Abstract
Over one-third of lung recipients have preexisting antibodies against lung-restricted antigens: collagen (Col) type V and K-α1 tubulin (KAT). Although clinical studies have shown association of these antibodies with primary graft dysfunction (PGD), their biological significance remains unclear. We tested whether preexisting lung-restricted antibodies can mediate PGD and prevent allotolerance. A murine syngeneic (C57BL/6) or allogeneic (C57BL/6 to BALB/c) left lung transplantation model was used. Rabbit polyclonal antibodies were produced against KAT and Col-V and injected pretransplantation. T cell frequency was analyzed using enzyme-linked immunospot, whereas alloantibodies were determined using flow cytometry. Wet:dry ratio, arterial oxygenation, and histology were used to determine PGD. Preexisting Col-V or KAT, but not isotype control, antibodies lead to dose-dependent development of PGD after syngeneic lung transplantation, as evidenced by poor oxygenation and increased wet:dry ratio. Histology confirmed alveolar and capillary edema. The native right lung remained unaffected. Epitope spreading was observed where KAT antibody treatment led to the development of IL-17–producing CD4+ T cells and humoral response against Col-V, or vice versa. In contrast, isotype control antibody failed to induce Col-V– or KAT-specific cellular or humoral immunity. In addition, none of the mice developed immunity against a non–lung antigen, collagen type II. Preexisting lung-restricted antibodies, but not isotype control, prevented development of allotolerance using the MHC-related 1 and cytotoxic T-lymphocyte-associated protein 4-Ig regimen. Lung-restricted antibodies can induce both early and delayed lung graft dysfunction. These antibodies can also cause spreading of lung-restricted immunity and promote alloimmunity. Antibody-directed therapy to treat preexisting lung-restricted antibodies might reduce PGD after lung transplantation.
Keywords: lung transplant, autoimmunity, rejection, primary graft dysfunction
Clinical Relevance
This research shows that lung-restricted autoimmunity can lead to primary graft dysfunction and prevent development of tolerance after lung transplantation. This work supports the role of testing for lung-restricted autoantibodies in patients undergoing lung transplantation.
The practice of cross-matching before lung and other solid organ transplants detects preexisting antibodies in the recipient against donor human leukocyte antigens (HLAs) (1). Although cross-matching prevents hyperacute rejection in solid organ transplantation, primary graft dysfunction (PGD) after lung transplantation remains very frequent, with a reported incidence of over 50% (2, 3). PGD after lung transplantation occurs within the first 24–72 hours, and is characterized clinically by hypoxemic respiratory failure and histologically by alveolar damage and edema. Although several donor and recipient risk factors have been postulated to contribute to PGD, the pathogenesis remains unknown (4).
Underlying pulmonary disease causes damage to the native lungs, which can reveal cryptic, tissue-restricted self-antigens (sAgs), which are normally protected from the host’s immune system (5). It has recently been shown that T cells specific for lung tissue-restricted sAgs are not deleted by the thymus, but are actively suppressed by thymically derived, antigen-specific forkhead box P3 (Foxp3)+ regulatory T cells (Tregs) (6). Loss of Tregs, for example, by respiratory viral infections, can lead to the expansion of lung tissue–restricted T cells and development of both cellular and humoral lung-restricted autoimmunity (7). We have previously shown that up to one-third of lung allograft recipients have preexisting IgG antibodies against collagen (Col) type V and K-α1 tubulin (KAT), cryptic sAgs present in the normal lungs (8). After lung transplantation, these antibodies are associated with a sevenfold-increased risk of PGD, development of donor-specific HLA antibodies, and chronic lung allograft rejection (bronchiolitis obliterans syndrome [BOS]) (8–12), the predominant cause of poor long-term lung allograft survival (13–15). sAgs, unlike HLA antigens, are nonpolymorphic, and do not differ between individuals within a species. Hence, preexisting antibodies can bind to the allograft when their target sAgs are revealed as a result of ischemia–reperfusion injury at the time of transplantation, leading to humoral rejection (16–18). Therefore, it is plausible that a subset of recipients affected by PGD represent a manifestation of humoral rejection caused by preexisting antibodies directed against sAgs. Because the current cross-match system uses donor lymphocytes that do not express tissue-restricted sAgs (19), these antibodies remain undetected. Here, using the well established murine model of lung transplantation, we investigate whether preexisting lung-restricted antibodies can induce PGD in syngeneic lung grafts and prevent development of allotolerance.
Materials and Methods
Mice and Lung Transplantation
Male C57BL/6 (H-2kb; 12–14 wk old), BALB/c (H-2kd), and CBA/J (H-2kk) were commercially obtained (Jackson Laboratories, Bar Harbor, ME). Orthotopic left lung transplantation was performed using the cuff technique (20). For sham experiments, mice were ventilated for 1 hour (duration of mouse lung transplantation). All animal studies were performed with sterile precautions and approved by the institutional animal studies committee.
Antibodies to KAT and Col-V
Rabbit polyclonal IgG antibodies to KAT and Col-V were produced against KAT and Col-V proteins as previously described (17). Specificity of the antibodies was determined by ELISA with plates coated with purified proteins (Col-V, Col-I, and Col-II; optical densities for: Col-V, 0.863 nm; Col-I, 0.124 nm; and Col-II, 0.109 nm). Purified antibodies were endotoxin free by limulus amebocyte lysate assay. Antibodies were administered intraperitoneally at specific doses before lung transplantation. Rabbit IgG was used as isotype control.
ELISA for Antibodies
Development of antibodies to KAT, Col-V, and Col-II (control) was determined by ELISA as previously described (21, 22). In brief, 1 μg/ml of Col-V, KAT, and Col-II suspended in PBS were coated onto an ELISA plate (Col-II and Col-V were obtained from Chemicon/Millipore [Billerica, MA], and recombinant KAT was expressed in our laboratory) and incubated overnight at 4°C. Diluted murine and normal sera were then added to these plates. Detection was done using anti-human IgG–horseradish peroxidase (1:10,000), developed using tetramethylbenzidine substrate and read at 450 nm. Antibody concentration was calculated using a standard curve from known concentrations of respective antibodies (Santa Cruz Biotechnology, Santa Cruz, CA).
Enzyme-Linked Immunospot for Cellular Immune Response
Cellular immune responses to KAT, Col-V, and Col-II were enumerated using enzyme-linked immunospot (21, 23). All antigens were endotoxin free. Briefly, multiscreen 96-well filtration plates were coated overnight at 4°C with 5.0 μg/ml of capture human cytokine-specific monoclonal antibodies (BD Biosciences, San Jose, CA). The plates were then blocked with 1% BSA for 2 hours. Subsequently, 3 × 105 C57BL/6 splenocytes were cultured in triplicate in the presence of irradiated (3,000 rads) syngeneic stimulators at a 1:1 ratio. The irradiated splenocytes were pulsed with the respective antigens overnight. After 48 hours, the plates were washed and 2.0 μg/ml of biotinylated cytokine-specific monoclonal antibody (BD Biosciences) was added to the wells. After an overnight incubation at 4°C, the plates were developed using horseradish peroxidase–labeled streptavidin (BD Biosciences) and 3-amino-9-ethylcarbazole substrate reagent (BD Biosciences). The spots were analyzed in an ImmunoSpot Series I analyzer (Cellular Technology, Shaker Heights, OH).
Wet:Dry Weight Ratio for Pulmonary Edema
The transplanted left lung was harvested after reperfusion at defined time points, weighed, and then placed at 54°C until a stable dry weight was achieved. The ratio of wet weight to dry weight was then calculated as an indicator of pulmonary edema.
Arterial Oxygen Analysis
To assess left lung graft function, arterial blood gases were measured using at a fraction of inspired oxygen of 100% after the right pulmonary hilum was clamped for 5 minutes. Blood was collected by left ventricular puncture.
Histology
Tissue sections were stained with hematoxylin and eosin and trichrome and analyzed blindly. Images were obtained on a Nikon Eclipse microscope (Nikon, Minato-ku, Tokyo) or Olympus DP-71 (Olympus, Center Valley, PA), and morphometric analysis performed using Nikon Elements software. Analysis was performed on 10 different areas of the lungs, and at least 10 high-powered fields were analyzed in each area.
Statistical Analysis
Data were analyzed in SPSS v.19 (IBM Inc., Chicago, IL) and GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA), and presented as mean (±SEM) with at least five mice in each cohort. χ2 tests, ANOVA, and Student’s t tests were used as appropriate. Results were considered significant at a P value of less than 0.05.
Results
Preexisting Lung-Restricted Antibodies Induce PGD after Syngeneic Lung Transplantation
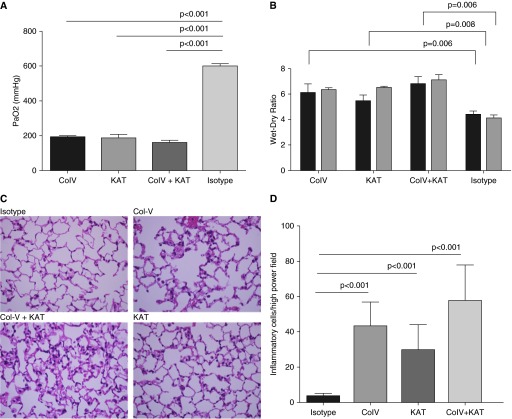
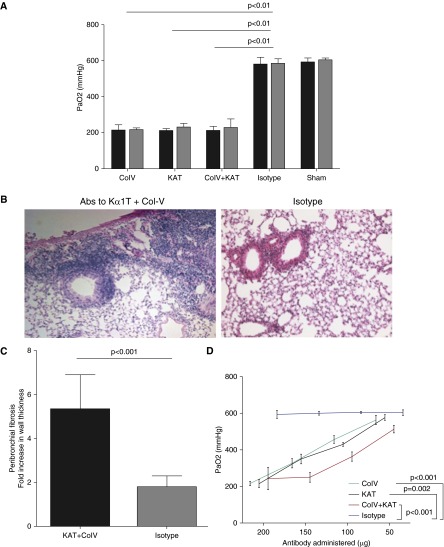
Preexisting antibodies against lung-restricted sAgs Col-V and KAT are associated with the development of PGD, characterized by hypoxemia and pulmonary edema, after human lung transplantation (8, 24). Histologically, PGD is associated with alveolar edema and damage. We tested the role of lung tissue–restricted antibodies against Col-V and KAT in the development of PGD after murine syngeneic lung transplantation. Recipient mice received 200 μg each of Col-V, KAT, Col-V+KAT, or isotype control antibodies daily starting at Day −7 until the day of transplantation. At 24 hours after transplant, arterial blood oxygenation was analyzed after clamping the right hilum. Lungs were harvested for determination of pulmonary edema and histological examination. We found a significant reduction in syngeneic lung graft function with Col-V (PaO2 193 ± 7 mm Hg), KAT (186 ± 20 mm Hg), and Col-V plus KAT (162 ± 11 mm Hg) antibodies, but not isotype control (593 ± 6 mm Hg, P < 0.001; Figure 1A). Each of the transplanted syngeneic grafts in murine recipients of lung-restricted antibodies revealed increased wet:dry ratio, indicative of pulmonary edema at 24 hours (Figure 1B). Histology revealed alveolar damage and edema in recipients treated with lung-restricted antibodies, but not isotype control (Figure 1C). Polymorphonuclear and mononuclear cells were counted in 10 high-power microscopy fields (40×) and averaged for each group. As shown in Figure 1D, recipients that were treated with lung-restricted antibodies revealed marked increase in inflammatory cells compared with those that only received isotype control. Native lungs did not reveal any pathological changes. Taken together, this indicated that lung-restricted antibodies induce graft dysfunction after syngeneic lung transplantation, and that the ischemia–reperfusion injury is necessary for the lung-restricted antigens to be exposed to the circulating antibodies.
Figure 1.
Preexisting lung-restricted antibodies against self-antigens lead to primary graft dysfunction after syngeneic murine lung transplantation. (A) Recipient mice (C57BL/6) received 200 μg each of collagen (Col) type V, K-α1 tubulin (KAT), Col-V plus KAT, or isotype control antibodies daily starting Day −7 until the day of transplantation. At 24 hours after transplant, arterial blood oxygenation was analyzed after clamping the right hilum. Each group consisted of five mice, and the P value between isotype control and each lung-restricted antibody group was less than 0.01. (B) Lungs were harvested for determination of pulmonary edema at 24 (black bars) and 48 (gray bars) hours. Each group consisted of five mice, and the P value between isotype control and each lung-restricted antibody group was less than 0.01. (C) Histology showing capillaritis and alveolar edema in mice treated with lung-restricted antibodies but not isotype control antibodies. (D) Inflammatory cells (both polymorpho- and mononuclear) were counted in 10 high-power fields (40×) and averaged for each group. Recipients treated with lung-restricted antibodies revealed a marked increase in inflammatory cells compared with isotype control. Data are presented as mean (±SEM). PaO2, arterial oxygen pressure.
Preexisting Lung-Restricted Antibodies Induce a Dose-Dependent PGD in Syngeneic Grafts
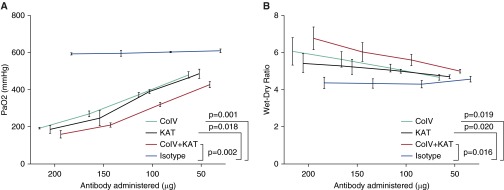
We next investigated whether PGD observed after syngeneic murine lung transplant was dependent on the dose of the lung-restricted antibodies. The mice were injected with varying doses of Col-V, KAT or isotype control antibodies daily starting Day −7 until the day of transplantation. At 24 hours after transplantation, graft function was analyzed by measuring arterial blood gases after clamping the right hilum for 5 minutes. Wet:dry ratios were calculated to determine pulmonary edema. As shown in Figure 2A, Col-V, KAT, or a combination of Col-V and KAT, but not isotype control, antibodies induced a dose-dependent decrease in lung graft function. In addition, lower doses of the lung-restricted antibodies were associated with decreased pulmonary edema (Figure 2B).
Figure 2.
The effect of lung-restricted antibodies is dose dependent. The mice were injected with varying doses of Col-V, KAT, or isotype control antibodies daily starting at Day −7 until the day of transplantation. (A) At 24 hours after transplantation, graft function was analyzed by measuring arterial blood gases after clamping the right hilum for 5 minutes, and (B) wet:dry ratios were calculated at 24 hours to determine pulmonary edema. Data are presented as mean (±SEM).
Preexisting Lung-Restricted Antibodies Perpetuate Lung-Restricted Autoimmunity
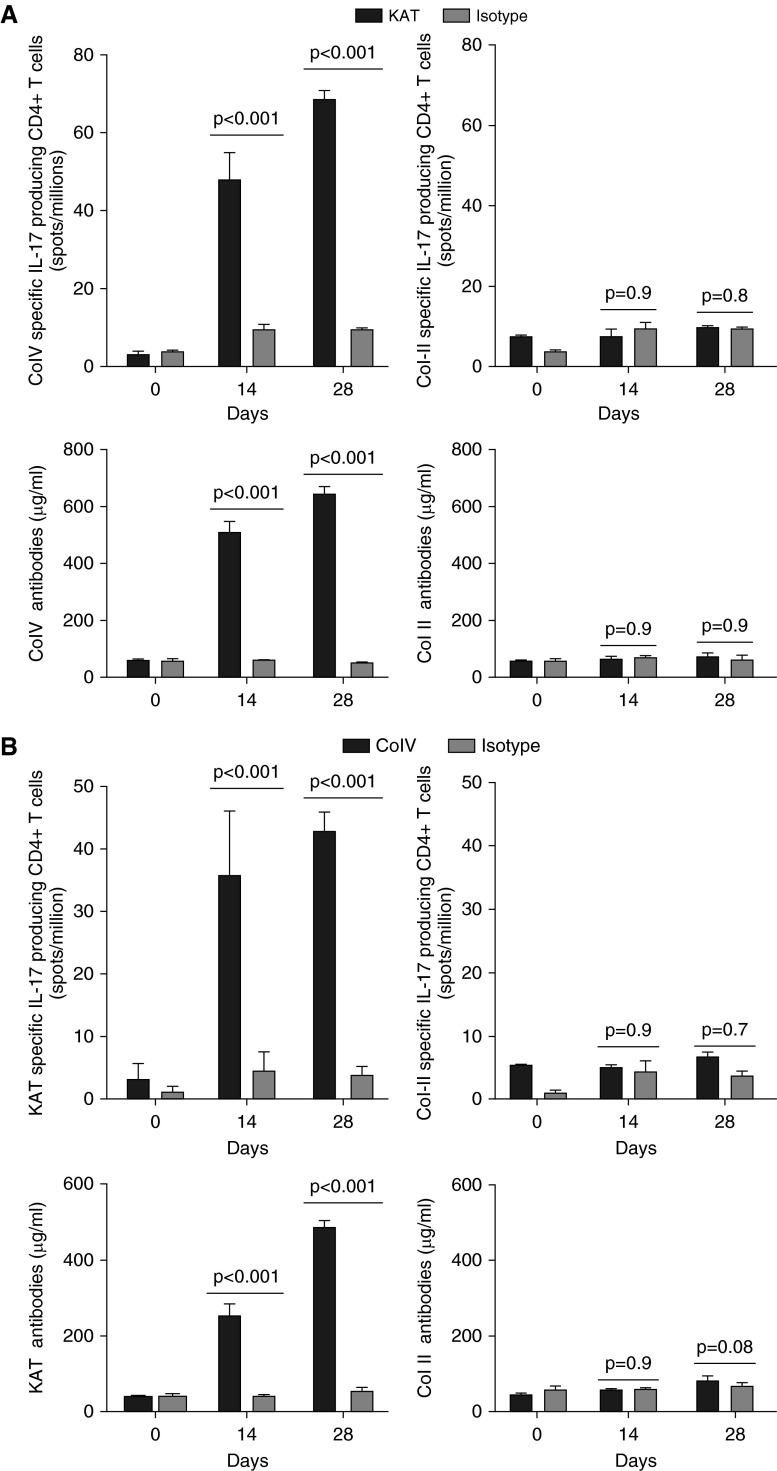
It has been recently demonstrated that T cells restricted to lung sAgs are not deleted by the thymus (6). Hence, we hypothesized that injury by one antibody against lung sAgs might lead to expansion of lung-restricted immunity by exposing the other sAgs in the lungs to the lung-restricted T cells. To investigate this, we treated mice with KAT, Col-V, or isotype control antibodies and serially tested for the development of Col-V (or KAT) specific IL-17–producing CD4+ T cells and de novo Col-V (or KAT) antibodies. We found that KAT antibody treatment led to the development of IL-17–producing CD4+ T cells (Day 28, 68.3 ± 1.5 spots/million; Figure 3A) and humoral response against Col-V (Day 28, 642 ± 16 μg/ml; Figure 3A). In contrast, isotype control antibody failed to induce Col-V–specific cellular (Day 28, 9.3 ± 0.3 spots/million, P < 0.001; Figure 3A) or humoral immunity (Day 28, 48.7 ± 2.9 μg/ml, P < 0.001; Figure 3A). In addition, none of the mice developed immunity against a non–lung antigen, collagen type II (Figure 3). Similarly, administration of Col-V antibodies, but not isotype control, resulted in development of IL-17–producing CD4+ T cells (Day 28, 42.7 ± 1.8 spots/million; Figure 3B) and humoral response against KAT (Day 28, 484 ± 20 μg/ml; Figure 3B).
Figure 3.
Preexisting lung-restricted antibodies perpetuate lung-restricted autoimmunity. Mice were treated with KAT, Col-V, or isotype control antibodies and serially tested for the development of Col-V–specific or KAT-specific IL-17–producing CD4+T cells and de novo Col-V or KAT antibodies after syngeneic lung transplant. (A) Five mice in each group were administered KAT or isotype control antibodies. On Days 0, 14, and 28, mice were killed and splenocytes as well as serum collected and analyzed for Col-V or Col-II–specific IL17–producing CD4+ T cells or de novo Col-V or Col-II antibodies using enzyme-linked immunospot (ELISPOT) and ELISA, respectively. The increase in Col-V–specific CD4+ T cells and Col-V antibodies by injection of KAT antibodies was statistically significant compared with isotype control (P < 0.01). (B) Five mice in each group were administered Col-V or isotype control antibodies. On Days 0, 14, and 28, mice were killed and splenocytes as well as serum collected and analyzed for KAT- or Col-II–specific IL17–producing CD4+ T cells or de novo KAT or Col-II antibodies using ELISPOT and ELISA, respectively. The increase in KAT-specific CD4+ T cells and KAT antibodies by injection of Col-V antibodies was statistically significant compared with isotype control (P < 0.01). Data are presented as mean (±SEM).
Preexisting Lung-Restricted Antibodies Induce Alloimmunity after Murine Lung Transplant
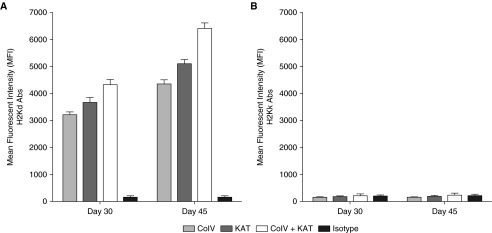
We previously found that human lung transplant recipients with preexisting antibodies against Col-V and KAT are associated with decreased freedom from de novo donor-specific HLA antibodies (8, 24, 25). However, in human patients, there are a number of confounding variables, and we cannot yet conclude that lung-restricted antibodies lead to donor-specific HLA antibodies. Therefore, we tested if antibodies against lung sAgs could induce alloantibodies and prevent development of tolerance in the well established murine lung transplant model. Toward this wild-type donor BALB/c (H2d) lungs were transplanted into C57BL/6 (H2b) recipients. We administered Col-V (200 μg), KAT (200 μg), Col-V plus KAT (200 μg each), or isotype control antibodies on Days −2, −1, 0, 6, 7, and 8, and weekly thereafter until Days 30 and 45. To induce tolerance, we used a combination of MHC-related 1 and cytotoxic T-lymphocyte-associated protein 4-Ig, as previously described (26, 27). Subsequently, development of allospecific antibodies was analyzed using flow cytometry by testing the recipient murine serum against donor or third-party splenocytes. We found that mice that received Col-V, KAT, or Col-V plus KAT, but not isotype control, revealed antibodies against donor major histocompatibility complex class I (anti-H-2kd), as shown in Figure 4A, but not against third-party splenocytes (anti-H-2kk), as evident from Figure 4B.
Figure 4.
Preexisting lung-restricted antibodies induce alloimmunity after murine lung transplantation. Wild-type donor BALB/c lungs were transplanted into C57BL/6 recipients. A combination of MHC-related 1 and cytotoxic T-lymphocyte–associated protein 4-Ig was used to induce tolerance. We administered Col-V (200 μg), KAT (200 μg), Col-V plus KAT (200 μg each), or isotype control antibodies on Days −2, −1, 0, 6, 7, and 8, and weekly thereafter until Post-transplant Days 30 and 45. Subsequently, development of allospecific antibodies was analyzed using flow cytometry by testing the recipient murine serum against (A) donor H-2kd or (B) third-party H-2kk splenocytes on Days 30 and 45. The increase in anti-H-2kd compared with anti-H-2kk was statistically significant (P < 0.01). Data are presented as mean (±SEM). Abs, antibodies.
Preexisting Lung-Restricted Antibodies Induce Allograft Dysfunction and Prevent Development of Tolerance
We next analyzed the allograft function on Days 30 and 45. To this end, the hilum of the native right lung was clamped for 5 minutes, after which we analyzed arterial oxygen content. As shown in Figure 5A, the group of mice that were treated with isotype control antibodies revealed normal oxygenation (Day 30, 578 ± 18 mm Hg; Day 45, 582 ± 13 mm Hg) compared with sham-treated mice (Day 30, 590 ± 10.7; Day 45, 602 ± 6 mm Hg, P = 0.9). In contrast, mice that received Col-V (Day 30, 211 ± 14; Day 45, 213 ± 5 mm Hg), KAT (Day 30, 208 ± 6; Day 45, 227 ± 10 mm Hg), or KAT plus Col-V (Day 30, 209 ± 11; Day 45, 226 ± 22 mm Hg) (P < 0.001 for all) antibodies revealed significantly reduced oxygenation, indicative of poor allograft function. Histology revealed development of peribronchial fibrosis on Day 45 in recipients treated with lung-restricted antibodies, but not isotype control (Figures 5B and 5C). We then performed a dose titration curve on Day 45. The loss of allograft function was dose dependent, as administration of less than 200 μg was associated with less dysfunction in the transplanted lung, as evident in Figure 5D.
Figure 5.
Preexisting lung-restricted antibodies induce allograft dysfunction and prevent development of tolerance. Wild-type donor BALB/c lungs were transplanted into C57BL/6 recipients. A combination of MHC-related 1 and cytotoxic T-lymphocyte–associated protein 4-Ig was used to induce tolerance. We administered Col-V (200 μg), KAT (200 μg), Col-V plus KAT (200 μg each), or isotype control antibodies on Days −2, −1, 0, 6, 7, and 8, and weekly thereafter. (A) On Days 30 (black bars) and 45 (gray bars) arterial blood gases were obtained to analyze allograft function. Sham mice underwent ventilation for 1 hour without lung transplantation. (B and C) Histology revealed developement of peribronchial fibrosis in mice treated with lung-restricted antibodies compared with isotype control. For calculation of peribronchial fibrosis, the average of maximum bronchial wall thickness in 10 high-power fields in mice within each treatment group was divided by average wall thickness in native lungs to determine fold increase. (D) The effects of lung-restricted antibodies were dose dependent. Mice underwent serial administration of lung-restricted antibodies at the time points described previously here. On Day 45, allograft function was analyzed by arterial blood gases. Data are presented as mean (±SEM).
Discussion
Long-term function of lung allografts and patient survival continues to be significantly affected by the development of chronic rejection (BOS) (13–15). Although various risk factors have been postulated, PGD has emerged as one of the most important risks, setting the stage for BOS early after transplantation. The pathogenesis of PGD, however, remains unknown. Antibodies against sAgs have been shown to bind graft epithelial cells, activating proinflammatory and complement cascades (8, 24, 25, 28, 29). Hence, it seems logical to hypothesize that antibodies against lung sAgs could mediate graft dysfunction. Accordingly, we demonstrate here that lung-restricted antibodies can contribute to the pathogenesis of PGD and prevent allotolerance. In addition, antibodies to lung-restricted sAgs can elicit alloimmune responses manifested by development of antibodies against MHC class I as well as cellular immunity to not only the same sAg (i.e., KAT or Col-V), but other lung-associated sAg (Figures 3 and 4). This response was lung restricted, as evident by the absence of immune responses to Col-II, an extracellular matrix sAg (30). Furthermore, other organs, including heart, liver, kidney, and native lung, remained undamaged (data not shown). These findings demonstrate that the effects of the lung-restricted antibodies are not global, but, rather, specific to the transplanted lung alone. We recently demonstrated that de novo lung-restricted antibodies, administered after lung transplantation, can mediate rejection of syngeneic lung grafts and predispose to chronic rejection (17). In the present article, we establish that preexisting lung-restricted antibodies lead to PGD in a dose-dependent manner.
Lung sAgs are usually sequestered, but ischemia–reperfusion injury can reveal these sAgs on the allograft to the immune system (21, 31–33). Hence, preexisting antibodies can bind to the sAgs on the allograft, augmenting the immune response and contributing to the pathogenesis of PGD. In the native lungs, because there is no ischemia–reperfusion and exposure of sAgs, there is no injury. This is supported by earlier studies using rat lung transplantation, where Col-V was found to be released into the bronchoalveolar lavage fluid after ischemia–reperfusion of both syngeneic and allogeneic grafts (34, 35).
The inflammatory response triggered by these antibodies may further perpetuate tissue-restricted immunity. In fact, over 96% of lung recipients will develop de novo antibodies within 3 years after lung transplantation (36). It is not entirely clear how the immune response spreads to other tissue-restricted antigens. We propose that, once antibodies are bound to the sAgs, the antigen-presenting cells can opsonize the membrane on which the sAgs are present. This, in turn, can cause phenotypic switch and activation of immune response to other antigens present on the phagocytized membrane, leading to epitope spreading to other sAgs, and likely to alloantigens. The development of PGD and associated inflammatory response can also lead to infiltration of neutrophils and activation of macrophages (37, 38). These macrophages have an important role for further induction of autoimmune responses. Activation of alveolar macrophages and their switch to an M2 phenotype, as well as interstitial macrophages, have been shown to be critical in the induction of IL-17–secreting cells (39, 40). In addition to macrophages, neutrophils also play a significant role in abrogating tolerance after administration of antibodies specific to lung sAgs. Neutrophil activation in the allograft leads to significant increase in CD4+ T cell activation, as well as increase in IL-17, IFN-γ, and IL-2 in the graft (41). Neutrophils are known to be an important source of IL-6 (42), and the matrix metalloproteinases secreted by neutrophils may influence tissue inflammation, leading to presentation of cryptic sAgs to the immune system. Neutrophil infiltration can also increase local inflammatory factors, including TNF-α and granulocyte colony-stimulating factor (38). These processes can lead to further exposure of sequestered sAgs, causing propagation of lung-restricted autoimmunity (41, 43).
A growing body of evidence supports the presence of lung-restricted immunity in patients with chronic lung disease (8–11, 44). Although the effects of lung-restricted antibodies in non–transplant patients remains unclear, they have been shown to significantly reduce the allograft survival by causing both early (8–11, 18) and chronic lung allograft rejection (45). In our previous study, pretransplant antibodies to KAT or Col-V increased the risk of PGD and BOS (9–11). It is also noteworthy that production of IL-17 in response to sAgs has been shown to be critical in the pathogenesis of chronic lung graft rejection (46), and neutralization of IL-17 prevented the development of obliterative airway disease (OAD), the pathological hallmark of BOS, both in the intrabronchial anti-MHC administration model of OAD and the minor histocompatibility mismatch lung transplant model (21, 47). Th17 cells specific to sAgs play an important role in development of sAg-specific immune responses. Although we specifically did not block Th17, previous studies from our laboratory, and others, have shown that blocking of Th17 can prevent OAD (17, 48). The phenomenon of tissue-restricted immunity leading to organ rejection has also been demonstrated in heart allograft, where antibodies against cardiac myosin have been shown to be pathogenic in chronic heart allograft rejection (49). Furthermore, immunization with cardiac myosin can lead to induction of chronic rejection in a syngeneic heart transplant model (50).
Although, in this study, we determined immune responses against only KAT and Col-V, it is possible that there may be development of autoimmune response against other unknown lung sAgs. The mechanisms by which antibodies against sAgs are induced, or how they act once formed, are not well understood. It is known that T cells against lung-restricted sAgs are not deleted in the thymus and are suppressed by CD4+CD25+Foxp3+ Tregs (6). These Tregs play an important role in both self- and allotolerance (9, 51–57). Tregs can be found in many different tissues, where they play an important role in suppressing inflammation and autoimmune responses (53–56). There is increasing evidence that alteration in cellular regulation of peripheral tolerance plays a crucial role in chronic allograft rejection (51, 52). We have shown that respiratory viruses can induce Treg apoptosis through up-regulation of Fas ligand on the epithelial cells (7). Therefore, ongoing injury from the underlying lung disease combined with loss of Treg can lead to the development of lung-restricted immunity.
In conclusion, this study demonstrates that immune responses to lung-associated sAgs can lead to PGD in murine syngeneic transplants and prevent development of allotolerance. Strategies to detect and deplete such antibodies may represent a novel approach to reduce PGD and subsequent BOS after lung transplantation. Prospective clinical trials are required to investigate the efficacy of antibody-directed therapy in patients with preexisting lung-restricted antibodies in preventing PGD and alloimmunity.
Supplementary Material
Acknowledgments
Acknowledgments
The authors thank Ms. Elena Susan (Northwestern University, Chicago) for the formatting and submission of this manuscript.
Footnotes
This work was supported by grant T32 DK077662, Transplant Scientist Training Program (S.C.), grant HL125940 (matching funds from Thoracic Surgery Foundation), and Gibbon Research Scholarship from the American Association for Thoracic Surgery (A.B.), grant NIA-AG049665, NHLBI-HL071643 (G.R.S.B.), and by grants HL092514 and HL056643 (T.M.).
Author Contributions: A.B., S.C., Z.Z., H.S., and H.P. contributed to the study design, data collection, and data analysis; all authors contributed to manuscript writing; T.M. contributed to data analysis, data collection, and providing reagents; A.Y. reviewed pathology specimens and contributed to data analysis; M.M.D. and G.R.S.B. contributed to data analysis, study supervision, and critical evaluation.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0077OC on May 4, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Campbell P. Clinical relevance of human leukocyte antigen antibodies in liver, heart, lung and intestine transplantation. Curr Opin Organ Transplant. 2013;18:463–469. doi: 10.1097/MOT.0b013e3283636c71. [DOI] [PubMed] [Google Scholar]
- 2.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, Patterson GA, Trulock EP, Hachem RR. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 3.Huang HJ, Yusen RD, Meyers BF, Walter MJ, Mohanakumar T, Patterson GA, Trulock EP, Hachem RR. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant. 2008;8:2454–2462. doi: 10.1111/j.1600-6143.2008.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arcasoy SM, Fisher A, Hachem RR, Scavuzzo M, Ware LB ISHLT Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part V: predictors and outcomes. J Heart Lung Transplant. 2005;24:1483–1488. doi: 10.1016/j.healun.2004.11.314. [DOI] [PubMed] [Google Scholar]
- 5.Valesini G, Gerardi MC, Iannuccelli C, Pacucci VA, Pendolino M, Shoenfeld Y. Citrullination and autoimmunity. Autoimmun Rev. 2015;14:490–497. doi: 10.1016/j.autrev.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Legoux FP, Lim JB, Cauley AW, Dikiy S, Ertelt J, Mariani TJ, Sparwasser T, Way SS, Moon JJ. CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory t cells rather than deletion. Immunity. 2015;43:896–908. doi: 10.1016/j.immuni.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharat A, Kuo E, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Respiratory virus–induced dysregulation of T-regulatory cells leads to chronic rejection. Ann Thorac Surg. 2010;90:1637–1644, discussion 1644. doi: 10.1016/j.athoracsur.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharat A, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90:1094–1101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6:1799–1808. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 10.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, et al. IL-17–dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of K-α1 tubulin–specific antibodies: role in chronic lung allograft rejection. J Immunol. 2008;180:4487–4494. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burlingham W, Wilkes DS, Sullivan JA. Why is the patient out of breath? Collagen V(α1) and K-α1-tubulin take center stage in lung transplantation. Am J Transplant. 2014;14:2201–2203. doi: 10.1111/ajt.12910. [DOI] [PubMed] [Google Scholar]
- 13.Hachem RR, Khalifah AP, Chakinala MM, Yusen RD, Aloush AA, Mohanakumar T, Patterson GA, Trulock EP, Walter MJ. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation. 2005;80:1406–1413. doi: 10.1097/01.tp.0000181161.60638.fa. [DOI] [PubMed] [Google Scholar]
- 14.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, Marchevsky A, Ohori NP, Ritter J, Stewart S, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 15.Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant. 1998;17:1255–1263. [PubMed] [Google Scholar]
- 16.Budding K, van de Graaf EA, Otten HG. Humoral immunity and complement effector mechanisms after lung transplantation. Transpl Immunol. 2014;31:260–265. doi: 10.1016/j.trim.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian V, Ramachandran S, Banan B, Bharat A, Wang X, Benshoff N, Kreisel D, Gelman AE, Mohanakumar T. Immune response to tissue-restricted self-antigens induces airway inflammation and fibrosis following murine lung transplantation. Am J Transplant. 2014;14:2359–2366. doi: 10.1111/ajt.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwata T, Philipovskiy A, Fisher AJ, Presson RG, Jr, Chiyo M, Lee J, Mickler E, Smith GN, Petrache I, Brand DB, et al. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol. 2008;181:5738–5747. doi: 10.4049/jimmunol.181.8.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tait BD, Süsal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, Reed EF, Bray RA, Campbell P, Chapman JR, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 20.Krupnick AS, Lin X, Li W, Okazaki M, Lai J, Sugimoto S, Richardson SB, Kornfeld CG, Garbow JR, Patterson GA, et al. Orthotopic mouse lung transplantation as experimental methodology to study transplant and tumor biology. Nat Protoc. 2009;4:86–93. doi: 10.1038/nprot.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, Mohanakumar T. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiriveedhi V, Gautam B, Sarma NJ, Askar M, Budev M, Aloush A, Hachem R, Trulock E, Myers B, Patterson AG, et al. Pre-transplant antibodies to Kα1 tubulin and collagen-V in lung transplantation: clinical correlations. J Heart Lung Transplant. 2013;32:807–814. doi: 10.1016/j.healun.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaramillo A, Majumder K, Manna PP, Fleming TP, Doherty G, Dipersio JF, Mohanakumar T. Identification of HLA-A3-restricted CD8+ T cell epitopes derived from mammaglobin-A, a tumor-associated antigen of human breast cancer. Int J Cancer. 2002;102:499–506. doi: 10.1002/ijc.10736. [DOI] [PubMed] [Google Scholar]
- 24.Bharat A, Mohanakumar T. Autoimmunity and lung transplantation. Front Biosci (Elite Ed) 2012;4:2378–2388. doi: 10.2741/e549. [DOI] [PubMed] [Google Scholar]
- 25.Bharat A, Kuo E, Steward N, Aloush A, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann Thorac Surg. 2008;86:189–195, discussion 196–197. doi: 10.1016/j.athoracsur.2008.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, Huang HJ, Das NA, Patterson GA, Gelman AE, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007;7:1672–1679. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 27.Krupnick AS, Lin X, Li W, Higashikubo R, Zinselmeyer BH, Hartzler H, Toth K, Ritter JH, Berezin MY, Wang ST, et al. Central memory CD8+ T lymphocytes mediate lung allograft acceptance. J Clin Invest. 2014;124:1130–1143. doi: 10.1172/JCI71359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiriveedhi V, Gelman AE, Mohanakumar T. HIF-1α signaling by airway epithelial cell K-α1-tubulin: role in fibrosis and chronic rejection of human lung allografts. Cell Immunol. 2012;273:59–66. doi: 10.1016/j.cellimm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bharat A, Narayanan K, Street T, Fields RC, Steward N, Aloush A, Meyers B, Schuessler R, Trulock EP, Patterson GA, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83:150–158. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 30.Laurent GJ. Lung collagen: more than scaffolding. Thorax. 1986;41:418–428. doi: 10.1136/thx.41.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludewig B, Zinkernagel RM, Hengartner H. Transgenic animal models for virus-induced autoimmune diseases. Exp Physiol. 2000;85:653–659. [PubMed] [Google Scholar]
- 33.Muñoz LE, Janko C, Schulze C, Schorn C, Sarter K, Schett G, Herrmann M. Autoimmunity and chronic inflammation—two clearance-related steps in the etiopathogenesis of SLE. Autoimmun Rev. 2010;10:38–42. doi: 10.1016/j.autrev.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, Baldridge LA, Heidler KM, Cummings OW, Fujisawa T, et al. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6:724–735. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 35.Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, Woods K, Smith GN, Cummings OW, Heidler KM, et al. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen–specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169:1542–1549. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- 36.Hachem RR, Tiriveedhi V, Patterson GA, Aloush A, Trulock EP, Mohanakumar T. Antibodies to K-α 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am J Transplant. 2012;12:2164–2171. doi: 10.1111/j.1600-6143.2012.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prakash A, Mesa KR, Wilhelmsen K, Xu F, Dodd-o JM, Hellman J. Alveolar macrophages and Toll-like receptor 4 mediate ventilated lung ischemia reperfusion injury in mice. Anesthesiology. 2012;117:822–835. doi: 10.1097/ALN.0b013e31826a4ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, Pless R, Gelman AE, Krupnick AS, Miller MJ. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci USA. 2010;107:18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takenaka M, Tiriveedhi V, Subramanian V, Hoshinaga K, Patterson AG, Mohanakumar T. Antibodies to MHC class II molecules induce autoimmunity: critical role for macrophages in the immunopathogenesis of obliterative airway disease. PLoS One. 2012;7:e42370. doi: 10.1371/journal.pone.0042370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasahara DI, Kim HY, Williams AS, Verbout NG, Tran J, Si H, Wurmbrand AP, Jastrab J, Hug C, Umetsu DT, et al. Pulmonary inflammation induced by subacute ozone is augmented in adiponectin-deficient mice: role of IL-17A. J Immunol. 2012;188:4558–4567. doi: 10.4049/jimmunol.1102363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto S, Nava RG, Zhu J, Huang HJ, Ibrahim M, Mohanakumar T, Miller MJ, Krupnick AS, Kreisel D, Gelman AE. Cutting edge: Pseudomonas aeruginosa abolishes established lung transplant tolerance by stimulating B7 expression on neutrophils. J Immunol. 2012;189:4221–4225. doi: 10.4049/jimmunol.1201683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ericson SG, Zhao Y, Gao H, Miller KL, Gibson LF, Lynch JP, Landreth KS. Interleukin-6 production by human neutrophils after Fc-receptor cross-linking or exposure to granulocyte colony-stimulating factor. Blood. 1998;91:2099–2107. [PubMed] [Google Scholar]
- 43.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 44.Liu M, Subramanian V, Christie C, Castro M, Mohanakumar T. Immune responses to self-antigens in asthma patients: clinical and immunopathological implications. Hum Immunol. 2012;73:511–516. doi: 10.1016/j.humimm.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saini D, Weber J, Ramachandran S, Phelan D, Tiriveedhi V, Liu M, Steward N, Aloush A, Hachem R, Trulock E, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30:624–631. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shilling RA, Wilkes DS. Role of Th17 cells and IL-17 in lung transplant rejection. Semin Immunopathol. 2011;33:129–134. doi: 10.1007/s00281-011-0257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan L, Benson HL, Vittal R, Mickler EA, Presson R, Fisher AJ, Cummings OW, Heidler KM, Keller MR, Burlingham WJ, et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011;11:911–922. doi: 10.1111/j.1600-6143.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saini D, Angaswamy N, Tiriveedhi V, Fukami N, Ramachandran S, Hachem R, Trulock E, Meyers B, Patterson A, Mohanakumar T. Synergistic effect of antibodies to human leukocyte antigens and defensins in pathogenesis of bronchiolitis obliterans syndrome after human lung transplantation. J Heart Lung Transplant. 2010;29:1330–1336. doi: 10.1016/j.healun.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nath DS, Ilias Basha H, Tiriveedhi V, Alur C, Phelan D, Ewald GA, Moazami N, Mohanakumar T. Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. J Heart Lung Transplant. 2010;29:1277–1285. doi: 10.1016/j.healun.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalache S, Dinavahi R, Pinney S, Mehrotra A, Cunningham MW, Heeger PS. Anticardiac myosin immunity and chronic allograft vasculopathy in heart transplant recipients. J Immunol. 2011;187:1023–1030. doi: 10.4049/jimmunol.1004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tosiek MJ, Gruber AD, Bader SR, Mauel S, Hoymann HG, Prettin S, Tschernig T, Buer J, Gereke M, Bruder D. CD4+CD25+Foxp3+ regulatory T cells are dispensable for controlling CD8+ T cell–mediated lung inflammation. J Immunol. 2011;186:6106–6118. doi: 10.4049/jimmunol.1000632. [DOI] [PubMed] [Google Scholar]
- 52.Zhou W, Zhou X, Gaowa S, Meng Q, Zhan Z, Liu J, Li J, Fan H, Liu Z. The critical role of induced CD4+ FoxP3+ regulatory cells in suppression of interleukin-17 production and attenuation of mouse orthotopic lung allograft rejection. Transplantation. 2015;99:1356–1364. doi: 10.1097/TP.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 53.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 54.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 55.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shevach EM. Biological functions of regulatory T cells. Adv Immunol. 2011;112:137–176. doi: 10.1016/B978-0-12-387827-4.00004-8. [DOI] [PubMed] [Google Scholar]
- 57.Mizobuchi T, Yasufuku K, Zheng Y, Haque MA, Heidler KM, Woods K, Smith GN, Jr, Cummings OW, Fujisawa T, Blum JS, et al. Differential expression of Smad7 transcripts identifies the CD4+CD45RChigh regulatory T cells that mediate type V collagen–induced tolerance to lung allografts. J Immunol. 2003;171:1140–1147. doi: 10.4049/jimmunol.171.3.1140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.