Abstract
Dysregulated activation of the inflammasome–caspase-1–IL-1β axis elicits damaging hyperinflammation during critical illnesses, such as pneumonia and sepsis. However, in critical illness models of Salmonella infection, burn, or shock, caspase-1 inhibition worsens outcomes. These paradoxical effects suggest that caspase-1 drives novel protective responses. Whether the protective effects of caspase-1 activation involve canonical immune cell and/or nonimmune cell responses is unknown. The objective of this study was to test the hypothesis that, in addition to its recognized proinflammatory function, caspase-1 initiates protective stress responses in nonimmune cells. In vivo, lung epithelial and endothelial barrier function and inflammation were assessed in mice infected with Pseudomonas aeruginosa in the presence or absence of a caspase-1 inhibitor. Lung endothelial barrier function was assessed ex vivo in isolated, perfused rat lungs infected with P. aeruginosa in the presence or absence of a caspase-1 inhibitor. Endothelial barrier function during P. aeruginosa infection was assessed in vitro in cultured rat wild-type pulmonary microvascular endothelial cells (PMVECs) or recombinant PMVECs engineered to decrease caspase-1 expression. We demonstrated in vivo that caspase-1 inhibition in P. aeruginosa–infected mice ameliorated hyperinflammation, but, counterintuitively, increased pulmonary edema. Ex vivo, caspase-1 inhibition increased pulmonary permeability in P. aeruginosa–infected isolated rat lungs. To uncouple caspase-1 from its canonical inflammatory role, we used cultured rat PMVECs in vitro and discovered that genetic knockdown of caspase-1 accelerated P. aeruginosa–induced barrier disruption. In conclusion, caspase-1 is a sentinel stress-response regulator that initiates proinflammatory responses and also initiates novel response(s) to protect PMVEC barrier function during pneumonia.
Keywords: pulmonary endothelial cells, endothelial permeability, pulmonary edema, acute respiratory distress syndrome, vascular stress responses
Clinical Relevance
Using animal models, we demonstrate that caspase-1 inhibition during Pseudomonas aeruginosa–induced pneumonia leads to decreased inflammation, but, paradoxically, increased lung permeability. Furthermore, we directly demonstrate that cultured pulmonary microvascular endothelial cells (PMVECs), critical determinants of lung barrier integrity, express and activate caspase-1 in response to P. aeruginosa infection to initiate a barrier-protective stress response. Together, our data represent a significant conceptual advance in understanding the multifactorial consequences of caspase-1 activation during critical illness by recognizing that, in addition to an inflammatory role, caspase-1 also plays a protective role in PMVECs, a nonimmune cell phenotype.
Living cells adapt to environmental stressors by transducing danger signals into coordinated stress responses. In the context of critical illnesses, such as pneumonia, stress signaled by localized cellular dysfunction and damage leads to systemic release of damage-associated molecular patterns (DAMPs) (1). These DAMPs, alone or in concert with pathogen-associated molecular patterns (PAMPs) released from an underlying microbial etiology, trigger the innate immune system to activate inflammation (2). Severe inflammatory responses, which devolve into systemic inflammatory response syndrome, sepsis, or septic shock, ultimately drive loss of lung epithelial and endothelial barrier function, leading to alveolar edema and evolution of the acute respiratory distress syndrome (ARDS) (3, 4).
The Inflammasome–caspase-1–IL-1β axis is a prominent signaling pathway through which the innate immune system senses and responds to stress-released DAMPs and PAMPs (5). Thornberry and colleagues (6) first identified caspase-1 as the IL-1β–converting enzyme, a cysteine-active site aspartate-specific protease that processes pro–IL-1β to its active, secreted form. Caspase-1 itself is a proenzyme that, in response to DAMPs and PAMPs, assembles as part of intracellular multiprotein inflammasome complexes to initiate pro–caspase-1 processing. In addition to activating inflammation via pro–IL-1β and pro–IL-18 processing, activated caspase-1 also initiates pyroptosis, a form of programmed cell death marked by rapid release of lactate dehydrogenase (LDH) (5). Dysregulated caspase-1 activation and IL-1β release are well recognized pathophysiological mechanisms associated with hyperinflammation and progression of critical illness (5, 7).
The consequences of stress-induced caspase-1 activation in immune cells and in animal models present an intriguing natural paradox. For example, in mice exposed to uric acid crystal DAMPs or exposed to respiratory tract infection with Pseudomonas aeruginosa, caspase-1 activation leads to exacerbated, deleterious inflammatory effects (8–10). Furthermore, caspase-1 levels are elevated in critically ill patients and correlate with poor outcomes (7). Conversely, mouse models of Salmonella enterica infection, burn, or shock implicate caspase-1 activation as beneficial and protective to the host (11–13). Importantly, a recent study revealed the commonly used caspase-1−/− murine model is also deficient in noncanonical inflammatory responses (caspase-11−/−) (14). Thus, the molecular mechanisms determining whether caspase-1 activation in response to stress elicits either beneficial or deleterious effects to the host remain unresolved. We hypothesized that disparate outcomes associated with stress-induced caspase-1 activation is indicative of unrecognized functions in nonimmune cell phenotypes. We tested this idea using a P. aeruginosa model of pneumonia and ARDS (15) and describe a novel, nonimmune role for caspase-1 in protecting pulmonary microvascular endothelial cell (PMVEC) barrier function. Our results identify caspase-1 as a sentinel stress-response regulator that (1) controls innate inflammation and (2) protects lung endothelial cell barrier function, which, collectively, minimize pulmonary edema and mitigate evolution of pneumonia-induced ARDS.
Materials and Methods
Detailed methods are included as online supplement.
Ethics Statement
Animal infection studies were approved by the University of South Alabama (Mobile, AL) Institutional Animal Care and Use Committee (protocol nos. 694483 and 694922).
Murine Infection Experiments
The model stress response studied here was based on pneumonia and/or sepsis induced by P. aeruginosa infection. We used strain PA103(UT+) encoding exoenzyme effectors ExoU (a phospholipase A2) and ExoT (a dual Rho GTPase–activating protein and ADP-ribosyltranferase) (15, 16).
Effects of caspase-1 inhibition on lung inflammation and lung epithelial and endothelial barrier function in response to P. aeruginosa infection were determined using a mouse infection model. Control treatments consisted of intratracheal saline solution (SS) instillation and intraperitoneal DMSO injection as vehicles of infection and caspase-1 inhibition, respectively. Experimental treatments consisted of PA103(UT+) infection (105 CFU) and caspase-1 inhibitor injection (a substrate mimetic, N-acetyl-L-tyrosyl-L-valyl-N-[(1S)-1-(carboxymethyl)-3-chloro-2-oxopropyl]-L-alaninamide, N-acetyl-tyrosyl-valyl-alanyl-aspartyl chloromethyl ketone [YVAD], at a stock concentration of 370 µM). Animals were allowed to recover from anesthesia and anesthetized again at either 24 or 48 hours for analyses.
Inflammation was measured as total IL-1β in bronchoalveolar lavage (BAL) fluid by ELISA. Total BAL neutrophils were measured by staining and light microscopy. Lung tissue neutrophils were counted by staining and light microscopy of tissues taken from paraffin-embedded sections. Total CFU in homogenized lung tissues was determined by plating and counting.
Lung histology was assessed by hematoxylin and eosin staining, and light microscopy of tissues taken from paraffin-embedded sections. All lungs were prepared under identical conditions to control for effects of fixation. Lung airway compliance was measured in mechanically ventilated mice. Lung epithelial and endothelial permeability was assessed by extravasation of Evan’s blue dye–labeled albumin (EBD-albumin) into the air space (collected in BAL fluid).
Isolated Rat Lung Infection Experiments
Effects of caspase-1 inhibition on lung endothelial barrier integrity in response to P. aeruginosa infection were determined in isolated, perfused rat lungs. Vehicle controls for infection and caspase-1 inhibition were SS and DMSO, respectively. Infections were initiated using 107 total CFU of PA103(UT+) and caspase-1 inhibitor (YVAD) was used at 30 µM. Experiments were initiated by addition of compounds directly to perfusate. Lung hydraulic permeability was measured and expressed as filtration coefficient (Kf), a well established and sensitive pulmonary endothelial permeability index (17, 18).
Cell Culture Infection Experiments
Effects of P. aeruginosa infection on caspase-1 activation were determined in cultured cells. Rat alveolar macrophages and PMVECs were infected with: (1) wild-type PA103(UT+); (2) isogenic mutant PA103(U+) expressing ExoU alone; (3) isogenic mutant PA103(T+) expressing ExoT alone; or (4) isogenic double-mutant PA103(ΔUT) expressing no exoenzymes. Experiments were performed at a multiplicity of infection of 40 bacteria per host cell.
Unprocessed, full-length pro–caspase-1 was detected in cell lysates by Western blotting and normalized to β-actin. Active forms of caspase-1, IL-1β, and IL-18 were detected in culture supernatants by Western blotting and normalized to total infection volume. Active IL-1β was also detected in culture supernatants by ELISA. Effects of caspase-1 activation on cytotoxicity and host cell viability were measured via LDH release assay and 4-[3-(4-iodophenyl)-2-(4-nitro-phenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1) metabolism assays, respectively.
Caspase-1 Knockdown and Transwell Flux Assays
Effects of caspase-1 knockdown on PMVEC barrier integrity in response to P. aeruginosa infection were determined using transwell flux assays. Knockdown was achieved via lentivirus-mediated expression of short hairpin RNAs (shRNAs) targeting the caspase-1 mRNA, and controls included vector only and a scrambled shRNA. Recombinant PMVECs were infected with PA103(UT+) or mutant PA103(ΔUT) at a 40:1 multiplicity of infection, and barrier function was assessed as FITC-dextran (40,000 molecular weight) flux across monolayers growing on transwell filters (0.4-µm pore size, 6.5-mm insert; Corning Inc., Corning, NY).
Statistical Analysis
GraphPad Prism v6.1 (GraphPad Software, Inc., La Jolla, CA) was used for all analyses. Data are reported as mean (±SE). Data were assessed for normality before analysis. Pairwise comparison of parametric data was performed using two-tailed paired or unpaired t test to compare two groups, as indicated. One-way ANOVA was used to compare two or more groups, and Newman-Keuls post hoc test applied as necessary. Differences with a P value less than 0.05 were considered significant.
Results
Inhibiting Caspase-1 in P. aeruginosa–Infected Mice Increases Pulmonary Edema
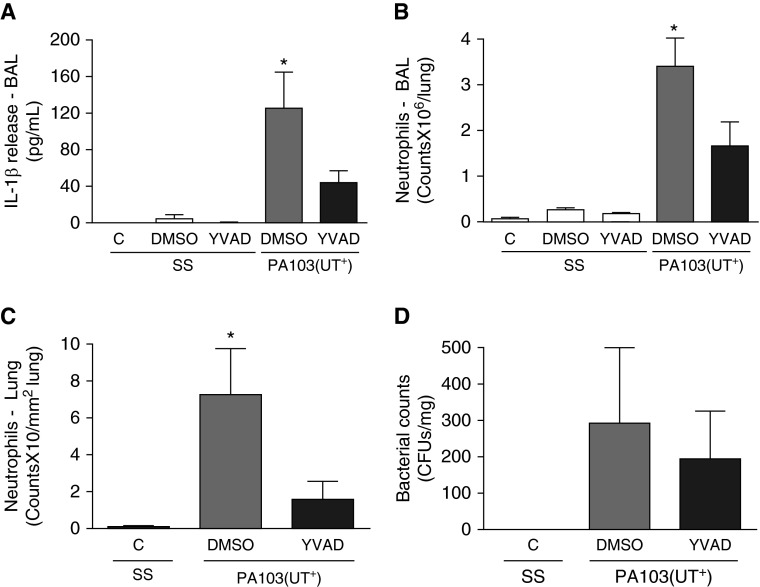
P. aeruginosa is an opportunistic pathogen and a common cause of severe pneumonia, associated with significant morbidity and mortality (19, 20). Here, we tested the potential utility of inhibiting the inflammasome–caspase-1–IL-1β pathway (1, 5) as an immune-modulatory therapy to treat hyperinflammation associated with P. aeruginosa–induced pneumonia. Considering that the available murine caspase-1–/– model is also deficient in the proinflammatory caspase-11 (14), we instead employed a pharmacologic approach to inhibit caspase-1 (13). In our model, murine pneumonia was established via intratracheal administration of P. aeruginosa strain PA103(UT+), which encodes two exoenzyme effectors, namely, ExoU (a phospholipase A2) and ExoT (a dual Rho GTPase–activating protein and ADP-ribosyltranferase). Upon infection, ExoU and ExoT are directly injected into host cell cytoplasm via a type 3 secretion system, where they alter cell physiology and elicit damage (15, 16). Figure 1 shows the effects of caspase-1 inhibition on lung inflammation in response to P. aeruginosa infection at 24 hours after inoculation. As expected, intratracheal instillation of SS alone (infection vehicle control) did not elicit an inflammatory response in mouse lungs, as reflected by a lack of measurable IL-1β in BAL fluid (Figure 1A, C/SS, white bar), lack of neutrophils in BAL fluid (Figure 1B, C/SS, white bar), and lack of neutrophils infiltrating the lung parenchyma and airspaces in tissue sections (Figure 1C, C/SS, white bar). Furthermore, instillation of SS and concomitant intraperitoneal injection of either DMSO (caspase-1 inhibitor vehicle control) or a caspase-1 small molecule inhibitor (YVAD) did not elicit inflammation (Figures 1A–1C, DMSO/SS and YVAD/SS, white bars, respectively). However, instillation of PA103(UT+) in mice concomitantly injected with DMSO elicited significant IL-1β activation (Figure 1A, gray bar) as well as neutrophil infiltration into lung parenchyma and airspaces (Figures 1B and 1C, gray bars). As expected, concomitantly injecting PA103(UT+)-infected mice with a caspase-1 inhibitor (YVAD) significantly reduced IL-1β activation (Figure 1A, black bar) and attenuated neutrophil infiltration into lung parenchyma and airspaces (Figures 1B and 1C, black bars). Importantly, administration of the caspase-1 inhibitor (YVAD) did not change the bacterial burden, as measured by total CFU grown out of lung homogenates (Figure 1D, compare gray and black bars), nor did it have an effect on 48-hour mortality (data not shown). Thus, caspase-1 inhibition during P. aeruginosa infection ameliorates inflammation.
Figure 1.
Caspase-1 inhibition in Pseudomonas aeruginosa–infected mice decreases lung inflammation. Mice were inoculated via intratracheal (I.T.→) route with 15 µl saline solution (SS) or with 105 CFU of P. aeruginosa suspended in 15 µl saline (PA103[UT+]). Concomitantly, mice were injected via intraperitoneal (I.P.→) route with nothing (C), 50 µl of a vehicle control (DMSO), or 50 µl of a caspase-1 inhibitor from a 370-µM stock solution (YVAD). Mice were analyzed at 24 hours after inoculation. (A) Effects of caspase-1 inhibition on IL-1β levels measured in bronchoalveolar lavage (BAL) fluid, where n = 3 mice from each control group (SS/C, SS/DMSO, SS/YVAD), 7 mice from the PA103(UT+)/DMSO experimental group, and 9 mice from the PA103(UT+)/YVAD experimental group. (B) Effects of caspase-1 inhibition on numbers of neutrophils counted in BAL fluid, where n = 7 mice from the SS/C control group, 3 mice from the SS/DMSO control group, 3 mice from the SS/YVAD control group, 7 mice from the PA103(UT+)/DMSO experimental group, and 9 mice from the PA103(UT+)/YVAD experimental group. (C) Effects of caspase-1 inhibition on numbers of neutrophils present in lung parenchyma and airspaces counted in paraffin-mounted tissue sections, where n = 4 mice from the SS/C control group, 4 mice from the PA103(UT+)/DMSO experimental group, and 5 mice from the PA103(UT+)/YVAD experimental group. (D) Effects of caspase-1 inhibition on numbers of viable P. aeruginosa (reported as CFU) recovered from whole-lung homogenates by plating and counting, where n = 3 mice from the SS/C control group, 3 mice from the PA103(UT+)/DMSO experimental group, and 3 mice from the PA103(UT+)/YVAD experimental group. Data are presented as mean ± SEM. *P < 0.05 by one-way ANOVA (Newman-Keuls post hoc test) when comparing within the group. YVAD, N-acetyl-L-tyrosyl-L-valyl-N-[(1S)-1-(carboxymethyl)-3-chloro-2-oxopropyl]-L-alaninamide, N-acetyl-tyrosyl-valyl-alanyl-aspartyl chloromethyl ketone.
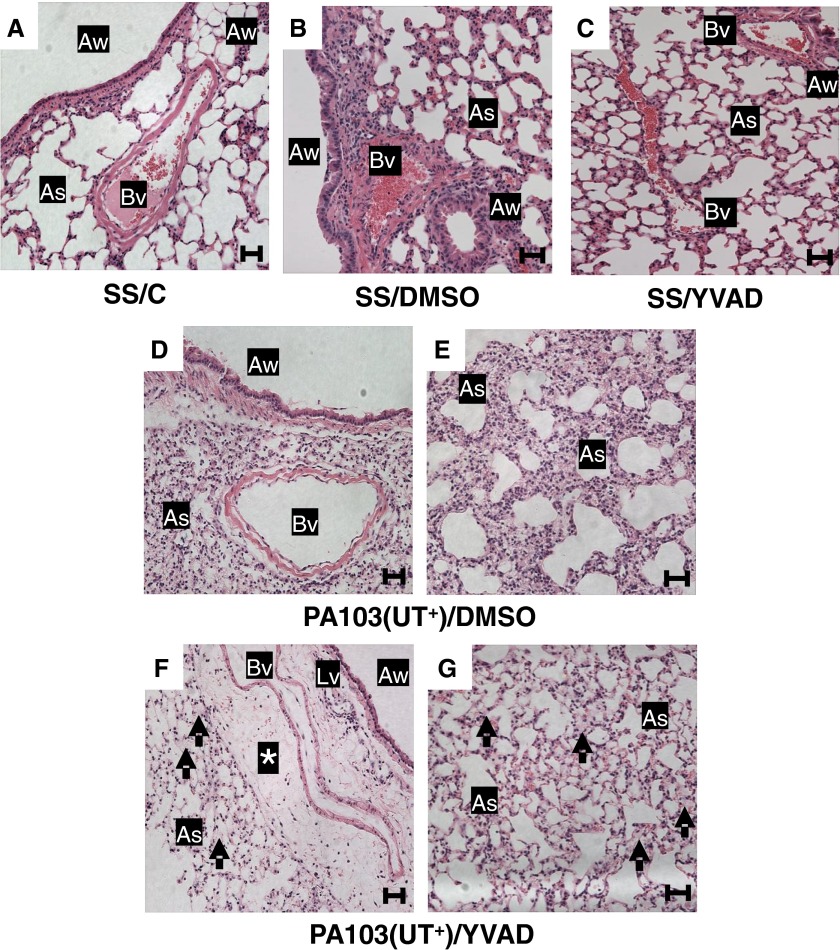
In our experimental model, the natural evolution of P. aeruginosa–induced pneumonia results in pulmonary epithelial and endothelial barrier disruption, interstitial and alveolar edema, and progression to acute lung injury (15, 21–23). Thus, we next examined the effects of caspase-1 inhibition on lung epithelial and endothelial barrier integrity, and lung function in response to P. aeruginosa infection. Histological analyses demonstrated that instillation of SS alone caused no overt damage to the lung, as determined by hematoxylin and eosin staining and light microscopy (Figure 2A, SS/C). Furthermore, instillation of SS and concomitant injection of either DMSO or a caspase-1 inhibitor (YVAD) did not elicit damage (Figures 2B and 2C, SS/DMSO and SS/YVAD, respectively). However, PA103(UT+) infection combined with DMSO injection elicited interstitial thickening and marked cellular infiltrates (Figures 2D and 2E). Unexpectedly, PA103(UT+) infection combined with caspase-1 inhibitor injection (YVAD) elicited interstitial (perivascular cuffing) and alveolar edema (Figures 2F and 2G), despite the expected reduction in cellular infiltrates into the lung parenchyma and airspaces (Figures 1B and 1C). All lungs were perfused and fixed in an identical manner before analyses to minimize the potential for spurious, fixation-induced fluid accumulation in the lungs. Thus, although administration of a caspase-1 inhibitor expectedly ameliorated inflammation (Figures 1A–1C), it paradoxically worsened the pulmonary epithelial and endothelial barrier–disruptive effects of P. aeruginosa infection (Figure 2).
Figure 2.
Caspase-1 inhibition in P. aeruginosa–infected mice increases pulmonary edema. Groups of mice were inoculated intratracheally and injected intraperitoneally, as described in the legend to Figure 1. At 24 hours after inoculation, lungs were harvested under identical conditions, whereby the trachea was tied-off at the peak of inspiration, and the lungs removed and immersed in 10% neutral buffered formalin solution for 48 hours. Subsequently, paraffin-embedded sections were prepared for hematoxylin and eosin staining, and tissues analyzed by light microscopy (20× objective). For all groups, n = 3 mice. Representative images from each group are shown. (A) SS/C control group lungs showed normal architecture, no inflammation, and open airspaces. (B) SS/DMSO control group lungs showed normal architecture, no inflammation, and open airspaces. (C) SS/YVAD control group lungs showed normal architecture, no inflammation, and open airspaces. (D and E) PA103(UT+)/DMSO experimental group lungs from two different fields of view showing the bronchioarterial bundle (D) and the septal network (E). Sections displayed septal thickening and substantial cell infiltration. (F and G) PA103(UT+)/YVAD experimental group lungs from two different fields of view showing the bronchioarterial bundle (F) and the septal network (G). Sections displayed fluid accumulation in perivascular cuffs (asterisk) and fluid accumulation in air spaces (arrows) with a marked decrease in the number of infiltrating cells when compared with PA103(UT+)/DMSO experimental group lungs (D and E). Scale bars: 50 μm. As, air space; Aw, airway; Bv, blood vessel; Lv, lymphatic vessel.
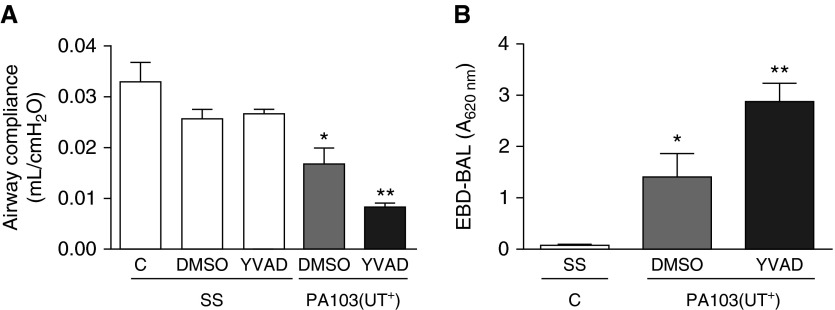
We next quantified the effects of caspase-1 inhibition on pulmonary epithelial and endothelial barrier function during P. aeruginosa infection. As a clinically relevant assessment of lung function, we measured airway compliance, which our group has previously shown to decrease in animal models as lung permeability increases (24, 25). None of the control conditions caused an overt effect on airway compliance (Figure 3A, C/SS, DMSO/SS, and YVAD/SS, white bars). However, PA103(UT+) infection combined with DMSO injection resulted in decreased airway compliance, indicative of pulmonary edema (Figure 3A, gray bar). Intriguingly, PA103(UT+) infection combined with caspase-1 inhibitor (YVAD) injection resulted in a significant, further decrease in airway compliance (Figure 3A, compare gray and black bars).
Figure 3.
Caspase-1 inhibition in P. aeruginosa–infected mice decreases lung compliance and increases lung permeability. Groups of mice were inoculated intratracheally and injected intraperitoneally, as described in the legend to Figure 1, and analyzed 24 hours after inoculation. (A) Effects of caspase-1 inhibition on lung airway compliance, where n = 6 mice from the SS/C control group, 3 mice from the SS/DMSO control group, 3 mice from the SS/YVAD control group, 6 mice from the PA103(UT+)/DMSO experimental group, and 6 mice from the PA103(UT+)/YVAD experimental group. (B) Effects of caspase-1 inhibition on lung permeability measured as the extravasation of systemically perfused Evan’s blue dye conjugated to albumin (EBD-albumin) into the air space, where n = 5 mice from the SS/C control group, 5 mice from the PA103/DMSO experimental group, and 5 mice from the PA103/YVAD experimental group. Ventilated mouse lungs were perfused, in situ, with EBD-albmuin, and lungs were subsequently washed and BAL fluid collected and measured by spectrophotometry, with spectrophotometric absorbance measured at a wavelength of 620 nm (EBD-BAL A620 nm). Data are presented as mean ± SEM. *P < 0.05 by one-way ANOVA (Newman-Keuls post hoc test) for experimental conditions (gray and black bars) compared with control conditions (white bars). **P < 0.05 by one-way ANOVA (Newman-Keuls post hoc test) when comparing within a group.
As a complementary, quantitative approach, we perfused lungs with EBD-albumin and measured its extravasation into the lung interstitium and airspace via collection in BAL fluid. Instillation of SS alone caused no measurable extravasation of EBD-albumin into the airspace (Figure 3B, white bar), whereas PA103(UT+) infection in mice concomitantly injected with DMSO elicited a significant increase in EBD-albumin extravasation, indicating disruption of the pulmonary epithelial and endothelial barrier (Figure 3B, gray bar). Again, PA103(UT+)-infected mice concomitantly injected with a caspase-1 inhibitor (YVAD) displayed a significant increase in pulmonary epithelial and endothelial barrier disruption (Figure 3B, compare gray and black bars). Taken together, our analyses strongly suggest that caspase-1 inhibition during P. aeruginosa infection causes lung barrier disruption via mechanism(s) involving both an inflammatory component and a novel noninflammatory component—that is, direct epithelial and/or endothelial cell dysfunction.
Inhibiting Caspase-1 in P. aeruginosa–Infected Rat Lungs Increases Pulmonary Edema
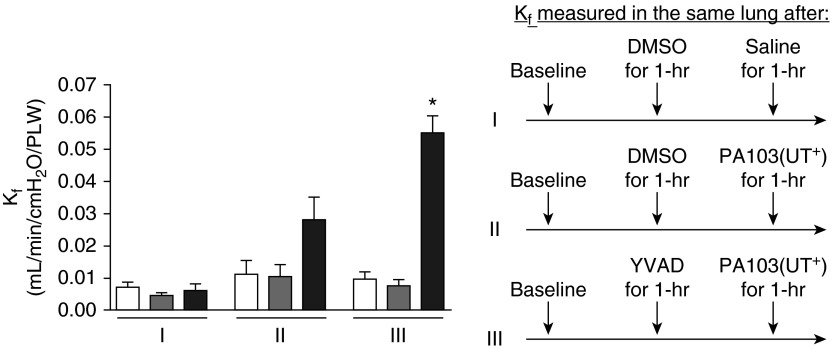
We next tested the effects of caspase-1 inhibition during P. aeruginosa infection using an isolated, perfused rat lung (15). In this model, the heart and lungs are isolated en bloc and perfused in a closed circuit, allowing additions to be made directly to the pulmonary circulation via the perfusate. Thus, here we are testing the effects of inhibiting caspase-1 in the context of P. aeruginosa bacteremia. The lungs are hung in a force transducer and lung hydraulic permeability is measured and expressed as Kf. Any manipulations of the perfusate causing pulmonary endothelial barrier disruption result in increased Kf. (17, 26). An advantage to this model is that circulating immune cells are not present, thus allowing for more refined testing of infection and its effects on pulmonary endothelial barrier function. As another advantage, this experimental protocol allows for serial repeated measures of Kf on the same isolated lung. Figure 4, segment I shows control lungs where a baseline Kf measurement was taken (segment I, white bar), followed by a second Kf measurement taken 60 minutes after addition of DMSO to the perfusate (inhibitor vehicle control, segment I, gray bar). A third Kf measurement was taken on the same lung 60 minutes after addition of SS (infection vehicle control, segment I, black bar). No deleterious effects on pulmonary endothelial barrier function were observed. Figure 4, segment II, shows the barrier-disruptive effect (i.e., increased Kf) of adding the wild-type PA103(UT+) strain to the perfusate and taking measurements along the same time course as described for segment I (compare black bar to white and gray bars). Strikingly, Figure 4, segment III, shows that perfusion of a caspase-1 inhibitor (YVAD) alone had no effect on basal pulmonary endothelial permeability, but significantly worsened (i.e., further increased Kf) the effect of PA103(UT+) infection (compare black bar to white and gray bars in segment III and to the black bar in segment II). To control for effects of exposure time, we performed separate experiments using the protocol described for segment III, except that no bacteria were added at the step before the final Kf measurement. Regardless of exposure time, caspase-1 inhibitor treatment alone never caused pulmonary edema (data not shown). Thus, these data lend further support to a model in which caspase-1 activation in pulmonary endothelial cells protects their barrier function in response to P. aeruginosa infection.
Figure 4.
Caspase-1 inhibition in P. aeruginosa–infected rat lung increases permeability. Endothelial barrier function in isolated rat lungs was measured as hydraulic permeability (filtration coefficient [Kf]). The schematic on the right depicts the serial treatment protocol for lungs assayed in each segment. Segment I: serial Kf measurements taken at baseline (white bar), 1 hour after addition of DMSO (inhibitor vehicle control) to the perfusate at a final concentration of 0.3% (gray bar), and 1 hour after addition of 200 µl saline (infection vehicle control) to the perfusate (black bar). Segment II: serial Kf measurements taken at baseline (white bar), 1 hour after addition of DMSO to the perfusate (gray bar), and 1 hour after addition of 107 CFU of PA103(UT+) suspended in 200 µl saline to the perfusate (black bar, infected control). Segment III: serial Kf measurements taken at baseline (white bar), 1 hour after addition of a caspase-1 inhibitor (YVAD) to the perfusate at a final concentration of 30 µM (gray bar), and 1 hour after PA103(UT+) infection (black bar, experimental). For each segment, n = 3 rat lungs. Data are presented as mean ± SEM. *P < 0.05 by one-way ANOVA (Newman-Keuls post hoc test) when comparing within segment III.
Caspase-1 Is Expressed in In Vitro–Cultured PMVECs, and P. aeruginosa–Secreted Type 3 Exoenzyme Effectors Inhibit Caspase-1 Activation
Our in vivo airway infection and ex vivo bacteremia data indicate that caspase-1 inhibition during P. aeruginosa infection exacerbated pulmonary epithelial and/or endothelial barrier disruption, raising the intriguing prospect that caspase-1 plays a protective role in these cells. PMVECs cover a vast surface area, interface with the entire cardiac output, and are the major contributors to lung endothelial barrier permeability. Thus, to uncouple caspase-1 from its canonical inflammatory role, we used in vitro–cultured PMVECs (27) to elucidate the role of caspase-1 during P. aeruginosa infection.
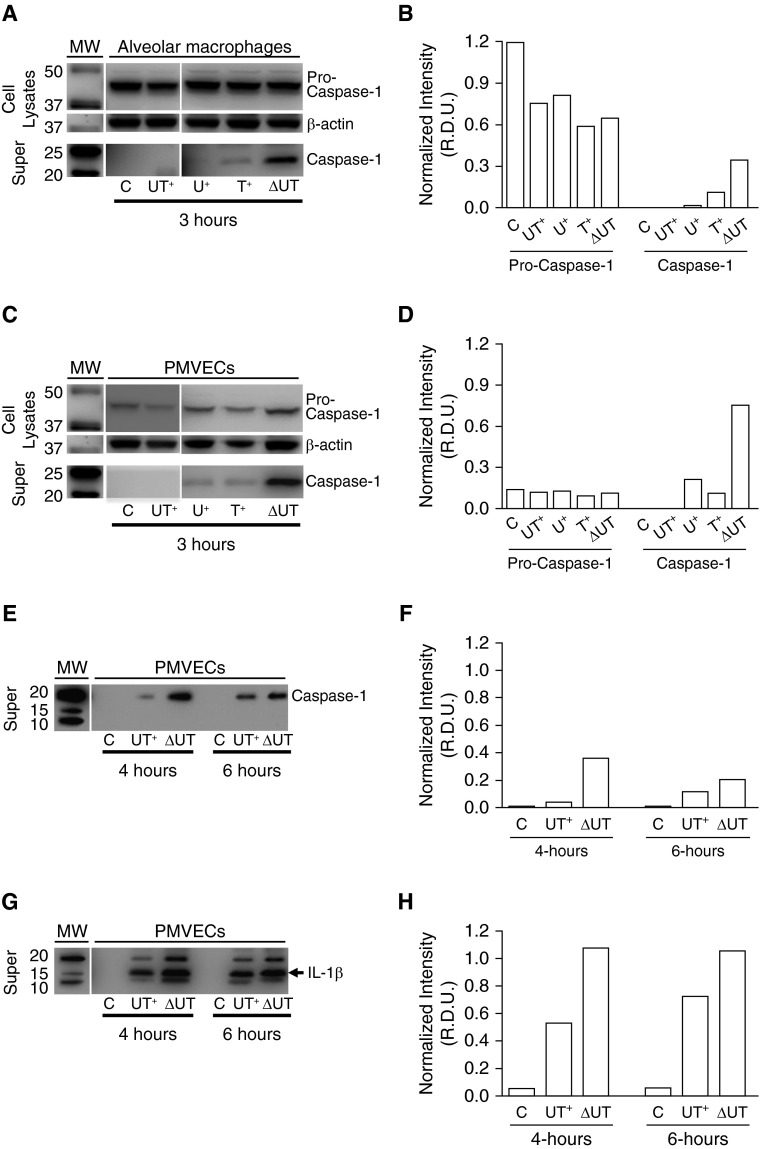
We first confirmed that in vitro–cultured rat PMVECs expressed caspase-1 mRNA (RT-PCR data not shown). Next, we used our previously described infection model (15), in which cultured rat alveolar macrophages (positive control cell line) or PMVECs were inoculated with saline as a control (C) or independently infected with: (1) wild-type PA103(UT+); (2) isogenic mutant PA103(U+) encoding only ExoU; (3) isogenic mutant PA103(T+) encoding only ExoT; or (4) isogenic double-mutant PA103(ΔUT), where both exoenzyme genes are deleted. Cultures were harvested for assay at 3 hours after treatment. Western blots and densitometry in Figures 5A–5D show that pro–caspase-1 (inactive form) was detectable both in macrophage and PMVEC whole-cell lysates under all conditions tested. Intriguingly, infection with the double-mutant PA103(ΔUT) strain resulted in the highest observable levels of activated caspase-1 assessed in culture supernatants (Figures 5A and 5C, bottom panel, labeled “Super”) (28). Infection with PA103 mutants expressing either secreted exoenzyme effector (U+ only or T+ only) resulted in modest caspase-1 activation (Figures 5A and 5C, bottom panel, labeled “Super”). Infection with wild-type PA103(UT+) did not elicit detectable caspase-1 activation at 3 hours after infection (Figures 5A and 5C, bottom panel, labeled “Super”).
Figure 5.
Caspase-1 is activated in cultured rat pulmonary microvascular endothelial cells (PMVECs) in response to P. aeruginosa infection, and exoenzyme effectors ExoU and ExoT delay onset of caspase-1 activation. Rat alveolar macrophages or PMVECs growing in culture were untreated or independently inoculated with different P. aeruginosa strains at a multiplicity of infection (MOI) of 40 bacteria per host cell. At indicated time points after inoculation, cell lysates and clarified culture supernatants (“Super”) were prepared and analyzed by Western blot. Cell lysates were probed with an antibody against caspase-1 and subsequently stripped and reprobed with an antibody against β-actin. Clarified culture supernatants were probed with an antibody against caspase-1 or an antibody against IL-1β. (A) Effects of P. aeruginosa infection on caspase-1 activation in macrophages at 3 hours after inoculation. Macrophages were untreated (C) or inoculated with wild-type PA103(UT+), mutant PA103(U+) expressing only ExoU, mutant PA103(T+) expressing only ExoT, or double-mutant PA103(ΔUT) expressing no exoenzyme effectors. The top panels show expression of the inactive pro–caspase-1 enzyme in whole-cell lysates. The middle panels show β-actin levels as a loading control. The bottom panels show activated caspase-1 released to the culture medium. MW, molecular weight markers. (B) Representative densitometric analysis of macrophage pro–caspase-1 was normalized to β-actin for cell lysates. Active caspase-1 was normalized to sample volume. Data are expressed as relative density units (R.D.U.). (C) Effects of P. aeruginosa infection on caspase-1 activation in PMVECs at 3 hours after inoculation under the untreated and infection conditions described for A above. (D) Representative densitometric analysis of PMVEC pro–caspase-1 was normalized to β-actin for cell lysates. Active caspase-1 was normalized to sample volume. (E) Effects of P. aeruginosa infection on caspase-1 activation 4 and 6 hours after inoculation in PMVECs that were untreated (C) or inoculated with either wild-type PA103(UT+) or double-mutant PA103(ΔUT). (F) Representative densitometric analysis of PMVEC active caspase-1 was normalized to sample volume. (G) Effects of P. aeruginosa infection on IL-1β activation 4 and 6 hours after inoculation in PMVECs that were untreated (C) or inoculated with either wild-type PA103(UT+) or double-mutant PA103(ΔUT). (H) Representative densitometric analysis of PMVEC active IL-1β was normalized to sample volume. (A–H); n = 3 experiments; representative blots and corresponding densitometry are shown.
To determine whether infection with wild-type PA103(UT+) permanently suppressed caspase-1 activation, we extended the infection time course. Interestingly, the extended time course revealed that caspase-1 activation in response to PA103(UT+) infection occurred, but was delayed compared with infection with the double-mutant PA103(ΔUT) strain (Figures 5E and 5F). As a secondary indicator of caspase-1 activation in PMVECs, we assayed for its canonical function, namely, IL-1β activation and secretion to the culture medium. Figures 5G and 5H show infection with either PA103(UT+)- or PA103(ΔUT)-stimulated release of activated IL-1β to the culture medium with PA103(UT+)-infected PMVECs, demonstrating delayed activation. We also observed that PA103(UT+) and PA103(ΔUT) both stimulated IL-1β activation at 3 hours after infection (data not shown). The P. aeruginosa ExoU and ExoS exoenzyme effectors have been previously reported to inhibit caspase-1 activation in macrophages (2, 10), and ours is the first report that the same is true of ExoT. The observed delay in caspase-1 activation in response to infection with P. aeruginosa expressing ExoU was also reported previously in a murine macrophage model (10). It is known that ExoU- and ExoS-mediated inhibition of caspase-1 activation requires their respective enzymatic activities, but the underlying mechanisms remain unknown. Inhibiting caspase-1 activation may confer an evolutionary advantage to the pathogen when colonizing a eukaryotic host by dampening inflammation and remodeling the lung endothelial growth niche.
Caspase-1 Activation in In Vitro–Cultured PMVECs Results in Modest IL-1β and IL-18 Activation and Does Not Induce Pyroptotic Cell Death
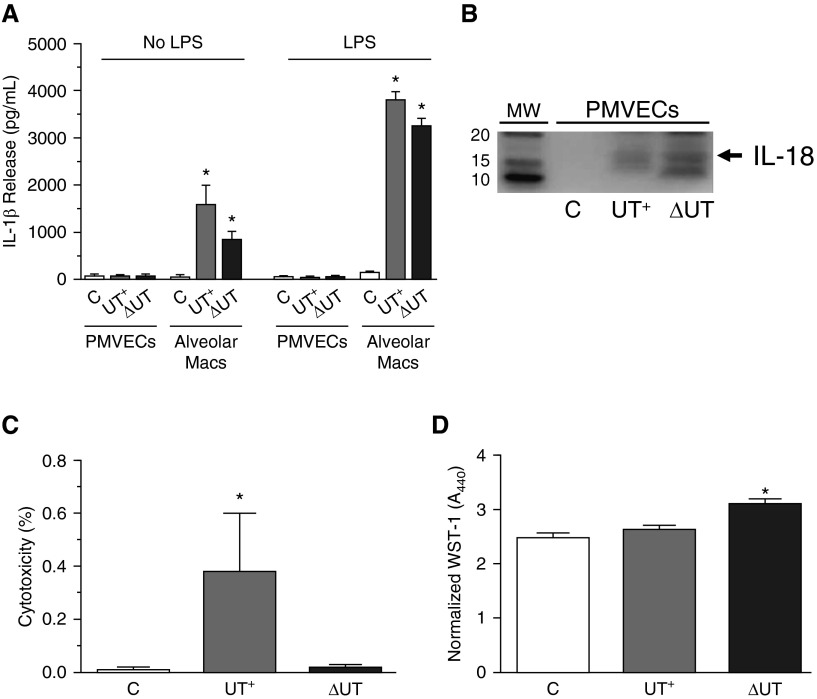
In addition to stimulating processing of pro–IL-1β, the other major consequences of caspase-1 activation in immune cells are processing of pro–IL-18 into its active form, and initiation of rapid programmed cell death (pyroptosis) (29). Figure 6A shows ELISA data comparing the levels of IL-1β activation displayed by P. aeruginosa–infected PMVECs versus macrophages at 3 hours after treatment. Regardless of P. aeruginosa strain background, the absolute levels of IL-1β activation by infected PMVECs were negligible when compared with infected macrophages.
Figure 6.
Caspase-1 activation in PMVECs results in modest IL-1β and IL-18 activation and does not induce pyroptotic cell death. Rat alveolar macrophages or PMVECs growing in culture were untreated or independently inoculated with either P. aeruginosa PA103(UT+) or the isogenic double-mutant PA103(ΔUT) strain at a MOI of 40 bacteria per host cell. Cells were analyzed at 3 hours after inoculation. (A) Culture supernatants from control (C) PMVECs and macrophages were collected and IL-1β activation/secretion was measured by ELISA. Note that the absolute levels of IL-1β secretion by PMVECs were negligible, even in the presence of LPS pretreatment (100 ng/ml, overnight incubation), a critical priming signal known to increase IL-1β and inflammasome component gene expression in macrophages. (B) Clarified culture supernatants from control (C) or infected PMVECs were prepared and analyzed for IL-18 levels by Western blot. (C) Clarified culture supernatants from control (C) or infected PMVECs were analyzed for lactate dehydrogenase (LDH) release into the culture medium as an indicator of cell death. Data represent percent released as normalized to total LDH assayed in the cell monolayer. (D) Control (C) or infected PMVECs were analyzed for metabolism of WST-1 as an indicator of cell viability. Normalized WST-1 conversion was measured spectrophotometrically at A440nm after 1 hour of incubation and corrected for background at A620nm. (A–D) n = 3 experiments. All data represent mean ± SEM. *P < 0.05 by one-way ANOVA (Newman-Keuls post hoc test) when compared within the group. Macs, macrophages; WST-1, 4-[3-(4-iodophenyl)-2-(4-nitro-phenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate.
Importantly, there were no observable differences in PMVECs when infections were performed with or without LPS pretreatment (overnight at 100 ng/ml final concentration), a critical priming signal known to increase expression of ILs and inflammasome components in macrophages (Figure 6A, compare No LPS to LPS treatment). In addition to stimulating IL-1β activation, P. aeruginosa infection of PMVECs also stimulated IL-18 processing and secretion (Figure 6B).
We next determined whether infection-mediated caspase-1 activation in PMVECs induced pyroptotic cell death. At 3 hours after infection, overall PMVEC health was largely unchanged by infection with either the PA103(UT+) or PA103(ΔUT) strain when assayed for cytotoxicity via LDH release to the culture medium (Figure 6C) or metabolism of WST-1 (a redox indicator of cell viability; Figure 6D). The low, but significantly different, amount of LDH release elicited by wild-type PA103(UT+) was most likely due to the documented cytolytic effects of ExoU phospholipase A2 activity (30, 31), and was not likely the result of pyroptosis, as reported in macrophages (5). These data indicate that the observed lung barrier–disruptive effects of caspase-1 inhibition during P. aeruginosa infection are not likely related to the canonical, inflammatory outcomes commonly associated with caspase-1 activation.
Caspase-1 Knockdown in PMVECs Increases the Rate of Barrier Disruption Caused by P. aeruginosa Infection
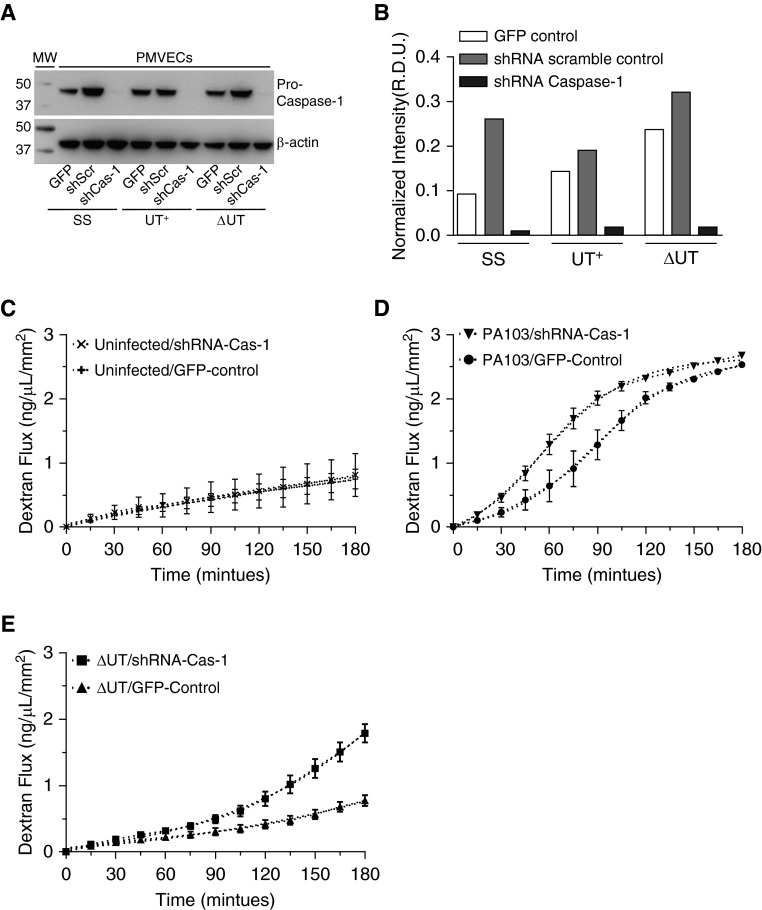
In vivo and ex vivo, caspase-1 inhibition reduced inflammation, but worsened the barrier-disruptive effects of P. aeruginosa infection; however, the specific cell type(s)affected were unknown. The data presented in Figures 5 and 6 show caspase-1 expression and activation in P. aeruginosa–infected PMVECs. Thus, we tested caspase-1 for a novel role in protecting PMVEC barrier function during P. aeruginosa infection. To assay PMVEC barrier function, cells were grown as confluent monolayers on transwell filters to experimentally mimic their in vivo function of forming barriers that limit the flux of large molecules across the monolayer (15). However, we have previously determined that the addition of DMSO (caspase-1 inhibitor vehicle control) adversely affects the integrity of PMVEC monolayers, thus precluding testing of the caspase-1 inhibitor in this functional permeability assay (unpublished data). Therefore, we used shRNA knockdown as an alternative genetic approach to the pharmacological inhibitor (YVAD). To this end, we used lentivirus-mediated transduction and generated three recombinant PMVEC lines: (1) expressing green fluorescent protein 2 from the copepod Pontellina plumata (copGFP) reporter alone (GFP); (2) coexpressing copGFP and a control, scrambled shRNA (shScr); and (3) coexpressing copGFP and two shRNAs targeting the caspase-1 mRNA (shCas-1). Figures 7A and 7B show Western blots for pro–caspase-1 in whole-cell lysates from all three recombinant PMVEC lines under uninfected (SS) or P. aeruginosa–infected (3-h infection) conditions. The shRNA directed against caspase-1 mRNA readily knocked down pro–caspase-1 levels. There were no measureable effects of P. aeruginosa infection in the pro–caspase-1 expression levels. Intriguingly, the caspase-1 knockdown PMVECs still exhibited IL-1β activation in response to P. aeruginosa infection (data not shown), indicating that only very low levels of activated caspase-1 are sufficient to activate IL-1β and/or that PMVECs possess redundant (or compensatory) pathways for IL-1β activation.
Figure 7.
Genetic knockdown of caspase-1 in cultured rat PMVECs increases the rate of barrier disruption during P. aeruginosa infection. Lentivirus-mediated transduction was used to generate three recombinant PMVEC lines: (1) expressing copGFP reporter alone (GFP); (2) coexpressing copGFP and a control, scrambled shRNA (shScr); and (3) coexpressing copGFP and two shRNAs targeting the caspase-1 mRNA (shCas-1). Recombinant PMVECs grown in culture were untreated (SS) or independently inoculated with either P. aeruginosa PA103(UT+) or the isogenic double-mutant PA103(ΔUT) strain at an MOI of 40 bacteria per host cell. Cells were analyzed after inoculation as described subsequently here. (A) At 3 hours after inoculation, cell lysates were collected and probed with an antibody against caspase-1. Blots were subsequently stripped and reprobed with an antibody against β-actin as a loading control. (B) Representative densitometric analysis of recombinant PMVEC pro–caspase-1 levels was normalized to β-actin. Data are expressed as R.D.U. Representative blots and corresponding densitometry are shown. (C) The recombinant control and shCas-1 PMVECs were grown as monolayers on transwell filters, and transendothelial flux of FITC-dextran was measured over a 180-minute time course. Caspase-1 knockdown did not affect PMVEC basal permeability. (D) Transendothelial FITC-dextran flux of recombinant control and shCas-1 PMVECs was assayed during infection with wild-type PA103(UT+). Compared with control PMVECs, caspase-1 knockdown PMVECs displayed an increased rate of dextran flux when infected with wild-type PA103(UT+), as evidenced by a left shift in the flux curve profile. (E) Transendothelial FITC-dextran flux of recombinant control and shCas-1 PMVECs was assayed during infection with PA103(ΔUT). Compared with control PMVECs, caspase-1 knockdown PMVECs displayed an increased rate of dextran flux when infected with PA103(ΔUT), as evidenced by a left shift in the flux curve profile. (A–E) n = 3 experiments. All data represent mean ± SEM. copGFP, green fluorescent protein 2 from the copepod Pontellina plumata; shRNA, short hairpin RNAs.
We next tested the cultured recombinant GFP and shRNA targeting the caspase-1 mRNA-1 PMVEC lines in the transwell macromolecular flux assay (15). Figure 7C shows that caspase-1 knockdown did not affect PMVEC basal permeability rate, as measured using a fluorescently labeled 40-kD dextran reporter. However, in response to infection with wild-type PA103(UT+), caspase-1 knockdown PMVEC monolayers demonstrated a left shift in the dextran flux permeability profile compared with control (Figure 7D), indicating that caspase-1 knockdown increased the rate of barrier disruption. Moreover, in response to infection with the PA103(ΔUT) mutant deleted for both exoenzymes, caspase-1 knockdown PMVECs were also more prone to barrier disruption compared with control PMVECs (Figure 7E). Together, these data suggest that caspase-1 activation in PMVECs is protective by delaying the onset of barrier disruption in response to P. aeruginosa infection.
Discussion
In this prospective, experimental study, we elucidated a novel nonimmune role for caspase-1 as a protective factor of pulmonary barrier function in the setting of P. aeruginosa–induced pneumonia and ARDS. Although administration of a caspase-1 inhibitor to P. aeruginosa–infected mice provided an expected amelioration of hyperinflammation, we also identified an unexpected deleterious effect on lung epithelial and endothelial barrier function. Specifically, P. aeruginosa infection and concomitant inhibition of caspase-1 increased pulmonary edema: histological findings were complemented by quantitative analyses of airway compliance and epithelial/endothelial permeability, which collectively indicated a marked loss of lung barrier function. Moreover, reductionist cell culture and recombinant genetics approaches determined that caspase-1 in PMVECs is activated in response to P. aeruginosa infection, and that caspase-1 is protective of PMVEC barrier function in a cell death–independent manner. Together, these data strongly suggest that caspase-1 activation in response to P. aeruginosa infection mounts an inflammatory response and an inflammation-independent, PMVEC barrier–protective response. A limitation of our study includes the selection of a pharmacological approach to inhibit caspase-1 in animals, which was necessitated by the existing genetic models harboring confounding mutations in proinflammatory pathways (i.e., caspase-1–/– caspase-11–/– double mutant) (14). Furthermore, potential protective roles for caspase-1 in airway epithelial or endothelial cells in other vascular segments (e.g., pulmonary artery, pulmonary vein) were not tested. Finally, mechanism(s) underlying caspase-1–mediated protection of PMVEC barrier function in response to P. aeruginosa infection remain unresolved.
Interestingly, our studies indicate that caspase-1 activation in PMVECs may protect barrier function via mechanism(s) working independently of the known, canonical caspase-1 pathways, namely, activation of IL-1β, IL-18, and pyroptosis (5). In support of this idea, IL-1β is known to elicit endothelial barrier disruption (32) and, thus, caspase-1 inhibition should have been protective rather than deleterious under the conditions tested in the present study. Moreover, it seems unlikely that IL-1β/IL-18 play protective roles, because infection of PMVECs with either wild-type PA103(UT+) or the attenuated virulent PA103(ΔUT) double mutant resulted in modest, but comparable, levels of IL-1β/IL-18 activation (Figures 5G, 5H, and 6B), whereas only wild-type PA103(UT+) elicited dramatic PMVEC barrier disruption (compare Figures 7D and 7E, infection of the GFP-control PMVEC line).
Alternatively, we postulate that the protective mechanism(s) of caspase-1 activation in PMVECs involve novel cellular caspase-1 cleavage substrates. Recent proteomics and bioinformatics studies globally identified enzymes involved in diverse physiological processes, such as glycolysis, as potential substrates for caspase-1 (33, 34). Intriguingly, the global proteomics studies of Shao and colleagues (33) showed cultured macrophages responding to Salmonella infection by up-regulating glycolysis, and that activated caspase-1 cleaved glycolytic enzymes to reduce the amounts of glucose consumed and lactate produced. However, the consequences of caspase-1 acting as a governor of cellular glycolytic flux were not determined. In our vertically integrated model of stress-induced ARDS (15), P. aeruginosa infection stimulates increased glycolytic flux in PMVECs (unpublished data). Moreover, inhibitors of eukaryotic glycolysis partially protect PMVECs from P. aeruginosa infection–induced barrier disruption (unpublished data). Thus, our future studies on PMVEC stress responses will focus on elucidating the protective role of caspase-1 as a governor of cellular glycolytic flux in the context of infection and adaptive response to injury (35).
In a broader context, our studies raise the intriguing notion that caspase-1 activation in response to stress and its consequences to the host involve inflammatory and noninflammatory pathways. It is highly likely that caspase-1 activation during critical illnesses, such as pneumonia and ARDS, is a complex and dynamic process, as highlighted by the fact that elevated caspase-1 is detected in critically ill patients (7). Indeed, the nucleotide oligomerization domain-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome complex has been associated with pleiotropic effects that are either beneficial or deleterious (36). Thus, akin to hormesis, we postulate that caspase-1–mediated stress responses in the lung are governed by the goldilocks effect, whereby too much or too little caspase-1 activation in either the inflammatory and/or noninflammatory pathway ultimately drives deleterious outcomes (37–39). This idea of optimal response windows or response thresholds has been offered as an explanation for the failure of immunomodulatory approaches to treat critical illnesses, such as sepsis and septic shock, whereby too much inflammation is damaging, but too little inflammation impairs pathogen clearance (40). Ultimately, caspase-1 in PMVECs may represent a novel sentinel stress-response regulator, the activation of which protects lung barrier function during critical illness, such as P. aeruginosa–induced ARDS. Future studies engineering molecular control over the magnitude of caspase-1 responses are required to further elucidate the proprotective window of caspase-1 activation in PMVECs, and other nonimmune cell phenotypes.
Supplementary Material
Acknowledgments
Acknowledgments
The authors thank Linn Ayers and the Center for Lung Biology cell culture core (University of South Alabama, Mobile, AL) for providing pulmonary microvascular endothelial cells used in these studies, Dara Frank (Medical College of Wisconsin, Milwaukee, WI) for providing bacterial strains and insights into the project, and Brian Fouty (University of South Alabama, Mobile, AL) for providing critical comments regarding this manuscript.
Footnotes
This work was supported by National Institutes of Health grants HL118334 (D.F.A. and J.P.A.), HL066299-Core B (D.F.A.), and AI085002 (J.P.A.).
Author Contributions: D.F.A. and J.P.A.—conception and design of the work, data acquisition and interpretation, and drafting the manuscript; N.H., A.K., C.Z., and K.O’D.—data acquisition; all authors—critical review of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0386OC on April 27, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulland TK, Ferguson PJ, Sutterwala FS. Evasion of inflammasome activation by microbial pathogens. J Clin Invest. 2015;125:469–477. doi: 10.1172/JCI75254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 4.Ware LB, Matthay MA. Clinical practice:acute pulmonary edema. N Engl J Med. 2005;353:2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 5.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 6.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. A novel heterodimeric cysteine protease is required for interleukin-1 β processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 7.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest. 2013;123:1630–1637. doi: 10.1172/JCI66142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 10.Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menzel CL, Sun Q, Loughran PA, Pape HC, Billiar TR, Scott MJ. Caspase-1 is hepatoprotective during trauma and hemorrhagic shock by reducing liver injury and inflammation. Mol Med. 2011;17:1031–1038. doi: 10.2119/molmed.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osuka A, Hanschen M, Stoecklein V, Lederer JA. A protective role for inflammasome activation following injury. Shock. 2012;37:47–55. doi: 10.1097/SHK.0b013e318234f7ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 15.Audia JP, Lindsey AS, Housley NA, Ochoa CR, Zhou C, Toba M, Oka M, Annamdevula NS, Fitzgerald MS, Frank DW, et al. In the absence of effector proteins, the Pseudomonas aeruginosa type three secretion system needle tip complex contributes to lung injury and systemic inflammatory responses. PLoS One. 2013;8:e81792. doi: 10.1371/journal.pone.0081792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Berre R, Nguyen S, Nowak E, Kipnis E, Pierre M, Quenee L, Ader F, Lancel S, Courcol R, Guery BP, et al. Pyopneumagen Group. Relative contribution of three main virulence factors in Pseudomonas aeruginosa pneumonia. Crit Care Med. 2011;39:2113–2120. doi: 10.1097/CCM.0b013e31821e899f. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4–mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res. 2006;99:988–995. doi: 10.1161/01.RES.0000247065.11756.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharya J. Interpreting the lung microvascular filtration coefficient. Am J Physiol Lung Cell Mol Physiol. 2007;293:L9–L10. doi: 10.1152/ajplung.00148.2007. [DOI] [PubMed] [Google Scholar]
- 19.Hauser AR, Cobb E, Bodi M, Mariscal D, Vallés J, Engel JN, Rello J. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 2002;30:521–528. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, Sawa T, Frank DW, Wiener-Kronish JP. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis. 2001;183:1767–1774. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]
- 21.Lange M, Hamahata A, Enkhbaatar P, Esechie A, Connelly R, Nakano Y, Jonkam C, Cox RA, Traber LD, Herndon DN, et al. Assessment of vascular permeability in an ovine model of acute lung injury and pneumonia-induced Pseudomonas aeruginosa sepsis. Crit Care Med. 2008;36:1284–1289. doi: 10.1097/CCM.0b013e318169ef74. [DOI] [PubMed] [Google Scholar]
- 22.Seeger W, Walmrath D, Neuhof H, Lutz F. Pulmonary microvascular injury induced by Pseudomonas aeruginosa cytotoxin in isolated rabbit lungs. Infect Immun. 1986;52:846–852. doi: 10.1128/iai.52.3.846-852.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teplitz C. Pathogenesis of Pseudomonas vasculitis and septic legions. Arch Pathol. 1965;80:297–307. [PubMed] [Google Scholar]
- 24.Lowe K, Alvarez D, King J, Stevens T. Phenotypic heterogeneity in lung capillary and extra-alveolar endothelial cells: increased extra-alveolar endothelial permeability is sufficient to decrease compliance. J Surg Res. 2007;143:70–77. doi: 10.1016/j.jss.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe K, Alvarez DF, King JA, Stevens T. Perivascular fluid cuffs decrease lung compliance by increasing tissue resistance. Crit Care Med. 2010;38:1458–1466. doi: 10.1097/CCM.0b013e3181de18f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor AE. Capillary fluid filtration: starling forces and lymph flow. Circ Res. 1981;49:557–575. doi: 10.1161/01.res.49.3.557. [DOI] [PubMed] [Google Scholar]
- 27.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res. 2004;67:139–151. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Broz P, Monack DM. Measuring inflammasome activation in response to bacterial infection. Methods Mol Biol. 2013;1040:65–84. doi: 10.1007/978-1-62703-523-1_6. [DOI] [PubMed] [Google Scholar]
- 29.Broz P, Monack DM. Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev. 2011;243:174–190. doi: 10.1111/j.1600-065X.2011.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee VT, Smith RS, Tümmler B, Lory S. Activities of Pseudomonas aeruginosa effectors secreted by the type III secretion system in vitro and during infection. Infect Immun. 2005;73:1695–1705. doi: 10.1128/IAI.73.3.1695-1705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato H, Frank DW. ExoU is a potent intracellular phospholipase. Mol Microbiol. 2004;53:1279–1290. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- 32.Du L, Dong F, Guo L, Hou Y, Yi F, Liu J, Xu D. Interleukin-1β increases permeability and upregulates the expression of vascular endothelial-cadherin in human renal glomerular endothelial cells. Mol Med Rep. 2015;11:3708–3714. doi: 10.3892/mmr.2015.3172. [DOI] [PubMed] [Google Scholar]
- 33.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282:36321–36329. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 34.Shen J, Yin Y, Mai J, Xiong X, Pansuria M, Liu J, Maley E, Saqib NU, Wang H, Yang XF. Caspase-1 recognizes extended cleavage sites in its natural substrates. Atherosclerosis. 2010;210:422–429. doi: 10.1016/j.atherosclerosis.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, Kellum JA. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S, Suh GY, Ryter SW, Choi AM. Regulation and function of the nucleotide binding domain leucine-rich repeat-containing receptor, pyrin domain–containing-3 inflammasome in lung disease. Am J Respir Cell Mol Biol. 2016;54:151–160. doi: 10.1165/rcmb.2015-0231TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alleman RJ, Katunga LA, Nelson MA, Brown DA, Anderson EJ. The “Goldilocks Zone” from a redox perspective—adaptive vs. deleterious responses to oxidative stress in striated muscle. Front Physiol. 2014;5:358. doi: 10.3389/fphys.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calabrese EJ, Mattson MP. Hormesis provides a generalized quantitative estimate of biological plasticity. J Cell Commun Signal. 2011;5:25–38. doi: 10.1007/s12079-011-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudensky AY, Chervonsky AV. A narrow circle of mutual friends. Immunity. 2011;34:697–699. doi: 10.1016/j.immuni.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.