Abstract
Supraphysiological concentrations of oxygen (hyperoxia) can compromise host defense and increase susceptibility to bacterial infections, causing ventilator-associated pneumonia. The phagocytic activity of macrophages is impaired by hyperoxia-induced increases in the levels of reactive oxygen species (ROS) and extracellular high-mobility group box protein B1 (HMGB1). Ascorbic acid (AA), an essential nutrient and antioxidant, has been shown to be beneficial in various animal models of ROS-mediated diseases. The aim of this study was to determine whether AA could attenuate hyperoxia-compromised host defense and improve macrophage functions against bacterial infections. C57BL/6 male mice were exposed to hyperoxia (≥98% O2, 48 h), followed by intratracheal inoculation with Pseudomonas aeruginosa, and simultaneous intraperitoneal administration of AA. AA (50 mg/kg) significantly improved bacterial clearance in the lungs and airways, and significantly reduced HMGB1 accumulation in the airways. The incubation of RAW 264.7 cells (a macrophage-like cell line) with AA (0–1,000 μM) before hyperoxic exposure (95% O2) stabilized the phagocytic activity of macrophages in a concentration-dependent manner. The AA-enhanced macrophage function was associated with significantly decreased production of intracellular ROS and accumulation of extracellular HMGB1. These data suggest that AA supplementation can prevent or attenuate the development of ventilator-associated pneumonia in patients receiving oxygen support.
Keywords: hyperoxia, ascorbic acid, pneumonia, high-mobility group box 1
Clinical Relevance
Patients receiving oxygen therapy are highly susceptible to pulmonary infections, which may lead to ventilator-associated pneumonia (VAP). Data in this study suggest that ascorbic acid (AA) levels can be significantly reduced in patients receiving oxygen therapy, and AA supplementation can prevent or attenuate the development of VAP in these patients. Thus, our findings demonstrate that boosting the host defense with a simple and inexpensive antioxidant, ascorbate, may help to reduce mortality and morbidity in patients receiving oxygen support.
Supraphysiological concentrations of oxygen (hyperoxia) are routinely used to treat patients with respiratory distress and those undergoing surgery (1–5). However, oxygen toxicity from prolonged exposure to hyperoxia can contribute to the complications, such as ventilator-associated pneumonia (VAP) and acute lung injury, observed in these patients (6–12). Prolonged exposure to hyperoxia can compromise the host defense against bacterial infections by impairing the efficacy of alveolar macrophages to migrate, phagocytose, kill, and clear bacteria (7, 13–18). Hyperoxia-induced deleterious effects are mainly attributable to an increase in the levels of reactive oxygen species (ROS), resulting in oxidative stress, which ultimately surmount the antioxidant defense mechanisms (19, 20). ROS can cause lipid peroxidation, protein, and DNA oxidation, and alter signal transduction pathways, leading to cell damage (21–25). Exposure of cultured macrophages to hyperoxia induces actin oxidation and subsequent cytoskeleton disorganization, resulting in a decrease in the ability to phagocytose bacteria (16, 18). Treatment of hyperoxia-exposed macrophages with antioxidants, such as superoxide dismutase and procysteine, can preserve actin cytoskeleton organization and increase the phagocytosis of bacteria (18, 26). Hyperoxia-exposed cells overexpressing antioxidant enzyme, manganese superoxide dismutase, exhibit reduced bacterial adherence, increased phagocytic activity (26, 27), and attenuated ROS-induced damage (28). These aforementioned findings suggest that antioxidants may be useful in attenuating ROS-induced cellular damage and impairment of macrophage functions.
Ascorbic acid (AA), an essential nutrient, is a well known reductant and scavenger of intracellular ROS (29–32). AA can donate two electrons that are lost sequentially, and is considered to be an efficacious antioxidant, as it reduces a reactive free radical with the formation of a less reactive compound (30, 33, 34).
Recent studies in our laboratory have demonstrated that high-mobility group box protein 1 (HMGB1), a late mediator of inflammation (35, 36), can significantly attenuate the phagocytic function of macrophages in airways (7, 37). In mice exposed to hyperoxia, elevated levels of airway HMGB1 are associated with an increase in both the lung bacterial burden and lung damage. Neutralizing or inhibiting HMGB1 by monoclonal antibodies or compounds, such as 3-(2,4-dimethoxybenzylidene)-anabaseine dihydrochloride (GTS)-21, respectively, in these mice, significantly decreases lung injury and hyperoxia-compromised bacterial clearance (7, 38).
In this study, we tested the hypothesis that AA supplementation can attenuate hyperoxia-compromised host defense by scavenging hyperoxia-induced excessive intracellular ROS and reducing HMGB1 levels in the airways. We show that AA significantly: (1) preserved macrophage phagocytic function and host defense against bacterial infections, leading to improved survival; (2) decreased intracellular oxidative stress in cultured macrophages by replenishing the intracellular levels of AA; and (3) reduced extracellular HMGB1 levels in both in vitro and in vivo model systems. Some of the results presented in this study have been previously reported in the form of an abstract (39).
Materials and Methods
Refer to the online supplement for detailed methods.
Animal Studies
C57BL/6 mice (male, 8–12 wk old; The Jackson Laboratory, Bar Harbor, ME) were used in this study, in accordance with the Institutional Animal Care and Use Committees of St. John’s University (Queens, NY). Mice were randomized to receive either sodium L-ascorbate (50 mg/kg dissolved in saline; Sigma-Aldrich, St. Louis, MO) or saline (200 µl), administered by intraperitoneal injection every 12 hours, starting 24 hours after the onset of hyperoxic exposure. After 48 hours of hyperoxic exposure, mice were anesthetized with sodium pentobarbital (60–70 mg/kg intraperitoneal) and inoculated with 1 × 107 CFUs of Pseudomonas aeruginosa PAO1 (PA) by making an approximately 1-cm incision on the neck to expose the trachea, and kept at room air (21% O2). Mice were either killed 18 hours later using intraperitoneal sodium pentobarbital (120 mg/kg) to obtain bronchoalveolar lavage fluids (BALFs) and lung tissues, or observed for survival after bacterial inoculation, as described previously (7).
Measurement of Phagocytic Activity, HMGB1 Levels, and Immunofluorescence Analyses
RAW 264.7 cells were seeded in 24-well plates and allowed to adhere overnight at 37°C. Cells were exposed to hyperoxia (95% O2) for 24 hours (7) while in media supplemented with AA (0–1,000 µM). The phagocytic activity was assessed by counting 200 consecutive individual macrophages per well in duplicates, as previously described (17, 18). The levels of HMGB1 in the culture media of RAW 264.7 cells and BALF samples obtained from mice were measured by Western blot analysis, as described previously (7). HMGB1 and NF-κB localization was observed, as mentioned previously (17).
Assay for Oxidative Stress
Oxidative stress was measured by assessing oxidation–reduction potential (ORP) using the RedoxSYS Diagnostic System (Luoxis Diagnostics, Inc., Englewood, CO). Cultured macrophages were exposed to hyperoxia and treated with AA, as indicated, and cell lysates were prepared. ORP of samples (30 μl) was measured at room temperature, using the protocol provided by the manufacturer.
Measurement of Intracellular Ascorbate
After the treatments of cultured RAW 264.7 macrophages in six-well plates, the cells were lysed as described previously (40), except that the adherent cell monolayer was rinsed with ice-cold PBS and the resulting lysate was centrifuged at 13,000 × g for 10 minutes at 3°C. Supernatants were analyzed by HPLC and intracellular concentrations of AA were calculated using the equation described previously (40, 41), with minor modifications. Samples (50 μl) were injected and separation was performed on Agilent HC-C18 (2) column (5 µm, 4.6 × 1.50 mm; Agilent Technologies, Santa Clara, CA). AA was detected by its ultraviolet absorption at a wavelength of 260 nm on a Waters 486 tunable absorbance detector (Waters, Milford, MA). At a flow rate of 1 ml/min, AA was eluted at roughly 3 minutes.
Statistical Analyses
The data are presented as the mean (±SEM) of at least two independent experiments. The data were analyzed using Student’s unpaired t test or ANOVA. The Kaplan-Meier survival curve was analyzed using the log-rank (Mantel-Cox) test. A P value of less than or equal to 0.05 was considered statistically significant. The post hoc analyses were conducted using Dunnett’s or Sidak’s multiple comparison test.
Results
AA Significantly Increases Hyperoxia-Compromised Bacterial Clearance
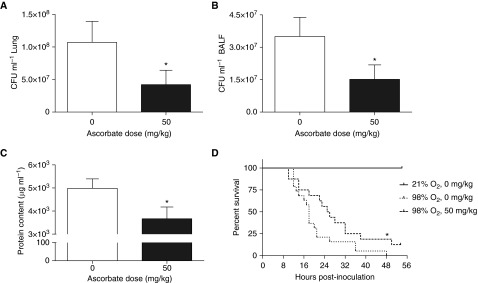
To determine whether AA can improve hyperoxia-compromised host defense against PA pneumonia, bacterial counts were assessed in the airways and lung tissues in a mouse model of VAP, as described previously (7). These mice were treated with either 50 mg/kg of AA or saline (control), as described in the Materials and Methods. Bacterial counts in the lungs of AA-treated mice were significantly less than that of saline-treated mice (0.4 ± 0.2 × 108 versus 1.1 ± 0.3 × 108/lung; t = 1.66, degrees of freedom [df] = 42, P = 0.05; Figure 1A). Similar results were obtained in the airways (1.5 ± 0.6 × 107 versus 3.5 ± 0.9 × 107/ml BALF; t = 1.78, df = 41, P < 0.05; Figure 1B). In addition, the total protein content in BALF, an important marker of lung injury, was significantly lower in AA-treated mice compared with the controls (3,666 ± 508.3 versus 4,983 ± 408.9 μg/ml BALF of saline-treated mice; t = 2.02, df = 42, P < 0.05; Figure 1C). Moreover, a significant decrease in animal mortality was observed in the AA-treated group (87.5 versus 100% in the saline-treated group; chi-square = 4.07, df = 1, P < 0.05; Figure 1D). These results indicate that AA improves bacterial clearance and acute lung injury, which results in an increase in survival in this mouse model of VAP. Interestingly, treatment of mice without prior exposure to hyperoxia with the same dose of AA did not significantly alter bacterial counts in either lung tissues or airways compared with that of saline-treated mice (lungs: 7.0 ± 5.4 × 107 versus 6.0 ± 4.7 × 106; t = 1.19, df = 14, P = 0.25; BALF: 1.1 ± 0.7 × 107 versus 8.6 ± 6.0 × 105/ml; t = 1.34, df = 13, P = 0.20; not shown in figures). Similar results were obtained for the total protein content in the airways (3,917 ± 843.5 versus 3,568 ± 597.5 µg/ml BALF of saline controls). These data suggest that the beneficial effects of AA in enhancing innate immunity against bacterial infections are limited to mice with hyperoxia-compromised host defense.
Figure 1.
Ascorbic acid (AA) decreases hyperoxia-compromised bacterial clearance in mice. Male C57BL/6 mice, exposed to 98% O2 or higher for 48 hours, were inoculated with Pseudomonas aeruginosa (PA; 1 × 107 CFU) intratracheally. Mice were randomized to receive either AA (50 mg/kg) or saline intraperitoneally. Bronchoalveolar lavage fluid (BALF) and lung tissues were harvested 18 hours after infection. Number of viable bacteria in lungs (A) and BALF (B) were determined by plating serial dilutions of either homogenized lung tissues or BALF, respectively. The total protein content in BALF (C) was measured using the bicinchoninic acid assay (n = 21 mice per group). In another set of experiments, mice were observed for survival after inoculation with PA (n = 13–18 mice per group) (D). Data represent the mean ± SEM of at least four independent experiments. *P ≤ 0.05 compared with control mice.
AA Significantly Attenuates the Impairment in Macrophage Phagocytosis Induced by Hyperoxia
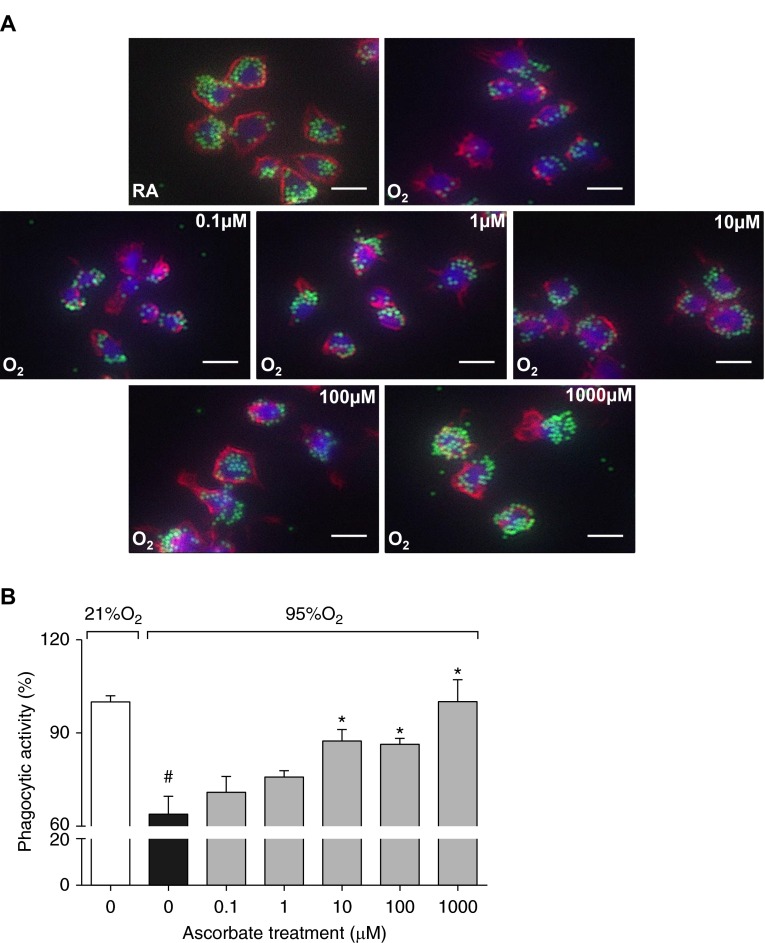
Hyperoxia-compromised macrophage functions play a critical role in the reduced host defense against bacterial infections (7). To determine the cellular mechanisms in AA-improved host defense, RAW 264.7 cells were exposed to hyperoxia in the presence of a series of AA concentrations (0–1,000 μM). Consistent with previous observations (7, 13, 16, 18, 26), macrophages exposed to hyperoxia in the absence of AA showed a significant decrease in phagocytosis, as indicated by a significant decrease in the number of phagocytosed beads compared with macrophages that remained in room air (21% O2 control; 63.9 versus 100%; F-statistic (F(6,35)) = 10.18, P < 0.0001; Figures 2A and 2B). Phagocytosis in hyperoxic macrophages cultured in AA-supplemented media was significantly increased compared with those exposed to hyperoxia in the absence of AA, indicated by higher numbers of phagocytosed beads at 10, 100, and 1,000 μM (87.4, 86.4 and 100.1, respectively, versus 63.9%; F(6,35) = 10.18, P < 0.01; Figures 2A and 2B). These results suggest that AA can enhance innate immunity against bacterial infections by improving macrophage phagocytic activity under hyperoxic conditions.
Figure 2.
AA attenuates hyperoxia-compromised macrophage phagocytic function. RAW 264.7 cells either remained at room air (RA; 21% O2) or were exposed to 95% O2 for 24 hours in the presence of AA (0–1,000 μM). Cells were then incubated with FITC-labeled latex mini-beads for 1 hour and stained with phalloidin and 4′,6-diamidino-2-phenylindole (DAPI) to visualize the actin cytoskeleton and nuclei, respectively. Representative immunofluorescent images of RAW 264.7 cells (red, F-actin cytoskeleton; green, latex mini-beads; blue, nuclei) are shown. Scale bars: 10 μm (A). For quantification of phagocytic activity, at least 200 cells per well were counted and the numbers of beads per cell were represented as a percentage of the 21% O2 (0 μM) control group (B). Each value represents the mean ± SEM of three independent experiments for each group. *P ≤ 0.05 compared with the 95% O2 (0 μM) control group; #P ≤ 0.05 compared with 21% O2 (0 μM) control group. O2, 95% oxygen; RA, 21% oxygen.
AA Significantly Decreases Oxidative Stress in Hyperoxia-Exposed Macrophages
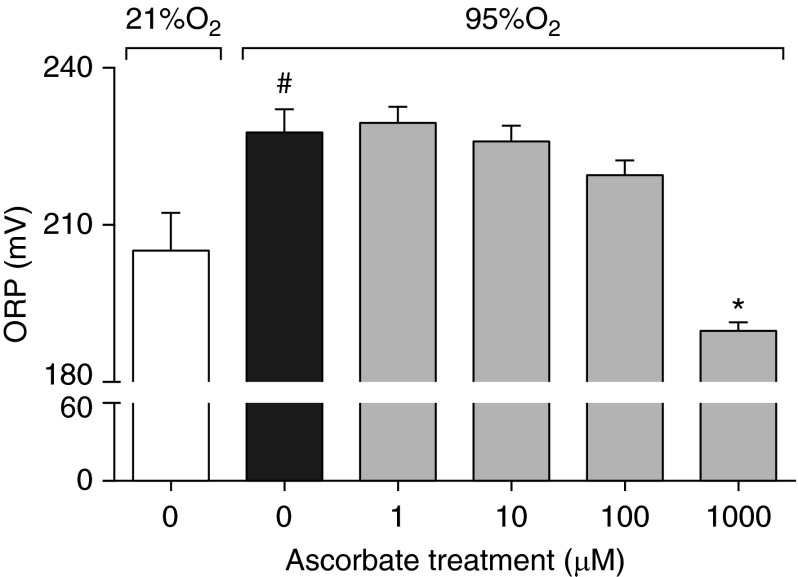
To determine the mechanisms underlying AA-attenuated hyperoxia-compromised macrophage phagocytic function (Figure 2), ORP, an indicator of oxidative stress, was measured in RAW 264.7 cells exposed to hyperoxia in the presence of a series of AA concentrations (0–1,000 μM). Macrophages exposed to hyperoxia had significantly higher ORP compared with those exposed to room air (21% O2; 227.7 versus 205.1 mV; F(5,32) = 11.19, P < 0.01; Figure 3). Cells incubated with AA showed a concentration-dependent decrease in ORP, which was significantly less at 1,000 µM, compared with macrophages exposed to hyperoxia in the absence of AA (189.8 versus 227.7 mV; F(5,32) = 11.19, P < 0.0001; Figure 3). These results suggest that AA enhances macrophage functions by blunting hyperoxia-induced oxidative stress.
Figure 3.
AA decreases oxidative stress in hyperoxia-exposed macrophages. RAW 264.7 cells either remained at RA (21% O2) or were exposed to 95% O2 for 24 hours in the presence of AA (0–1,000 μM). Cell lysates were prepared and used to measure oxidation–reduction potential (ORP). Values represent the mean ± SEM of three independent experiments. *P ≤ 0.05 compared with 95% O2 (0 μM) control group; #P ≤ 0.05 compared with 21% O2 (0 µM) control group.
Exposure to Hyperoxia Reduces Intracellular AA Levels in Macrophages
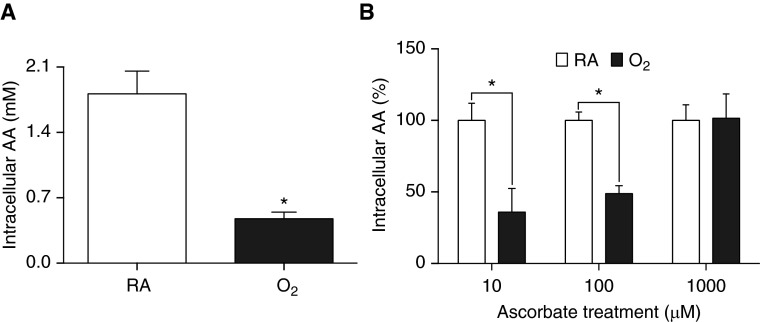
Immune cells, especially macrophages, maintain very high concentrations of AA compared with plasma (40). To determine if exposure to hyperoxia alters AA levels in macrophages, the intracellular concentrations of AA were measured. Because AA was undetectable in cultured RAW 264.7 cells (data not shown), RAW cells were incubated with 1,000 µM AA for 1 hour before hyperoxia exposure. Exposure to hyperoxia significantly reduced the intracellular AA levels compared with control RAW cells exposed to room air (0.47 ± 0.07 versus 1.81 ± 0.24 arbitrary unit (AU); t = 5.66, df = 11, P < 0.001; Figure 4A). These data indicate that intracellular AA levels in macrophages are rapidly exhausted upon hyperoxia exposure.
Figure 4.
Exposure to hyperoxia reduces intracellular AA levels in macrophages. RAW 264.7 cells were pretreated with AA (1,000 μM) for 1 hour, followed by replacing media with fresh media, and cells were exposed to either RA (21% O2) or 95% O2 for 24 hours (A). In a different set of experiments, RAW 264.7 cells either remained at RA or were exposed to 95% O2 for 24 hours in the presence of AA (0–1,000 μM) (B). Intracellular AA levels were determined using HPLC–ultraviolet spectrometry. Values represent the mean ± SEM of three independent experiments. *P ≤ 0.05 compared with RA control group.
We further investigated whether continuous AA (10–1,000 µM) supplementation for 24 hours with simultaneous exposure of RAW cells to hyperoxia can maintain intracellular AA levels. Data presented in Figure 4B show that RAW cells with supplementation of 1,000 µM AA had similar intracellular AA levels upon exposure to hyperoxia as that of room air–exposed cells (101.5 versus 100%; t = 0.08, df = 24; Figure 4B). However, intracellular AA levels were significantly lower in hyperoxia-exposed cells when RAW cells were supplemented with 10 or 100 µM AA as compared with the room air control groups (35.94 and 48.81 versus 100%; t = 3.72 and 2.97, respectively, df = 24, P < 0.05; Figure 4B). These results suggest that a continuous supply with appropriate concentrations of AA mitigates hyperoxia-induced decrease in intracellular AA levels and maintains the intracellular antioxidant capacity.
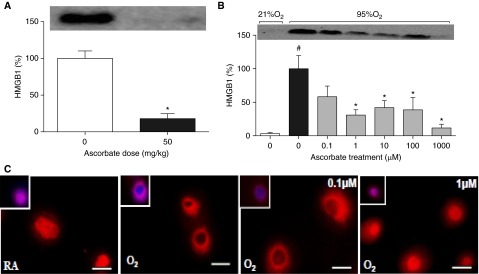
AA Significantly Reduces the Extracellular Accumulation of HMGB1 in Hyperoxia by Inhibiting Its Translocation
Previous studies in our laboratory have shown that elevated levels of airway HMGB1 contribute to hyperoxia-compromised macrophage functions (7, 17). To determine whether or not AA enhances the functions of hyperoxic macrophages by reducing the levels of extracellular HMGB1, we determined the levels of HMGB1 in the airways of mice exposed to hyperoxia, as described in the Materials and Methods section. Figure 5A illustrates that mice receiving AA supplementation had significantly lower HMGB1 levels in BALF compared with the unsupplemented controls (18 ± 6.7 versus 100 ± 10.1%; t = 7.26, df = 4, P < 0.0001; Figure 5A). To further delineate the AA-induced increase in macrophage function, the levels of extracellular HMGB1 were assessed in the culture media of RAW 264.7 cells that were exposed to hyperoxia in the presence of AA (0–1,000 μM). Macrophages exposed to hyperoxia had a significant increase in the levels of extracellular HMGB1 accumulation as compared with the 21% O2 control (100 versus 3.2%; F(6,14) = 11.92, P < 0.0001; Figure 5B). AA at 1, 10, 100, and 1,000 μM significantly reduced the levels of extracellular HMGB1 as compared with cells exposed to hyperoxia in the absence of AA (30.9, 42.0, 38.7, and 11.7 versus 100%, respectively; F(6,14) = 11.92, P < 0.01; Figure 5B).
Figure 5.
AA decreases the hyperoxia-induced release of high-mobility group box protein B1 (HMGB1) by inhibiting its translocation from the nucleus to the cytoplasm. Male C57BL/6 mice, exposed to 98% O2 or higher for 48 hours, were inoculated with PA (1 × 107 CFU) intratracheally. Mice were randomized to receive either AA (50 mg/kg) or saline intraperitoneally. HMGB1 levels in the BALF were analyzed by Western blot analysis. *P ≤ 0.05 compared with hyperoxia control group (A). RAW 264.7 cells either remained at RA (21% O2) or were exposed to 95% O2 for 24 hours with AA (0–1,000 μM). HMGB1 levels in cell culture media were analyzed by Western blot analysis. The data obtained are represented in percentage of the 95% O2 control group. *P ≤ 0.05 compared with 95% O2 (0 μM) control group; #P ≤ 0.05 compared with 21% O2 (0 μM) control group (B). The translocation of HMGB1 was assessed by immunostaining the cells with anti-HMGB1 antibody (red). DAPI stain was used to visualize the nuclei (blue). Scale bars: 10 μm (C). Each value represents the mean ± SEM of three independent experiments.
The translocation of HMGB1 from the nucleus to the cytoplasm is a critical step in the extracellular secretion of HMGB1 (42, 43). Therefore, we determined the effect of AA on the nucleocytoplasmic translocation of HMGB1 in hyperoxic macrophages. Macrophages exposed to hyperoxia exhibited intense staining of HMGB1 (red) in the cytoplasm as compared with control cells that remained in room air (21% O2), where the predominant stain was in the nucleus (Figure 5C). The macrophages incubated with 1–1,000 μM of AA displayed distinct staining of HMGB1 in the nucleus compared with the negligible nuclear staining in macrophages incubated with either hyperoxia alone or in the presence of 0.1 µM AA (Figure 5C; images for 10, 100, and 1,000 µM not shown). These results indicate that AA significantly reduces the hyperoxia-elevated HMGB1 accumulation in the extracellular milieu by inhibiting its translocation from the nucleus to the cytoplasm.
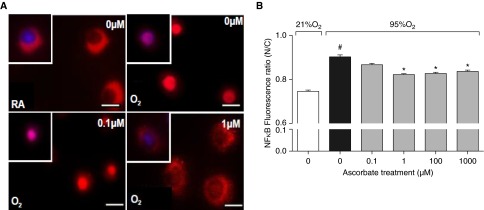
AA Inhibits Nuclear Translocation of NF-κB in Hyperoxic Macrophages
NF-κB activation plays a critical role in mediating the release of HMGB1 from cultured macrophages under stress stimuli, including exposure to hyperoxia (17, 38, 44). NF-κB activation was assessed by immunostaining the p65 subunit of NF-κB, as described in the published studies. In nonactivated cells, NF-κB is primarily localized in the cytoplasm, as indicated by a prominent cytoplasmic stain and a distinct hollow nucleus in cells that remained at 21% O2 (Figure 6A). Upon hyperoxic exposure, NF-κB translocates into the nucleus, as indicated by a profound nuclear staining (Figure 6A; images for 100 and 1,000 µM not shown). However, macrophages exposed to hyperoxia and incubated with AA at 1, 100, and 1,000 µM had significantly reduced nuclear staining compared with cells exposed to hyperoxia alone (0.82, 0.83, and 0.84 versus 0.90, respectively; F(5,2374) = 49.58, P < 0.0001; Figures 6A and 6B). These results suggest that AA inhibits HMGB1 release, at least in part, by preventing hyperoxia-induced NF-κB activation.
Figure 6.
AA inhibits the nuclear translocation of NF-κB in hyperoxic macrophages. RAW 264.7 cells either remained at RA (21% O2) or were exposed to 95% O2 for 24 hours in the presence of AA (0–1,000 μM). The localization of NF-κB was assessed by immunostaining the cells with anti-p65 antibody (red). DAPI stain was used to visualize nuclei (blue). Scale bars: 10 μm (A). Fluorescence was quantified using ImageJ software (National Institutes of Health, Bethesda, MD) and represented as the fluorescence ratio of nucleus to cytoplasm (N/C) (B). *P ≤ 0.05 compared with 95% O2 (0 µM) control group; #P ≤ 0.05 compared with 21% O2 (0 μM) control group.
Discussion
The results of this study indicate that AA protects against hyperoxia-induced lung injury and enhances innate immunity against pulmonary PA infection in a mouse model of VAP. In addition, AA can effectively restore hyperoxia-compromised phagocytic function of cultured macrophages. AA-increased macrophage phagocytic function was associated with a decrease in hyperoxia-induced: (1) elevation in intracellular oxidative stress; and (2) accumulation of extracellular HMGB1. The AA-mediated decrease in the extracellular accumulation of HMGB1 was associated with inhibition of the translocation of nuclear HMGB1 to the cytoplasm, which was associated with attenuated NF-κB activation.
Prolonged exposure to hyperoxia increases the susceptibility of subjects to lung microbial infections (7, 13, 45). The results in Figure 1 show that AA significantly increases bacterial clearance in the airways and lungs of mice exposed to hyperoxia (Figures 1A and 1B). Hyperoxia-induced lung permeability, a marker of tissue injury, can also be reduced by AA (Figure 1C). This increase in host immunity was associated with an improved survival of these animals (Figure 1D). Interestingly, AA did not significantly increase bacterial clearance and lung injury under normoxic (healthy) conditions. Similarly, AA (100 mg/kg/12 h) does not significantly increase the clearance of Streptococcus pneumoniae in normoxic, healthy mice (46). These results may provide further explanations, other than the route of administration of AA, for the difference in the incidence of infections observed in clinical trials of AA administration. Glazebrook and Thomson (47) reported that AA administration did not affect the incidence of viral infections in healthy youth, whereas Heyland and colleagues (48) found that patients on mechanical ventilation receiving supplementation of AA, in combination with other antioxidants, had reduced occurrence of intensive care unit–acquired pneumonia (71 [∼42.3%] versus 95 patients [∼52.5%] in the control group without antioxidant supplementation, P = 0.06). A similar protective effect of AA (50 or 200 mg/kg/24 h) against organ failure and tissue injury has also been observed in patients with severe sepsis (49). These findings suggest that the protective effect of AA is specific to pathological conditions with AA deficiency. Oxidative stress induced by prolonged exposure to hyperoxia or by pronounced inflammatory responses may cause a critical reduction or even depletion of the AA reservoir in the system. AA supplementation brings about repletion to attenuate the adverse effects of oxidative stress. This hypothesis is supported by the results presented in Figures 4A and 4B, which show that exposure to hyperoxia depletes intracellular AA levels in cultured macrophages, and continuous supplementation with 1,000 µM AA can replenish and maintain the levels of intracellular AA.
Prolonged exposure to hyperoxia has been shown to result in excessive production of mitochondrial ROS in lung cells (20). Our results indicate that hyperoxia increases intracellular oxidative stress in cultured macrophages (Figure 3). Supplementation with AA can reduce hyperoxia-increased levels of ORP to similar levels observed under normoxic conditions (Figure 3). In hyperoxic macrophages without AA supplementation, oxidative stress can cause actin oxidation and subsequently increase stress fiber formation (16, 18), and oxidation of actin’s regulatory proteins, thereby causing dysfunction in normal actin polymerization (24, 50), leading to the compromised macrophage phagocytic function. AA has also been shown to decrease the levels of the hydrogen peroxide–induced ROS (51). Interestingly, plasma AA provides protection against aqueous peroxyl radicals in healthy human subjects (29). Macrophages normally accumulate and maintain very high intracellular levels of AA compared with plasma via specific sodium-dependent transporters, thus providing an effective mechanism to neutralize excessive ROS in these cells (40). However, levels of intracellular AA can be significantly decreased after activation of phagocytosis (52). Thus, AA supplementation under oxidative stress may help to maintain the levels of intracellular AA, and subsequently the antioxidant capacity of the alveolar macrophages during phagocytosis, leading to the improvement in phagocytic activity, as in the results shown in Figure 2. Similar to these observations in macrophages, AA has also been shown to improve phagocytic functions of monocytes and neutrophils in humans (53, 54) and murine peritoneal macrophages (55, 56). These results demonstrate that AA can effectively maintain the phagocytic functions of macrophages under oxidative stress.
HMGB1, a potent proinflammatory cytokine, can impair macrophage phagocytosis when it is present in the extracellular milieu (7, 37). High levels of airway HMGB1 are observed in animals exposed to hyperoxia and/or mechanical ventilation, as well as in patients with VAP (7, 11, 57–59). The results of this study indicate that AA can inhibit hyperoxia-induced HMGB1 accumulation in the extracellular milieu (Figure 5). This effect was associated with an increase in macrophage phagocytosis function (Figure 2), and a decrease in the cytoplasmic translocation of HMGB1 from nuclei in hyperoxic macrophages, which is the first step in the release of HMGB1 into the extracellular milieu (Figure 5). Consistent with these results, it has been reported that the administration of AA significantly inhibited LPS-stimulated HMGB1 secretion from cultured macrophages (60, 61). These results suggest that AA significantly increases macrophage phagocytosis not only by reducing the intracellular oxidative stress, but also by inhibiting the translocation and the release of nuclear HMGB1 from these cells.
NF-κB is a redox-sensitive transcription factor (62–65) that is activated under oxidative stress conditions, including hyperoxia (66–68). Activation of NF-κB has been shown to play a critical role in the release of HMGB1 from macrophages exposed to hyperoxia or LPS (17, 44, 69). Our results indicate that AA can significantly inhibit NF-κB activation by attenuating the hyperoxia-induced nuclear translocation of NF-κB in cultured macrophages (Figure 6). This effect is accompanied by the inhibition of cytoplasmic translocation and extracellular accumulation of HMGB1 in these cells (Figure 5). Previously, it has been reported that AA significantly inhibits NF-κB activation produced by TNF-α in cultured human epithelia, endothelia, and monocytes (70). Cárcamo and colleagues (70) showed that AA can block the phosphorylation of IκBα, a key step that allows for the nuclear translocation of NF-κB and subsequent activation of gene expression (70, 71). Our results suggest that the AA-induced decrease in the accumulation of HMGB1 in extracellular milieu is mediated, at least in part, by suppressing NF-κB activation.
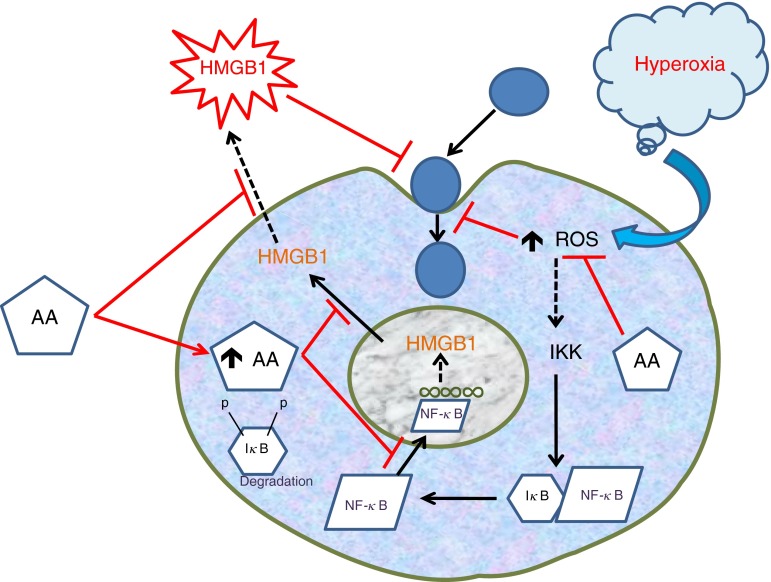
In this study, we demonstrate the protective effects of AA against PA infection under hyperoxic conditions. The data presented implicate the involvement of parallel pathways, consisting of hyperoxia-induced elevation of both intracellular levels of ROS and extracellular HMGB1, in hyperoxia-compromised macrophage functions. Through these pathways, AA effectively enhanced macrophage functions that were compromised by prolonged exposure to hyperoxia (Figure 7). Although other antioxidants have been shown to be effective in rescuing hyperoxia-compromised macrophage functions (18, 26), AA might be more advantageous, due to its relatively nontoxic properties, affordable cost, and easy commercial availability (30, 72). Thus, our findings suggest that supplementation with AA during supportive oxygen therapy may be an effective intervention to attenuate or prevent the development of VAP in these patients.
Figure 7.
Graphical summary of AA’s effects on hyperoxia-compromised phagocytic function. The prolonged exposure of macrophages to hyperoxia leads to elevated levels of intracellular reactive oxygen species (ROS) and extracellular HMGB1. Hyperoxia-induced HMGB1 translocation is mediated by the activation of the NF-κB pathway, which further induces the release of HMGB1. AA not only significantly attenuates ROS levels but also inhibits HMGB1 release via inhibiting NF-κB activation. Hence, AA attenuates the hyperoxia-induced impairment of macrophage phagocytic function by decreasing the levels of ROS and extracellular HMGB1.
Supplementary Material
Acknowledgments
Acknowledgments
The authors thank Dr. Lokesh Sharma, Suhas Gumaste, and Wenjun Wu for their help with experiments, and Retina Kundu for her help with editing the manuscript.
Footnotes
This work was supported by National Heart, Lung, and Blood Institute grant HL093708 (L.L.M.) and a grant from St. John’s University.
Author Contributions: Conception and design—V.S.P., V.S., M.G.E., H.W., L.L.M.; analysis and interpretation—V.S.P., V.S., M.G.E., R.S., X.Y., C.R.A., D.D.T., and L.L.M.; drafting the manuscript for important intellectual content—V.S.P., V.S., M.G.E., R.S., H.W., X.Y., C.R.A., D.D.T., and L.L.M.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0310OC on April 27, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gore A, Muralidhar M, Espey MG, Degenhardt K, Mantell LL. Hyperoxia sensing: from molecular mechanisms to significance in disease. J Immunotoxicol. 2010;7:239–254. doi: 10.3109/1547691X.2010.492254. [DOI] [PubMed] [Google Scholar]
- 2.Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B Long-term Oxygen Treatment Trial Research Group. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest. 2010;138:179–187. doi: 10.1378/chest.09-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saguil A, Fargo M. Acute respiratory distress syndrome: diagnosis and management. Am Fam Physician. 2012;85:352–358. [PubMed] [Google Scholar]
- 4.O’Donohue WJ, Jr, Plummer AL. Magnitude of usage and cost of home oxygen therapy in the United States. Chest. 1995;107:301–302. doi: 10.1378/chest.107.2.301. [DOI] [PubMed] [Google Scholar]
- 5.Habre W, Peták F. Perioperative use of oxygen: variabilities across age. Br J Anaesth. 2014;113:ii26–ii36. doi: 10.1093/bja/aeu380. [DOI] [PubMed] [Google Scholar]
- 6.Koenig SM, Truwit JD. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin Microbiol Rev. 2006;19:637–657. doi: 10.1128/CMR.00051-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel VS, Sitapara RA, Gore A, Phan B, Sharma L, Sampat V, Li JH, Yang H, Chavan SS, Wang H, et al. High mobility group box-1 mediates hyperoxia-induced impairment of Pseudomonas aeruginosa clearance and inflammatory lung injury in mice. Am J Respir Cell Mol Biol. 2013;48:280–287. doi: 10.1165/rcmb.2012-0279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito K, Kimura S, Saga T, Misonoo Y, Yoshizawa S, Akasaka Y, Ishii T, Kuwano K, Yamaguchi K, Tateda K. Protective effect of procysteine on Acinetobacter pneumonia in hyperoxic conditions. J Antimicrob Chemother. 2013;68:2305–2310. doi: 10.1093/jac/dkt192. [DOI] [PubMed] [Google Scholar]
- 9.Coalson JJ, King RJ, Winter VT, Prihoda TJ, Anzueto AR, Peters JI, Johanson WG., Jr O2- and pneumonia-induced lung injury. I. Pathological and morphometric studies. J Appl Physiol (1985) 1989;67:346–356. doi: 10.1152/jappl.1989.67.1.346. [DOI] [PubMed] [Google Scholar]
- 10.Crapo JD. Morphologic changes in pulmonary oxygen toxicity. Annu Rev Physiol. 1986;48:721–731. doi: 10.1146/annurev.ph.48.030186.003445. [DOI] [PubMed] [Google Scholar]
- 11.Entezari M, Javdan M, Antoine DJ, Morrow DM, Sitapara RA, Patel V, Wang M, Sharma L, Gorasiya S, Zur M, et al. Inhibition of extracellular HMGB1 attenuates hyperoxia-induced inflammatory acute lung injury. Redox Biol. 2014;2:314–322. doi: 10.1016/j.redox.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013;58:123–141. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baleeiro CE, Wilcoxen SE, Morris SB, Standiford TJ, Paine R., III Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol. 2003;171:955–963. doi: 10.4049/jimmunol.171.2.955. [DOI] [PubMed] [Google Scholar]
- 14.Rister M. Effects of hyperoxia on phagocytosis. Blut. 1982;45:157–166. doi: 10.1007/BF00320800. [DOI] [PubMed] [Google Scholar]
- 15.Raffin TA, Simon LM, Braun D, Theodore J, Robin ED. Impairment of phagocytosis by moderate hyperoxia (40 to 60 per cent oxygen) in lung macrophages. Lab Invest. 1980;42:622–626. [PubMed] [Google Scholar]
- 16.O’Reilly PJ, Hickman-Davis JM, Davis IC, Matalon S. Hyperoxia impairs antibacterial function of macrophages through effects on actin. Am J Respir Cell Mol Biol. 2003;28:443–450. doi: 10.1165/rcmb.2002-0153OC. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Gorasiya S, Antoine DJ, Sitapara RA, Wu W, Sharma L, Yang H, Ashby CR, Jr, Vasudevan D, Zur M, et al. The compromise of macrophage functions by hyperoxia is attenuated by ethacrynic acid via inhibition of NF-κB–mediated release of high-mobility group box-1. Am J Respir Cell Mol Biol. 2015;52:171–182. doi: 10.1165/rcmb.2013-0544OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrow DM, Entezari-Zaher T, Romashko J, III, Azghani AO, Javdan M, Ulloa L, Miller EJ, Mantell LL. Antioxidants preserve macrophage phagocytosis of Pseudomonas aeruginosa during hyperoxia. Free Radic Biol Med. 2007;42:1338–1349. doi: 10.1016/j.freeradbiomed.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman BA, Topolosky MK, Crapo JD. Hyperoxia increases oxygen radical production in rat lung homogenates. Arch Biochem Biophys. 1982;216:477–484. doi: 10.1016/0003-9861(82)90236-3. [DOI] [PubMed] [Google Scholar]
- 20.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- 21.Huot J, Houle F, Marceau F, Landry J. Oxidative stress–induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- 22.Gaté L, Paul J, Ba GN, Tew KD, Tapiero H. Oxidative stress induced in pathologies: the role of antioxidants. Biomed Pharmacother. 1999;53:169–180. doi: 10.1016/S0753-3322(99)80086-9. [DOI] [PubMed] [Google Scholar]
- 23.Martin KR, Barrett JC. Reactive oxygen species as double-edged swords in cellular processes: low-dose cell signaling versus high-dose toxicity. Hum Exp Toxicol. 2002;21:71–75. doi: 10.1191/0960327102ht213oa. [DOI] [PubMed] [Google Scholar]
- 24.Dalle-Donne I, Rossi R, Giustarini D, Gagliano N, Lusini L, Milzani A, Di Simplicio P, Colombo R. Actin carbonylation: from a simple marker of protein oxidation to relevant signs of severe functional impairment. Free Radic Biol Med. 2001;31:1075–1083. doi: 10.1016/s0891-5849(01)00690-6. [DOI] [PubMed] [Google Scholar]
- 25.Ayala A, Muñoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arita Y, Kazzaz JA, Joseph A, Koo HC, Li Y, Davis JM. Antioxidants improve antibacterial function in hyperoxia-exposed macrophages. Free Radic Biol Med. 2007;42:1517–1523. doi: 10.1016/j.freeradbiomed.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arita Y, Joseph A, Koo HC, Li Y, Palaia TA, Davis JM, Kazzaz JA. Superoxide dismutase moderates basal and induced bacterial adherence and interleukin-8 expression in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1199–L1206. doi: 10.1152/ajplung.00457.2003. [DOI] [PubMed] [Google Scholar]
- 28.Ilizarov AM, Koo HC, Kazzaz JA, Mantell LL, Li Y, Bhapat R, Pollack S, Horowitz S, Davis JM. Overexpression of manganese superoxide dismutase protects lung epithelial cells against oxidant injury. Am J Respir Cell Mol Biol. 2001;24:436–441. doi: 10.1165/ajrcmb.24.4.4240. [DOI] [PubMed] [Google Scholar]
- 29.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 31.Lutsenko EA, Cárcamo JM, Golde DW. Vitamin C prevents DNA mutation induced by oxidative stress. J Biol Chem. 2002;277:16895–16899. doi: 10.1074/jbc.M201151200. [DOI] [PubMed] [Google Scholar]
- 32.Jain A, Mårtensson J, Mehta T, Krauss AN, Auld PA, Meister A. Ascorbic acid prevents oxidative stress in glutathione-deficient mice: effects on lung type 2 cell lamellar bodies, lung surfactant, and skeletal muscle. Proc Natl Acad Sci USA. 1992;89:5093–5097. doi: 10.1073/pnas.89.11.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, α-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 34.Bielski BH, Richter HW, Chan PC. Some properties of the ascorbate free radical. Ann N Y Acad Sci. 1975;258:231–237. doi: 10.1111/j.1749-6632.1975.tb29283.x. [DOI] [PubMed] [Google Scholar]
- 35.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 37.Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li J, Wang H, Yang H, Sharma L, Phan BD, et al. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in cystic fibrosis. Mol Med. 2012;18:477–485. doi: 10.2119/molmed.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sitapara RA, Antoine DJ, Sharma L, Patel VS, Ashby CR, Jr, Gorasiya S, Yang H, Zur M, Mantell LL. The α7 nicotinic acetylcholine receptor agonist GTS-21 improves bacterial clearance in mice by restoring hyperoxia-compromised macrophage function. Mol Med. 2014;20:238–247. doi: 10.2119/molmed.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Patel VS, Sampat V, Sharma LK, Sitapara RA, Wu W, Wang H, Espey M, Mantell L. Ascorbic acid improves hyperoxia-compromised host defense against Pseudomonas aeruginosa infection [abstract] The Toxicologist: Supplement to Toxicological Sciences. 2015;144:1346. [Google Scholar]
- 40.May JM, Huang J, Qu ZC. Macrophage uptake and recycling of ascorbic acid: response to activation by lipopolysaccharide. Free Radic Biol Med. 2005;39:1449–1459. doi: 10.1016/j.freeradbiomed.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 41.May JM, Qu ZC, Mendiratta S. Protection and recycling of α-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys. 1998;349:281–289. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- 42.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 44.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunn MM, Smith LJ. The effects of hyperoxia on pulmonary clearance of Pseudomonas aeruginosa. J Infect Dis. 1986;153:676–681. doi: 10.1093/infdis/153.4.676. [DOI] [PubMed] [Google Scholar]
- 46.Esposito AL. Ascorbate modulates antibacterial mechanisms in experimental pneumococcal pneumonia. Am Rev Respir Dis. 1986;133:643–647. doi: 10.1164/arrd.1986.133.4.643. [DOI] [PubMed] [Google Scholar]
- 47.Glazebrook AJ, Thomson S. The administration of vitamin C in a large institution and its effect on general health and resistance to infection. J Hyg (Lond) 1942;42:1–19. doi: 10.1017/s0022172400012596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heyland D, Wischmeyer PE, Day AG Canadian Clinical Care Trials Group. Glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;369:484–485. doi: 10.1056/NEJMc1306658. [DOI] [PubMed] [Google Scholar]
- 49.Fowler AA, III, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, Farthing CA, Larus TL, Martin E, Brophy DF, et al. Medical Respiratory Intensive Care Unit Nursing. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milzani A, DalleDonne I, Colombo R. Prolonged oxidative stress on actin. Arch Biochem Biophys. 1997;339:267–274. doi: 10.1006/abbi.1996.9847. [DOI] [PubMed] [Google Scholar]
- 51.Guaiquil VH, Vera JC, Golde DW. Mechanism of vitamin C inhibition of cell death induced by oxidative stress in glutathione-depleted HL-60 cells. J Biol Chem. 2001;276:40955–40961. doi: 10.1074/jbc.M106878200. [DOI] [PubMed] [Google Scholar]
- 52.Oberritter H, Glatthaar B, Moser U, Schmidt KH. Effect of functional stimulation on ascorbate content in phagocytes under physiological and pathological conditions. Int Arch Allergy Appl Immunol. 1986;81:46–50. doi: 10.1159/000234106. [DOI] [PubMed] [Google Scholar]
- 53.Ciocoiu M, Lupuşoru EC, Colev V, Bădescu M, Păduraru I. The involvement of vitamins C and E in changing the immune response [article in Romanian] Rev Med Chir Soc Med Nat Iasi. 1998;102:93–96. [PubMed] [Google Scholar]
- 54.Bergman M, Salman H, Djaldetti M, Fish L, Punsky I, Bessler H. In vitro immune response of human peripheral blood cells to vitamins C and E. J Nutr Biochem. 2004;15:45–50. doi: 10.1016/j.jnutbio.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Del Rio M, Ruedas G, Medina S, Victor VM, De la Fuente M. Improvement by several antioxidants of macrophage function in vitro. Life Sci. 1998;63:871–881. doi: 10.1016/s0024-3205(98)00344-0. [DOI] [PubMed] [Google Scholar]
- 56.Victor VV, Guayerbas N, Puerto M, Medina S, De la Fuente M. Ascorbic acid modulates in vitro the function of macrophages from mice with endotoxic shock. Immunopharmacology. 2000;46:89–101. doi: 10.1016/s0162-3109(99)00162-9. [DOI] [PubMed] [Google Scholar]
- 57.Feng J, Deng C, Yu JL, Guo CB, Zhao QQ. Expression of high mobility group protein-B1 in mice with hyperoxia-induced bronchopulmonary dysplasia [article in Chinese] Zhongguo Dang Dai Er Ke Za Zhi. 2010;12:219–223. [PubMed] [Google Scholar]
- 58.Li LF, Yang CT, Huang CC, Liu YY, Kao KC, Lin HC. Low–molecular-weight heparin reduces hyperoxia-augmented ventilator-induced lung injury via serine/threonine kinase–protein kinase B. Respir Res. 2011;12:90. doi: 10.1186/1465-9921-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Zoelen MA, Ishizaka A, Wolthuls EK, Choi G, van der Poll T, Schultz MJ. Pulmonary levels of high-mobility group box 1 during mechanical ventilation and ventilator-associated pneumonia. Shock. 2008;29:441–445. doi: 10.1097/SHK.0b013e318157eddd. [DOI] [PubMed] [Google Scholar]
- 60.Shingu C, Hagiwara S, Iwasaka H, Matsumoto S, Koga H, Yokoi I, Noguchi T. EPCK1, a vitamin C and E analogue, reduces endotoxin-induced systemic inflammation in mice. J Surg Res. 2011;171:719–725. doi: 10.1016/j.jss.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 61.Kim SR, Ha YM, Kim YM, Park EJ, Kim JW, Park SW, Kim HJ, Chung HT, Chang KC. Ascorbic acid reduces HMGB1 secretion in lipopolysaccharide-activated RAW 264.7 cells and improves survival rate in septic mice by activation of Nrf2/HO-1 signals. Biochem Pharmacol. 2015;95:279–289. doi: 10.1016/j.bcp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-κB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395–403. doi: 10.1089/ars.2005.7.395. [DOI] [PubMed] [Google Scholar]
- 63.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li N, Karin M. Is NF-κB the sensor of oxidative stress? FASEB J. 1999;13:1137–1143. [PubMed] [Google Scholar]
- 65.Meyer M, Pahl HL, Baeuerle PA. Regulation of the transcription factors NF-κB and AP-1 by redox changes. Chem Biol Interact. 1994;91:91–100. doi: 10.1016/0009-2797(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 66.Shea LM, Beehler C, Schwartz M, Shenkar R, Tuder R, Abraham E. Hyperoxia activates NF-κB and increases TNF-α and IFN-γ gene expression in mouse pulmonary lymphocytes. J Immunol. 1996;157:3902–3908. [PubMed] [Google Scholar]
- 67.Liu YY, Liao SK, Huang CC, Tsai YH, Quinn DA, Li LF. Role for nuclear factor-κB in augmented lung injury because of interaction between hyperoxia and high stretch ventilation. Transl Res. 2009;154:228–240. doi: 10.1016/j.trsl.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 68.Pepperl S, Dörger M, Ringel F, Kupatt C, Krombach F. Hyperoxia upregulates the NO pathway in alveolar macrophages in vitro: role of AP-1 and NF- B. Am J Physiol Lung Cell Mol Physiol. 2001;280:L905–L913. doi: 10.1152/ajplung.2001.280.5.L905. [DOI] [PubMed] [Google Scholar]
- 69.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 70.Cárcamo JM, Pedraza A, Bórquez-Ojeda O, Golde DW. Vitamin C suppresses TNFα–induced NFκB activation by inhibiting IκBα phosphorylation. Biochemistry. 2002;41:12995–13002. doi: 10.1021/bi0263210. [DOI] [PubMed] [Google Scholar]
- 71.Cárcamo JM, Pedraza A, Bórquez-Ojeda O, Zhang B, Sanchez R, Golde DW. Vitamin C is a kinase inhibitor: dehydroascorbic acid inhibits IκBα kinase β. Mol Cell Biol. 2004;24:6645–6652. doi: 10.1128/MCB.24.15.6645-6652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naidu KA. Vitamin C in human health and disease is still a mystery? An overview. Nutr J. 2003;2:7. doi: 10.1186/1475-2891-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.