Abstract
In the yeast Saccharomyces diastaticus, expression of the STA1 gene, which encodes an extracellular glucoamylase, is negatively regulated by glucose. Here we demonstrate that glucose-dependent repression of STA1 expression is imposed by both Sfl1 and Nrg1, which serve as direct transcriptional repressors. We show that Nrg1 acts only on UAS1, and Sfl1 acts only on UAS2, in the STA1 promoter. When bound to its specific site, Sfl1 (but not Nrg1) prevents the binding to UAS2 of two transcriptional activators, Ste12 and Tec1, required for STA1 expression. We also found that Sfl1 contributes to STA1 repression by binding to the promoter and inhibiting the expression of FLO8, a gene that encodes a third transcriptional activator involved in STA1 expression. In addition, we show that the levels of Nrg1 and Sfl1 increase in glucose-grown cells, suggesting that the effects of glucose are mediated, at least in part, through an increase in the abundance of these repressors. NRG1 and SFL1 expression requires the Srb8-11 complex, and correspondingly, the Srb8-11 complex is also necessary for STA1 repression. However, our evidence indicates that the Srb8-11 complex does not associate with either the SFL1 or the NRG1 promoter and thus plays an indirect role in activating NRG1 and SFL1 expression.
In yeast, glucose negatively regulates the expression of genes involved in the metabolism of alternative carbon sources. This phenomenon, known as glucose repression, involves complex interactions between DNA-binding repressors, their cognate elements, and components of the transcriptional machinery. Several zinc finger proteins, such as Mig1, Mig2, Nrg1, and Nrg2, repress transcription of the SUC2, GAL1, MAL, FLO11, and STA1 genes by binding to specific elements and recruiting the general corepressor Ssn6-Tup1 to the promoters of the target genes (8, 14, 19, 20, 23, 26, 27, 31, 38).
In the yeast Saccharomyces diastaticus, three unlinked homologous STA genes (STA1, STA2, and STA3) encode glucoamylase isozymes (GAI, GAII, and GAIII) that degrade starch to glucose. The expression of STA1 is repressed at three different levels: (i) glucose repression (9, 33), (ii) repression by STA10 (32), and (iii) diploid cell-specific repression (9, 33). It has been reported that STA10 repression is due to a mutation in the activator FLO8, which is critical for STA1 expression (12, 21). In glucose repression, Nrg1 is thought to bind directly to UAS1-1 of the STA1 promoter and to recruit the Ssn6-Tup1 corepressor (31).
The STA1 promoter is almost identical to the FLO11 promoter, which encodes a mucin-like cell surface glycoprotein essential for pseudohyphal differentiation, invasive growth, and flocculation (12). STA1 and FLO11 are coregulated in response to various environmental signals, and their expression is controlled in a complicated manner by several transcriptional activators, e.g., Flo8, Mss11, Ste12, and Tec1 (11-13, 30, 35), and transcriptional repressors (23, 30, 31, 34). Glucose depletion causes derepression of FLO11 expression in haploid cells, whereas nitrogen starvation causes derepression in diploid cells (6, 23, 29, 40). The 5′ upstream region of FLO11 contains an Nrg1 binding site, and transcription is repressed by Nrg1 and Nrg2 as well as by Sfl1 (23, 30, 34). However, it is not clear whether Sfl1 is also involved in glucose repression of STA1 expression.
Sfl1 represses the transcription of several genes, including SUC2 and FLO11, and interacts physically and functionally with Srb and other mediator proteins to repress the transcription of target genes. DNA-bound LexA-Sfl1 represses the transcription of a reporter gene, and Ssn6-Tup1 is required for Sfl1-mediated repression (5, 30, 37). Sfl1 forms multimers via a coiled-coil domain, and this multimerization is thought to be important for binding to DNA. Tpk2, a catalytic subunit of cyclic AMP (cAMP)-dependent protein kinase A (PKA), inhibits multimerization and Sfl1 binding to DNA (30).
Srb proteins, such as Srb8, -9, -10, and -11, form a large complex and play an important role in the activation and repression of gene expression (2). The Srb8-11 complex is also somewhat involved in transcriptional repression by DNA-bound LexA-Ssn6 and LexA-Tup1 (22, 24, 25). The purified complex phosphorylates the C-terminal domain of RNA polymerase II on serines 2 and 5 prior to initiating the formation of a complex on the target promoter (2, 17). The complex is also involved in the transcription of several genes, including GAL1 and gluconeogenic genes, by positively regulating gene-specific activators (22, 39). Moreover, this complex phosphorylates transcriptional activators, such as Ste12, Sip4, Gal4, Msn2, and Gcn4, and regulates their activity. It also promotes the degradation of Ste12 and Gcn4 and is important for activation by Sip4 (3, 18, 28, 39).
In this study, we have used a UASSTA1-CYC1TATA-lacZ expression system to examine the roles of different regions of the STA1 promoter in STA1 transcription, and we have isolated SFL1 as a multicopy inhibitor of the STA1 promoter. We found that two of the upstream elements of the STA1 promoter, UAS1-1 and UAS2-2, mediate glucose repression of STA1 expression and are the targets of Nrg1 and Sfl1, respectively. We also provide evidence that Sfl1 competes with Ste12 and Tec1 for binding of the UAS2 region of the STA1 promoter and represses FLO8 expression. In glucose repression, Nrg1 and Sfl1 inhibit STA1 expression through different mechanisms. Furthermore, we show that the Srb8-11 complex plays critical roles in glucose repression of STA1 expression and that this complex indirectly activates NRG1 and SFL1 expression. Finally, we suggest that increased levels of the repressors Nrg1 and Sfl1 are important in mediating glucose repression of STA1 expression.
MATERIALS AND METHODS
Strains and media.
The S. diastaticus strains used in this work are listed in Table 1. KHS 182 was constructed by mating YHP499 (MATα ade2 his3 leu2 lys2 trp1 ura3 flo8-1) with SPX15-3D(MATa leu1 thr1 STA1 FLO8). Yeast cells were grown at 30°C in YPD (1% yeast extract, 2% tryptone, and 2% glucose) or a synthetic medium containing 0.67% yeast nitrogen base supplemented with appropriate amino acids and carbon sources (2% glucose or 2% glycerol-ethanol). Mutant strains were constructed by replacing the open reading frames (ORFs) with TRP1, HIS3, or URA3 by PCR-mediated disruption, and mutations were confirmed by PCR. To construct the hemagglutinin (HA)-tagged strains, pRS305-FLO8-HA, pRS305-MSS11-HA, pRS305-STE12-HA, pRS305-TEC1-HA, and pRS305-SRB10-HA were linearized with BglII, SphI, NcoI, NheI, and SphI, respectively, and the linearized DNA fragments were integrated into their respective genomic loci. Tagged strains were confirmed by PCR analysis, glucoamylase assay, and Western blot analysis with an anti-HA (α-HA) antibody.
TABLE 1.
S. diastaticus strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| SPX15-3D | MATaSTA1 leu1 thr1 FLO8 | 32 |
| YPH499 | MATα ade2 his3 leu2 thr1 lys2 trp1 ura3 flo8-1 | 35 |
| KHS 182 | MATα STA1 leu2 his3 trp1 ura3 FLO8 | 21 |
| KHS 182-11 | MATα STA1 leu2 his3 trp1 ura3 nrg1Δ::TRP1 | This study |
| KHS 182-12 | MATα STA1 leu2 his3 trp1 ura3 sfl1Δ::TRP1 | This study |
| KHS 182-13 | MATα STA1 leu2 his3 trp1 ura3 nrg1 Δ::HIS3 sfl1Δ::TRP1 | This study |
| KHS 182-14 | MATα STA1 leu2 his3 trp1 ura3 ssn6Δ::URA3 | This study |
| KHS 182-15 | MATα STA1 leu2 his3 trp1 ura3 sin4Δ::TRP1 | This study |
| KHS 182-16 | MATα STA1 leu2 his3 trp1 ura3 srb8Δ::TRP1 | This study |
| KHS 182-17 | MATα STA1 leu2 his3 trp1 ura3 srb9Δ::TRP1 | This study |
| KHS 182-18 | MATα STA1 leu2 his3 trp1 ura3 srb10Δ::TRP1 | This study |
| KHS 182-19 | MATα STA1 leu2 his3 trp1 ura3 sfl1Δ::URA3 srb10Δ::TRP1 | This study |
| KHS 182-20 | MATα STA1 leu2 his3 trp1 ura3 nrg1Δ::HIS3 srb10Δ::TRP1 | This study |
| KHS 182-21 | MATα STA1 leu2 his3 trp1 ura3 srb11Δ::TRP1 | This study |
| KHS 182-22 | MATα STA1 leu2 his3 trp1 ura3 sfl1Δ::URA3 srb11Δ::TRP1 | This study |
| KHS 182-23 | MATα STA1 leu2 his3 trp1 ura3 nrg1Δ::HIS3 srb11Δ::TRP1 | This study |
| KHS 182-30 | MATα STA1 leu2 his3 trp1 ura3 FLO8-HA::LEU2 | This study |
| KHS 182-31 | MATα STA1 leu2 his3 trp1 ura3 MSS11-HA::LEU2 | This study |
| KHS 182-32 | MATα STA1 leu2 his3 trp1 ura3 STA12-HA::LEU2 | This study |
| KHS 182-33 | MATα STA1 leu2 his3 trp1 ura3 TEC1-HA::LEU2 | This study |
| KHS 182-40 | MATα STA1 leu2 his3 trp1 ura3 SRB10-HA::LEU2 | This study |
| KHS 182-40-1 | MATα STA1 leu2 his3 trp1 ura3 nrg1Δ::TRP1 SRB10-HA::LEU2 | This study |
| KHS 182-40-2 | MATα STA1 leu2 his3 trp1 ura3 sfl1Δ::TRP1 SRB10-HA::LEU2 | This study |
| KHS 182-40-3 | MATα STA1 leu2 his3 trp1 ura3 nrg1Δ::HIS3 sfl1Δ::TRP1 SRB10-HA::LEU2 | This study |
Plasmids.
The plasmids used in this study are listed in Table 2. To construct the pRS-HA plasmids, the triple-HA tag was amplified by using primers that create terminal SalI/XhoI sites, and the resulting HA fragment was subcloned into SalI/XhoI sites in pRS305 and pRS325. To construct pRS305-ORF-HA, the entire FLO8, MSS11, STE12, TEC1, and SRB10 ORFs were amplified by PCR using primers that create terminal BamHI/XhoI (for FLO8) and BamHI/SalI (for the other ORFs) sites and then subcloned into pRS305-HA at the corresponding sites. pLG-UAS2a containing a mutated Sfl1 binding site (CGCA) was constructed by PCR using Pfu polymerase (Stratagene) and the mutagenic primer pairs 5′-GGTTTTTTCTTCTGTTTCTTGACACGCAAAT GTTG CCCAAAGAGTTTCG-3′ and 5′-CGAAACTCTTTGGGCAACATTTGCGTGTCAAGAAACAGAAGAAAAAACC-3′. The construct generated was confirmed by sequencing.
TABLE 2.
Plasmids used in this study
| Plasmid | Description | Reference or source |
|---|---|---|
| pRS305 | LEU2 | 36 |
| pRS305-HA | Triple HA tags in pRS305 (SalI/XhoI) | This study |
| pRS325 | 2μm LEU2 | 4 |
| pRS323 | 2μm HIS3 | 4 |
| pRS325-HA | Triple HA tags in pRS325 (SalI/XhoI) | This study |
| pRS325-ADH1p-HA | ADH1 promoter (−1500 to −1) in pRS325-HA (SacI/NotI) | This study |
| pRS325-lacZ | lacZ in pRS325 (BamHI) | This study |
| pLG 669-Z | 2μm URA3 UASCYC1-CYC1-lacZ | 15 |
| pLG 670-Z | 2μm UAS3 CYCI-lacZ | 15 |
| JK1621 | 2μm URA3 4lexAop-UASCYCI-CYC1-lacZ | 20 |
| pLG-STA1-1 | −2105 to −1 region of the STA1 promoter in pLG-670Z | This study |
| pLG-STA1-2 | −2105 to −882 region of the STA1 promoter in pLG-670Z | This study |
| pLG-STA1-3 | −1642 to −882 region of the STA1 promoter in pLG-670Z | This study |
| pLG-STA1-4 | −1642 to −1380 region of the STA1 promoter in pLG-670Z | This study |
| pLG-UAS1 | −2105 to −1642 region of the STA1 promoter in pLG-670Z | This study |
| pLG-UAS1-1 | −2105 to −1905 region of the STA1 promoter in pLG-670Z | This study |
| pLG-UAS1-2 | −1905 to −1642 region of the STA1 promoter in pLG-670Z | This study |
| pLG-UAS2 | −1380 to −882 region of the STA1 promoter in pLG-670Z | This study |
| pLG-UAS2-1 | −1380 to −1147 region of the STA1 promoter in pLG-670Z | This study |
| pLG-UAS2-2 | −1147 to −882 region of the STA1 promoter in pLG-670Z | This study |
| pLG-UAS2A | BglII linker in pLG-UAS2 (SmaI) | This study |
| pLG-UAS2a | Replacement of AGAA with CGCA in pLG-UAS2 | This study |
| pLG-UAS1-NRGI | 2.0-kb fragment containing NRG1 ORF in pLG-UAS1 | This study |
| pLG-UAS2-SFL1 | 3.5-kb fragment containing SFL1 ORF in pLG-UAS2 | This study |
| pLG-UAS2a-SFL1 | 3.5-kb fragment containing SFL1 ORF in pLG-UAS2a | This study |
| pRS323-NRG1 | 2.0-kb-fragment containing NRG1 ORF in pRS323 | This study |
| pRS323-SFL1 | 3.5-kb fragment containing SFL1 ORF in pRS323 | This study |
| pRS325-NRG1-HA | 2.0-kb fragment containing NRG1 ORF in pRS325-HA | This study |
| pRS325-SFL1-HA | 3.5-kb fragment containing SFL1 ORF in pRS325-HA | This study |
| pRS325-ADH1p-NRGI-HA | NRGI ORF in pRS325-ADHIp-HA | This study |
| pRS325-ADH1p-SFL1-HA | SFL1 ORF in pRS325-ADH1p-HA | This study |
| pNRG1-lacZ | NRG1 promoter (bp − 1200 to −1) in pRS325-lacZ (NotI) | This study |
| pSFL1-lacZ | SFL1 promoter (bp −1500 to −1) in pRS325-lacZ (NotI) | This study |
| pLexA-NRG1 | NRG1 in pSH2-1 (BamHI/XhoI) | 31 |
| pLexA-SFL1 | SFL1 in pSH2-1 (BamHI/XhoI) | This study |
| pRS305-FLO8-HA | FLO8 in pRS305-HA (BamHI/SalI) | This study |
| pRS305-MSS11-HA | MSS11 in pRS305-HA (BamHI/XhoI) | This study |
| pRS305-STE12-HA | STE12 in pRS305-HA (BamHI/XhoI) | This study |
| pRS305-TEC1-HA | TEC1 in pRS305-HA (BamHI/XhoI) | This study |
| pRS305-SRB10-HA | SRB10 in pRS305-HA (BamHI/XhoI) | This study |
Cloning of SFL1.
To isolate a multicopy inhibitor(s) that acts on the UAS2 of the STA1 promoter, pLG-UAS2A, which bears a BglII linker at the SmaI site of pLG-UAS2, was used to generate a genomic library. Yeast genomic DNA isolated from KHS 182 was partially digested with Sau3AI, and fragments with an average size of 5 to 10 kb were collected by sucrose velocity sedimentation. The resulting fragments were inserted at the BglII site of pLG-UAS2A. The genomic library was transformed into KHS 182, and the resulting transformants were incubated on BMM-X-Gal plates (1). Under these conditions, transformants containing the control pLG-UAS2 plasmid form blue colonies, whereas cells bearing a repressor acting on UAS2 would generate white colonies. On this basis, we isolated 3 white colonies from about 100,000 transformants on BMM-X-Gal plates. Plasmids were recovered from these three candidates, B42, C13, and C33, and retransformed into KHS 182 to check their effect. Transformants harboring the control plasmid, pLG-UAS2, had high glucoamylase and β-galactosidase activities, whereas transformants bearing plasmid B42, C13, or C33 all had reduced levels of both activities (data not shown).
Glucoamylase assay, Northern blot analysis, and β-galactosidase assay.
Glucoamylase assays and Northern blot analyses were performed as described previously (31). β-Galactosidase was assayed as described previously (1).
GST pull-down assay.
Total extracts (1 mg) prepared from the integrated SRB10-HA strain were incubated for 3 h on ice with 5 μg of purified glutathione S-transferase (GST)-fused proteins. Glutathione-agarose beads (25 μl) were added and incubated for 2 h at 4°C with constant agitation. The beads were pelleted and washed four times. Proteins in the pellet were eluted by boiling the beads in sample buffer and were analyzed by Western blotting with monoclonal α-HA antibodies (Santa Cruz).
Preparation of protein extracts and immunoblot analysis.
Total proteins were extracted as described previously (42). The extraction buffer was 50 mM HEPES (pH 7.5), 150 mM NaCl, 0.1% Triton X-100, 1 mM dithiothreitol, and 10% glycerol, containing 2 mM phenylmethylsulfonyl fluoride and Complete protease inhibitor cocktail (Sigma). Proteins were separated by sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE; 8 to 10% acrylamide) and analyzed by immunoblotting with α-HA (Santa Cruz) and α-actin (Sigma) antibodies.
ChIP assays.
To detect protein-DNA interaction, chromatin immunoprecipitation (ChIP) assays were performed as described by Hecht et al. (16) with minor modifications. Cells were grown in a 2% glucose or 2% glycerol-ethanol medium to an optical density at 600 nm (OD600) of 1.0 and were treated with formaldehyde (1%) to cross-link DNA and proteins. Extracts were sonicated, and equal amounts of extract were incubated with an α-HA antibody at 4°C overnight. GammaBind G Sepharose beads (Amersham) were added to precipitate DNA-HA-tagged proteins, and the beads were washed four times. Elution buffer was added and incubated at 65°C overnight. DNA fragments were purified with a QiaQuick PCR column (QIAGEN), and the immunoprecipitated DNAs were amplified by 30 cycles of PCR to detect the upstream regions of the STA1 promoter in the pLG vector series and the endogenous STA1 promoter by using the following primer pairs: for CYC1, 5′-GAAAGGAAAGCAGGAAAGG-3′ and 5′-TATACACGCCTGGCGGATCTG-3′; for UAS1-2, 5′-CCTATTCTCATCGAGAGCCGAG-3′ and 5′-CAAGTACTGCAGTGCATGTCC-3′); for UAS2-1, 5′-GGTAAGATTTGTTCTATG-3′ and 5′-GAACTTTCCAGGCTCACC-3′; for UAS2-2, 5′-GGTGTGCCTGGAAAGTTC-3′ and 5′-GAGCAATCAGCAGTTCTTTG-3′; and for TATA, 5′-CTTAACAAATATGTTCAAGC-3′ and 5′-TGGATTTTTGAGGCCTACC-3′. The CYC1 primer pairs were used to detect the upstream activation sequence (UAS) of the STA1 promoter within the pLG series. The PCR products were separated by 2% agarose gel electrophoresis and photographed.
RESULTS
Sfl1 and Nrg1 are transcriptional repressors for glucose repression of STA1 expression.
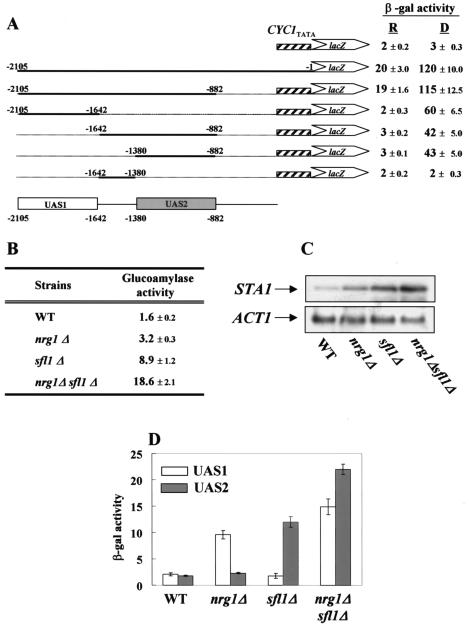
It was previously reported that Nrg1 is a transcriptional repressor that acts on the UAS1 region (−2105 to −1642) of the STA1 promoter and mediates glucose repression of STA1 expression (31). However, nrg1Δ did not greatly relieve glucose repression of STA1 in the wild-type strain KHS 182 (Fig. 1B), suggesting that some additional transcriptional repressor(s) exists to repress STA1 expression. To explore this possibility, we tested whether another upstream region besides UAS1 is also involved in glucose repression. To identify the UAS(s), we determined the β-galactosidase activities of plasmid-based UASSTA1-CYC1TATA-lacZ reporter constructs. As shown in Fig. 1A, not only UAS1 (−2105 to −1642) but also the 498-bp fragment (−1380 to −882) referred to as UAS2 causes strong expression of the reporter gene under derepressed conditions (2% glycerol-ethanol) but complete repression under repressed conditions (2% glucose). This result indicates that UAS2 is also involved in glucose repression of STA1 and may be a target for a repressor(s).
FIG. 1.
Nrg1 and Sfl1 act on UAS1 and UAS2, respectively. (A) DNA fragments carrying the STA1 promoter were inserted into pLG 670-Z containing a CYC1TATA-lacZ reporter gene, yielding the pLG-UAS series. These vectors were transformed separately into KHS 182. Three independent colonies obtained with each plasmid were tested for β-galactosidase activity under repressed (2% glucose) (R) or derepressed (2% glycerol-ethanol) (D) conditions. (B) Wild-type (WT) and mutant strains were grown in synthetic medium containing 2% glucose as a carbon source. Glucoamylase activities are averages from three independent experiments. (C) Total RNA was prepared from the same strains for Northern blot analysis. The yeast actin gene (ACT1) was used as an internal control. (D) pLG-UAS1 or pLG-UAS2 was introduced into wild-type, nrg1Δ, sfl1Δ, or nrg1Δ sfl1Δ cells, and three independent transformants were tested for β-galactosidase activity under repressed conditions (2% glucose).
To isolate a multicopy inhibitor(s) that acts on UAS2, we used a method modified from that of Ahn et al. (1) (for details, see Materials and Methods). From this screening, candidate plasmids B42, C13, and C33 were isolated and sequenced. Overlapping regions of the three clones contained the same gene, SFL1, which is known to repress SUC2 and FLO11 transcription (5, 34, 37).
To examine the effect of SFL1 on STA1 expression in comparison to that of the previously characterized NRG1 gene, isogenic nrg1Δ, sfl1Δ, and nrg1Δ sfl1Δ double mutants were generated. As shown in Fig. 1B, deletion of SFL1 increased glucoamylase activity from 1.6 to 8.9 U under repressed conditions, whereas deletion of NRG1 increased activity only twofold (from 1.6 to 3.2 U). Furthermore, sfl1Δ synergized with nrg1Δ to completely relieve glucose repression; the level of glucoamylase activity of nrg1Δ sfl1Δ cells increased under repressed conditions to 18.7 U, a level similar to that of wild-type cells under derepressed conditions (Fig. 1B). To examine whether the increased glucoamylase activity correlates with the level of STA1 mRNA in wild-type and mutant cells, we carried out Northern blot analysis. In a glucose medium, the level of the STA1 transcript was slightly higher in nrg1Δ cells than in wild-type cells, whereas it was approximately fivefold higher in sfl1Δ cells. Consistent with the synergistic elevation of glucoamylase activity, the nrg1Δ sfl1Δ double mutant exhibited a STA1 mRNA level approximately 10-fold higher than that of the isogenic wild type (Fig. 1C).
Nrg1 and Sfl1 specifically bind to UAS1 and UAS2, respectively.
As mentioned above, Nrg1 and Sfl1 were isolated as multicopy inhibitors acting on UAS1 and UAS2, respectively. Thus, we asked if Nrg1 and Sfl1 act on these sequences specifically. To this end, we first transformed plasmids containing UAS1-CYC1TATA-lacZ (pLG-UAS1) or UAS2-CYC1TATA-lacZ (pLG-UAS2) reporter genes into wild-type, nrg1Δ, sfl1Δ, and nrg1Δ sfl1Δ cells and then measured β-galactosidase activity under repressed conditions. Expression of lacZ from pLG-UAS1 or pLG-UAS2 in wild-type cells was very low under repressed conditions (Fig. 1D). The β-galactosidase activity of pLG-UAS1 was fourfold higher in nrg1Δ cells than in wild-type cells, whereas the β-galactosidase activity of pLG-UAS2 was unaffected (Fig. 1D). This suggested that Nrg1 acts on UAS1 but not on UAS2. In stark contrast, lacZ expression from UAS2 but not from UAS1 was derepressed about fivefold in sfl1Δ cells (Fig. 1D), indicating that Sfl1 specifically functions on UAS2 but not UAS1. Consistent with the synergistic increases in the level of the STA1 transcript and the glucoamylase activity, the nrg1Δ sfl1Δ double mutant exhibited greatly enhanced β-galactosidase activities derived from UAS1 and UAS2. Taken together, these data indicate that Nrg1 specifically functions through UAS1 whereas Sfl1 is specific to UAS2.
Sfl1 acts on the heat shock elements in UAS2.
It has been reported that Sfl1 has a DNA binding domain at its N terminus that is similar to that of the yeast heat shock transcription factor, and it has been proposed that Sfl1 binds to an inverted repeat, 5′ AGAA-n-TTCT 3′, of the heat shock factor element (5). Analysis of the UAS2 sequence revealed a conserved Sfl1 binding motif. To examine whether Sfl1 acts on this conserved motif, the AGAA sequence in pLG-UAS2 was replaced with CGCA by site-directed mutagenesis. The resulting plasmid, pLG-UAS2a, was transformed into wild-type and sfl1Δ cells, and β-galactosidase activity was determined under repressed conditions. Whereas lacZ expression from UAS2 was repressed in wild-type cells, lacZ expression from the mutated UAS2a (CGCA) was completely derepressed: the β-galactosidase activity of pLG-UAS2a (CGCA) in wild-type cells was similar to that of pLG-UAS2 in sfl1Δ cells (Fig. 2A). This result indicates that Sfl1 confers glucose repression by acting on the inverted repeat sequence AGAA-n-TTCT.
FIG. 2.
Specific binding of Sfl1 to UAS2. (A). The AGAA sequences (bp −1106 to −1103) in pLG-UAS2 were replaced with CGCA by site-directed mutagenesis. The resulting construct, pLG-UAS2a, and also the parental pLG-UAS2 construct were transformed into wild type (WT) and sfl1Δ cells. β-Galactosidase activity was determined under repressed conditions by using three independent colonies. (B) ChIP assays for Sfl1. pLG-UAS2 or pLG-UAS2a and plasmid pRS325-SFL1-HA or pRS323-SFL1, expressing Sfl1-HA or Sfl1 from its own promoter, respectively, were cotransformed into wild-type cells. Transformants were grown to mid-log phase in synthetic medium with 2% glucose and were treated with formaldehyde to cross-link DNA and proteins. An α-HA ChIP assay was performed, and the UAS2 region was PCR amplified by using purified DNA to determine Sfll binding to UAS2.
To examine whether Sfl1 was unable to bind to UAS2a, we performed a ChIP assay. Plasmids pRS325-SFL1-HA and pRS325-SFL1, expressing Sfl1-HA and Sfl1, respectively, were transformed into wild-type cells bearing pLG-UAS2 or pLG-UAS2a, and a ChIP assay was performed under repressed conditions. Sfl1-HA coimmunoprecipitated a UAS2 fragment, indicating that Sfl1 interacts with this fragment in vivo. Under the same condition, Sfl1-HA failed to coimmunoprecipitate UAS2a (Fig. 2B). These results indicate that Sfl1 binds to, and acts on, the conserved sequence AGAA-n-TTCT in UAS2, to repress STA1 transcription.
Two independent UASs and URSs are involved in STA1 expression.
Both the UAS1 and UAS2 segments of the STA1 promoter mediate glucose repression as well as activation of STA1 expression in response to different carbon sources (Fig. 1A). These observations suggest that each of these DNA fragments acts as a UAS as well as an upstream repression sequence (URS). To determine whether UAS1 and UAS2 have independent functions as UASs and URSs, we subdivided UAS1 into UAS1-1 (−2105 to −1906) and UAS1-2 (−1905 to −1642) and UAS2 into UAS2-1 (−1380 to −1148) and UAS2-2 (−1147 to −882) (Fig. 3). As shown in Fig. 3, lacZ expression mediated by the complete UAS1 and UAS2 regions was totally repressed in cells grown in glucose-containing medium. However, when the Nrg1 and Sfl1 binding sequences, UAS1-1 and UAS2-2, were removed from the respective UAS1 and UAS2 regions (pLG-UAS1-2 and pLG-UAS2-1, respectively), lacZ was expressed even under repressed conditions. Furthermore, the β-galactosidase levels generated from UAS1-2 and UAS2-1 were similar to those generated by UAS1 and UAS2 under derepressed conditions (Fig. 3). These results demonstrate that activation of STA1 is mediated by UAS1-2 and UAS2-1 and that the activators bind to them under both repressed and derepressed conditions. These results also suggest that UAS1-1 and UAS2-2 act as URSs mediating glucose repression of STA1.
FIG. 3.
Effects of carbon source on lacZ expression mediated by UAS1, UAS2, and their subregions. UAS1 is subdivided into UAS1-1 and UAS1-2, whereas UAS2 is subdivided into UAS2-1 and UAS2-2. Nrg1 and Sfl1 act on UAS1-1 and UAS2-2, respectively, and the transcriptional activators required for activation of UAS1-2 and UAS2-1 are indicated. The pLG-UAS series was transformed into wild-type cells, and the resulting transformants were grown to mid-log phase in synthetic medium containing 2% glucose (R) or 2% glycerol-ethanol (D). β-Galactosidase activity was determined on three independent colonies.
Binding of Sfl1 to UAS2-2 interferes with the access of Ste12 and Tec1.
Kim et al. have presented evidence that all four of the transcriptional activators Flo8, Mss11, Ste12, and Tec1 are required for activation of STA1 expression, since deletion of any one of them abolishes STA1 expression (21). Furthermore, it has been shown that Ste12 and Tec1 act specifically on UAS2-1, which then enhances Flo8 and Mss11 binding to UAS1-2 and UAS2-1 to activate STA1 expression (Fig. 3) (T. S. Kim, H. Y. Kim, J. H. Yoon, and H. S. Kang, submitted for publication). These results suggest that interactions between the repressors and the upstream elements, UAS1-1 and UAS2-2, may have hindered the accessibility of the transcriptional activators to UAS1-2 and UAS2-1 and thus resulted in repression. To examine this possibility, we first constructed FLO8, MSS11, STE12, and TEC1 tagged with triple HA at their C termini and integrated them into the respective genomic loci. These integrated fusion proteins were functional, since the integrated strains showed normal STA1 expression patterns under both repressed and derepressed conditions (data not shown). To examine whether overexpressed Nrg1 or Sfl1 is able to repress STA1 expression and inhibit binding of the activators to UASs under derepressed conditions, the integrated strains were transformed with either a multicopy NRG1 plasmid, a multicopy SFL1 plasmid, or an empty vector, and their glucoamylase activities were determined. As shown in Fig. 4A, STA1 expression was repressed in cells containing the multicopy NRG1 or multicopy SFL1 plasmid despite the fact that they were grown under derepressed conditions. We performed a ChIP assay to test whether overexpressed Nrg1 and Sfl1 inhibit binding of the activators to the endogenous UASs of the STA1 promoter in these strains. The multicopy NRG1 plasmid did not affect the binding of the activators to UAS1-2 or UAS2-1 at all (Fig. 4B), indicating that overexpression of Nrg1 does not inhibit binding of the activators to the UASs even though it reduces STA1 expression. However, the multicopy SFL1 plasmid prevented the activators from binding to UAS2-1; UAS2-1 was hardly immunoprecipitated at all with Flo8-HA, Mss11-HA, Ste12-HA, and Tec1-HA in cells bearing the multicopy SFL1 plasmid, whereas it was immunoprecipitated in cells containing the control vector (Fig. 4B).
FIG. 4.
Sfl1 inhibits access of Ste12 and Tec1 to UAS2-1. (A) Cells containing a control, multicopy NRG1, or multicopy SFL1 plasmid were grown in 2% glycerol-ethanol medium to mid-log phase and subjected to a glucoamylase activity assay. (B) pRS323 (−), pRS323-NRG1 (+), or pRS323-SFL1 (+) was transformed into integrated HA-tagged strains. Transformants were grown to mid-log phase in synthetic medium containing 2% glycerol-ethanol and were fixed with formaldehyde. After α-HA ChIP, UAS1-2 and UAS2-1 were PCR amplified by using purified DNA. (C) Cells containing the multicopy SFL1 plasmid (+) or controls (−) were grown in 2% glycerol-ethanol medium to mid-log phase, and total proteins were extracted from these cells. Levels of HA-tagged transcriptional activators from 50 or 100 μg of protein extract were determined. (D) pLG-UAS1 (lane 1), pLG-UAS1-NRG1 (lane 2), pLG-UAS2 (lane 3), or pLG-UAS2-SFL1 (lane 4) was introduced into wild-type cells. The resulting transformants were grown in synthetic medium containing 2% glycerol-ethanol before being subjected to a β-galactosidase activity assay. (E) pLG-UAS2 (−) or pLG-UAS2-SFL1 (+) was transformed into wild-type, STE12-HA, or TEC1-HA cells. The resulting transformants were cultured and subjected to an α-HA ChIP assay as in panel B. (F) pLG-UAS2a (−) and pLG-UAS2a-SFL1 (+) were transformed into the same strains. A ChIP assay was performed with an α-HA antibody as above, and Ste12 or Tec1 binding to UAS2-1 was detected by PCR.
We next compared the levels of the activators under the same condition to determine whether the effect of the multicopy SFL1 plasmid was due to reduced levels of activators. Western blot analyses showed that the presence of the multicopy SFL1 plasmid did not affect the amount of Mss11, Ste12, or Tec1 but completely abolished expression of FLO8 (Fig. 4C). Furthermore, we revealed that the FLO8 promoter contained the heat shock factor element recognized by Sfl1 and that Sfl1 bound to this region in vivo (data not shown). These results suggest that overexpression of Sfl1 reduces STA1 expression via two different mechanisms: by inhibition of FLO8 expression and also by inhibition of the binding of Ste12 and Tec1 to UAS2.
Since the binding of Ste12 and Tec1 was not affected by deletion of FLO8 and MSS11 (Kim et al., submitted), we next examined whether the inhibitory effect of overexpressed Sfl1 on Ste12 and Tec1 binding is direct, by using lacZ reporter plasmids containing either wild-type UAS2 (pLG-UAS2) or the mutant UAS2a (pLG-UAS2a). If the effect of Sfl1 is indirect, the activators will not bind to UAS2-1 in Sfl1-overexpressing cells, whether Sfl1 binds to UAS2 or not. However, if Sfl1 inhibits the binding of Ste12 and Tec1 to UAS2-1 directly, these activators will bind to UAS2a, since Sfl1 cannot bind to it. As expected, overexpression of Sfl1 repressed UAS2 on the plasmid as well as on the endogenous STA1 promoter; the β-galactosidase activity of pLG-UAS2 was reduced by the multicopy SFL1 plasmid but not by the control empty plasmid (Fig. 4D). Next we performed a ChIP assay. Like the endogenous UAS2-1, UAS2-1 on pLG-UAS2 was immunoprecipitated together with Ste12-HA and Tec1-HA in cells containing the control plasmid, but hardly at all in cells bearing the multicopy SFL1 plasmid (Fig. 4E). However, Ste12 and Tec1 still bound to UAS2a containing the mutated Sfl1 binding site, even though SFL1 was overexpressed (Fig. 4F). These results indicate that the binding of Sfl1 to UAS2-2 directly prevents Ste12 and Tec1 from binding to UAS2-1 to repress STA1 expression and that the effect of Sfl1 overexpression is not an indirect consequence of altered expression of another factor(s) that may influence the binding of Ste12 and Tec1. We conclude that Sfl1 competes with Ste12 and Tec1 for occupation of UAS2.
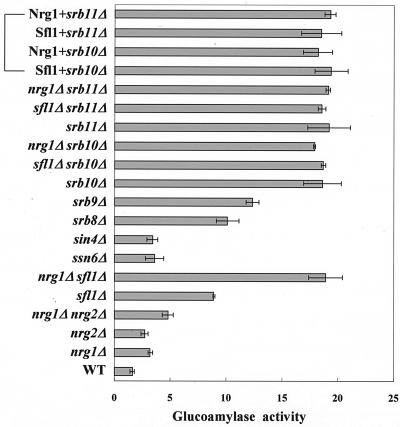
The Srb8-11 complex is critical for glucose repression of STA1 expression.
It has been reported that the function of Sfl1 is related to that of Ssn6-Tup1 and Srb proteins, such as Srb8, Srb9, and Srb11, or to that of Sin4 (5, 37). Nrg1 also requires Ssn6-Tup1 to repress STA1 transcription (31). We therefore disrupted the SRB genes, SIN4 and SSN6, to investigate whether these proteins are also involved in glucose repression. In sin4Δ cells and ssn6Δ cells, STA1 expression was slightly derepressed under repressed conditions (Fig. 5). On the other hand, glucose repression was greatly relieved in srb8Δ, srb9Δ, srb10Δ, and srb11Δ cells; notably, srb10Δ and srb11Δ completely reversed the glucose repression. Interestingly, neither srb10Δ nor srb11Δ synergized with nrg1Δ or sfl1Δ to relieve repression. In addition, the effects of srb10Δ and srb11Δ were not suppressed by multicopy plasmids bearing NRG1 or SFL1, even though NRG1 and SFL1 were originally isolated as multicopy inhibitors of STA1 expression under derepressed conditions (Fig. 5). Taken together, these results suggest that the function of Nrg1 and Sfl1 is closely related to that of the Srb8-11 complex.
FIG. 5.
Effects of Srb and mediator proteins on glucose repression of STA1 expression. Both wild-type (WT) and mutant strains were grown in synthetic medium with 2% glucose. Nrg1+ and Sfl1+ indicate the presence of multicopy plasmids bearing NRG1 or SFL1 in the srb10Δ or srb11Δ cells. Average glucoamylase activities from three independent experiments are presented.
NRG1 and SFL1 expression requires the Srb8-11 complex.
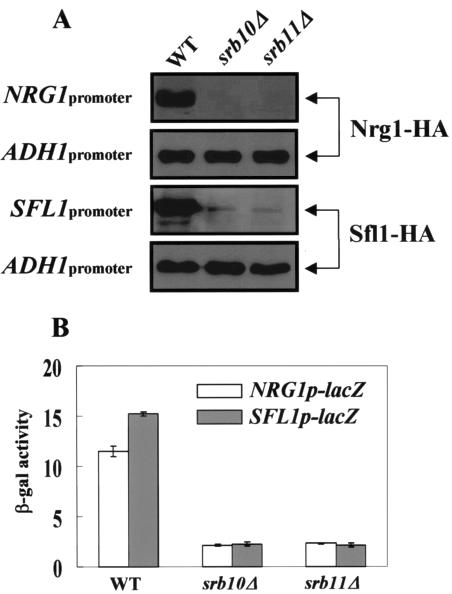
As mentioned above, the effect of multicopy NRG1 and SFL1 plasmids was not observed in either srb10Δ or srb11Δ cells, although Nrg1 and Sfl1 were isolated as multicopy inhibitors of STA1 expression (Fig. 5). One possible explanation is that Nrg1 and Sfl1 do not function as transcriptional repressors in these deletion mutants because the Srb8-11 complex is critical for Nrg1- and Sfl1-mediated repression (5, 37). Alternatively, the failure of repression by multicopy NRG1 and SFL1 may have resulted from reduced levels of these repressors: the Nrg1 and Sfl1 could be unstable, or NRG1 and SFL1 expression might have been blocked, in srb10Δ or srb11Δ mutants.
To investigate these possibilities, we first determined the levels of Nrg1 and Sfl1 in the srb10Δ and srb11Δ mutants by immunoblotting with an α-HA antibody. Cells carrying plasmids expressing HA-tagged Nrg1 or Sfl1 from its own promoter or the ADH1 promoter were grown in glucose medium. Western blot analyses showed that the levels of the two repressors were substantially reduced in srb10Δ and srb11Δ cells compared to those in wild-type cells (Fig. 6A). However, when the repressors were expressed from the ADH1 promoter, levels were almost the same in wild-type and mutant cells. These results indicate that the reduction of Nrg1 and Sfl1 levels in the srb10Δ or srb11Δ background is due not to instability but to reduced transcription. To confirm this result, we examined the activities of the SFL1 and NRG1 promoter regions by using a lacZ reporter fused to an SFL1 or NRG1 promoter. As expected, expression of lacZ from the SFL1 and NRG1 promoter regions was also reduced significantly in srb10Δ and srb11Δ cells from that in wild-type cells (Fig. 6B). We conclude from these results that the defect in repression by introduction of multicopy NRG1 and SFL1 plasmids in srb10Δ or srb11Δ cells is due to reduced Nrg1 and Sfl1 levels caused by repressed transcription of NRG1 and SFL1. Thus, these results strongly suggest that the Srb8-11 complex is required to promote NRG1 and SFL1 expression.
FIG. 6.
The Srb8-11 complex is required for NRG1 and SFL1 expression. (A) Plasmids expressing Nrg1-HA or Sfl1-HA, from their own promoters or from the ADH1 promoter, were transformed into wild-type (WT) and mutant cells. Transformants were grown to mid-log phase in synthetic medium containing 2% glucose. Protein extracts were prepared from each transformant, and 50 μg (own promoter) or 20 μg (ADH1 promoter) of extract was separated by SDS-PAGE for immunoblotting with an α-HA antibody. The same membranes were probed with an α-actin monoclonal antibody. (B) Plasmids containing NRG1p-lacZ and SFL1p-lacZ were transformed into wild-type and mutant cells. The cells were grown in synthetic medium containing 2% glucose, and β-galactosidase activity was measured with three independent colonies.
Our attempt to determine whether the Srb8-11 complex directly regulates the expression of NRG1 and SFL1 or indirectly affects the function of the activator(s) involved in the transcription of the two repressor genes failed. However, it seems likely that the Srb8-11 complex activates expression of NRG1 and SFL1 indirectly, because we did not find that Srb10-HA bound to the NRG1 or SFL1 promoter in ChIP assays (data not shown). Nevertheless, our data show that the Srb8-11 complex functions to promote NRG1 and SFL1 expression.
Nrg1 and Sfl1 recruit the Srb8-11 complex to the STA1 promoter.
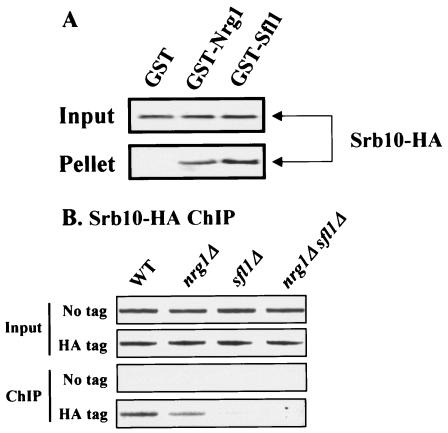
It has been reported that the functions of Sfl1, the Ssn6-Tup1 corepressor, and the Srb8-11 complex are closely related and that Sfl1 interacts physically with the Ssn6-Tup1 and the Srb8-11 complex (5, 22, 25, 37). We found that repression by LexA-Nrg1 also requires the Srb8-11 complex (data not shown). These results suggest that Nrg1 as well as Sfl1 interacts with the Srb8-11 complex. To examine this possibility, we performed a GST pull-down assay using yeast whole-cell lysates. Cellular lysates prepared from the integrated SRB10-HA strain were incubated with purified GST, GST-Nrg1, or GST-Sfl1. As shown in Fig. 7A, Srb10-HA was coprecipitated with GST-Nrg1 and GST-Sfl1 but not with GST alone.
FIG. 7.
Recruitment of the Srb8-11 complex to the STA1 promoter requires Nrg1 and Sfl1. (A) Cell extracts prepared from the integrated SRB10-HA strain were incubated with 5 μg of GST, GST-Nrg1, or GST-Sfl1. GST proteins and their interacting proteins were precipitated with glutathione-agarose beads. Fractions of the input (1/10) and pellet (1/2) were analyzed by Western blot analysis with an α-HA antibody. (B) Cells tagged with integrated SRB10-HA, isogenic mutants, or nontagged strains were grown to mid-log phase in synthetic medium containing 2% glucose and were then fixed with formaldehyde. An α-HA ChIP assay was performed, and the core promoter region of the STA1 promoter was amplified by PCR using purified DNA to detect Srb10 binding to the STA1 promoter.
Since our finding, together with the data reported previously (37), suggests that Nrg1 and Sfl1 may directly recruit the Srb8-11 complex to the STA1 promoter, we performed a ChIP assay using the integrated SRB10-HA strain. As expected, Srb10 bound to the STA1 promoter in wild-type cells. In contrast, Srb10 binding was marginally reduced in the nrg1Δ mutant and greatly diminished in the sfl1Δ mutant. Furthermore, the interaction between Srb10 and the STA1 promoter was completely abolished in the nrg1Δ sfl1Δ double mutant (Fig. 7B). These results indicate that both Nrg1 and Sfl1 recruit the Srb8-11 complex to the STA1 promoter to repress STA1 expression.
SFL1 expression is regulated posttranscriptionally.
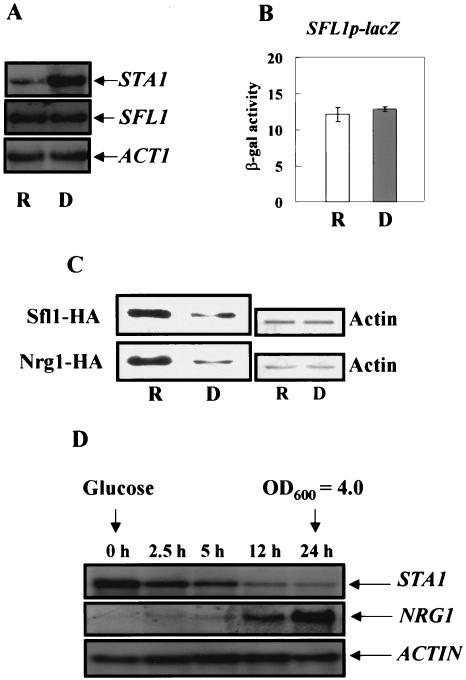
It has been reported previously that NRG1 is transcriptionally induced by glucose (31). However, it was not known whether SFL1 transcription is also induced in the presence of glucose. Thus, we examined levels of SFL1 mRNA in the wild-type strain under both repressed and derepressed conditions. The level of the SFL1 transcript in glucose medium was similar to that in glycerol-ethanol medium (Fig. 8A). Consistent with these observations, β-galactosidase activities of the SFL1 promoter were also similar (Fig. 8B), indicating that SFL1 transcription is not regulated in response to different carbon sources.
FIG. 8.
Levels of Nrg1 and Sfl1 increase in the presence of glucose. (A) Wild-type cells were grown in a synthetic medium containing 2% glucose (R) or 2% glycerol-ethanol (D) as carbon sources, and total RNA was prepared for Northern blot analysis. The yeast actin gene (ACT1) was used as an internal control. (B) A plasmid containing SFL1p-lacZ was transformed into wild-type cells, and β-galactosidase activity was determined under the same conditions from three independent colonies. (C) Nrg1-HA and Sfl1-HA were expressed from their own promoters. Cells were grown in synthetic medium with 2% glucose or 2% glycerol-ethanol to mid-log phase, and total cellular proteins were prepared. Western blot analysis was performed to determine the levels of Sfl1-HA and Nrg1-HA from 50 μg of protein extract. The same membranes were probed with an α-actin monoclonal antibody. (D) Cells were grown to mid-log phase in synthetic medium with 2% glycerol-ethanol and shifted to synthetic medium with 4% glucose until they reached an OD600 of 4.0. Total RNA was prepared at the indicated time points for Northern blot analysis. The blot was hybridized with an NRG1 probe and then stripped and rehybridized with STA1 and ACT1 probes.
Next, we examined Sfl1 protein levels in cells carrying plasmid pRS325-SFL1-HA, which expresses Sfl1 tagged with 3 HA molecules at its C terminus from its own promoter. This Sfl1-HA fusion was functional, since it complemented sfl1Δ, and the level of SFL1-HA transcription was not affected by glucose (data not shown). Although levels of SFL1 transcription were similar in glucose- and glycerol-ethanol-grown cells, cells cultured in glucose medium exhibited approximately fourfold-higher Sfl1-HA protein levels than cells cultured in glycerol-ethanol medium (Fig. 8C). These data indicate that SFL1 expression is regulated posttranscriptionally.
Since it has been reported that NRG1 transcription is also induced during diauxic shift (7), we performed Northern blot analysis in order to investigate whether the increase in NRG1 mRNA levels is due to the presence of glucose. Cells were grown to an OD600 of 1.0 in 2% glycerol-ethanol medium, and 4% glucose was then added. Figure 8D shows that transcription of NRG1 was barely detectable in glycerol-ethanol but was initiated upon addition of glucose and continued to increase up to 24 h. About 0.5% glucose was still detected in the medium at this point, indicating that the increase in NRG1 transcript levels is not due to depletion of the glucose. In contrast, the STA1 mRNA level fell after 4% glucose was added. Under the same conditions, the level of Nrg1-HA is fourfold higher in glucose-grown cells than in glycerol-ethanol-grown cells. Furthermore, there was no detectable mobility shift of Nrg1-HA in either the glucose- or glycerol-ethanol-grown cells. These results suggest that glucose regulates NRG1 at the transcriptional level, whereas it regulates SFL1 at the posttranscriptional level.
DISCUSSION
In this study, we provide evidence that the transcriptional repressors Nrg1 and Sfl1 and the members of the Srb8-11 complex are required for glucose repression of STA1 expression. Our data showed that Sfl1 acts specifically on UAS2 of the STA1 promoter, indicating that this region contains a binding site for Sfl1. It has been reported that Sfl1 has an N-terminal DNA binding domain similar to that of the yeast heat shock transcription factor (5, 10). Based on these observations, Conlan and Tzamarias proposed that Sfl1 might bind to an inverted repeat of the heat shock factor element, 5′ AGAA-n-TTCT 3′, and later showed that Sfl1 bound to the region from −475 to −316 of the FLO11 promoter, containing a putative heat shock factor element, in vivo (5). However, they did not confirm whether Sfl1 directly interacts with this conserved binding element or not. Recently, Pan and Heitman reported that Sfl1 bound directly to the region from −1400 to −1150 of the FLO11 promoter in vitro (30). However, even though this region is very similar to the region from −1316 to −1088 of the STA1 promoter, except for a 20-bp insert, 2-bp deletions, and a few small substitutions, we failed to find the heat shock element in this region. Instead, we found that a heat-shock factor element (5′ −1106AGAA-n-TTCT−1034,−975,−954,−919 3′ [n = 64, 123, 144, and 179 bp]) exists in UAS2-2 (−1147 to −882) and appears to be critical for in vivo Sfl1 binding and for glucose repression of STA1.
Previous reports showed that the activators, such as Flo8, Mss11, Ste12, and Tec1, are required for activation of STA1 and FLO11 expression (11, 12, 13, 21). Flo8 and Sfl1, which antagonistically control FLO11 expression, are direct targets of PKA (30). Phosphorylation by the PKA catalytic subunit Tpk2 activates FLO11 transcription by inhibiting Sfl1 binding and promoting Flo8 binding to the region from −1400 to −1150 of the FLO11 promoter. It has been suggested that Sfl1 competes with the activator Flo8 for occupation of this region (30). The 5′ upstream regions of the FLO11 and STA1 genes are quite similar, and these genes are coregulated in response to environmental signals (12). Thus, we speculated that STA1 expression might be regulated positively or negatively by Tpk2 and that Sfl1 and Flo8 might compete with each other to occupy the STA1 promoter. However, STA1 expression was not affected by deletion of TPK2 (unpublished data). Furthermore, we failed to observe that Sfl1 competes with Flo8 for binding to the STA1 promoter. Rather, the Sfl1 repressor appears to inhibit FLO8 expression. We have evidence that the level of Flo8 is reduced in glucose-grown cells (Kim et al., submitted). Here we also show that Sfl1 overexpression reduces FLO8 expression (Fig. 4). In fact, we reveal that the FLO8 promoter contains the heat shock factor element and that Sfl1 binds to the FLO8 promoter in vivo. These results indicate that Sfl1 represses FLO8 expression, and FLO8 is a new target of Sfl1. On the other hand, we also present direct evidence that the repressor Sfl1 competes with the activators Ste12 and Tec1 to occupy UAS2 of the STA1 promoter. Thus, our results indicate that Sfl1 represses STA1 expression via two distinct mechanisms: by directly preventing the binding of Ste12 and Tec1 to UAS2-1 and by repressing the expression of FLO8.
In UAS1, in contrast, competition between the repressor and activators was not observed (data not shown). Thus, Nrg1 binding to UAS1-1 does not prevent Flo8 and Mss11 from binding to the UAS of the STA1 promoter, and a different mechanism must exist to account for the Nrg1-dependent repression. We suggest that Nrg1 recruits a general corepressor, such as Ssn6-Tup1, perhaps together with Srb proteins, and that this inhibits the interaction between the transcriptional activators and their coactivators or RNA polymerase II. It is also possible that Tup1, which is recruited by Nrg1, alters the chromatin structure of the STA1 promoter.
Mutants with mutations of SRB genes, such as SRB8, SRB9, SRB10, and SRB11, have significantly increased glucoamylase activity and hyperinvasive phenotypes (Fig. 5) (data not shown). In contrast, mutation of SIN4, a component of the RNA polymerase II subcomplex that interacts physically with Sfl1, also induces the hyperinvasive phenotype (data not shown), but the glucoamylase activity of this mutant is only marginally elevated under repressed conditions (Fig. 5). These results indicate that although STA1 and FLO11 are to a large extent coregulated, certain factors act differentially on them. The functions of Nrg1 and Sfl1 are closely related to those of the Ssn6-Tup1 and the Srb8-11 complex (5, 31, 37). Furthermore, repression by LexA-Ssn6 and LexA-Tup1 requires the Srb8-11 complex (22, 25). However, our findings suggest that the Srb8-11 complex is more important than Ssn6-Tup1 in glucose repression of STA1 expression.
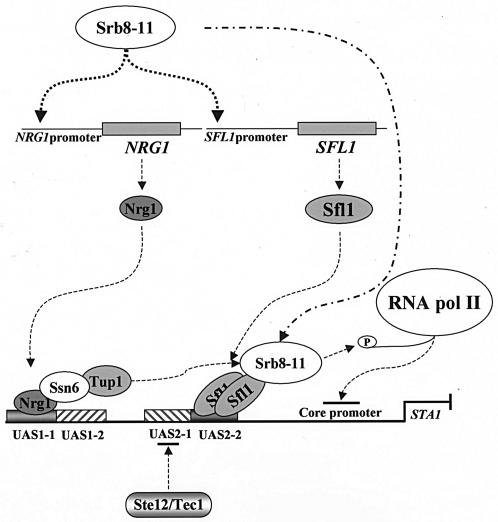
We also showed that the Srb8-11 complex was required for expression of both NRG1 and SFL1, though the molecular mechanism by which it mediates this regulation is not clear at present. Since we did not find that Srb10-HA bound to the NRG1 or SFL1 promoter (data not shown), it is possible that the Srb8-11 complex positively regulates transcriptional activators that are involved in NRG1 and SFL1 transcription. Previous reports showed that Srb10 both positively and negatively regulates gene-specific activators, such as Gal4, Sip4, Gcn4, and Ste12 (3, 18, 28, 39). The transcriptional activation activity of Sip4 is stimulated by Srb10, but Srb10 phosphorylates Ste12 and Gcn4 and inhibits their function by promoting their turnover. Thus, in glucose-grown cells, the Srb8-11 complex represses the transcription of STA1 by activating NRG1 and SFL1 expression. Furthermore, we found that the interaction between Srb10 and the STA1 promoter required functional Nrg1 and Sfl1 (Fig. 7), strongly suggesting that the Srb8-11 complex is recruited to the STA1 promoter by Nrg1 and Sfl1. Our data suggest that the Srb8-11 complex plays essential roles in glucose repression of STA1 expression by activating NRG1 and SFL1 expression and also by participating in Nrg1- and Sfl1-dependent repression (Fig. 9).
FIG. 9.
Model of the molecular mechanism of glucose repression of STA1 expression. In glucose-grown cells, the Srb8-11 complex activates NRG1 and SFL1 expression. The increased levels of Nrg1 and Sfl1 bind to UAS1-1 and UAS2-2, respectively. DNA-bound Nrg1 and Sfl1 recruit the Ssn6-Tup1 corepressor and the Srb8-11 complex to the STA1 promoter. In addition, DNA-bound Sfl1 prevents access of transcriptional activators Ste12 and Tec1 to UAS2-1. The Ssn6-Tup1 or Srb8-11 complex may alter chromatin structure or phosphorylate the C-terminal domain of RNA polymerase II prior to the formation of an initiation complex.
We showed that the level of Sfl1 was higher in glucose-grown cells than in glycerol-ethanol-grown cells, although SFL1 transcription is neither significantly induced nor repressed with different carbon sources. In addition, an increased dosage of Sfl1 by a multicopy plasmid reduces STA1 expression, even under derepressed conditions. Thus, it is likely that the level of Sfl1 is a more important factor in glucose repression of STA1 than its modification. However, the question of whether another protein kinase besides Tpk2 also regulates Sfl1 function or not remains to be investigated.
Snf1 kinase was known to interact with the Nrg1 repressor and to act as an antagonist of Nrg1 (23, 41). Interestingly, it has been shown that Nrg1 localization is not affected by different carbon sources (41). However, the molecular mechanism by which Snf1 regulates the function of Nrg1 is largely unknown. It was observed previously that induction of NRG1 transcription was enhanced about 6-fold by glucose (31), but it was also reported to be enhanced 2.7-fold during a diauxic shift (7). In the present work, we showed that NRG1 transcription was induced after glucose addition but before there was any glucose depletion (Fig. 8D). Furthermore, the level of Nrg1 is also about fourfold higher in glucose-grown cells than in glycerol-ethanol-grown cells (Fig. 8C). These data suggest that NRG1 expression is induced by glucose, although it is also induced during a diauxic shift. In addition, the levels of Flo8 and Tec1 that are required for activation of STA1 expression are significantly reduced in the presence of glucose (Kim et al., submitted). We therefore suggest that the increased quantities of Nrg1 and Sfl1 and the reduced levels of Flo8 and Tec1 repress the transcription of STA1 and FLO11 in glucose-grown cells.
In conclusion, glucose repression of STA1 expression requires a series of complex regulatory elements (Fig. 9). The Srb8-11 complex activates NRG1 and SFL1 expression under repressed conditions; Nrg1 binds directly to UAS1-1, whereas Sfl1 binds to UAS2-2 after forming multimers through the coiled-coil domains. When bound to their specific sites, Nrg1 and Sfl1 can recruit the Ssn6-Tup1 corepressor or the Srb8-11 complex, resulting in alteration of the chromatin structures and/or perhaps hindering the formation of the initiation complex on the STA1 promoter. Furthermore, Sfl1 represses FLO8 expression and inhibits the access of Ste12 and Tec1 to the STA1 promoter.
Acknowledgments
We thank Kyung S. Lee (NIH) for preparation of the manuscript.
This work was supported by a grant from the Korea Science and Engineering Foundation [R01-2002-000-00221-0(2002)]. T. S. Kim and S. B. Lee are supported by a BK21 Research Fellowship from the Ministry of Education and Human Resources Development.
REFERENCES
- 1.Ahn, J. H., S. H. Park, and H. S. Kang. 1995. Inactivation of the UAS1 of STA1 by glucose and STA10 and identification of two loci, SNS1 and MSS1, involved in STA10-dependent repression in Saccharomyces cerevisiae. Mol. Gen. Genet. 246:529-537. [DOI] [PubMed] [Google Scholar]
- 2.Borggrefe, T., R. Davis, H. Erdjument-Bromage, P. Tempst, and R. D. Kornberg. 2002. A complex of the Srb8, -9, -10, and -11 transcriptional regulatory proteins from yeast. J. Biol. Chem. 277:44202-44207. [DOI] [PubMed] [Google Scholar]
- 3.Chi, Y., M. J. Huddleston, X. Zhang, R. A. Young, R. S. Annan, S. A. Carr, and R. J. Deshaies. 2001. Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev. 15:1078-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christianson, T. W., R. S. Sikorski, M. Dante, J. H. Shero, and P. Hieter. 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119-122. [DOI] [PubMed] [Google Scholar]
- 5.Conlan, R. S., and D. Tzamarias. 2001. Sfl1 functions via the co-repressor Ssn6-Tup1 and the cAMP-dependent protein kinase Tpk2. J. Mol. Biol. 309:1007-1015. [DOI] [PubMed] [Google Scholar]
- 6.Cullen, P. J., and G. F. Sprague, Jr. 2000. Glucose depletion causes haploid invasive growth in yeast. Proc. Natl. Acad. Sci. USA 97:13619-13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeRisi, J. L., V. R. Iyer, and P. O. Brown. 1997. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278:680-686. [DOI] [PubMed] [Google Scholar]
- 8.De Vit, M. J., J. A. Waddle, and M. Johnston. 1997. Regulated nuclear translocation of the Mig1 glucose repressor. Mol. Biol. Cell 8:1603-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dranginis, A. M. 1989. Regulation of STA1 gene expression by MAT during the life cycle of Saccharomyces cerevisiae. Mol. Cell. Biol. 9:3992-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita, A., Y. Kikuchi, S. Kuhara, Y. Misumi, S. Matsumoto, and H. Kobayashi. 1989. Domains of the SFL1 protein of yeasts are homologous to Myc oncoproteins or yeast heat-shock transcription factor. Gene 85:321-328. [DOI] [PubMed] [Google Scholar]
- 11.Gagiano, M., M. Bester, D. van Dyk, J. Franken, F. F. Bauer, and I. S. Pretorius. 2003. Mss11p is a transcription factor regulating pseudohyphal differentiation, invasive growth and starch metabolism in Saccharomyces cerevisiae in response to nutrient availability. Mol. Microbiol. 47:119-134. [DOI] [PubMed] [Google Scholar]
- 12.Gagiano, M., D. Van Dyk, F. F. Bauer, M. G. Lambrechts, and I. S. Pretorius. 1999. Divergent regulation of the evolutionarily closely related promoters of the Saccharomyces cerevisiae STA2 and MUC1 genes. J. Bacteriol. 181:6497-6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagiano, M., D. van Dyk, F. F. Bauer, M. G. Lambrechts, and I. S. Pretorius. 1999. Msn1p/Mss10p, Mss11p and Muc1p/Flo11p are part of a signal transduction pathway downstream of Mep2p regulating invasive growth and pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Microbiol. 31:103-116. [DOI] [PubMed] [Google Scholar]
- 14.Griggs, D. W., and M. Johnston. 1991. Regulated expression of the GAL4 activator gene in yeast provides a sensitive genetic switch for glucose repression. Proc. Natl. Acad. Sci. USA 88:8597-8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarente, L., and M. Ptashne. 1981. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 78:2199-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hecht, A., S. Strahl-Bolsinger, and M. Grunstein. 1996. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature 383:92-96. [DOI] [PubMed] [Google Scholar]
- 17.Hengartner, C. J., V. E. Myer, S. M. Liao, C. J. Wilson, S. S. Koh, and R. A. Young. 1998. Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol. Cell 2:43-53. [DOI] [PubMed] [Google Scholar]
- 18.Hirst, M., M. S. Kobor, N. Kuriakose, J. Greenblatt, and I. Sadowski. 1999. GAL4 is regulated by the RNA polymerase II holoenzyme-associated cyclin-dependent protein kinase SRB10/CDK8. Mol. Cell 3:673-678. [DOI] [PubMed] [Google Scholar]
- 19.Hu, Z., J. O. Nehlin, H. Ronne, and C. A. Michels. 1995. MIG1-dependent and MIG1-independent glucose regulation of MAL gene expression in Saccharomyces cerevisiae. Curr. Genet. 28:258-266. [DOI] [PubMed] [Google Scholar]
- 20.Keleher, C. A., M. J. Redd, J. Schultz, M. Carlson, and A. D. Johnson. 1992. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell 68:709-719. [DOI] [PubMed] [Google Scholar]
- 21.Kim, T. S., J. Y. Ahn, J. H. Yoon, and H. S. Kang. 2003. STA10 repression of STA gene expression is caused by a defective activator, flo8, in Saccharomyces cerevisiae. Curr. Genet. 44:261-267. [DOI] [PubMed] [Google Scholar]
- 22.Kuchin, S., and M. Carlson. 1998. Functional relationships of Srb10-Srb11 kinase, carboxy-terminal domain kinase CTDK-I, and transcriptional corepressor Ssn6-Tup1. Mol. Cell. Biol. 18:1163-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuchin, S., V. K. Vyas, and M. Carlson. 2002. Snf1 protein kinase and the repressors Nrg1 and Nrg2 regulate FLO11, haploid invasive growth, and diploid pseudohyphal differentiation. Mol. Cell. Biol. 22:3994-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuchin, S., P. Yeghiayan, and M. Carlson. 1995. Cyclin-dependent protein kinase and cyclin homologs SSN3 and SSN8 contribute to transcriptional control in yeast. Proc. Natl. Acad. Sci. USA 92:4006-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, M., S. Chatterjee, and K. Struhl. 2000. Genetic analysis of the role of Pol II holoenzyme components in repression by the Cyc8-Tup1 corepressor in yeast. Genetics 155:1535-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nehlin, J. O., M. Carlberg, and H. Ronne. 1991. Control of yeast GAL genes by MIG1 repressor: a transcriptional cascade in the glucose response. EMBO J. 10:3373-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nehlin, J. O., and H. Ronne. 1990. Yeast MIG1 repressor is related to the mammalian early growth response and Wilms' tumour finger proteins. EMBO J. 9:2891-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson, C., S. Goto, K. Lund, W. Hung, and I. Sadowski. 2003. Srb10/Cdk8 regulates yeast filamentous growth by phosphorylating the transcription factor Ste12. Nature 421:187-190. [DOI] [PubMed] [Google Scholar]
- 29.Pan, X., and J. Heitman. 1999. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:4874-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan, X., and J. Heitman. 2002. Protein kinase A operates a molecular switch that governs yeast pseudohyphal differentiation. Mol. Cell. Biol. 22:3981-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park, S. H., S. S. Koh, J. H. Chun, H. J. Hwang, and H. S. Kang. 1999. Nrg1 is a transcriptional repressor for glucose repression of STA1 gene expression in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:2044-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polaina, J., and M. Wiggs. 1983. STA10: a gene involved in the control of starch utilization by Saccharomyces. Curr. Genet. 7:109-112. [DOI] [PubMed] [Google Scholar]
- 33.Pretorius, I. S., D. Modena, M. Vanoni, S. Englard, and J. Marmur. 1986. Transcriptional control of glucoamylase synthesis in vegetatively growing and sporulating Saccharomyces species. Mol. Cell. Biol. 6:3034-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson, L. S., and G. R. Fink. 1998. The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc. Natl. Acad. Sci. USA 95:13783-13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rupp, S., E. Summers, H. J. Lo, H. Madhani, and G. Fink. 1999. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 18:1257-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song, W., and M. Carlson. 1998. Srb/mediator proteins interact functionally and physically with transcriptional repressor Sfl1. EMBO J. 17:5757-5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Treitel, M. A., and M. Carlson. 1995. Repression by SSN6-TUP1 is directed by MIG1, a repressor/activator protein. Proc. Natl. Acad. Sci. USA 92:3132-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincent, O., S. Kuchin, S. P. Hong, R. Townley, V. K. Vyas, and M. Carlson. 2001. Interaction of the Srb10 kinase with Sip4, a transcriptional activator of gluconeogenic genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:5790-5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vyas, V. K., S. Kuchin, C. D. Berkey, and M. Carlson. 2003. Snf1 kinases with different beta-subunit isoforms play distinct roles in regulating haploid invasive growth. Mol. Cell. Biol. 23:1341-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vyas, V. K., S. Kuchin, and M. Carlson. 2001. Interaction of the repressors Nrg1 and Nrg2 with the Snf1 protein kinase in Saccharomyces cerevisiae. Genetics 158:563-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang, X., E. J. Hubbard, and M. Carlson. 1992. A protein kinase substrate identified by the two-hybrid system. Science 257:680-682. [DOI] [PubMed] [Google Scholar]