Abstract
Formaldehyde, a common indoor air pollutant, exacerbates asthma and synergizes with allergen to induce airway hyperresponsiveness (AHR) in animal models. The mechanisms mediating formaldehyde-induced AHR remain poorly understood. We posit that formaldehyde modulates agonist-induced contractile response of human airway smooth muscle (HASM) cells to elicit AHR. HASM cells were exposed to formaldehyde or vehicle and agonist-induced intracellular Ca2+ ([Ca2+]i) and myosin light-chain phosphatase (MYPT1) phosphorylation were determined. Air–liquid interface–differentiated human bronchial epithelial (HBE) cells were exposed to formaldehyde or vehicle and cocultured with HASM cells. Agonist-induced [Ca2+]i and MYPT1 phosphorylation were determined in the cocultured HASM cells. Precision-cut human lung slices were exposed to PBS or varying concentrations of formaldehyde, and then carbachol-induced airway narrowing was determined 24 hours after exposure. HASM cells were transfected with nontargeting or nuclear factor erythroid-derived 2, like 2 (Nrf-2)-targeting small interfering RNA and exposed to formaldehyde or vehicle, followed by determination of antioxidant response (quinone oxido-reductase 1 and thioredoxin 1) and basal and agonist-induced MYPT1 phosphorylation. Formaldehyde enhanced the basal Rho-kinase activity and MYPT1 phosphorylation with little effect on agonist-induced [Ca2+]i in HASM cells. Formaldehyde induced Nrf-2–dependent antioxidant response in HASM cells, although the MYPT1 phosphorylation was independent of Nrf-2 induction. Although HBE cells exposed to formaldehyde had little effect on agonist-induced [Ca2+]i or MYPT1 phosphorylation in cocultured HASM cells, formaldehyde enhanced carbachol-induced airway responsiveness in precision-cut human lung slices. In conclusion, formaldehyde induces phosphorylation of the regulatory subunit of MYPT1, independent of formaldehyde-induced Nrf-2 activation in HASM cells. The findings suggest that the Rho kinase-dependent Ca2+ sensitization pathway plays a role in formaldehyde-induced AHR.
Keywords: formaldehyde, airway hyperresponsiveness, asthma, airway smooth muscle, Ca2+ sensitization
Clinical Relevance
Formaldehyde, an indoor air pollutant, enhances asthma symptoms, although the mechanisms involved remain poorly understood. We examined whether formaldehyde modulates procontractile signaling in human airway smooth muscle (ASM) cells. We report that formaldehyde modulates a key Ca2+ sensitization pathway in ASM cells and enhances agonist-induced airway narrowing in precision-cut human lung slices. The study sheds light on airway structural cells as the potential mediators of formaldehyde-induced airway hyperresponsiveness.
Asthma, a chronic airway disorder, manifests as airway inflammation, hyperresponsiveness, and remodeling. Higher incidence of airway disorders, such as asthma, in some populations is attributed to increased air pollution (1). Evidence also shows that air pollution adversely affects lung health in healthy individuals and in patients with asthma (2, 3). Although outdoor air pollutants, such as ozone, nitric oxide, and particulate matter of 2.5 μm or less, induce exacerbations of asthma and chronic obstructive pulmonary disease (4–6); formaldehyde, an indoor air pollutant, exacerbates asthma in children and adults (7–10). In animal models of asthma, formaldehyde exposure enhances the allergic airway inflammation and airway hyperresponsiveness (AHR) (11, 12). Studies in animal models suggest that airway inflammation from irritation and oxidative stress may be the mechanisms enhancing formaldehyde-induced AHR. Whether formaldehyde modulates airway structural cell functions, airway epithelium, and airway smooth muscle (ASM) remains unknown.
Shortening of ASM cells directly regulates airway resistance and hyperresponsiveness in asthma (13, 14). Agonist-induced Ca2+ mobilization and subsequent activation of actin–myosin cross-bridge formation are required for ASM cell shortening. In ASM cells isolated from subjects with asthma, expression and function of proteins involved in Ca2+ homeostasis are dysregulated (14–16). A second pathway that does not directly involve Ca2+ mobilization can also amplify ASM responses to contractile agonists.
Ca2+ sensitization pathways amplify the contractile response to agonists through signaling entities, such as RhoA/RhoA-associated kinase (ROCK) (17). ROCK signaling pathway regulates Ca2+ sensitization in smooth muscle cells by phosphorylating the regulatory subunit of myosin light-chain phosphatase (myosin phosphatase target [MYPT] subunit 1) (18, 19). Phosphorylation of T696 in the regulatory subunit MYPT1 inactivates the phosphatase activity of myosin light chain phosphatase (20), thus maintaining the phosphorylation of myosin light chain (MLC) and enhancing bronchomotor tone. In animal models of allergen-induced AHR, ROCK-dependent Ca2+ sensitization plays an important role in enhancing airway resistance (19). Fungal allergens from Aspergillus fumigatus (Af) enhance MYPT1 phosphorylation in human ASM (HASM) cells, suggesting that ROCK-dependent Ca2+ sensitization is an important mechanism in A. fumigatus–induced AHR (21). Evidence suggests that toxicants can impact signaling pathways in airway structural cells, such as airway epithelial and smooth muscle cells, to modulate lung functions. In addition to the protective role, airway epithelial cells also act as mediators of intercellular signals to modulate the other cells, such as ASM cells (22, 23). Environmental toxicants also can directly act on ASM cells, especially in pathophysiological conditions that disrupt the epithelial barrier (24). The environmental toxicants, acrolein and ozone, modulate agonist-induced Ca2+ homeostasis, which impacts the contractile functions of ASM, thereby altering pulmonary function (25, 26). These observations underscore the importance of airway structural cells and in mediating the adverse effects of respiratory toxicants. We hypothesized that formaldehyde modulates agonist-induced contractile response of HASM cells to elicit AHR. Our findings show that formaldehyde enhances agonist-induced airway narrowing in human lung slices without significant changes in HASM cellular Ca2+ response. Collectively, these findings suggest that ROCK activation mediates formaldehyde-induced AHR in human small airways.
Materials and Methods
Reagents
Ham’s F-12 medium, PBS, FBS, 0.05% trypsin and EDTA, Lipofectamine RNAimax, Opti-MEM, cDNA synthesis kit, TriZol, SYBR green quantitative PCR reaction mixture, and all PAGE/immune blotting supplies were purchased from Life Technologies (Grand Island, NY). Primers for ROCK1, ROCK2, and cyclophilin were purchased from IDT (Coralville, IA). Carbachol, bradykinin, and thrombin were purchased from Sigma-Aldrich (St. Louis, MO). Nontargeting small interfering RNA (siRNA) and nuclear factor erythroid-derived 2, like 2 (Nrf-2)–targeting siRNA were purchased from ThermoFisher Scientific (Dharmacon, Lafayette, CO). Antibodies against phosphorylated MYPT1 (pMYPT1) were obtained from Millipore (Billerica, MA). pMLC and α-tubulin were from Cell Signaling Technology (Danvers, MA) and quinone oxido-reductase (NQO) 1 and thioredoxin (Trx) 1 were from Santa Cruz Biotechnology (Santa Cruz, CA; ). Y27642 was purchased from Cayman Chemicals (Ann Arbor, MI). Chemiluminescent reagent was obtained from ThermoFisher Scientific (Pierce, Rockford, IL).
Culture of HASM and Human Bronchial Epithelial Cells
Primary HASM cells were isolated from donor lung trachea as described previously (27, 28). HASM cells (passage 2–4) were grown in Ham’s F-12 medium containing 10% FBS to confluence. Before experiments, the cells were serum starved for 48 hours. Human bronchial epithelial (HBE) cells were cultured and differentiated in air–liquid interface, as previously described (29). Differentiated cells were exposed to vehicle or formaldehyde for 1 hour and placed on serum-starved HASM cells for 24 hours. pMLC/pMYPT1 or agonist-induced intracellular Ca2+ ([Ca2+]i) were determined in cocultured HASM cells.
Formaldehyde Exposure
Neutral buffered formalin solution (10%; VWR International, Radnor, PA) in PBS was placed in a glass impinger and atmospheric air was drawn by vacuum force (5 L/min) to generate formaldehyde vapor. Formaldehyde vapor was delivered into a chamber containing precision-cut human lung slice (PCLSs) or cells. Various concentrations of formalin solutions were used in preliminary trials to obtain vapor concentrations of 0.2, 0.8, or 2 ppm formaldehyde within the exposure chamber. Chamber formaldehyde levels were measured by passive formaldehyde monitors (product no. 3721; 3M, St. Paul, MN) placed in the chamber and submitted for analysis (Bureau Veritas, Novi, MI). PCLSs or cells were exposed to PBS or formaldehyde vapor (0.2, 0.8, or 2 ppm) for 1 hour and readouts were determined 24 hours after exposure.
Determination of [Ca2+]i in HASM Cells
Agonist-induced [Ca2+]i in HASM cells was determined as previously described (21) with some modifications. Bradykinin (1 nM) or histamine (1 μM) was used as agonist.
Mediator Release from PCLSs and HASM Cells
IL-6 and IL-8 levels were determined in culture supernatants of lung slices or HASM cells following the manufacturer’s instructions (R&D Systems, Minneapolis, MN). Cytokine levels were normalized to the total protein of the supernatants.
ROCK Assay
Lysates were collected from HASM cells in Tris-sucrose lysis buffer with protease and phosphatase inhibitors. Protein (10 μg) was used in duplicate to determine ROCK activity following the manufacturer’s instructions (catalog no. CSA001; Millipore).
Immunoblotting
Lysates were collected from HASM cells in Tris-sucrose buffer with protease and phosphatase inhibitors. Protein (10 μg) was run in SDS-PAGE for immunoblotting. The membrane was blocked with 3% BSA solution and probed for nicotinamide adenine dinucleotide phosphate reduced–NQO1, Trx, and α-tubulin. To detect phosphoproteins, lysates were collected in 0.6 N HClO4.
siRNA Transfection
HASM cells were transfected with nontargeting or Nrf-2 siRNA using Lipofectamine RNAiMAX as previously described (30). Nrf-2 silencing was confirmed by NQO1 and Trx1 expression. HASM cells were exposed to formaldehyde at 72 hours after transfection and cell lysates were collected 24 hours after formaldehyde exposure.
Human PCLS and Carbachol Dose–Response
Human lungs were obtained through NDRI (Philadelphia, PA) or IIAM (Edison, NJ). Samples were deidentified and therefore exempted by the University of Pennsylvania (Philadelphia, PA) Institutional Review Board. PCLSs were prepared and carbachol (10−8–10−4 M) dose–response parameters (half-maximal effective concentration [log EC50], area under the curve, and maximal effect) were determined as previously described (31).
Statistical Analysis
HASM cells from at least three donors or PCLSs from at least five donors were used in the studies. In PCLS studies, from each donor, a minimum of three slices per experimental group were used. Data are expressed as mean or mean (±SEM). GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA) was used for statistical analysis (one way ANOVA or Student’s t test) and means were considered significantly different when P was less than or equal to 0.05.
Results
Formaldehyde Has Little Effect on Agonist-Induced [Ca2+]i, but Enhances ROCK Activity in HASM Cells
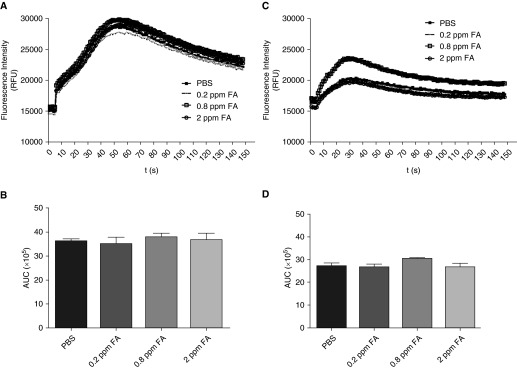
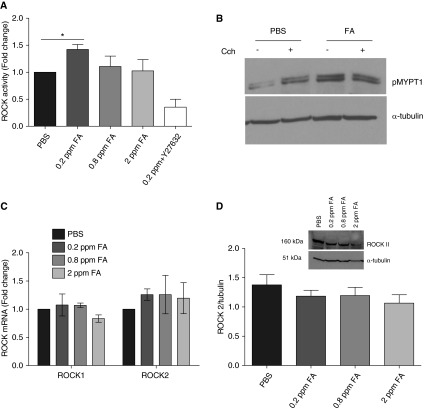
Mobilization of [Ca2+]i in ASM cells is a pivotal signaling event in regulating agonist-induced ASM shortening. To determine whether formaldehyde exposure alters agonist-induced [Ca2+]i in HASM cells, bradykinin or histamine-induced [Ca2+]i was determined in HASM cells 24 hours after exposure to formaldehyde. The magnitude of bradykinin-induced [Ca2+]i (area under the curve of the Ca2+ transients) was comparable in PBS and formaldehyde-treated HASM cells (Figures 1A and 1B). Similarly, histamine (1 μM) evoked comparable magnitudes of [Ca2+]i responses in HASM cells exposed to PBS and formaldehyde (Figures 1C and 1D). Furthermore, formaldehyde had little effect on basal and thrombin-induced [Ca2+]i in HASM cells (Figure E1). Ca2+ sensitization is a complementary mechanism that sensitizes the contractile apparatus to an existing [Ca2+]i level so that the contractile response is enhanced without an increase in agonist-induced [Ca2+]i. ROCK pathway is a key modulator of Ca2+ sensitization in smooth muscle cells (19). We next determined ROCK activity in HASM cell lysates by assessing the pMYPT1 level, either by ELISA or by immunoblotting. In ELISA assays, ROCK activity was significantly increased in HASM cells exposed to 0.2 ppm formaldehyde compared with PBS-treated cells (Figure 2A). We confirmed increased MYPT1 phosphorylation by immunoblotting lysates from HASM cells exposed to formaldehyde. The basal MYPT1 phosphorylation was increased by formaldehyde exposure, even in the absence of a contractile agonist (Figure 2B). To determine whether formaldehyde alters ROCK gene expression, we examined ROCK I and 2 mRNA expression levels in HASM cells 24 hours after exposure to PBS or formaldehyde. Formaldehyde has little effect on ROCK1 or ROCK2 mRNA levels in HASM cells (Figure 2C). Similar to ROCK mRNA levels, formaldehyde had little effect on ROCK2 protein expression in HASM cells (2 D). In ASM cells, ROCK activation is regulated by the upstream small molecular weight G protein RhoA. To determine whether formaldehyde induces MYPT1 phosphorylation via Rho activation, HASM cells were exposed to formaldehyde in the presence of Rho Inhibitor I (CT04 or exoenzyme C3 transferase of C.botulinum). Rho Inhibitor has little effect on formaldehyde-induced MYPT1 phosphorylation (Figure E2).
Figure 1.
Formaldehyde has little effect on agonist-induced intracellular Ca2+ [Ca2+]i in human airway smooth muscle (HASM) cells. HASM cells were loaded with the Ca2+-binding dye fluo-8 and stimulated with agonists to determine the global changes in [Ca2+]i. There was no significant effect on the (A) bradykinin-induced (1 nM) or (C) histamine-induced (1 μM) [Ca2+]i in cells exposed to formaldehyde. Area under the curves (AUCs) of [Ca2+]i transients induced by (B) bradykinin or (D) histamine were comparable between PBS-treated and formaldehyde-treated HASM cells (n = 3 donors with 3 technical replicates per condition); A and C, mean RFU; B and D, mean ± SEM. FA, formaldehyde; ppm, parts per million; RFU, relative fluorescence unit.
Figure 2.
Formaldehyde enhances myosin light-chain phosphatase (MYPT1) phosphorylation in HASM cells. Rho-associated kinase (ROCK) activity was determined in HASM cell lysates by assessing phosphorylated (p) MYPT1 (pMYPT1) levels, either by ELISA or immune blotting. (A) Formaldehyde (0.2 ppm) increased the basal ROCK activity in HASM cells compared with PBS-treated control (mean ± SEM; n = 3 donors; *P < 0.05). (B) Phosphorylation of MYPT1, the downstream target of ROCK, was increased in HASM cells exposed to 0.2 ppm formaldehyde. Stimulation with the contractile agonist carbachol (10 μM for 10 min) increased the pMYPT1 in PBS-treated HASM cells but not in formaldehyde-treated cells (blot representative of five experiments). Formaldehyde exposure has little effect on the expression of (C) ROCK1/2 mRNA (mean ± SEM, n = 3) or (D) ROCK2 protein in HASM cells (representative blot for n = 3; mean ± SEM). CCh, carbachol concentration.
Formaldehyde Has Little Effect on Inflammatory Mediator Release from PCLSs or HASM Cells
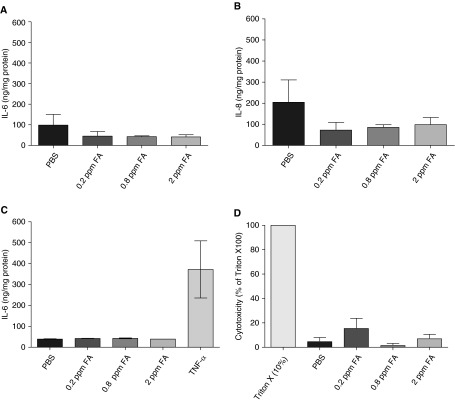
ASM cells contribute to asthma pathogenesis, not only by modulating bronchomotor tone, but also by releasing inflammatory mediators. IL-8 and IL-6 are two major inflammatory mediators released by ASM cells. Ozone, an important environmental pollutant, induces IL-6 release from HASM and airway epithelial cells (29). To determine whether formaldehyde induces cytokine or chemokine secretion, PCLSs or HASM cells were exposed to PBS or formaldehyde and the culture supernatants were analyzed for IL-6 and IL-8. In PCLSs, supernatant IL-6 or IL-8 levels were unaffected in response to formaldehyde exposure (Figures 3A and 3B). Furthermore, formaldehyde has little effect on IL-6 release from HASM cells, while TNF-α (10 ng/ml) increased IL-6 levels (Figure 3C). IL-8 levels followed a similar pattern in HASM cell culture supernatant after formaldehyde exposure (data not shown). To address whether formaldehyde induces cytotoxicity in lung slices, lactate dehydrogenase (LDH) activity was determined in the slice supernatants. Formaldehyde-induced LDH release was minimal in slices when compared with the Triton X-100, which was the positive inducer of toxicity (Figure 3D).
Figure 3.
Formaldehyde has little effect on inflammatory mediator release from precision-cut human lung slices (PCLSs) or HASM cells. In the supernatants of lung slices, formaldehyde reduced the (A) IL-6 and (B) IL-8 levels, although the reduction was not statistically significant (mean ± SEM; n = 3 donors). (C) IL-6 levels in the culture supernatants of HASM cells were not altered by formaldehyde exposure. TNF-α (10 ng/ml, 24 h) induced a robust IL-6 level in the HASM cells (mean ± SEM; n = 4 donors). To confirm whether formaldehyde adversely affects the viability of slices, lactate dehydrogenase (LDH) activity was determined in supernatants. (D) Formaldehyde caused marginal LDH release compared with 10% Triton X 100 (six slices from two independent donors).
Formaldehyde-Treated HBE Cells Have Little Effect on Agonist-Induced [Ca2+]i or MYPT1 Phosphorylation in HASM Cells
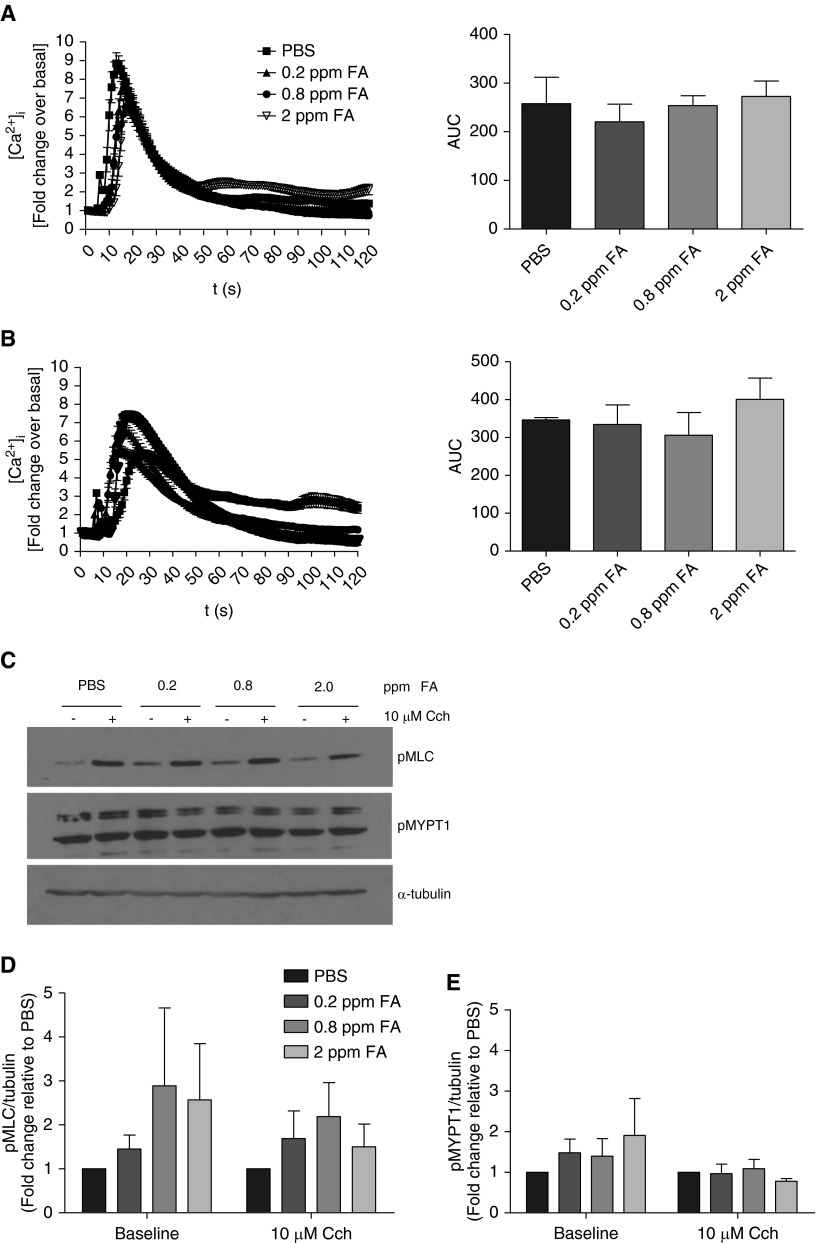
Epithelial cells that line the airway are likely some of the first cells exposed to inhaled formaldehyde. In vitro, airway epithelial cells exposed to formaldehyde exhibit altered cell structure and functions (32).To determine whether epithelial cells mediate formaldehyde effects on the HASM cells, air–liquid interface–differentiated HBE cells were exposed to formaldehyde and then co-cultured with HASM cells for 24 hours. Coculture of formaldehyde-treated HBE cells with HASM cells has little effect on agonist-induced [Ca2+]i (Figures 4A and 4B). There was a trend toward enhancement of basal MLC phosphorylation in HASM cells co-cultured with formaldehyde-treated HBE cells (Figures 4C and 4D). Formaldehyde-treated HBE cells had little effect on the phosphorylation of MYPT1 (Figures 4C and 4E).
Figure 4.
Formaldehyde-treated human bronchial epithelial (HBE) cells have little effect on agonist-induced [Ca2+]i or MYPT1 phosphorylation in HASM cells. Air–liquid interface–differentiated HBE cells were exposed to formaldehyde and co-incubated with HASM cells for 24 hours. Agonist-induced [Ca2+]i and phosphorylation status of myosin light chain (MLC) and MYPT1 were determined in the HASM cells. Co-culture of HASM cells with formaldehyde-treated HBE cells had little effect on (A) histamine- or (B) thrombin-induced [Ca2+]i in the HASM cells (left panels, baseline-corrected fluorescence intensity; right panels, AUCs of the traces; mean ± SEM, n = 3 donors, 60 cells/experimental condition). (C) There were marginal elevations in the baseline phosphorylation of MLC and MYPT1 in the HASM cells cocultured with formaldehyde-treated HBE cells (immune blot representative of five experiments). (D) At the baseline (without carbachol), phosphorylated MLC levels trended toward an increase in HASM cells cocultured with formaldehyde-treated HBE cells, although the increase was not statistically significant (mean ± SEM; n = 5 donors). (E) At baseline, phosphorylated MYPT1 levels were marginally elevated in HASM cells upon coculture with formaldehyde-treated HBE cells, without statistical significance (mean ± SEM; n = 5 donors).
Formaldehyde Elicits Nrf-2–Dependent Antioxidant Response in HASM Cells
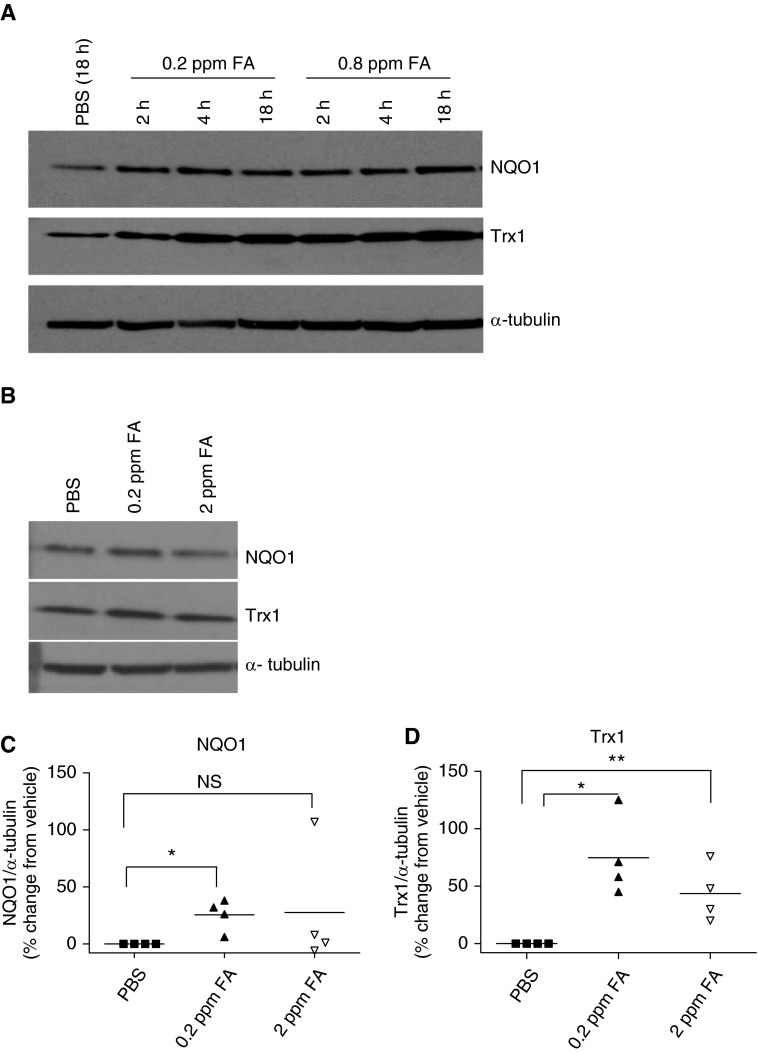
In other cells types, formaldehyde effects are partly attributed to oxidative injury (33). Typical cellular antioxidant response is mediated by stabilization and activation of nuclear factor erythroid-derived 2, like 2 (Nrf-2) and subsequent up-regulation of antioxidant and cytoprotective genes (34). We sought to determine whether formaldehyde induces Nrf-2 activation in HASM cells. The cytoprotective enzyme, nicotinamide adenine dinucleotide phosphate reduced–NQO1, and the antioxidant protein, Trx1, served as the surrogates of Nrf-2 activation in these experiments. NQO1 and Trx1 expression levels increased as early as 2 hours after formaldehyde exposure and remained elevated 18 hours after exposure (Figure 5A). In subsequent experiments, NQO1 and Trx1 expression levels were determined after 18 hours exposure to PBS or formaldehyde. The lowest concentration of formaldehyde (0.2 ppm) increased NQO1 and Trx1 levels in HASM cells (Figures 5C and 5D) while exposure to higher concentration (2 ppm) also increased, albeit to a lesser level (Figure 5D).
Figure 5.
Formaldehyde elicits nuclear factor erythroid-derived 2, like 2 (Nrf-2)–dependent antioxidant response in HASM cells. Whole-cell lysates from HASM cells exposed to PBS or formaldehyde were immune blotted for the markers of antioxidant (thioredoxin [Trx] 1) and cytoprotective (quinone oxido-reductase [NQO] 1) responses, as surrogate measures of Nrf-2 activation. (A) In response to formaldehyde exposure (0.2 and 0.8 ppm), NQO1 and Trx1 were elevated compared with the PBS-treated cells. NQO1 and Trx1 increases were noticed as early as 2 hours after exposure and remained elevated 18 hours after exposure (representative immune blot, n = 2). (B–D) At 18 hours after exposure, the lowest concentration of formaldehyde (0.2 ppm) caused a significant increase in NQO1 (*P = 0.017, n = 4) and Trx1 (*P = 0.0054, n = 4 donors), whereas a higher level of formaldehyde (2 ppm) caused relatively subdued induction of NQO1 and Trx1 (**P = 0.012, n = 4 donors). (B) Representative immune blot for n = 4 donors; (C and D) densitometry ratio; black lines show the mean for n = 4 donors. NS, not significant.
Formaldehyde-Induced MYPT1 Phosphorylation Is Independent of Nrf-2 Induction in HASM Cells
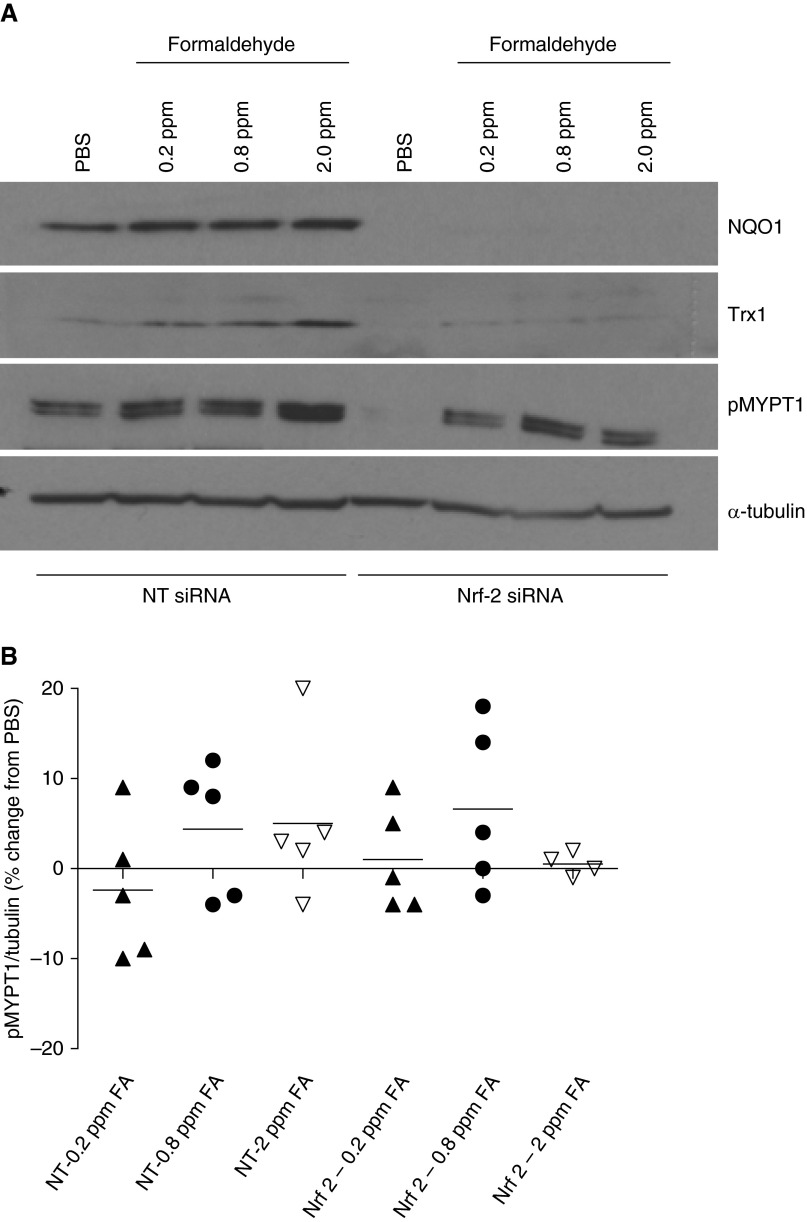
Cellular antioxidant response elicited by Nrf-2 plays a protective role against oxidants and environmental toxicants (35). To determine whether Nrf-2 has a role in formaldehyde-induced MYPT1 phosphorylation, HASM cells were exposed to PBS or formaldehyde after transiently transfecting them with Nrf-2–targeted siRNA. After transfection of Nrf-2–targeting siRNA, both basal and formaldehyde-induced NQO1 or Trx1 expressions were completely abolished (Figure 6A). Although there was a variable response to Nrf-2 silencing among the 5 donors, on average, silencing of Nrf-2 has little effect on formaldehyde-induced MYPT1 phosphorylation in HASM cells (Figure 6B).
Figure 6.
Formaldehyde-induced MYPT1 phosphorylation is independent of Nrf-2 induction in HASM cells. HASM cells were transiently transfected with nontargeting (NT) or Nrf-2–trageting (Nrf-2) small interfering RNA (siRNA) (40 nM) and exposed to PBS or formaldehyde 72 hours after transfection. Phosphorylated MYPT1 levels were determined at baseline 24 hours after formaldehyde exposure. (A) NQO1 and Trx1 expression levels were significantly down-regulated 72 hours after transfection with Nrf-2 siRNA (representative immune blot; n = 5 donors). (B) Formaldehyde-induced MYPT1 phosphorylation was comparable in HASM cells transfected with NT or Nrf-2 siRNA (each point denotes an independent donor; horizontal black lines mark means; n = 5 donors).
Formaldehyde Enhances Carbachol-Induced Airway Narrowing in PCLSs
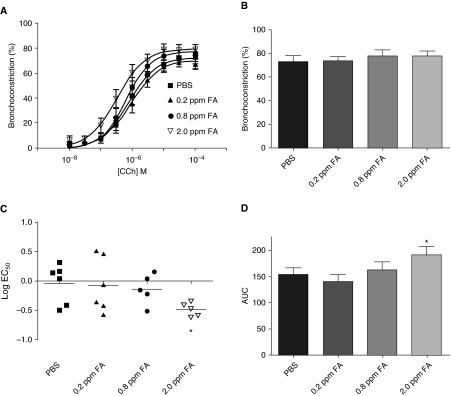
Our findings show that formaldehyde enhances a key Ca2+ sensitization pathway in HASM cells, potentially enhancing agonist-induced excitation-contraction coupling in HASM cells. To determine whether formaldehyde enhanced airway responsiveness, human PCLSs were exposed to PBS or formaldehyde and carbachol-induced contractile response was determined 24 hours after exposure. Exposure to 2 ppm formaldehyde enhanced carbachol-induced contractile response in PCLSs (Figure 7A). The maximal contractile response was comparable in PBS- and formaldehyde-treated slices (Figure 7B). Sensitivity to carbachol, as measured by log EC50, was significantly increased in 2 ppm formaldehyde-treated slices compared with the PBS-treated slices (Figure 7C). Area under the curve of the carbachol dose–response curve was significantly increased in slices exposed to 2 ppm formaldehyde compared with that of PBS-treated slices (Figure 7D).
Figure 7.
Formaldehyde enhances carbachol-induced airway narrowing in PCLSs. PCLSs were exposed to PBS or formaldehyde (0.2, 0.8, or 2 ppm) for 1 hour and then incubated for 24 hours before determining the carbachol-induced contractile response. (A) Slices exposed to 2 ppm carbachol (inverted open triangles) showed a left shift in the carbachol dose–response curve, indicating enhanced sensitivity (mean ± SEM; n = 5–6 donors). (B) There was little effect on the maximal response to carbachol after formaldehyde exposure (mean ± SEM; n = 5–6 donors). (C) The half-maximal effective concentration (log EC50) of the carbachol dose–response curve was significantly decreased in slices exposed to 2 ppm formaldehyde compared with that of PBS-treated slices (mean denoted by black horizontal lines; n = 5–6 donors; *P = 0.019). (D) The AUC of carbachol dose–response curve was significantly elevated in slices exposed to 2 ppm formaldehyde compared with that of slices exposed to PBS (mean ± SEM; n = 5–6 donors; *P = 0.049).
Discussion
Formaldehyde induces asthma exacerbations in humans and AHR in animal models of asthma (7, 9, 11). The underlying mechanisms, however, remain poorly understood. We hypothesized that formaldehyde induces AHR and asthma exacerbation through modulating the contractile response of ASM cells to agonists. Our findings show that formaldehyde enhances contractile response of human lung slices to agonist. Furthermore, formaldehyde exposure enhanced MYPT1 phosphorylation in HASM cells, suggesting Ca2+ sensitization as a possible mechanism causing AHR. To the best of our knowledge, this is the first report on the effect of formaldehyde on a signaling pathway that leads to enhanced contractile response in ASM cells.
Ca2+ mobilization and elevated [Ca2+]i regulate excitation–contraction coupling in various types of smooth muscle cells (reviewed in Ref. 36). Altered Ca2+ mobilization mechanisms are reported in ASM cells isolated from donors with asthma, potentially accounting for enhanced agonist-induced ASM cell shortening in asthma (15, 16). Toxicants, such as acrolein and ozone, enhance agonist-induced airway narrowing by altering the Ca2+ mobilization in ASM cells (25, 37). Lack of significant effects by formaldehyde on ASM cell Ca2+ dynamics prompted us to focus on an alternate mechanism of hypercontractility in ASM cells.
Ca2+ sensitization, a mechanism in which the contractile apparatus of smooth muscle cells is rendered more sensitive to [Ca2+]i levels, amplifies even smaller increments in [Ca2+]i to cause a larger magnitude of ASM cell shortening (reviewed in Ref. 38). In smooth muscle cells, RhoA/RhoA-associated kinase (RhoA/ROCK) signaling is a key regulator of Ca2+ sensitization (39). Our results show that ROCK activity is increased 24 hours after formaldehyde exposure. This observation, taken together with unaltered ROCKI/II levels, implied either: (1) an enhancing effect on ROCK enzyme activity by formaldehyde; or (2) increased RhoA activity upstream of ROCK. Our studies, using a cell-permeant Rho inhibitor, suggested that formaldehyde-induced MYPT1 phosphorylation is Rho-independent. Studies in vascular smooth muscle, however, show that oxidative stress and reactive oxygen species modulate ROCK-dependent Ca2+ sensitization (40). Furthermore, in rodent models of AHR, formaldehyde exposure increased oxidative stress and attenuated antioxidant responses (33). These observations prompted us to determine whether similar mechanisms are functional in HASM cells exposed to formaldehyde.
Formaldehyde induced a typical Nrf-2–dependent antioxidant response in HASM cells. However, the effect of Nrf-2 silencing on formaldehyde-induced MYPT1 phosphorylation was heterogeneous among the five donors used in the experiments. The variability among donors may be due to differential basal redox capacity. It is difficult to speculate on specific signaling molecules involved in this redox status heterogeneity, although various redox-related polymorphisms have been reported in humans. For instance, polymorphisms in γ glutamyl cysteine synthetase, the key enzyme of glutathione synthesis, are known to be associated with cystic fibrosis and certain lung cancers (41, 42). Alternatively, formaldehyde-induced MYPT1 phosphorylation and oxidative injury may be independent events in HASM cells. Because formaldehyde is an electrophile, the enhancing effects on ROCK activity may be mediated directly by electrophile attack and be independent of reactive oxygen species generation. Our findings, that formaldehyde-induced Rho kinase activity is independent of Rho activation, lend further support to the aforementioned hypothesis. Although it remains to be determined whether this is the mechanism, the physicochemical properties of formaldehyde, such as higher solubility and smaller molecular weight, support this hypothesis.
Airway epithelial cell–ASM interaction is another plausible mechanism for formaldehyde-induced AHR that we observed in lung slices. HBE cell–HASM cell coculture studies were designed to test this possibility. We showed that HBE cells exposed to formaldehyde have little effect on agonist-induced Ca2+ mobilization in HASM cells. However, formaldehyde-treated HBE cells trended toward enhancing pMLC levels in the HASM cells, suggesting that the epithelial cells may be an integral component in the formaldehyde-enhanced airway responsiveness seen in PCLSs.
Formaldehyde is primarily an indoor air pollutant, although it is an important component in outdoor emissions from automobiles and industries (43). In the developed countries, indoor formaldehyde levels in households range between 0.005 and 0.136 ppm, depending on the method of measurement (7, 10, 44–46). In the United States, the National Institute for Occupational Safety and Health recommended exposure limit over an 8-hour period is 0.016 ppm. A recent study by Dannemiller and colleagues (7) found that a significant proportion of households in their study population had formaldehyde levels well above the National Institute for Occupational Safety and Health standard. We chose 0.2 ppm as our minimum formaldehyde level to account for the shorter exposure time (1 h) in the cells and PCLSs. The lowest level of formaldehyde (0.2 ppm) induced antioxidant response and ROCK activation in HASM cells, whereas only the highest dose (2 ppm) elicited enhanced airway narrowing in lung slices. It is likely that, compared with a monolayer of cells, the lung slices (∼350 μm in thickness) had additional barriers to the distribution of formaldehyde, requiring 10-fold higher formaldehyde concentration to elicit a response.
Most of the studies examining the effects of formaldehyde have focused on in vitro cultures of airway epithelial cells. These studies showed that formaldehyde exposure alters gene expression profile of airway epithelial cells and enhances inflammatory mediator release (47–50). Evidence on the direct effects of formaldehyde on ASM cells are few (51). Studies show that inhaled toxicants, including formaldehyde, however, impair the structural integrity of the airway epithelial cells, thus allowing diffusion of toxicants to submucosal sites (32, 52, 53). These observations provide a rationale for directly exposing HASM cells to formaldehyde vapor to examine the outcomes. To account for the role of airway epithelial cells in mediating formaldehyde-induced AHR, we also used HBE–HASM cell coculture system in our studies. Furthermore, human PCLSs are an integrative platform to study the effects of inhaled toxicants, and our findings provide physiological significance to the effects of formaldehyde on AHR in humans.
In summary, the current study identifies MYPT1 phosphorylation and ROCK-dependent Ca2+ sensitization in HASM cells as plausible mechanisms for formaldehyde-induced AHR. Although not related to MYPT1 phosphorylation in our studies, an Nrf-2–dependent antioxidant response is elicited by formaldehyde in HASM cells. In human PCLSs, formaldehyde exposure enhanced airway narrowing, indicating that the augmented MYPT1 phosphorylation manifests into AHR. The findings suggest that formaldehyde-induced AHR, and potentially asthma exacerbations that result from formaldehyde exposure, may be therapeutically targeted at the level of ROCK-mediated Ca2+ sensitization.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants T32-ES019851 and P30-ES013508.
Author Contributions: Conception and design— J.J., C.K.-W., E.Y., P.D., and R.P.; analysis and interpretation— J.J., C.K.-W., E.Y., W.J., and R.P.; execution of experiments—J.J., C.K.-W., J.S., and C.M.; drafting manuscript for important intellectual content—J.J., C.K.-W., C.M., P.D., and R.P.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0254OC on May 5, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, Williams H ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Künzli N, Bridevaux PO, Liu LJ, Garcia-Esteban R, Schindler C, Gerbase MW, Sunyer J, Keidel D, Rochat T Swiss Cohort Study on Air Pollution and Lung Diseases in Adults. Traffic-related air pollution correlates with adult-onset asthma among never-smokers. Thorax. 2009;64:664–670. doi: 10.1136/thx.2008.110031. [DOI] [PubMed] [Google Scholar]
- 3.Laumbach RJ, Kipen HM. Respiratory health effects of air pollution: update on biomass smoke and traffic pollution. J Allergy Clin Immunol. 2012;129:3–11, quiz 12–13. doi: 10.1016/j.jaci.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunekreef B, Dockery DW, Krzyzanowski M. Epidemiologic studies on short-term effects of low levels of major ambient air pollution components. Environ Health Perspect. 1995;103:3–13. doi: 10.1289/ehp.95103s23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagorio S, Forastiere F, Pistelli R, Iavarone I, Michelozzi P, Fano V, Marconi A, Ziemacki G, Ostro BD. Air pollution and lung function among susceptible adult subjects: a panel study. Environ Health. 2006;5:11. doi: 10.1186/1476-069X-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trenga CA, Sullivan JH, Schildcrout JS, Shepherd KP, Shapiro GG, Liu LJ, Kaufman JD, Koenig JQ. Effect of particulate air pollution on lung function in adult and pediatric subjects in a Seattle panel study. Chest. 2006;129:1614–1622. doi: 10.1378/chest.129.6.1614. [DOI] [PubMed] [Google Scholar]
- 7.Dannemiller KC, Murphy JS, Dixon SL, Pennell KG, Suuberg EM, Jacobs DE, Sandel M. Formaldehyde concentrations in household air of asthma patients determined using colorimetric detector tubes. Indoor Air. 2013;23:285–294. doi: 10.1111/ina.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krzyzanowski M, Quackenboss JJ, Lebowitz MD. Chronic respiratory effects of indoor formaldehyde exposure. Environ Res. 1990;52:117–125. doi: 10.1016/s0013-9351(05)80247-6. [DOI] [PubMed] [Google Scholar]
- 9.Casset A, Marchand C, Purohit A, le Calve S, Uring-Lambert B, Donnay C, Meyer P, de Blay F. Inhaled formaldehyde exposure: effect on bronchial response to mite allergen in sensitized asthma patients. Allergy. 2006;61:1344–1350. doi: 10.1111/j.1398-9995.2006.01174.x. [DOI] [PubMed] [Google Scholar]
- 10.Garrett MH, Hooper MA, Hooper BM, Rayment PR, Abramson MJ. Increased risk of allergy in children due to formaldehyde exposure in homes. Allergy. 1999;54:330–337. doi: 10.1034/j.1398-9995.1999.00763.x. [DOI] [PubMed] [Google Scholar]
- 11.Lino-dos-Santos-Franco A, Domingos HV, de Oliveira AP, Breithaupt-Faloppa AC, Peron JP, Bolonheis S, Muscará MN, Oliveira-Filho RM, Vargaftig BB, Tavares-de-Lima W. Differential effects of formaldehyde exposure on the cell influx and vascular permeability in a rat model of allergic lung inflammation. Toxicol Lett. 2010;197:211–218. doi: 10.1016/j.toxlet.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Swiecichowski AL, Long KJ, Miller ML, Leikauf GD. Formaldehyde-induced airway hyperreactivity in vivo and ex vivo in guinea pigs. Environ Res. 1993;61:185–199. doi: 10.1006/enrs.1993.1063. [DOI] [PubMed] [Google Scholar]
- 13.Woolcock AJ, Salome CM, Yan K. The shape of the dose–response curve to histamine in asthmatic and normal subjects. Am Rev Respir Dis. 1984;130:71–75. doi: 10.1164/arrd.1984.130.1.71. [DOI] [PubMed] [Google Scholar]
- 14.Ma X, Cheng Z, Kong H, Wang Y, Unruh H, Stephens NL, Laviolette M. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1181–L1189. doi: 10.1152/ajplung.00389.2001. [DOI] [PubMed] [Google Scholar]
- 15.Mahn K, Hirst SJ, Ying S, Holt MR, Lavender P, Ojo OO, Siew L, Simcock DE, McVicker CG, Kanabar V, et al. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc Natl Acad Sci USA. 2009;106:10775–10780. doi: 10.1073/pnas.0902295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jude JA, Solway J, Panettieri RA, Jr, Walseth TF, Kannan MS. Differential induction of CD38 expression by TNF-α in asthmatic airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2010;299:L879–L890. doi: 10.1152/ajplung.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitazawa T, Kobayashi S, Horiuti K, Somlyo AV, Somlyo AP. Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+ J Biol Chem. 1989;264:5339–5342. [PubMed] [Google Scholar]
- 18.Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiba Y, Ueno A, Shinozaki K, Takeyama H, Nakazawa S, Sakai H, Misawa M. Involvement of RhoA-mediated Ca2+ sensitization in antigen-induced bronchial smooth muscle hyperresponsiveness in mice. Respir Res. 2005;6:4. doi: 10.1186/1465-9921-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- 21.Balenga NA, Klichinsky M, Xie Z, Chan EC, Zhao M, Jude J, Laviolette M, Panettieri RA, Jr, Druey KM. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat Commun. 2015;6:6763. doi: 10.1038/ncomms7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flavahan NA, Aarhus LL, Rimele TJ, Vanhoutte PM. Respiratory epithelium inhibits bronchial smooth muscle tone. J Appl Physiol (1985) 1985;58:834–838. doi: 10.1152/jappl.1985.58.3.834. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, Vanhoutte PM. Epithelium acts as a modulator and a diffusion barrier in the responses of canine airway smooth muscle. J Appl Physiol (1985) 1994;76:1843–1847. doi: 10.1152/jappl.1994.76.5.1843. [DOI] [PubMed] [Google Scholar]
- 24.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc Am Thorac Soc. 2009;6:655–659. doi: 10.1513/pats.200907-072DP. [DOI] [PubMed] [Google Scholar]
- 25.Hyvelin JM, Roux E, Prévost MC, Savineau JP, Marthan R. Cellular mechanisms of acrolein-induced alteration in calcium signaling in airway smooth muscle. Toxicol Appl Pharmacol. 2000;164:176–183. doi: 10.1006/taap.1999.8879. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida M, Aizawa H, Inoue H, Koto H, Nakano H, Komori M, Fukuyama S, Hara N. Ozone exposure may enhance airway smooth muscle contraction by increasing Ca2+ refilling of sarcoplasmic reticulum in guinea pig. Pulm Pharmacol Ther. 2002;15:111–119. doi: 10.1006/pupt.2001.0324. [DOI] [PubMed] [Google Scholar]
- 27.Amrani Y, Ammit AJ, Panettieri RA., Jr Tumor necrosis factor receptor (TNFR) 1, but not TNFR2, mediates tumor necrosis factor-alpha-induced interleukin-6 and RANTES in human airway smooth muscle cells: role of p38 and p42/44 mitogen-activated protein kinases. Mol Pharmacol. 2001;60:646–655. [PubMed] [Google Scholar]
- 28.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol. 1989;256:C329–C335. doi: 10.1152/ajpcell.1989.256.2.C329. [DOI] [PubMed] [Google Scholar]
- 29.Damera G, Zhao H, Wang M, Smith M, Kirby C, Jester WF, Lawson JA, Panettieri RA., Jr Ozone modulates IL-6 secretion in human airway epithelial and smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L674–L683. doi: 10.1152/ajplung.90585.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jude JA, Tirumurugaan KG, Kang BN, Panettieri RA, Walseth TF, Kannan MS. Regulation of CD38 expression in human airway smooth muscle cells: role of class I phosphatidylinositol 3 kinases. Am J Respir Cell Mol Biol. 2012;47:427–435. doi: 10.1165/rcmb.2012-0025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper PR, Panettieri RA., Jr Steroids completely reverse albuterol-induced β2-adrenergic receptor tolerance in human small airways. J Allergy Clin Immunol. 2008;122:734–740. doi: 10.1016/j.jaci.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 32.Kastner PE, Casset A, Pons F. Formaldehyde interferes with airway epithelium integrity and functions in a dose- and time-dependent manner. Toxicol Lett. 2011;200:109–116. doi: 10.1016/j.toxlet.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Lino-dos-Santos-Franco A, Correa-Costa M, Durão AC, de Oliveira AP, Breithaupt-Faloppa AC, Bertoni JdeA, Oliveira-Filho RM, Câmara NO, Marcourakis T, Tavares-de-Lima W. Formaldehyde induces lung inflammation by an oxidant and antioxidant enzymes mediated mechanism in the lung tissue. Toxicol Lett. 2011;207:278–285. doi: 10.1016/j.toxlet.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 34.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26:175–182. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 35.Heber D, Li Z, Garcia-Lloret M, Wong AM, Lee TY, Thames G, Krak M, Zhang Y, Nel A. Sulforaphane-rich broccoli sprout extract attenuates nasal allergic response to diesel exhaust particles. Food Funct. 2014;5:35–41. doi: 10.1039/c3fo60277j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586:5047–5061. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montaño LM, Jones GL, O’Byrne PM, Daniel EE. Effect of ozone exposure in vivo on response of bronchial rings in vitro: role of intracellular Ca2+ J Appl Physiol (1985) 1993;75:1315–1322. doi: 10.1152/jappl.1993.75.3.1315. [DOI] [PubMed] [Google Scholar]
- 38.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 39.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 40.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase–induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–L529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marson FA, Bertuzzo CS, Ribeiro AF, Ribeiro JD. Polymorphisms in the glutathione pathway modulate cystic fibrosis severity: a cross-sectional study. BMC Med Genet. 2014;15:27. doi: 10.1186/1471-2350-15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nichenametla SN, Muscat JE, Liao JG, Lazarus P, Richie JP., Jr A functional trinucleotide repeat polymorphism in the 5′-untranslated region of the glutathione biosynthetic gene GCLC is associated with increased risk for lung and aerodigestive tract cancers. Mol Carcinog. 2013;52:791–799. doi: 10.1002/mc.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcon A, Fracasso ME, Marchetti P, Doria D, Girardi P, Guarda L, Pesce G, Pironi V, Ricci P, de Marco R. Outdoor formaldehyde and NO2 exposures and markers of genotoxicity in children living near chipboard industries. Environ Health Perspect. 2014;122:639–645. doi: 10.1289/ehp.1307259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franklin P, Dingle P, Stick S. Raised exhaled nitric oxide in healthy children is associated with domestic formaldehyde levels. Am J Respir Crit Care Med. 2000;161:1757–1759. doi: 10.1164/ajrccm.161.5.9905061. [DOI] [PubMed] [Google Scholar]
- 45.Gilbert NL, Gauvin D, Guay M, Héroux ME, Dupuis G, Legris M, Chan CC, Dietz RN, Lévesque B. Housing characteristics and indoor concentrations of nitrogen dioxide and formaldehyde in Quebec City, Canada. Environ Res. 2006;102:1–8. doi: 10.1016/j.envres.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 46.Hodgson AT, Rudd AF, Beal D, Chandra S. Volatile organic compound concentrations and emission rates in new manufactured and site-built houses. Indoor Air. 2000;10:178–192. doi: 10.1034/j.1600-0668.2000.010003178.x. [DOI] [PubMed] [Google Scholar]
- 47.Persoz C, Achard S, Momas I, Seta N. Inflammatory response modulation of airway epithelial cells exposed to formaldehyde. Toxicol Lett. 2012;211:159–163. doi: 10.1016/j.toxlet.2012.03.799. [DOI] [PubMed] [Google Scholar]
- 48.Persoz C, Achard S, Leleu C, Momas I, Seta N. An in vitro model to evaluate the inflammatory response after gaseous formaldehyde exposure of lung epithelial cells. Toxicol Lett. 2010;195:99–105. doi: 10.1016/j.toxlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Rager JE, Moeller BC, Doyle-Eisele M, Kracko D, Swenberg JA, Fry RC. Formaldehyde and epigenetic alterations: microRNA changes in the nasal epithelium of nonhuman primates. Environ Health Perspect. 2013;121:339–344. doi: 10.1289/ehp.1205582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rager JE, Smeester L, Jaspers I, Sexton KG, Fry RC. Epigenetic changes induced by air toxics: formaldehyde exposure alters miRNA expression profiles in human lung cells. Environ Health Perspect. 2011;119:494–500. doi: 10.1289/ehp.1002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richards IS, DeHate RB. Formalin produces depolarizations in human airway smooth muscle in vitro. Toxicol Ind Health. 2006;22:59–63. doi: 10.1191/0748233706th243oa. [DOI] [PubMed] [Google Scholar]
- 52.Shaykhiev R, Otaki F, Bonsu P, Dang DT, Teater M, Strulovici-Barel Y, Salit J, Harvey BG, Crystal RG. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell Mol Life Sci. 2011;68:877–892. doi: 10.1007/s00018-010-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heijink IH, Brandenburg SM, Postma DS, van Oosterhout AJ. Cigarette smoke impairs airway epithelial barrier function and cell–cell contact recovery. Eur Respir J. 2012;39:419–428. doi: 10.1183/09031936.00193810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.