Abstract
The stomach of pigs at slaughter age is often colonized by Helicobacter (H.) suis, which is also the most prevalent gastric non-H. pylori Helicobacter (NHPH) species in humans. It is associated with chronic gastritis, gastric ulceration and other gastric pathological changes in both hosts. Parietal cells are highly specialized, terminally differentiated epithelial cells responsible for gastric acid secretion and regulation. Dysfunction of these cells is closely associated with gastric pathology and disease. Here we describe a method for isolation and culture of viable and responsive parietal cells from slaughterhouse pigs. In addition, we investigated the interactions between H. suis and gastric parietal cells both in H. suis-infected six-month-old slaughter pigs, as well as in our in vitro parietal cell model. A close interaction of H. suis and parietal cells was observed in the fundic region of stomachs from H. suis positive pigs. The bacterium was shown to be able to directly interfere with cultured porcine parietal cells, causing a significant impairment of cell viability. Transcriptional levels of Atp4a, essential for gastric acid secretion, showed a trend towards an up-regulation in H. suis positive pigs compared to H. suis-negative pigs. In addition, sonic hedgehog, an important factor involved in gastric epithelial differentiation, gastric mucosal repair, and stomach homeostasis, was also significantly up-regulated in H. suis positive pigs. In conclusion, this study describes a successful approach for the isolation and culture of porcine gastric parietal cells. The results indicate that H. suis affects the viability and function of this cell type.
Introduction
Helicobacter (H.) suis is a Gram-negative bacterium with a typical spiral-shaped morphology, which frequently colonizes the stomach of pigs as well as a minority of humans [1–3]. Indeed, gastric non-H. pylori helicobacters (NHPH) are found in 0.2–6% of gastric biopsies, depending on the study [4], and H. suis is considered to be the most prevalent NHPH in humans [3–5]. In humans, infection with H. suis has been described to cause gastritis, gastric ulceration, as well as gastric mucosa-associated lymphoid tissue (MALT) lymphoma and sporadically gastric adenocarcinoma [6–8]. In naturally infected or experimentally infected pigs, H. suis infection has been shown to cause gastritis, reduced daily weight gain and other gastric pathological changes [9, 10].
The gastric mucosa is composed of various cell types. Parietal (oxyntic) cells are abundant in the fundic gland region. They are responsible for the secretion of gastric acid and play a vital role in the maintenance of the normal structure and function of the gastric mucosa [11]. In some species, including humans, pigs, rabbits and cats, parietal cells can also secrete intrinsic factor which plays an important role in the absorption of vitamins and other nutrients by the small intestine [12]. Hydrogen potassium ATPase (H+/K+ ATPase) is the proton pump composed of a catalytic subunit (α-subunit) and an accessory subunit (β-subunit) in parietal cells, and it mediates secretion of acid into the gastric lumen [11]. Various studies have shown that atrophic gastritis induced by H. pylori infection is characterized by the dysfunction or loss of parietal cells [13, 14]. While H. pylori is mainly observed in the mucus layer or close to mucus-producing cells, H. suis is often observed near or even inside the canaliculi of parietal cells in experimentally infected Mongolian gerbils and mice. Similar observations have been made in humans [15]. Both in rodent models and humans, these parietal cells can show signs of degeneration [15, 16].
Besides H+/K+ ATPase, sonic hedgehog (Shh) is another identified factor playing an important role in the regulation of gastric acid secretion, as well as maturation and differentiation of gastric epithelial cells and fundic glands in mice and humans under normal conditions [17, 18]. It has also been described to play a role in the pathogenesis of H. pylori infection and in the development of gastric cancer [19, 20]. Currently, no information is available on potential effects of H. suis infection on the expression of Shh.
To date, there is no report illustrating the interactions between H. suis and parietal cells in pigs. Therefore, the aim of this study was to examine the direct effects of H. suis on porcine parietal cells, both using a newly developed in vitro parietal cell culture method and tissues from H. suis-infected pigs.
Materials and methods
Collection of pig stomachs
All pig stomachs were collected from 6 month-old slaughter pigs, brought to the laboratory immediately, and kept at 4 °C until further use.
Isolation and culture of primary porcine parietal cells
Pig stomachs were opened, and washed successively several times with water (37 °C) and phosphate buffered saline (PBS; 37 °C). The mucus was removed using a glass slide, and the fundic region of the stomach was collected and kept in ice-cold PBS. The mucosa was separated gently from the underlying tunica submucosa and tunica muscularis, using the sharp side of a scalpel, and minced into small fragments. After washing the minced mucosa several times with PBS (37 °C) and minimal essential medium-glutamax (37 °C) (MEM; Invitrogen, Carlsbad, CA, USA), it was placed in MEM supplemented with dispase (1 mg/mL, Invitrogen) and BSA (5 mg/mL). This mixture was transferred to a tissue culture flask, and the tissue was digested at 37 °C for 25 min on a rotational shaker. The digestion was stopped by three-fold dilution with MEM, and the sample was subjected to centrifugation at 200 g for 10 min. The supernatant was discarded and the tissue was placed in MEM supplemented with collagenase type 1 (2.5 mg/mL, Invitrogen) and BSA (5 mg/mL) and incubated for another 50 min under the same conditions as described above. The resulting mixture was filtered through a 150 µm metal sieve, and centrifuged at 200g for 10 min. The supernatant was removed carefully. The remaining cells were washed with MEM, and then filtered using a 70 and 40 µm cell strainer for two times each. The cell suspension was washed two times in MEM, and further purified using an OptiPrep™ gradient (Sigma-Aldrich St. Louis, MO, USA) according to the procedure described by Chew and Brown [21]. The purified cells were washed in MEM and incubated in cell culture flasks containing medium A [DMEM/F12 (Sigma-Aldrich) supplemented with 20 mM Hepes, 0.2% BSA, 10 mM glucose, 8 nM EGF (Sigma-Aldrich), 1× Insulin, Transferrin, Selenium Solution (ITS) (Invitrogen), 1% penicillin–streptomycin, 50 μg/mL amphotericin B and 25 μg/mL gentamicin (Invitrogen)] for 40 min to eliminate contaminating bacteria and fungi. Subsequently, the cells were washed in DMEM/F12 supplemented with 0.2% BSA and 10 mM glucose, and incubated in medium A without amphotericin B in 24-well flat-bottom cell-culture plates (Greiner Bio-One, Frickenhausen, Germany) containing Matrigel®-coated glass coverslips (circular diameter 12 mm; Thermo Scientific, Leicestershire, UK). To coat these coverslips, Matrigel® basement membrane matrix (Corning B.V. Life Sciences, Amsterdam, LJ, Netherlands) was thawed on ice for at least 12 h. Subsequently, the glass coverslips were coated with Matrigel® matrix, diluted six times in ice-cold sterile water, and left to dry in a laminar air flow over night.
Activation of parietal cells and visualization of gastric acid secretion
Twelve hours after seeding of parietal cells on coverslips, the medium was replaced by fresh medium. In order to stimulate cells to secrete HCl, they were incubated in medium supplemented with histamine (400 μM; Sigma-Aldrich) and 3-isobutyl-1-methylxanthine (IBMX) (30 μM; Sigma). Control cells were held in a resting state by administering cimetidine (100 μM; Sigma-Aldrich). After 30 min of incubation at 37 °C, cells were incubated in medium A without amphotericin B and supplemented with 2 µM LysoSensor™ Yellow/Blue DND-160 (Invitrogen) and 2 µM Cell Tracker Red CMTPX (Invitrogen) at 37 °C for 30 min. Subsequently, cells were washed 3 times, immediately mounted in a small volume of PBS (50% glycerol, v/v) on glass slides at room temperature, and analyzed using a confocal microscopy within 30 min.
Preparation of H. suis and bacterial lysate
H. suis strain HS5cLP was grown on Brucella agar (BD, Franklin Lakes, NJ, USA) plates with a pH of 5 and supplemented with 20% fetal calf serum (HyClone), 5 mg/L amphotericin B (Fungizone; Bristol-Myers Squibb, Epernon, France), Campylobacter selective supplement (Oxoid, Basingstoke, UK) and Vitox supplement (Oxoid) under microaerobic and biphasic conditions (37 °C; 85% N2, 10% CO2, 5% O2) as described elsewhere [22]. This strain was isolated in 2008 from the stomach of a slaughterhouse pig [23]. Bacterial lysate was prepared as described previously [24].
Treatment of parietal cells and determination of cell viability
Parietal cells were cultured as described above in fresh medium without antibiotics and amphotericin B. Parietal cells were inoculated with viable H. suis bacteria at a multiplicity of infection (MOI) of 100 or 200 or with whole bacterial lysate at a final concentration of 100 µg/mL or 200 µg/mL in 24-well plates. Parietal cells incubated with Hank’s buffered salt solution (HBSS) with Ca2+ and Mg2+ (Gibco, Life Technologies, Paisley, Scotland) were used as controls. For the first 4 h, incubation was done at 37 °C under microaerobic conditions, after which the cells were transferred to normal conditions (5% CO2) for another 20 h. Parietal cell viability was determined using the neutral red (3-amino-7-dimethylamino-2-methyl-phenazine hydrochloride) uptake assay as described previously with some minor modifications [25]. Briefly, 400 μL of pre-warmed neutral red solution (33 μg/mL in DMEM without phenol red) was added to each well and the plate was incubated at 37 °C for 3 h. The cells were then washed twice with HBSS. Two hundred microliter of extracting solution [ethanol/water/acetic acid, 50/49/1 (v/v/v)] was added to each well to release the dye, and the plate was shaken for another 30 min. The absorbance was then read at 540 nm with a microplate ELISA reader (Multiscan MS, Thermo Labsystems, Helsinki, Finland). The percentage of viable cells was estimated using the following formula:
with a = OD540 derived from the wells incubated with live bacteria or lysate, b = OD540 derived from blank wells, c = OD540 derived from untreated control wells.
Indirect immunofluorescent staining
Cultured parietal cells treated as described above were fixed with 4% paraformaldehyde in PBS for 15 min at room temperature. After fixation, the cells were washed three times with PBS, and permeabilized with 0.3% Triton X-100 in PBS (2% BSA) for 20 min followed by incubation in PBS (2% BSA) for another 30 min. The cells were washed 3 times with PBS. Subsequently, cells were incubated with a primary mouse monoclonal anti-H+/K+ ATPase β-subunit antibody (1/200; Abcam Ltd, Cambridge, UK) and a polyclonal rabbit anti-H. pylori antibody (1/320; Dako, Glostrup, Denmark) for 1 h at 37 °C, followed by an Alexa Fluor 633-conjugated goat anti-mouse secondary antibody (1/200; Invitrogen) and Alexa Fluor 488-conjugated goat anti-rabbit IgG (1/100; Invitrogen) for 1 h at 37 °C. All antibodies were diluted in PBS and the cells were washed 5 times after incubation with the primary and secondary antibodies. Incubation for 15 min with DAPI (0.5 μg/mL; Sigma) was performed to counterstain the nuclei and the cells were rinsed 5 times in PBS. Stained cells were mounted in ProLong ® Gold antifade reagent medium (Invitrogen) and imaged by an Olympus BX61 fluorescence microscope (Olympus Belgium N.V.).
Immunohistochemical (IHC) and immunofluorescent staining of pig gastric tissue slides
Stomachs from slaughterhouse pigs were opened along the greater curvature. For detection of H. suis colonization, a small piece of tissue from the fundic region of the stomach was collected, followed by DNA extraction and H. suis-specific Quantitative Real-Time PCR (qRT-PCR) as described previously [26].
Gastric samples from the fundic gland zone were fixed in 10% phosphate-buffered formalin, processed by routine methods and embedded in paraffin. Consecutive sections of 5 µm were cut, and IHC staining for the identification and visualization of parietal cells was performed with these sections as described previously [16]. Immunofluorescent staining was also performed to visualize co-localization of parietal cells and H. suis. Briefly, 5 μm formaldehyde-fixed tissue sections were deparaffinized in xylene and rehydrated in graded ethanol. Sections were boiled in antigen retrieval solution (850 W, 1.5 min; 300 W, 10 min) and washed respectively for 15 min in water and 5 min in PBS. Sections were permeabilized with 0.3% TritonX-100 in PBS (2% goat serum) for 15 min, and incubated in PBS (10% goat serum) for 45 min. Tissue sections were incubated with a primary mouse monoclonal anti-H+/K+ ATPase β-subunit antibody (1/3125; Abcam) and a polyclonal rabbit anti-H. pylori (1/320; Dako) antibody overnight at 4 °C. After washing with PBS, sections were incubated for 1 h with secondary Alexa Fluor 633 goat anti-mouse IgG (1/100; Invitrogen) and Alexa Fluor 488 goat anti-rabbit IgG (1/100; Invitrogen). DAPI (0.5 μg/mL) was used to counterstain the nuclei. Tissue sections were washed extensively with PBS, mounted in ProLong® Gold antifade reagent medium and examined by fluorescence or confocal microscopy.
RNA extraction, reverse transcription and qRT-PCR
qRT-PCR was used to compare gene expression levels of gastric tissue from H. suis negative pigs (n = 15) and H. suis positive pigs (n = 15). RNA was extracted and cDNA was prepared as described previously [22]. Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The concentration of RNA was measured using a NanoDrop spectrophotometer (Isogen Life Science, PW De Meern, Utrecht, The Netherlands). The purity of the RNA was evaluated with the Experion automated electrophoresis system using StdSens RNA chips (Bio-Rad, Hercules CA, USA). The RNA concentration from all samples was adjusted to 1 μg/μL and cDNA was synthesized immediately using the iScript™ cDNA Synthesis Kit (Bio-Rad).
The housekeeping genes ACTB, Cyc-5 and HPRT were included as reference genes (Bosschem et al. unpublished data). Primers for Atp4a were referenced elsewhere [27], and primers for Shh were designed based on the conserved complete or partial coding sequences of Shh available for humans, pigs, mice and rats on GenBank. The mRNA expression levels of reference genes and target genes were quantified using SYBR Green based RT-PCR with iQ™ SYBR Green Supermix. Reactions were performed using a CFX96 RT PCR System in a C1000 Thermal Cycler (Bio-Rad). qRT-PCR was performed as described elsewhere [22]. Sequence information of the primers is shown in Table 1.
Table 1.
Primers used in qRT-PCR
| Gene | Primer | Sequence (5′-3′) | Reference |
|---|---|---|---|
| Atp4a | Sense | GCATATGAGAAGGCCGAGAG | [27] |
| Antisense | TGGCCGTGAAGTAGTCAGTG | ||
| Sonic hedgehog | Sense | TGACCCCTTTAGCCTACAAGCA | This study |
| Antisense | TGGGGGTGAGTTCCTTAAATCG |
Results
Activation of parietal cells and stimulation of gastric acid secretion
Cultured parietal cells responded to stimulation with histamine/IBMX, as shown by the presence of more and bigger vacuoles observed by light microscopy (data not shown). In order to confirm the secretion of gastric acid by parietal cells after stimulation, a fluorescent acidic pH indicator, LysoSensor, was loaded both to resting and stimulated parietal cells.
An accumulation of LysoSensor was observed in the stimulated parietal cells, characterized by a strong yellow fluorescence (Figure 1B). Parietal cells in resting stage also showed several small areas with weak yellow fluorescence, indicating that vacuoles in parietal cells had a basal acid production (Figure 1A). Upon the stimulation by histamine/IBMX, an increase in the fluorescence intensity of LysoSensor in the vacuoles was observed and the size of the vacuoles was increased as well (Figure 1B), indicating the enhancement of gastric acid secretion.
Figure 1.

Acid secretion by parietal cells. LysoSensor™ Yellow/Blue DND-160 was used to monitor the acid secretion by live parietal cells incubated either by Cim or His/IBMX. Cell Tracker Red CMTPX was used to track all live cells. Some apical vacuoles of parietal cells in resting stage revealed several small areas with weak yellow fluorescence, indicating basal acid secretion (A). Parietal cell treated with His/IBMX showed an expansion of apical vacuoles, as shown by strongly enlarged zones with yellow fluorescence, indicating the continuous secretion of acid (B). Cim: Cimitidine; His: histamine; IBMX: 3-isobutyl-1-methylxanthine.
H. suis bacteria interact with cultured parietal cells
Immunofluorescence staining showed adhesion of H. suis to parietal cells after incubation of cells with H. suis at an MOI of 10 or 100:1 for 6 h (Figure 2). Longer incubation time (12 h) and a higher MOI (200) exhibited similar results (data not shown).
Figure 2.

The presence of H. suis near or inside the cultured parietal cell. Cultured parietal cells were inoculated with live H. suis at an MOI of 10 for 6 h, and a close relationship between H. suis (green) and parietal cells (red) could be observed. Cell nuclei were stained by DAPI (blue). Representative fluorescence micrographs were shown. Scale bars: 50 µm. MOI: multiplicity of infection.
Cell viability assay
A neutral red assay was used to determine the effect of live H. suis bacteria and whole cell lysate of H. suis on parietal cell viability. Parietal cells were treated with live bacteria or bacterial lysate for 24 h. Compared to untreated control cells, a significant decrease of cell viability was observed in live bacteria-treated cells (MOI: 100, 200) and lysate-treated cells (200 µg/mL) (Figure 3, p < 0.05), confirming that both live bacteria and lysate can affect parietal cell viability in vitro.
Figure 3.
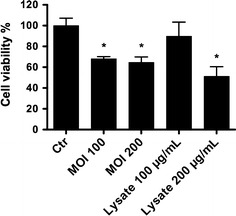
Effect of H. suis on parietal cell viability. Parietal cells were treated with live H. suis (MOI: 100:1, 200:1) or whole bacterial lysate (100 µg/mL, 200 µg/mL), and control cells were treated with HBSS. After 24 h, cell viability was determined by a neutral red assay. Results of one representative experiment (out of 3 performed in total) are shown (n = 5). An * represents a statistically significant difference between bacteria or lysate treated cells and HBSS treated cells (Student t test, p < 0.05). MOI: multiplicity of infection; HBSS: Hank’s buffered salt solution.
Interaction between H. suis and porcine parietal cells in vivo
IHC staining did not reveal a clear change of parietal cell numbers in the stomach of H. suis-infected pigs compared to H. suis-negative pigs (data not shown). However, a close relationship between parietal cells and H. suis was observed in the fundic region of the pig stomach (Figure 4A), and H. suis bacteria could also be observed amidst the debris of parietal cells (Figure 4A, right panel). In order to further investigate the co-localization of parietal cells and H. suis, a double immunofluorescence staining for H+/K+ ATPase and H. suis was performed. Confocal microscopic analysis showed that the majority of the bacteria were observed in the vicinity of or inside the canaliculi or cytoplasm of parietal cells (Figure 4B).
Figure 4.
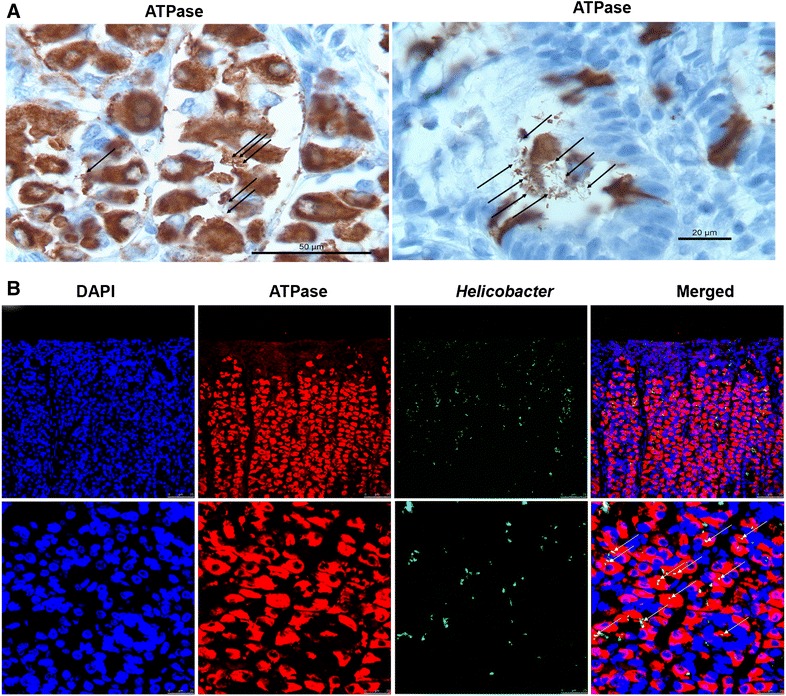
Co-localization of H. suis and porcine parietal cells in the stomach from slaughterhouse pigs. Shown are representative IHC (A) and fluorescent micrographs (B) of H. suis bacteria near or potentially in the canaliculi of the parietal cells in the fundic region of the stomach from slaughterhouse pigs. IHC staining (A) showed that H. suis bacteria (black arrows) were observed next to parietal cells (brown), sometimes showing signs of degeneration (right panel). Immunofluorescence staining (B) revealed that a substantial number of H. suis bacteria (green, white arrows) can be found in close association or in the canaliculi of the parietal cells (red). Nuclei are stained with DAPI (blue). IHC: immunohistochemistry.
qRT-PCR
Transcriptional changes of crucial genes involved in parietal cell function and gastric epithelial cell homeostasis were determined using qRT-PCR. A tendency towards an up-regulation of Atp4a was observed in H. suis positive pigs compared to negative animals (Figure 5, p = 0.14). Compared to H. suis negative pigs, a significant up-regulation of Shh was observed in H. suis-infected slaughter pigs (Figure 5, p = 0.012).
Figure 5.
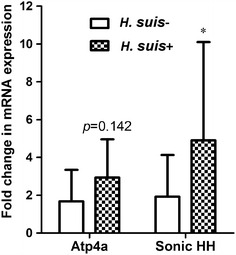
mRNA expression analysis. Shown are the mean fold changes of mRNA expression in H. suis positive pigs (n = 15) for Atp4a and Sonic HH, compared to that in H. suis negative pigs (n = 15). An * indicates a statistically significant difference (Student t test, p < 0.05) between H. suis positive pigs (H. suis−) and H. suis negative pigs (H. suis+). Sonic HH: sonic hedgehog.
Discussion
Pig stomachs are frequently inhabited by H. suis, a zoonotic bacterium, raising concerns regarding animal welfare, economic interests, public health and food safety [4, 10, 28]. H. suis infection can cause a decreased body weight gain and gastritis in pigs [10], and chronic gastritis, peptic ulceration and the development of MALT lymphoma-like lesions in humans and rodent models of human gastric disease [8, 16, 29, 30]. In the latter, a close association between H. suis and parietal cells has been observed and these cells can show signs of degeneration or malfunction [16]. In previous studies, it has been described that malfunction of acid secretion by parietal cells is closely associated with the development of gastritis [31], indicating that the function of parietal cells might be influenced by gastritis. On the other hand, a direct effect of H. suis on the health and function of parietal cells might also be involved. At the onset of this study, very little information was available on the interactions between H. suis and parietal cells in its natural host, the pig.
In the present study, we explored and described an effective method for isolation and culture of porcine parietal cells. This cell type is highly specialized and differentiated, requiring a specific approach. Our method was based on previously described methods for the isolation of rabbit parietal cells [32], and to a lesser extent on those described for dogs, rats and mice [33–35]. At first, we followed the protocols described for isolation of rabbit parietal cells, however without a great deal of success. Compared to rabbit stomach mucosa, it is more difficult to separate the pig stomach mucosa from the deeper layers, enzymatic digestion is less efficient, and the mucosa is covered by a thick layer of mucus, all of which give rise to some obstacles during the initial isolation of parietal cells. Some reagents that have previously been shown to be useful for the removal of mucus, including N-acetylcysteine and DTT [36, 37], did not contribute a lot to successful parietal cell isolation in the current study. In addition, some studies have shown that the use of EDTA can disrupt tight junctions between gastric epithelial cells, further facilitating the release of parietal cells from the gastric glands. In our study, however, the administration of EDTA did not exhibit beneficial effects. In view of the existing difficulties, we have optimized some steps that appeared to be essential for isolation of porcine parietal cells. These include an adequate removal of mucus by scraping, separating the mucosa in small pieces from the underlying tissue using a sharp blade and taking care to minimize the presence of submucosa and other connective tissues. Finally, using a combination of dispase and collagenase also proved to contribute to the release of parietal cells from the mucosa. Several matrices were tested for their ability to stimulate adhesion of parietal cells to coverslips, including fibronectin, collagen type I, collagen type IV, gelatine and Matrigel (data not shown). The latter was shown to provide the best results. In general, the majority of the cultured parietal cells existed in the form of single cells or small cellular clumps, and they were shown to remain viable under the described conditions for up to 5 days with a purity of ~80%.
In the present study, histological analysis of the stomachs of H. suis-infected pigs at slaughter age, revealed that H. suis bacteria are often observed in close vicinity of parietal cells and they even can be observed inside the canaliculi of parietal cells, which reveals a direct interaction of H. suis and parietal cells in situ. Upon co-incubation of isolated parietal cells with live H. suis, a considerable number of H. suis bacteria were found near or potentially in the canaliculi of the isolated parietal cells, which further confirmed the direct interplay between this bacterium and parietal cells in vivo and in vitro. Longer times of incubation of H. suis with isolated parietal cells showed similar results, and the most plausible explanation for this may be that a longer incubation time decreases the bacterial viability due to the improper medium and gas environment for this fastidious bacterium, requiring vigorous culture conditions. Future experiments should attempt to identify the possible mechanisms of adhesion.
IHC and immunofluorescent analysis revealed that H. suis infection did not greatly affect parietal cell numbers in the stomach of naturally infected pigs. We were, nevertheless, able to show for the first time a direct effect of H. suis on the viability of cultured parietal cells. This confirms previous findings that long-term H. suis infection can induce necrosis of parietal cells in the stomach of experimentally infected mice and Mongolian gerbils [16] and that swollen and degenerated parietal cells are often found in NHPH-infected patients with chronic gastritis [15]. Future experiments should aim to characterize the mechanisms involved. For H. pylori, it has been shown that infection can induce apoptosis of cultured rat parietal cells in a nuclear factor-κB- and nitric oxide-dependent manner [38].
Other gastric Helicobacter species, including H. pylori, H. heilmannii, and H. felis, have been described to cause massive parietal cell loss in rodent models, leading to the deregulation of gastric morphology and the development of intestinal metaplasia [39–41]. Most likely, the development of gastritis in the corpus region, which is more pronounced compared to H. suis infection in these same animal models, contributes largely to this massive loss of parietal cells. Indeed, Feldman et al., have demonstrated a positive correlation between the severity of H. pylori-related corpus gastritis and the degree of reduction in acid secretion function of parietal cells [42], and other reports have shown that the development of chronic gastritis in patients with H. pylori infection is associated with or causes the loss of parietal cells [43–45].
Besides an effect on the viability of parietal cells, H. suis may also affect the normal function and homeostasis of parietal cells in particular and the gastric epithelium in general. In the present study, mRNA expression levels of Atp4a, part of the proton pump, showed a trend towards being higher in H. suis positive pigs, which may be somewhat surprising, since other studies have shown that H. pylori infection can inhibit acid secretion through down-regulation of the expression of H+/K+ ATPase, resulting in hypochlorhydria [14, 46, 47]. However, yet another group of studies have described that H. pylori infection can in fact also cause hyperchlorhydria [48, 49], depending on the distribution of bacteria within the stomach, the infection stage, the profile of cytokines produced by the local epithelial cells or immune cells, and the pattern of gastritis [31, 50]. Therefore, the effect of H. suis infection on the dynamic changes of expression of H+/K+ ATPase as well as the function of parietal cells in the pig stomach needs to be further explored in future experimental studies.
Interestingly, significantly elevated expression levels of Shh were demonstrated in H. suis positive animals compared to animals free of H. suis, suggesting that H. suis infection affects the Shh signalling pathway. Sonic, India, and Desert hedgehog are important members of the Hedgehog family, playing an essential role during regulation of differentiation and growth of many tissues and cells [51]. In the stomach of mammals, and especially in the stomachs of mice and humans, Shh has been described to serve as an important regulator in the differentiation of gastric epithelium and immune cells as well as gastric gland morphogenesis [17, 52]. An exclusive expression of Shh is detected in the parietal cells located at the gland-pit boundary in the human stomach, which has been proven to be co-localized with ATPase [17, 53]. H. pylori infection has been described to induce an overexpression of Shh in mice during the early stage of infection and Shh may have a progressive role in the development of gastric cancer [54–56]. In addition, other studies have provided evidence that gastrin and gastric acid can stimulate the expression of Shh, while Shh in turn is also important for maintaining acid secretion, suggesting a feedback mechanism between gastric acid and Shh expression [57, 58]. It is also worth noting that Shh signalling is crucial for macrophage infiltration in the stomach [55]. Indeed, higher numbers of macrophages have been detected in the fundic region of the stomach from BALB/c mice during the initial stages of H. suis infection [16].
In summary, an effective method for the isolation and culture of porcine parietal cells was established. Direct interactions between H. suis and parietal cells were investigated using this in vitro cell model as well as in vivo in the stomach of pigs at slaughter age. H. suis was shown to interfere with parietal cells, by directly affecting their viability in vitro. H. suis infection triggers abnormal mRNA expression levels of Atp4a, responsible for acid production and regulation. In addition, H. suis infection was shown to induce a marked up-regulation of transcriptional levels of Shh, a critical factor involved in gastric organogenesis, glandular differentiation, and gastric homeostasis.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GZ, BM, AS and BF participated in the design of the study. GZ, BM and BF carried out the experiments, analysed the data and drafted the manuscript. FH and RD coordinated the study and participated in the design of the study, analysis of the data and drafting of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by grants from the Research Fund of Ghent University, The Research Foundation Flanders (FWO Vlaanderen), Ghent, Belgium (Grant No. GOA01G00408 and 01SC2411) and China Scholarship Council (CSC) (Grant No. 2010676001). We thank Iris Bosschem and Ellen De Bruyne for the kind help with qRT-PCR, Sarah Loomans and Elin Verbrugghe for their technical support with the tissue staining, Prof. Wim Van den Broeck for providing help with fluorescence microscopy, and Shunchuan Zhang and Xiaoyun Yang for their assistance with the cell work.
Footnotes
Bram Flahou and Freddy Haesebrouck shared senior authorship
Contributor Information
Guangzhi Zhang, Email: guangzhi.zhang@berkeley.edu.
Richard Ducatelle, Email: richard.ducatelle@ugent.be.
Belgacem Mihi, Email: belgacem.mihi@chiba-u.jp.
Annemieke Smet, Email: annemieke.smet@ugent.be.
Bram Flahou, Email: bramflahou@hotmail.com.
Freddy Haesebrouck, Email: freddy.haesebrouck@ugent.be.
References
- 1.Grasso GM, Ripabelli G, Sammarco ML, Ruberto A, Iannitto G. Prevalence of Helicobacter-like organisms in porcine gastric mucosa: a study of swine slaughtered in Italy. Comp Immunol Microbiol Infect Dis. 1996;19:213–217. doi: 10.1016/0147-9571(96)00007-0. [DOI] [PubMed] [Google Scholar]
- 2.Hellemans A, Chiers K, De Bock M, Decostere A, Haesebrouck F, Ducatelle R, Maes D. Prevalence of ‘Candidatus Helicobacter suis’ in pigs of different ages. Vet Rec. 2007;161:189–192. doi: 10.1136/vr.161.6.189. [DOI] [PubMed] [Google Scholar]
- 3.Joosten M, Flahou B, Meyns T, Smet A, Arts J, De Cooman L, Pasmans F, Ducatelle R, Haesebrouck F. Case report: Helicobacter suis infection in a pig veterinarian. Helicobacter. 2013;18:392–396. doi: 10.1111/hel.12054. [DOI] [PubMed] [Google Scholar]
- 4.Haesebrouck F, Pasmans F, Flahou B, Chiers K, Baele M, Meyns T, Decostere A, Ducatelle R. Gastric helicobacters in domestic animals and nonhuman primates and their significance for human health. Clin Microbiol Rev. 2009;22:202–223. doi: 10.1128/CMR.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Groote D, Van Doorn LJ, Van den Bulck K, Vandamme P, Vieth M, Stolte M, Debongnie JC, Burette A, Haesebrouck F, Ducatelle R. Detection of non-pylori Helicobacter species in “Helicobacter heilmannii”-infected humans. Helicobacter. 2005;10:398–406. doi: 10.1111/j.1523-5378.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 6.Morgner A, Bayerdorffer E, Meining A, Stolte M, Kroher G. Helicobacter heilmannii and gastric cancer. Lancet. 1995;346:511–512. doi: 10.1016/S0140-6736(95)91364-5. [DOI] [PubMed] [Google Scholar]
- 7.Debongnie JC, Donnay M, Mairesse J, Lamy V, Dekoninck X, Ramdani B. Gastric ulcers and Helicobacter heilmannii. Eur J Gastroenterol Hepatol. 1998;10:251–254. doi: 10.1097/00042737-199803000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Morgner A, Lehn N, Andersen LP, Thiede C, Bennedsen M, Trebesius K, Neubauer B, Neubauer A, Stolte M, Bayerdorffer E. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118:821–828. doi: 10.1016/S0016-5085(00)70167-3. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Lee BJ, Lee YS, Park JH. Association of tightly spiraled bacterial infection and gastritis in pigs. J Vet Med Sci. 2000;62:725–729. doi: 10.1292/jvms.62.725. [DOI] [PubMed] [Google Scholar]
- 10.De Bruyne E, Flahou B, Chiers K, Meyns T, Kumar S, Vermoote M, Pasmans F, Millet S, Dewulf J, Haesebrouck F, Ducatelle R. An experimental Helicobacter suis infection causes gastritis and reduced daily weight gain in pigs. Vet Microbiol. 2012;160:449–454. doi: 10.1016/j.vetmic.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Yao X, Forte JG. Cell biology of acid secretion by the parietal cell. Annu Rev Physiol. 2003;65:103–131. doi: 10.1146/annurev.physiol.65.072302.114200. [DOI] [PubMed] [Google Scholar]
- 12.Chew CS. Parietal cell culture: new models and directions. Annu Rev Physiol. 1994;56:445–461. doi: 10.1146/annurev.ph.56.030194.002305. [DOI] [PubMed] [Google Scholar]
- 13.Nozaki K, Ogawa M, Williams JA, Lafleur BJ, Ng V, Drapkin RI, Mills JC, Konieczny SF, Nomura S, Goldenring JR. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511–522. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saha A, Hammond CE, Beeson C, Peek RM, Jr, Smolka AJ. Helicobacter pylori represses proton pump expression and inhibits acid secretion in human gastric mucosa. Gut. 2010;59:874–881. doi: 10.1136/gut.2009.194795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joo M, Kwak JE, Chang SH, Kim H, Chi JG, Kim KA, Yang JH, Lee JS, Moon YS, Kim KM. Helicobacter heilmannii-associated gastritis: clinicopathologic findings and comparison with Helicobacter pylori-associated gastritis. J Korean Med Sci. 2007;22:63–69. doi: 10.3346/jkms.2007.22.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flahou B, Haesebrouck F, Pasmans F, D’Herde K, Driessen A, Van Deun K, Smet A, Duchateau L, Chiers K, Ducatelle R. Helicobacter suis causes severe gastric pathology in mouse and mongolian gerbil models of human gastric disease. PLoS One. 2010;5:e14083. doi: 10.1371/journal.pone.0014083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Brink GR, Hardwick JC, Tytgat GN, Brink MA, Ten Kate FJ, Van Deventer SJ, Peppelenbosch MP. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–328. doi: 10.1053/gast.2001.26261. [DOI] [PubMed] [Google Scholar]
- 18.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 19.Katoh Y, Katoh M. Hedgehog signaling pathway and gastric cancer. Cancer Biol Ther. 2005;4:1050–1054. doi: 10.4161/cbt.4.10.2184. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen CM, Williams J, van den Brink GR, Lauwers GY, Roberts DJ. Hh pathway expression in human gut tissues and in inflammatory gut diseases. Lab Invest. 2004;84:1631–1642. doi: 10.1038/labinvest.3700197. [DOI] [PubMed] [Google Scholar]
- 21.Chew CS, Brown MR. Release of intracellular Ca2 + and elevation of inositol trisphosphate by secretagogues in parietal and chief cells isolated from rabbit gastric mucosa. Biochim Biophys Acta. 1986;888:116–125. doi: 10.1016/0167-4889(86)90077-7. [DOI] [PubMed] [Google Scholar]
- 22.Flahou B, Deun KV, Pasmans F, Smet A, Volf J, Rychlik I, Ducatelle R, Haesebrouck F. The local immune response of mice after Helicobacter suis infection: strain differences and distinction with Helicobacter pylori. Vet Res. 2012;43:75. doi: 10.1186/1297-9716-43-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baele M, Decostere A, Vandamme P, Ceelen L, Hellemans A, Mast J, Chiers K, Ducatelle R, Haesebrouck F. Isolation and characterization of Helicobacter suis sp. nov. from pig stomachs. Int J Syst Evol Microbiol. 2008;58:1350–1358. doi: 10.1099/ijs.0.65133-0. [DOI] [PubMed] [Google Scholar]
- 24.Flahou B, Haesebrouck F, Chiers K, Van Deun K, De Smet L, Devreese B, Vandenberghe I, Favoreel H, Smet A, Pasmans F, D’Herde K, Ducatelle R. Gastric epithelial cell death caused by Helicobacter suis and Helicobacter pylori gamma-glutamyl transpeptidase is mainly glutathione degradation-dependent. Cell Microbiol. 2011;13:1933–1955. doi: 10.1111/j.1462-5822.2011.01682.x. [DOI] [PubMed] [Google Scholar]
- 25.Verbrugghe E, Vandenbroucke V, Dhaenens M, Shearer N, Goossens J, De Saeger S, Eeckhout M, D’Herde K, Thompson A, Deforce D, Boyen F, Leyman B, Van Parys A, De Backer P, Haesebrouck F, Croubels S, Pasmans F. T-2 toxin induced Salmonella Typhimurium intoxication results in decreased Salmonella numbers in the cecum contents of pigs, despite marked effects on Salmonella-host cell interactions. Vet Res. 2012;43:22. doi: 10.1186/1297-9716-43-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaecher C, Smet A, Flahou B, Pasmans F, Ducatelle R, Taylor D, Weller C, Bjarnason I, Charlett A, Lawson AJ, Dobbs RJ, Dobbs SM, Haesebrouck F. Significantly higher frequency of Helicobacter suis in patients with idiopathic parkinsonism than in control patients. Aliment Pharmacol Ther. 2013;38:1347–1353. doi: 10.1111/apt.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosi P, Mazzoni M, De Filippi S, Trevisi P, Casini L, Petrosino G, Lalatta-Costerbosa G. A continuous dietary supply of free calcium formate negatively affects the parietal cell population and gastric RNA expression for H+/K+-ATPase in weaning pigs. J Nutr. 2006;136:1229–1235. doi: 10.1093/jn/136.5.1229. [DOI] [PubMed] [Google Scholar]
- 28.De Cooman L, Flahou B, Houf K, Smet A, Ducatelle R, Pasmans F, Haesebrouck F. Survival of Helicobacter suis bacteria in retail pig meat. Int J Food Microbiol. 2013;166:164–167. doi: 10.1016/j.ijfoodmicro.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 29.O’Rourke JL, Dixon MF, Jack A, Enno A, Lee A. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma in an animal model of ‘Helicobacter heilmannii’ infection. J Pathol. 2004;203:896–903. doi: 10.1002/path.1593. [DOI] [PubMed] [Google Scholar]
- 30.Zhang G, Ducatelle R, De Bruyne E, Joosten M, Bosschem I, Smet A, Haesebrouck F, Flahou B. Role of gamma-glutamyltranspeptidase in the pathogenesis of Helicobacter suis and Helicobacter pylori infections. Vet Res. 2015;46:31. doi: 10.1186/s13567-015-0163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu S, Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2012;28:587–593. doi: 10.1097/MOG.0b013e328358e5cc. [DOI] [PubMed] [Google Scholar]
- 32.Mihi B, Van Meulder F, Rinaldi M, Van Coppernolle S, Chiers K, Van den Broeck W, Goddeeris B, Vercruysse J, Claerebout E, Geldhof P. Analysis of cell hyperplasia and parietal cell dysfunction induced by Ostertagia ostertagi infection. Vet Res. 2013;44:121. doi: 10.1186/1297-9716-44-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidtler J, Dehne K, Offermanns S, Rosenthal W, Classen M, Schepp W. Stimulation of rat parietal cell function by histamine and GLP-1-(7-36) amide is mediated by Gs alpha. Am J Physiol. 1994;266:G775–782. doi: 10.1152/ajpgi.1994.266.5.G775. [DOI] [PubMed] [Google Scholar]
- 34.Gliddon BL, Nguyen NV, Gunn PA, Gleeson PA, van Driel IR. Isolation, culture and adenoviral transduction of parietal cells from mouse gastric mucosa. Biomed Mater. 2008;3:034117. doi: 10.1088/1748-6041/3/3/034117. [DOI] [PubMed] [Google Scholar]
- 35.Stepan V, Pausawasdi N, Ramamoorthy S, Todisco A. The Akt and MAPK signal-transduction pathways regulate growth factor actions in isolated gastric parietal cells. Gastroenterology. 2004;127:1150–1161. doi: 10.1053/j.gastro.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 36.Alemka A, Clyne M, Shanahan F, Tompkins T, Corcionivoschi N, Bourke B. Probiotic colonization of the adherent mucus layer of HT29MTXE12 cells attenuates Campylobacter jejuni virulence properties. Infect Immun. 2010;78:2812–2822. doi: 10.1128/IAI.01249-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Risack LE, Vandevelde ME, Gobert JG. Effect of thiol derivatives on mixed mucus and blood clots in vitro. Resuscitation. 1978;6:9–20. doi: 10.1016/0300-9572(78)90031-X. [DOI] [PubMed] [Google Scholar]
- 38.Neu B, Randlkofer P, Neuhofer M, Voland P, Mayerhofer A, Gerhard M, Schepp W, Prinz C. Helicobacter pylori induces apoptosis of rat gastric parietal cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G309–318. doi: 10.1152/ajpgi.00546.2001. [DOI] [PubMed] [Google Scholar]
- 39.Murakami M, Fukuzawa M, Yamamoto M, Hamaya K, Tamura Y, Sugiyama A, Takahashi R, Murakami T, Amagase K, Takeuchi K. Effects of Helicobacter pylori infection on gastric parietal cells and E-cadherin in Mongolian gerbils. J Pharmacol Sci. 2013;121:305–311. doi: 10.1254/jphs.12191FP. [DOI] [PubMed] [Google Scholar]
- 40.De Bock M, Decostere A, Hellemans A, Haesebrouck F, Ducatelle R. Helicobacter felis and Helicobacter bizzozeronii induce gastric parietal cell loss in Mongolian gerbils. Microbes Infect. 2006;8:503–510. doi: 10.1016/j.micinf.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Liu C, Smet A, Blaecher C, Flahou B, Ducatelle R, Linden S, Haesebrouck F. Gastric de novo Muc13 expression and spasmolytic polypeptide-expressing metaplasia during Helicobacter heilmannii infection. Infect Immun. 2014;82:3227–3239. doi: 10.1128/IAI.01867-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feldman M, Cryer B, McArthur KE, Huet BA, Lee E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: a prospective study. Gastroenterology. 1996;110:1043–1052. doi: 10.1053/gast.1996.v110.pm8612992. [DOI] [PubMed] [Google Scholar]
- 43.Correa P. New strategies for the prevention of gastric cancer: Helicobacter pylori and genetic susceptibility. J Surg Oncol. 2005;90:134–138. doi: 10.1002/jso.20216. [DOI] [PubMed] [Google Scholar]
- 44.Oh JD, Kling-Backhed H, Giannakis M, Engstrand LG, Gordon JI. Interactions between gastric epithelial stem cells and Helicobacter pylori in the setting of chronic atrophic gastritis. Curr Opin Microbiol. 2006;9:21–27. doi: 10.1016/j.mib.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 45.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF, Jr, Rabkin CS. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 46.Smolka AJ, Backert S. How Helicobacter pylori infection controls gastric acid secretion. J Gastroenterol. 2012;47:609–618. doi: 10.1007/s00535-012-0592-1. [DOI] [PubMed] [Google Scholar]
- 47.Saha A, Hammond CE, Trojanowska M, Smolka AJ. Helicobacter pylori-induced H, K-ATPase alpha-subunit gene repression is mediated by NF-kappaB p50 homodimer promoter binding. Am J Physiol Gastrointest Liver Physiol. 2008;294:G795–807. doi: 10.1152/ajpgi.00431.2007. [DOI] [PubMed] [Google Scholar]
- 48.Malfertheiner P. The intriguing relationship of Helicobacter pylori infection and acid secretion in peptic ulcer disease and gastric cancer. Dig Dis. 2011;29:459–464. doi: 10.1159/000332213. [DOI] [PubMed] [Google Scholar]
- 49.Smith JT, Pounder RE, Nwokolo CU, Lanzon-Miller S, Evans DG, Graham DY, Evans DJ., Jr Inappropriate hypergastrinaemia in asymptomatic healthy subjects infected with Helicobacter pylori. Gut. 1990;31:522–525. doi: 10.1136/gut.31.5.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jaup BH. Helicobacter pylori and altered parietal cell morphology and function. Helicobacter. 2001;6:77–78. doi: 10.1046/j.1523-5378.2001.00011.x. [DOI] [PubMed] [Google Scholar]
- 51.Hui CC, Angers S. Gli proteins in development and disease. Annu Rev Cell Dev Biol. 2011;27:513–537. doi: 10.1146/annurev-cellbio-092910-154048. [DOI] [PubMed] [Google Scholar]
- 52.Lowrey JA, Stewart GA, Lindey S, Hoyne GF, Dallman MJ, Howie SE, Lamb JR. Sonic hedgehog promotes cell cycle progression in activated peripheral CD4(+) T lymphocytes. J Immunol. 2002;169:1869–1875. doi: 10.4049/jimmunol.169.4.1869. [DOI] [PubMed] [Google Scholar]
- 53.Zavros Y, Orr MA, Xiao C, Malinowska DH. Sonic hedgehog is associated with H+-K+-ATPase-containing membranes in gastric parietal cells and secreted with histamine stimulation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G99–G111. doi: 10.1152/ajpgi.00389.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marwaha S, Schumacher MA, Zavros Y, Eghbalnia R. Crosstalks between cytokines and sonic hedgehog in Helicobacter pylori infection: a mathematical model. PLoS One. 2014;9:e111338. doi: 10.1371/journal.pone.0111338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schumacher MA, Donnelly JM, Engevik AC, Xiao C, Yang L, Kenny S, Varro A, Hollande F, Samuelson LC, Zavros Y. Gastric sonic hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. Gastroenterology. 2012;142(1150–1159):e6. doi: 10.1053/j.gastro.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shiotani A, Kamada T, Yamanaka Y, Manabe N, Kusunoki H, Hata J, Haruma K. Sonic hedgehog and CDX2 expression in the stomach. J Gastroenterol Hepatol. 2008;23(Suppl 2):S161–166. doi: 10.1111/j.1440-1746.2008.05406.x. [DOI] [PubMed] [Google Scholar]
- 57.El-Zaatari M, Zavros Y, Tessier A, Waghray M, Lentz S, Gumucio D, Todisco A, Merchant JL. Intracellular calcium release and protein kinase C activation stimulate sonic hedgehog gene expression during gastric acid secretion. Gastroenterology. 2010;139(2061–2071):e2. doi: 10.1053/j.gastro.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zavros Y, Waghray M, Tessier A, Bai L, Todisco A, Gumucio DL, Samuelson LC, Dlugosz A, Merchant JL. Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem. 2007;282:33265–33274. doi: 10.1074/jbc.M707090200. [DOI] [PubMed] [Google Scholar]


