Abstract
Increased vascular endothelial cell (EC) permeability is a result of intercellular gap formation that may be induced by contraction-dependent and contraction-independent mechanisms. This study investigated a role of the adaptor protein vinculin in EC permeability induced by contractile (thrombin) and noncontractile (IL-6) agonists. Although thrombin and IL-6 caused a similar permeability increase in human pulmonary ECs and disrupted the association between vinculin and vascular endothelial–cadherin, they induced different patterns of focal adhesion (FA) arrangement. Thrombin, but not IL-6, caused formation of large, vinculin-positive FAs, phosphorylation of FA proteins, FA kinase and Crk-associated substrate, and increased vinculin–talin association. Thrombin-induced formation of talin-positive FA and intercellular gaps were suppressed in ECs with small interfering RNA–induced vinculin knockdown. Vinculin knockdown and inhibitors of Rho kinase and myosin-II motor activity also attenuated thrombin-induced EC permeability. Importantly, ectopic expression of the vinculin mutant lacking the F-actin–binding domain decreased thrombin-induced Rho pathway activation and EC permeability. In contrast, IL-6–induced EC permeability did not involve RhoA- or myosin-dependent mechanisms but engaged Janus kinase/signal transducer and activator of transcription–mediated phosphorylation and internalization of vascular endothelial–cadherin. This process was vinculin independent but Janus kinase/tyrosine kinase Src-dependent. These data suggest that vinculin participates in a contractile-dependent mechanism of permeability by integrating FA with stress fibers, leading to maximal RhoA activation and EC permeability response. Vinculin inhibition does not affect contractile-independent mechanisms of EC barrier failure. This study provides, for the first time, a comparative analysis of two alternative mechanisms of vascular endothelial barrier dysfunction and defines a specific role for vinculin in the contractile type of permeability response.
Keywords: cyclic stretch, Rho, focal adhesions, vascular permeability, endothelium
Clinical Relevance
This study investigates the involvement of vinculin in mechanisms of agonist-induced endothelial permeability activated by contractile and noncontractile agonists and defines the role for vinculin-driven focal adhesion–actin cytoskeleton anchoring in the propagation of Rho-dependent endothelial permeability caused by contractile agonists.
The lung endothelial cells (ECs) form a semiselective barrier between circulating blood and interstitial fluid. The contractile model of EC barrier regulation (1, 2) suggests that paracellular gap formation is controlled by the balance of competing contractile forces imposed by the actomyosin cytoskeleton anchored to focal adhesions (FAs), which generate centripetal tension, and adhesive cell–cell and cell–matrix tethering forces imposed by peripheral FAs and adherens junctions (AJs). In this model, agonist-induced EC permeability requires activation of myosin light chain (MLC) kinase, RhoA GTPase, and other signaling kinases, which trigger actomyosin cytoskeletal rearrangement, phosphorylation of regulatory MLC, activation of EC contraction, disruption of intercellular AJs, and formation of paracellular gaps (1, 3).
FAs are multimolecular complexes that form a bidirectional linkage between the actin cytoskeleton and the cell–extracellular matrix adhesions formed by integrins (4, 5). FAs provide additional tethering forces that help maintain EC barrier integrity. Integrins and integrin-associated intracellular FA proteins not only physically connect the cytoskeleton to the extracellular matrix, but also function as the important transmitters of physical forces into chemical signals (6–8).
Enhancement of FAs experiencing increased mechanical loads requires additional recruitment and activation of FA proteins. Indeed, early studies that evaluated force development by agonist-stimulated cultured cells using traction force microscopy showed that cytoskeletal tension induced by thrombin caused tension-associated enlargement of vinculin-positive FAs, whereas inhibitors of contractility promoted FA disassembly (9). Vinculin is a globular FA protein consisting of five helical domains (D1–D5). D1–D4 form the vinculin head, which is connected to the vinculin tail domain (Vt) by a flexible linker region (10). Direct interaction of the head domain (D1) with the Vt renders a closed, autoinhibited vinculin conformation. The Vt contains binding sites for F-actin, paxillin, and phosphatidylinositol 4,5-bisphosphate, whereas the head domain, D1, holds binding sites for talin, α-actinin, and α-catenin, which are essential for vinculin targeting to FAs. In the closed conformation, vinculin is unable to bind both filamentous actin at Vt and talin at D1. The fraction of vinculin stably incorporated in FAs increases with cell forces and believed to be mediated by force-dependent change of vinculin conformation (11). Extracellular and intracellular mechanical forces stimulate formation of a stable complex between vinculin and its FA-associated partner, talin, as well as vinculin interaction with the actin cytoskeleton. This FA-associated mechanosensitive module stabilizes FA complexes and cell adhesion, and is essential for the proper functioning of the intracellular contractile machinery (12–14).
Thrombin is a serine protease produced on the surface of the injured endothelium from circulating prothrombin. Thrombin rapidly activates EC actomyosin contractile machinery (15) and increases pulmonary vascular permeability via activation of protease-activated receptor, protease activated receptor 1, and direct activation of RhoA through G12/13-coupled p115Rho-guanosine nucleotide exchange factor (16), which leads to increased MLC phosphorylation, stress fiber formation, increased actomyosin contraction, and EC barrier dysfunction (15). In addition to the contractile mechanism of EC permeability, an alternative mechanism of increased permeability involves partial disassembly of AJs dependent on tyrosine phosphorylation of vascular endothelial (VE)-cadherin (17, 18) and leading to the opening of intercellular junctions and increased macromolecule flux. AJs, composed of cadherins bound together in a homotypic and Ca2+-dependent fashion, serve to link adjacent ECs. Transmembrane AJ cadherins interact through the cytoplasmic tail with the catenin family of intracellular proteins (α, β, γ, p120) and adaptor protein vinculin, providing anchorage to the actin cytoskeleton (19, 20).
IL-6 is an inflammatory mediator up-regulated in septic and ventilator-induced lung injury (21, 22). The IL-6 receptor (IL-6R) consists of two polypeptide chains: an 80-kD IL-6R and a 130-kD signal transducer (glycoprotein 130 [gp130]). IL-6 associates with the gp130 coreceptor on the EC membrane surface and triggers the activation of Janus kinase/signal transducer and activator of transcription–mediated transcription, phosphatidylinositol-3-kinase-kinase/AKT cascade, and mitogen-activated protein kinase pathways (23). The signaling pathways activated in pulmonary endothelium by IL-6 are not clearly understood, and the precise mechanisms of IL-6–mediated EC barrier dysfunction remain to be elucidated. Our previous study suggests that IL-6–induced EC permeability is independent of RhoA signaling (24). In the current study, we used thrombin and IL-6 as model agents to investigate a role of vinculin in contractile-dependent and contractile-independent mechanisms of EC barrier failure relevant to pathological conditions of lung vascular barrier dysfunction caused by circulating vasoactive agonists and proinflammatory cytokines.
Materials and Methods
Cell Culture and Reagents
Human pulmonary artery ECs (HPAECs) were obtained from Lonza (Allendale, NJ). Human IL-6 and IL-6–soluble receptor were from R&D Systems (Minneapolis, MN). MLC-threonine18/serine19, STAT3-Y705, FA kinase (FAK)-Y576/577, Crk-associated substrate (p130Cas)-Y410, tyrosine kinase Src-Y416 antibodies were from Cell Signaling (Beverly, MA); VE-cadherin was from Santa Cruz Biotechnology (Santa Cruz, CA); vinculin and β-actin were from Sigma (St. Louis, MO); myosin-A was from Covance (Berkley, CA), phospho-VE-cadherin-Y658 was from Invitrogen (Carlsbad, CA). MLC phosphatase (MYPT) 1–threonine850 and talin antibodies, blebbistatin, JAK inhibitor-I, Y27632, and PP2 kinase inhibitor were purchased from EMD Millipore (Billerica, MA). Reagents for immunofluorescence were from Molecular Probes (Eugene, OR). Unless otherwise specified, biochemical reagents were obtained from Sigma. YFP-vinculin and YFP-VNC-880 mutant lacking C-terminal F-actin binding domain were provided by C. Ballestrem (13). Transient DNA transfections of HPAECs were performed using PolyJet reagent from Signagen Laboratories (Rockville, MD), as recommended by the manufacturer. Cells were used 24 hours after transfection. In experiments with vinculin knockdown, predesigned, standard-purity, vinculin-specific small interfering RNA sets were ordered from Ambion (Austin, TX), and transfection of ECs was performed as described previously (25). After 48–72 hours of transfection, cells were used for experiments or harvested for Western blot verification of specific protein depletion. Nonspecific, nontargeting siRNA (Dharmacon, Lafayette, CO) was used as a control. Cell viability assay was performed using the Viability/Cytotoxicity kit from Molecular Probes according to the manufacturer’s protocol.
Measurement of EC Permeability
Transendothelial electrical resistance (TER) across confluent human pulmonary artery endothelial monolayers was measured using an electrical cell-substrate impedance–sensing system (Applied Biophysics, Troy, NY) (26). Measurement of EC permeability for macromolecules was performed using Express Permeability Testing assay (XPerT), recently developed by our group (27). Images of were acquired using Nikon video imaging system Eclipse TE 300 (Nikon, Tokyo, Japan) equipped with a digital camera (DKC 5000; Sony, Tokyo, Japan) and processed with Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA).
GTPase Activation, Protein Fractionation, and Immunoprecipitation
Rho activation was assessed using the GTP-bound GTPase pulldown assays as described previously (15). Cytosolic and membrane fractions were separated using a subcellular protein fractionation kit (Thermo Fisher Scientific, Rockford, IL). Coimmunoprecipitation studies and Western blot analysis were performed using confluent HPAEC monolayers, as described elsewhere (28). Protein extracts were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes, and the membranes were incubated with specific antibodies of interest.
Immunofluorescence
Endothelial monolayers plated on glass coverslips were subjected to double immunofluorescence staining with the appropriate antibody, as described previously (28). Texas red phalloidin was used to visualize F-actin. After immunostaining, slides were analyzed using a Nikon video imaging system (Nikon Instech Co., Tokyo, Japan). Images were processed with Adobe Photoshop 7.0 software. Agonist-induced gap formation was quantified in EC monolayers after affinity staining with Texas red phalloidin, as described previously (15).
Statistical Analysis
Results are expressed as means (±SD). Experimental samples were compared with controls by the unpaired Student’s t test. For multiple-group comparisons, one-way variance analysis (ANOVA) and post hoc multiple comparison tests were used. P less than 0.05 was considered statistically significant.
Results
EC Permeability Caused by Thrombin and IL-6 Is Associated with Different Patterns of FA Remodeling and Signaling
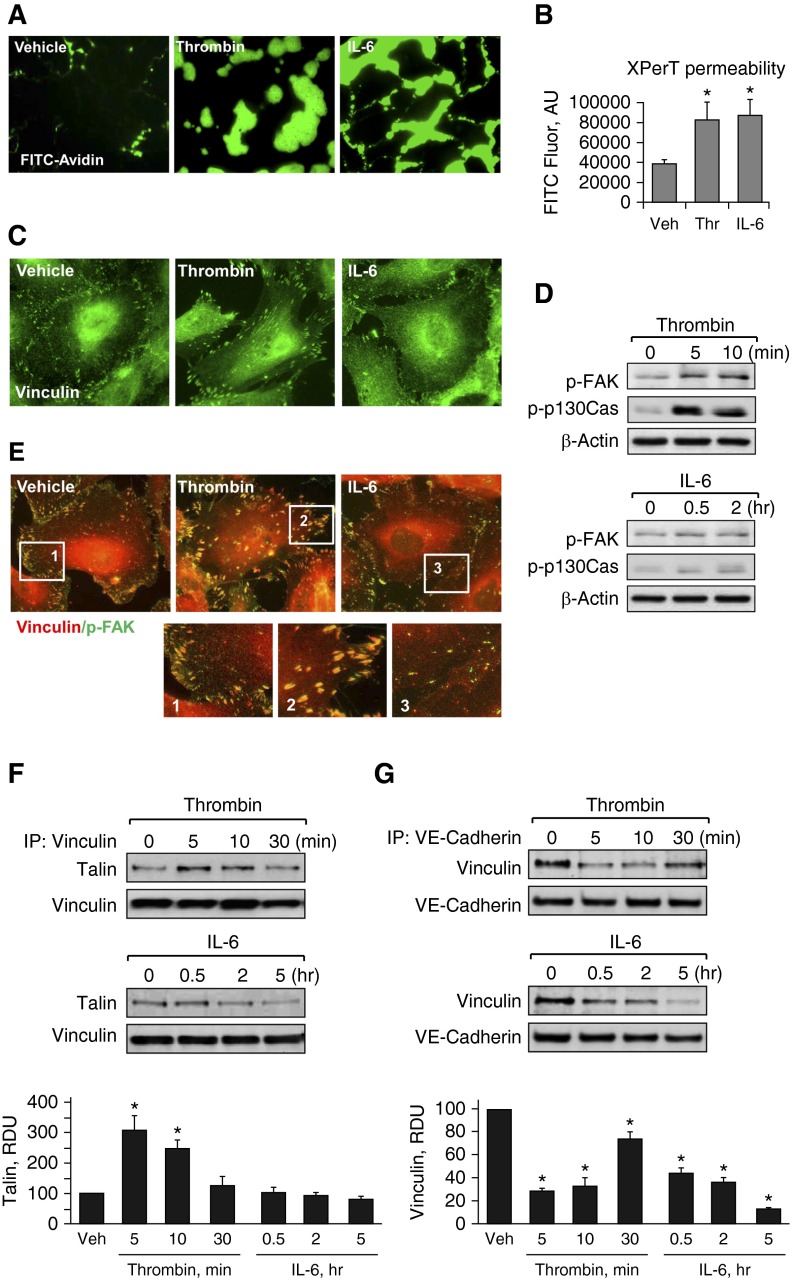
Human pulmonary ECs were treated with IL-6 in the presence of IL-6–soluble receptor or thrombin. Both agonists increased EC paracellular permeability (Figures 1A and 1B) monitored by the previously described XPerT assay (27). Thrombin-induced permeability was associated with significant enlargement of vinculin-positive FAs. By contrast, the EC permeability response to IL-6 was not accompanied by significant changes in FA arrangement (Figure 1C). Previous studies have shown more delayed EC permeability response to IL-6, which developed after 0.5–5.0 hours of stimulation (24) in contrast to more rapid (5–20 min) development of permeability caused by thrombin, which also was followed by recovery of EC barrier by 60 minutes after treatment (29). These time points were further used for analysis of thrombin- and IL-6–induced EC signaling and cytoskeletal remodeling.
Figure 1.
Effects of thrombin and IL-6 on endothelial permeability and focal adhesion (FA) remodeling. (A and B) Human pulmonary artery endothelial cells (HPAECs) grown on glass coverslips (A) or in 96-well plates (B) with immobilized, biotinylated gelatin (0.25 mg/ml) were treated with thrombin (0.2 U/ml, 10 min) or IL-6 and soluble receptor (SR; 30 ng/ml and 60 ng/ml, 5 h), followed by addition of fluorescein isothiocyanate (FITC)-avidin (25 µg/ml, 3 min). Unbound FITC-avidin was removed, and FITC fluorescence (Fluor) was measured; n = 4; *P < 0.05 versus vehicle. (C) Cells grown on coverslips were stimulated with thrombin (0.2 U/ml, 10 min) or IL-6 and SR (30 ng/ml and 60 ng/ml, 5 h). FA remodeling was analyzed by immunofluorescence staining for vinculin. (D) Levels of phosphorylated FA kinase (p-FAK) and Crk-associated substrate (p130Cas) were determined by Western blot analysis using specific antibodies. Equal protein loading was confirmed by determination of β-actin content in total cell lysates. (E) HPAECs were stimulated with thrombin or IL-6/SR (10 min and 5 h, respectively) followed by double immunofluorescence staining with antibodies against vinculin (red) and p-FAK (green). Merged images depict areas of vinculin and p-FAK colocalization, which appear in yellow. Higher-magnification insets show details of FA localization. (F and G) Co-immunoprecipitation assays using antibodies to vinculin (F) or vascular endothelial (VE)-cadherin (G) were performed, and talin or vinculin content in the immunoprecipitates was detected using the appropriate antibody. Bar graphs depict quantitative analysis of Western blot data; n = 3; *P < 0.05 versus vehicle. AU, arbitrary units; IP, immunoprecipitation; RDU, relative density unit; Thr, thrombin; Veh, vehicle.
Biochemical analysis of agonist-induced activation of FA proteins showed increased levels of phosphorylated FA-associated signaling proteins, FAK and p130Cas, after thrombin stimulation. In contrast, IL-6 treatment did not induce noticeable FAK or p130Cas phosphorylation (Figure 1D). In addition to activation of FAK and p130Cas, and FA remodeling, thrombin stimulation also induced colocalization of vinculin and activated FAK at the enlarged FAs (Figure 1E), which is consistent with activation of vinculin by tyrosine phosphorylation in mechanically loaded FAs (30). In contrast, colocalization of vinculin and phospho-FAK was not observed in IL-6–stimulated cells. These data illustrate the fundamental differences in FA remodeling in the contractile and noncontractile models of EC permeability.
Increased accumulation of vinculin at FAs or AJs has been linked to generation of intracellular tension (20, 31), suggesting a role for vinculin as a force sensor and mechanotransducer at FAs and AJs. The next tests examined vinculin interactions with FA and AJ protein complexes in ECs treated with thrombin and IL-6. Thrombin stimulated interactions between vinculin and its FA-associated binding partner, talin, as detected by co-immunoprecipitation assays. In contrast, vinculin–talin interaction was not stimulated by cell treatment with IL-6 (Figure 1F).
Both, thrombin and IL-6 decreased co-immunoprecipitation of vinculin and AJ protein VE-cadherin (Figure 1G). The data show that thrombin caused transient disruption of the vinculin–VE-cadherin complex, whereas dissociation of vinculin from VE-cadherin caused by IL-6 was more sustained and was observed at up to 6 hours after IL-6 treatment.
Involvement of Vinculin in Contractile and Noncontractile Mechanisms of EC Permeability
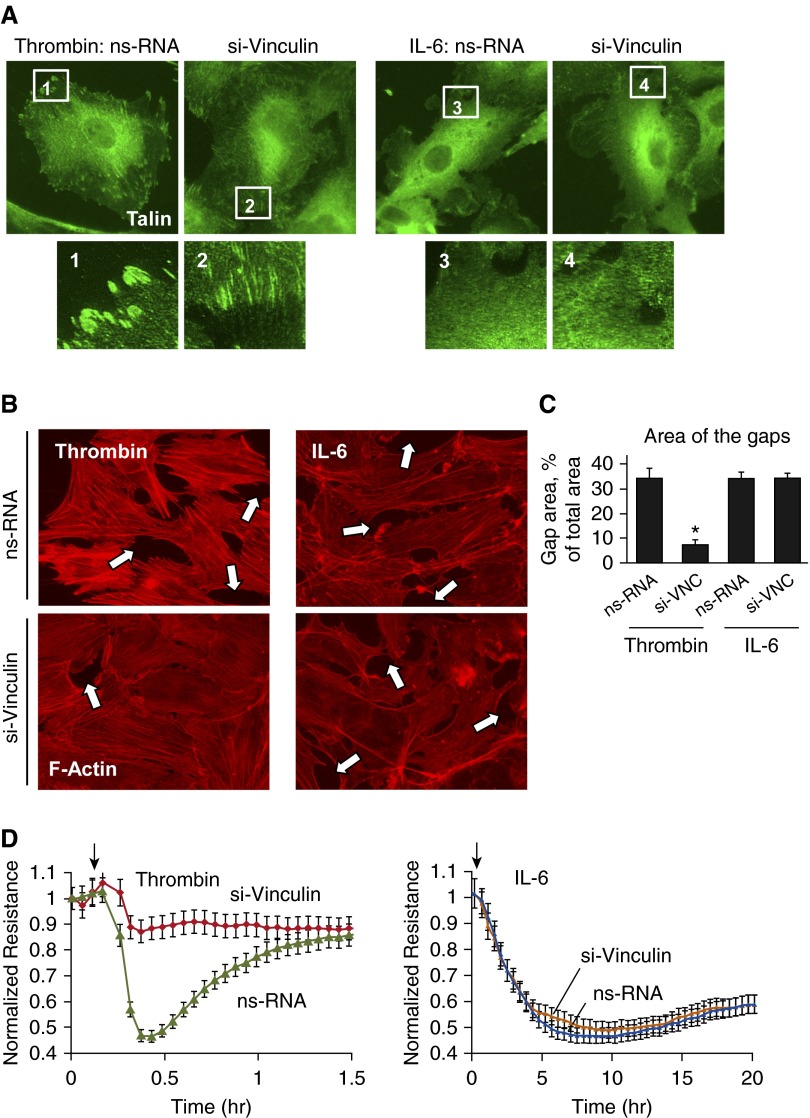
The role of vinculin in the modulation of the EC permeability response was examined using vinculin depletion by gene-specific siRNA. Vinculin knockdown significantly decreased the size of talin-positive FAs in thrombin-stimulated ECs, without a noticeable effect on IL-6–stimulated cells (Figure 2A).
Figure 2.
Effects of vinculin knockdown on thrombin– and IL-6–induced FA rearrangement, actin cytoskeletal remodeling, and endothelial permeability. Human pulmonary ECs were transfected with vinculin-specific (si-VNC) or nonspecific small interfering RNA. (A and B) FA (A) and cytoskeletal (B) remodeling in thrombin– (0.2 U/ml, 10 min) or IL-6/SR (30 ng/ml and 60 ng/ml, 5 h)–treated cells was analyzed by immunofluorescence staining for talin or F-actin, respectively. Paracellular gaps are marked by arrows. (C) Quantitative analysis of paracellular gap formation in control and treated HPAECs. Data are expressed as mean ± SD; n = 4; *P < 0.05 versus nonspecific RNA (ns-RNA). (D) HPAECs plated on microelectrodes were treated with thrombin or IL-6/SR followed by measurements of transendothelial electrical resistance (TER). Arrows in panel D indicate time point of agonist.
Vinculin knockdown attenuated thrombin-induced actin stress fiber formation and EC monolayer disruption, but was without an effect on F-actin arrangement or formation of paracellular gaps in ECs treated with IL-6 (Figures 2B and 2C). Measurements of TER showed significant attenuation of the TER drop caused by thrombin in ECs with vinculin knockdown. In contrast, vinculin knockdown did not affect the TER drop in IL-6–stimulated ECs (Figure 2D).
Differential Effects of Rho and Myosin Inhibitors on EC Permeability Caused by Thrombin and IL-6
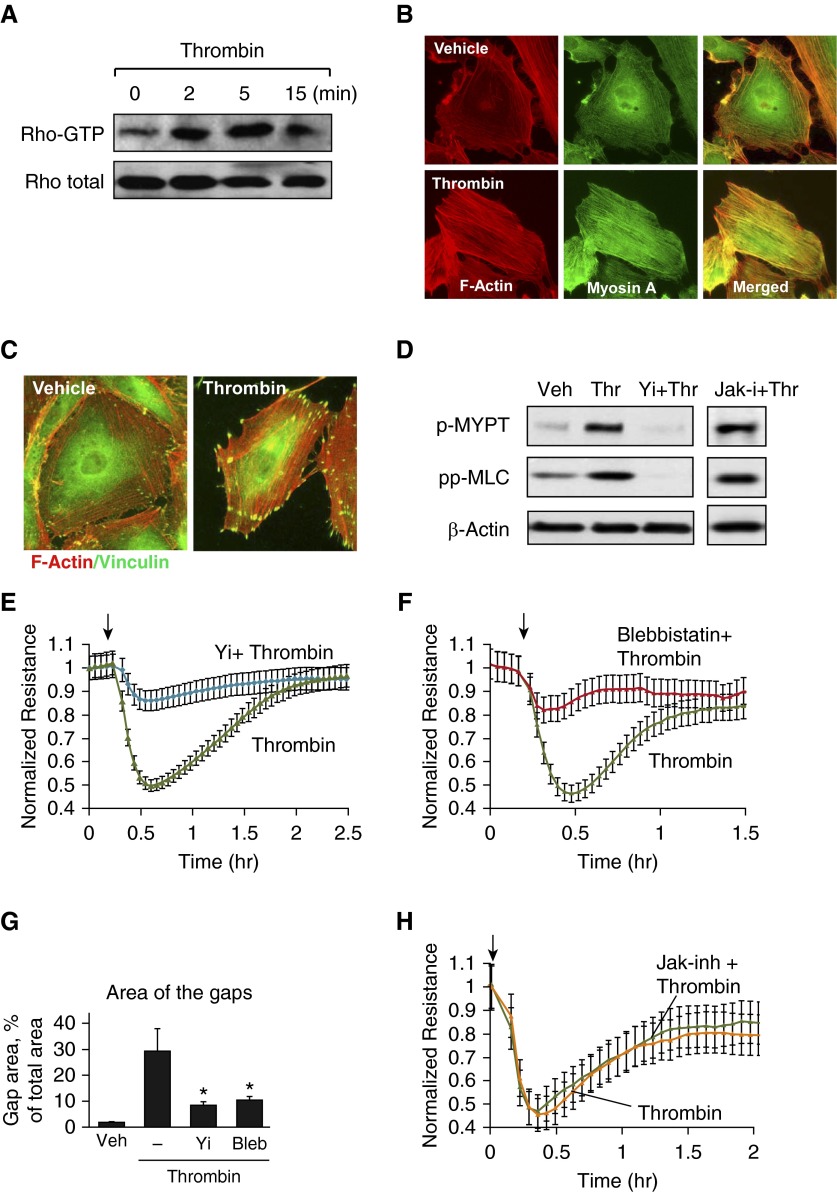
Activation of actomyosin contractility in vascular endothelium is triggered by the Rho pathway, which causes Rho kinase–dependent phosphorylation and inactivation of MYPT1 and increased phosphorylation of regulatory MLCs (15). As a reflection of the classical mechanism of Rho-dependent remodeling, thrombin stimulation rapidly activated RhoA (Figure 3A), and stimulated formation of central stress fibers containing F-actin and myosin A, essential for activation of cell contractile response (Figure 3B), and promoted anchoring of contractile stress fibers to FAs (Figure 3C). Cell pretreatment with Rho kinase inhibitor Y-27632 abolished thrombin-induced phosphorylation of MYPT1 and MLC, whereas inhibition of JAK was without effect (Figure 3D). Inhibition of Rho signaling by Y-27632 (Figure 3E) or inhibition of myosin A motor activity by cell treatment with blebbistatin (Figure 3F) attenuated a thrombin-induced increase in EC permeability, reflected by TER decline and suppressed thrombin-induced formation of paracellular gaps (Figure 3G). In contrast, thrombin-induced EC permeability was not affected by cell pretreatment with JAK inhibitor (Figure 3H).
Figure 3.
Analysis of contractile signaling activated by thrombin. (A) Thrombin-induced Rho activation was assessed by RhoGTP pulldown assay. (B) Cytoskeletal remodeling in response to thrombin treatment (0.2 U/ml, 10 min) was analyzed by immunofluorescence staining for F-actin (red) and myosin A (green). Merged images depict areas of actin and myosin colocalization, appearing in yellow. (C) Immunofluorescence analysis of F-actin (red) and vinculin (green) localization in control and thrombin-treated ECs. Areas of colocalization appear in yellow. (D) HPAECs were pretreated with vehicle, Y-27632 (2 µM, 30 min), or Janus kinase (JAK) inhibitor I (5 µM, 30 min), followed by stimulation with thrombin. Myosin light chain (MLC) phosphatase (MYPT) and MLC phosphorylation was analyzed by Western blotting with the corresponding antibody. Probing for β-actin was used as a normalization control. (E and F) HPAECs were pretreated with Y-27632 (E) or blebbistatin (15 µM, 30 min) (F) followed by stimulation with thrombin, and TER was measured over time. (G) Quantitative analysis of paracellular gap formation in control and thrombin-treated HPAECs with or without Y-27632 or blebbistatin (Bleb) preconditioning. Data are expressed as mean ± SD; n = 3; *P < 0.05 versus thrombin. (H) HPAECs were pretreated with JAK inhibitor I (5 µM, 30 min) followed by stimulation with thrombin, and TER was measured over time. Arrows in panels E, F, and H indicate time point of thrombin. Jak-I, Janus kinase inhibitor; Yi, Y-27632.
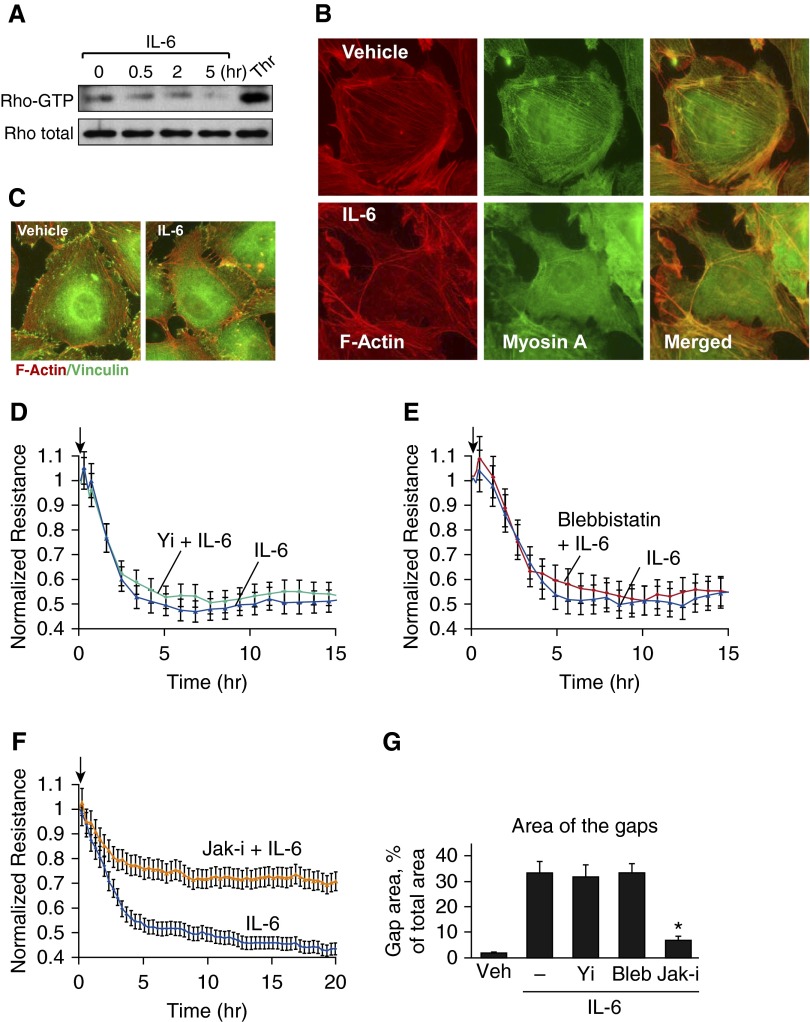
Unlike thrombin, IL-6 neither activated Rho (Figure 4A) nor stimulated formation of central stress fibers containing F-actin and myosin A, the complex essential for contractile mechanism of EC permeability (Figure 4B). Also in contrast with thrombin-stimulated conditions, IL-6 did not induce colocalization of vinculin with tips of actin filaments (Figure 4C). Y-27632 (Figure 4D) and blebbistatin (Figure 4E) were ineffective in the attenuation of EC permeability and paracellular gap formation caused by IL-6. However, in control experiments, EC barrier dysfunction caused by IL-6 was blocked by EC pretreatment with the pharmacologic inhibitor of Jak kinase (Figures 4F and 4G).
Figure 4.
Analysis of contractile signaling in response to IL-6. (A) IL-6–induced Rho activation was assessed by RhoGTP pulldown assay. Thrombin treatment (0.2 U/ml, 5 min) was used as a positive control. (B) Cytoskeletal remodeling in response to IL-6 and SR (30 ng/ml and 60 ng/ml, 5 h) was analyzed by immunofluorescence staining for F-actin (red) and myosin A (green). Merged images depict areas of actin and myosin colocalization, which appear in yellow. (C) Immunofluorescence analysis of F-actin (red) and vinculin (green) localization in control and IL-6/SR–treated ECs. Areas of colocalization appear in yellow. (D–F) HPAECs were pretreated with vehicle or Y-27632 (2 µM, 30 min) (D), blebbistatin (15 µM, 30 min) (E), or JAK inhibitor I (5 µM, 30 min) (F), followed by stimulation with IL-6 and SR. TER was measured over a 15- to 20-hour time period. (G) Quantitative analysis of paracellular gap formation in control and IL-6/SR–treated HPAECs with or without Y-27632, blebbistatin, or JAK inhibitor preconditioning. Data are expressed as mean ± SD; n = 3; *P < 0.05 versus IL-6/SR. Arrows in panels D, E, and F indicate time point of IL-6 addition.
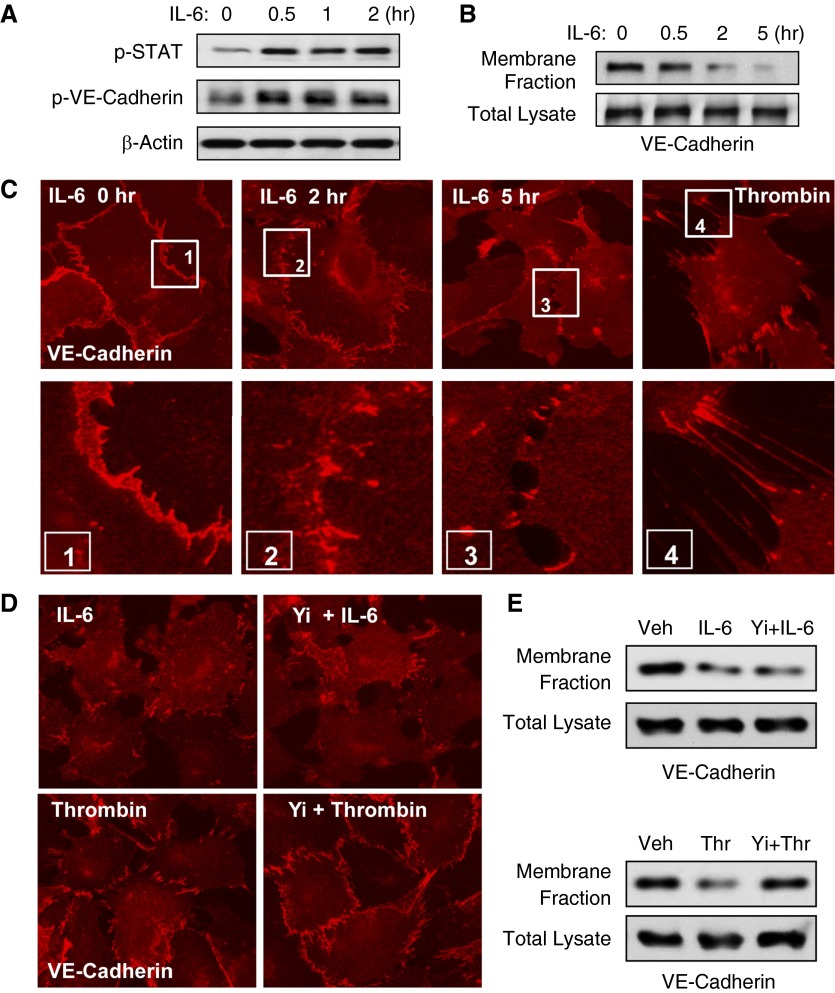
IL-6 activated JAK stress kinase (Figure 5A, upper panel); this activation led to phosphorylation of JAK substrates, STAT and VE-cadherin (Figure 5A, middle panel). IL-6–induced VE-cadherin phosphorylation caused its disappearance from the cell membrane fraction (Figure 5B), which reflects VE-cadherin internalization and disassembly of AJ complexes in IL-6–challenged ECs. Immunofluorescence staining of IL-6–treated ECs showed time-dependent disappearance of the continuous VE-cadherin–positive rim, reflecting disassembly of AJs (Figure 5C). In contrast to uniform VE-cadherin disappearance from the cell junctions of IL-6–stimulated cells, VE-cadherin disappearance in thrombin-stimulated cells was observed at sites of cell retraction (Figure 5C and high-magnification inset), but was still preserved in nonretracting regions. Pretreatment with Rho kinase inhibitor Y-27632 did not affect the IL-6–induced disassembly of VE-cadherin–positive AJs, which was monitored by immunofluorescence staining (Figure 5D) and VE-cadherin disappearance from the membrane fraction (Figure 5E), but inhibited VE-cadherin disappearance from the cell junctions and the membrane fractions of thrombin-stimulated ECs.
Figure 5.
Analysis of adherens junction (AJ) remodeling in response to IL-6. HPAECs were treated with IL-6 and SR (30 ng/ml and 60 ng/ml). (A) Levels of phosphorylated signal transducer and activator of transcription (STAT) and VE-cadherin in the cell lysates were determined by Western blot analysis using specific antibodies. Equal protein loading was confirmed by determination of β-actin content in total cell lysates. (B) Membrane translocation of VE-cadherin was analyzed by Western blot analysis of EC membrane fractions. Lower panel shows Western blot detection of VE-cadherin in total cell lysates. (C) AJ disassembly in control and IL-6/SR–stimulated EC monolayers was analyzed over a 5-hour time period by immunofluorescence staining for VE-cadherin. In contrast, thrombin treatment (0.2 U/ml, 10 min) caused acute disruption of adherence junctions initiated by cell contraction. Higher-magnification insets (boxes 1 to 4) show details of AJ remodeling. (D and E) HPAECs were pretreated with vehicle or Y-27632 (2 µM, 30 min) followed by stimulation with thrombin or IL-6/SR (10 min and 5 h, respectively). (D) AJ disassembly was analyzed by immunofluorescence staining for VE-cadherin. Membrane translocation of VE-cadherin was analyzed by Western blot analysis of the EC membrane fractions. (E) Lower panels in upper and lower gel sets show Western blot detection of VE-cadherin in total cell lysates.
Role of Vinculin in Thrombin- and IL-6–Mediated EC Barrier–Disruptive Effects
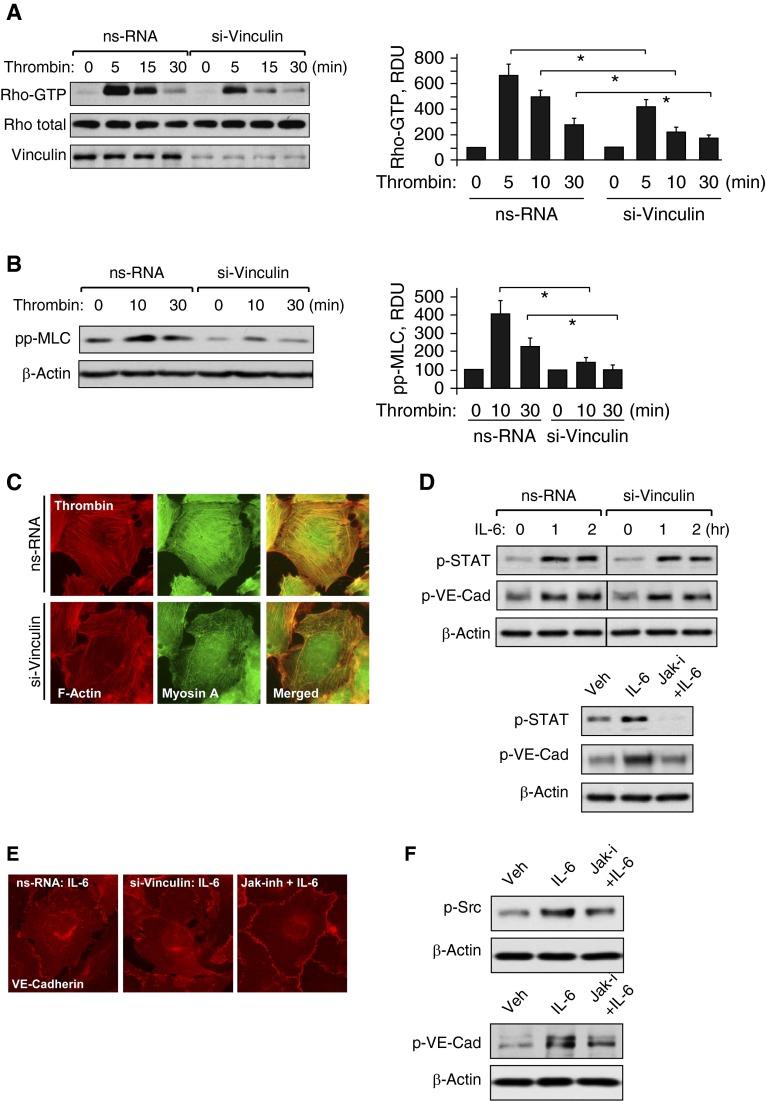
The results of this study suggest the role of vinculin in the contractile mechanism of actomyosin remodeling and EC permeability driven by Rho signaling. The next experiments tested the direct role of vinculin in barrier-disruptive mechanisms activated by thrombin and IL-6. Knockdown of vinculin using gene-specific siRNA dramatically attenuated thrombin-induced activation of Rho (Figure 6A) and phosphorylation of MLC (Figure 6B). Such vinculin-mediated inhibition of Rho signaling caused attenuation of thrombin-induced formation of stress fibers containing F-actin and myosin A (Figure 6C). In contrast, molecular inhibition of vinculin did not affect IL-6–induced phosphorylation of STAT3 and VE-cadherin (Figure 6D). IL-6–induced activation of STAT3, phosphorylation of VE-cadherin and disruption of VE-cadherin AJs was rescued by inhibitor of Jak kinase (Figures 6D and 6E). Further analysis of IL-6–induced signaling leading to VE-cadherin phosphorylation showed that pharmacological inhibition of JAK suppressed IL-6–induced activation of Src (Figure 6F, left panels), suggesting Src to be downstream of JAK. In turn, inhibition of Src activity by PP2 kinase inhibitor abolished IL-6–induced phosphorylation of VE-cadherin (Figure 6F, right panels).
Figure 6.
Effect of vinculin knockdown on cell signaling induced by thrombin and IL-6. Human pulmonary ECs were transfected with vinculin-specific or nonspecific siRNA. (A) Thrombin-induced Rho activation was assessed by RhoGTP pulldown assay. Bar graphs depict quantitative analysis of Western blot data; n = 3; *P < 0.05 versus ns-RNA. (B) Western blot analysis of thrombin-induced MLC phosphorylation. Probing for β-actin was used as a normalization control. Bar graphs depict quantitative analysis of Western blot data; n = 3; *P < 0.05 versus ns-RNA. (C) Cytoskeletal remodeling in response to thrombin was analyzed by double immunofluorescence staining for F-actin (red) and myosin A (green). Areas of actin and myosin colocalization appear in yellow. (D) Western blot analysis of STAT and VE-cadherin (VE-Cad) phosphorylation caused by IL-6 and SR (30 ng/ml and 60 ng/ml) in the cells transfected with nonspecific or vinculin-specific siRNA. In control experiments cells were pretreated with JAK inhibitor I (5 µM, 30 min) before IL-6/SR stimulation. (E) IL-6/SR–induced AJ disassembly in control (left panel) and vinculin-depleted cells (middle panel) was analyzed by immunofluorescence staining for VE-cadherin. Control experiments were performed with JAK inhibitor I (5 µM, 30 min) prior to IL-6/SR treatment (right panel). (F) IL-6/SR–induced (30 ng/ml and 60 ng/ml, 30 min) tyrosine kinase Src phosphorylation in cells pretreated with JAK inhibitor I (5 µM, 30 min) (left panels) and VE-cadherin phosphorylation in ECs pretreated with Src inhibitor PP-2 (2 µM, 30 min; right panels) were evaluated by Western blot.
Role of Vinculin Interaction with Actin Cytoskeleton in Thrombin-Induced Rho Signaling, EC Permeability, and Actomyosin Assembly
Vinculin head region participates in the stabilization of cell adhesion sites independently of actomyosin-mediated tension; however, its interaction with talin is crucial in maintaining this function (13). In contrast, tensile forces mediated by actomyosin are required for conformational changes and activation of native vinculin in living cells (32), which is a critical step in force-induced enhancement of cell adhesion to the substrate. The next experiments tested if vinculin linkage to both the FA complex and actin cytoskeleton is required for full activation of signaling, and cytoskeletal and permeability responses by pulmonary ECs to thrombin and IL-6. Cells were transfected with plasmids encoding the full-length vinculin (vinc-FL) and the truncated vinculin mutant (VNC-880) lacking the C-terminal domain responsible for interaction with the actin cytoskeleton (13).
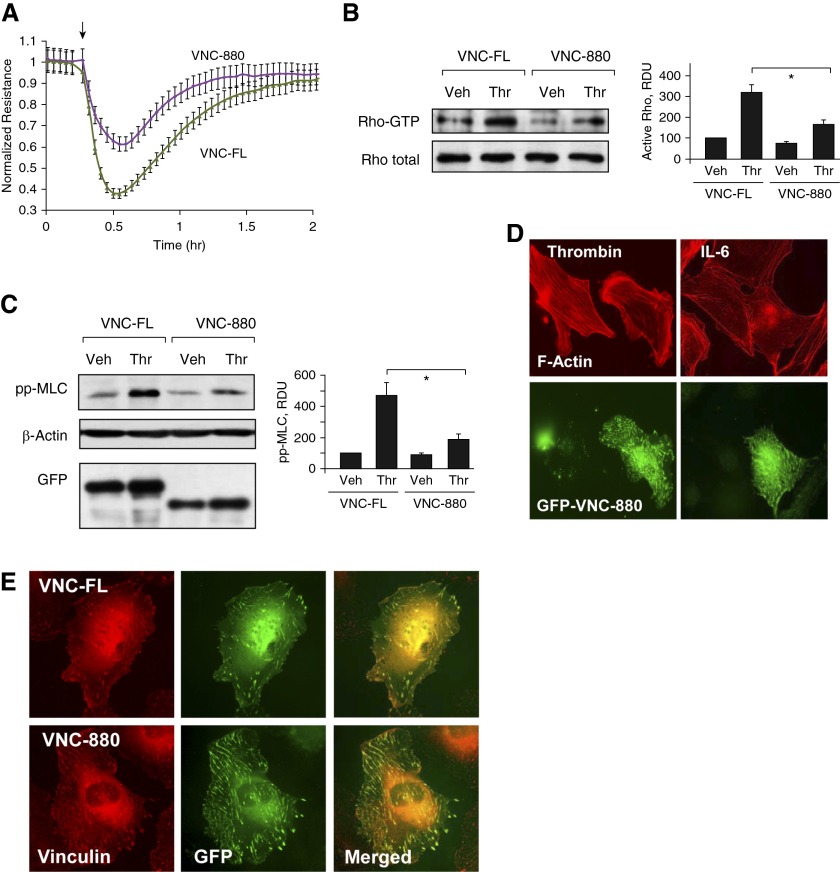
Expression of the VNC-880 mutant with preserved talin-binding domain, but missing actin-binding domain, attenuated thrombin-induced TER decline (Figure 7A), which was linked to suppression of Rho activation (Figure 7B) and MLC phosphorylation in ECs stimulated with thrombin (Figure 7C). Consistent with the observed attenuation of thrombin-induced permeability and signaling response, expression of VNC-880 attenuated thrombin-induced stress fiber formation (Figure 7D, left panels). In contrast, expression of VNC-880 did not affect the F-actin arrangement in the ECs stimulated with IL-6 (Figure 7D, right panels). Immunofluorescence analysis demonstrated colocalization of ectopically expressed recombinant vinc-FL and VNC-880 mutant with endogenous vinculin at the FAs (Figure 7E).
Figure 7.
Effect of VNC-880 mutant on thrombin-induced permeability and Rho signaling. HPAECs were transfected with full-length vinculin (VNC-FL) or vinculin-880 (VNC-880) mutant followed by stimulation with thrombin (0.2 U/ml). (A) TER measurements were performed over the time indicated. Arrow in panel A indicates time point of thrombin. (B and C) Rho activation pulldown assay (B) and Western blot analysis of thrombin-induced MLC diphosphorylation (C). Probing for β-actin was used as a normalization control. Ectopic expression of GFP-tagged wild-type and vinculin-880 mutant in pulmonary ECs was verified by immunoblotting using anti-GFP antibodies. Bar graphs depict quantitative analysis of Western blot data; n = 3; *P < 0.05 versus VNC-FL. (D) Cytoskeletal remodeling in response to thrombin (0.2 U/ml, 10 min) or IL-6 and SR (30 ng/ml and 60 ng/ml, 5 h) was analyzed in VNC-880–transfected ECs by immunofluorescence staining for F-actin. Costaining with green fluorescent protein (GFP) antibody was performed to detect VNC-880–expressing cells. (E) Analysis of VNC-FL and VNC-880 colocalization with endogenous vinculin at FAs. Areas of colocalization appear in yellow.
Discussion
This study tested, for the first time, side by side, the role of FA-associated mechanosensitive protein vinculin in the mediation of EC permeability responses to contractile and noncontractile agonists. The results show the direct involvement of vinculin in the contractile mechanism of agonist-induced EC permeability induced by thrombin. In contrast, vinculin inhibition did not affect EC barrier dysfunction caused by IL-6, which was independent of the Rho pathway and cell contractile forces. Instead, IL-6–induced permeability was associated with phosphorylation and internalization of VE-cadherin, leading to disassembly of AJs, which was not affected by inhibition of Rho-dependent contractile mechanisms. In support of the contractile mechanism of thrombin-induced EC barrier dysfunction, pharmacologic inhibition of Rho kinase or myosin motor activity abolished EC permeability caused by thrombin, but did not affect permeability caused by IL-6. On the other side, inhibition of JAK kinase did not affect permeability caused by thrombin, but abolished VE-cadherin phosphorylation, AJ disassembly and barrier dysfunction caused by IL-6.
The molecular inhibition of vinculin in this study not only suppressed thrombin-induced formation of enlarged FAs linked to actomyosin stress fibers and EC permeability response. Vinculin localization to FAs and transition to the activated state is regulated by external mechanical forces applied to focal contacts (33) or by internal forces generated by actomyosin contraction (14). External or internal actomyosin-generated mechanical strain also triggers Rho signaling at FAs (8, 33, 34), suggesting a role of FAs and vinculin in particular, in feedback regulation of agonist-induced Rho signaling, cell contractility, and EC permeability response.
Interestingly, siRNA-induced vinculin depletion only partially attenuated RhoA GTPase activity, but almost completely blocked the thrombin-induced permeability and stress fiber formation. Primary activation of RhoA by thrombin is mediated by a protease-activated receptor 1 receptor–dependent mechanism (35, 36) and is therefore independent on vinculin. However, thrombin-induced, RhoA-dependent stress fiber formation and internal force generation depends on assembly of FA complexes and triggers a positive feedback mechanism of RhoA activation (8, 34). Vinculin has been proposed to contribute to the mechanical stability under large external forces by regulating contractile stress generation (37). These findings may explain incomplete inhibition of thrombin-induced RhoA activation, but almost complete abrogation of permeability in ECs with vinculin knockdown.
Our results also show that expression of the VNC-880 mutant, which uncouples FA complexes from actomyosin fibers (13), attenuated thrombin-induced Rho signaling and EC permeability response. These results highlight the importance of vinculin as a linker integrating FAs with the actin cytoskeleton and point out the essential role for the vinculin-dependent mechanotransduction module in the development of a full EC permeability response to contractile agonists. Vinculin-mediated FA-actin cytoskeleton linkage was critical for the mediation of the contractile mechanism of EC permeability and FA-dependent activation of Rho signaling. A recent report by Dumbauld and colleagues (38) further supports a role of vinculin in contractile responses by demonstrating absolute requirement for a vinc-FL and activated vinculin molecule for the development of vinculin-dependent traction forces in adherent cells.
In relation to the role of vinculin as a tension-bearing element at the cell junctions, the study by Huveneers and colleagues (39) described VE-cadherin containing focal cell–cell adhesive complexes called focal AJs, which appeared in thrombin-stimulated ECs after disruption of a continuous line of classical AJs surrounding the cell borders. Focal AJs were attached to radial F-actin bundles and shown to contain vinculin (39). These structures do not protect against the acute phase of thrombin-induced permeability, but rather play an important role in EC barrier restoration, as they serve as initiation points for reannealing of AJs and restoration of EC monolayer integrity.
On the other hand, noncontractile mechanisms of EC barrier dysfunction caused by IL-6 were independent of vinculin and were not affected by vinculin knockdown. Instead, IL-6 caused JAK-dependent phosphorylation of Stat and VE-cadherin, VE-cadherin internalization, disassembly of AJs, and increased EC permeability. VE-cadherin internalization is triggered by serine/threonine or tyrosine phosphorylation mediated by p21 activated kinase (40) or Src kinase family (41, 42). The JAK-dependent mechanism of IL-6–induced endothelial permeability involved downstream activation of Src, as Jak inhibitor abolished Src phosphorylation at Y-416, reflecting its activation, whereas both Jak and Src inhibitors suppressed phosphorylation of VE-cadherin (Figure 6F) and VE-cadherin disappearance from cell junctions (data not shown). The precise mechanism of IL-6–JAK–mediated Src activation awaits further investigation. It has been reported that ligation of IL-6R gp130 recruits and activates JAK, which, in turn, phosphorylates and additionally activates gp130 (43). Full activation of gp130 stimulates association of the related tyrosine kinases, Src and tyrosine protein kinase Yes1, which are activated on receptor engagement (44). Experiments with pharmacological kinase inhibitors performed in this study support the JAK–Src mechanism of VE-cadherin phosphorylation in IL-6–stimulated ECs. It is important to note that the noncontractile mechanism of EC permeability described for IL-6 in this study may be applicable for other proinflammatory agonists. For example, Rho-independent, VE-cadherin phosphorylation–dependent permeability responses have been observed in ECs treated with truncated, oxidized phospholipids (45) and platelet-activating factor (46).
The goal of this study was to dissect contractile (modulation of the cytoskeleton) and noncontractile (modulation of the cell junctions) mechanisms of endothelial permeability. Endothelial culture is an ideal model for the separation and molecular analysis of these mechanisms. In turn, the lung endothelial environment in vivo is more complex and includes mechanical forces (shear forces and mechanical strain), circulating barrier-protective and barrier-disruptive agonists, mechanical stretch, pressure, et cetera. These factors lead to a more complex pattern of endothelial response, which will engage both contractile and noncontractile mechanisms. Thus, the translational impact of this study is a demonstration of the necessity of inhibiting the two mechanisms (contractile and noncontractile) to restore lung vascular barrier in contrast to monotherapies with agonists acting solely on EC cytoskeleton or cell junctions.
In summary, this study provides, for the first time, a comparative analysis of contractile and noncontractile mechanisms of agonist-induced EC permeability, and defines a role for vinculin-driven FA–actin cytoskeleton anchoring in the propagation of Rho-dependent EC permeability caused by contractile agonists. The data also show that alteration of vinculin does not affect noncontractile mechanism of vascular endothelial leak. This study expands our understanding of contractile and noncontractile mechanisms of vascular permeability, which might explain the diversity of pathways leading to vascular dysfunction in acute lung injury and other conditions associated with vascular inflammation. The results of this study also suggest the necessity of targeting both contractile and noncontractile mechanisms to restore lung vascular barrier in contrast to monotherapies acting on one mechanism of EC barrier dysfunction.
Supplementary Material
Acknowledgments
Acknowledgments
The authors thank Christopher Ballestrem (University of Manchester, Manchester, UK) for sharing vinculin constructs.
Footnotes
This work was supported by National Heart, Lung, and Blood Institute Public Health Service grants HL76259, HL87283, and HL107920, and National Institute of General Medical Sciences grant GM114171.
Author Contributions: Conception and design—A.A.B. and K.G.B.; analysis and interpretation—A.A.B., A.S.S., Y.T., G.G., N.S., and K.G.B.; drafting the manuscript for important intellectual content—A.A.B., A.S.S., and K.G.B.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0328OC on April 26, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol (1985) 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 2.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 3.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 4.Critchley DR. Focal adhesions—the cytoskeletal connection. Curr Opin Cell Biol. 2000;12:133–139. doi: 10.1016/s0955-0674(99)00067-8. [DOI] [PubMed] [Google Scholar]
- 5.Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 8.Gawlak G, Tian Y, O’Donnell JJ, III, Tian X, Birukova AA, Birukov KG. Paxillin mediates stretch-induced Rho signaling and endothelial permeability via assembly of paxillin-p42/44MAPK-GEF-H1 complex. FASEB J. 2014;28:3249–3260. doi: 10.1096/fj.13-245142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE. Cell shape provides global control of focal adhesion assembly. Biochem Biophys Res Commun. 2003;307:355–361. doi: 10.1016/s0006-291x(03)01165-3. [DOI] [PubMed] [Google Scholar]
- 10.Bakolitsa C, Cohen DM, Bankston LA, Bobkov AA, Cadwell GW, Jennings L, Critchley DR, Craig SW, Liddington RC. Structural basis for vinculin activation at sites of cell adhesion. Nature. 2004;430:583–586. doi: 10.1038/nature02610. [DOI] [PubMed] [Google Scholar]
- 11.Möhl C, Kirchgessner N, Schäfer C, Küpper K, Born S, Diez G, Goldmann WH, Merkel R, Hoffmann B. Becoming stable and strong: the interplay between vinculin exchange dynamics and adhesion strength during adhesion site maturation. Cell Motil Cytoskeleton. 2009;66:350–364. doi: 10.1002/cm.20375. [DOI] [PubMed] [Google Scholar]
- 12.Himmel M, Ritter A, Rothemund S, Pauling BV, Rottner K, Gingras AR, Ziegler WH. Control of high affinity interactions in the talin C terminus: how talin domains coordinate protein dynamics in cell adhesions. J Biol Chem. 2009;284:13832–13842. doi: 10.1074/jbc.M900266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007;179:1043–1057. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carisey A, Tsang R, Greiner AM, Nijenhuis N, Heath N, Nazgiewicz A, Kemkemer R, Derby B, Spatz J, Ballestrem C. Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr Biol. 2013;23:271–281. doi: 10.1016/j.cub.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004;67:64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost. 2010;103:40–55. doi: 10.1160/TH09-06-0403. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds AB, Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene. 2004;23:7947–7956. doi: 10.1038/sj.onc.1208161. [DOI] [PubMed] [Google Scholar]
- 18.Bogatcheva NV, Verin AD. The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc Res. 2008;76:202–207. doi: 10.1016/j.mvr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Leerberg JM, Yap AS. Vinculin, cadherin mechanotransduction and homeostasis of cell–cell junctions. Protoplasma. 2013;250:817–829. doi: 10.1007/s00709-012-0475-6. [DOI] [PubMed] [Google Scholar]
- 21.Villar J, Cabrera NE, Casula M, Flores C, Valladares F, Díaz-Flores L, Muros M, Slutsky AS, Kacmarek RM. Mechanical ventilation modulates TLR4 and IRAK-3 in a non-infectious, ventilator-induced lung injury model. Respir Res. 2010;11:27. doi: 10.1186/1465-9921-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YL, Malik AB, Sun Y, Hu S, Reynolds AB, Minshall RD, Hu G. Innate immune function of the adherens junction protein p120-catenin in endothelial response to endotoxin. J Immunol. 2011;186:3180–3187. doi: 10.4049/jimmunol.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Birukova AA, Tian Y, Meliton A, Leff A, Wu T, Birukov KG. Stimulation of Rho signaling by pathologic mechanical stretch is a “second hit” to Rho-independent lung injury induced by IL-6. Am J Physiol Lung Cell Mol Physiol. 2012;302:L965–L975. doi: 10.1152/ajplung.00292.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singleton PA, Chatchavalvanich S, Fu P, Xing J, Birukova AA, Fortune JA, Klibanov AM, Garcia JG, Birukov KG. Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ Res. 2009;104:978–986. doi: 10.1161/CIRCRESAHA.108.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birukova AA, Moldobaeva N, Xing J, Birukov KG. Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am J Physiol Lung Cell Mol Physiol. 2008;295:L612–L623. doi: 10.1152/ajplung.90236.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubrovskyi O, Birukova AA, Birukov KG. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab Invest. 2013;93:254–263. doi: 10.1038/labinvest.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin–β-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier–protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2007;293:L199–L211. doi: 10.1152/ajplung.00020.2007. [DOI] [PubMed] [Google Scholar]
- 29.Birukova AA, Tian X, Tian Y, Higginbotham K, Birukov KG. Rap–afadin axis in control of Rho signaling and endothelial barrier recovery. Mol Biol Cell. 2013;24:2678–2688. doi: 10.1091/mbc.E13-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tolbert CE, Thompson PM, Superfine R, Burridge K, Campbell SL. Phosphorylation at Y1065 in vinculin mediates actin bundling, cell spreading, and mechanical responses to force. Biochemistry. 2014;53:5526–5536. doi: 10.1021/bi500678x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubashkin MG, Cassereau L, Bainer R, DuFort CC, Yui Y, Ou G, Paszek MJ, Davidson MW, Chen YY, Weaver VM. Force engages vinculin and promotes tumor progression by enhancing PI3K activation of phosphatidylinositol (3,4,5)-triphosphate. Cancer Res. 2014;74:4597–4611. doi: 10.1158/0008-5472.CAN-13-3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011;13:722–727. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells CD, Liu MY, Jackson M, Gutowski S, Sternweis PM, Rothstein JD, Kozasa T, Sternweis PC. Mechanisms for reversible regulation between G13 and Rho exchange factors. J Biol Chem. 2002;277:1174–1181. doi: 10.1074/jbc.M105274200. [DOI] [PubMed] [Google Scholar]
- 36.Birukova AA, Birukov KG, Smurova K, Adyshev D, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 2004;18:1879–1890. doi: 10.1096/fj.04-2328com. [DOI] [PubMed] [Google Scholar]
- 37.Mierke CT, Kollmannsberger P, Zitterbart DP, Smith J, Fabry B, Goldmann WH. Mechano-coupling and regulation of contractility by the vinculin tail domain. Biophys J. 2008;94:661–670. doi: 10.1529/biophysj.107.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dumbauld DW, Lee TT, Singh A, Scrimgeour J, Gersbach CA, Zamir EA, Fu J, Chen CS, Curtis JE, Craig SW, et al. How vinculin regulates force transmission. Proc Natl Acad Sci USA. 2013;110:9788–9793. doi: 10.1073/pnas.1216209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012;196:641–652. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the β-arrestin–dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 41.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G, Maddaluno L, Koh GY, Franco D, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Fuller GM. Phosphorylation and internalization of gp130 occur after IL-6 activation of Jak2 kinase in hepatocytes. Mol Biol Cell. 1994;5:819–828. doi: 10.1091/mbc.5.7.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birukova AA, Starosta V, Tian X, Higginbotham K, Koroniak L, Berliner JA, Birukov KG. Fragmented oxidation products define barrier disruptive endothelial cell response to OxPAPC. Transl Res. 2013;161:495–504. doi: 10.1016/j.trsl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knezevic II, Predescu SA, Neamu RF, Gorovoy MS, Knezevic NM, Easington C, Malik AB, Predescu DN. Tiam1 and Rac1 are required for platelet-activating factor-induced endothelial junctional disassembly and increase in vascular permeability. J Biol Chem. 2009;284:5381–5394. doi: 10.1074/jbc.M808958200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.