there was a significant difference of nodal centrality. To further explore the relationship among these areas, and to test whether these findings were consistent with previous theories of the neural mechanism of depression, we calculated the functional connectivity among them. Blood oxygen level-dependent signals were extracted from all significant clusters, and Pearson correlation was calculated in a pair-wise manner. The correlation coefficients were transformed into z distribution using Fisher r-to-z transformation.
Statistical analysis
Age was compared between the two groups using a two-sample t-test. A X2 test was performed to compare the sex differences. For image data, motion correction was assessed using frame wise displacement (FD), which measures the motion of each brain volume compared with the previous volume.29 Mean FD was compared between the two groups using an independent two-sample t-test. Eigenvector centrality differences between the two groups were compared using a two-sample t-test performed in SPM8, during which age and sex were included as covariates. The threshold for ECM analysis was set at P<0.001, voxel size >10, corrected using the false discovery rate method. Significant clusters were used to extract connectivity values from the two groups and to calculate correlation with scale scores. For connectivities among significant clusters, a two-sample t-test was used to examine the difference between the two groups. Multiple comparison correction was performed using the Bonferroni method.
Results
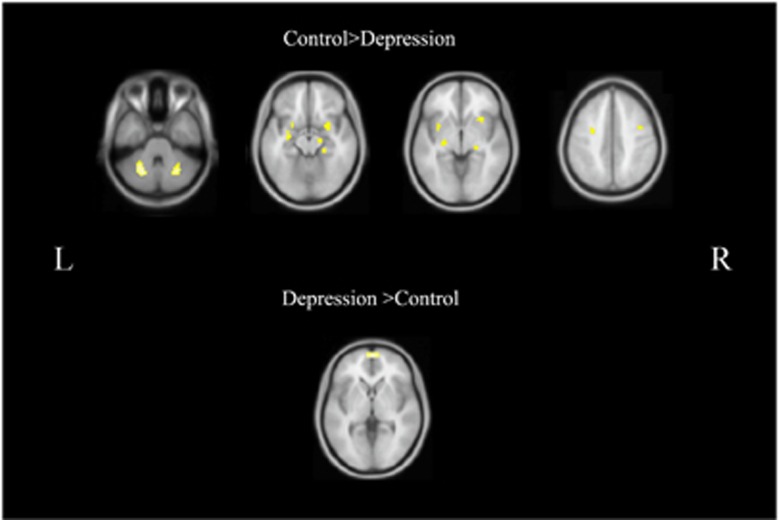
Statistical analysis showed no significant differences in age and sex between the two groups. Analysis of mean FD demonstrated no significant differences between groups. ECM analysis showed that compared with NCs, the patients groups had lower functional connectivity in the bilateral middle frontal gyrus (MFG), insula, hippocampus, amygdala and cerebellum (Figure 1, Table 2). Meanwhile, the patients had increased functional connectivity in the mPFC. The effect size of this comparison was quite high, ranging from 1.33 to 1.69 for each significant cluster. Furthermore, functional connectivity strength at the right hippocampus (r=−0.413, P=0.032) and the right insula (r=−0.372, P=0.041) negatively correlated with the severity of the disease (HRSD). To test whether these alterations were in line with previous hypotheses about depression, we explored the frontal–subcortical connectivity and insula–mPFC connectivity. Six connections were calculated and compared between the two groups. As shown in Figure 2, we found that frontal–subcortical connections were significantly reduced in the patient group (Table 3). In contrast, the connection between left insula and mPFC was significantly increased in the patients group (P=0.003).
Figure 1.
Functional connectivity strength differences between the MDD patients and normal controls. Warm colors indicate areas with higher functional connectivity in normal controls. Cold colors indicate higher functional connectivity in patients. MDD, major depressive disorder.
Table 2. Areas showing significant difference of functional connectivity strength between the two groups.
| Contrast | Voxels | Brain regions | MNI coordinate | Peak t |
|---|---|---|---|---|
| NC>MDD | 145 | Left cerebellum | −21 −63 −30 | 7.18 |
| 79 | Right cerebellum | 27 −63 −33 | 6.47 | |
| 35 | Right hippocampus Right parahippocampal gyrus | 21 −27 −9 | 5.60 | |
| 51 | Left hippocampus Left parahippocampal gyrus Left amygdala | −30 −12 −15 | 5.23 | |
| 65 | Right insula | 30 18 −3 | 5.39 | |
| 22 | Left insula | −33 3 −3 | 5.47 | |
| 12 | Left middle frontal gyrus | −27 −6 42 | 5.21 | |
| 10 | Right middle frontal gyrus | 42 0 42 | 5.19 | |
| NC<MDD | 241 | Medial frontal gyrus | 3 63 3 | 6.41 |
Abbreviations: NC, normal control; MDD, major depressive disorder.
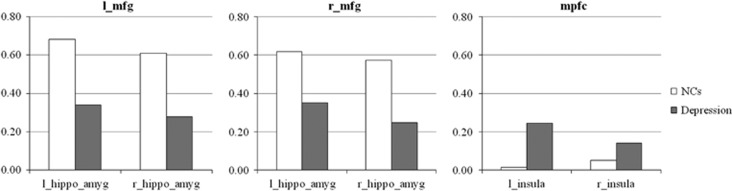
Figure 2.
Frontal–subcortical and mPFC–insula functional connectivities of the two groups. Frontal–subcortical connections were significantly reduced in the patient group. The connection between left insula and mPFC significantly increased in the patients group. hippo_amyg, hippocampus and amygdala; l, left; mfg, left middle frontal gyrus; mPFC, medial prefrontal cortex; r, right.
Table 3. Frontal–subcortical connectivity and insula–mPFC connectivity in the two groups.
| NC | Depression | P-value | |
|---|---|---|---|
| l_middle_frontal_gyrus | |||
| l_hippocampus_amygdala | 0.69±0.29 | 0.34±0.22 | 0.000 |
| r_hippocampus_amygdala | 0.61±0.26 | 0.28±0.17 | 0.000 |
| r_middle_frontal_gyrus | |||
| l_hippocampus_amygdala | 0.62±0.26 | 0.35±0.14 | 0.000 |
| r_hippocampus_amygdala | 0.57±0.23 | 0.25±0.18 | 0.000 |
| medial prefrontal cortex | |||
| l_insula | 0.02±0.26 | 0.25±0.22 | 0.003 |
| r_insula | 0.05±0.28 | 0.14±0.22 | 0.15 |
Abbreviation: NC, normal control.
Ten comparisons had been made, and the threshold for significance is set at P=0.005.
Discussion
To the best of our knowledge, this is the first study using the voxel-based ECM method to assess the whole brain network connectivity of MDD patients. Compared with NCs, depressed patients showed decreased functional connectivity in the frontal lobes, amygdala, hippocampus and cerebellum, and increased functional connectivity in the mPFC. Correlation between functional connectivity strength and HRSD scores was found in the right hippocampus and right insula. Furthermore, we verified aberrant frontal–subcortical coordination and insula–default mode network (DMN) connection in the patient group, which is consistent with previous theories about the mechanism of depression. These results suggest that without choosing ROIs subjectively, we can still detect important emotion network changes in depressed patients using the ECM method, which may have great potential in future studies on network changes of MDD.
First, we found decreased functional connectivity in the bilateral MFG; part of the dorsolateral prefrontal cortex (DLPFC). The DLPFC is primarily responsible for cognitive and executive functions, though it also plays important roles in regulating emotions through reappraisal/suppression strategies.30 Abnormal DLPFC structures and functions have been consistently reported in early MDD patients31, 32 and those with familial risk for MDD.33, 34 It is believed that deficits in the DLPFC may confer vulnerability to depression.35 Here the decreased functional connectivity in the DLPFC implies that its interaction with other brain areas is weakened. The disruption of DLPFC connections has also been documented in several previous studies36, 37 using ROI-based functional connectivity method.
Second, we found decreased functional connectivity in the limbic regions, including amygdala and hippocampus. Limbic structures are very important for spontaneous response to external emotional information. However, the reappraisal and regulation of spontaneous activities in these areas rely on cognitive analysis in higher cortices. Depressed patients often have chaotic and disorganized limbic activities,38, 39 which may be why they more often show uncategorized, biased responses than normal subjects when facing emotional stimuli.40 Besides, there were negative correlations between the functional connectivity strength of the amygdala, hippocampus and HRSD scores, suggesting that the more disorganized they were, the greater the chance the disease would become worse.
Taken together, the decreased frontal–subcortical functional connectivity is consistent with the previous hypothesis about the frontal–subcortical uncoupling in depressed patients.41, 42 When the higher cognitive functions of the DLPFC were damaged, or frontal–subcortical connections were interrupted, activities in subcortical areas were no longer constrained, and became chaotic and blunted. This hypothesis is quite popular and has been tested in several studies using functional imaging methods to study the mechanism of MDD.37, 43, 44 A recent effective connectivity study showed that top–down regulation from the DLPFC to the amygdala was greatly impaired in depressed patients and bottom–up connections were increased.37 To explore whether the changes we found were in line with this hypothesis, we tested the connectivity among bilateral MFG and subcortical structures. The result showed significantly decreased connectivity between the frontal clusters and subcortical areas in depressed patients, confirming frontal–subcortical uncoupling.
Insula connectivity was found to be reduced. The insula also has a well-established role in processing affect and emotion, and has been frequently found to be abnormal in depressed patients.45, 46 Specifically, the anterior insula has rich connections with limbic and cortical regions. Together with the anterior cingulate gyrus, they form an important network called the salience network (SN). The SN is of specific importance for the processing of a stimulus salience47 and regulating balance between the DMN and executive control network,48 drawing much attention from researchers worldwide. As part of the SN, disturbance in the insula may greatly impair its ability in coordinating the patient's attention being directed either toward the external world or the internal perception of self-related processes, resulting in depressive symptoms. A previous study also found decreased anterior insula connectivity, which negatively correlated with disease severity.49
Interestingly, besides decreased functional connectivity, we also found increased functional connectivity in the medial prefrontal cortex of the depressed patients.50 The medial prefrontal cortex is a central hub of the DMN, which has an important role in introspection. The increased functional connectivity strength within the DMN has been consistently reported in studies on depression,51, 52 which could lead to the patient's clinical symptoms, such as rumination.53 Sheline et al.54 reported that depressed patients were characterized by the inability to reduce DMN activity when facing negative stimuli. Using independent component analysis, Zhu et al.55 also revealed increased functional connectivity in anterior medial regions of the depressed patients. Furthermore, after antidepressant treatment, the elevated DMN connectivity in MDD patients could be normalized.56 In general, previous studies mostly agreed that depressed patients had hyper-connectivity in the DMN.51, 53
DMN hyper-connectivity has been ascribed to SN dysfunctions in several previous studies on MDD patients.49, 51 Increased communication between DMN and SN may have helped to form an aberrant circuit causing biased self-referential processes. To further test this theory, we extracted signals from the bilateral insula and mPFC, and calculated their correlations. The results showed that the coordination between the left insula and mPFC was significantly increased in the patient groups, despite that the overall connectivity strength was reduced. Therefore, our results support the theory that biased SN modulation on DMN contribute to depression.
There are several limitations in the present study. First, due to the heavy work load in our hospital, all of our patients were evaluated by only one psychiatrist, instead of two or more, through structured interviewing to diagnose MDD using the SCID (Structured Clinical Interview for DSM Disorders). Hence, some subjective bias might have been introduced during the diagnosis and scale evaluation procedure. Second, although our study had revealed brain network alterations in the patients group, the results were limited to the resting-state brain activities. How these brain areas activate and interact during emotional processing is important and need task-based fMRI investigations. The third limitation is the relatively small sample size. The findings of the present study still need further validation in a larger patient cohort.
In general, our study revealed reduced functional connectivity within several important nodes of emotional networks. These results are consistent with two main theories about brain network disruptions in MDD patients, and suggest that functional brain network had already been interrupted in the early stages of MDD. The ECM method has proven to be a powerful tool in detecting brain network changes, and is of great potential in studying the neuropathology underlying MDD, without the introduction of subjective ROI bias. Therefore, future studies using the ECM method to observe the brain network dynamics related to the development and treatment of MDD are warranted.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81301190).
Author contributions
ZS enrolled the patients; PH analyzed the data and wrote the paper; MZ directed the research. All authors reviewed the manuscript.
The authors declare no conflict of interest.
References
- Belmaker R, Agam G. Major depressive disorder. N Engl J Med 2008; 358: 55–68. [DOI] [PubMed] [Google Scholar]
- Parker G, Gladstone G, Chee KT. Depression in the planet's largest ethnic group: the Chinese. Am J Psychiatry 2001; 158: 857–864. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289: 3095–3105. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry 2003; 64: 1465–1475. [DOI] [PubMed] [Google Scholar]
- Zou K, Deng W, Li T, Zhang B, Jiang L, Huang C et al. Changes of brain morphometry in first-episode, drug-naive, non–late-life adult patients with major depression: an optimized voxel-based morphometry study. Biol Psychiatry 2010; 67: 186–188. [DOI] [PubMed] [Google Scholar]
- Guo W-B, Liu F, Xun G-L, Hu M-R, Guo X-F, Xiao C-Q et al. Reversal alterations of amplitude of low-frequency fluctuations in early and late onset, first-episode, drug-naive depression. Prog Neuropsychopharmacol Biol Psychiatry 2013; 40: 153–159. [DOI] [PubMed] [Google Scholar]
- Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci 2013; 38: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RC et al. Resting-state functional connectivity in treatment-resistant depression. Am J Psychiatry 2014; 168: 642–648. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci 2007; 27: 8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer IM, Beckmann CF, Van Tol M-J, Ferrarini L, Milles J, Veltman DJ et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci 2010; 4: eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry 2011; 70: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Mackay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry 2009; 66: 814–823. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang J, Wu Q, Kuang W, Huang X, He Y et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry 2011; 70: 334–342. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 2008; 213: 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci 2008; 9: 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 2007; 62: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Liu Z, Xu C, Li J, Gao Q, Sun N et al. Disrupted resting-state functional connectivity of the hippocampus in medication-naive patients with major depressive disorder. J Affect Disord 2012; 141: 194–203. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, Lewis DV et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage 2009; 45: 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov R, Chupin M, Williams E, Hammers A, Kesavadas C, Lemieux L. Evaluation of atlas-based segmentation of hippocampi in healthy humans. Magn Reson Imaging 2009; 27: 1104–1109. [DOI] [PubMed] [Google Scholar]
- Marrelec G, Fransson P. Assessing the influence of different ROI selection strategies on functional connectivity analyses of fMRI data acquired during steady-state conditions. PLoS One 2011; 6: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang L, Zang Y, Yang H, Tang H, Gong Q et al. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum Brain Mapp 2009; 30: 1511–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott CC, Lemke NT, Gopal S, Thoma RJ, Bustillo J, Calhoun VD et al. Electroconvulsive therapy response in major depressive disorder: a pilot functional network connectivity resting state FMRI investigation. Front Psychiatry 2013; 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Honey CJ. Small worlds inside big brains. Proc Natl Acad Sci USA 2006; 103: 19219–19220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G, Margulies DS, Horstmann A, Pleger B, Lepsien J, Goldhahn D et al. Eigenvector centrality mapping for analyzing connectivity patterns in fMRI data of the human brain. PLoS One 2010; 5: e10232–e10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, Larry et al. PageRank: Bringing order to the web. Vol. 72. Stanford Digital Libraries Working Paper, 1997.
- Di Martino A, Zuo X-N, Kelly C, Grzadzinski R, Mennes M, Schvarcz A et al. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol Psychiatry 2013; 74: 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnewijzend MA, Adriaanse SM, Flier WM, Teunissen CE, Munck JC, Stam CJ et al. Brain network alterations in Alzheimer's disease measured by Eigenvector centrality in fMRI are related to cognition and CSF biomarkers. Hum Brain Mapp 2014; 35: 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink AM, de Munck JC, van der Werf YD, van den Heuvel OA, Barkhof F. Fast eigenvector centrality mapping of voxel-wise connectivity in functional magnetic resonance imaging: implementation, validation, and interpretation. Brain Connect 2012; 2: 265–274. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 2012; 59: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res 2009; 201: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Liu J, Nie B, Li Y, Shan B, Wang G et al. Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. Eur J Radiol 2011; 80: 395–399. [DOI] [PubMed] [Google Scholar]
- Liu F, Guo W, Liu L, Long Z, Ma C, Xue Z et al. Abnormal amplitude low-frequency oscillations in medication-naive, first-episode patients with major depressive disorder: a resting-state fMRI study. J Affect Disord 2013; 146: 401–406. [DOI] [PubMed] [Google Scholar]
- Mannie ZN, Taylor MJ, Harmer CJ, Cowen PJ, Norbury R. Frontolimbic responses to emotional faces in young people at familial risk of depression. J Affect Disord 2011; 130: 127–132. [DOI] [PubMed] [Google Scholar]
- Carballedo A, Lisiecka D, Fagan A, Saleh K, Ferguson Y, Connolly G et al. Early life adversity is associated with brain changes in subjects at family risk for depression. World J Biol Psychiatry 2012; 13: 569–578. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. J Neurosci 2008; 28: 12341–12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T, Peng J, Nie B, Gao J, Liu J, Li Y et al. Altered functional connectivity of the dorsolateral prefrontal cortex in first-episode patients with major depressive disorder. Eur J Radiol 2012; 81: 4035–4040. [DOI] [PubMed] [Google Scholar]
- Lu Q, Li H, Luo G, Wang Y, Tang H, Han L et al. Impaired prefrontal–amygdala effective connectivity is responsible for the dysfunction of emotion process in major depressive disorder: a dynamic causal modeling study on MEG. Neurosci Lett 2012; 523: 125–130. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry 2001; 50: 651–658. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry 2002; 51: 693–707. [DOI] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Öhman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry 2010; 67: 1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Leow A, Ajilore O, Lamar M, Yang S, Joseph J et al. Quantitative tract-specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology 2012; 37: 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz D, Mayberg H, McIntosh A, Goldapple K, Kennedy S, Segal Z et al. Limbic–frontal circuitry in major depression: a path modeling metanalysis. Neuroimage 2004; 22: 409–418. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Zhang Z, Liu J, Yu M, Zhang J et al. Unidirectionally affected causal connectivity of cortico-limbic-cerebellar circuit by structural deficits in drug-naive major depressive disorder. J Affect Disord 2015; 172: 410–416. [DOI] [PubMed] [Google Scholar]
- Sheng K, Fang W, Su M, Li R, Zou D, Han Y et al. Altered spontaneous brain activity in patients with Parkinson's disease accompanied by depressive symptoms, as revealed by regional homogeneity and functional connectivity in the prefrontal-limbic system. PLoS One 2014; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Xu C, Xu Y, Wang Y, Zhao B, Lv Y et al. Decreased regional homogeneity in insula and cerebellum: a resting-state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry Res 2010; 182: 211–215. [DOI] [PubMed] [Google Scholar]
- Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A et al. Glutamatergic and resting-state functional connectivity correlates of severity in major depression–the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci 2010; 4: eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 2011; 15: 483–506. [DOI] [PubMed] [Google Scholar]
- Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci 2013; 7: 930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F, Wolf N, Pennuto M, Vasic N, Wolf R. Revisiting default mode network function in major depression: evidence for disrupted subsystem connectivity. Psychol Med 2014; 44: 2041–2051. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry 2015; 78: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleau EL, Taubitz LE, Larson CL. Imbalance of default mode and regulatory networks during externally focused processing in depression. Soc Cogn Affect Neurosci 2015; 10: 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Ann Rev Clin Psychol 2012; 8: 49–76. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA 2009; 106: 1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry 2012; 71: 611–617. [DOI] [PubMed] [Google Scholar]
- Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z et al. Antidepressants normalize the default mode network in patients with dysthymia. JAMA psychiatry 2013; 70: 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]