Abstract
Background
Antiepidermal growth factor receptor (EGFR)-targeted therapy is widely used in many epithelial cancer types. We investigated lapatinib effects on cutaneous squamous cell carcinoma (cSCC) scheduled for resection and in coexisting precursor lesions (actinic keratosis (AK) and Bowen's disease (BD)) in a phase 2 mode of action clinical trial including a histological workup of the cSCC.
Patients and methods
We initiated a prospective single-centre, open-label, non-controlled clinical study with translational intentions to investigate changes in size and histopathological features in cSCC after a 14-day period of neoadjuvant lapatinib therapy at a dose of 1500 mg/day prior to surgery, to quantify the impact on AK and BD in the same patient after 56 days and to evaluate the tolerability in patients with cSCC and precursor lesions.
Results
10 immunocompetent male patients were included with a mean age of 73 years (range 59–87). 8 patients were treated with the study medication lapatinib 1500 mg/day for a total duration of 56 days according to the protocol and were available for full analysis, whereas 2 patients had to discontinue treatment during the first 2 weeks because of adverse events (diarrhoea, pancreatitis). Tolerability was acceptable with only 1 related grade III adverse event. A reduction in tumour size of cSCC was documented in 2 of 8 evaluable patients after 14 days of treatment. The mean regression of captured precursor lesions was 30% after 56 days of treatment and 36% 28 days after therapy cessation.
Conclusions
Short-term lapatinib resulted in a cSCC tumour reduction in 2 of 8 patients. In addition, there was a clinically documented reduction of AK in 7 of 8 patients encouraging larger clinical trials, especially in high-risk patients with cSCC such as organ transplant recipients.
Trial registration number
NCT0166431.
Keywords: Advanced cutaneous squamous cell carcinoma, EGFR-inhibitors, lapatinib, options beyond chemotherapy, primary and secondary preve
Key questions.
What is already known about this subject?
Antiepithelial growth factor receptor (EGFR) antibodies have been successfully used in patients with cutaneous squamous cell carcinomas (SCC). EGFR targeting kinase inhibitors have not been studied as yet.
What does this study add?
The use of the EGFR inhibitor lapatinib resulted in regressions of SCC in the neoadjuvant setting and improvement of SCC precursor lesions.
How might this impact on clinical practice?
In high-risk patients with SCC such as organ transplant recipients, EGFR inhibitors should be investigated as a chemopreventive strategy.
Introduction
Cutaneous squamous cell carcinoma (cSCC) is the second most common skin cancer with rising incidence associated with high cumulative ultraviolet light exposure.1 Immunosuppressed patients, such as organ transplant recipients (OTR) or patients with lymphoma/leukaemia, have a higher risk for the development of cSCC and their precursor lesions.2
The number of patients with nodal and distant metastases, as well as locally advanced cSCC, remains unclear. Factors associated with aggressive behaviour and metastatic potential of cSCC include anatomical site, tumour size (diameter >2 cm) and depth, histological differentiation and host-immunosuppression.3
Surgical treatment is the standard treatment for the majority of invasive cSCC.4 5 Irradiation therapy is reserved for patients over 60 years.4–6 Systemic treatment options for locally advanced or metastasising SCC comprise cisplatin, doxorubicin, 5-fluorouracil, bleomycin, methotrexate, interferon and combination therapies. Response rates vary widely from 17% to 78%, but data are scarce.7
The epidermal growth factor receptor (EGFR) and its associated signalling pathways have been implicated in the development of SCC.8 9 EGFR is targeted by EGFR-monoclonal antibodies (EGFRab), such as cetuximab and panitumumab, and small-molecule tyrosine kinase inhibitors, such as erlotinib, lapatinib and gefitinib. Either approach aims to interrupt signal transduction downstream of the EGFR and results in the arrest of tumour proliferation.10 11 The human EGFR family encompasses four receptor tyrosine kinases (ErbB1/EGFR, ErbB2/HER2, ErbB3, ErbB4). Each receptor has an extracellular domain, a hydrophobic transmembrane and an intracellular domain, which contains a protein tyrosine kinase. Seven ligands bind to EGFR including the epidermal growth factor (EGF) and transforming growth factor β (TFGβ), none binds to ErbB2, two bind to ErbB3, and seven bind to ErbB4. The PI3K kinase is a major intracellular target of EGFRs, resulting in an activation of Akt kinase, which then phosphorylates mechanistic target of rapamycin and can provide many signals, important for proliferation and survival of malignant cells.12 13 Small kinase inhibitors are poorly studied in cSCC and their precursor lesions. Therefore, we investigated lapatinib in cutaneous squamous neoplasias.
Lapatinib is an orally active, low molecular weight, dual tyrosine kinase inhibitor targeting two members of the HER family receptors HER1 and HER2/c-neu.14 It acts intracellularly by blocking the cytoplasmic ATP-binding site of the kinase domain, inhibiting phosphorylation and receptor activation. This inhibits various downstream signalling cascades, including the phosphatidylinositol 3-kinase/Akt (PKB) and the Ras/Raf/MEK/ERK1/2 pathways. Lapatinib's advantage in comparison with other EGFR-targeted drugs is its small size and interference with more than one member of the HER family. Consequently, lapatinib is expected to suppress cancer cell growth more effectively than monotarget tyrosine kinase inhibitors.15 16
EGFRab-targeted therapy is used for the treatment of advanced cSCC with a response rate of 28–64%, either in combination with radiotherapy or as a single agent.17 To the best of our knowledge, lapatinib has not yet been investigated in cSCC.
We conducted a pilot study to determine the impact of oral treatment with the EGFR-antagonist lapatinib on cSCC and the coexisting actinic keratosis (AK) and Bowen's disease (BD).
Methods
Study design
Some financial support and the drug was assured by Glaxo Smith Kline (GSK).
The study design was a single-centre, investigator-initiated, open-label study (figure 1A). We included patients with advanced cSCC with concomitant AK or BD. All patients were informed about the investigative nature of this treatment and signed a written informed consent in accordance with the local institutional board and guidelines.
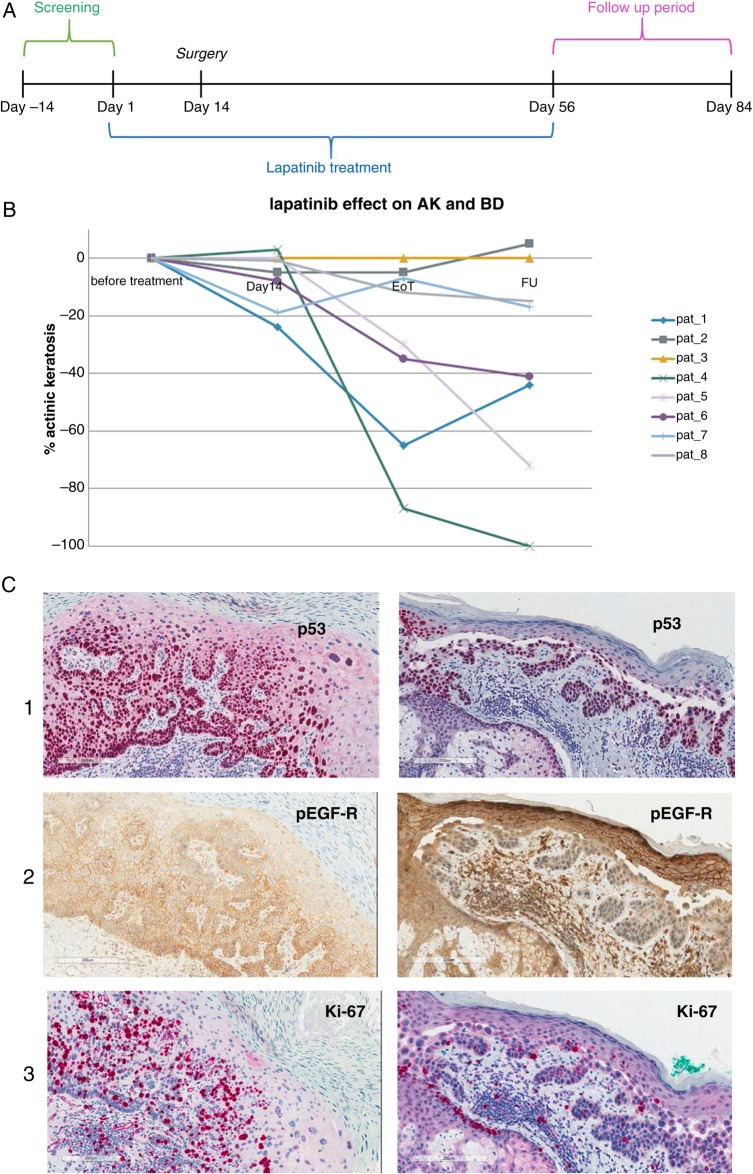
Figure 1.
(A) Study scheme graph. (B) Lapatinib effect on coexisting precursor lesions at 14 and 56 days under lapatinib treatment as well as 28 days after end of treatment. (C) Immunohistology of a pretherapy left and post-therapy biopsy right. (1) p53 identifies the malignant cell population. (2) pEGFR reduction in tumour cells suggest on-target activity of lapatinib. Note that normal keratinocytes still present pEGFR immunoreactivity. (3) Immunoreactivity of Ki67 reflecting proliferation is also reduced in malignant but not normal keratinocytes (see sebaceous gland epithelium) (AK, actinic keratosis; BD, Bowen's disease).
The primary objective was to evaluate the tumour response macroscopically in patients with primary cutaneous SCC lesions after 14 days of oral treatment, to investigate the histological features of regressive cSCC and to demonstrate the impact of the therapy on concomitant in situ lesions such as AK or BD. The secondary objective was to evaluate the tolerability of lapatinib therapy in patients with cSCC.
Eligibility
Patients with non-metastasising cSCC (at an age over 18) who were amenable to surgical resection were eligible. The diagnosis of histologically confirmed cSCC stage Tis-T2N0 was necessary for inclusion in the study with a minimum of two coexisting in situ lesions. Patients were required to have a performance status of 0 or 1 according to Eastern Cooperative Oncology Group (ECOG) and adequate organ system functions defined by laboratory assessment and echocardiography. Patients with active hepatic or biliary disease, with any anticancer therapy 14 days prior to the first dose of lapatinib or any topical therapy of AK <4 weeks prior to study initiation, were excluded.
Treatment
All patients received the study drug lapatinib at a dose of 1500 mg taken orally once daily for a total duration of 56 days (figure 1A). Patients were instructed to take lapatinib at least 1 h before or at least 1 h after meals. In order to gain insight into the mechanism of tumour regression induced by lapatinib, the primary tumour was excised 14 days after treatment start as it would have been done outside the clinical trial.
Study evaluation and follow-up
All patients underwent a complete medical history and physical examination within 14 days prior to treatment start. Evaluation of cardiac ejection fraction by echocardiogram and ECG were performed before study enrolment. A biopsy of the primary tumour for histological confirmation of cSCC clinical diagnosis was done during screening. Diagnosis of AK and BD was made clinically. Surgical excision of cSCC had to be performed 14 days after lapatinib initiation. Physical examinations and laboratory assessments were scheduled on days 14, 56 and 84. Clinical assessment of the cSCC and the precursor lesions was performed and photographs of all involved sites were taken on every study visit. We analysed tumour response of all primary tumours as well as of the coexisting AK or BD lesions by bi-dimensional perpendicular measurement of macroscopic tumour size, adding up the sum of the two diameters. Analysis of tumour response was done for all lesions before surgical excision on day 14 as well as by decrease of size in the at least two predefined coexisting reference AK or BD lesions present in the individual patient at day 56 and on the follow-up visit at day 84.
Histopathology
In total, formalin fixed paraffin-embedded (FFPE) blocks with tumour samples from eight patients enrolled in the trial were included in our histopathological evaluation. Each biopsy needed to have a diameter of at least 4 mm to be included in the analysis. FFPE samples were stained with H&E and various immunohistochemical protocols before independent evaluation by two dermatopathologists (RD and BM). Normal epithelium served as a positive internal control.
Samples were stained using the heat-induced epitope retrieval technique (HIER) as previously described.18 Unspecific Fc-receptor binding of antibodies was compared to isotype-matched controls. Stainings were performed on 4 μm sections counterstained with haematoxylin using an AutostainerLink 48 (Dako).
The following antibiodies were used for immunohistochemical protein expression analysis: to evaluate the activation of the EGF signalling pathway, anti-pEGFR clone 53A5 (Cat.Nr. 4407, Sell Signaling) and anti-HER2 clone 4B5 (Cat.Nr: 790–2991, Ventana) were used; to evaluate the activation of the PI3K/AKT signalling pathway, anti-AKT1 clone Y89 (Cat.Nr: ab32505, Abcam) and anti-pAKT (Cat.Nr: ab8932, Abcam) were used; to evaluate cell proliferation and UV-induced alterations in cell cycle anti-Ki67 clone MIB-1 (Cat.Nr: M7240, Dako), anti-p53 (Cat.Nr: E06125, Ventana) and anti-p16 clone G175–405 (Cat.Nr: 550834, BD Pharmogen) were used.
Immunohistochemical scoring of positive cells was graded as follows: 0–5%, no positive cells found in the tumour area; 6–25% for low expression; 26–50% for average expression, 51–75% for high expression and 76–100% for very high expression of the tumour area.
Statistical analysis
Owing to the low patient numbers, statistical analysis was performed using descriptive statistics. To compare changes in lesion size, Student t test was used.
Results
Patients
A total of 12 patients were screened from September 2012 to August 2013 and 10 patients were enrolled in the study. All enrolled patients were Caucasians of male gender. The mean age was 73 years (range 59–87). Several patients suffered from concomitant diseases such as diabetes mellitus, arterial hypertension, coronary or hypertensive heart disease, rheumatic polymyalgia, carcinoma of the prostate and coeliac disease, etc. Except for one patient, all patients had a history of previous skin cancer. Two patients discontinued the study due to adverse events: one after 2 weeks of lapatinib treatment due to diarrhoea and abdominal pain related to the study medication as grade 3 toxicity, and one due to an acute pancreatitis, judged by the investigator as not related to study medication. The follow-up examination on day 84 (28 days after end of treatment) was performed in eight patients who were treated with the study medication lapatinib for 56 days.
Most common toxicities were diarrhoea (6/10 patients), the typical papulopustular rash reported for EGFR and MEK inhibitors (6/10 patients) and fatigue (5/10 patients).19 Two patients reported abdominal pain, two olecranon bursitis, one reported nausea, one loss of appetite and one mucositis of the oral mucosa. There was only one related adverse event of grade 3 (diarrhoea), all other were mild adverse events of grades 1 and 2.
The localisation of the cSCC was on the head in six of our included patients, on the leg in two patients and on the trunk in two other patients. All of the patients had at least two additional clinically diagnosed AK or BD lesions (tables 1 and 2).
Table 1.
Size of cutaneous squamous cell carcinoma (cSCC) by calculation of the sum of the perpendicular diameter in mm
| Size of cSCC in mm (%) | ||
|---|---|---|
| Patient | Baseline | Day 14 |
| 1 | 50 (100) | 58 (116) |
| 2 | 16 (100) | 16 (100) |
| 3 | 30 (100) | 30 (100) |
| 4 | 28 (100) | 12 (43) |
| 5 | 37 (100) | 37 (100) |
| 6 | 350 (100) | 190 (54) |
| 7 | 16 (100) | 16 (100) |
| 8 | 22 (100) | 28 (127) |
Table 2.
Tumour size by sum of the perpendicular diameter in mm and percentage
| Actinic keratosis and Bowen's disease | ||||||
|---|---|---|---|---|---|---|
| Patient | Baseline | Day 56 | Day 84 | |||
| Number of lesions | Size in mm (%) | Number of lesions | Size in mm (%) | Number of lesions | Size in mm (%) | |
| 1 | 3 | 68 (100) | 2 | 24 (35) | 3 | 38 (56) |
| 2 | 2 | 19 (100) | 2 | 18 (95) | 2 | 20 (105) |
| 3 | 2 | 50 (100) | 2 | 50 (100) | 2 | 50 (100) |
| 4 | 3 | 30 (100) | 1 | 4 (13) | 0 | 0 (0) |
| 5 | 3 | 99 (100) | 3 | 69 (70) | 3 | 28 (28) |
| 6 | 7 | 172 (100) | 6 | 112 (65) | 6 | 102 (59) |
| 7 | 5 | 111 (100) | 5 | 103 (93) | 5 | 92 (83) |
| 8 | 6 | 163 (100) | 6 | 144 (88) | 6 | 139 (85) |
Macroscopic tumour evaluation
The median tumour size in the cSCC before treatment initiation was 69 mm (range 26–350 mm, n=8). On the day of surgical excision, 14 days after treatment start we observed a reduction in tumour size in 2 of 8 primary tumours with a tumour reduction of 16 mm (−42%), and 160 mm (−54%), respectively. In 2 out of 8 cSCC, we observed an increase in tumour size by 8 mm (+16%) respectively 6 mm (+27%). In four of eight patients, there was no change in tumour size (table 1).
In order to evaluate the effect on precursor lesions such as AK and BD, we analysed the sum of all precursor lesions in every patient after 56 days of treatment, as well as 28 days after the end of treatment at the follow-up visit (table 2). In seven of eight patients, we observed at least a partial remission of precancerous lesions, with a complete remission of 1 AK in two of eight patients (figure 1B and figure 2) and a complete remission of three AK in one patient. Only in one of eight patients, there was no change in the number and size of the coexisting lesions. The mean percentage of the regression of all precancerous lesions was 30% (range 0–87%) on day 56 and 36% (range 5–100%) on day 84 (table 2).
Figure 2.
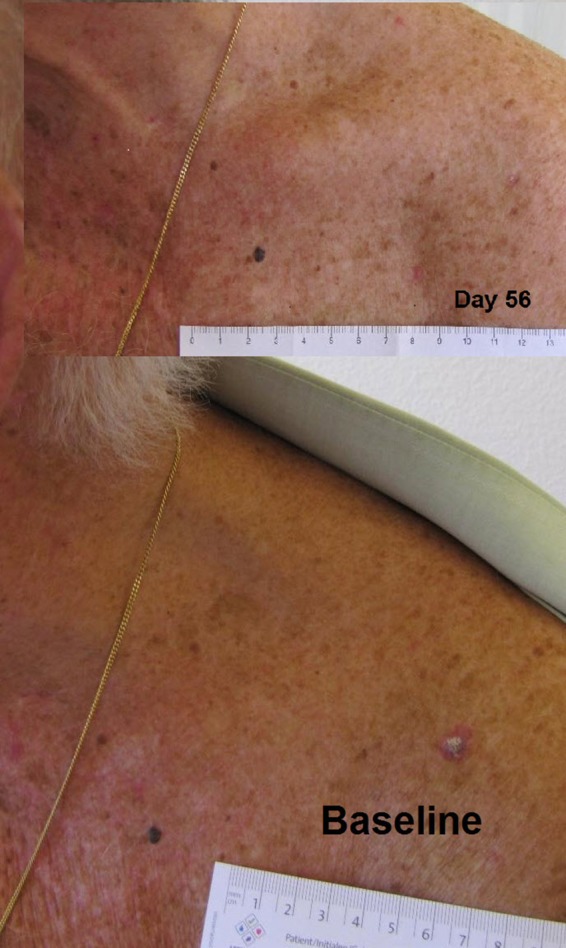
Clinical response of actinic keratosis (AK) during lapatinib treatment.
Histology and immunohistochemistry of pretreated and post-treated cSCC samples
In a total of eight patients, excision of the primary tumour was performed according to the study protocol for a comparison of the pretreatment sample with the resected tumours on day 14. The findings demonstrated profound intraindividual differences.
As expected, all tumour samples showed increased expression of the proliferation marker Ki67 and tumour suppressor p53. Strong immunoreactivity for p16 (>50% of the tumour cells were positive) was found in three patients. Strong immunoreactivity for pAKT (>50% of the tumour cells were positive) was detected in two patients. Her2 was not expressed. In five of nine patients, there was a strong immunoreactivity for pEGFR (>50% of the tumour cells were positive).
A clear alteration in EGFR phosphorylation and in proliferation reflected by Ki67 expression was found in one biopsy pair. Corresponding stainings are shown in figure 1C. All other stainings did not show profound differences during therapy.
Discussion
This study took advantage of the fact that clinical diagnosis of invasive cSCC is routinely confirmed by histology and patients who typically present concomitant in situ lesions such as AK and BD and undergo surgery a few weeks later. This allowed us to study alterations in key cellular processes induced by lapatinib in cSCC by histology and immunohistochemistry without additional biopsies, as well as to assess clinical effects on AK and BD.
Clinically, we only observed a clear detectable shrinkage in the cSCC of two patients. This low regression rate might be due to the short 14-day treatment period before resection. We did observe a notable change in the palpable tumoural infiltration of the cSCC during lapatinib treatment, which could not be captured by macroscopic measurements. Furthermore, there was a marked response in the coexisting precursor lesions such as AK and BD after 2 months of treatment. Seven patients presented a clear reduction of the size of AK. In two patients, there was a complete clearing of one AK (figure 1C), and in one patient we observed a complete clearance of three AK. The 1-month follow-up after treatment discontinuation per protocol revealed a lasting clinical remission, as well as a further decrease of the total lesion size in five patients (table 2). These observations may suggest a possible antitumour efficacy for lapatinib in keratinocytic carcinomas.
Our patient cohort showed a relatively high expression of pEGFR with positivity of >50% in five of eight evaluable patients. Fogarty et al20 found EGFR expression in 9 of 21 (43%) locally advanced cSCC with only five of them expressing phosphorylated EGFR. In our stainings, one biopsy pair supports the principle of lapatinib's value in patients with multiple AK. However, there is a need for biomarkers to enrich for patients who would benefit from the treatment. Comparing pretreatment to day 14 SCC tissue, there was one patient with a profound downregulation of pEGFR and reduced proliferation. The immunohistology results were very heterogeneous from patient to patient, which supports the concept that multiple signalling pathways are involved in UV-induced carcinogenesis.
Two-month treatment was associated with acceptable toxicity. There was no severe cardiac reaction in this patient population of advanced age with several comorbidities. Drug-related adverse reactions caused treatment discontinuation in only one patient. Of note, this patient had suffered from diarrhoea already before and also after the 2 weeks off lapatinib exposure, suggesting a pre-existing gastrointestinal problem. Adverse effects of the skin are the most common adverse events reported in treatment with EGFR inhibitors, followed by diarrhoea. Development of rash, mostly papolopustular, is a frequent problem in treatment with EGFR inhibitors. Still, HER1/2 inhibition by lapatinib is known to show a lower incidence of rash (41% of treated patients) compared to HER1 inhibition with cetuximab, erlotinib and panitumumab (75–90%).21 22 Diarrhoea in lapatinib treatment is a frequent adverse event but is usually mild (grade 1 or 2) and limited to a duration of 4–5 days. Even if most studies report a favourable adverse effect profile of EGFR inhibitors including lapatinib, the cutaneous and gastrointestinal adverse reactions cause significant discomfort that would impact on the long-term patient compliance. Lucchini et al23 describe a rate of up to 33% of treatment discontinuation in patients treated with EGFR inhibitors. Since epidermal growth factor inhibitors have become the standard of care in several solid malignancies, strategies for management of a number of adverse events have been developed in the past years.24
Drugs targeting EGFR have been approved for several types of malignancy and are investigated in numerous other tumour types, including advanced cSCC. At present, no targeted therapy has been approved in cSCC. Notably, one of the currently licensed indications for anti-EGFR therapies is advanced SCC of the head and neck (HNSCC). Several case reports and smaller trials have shown a remarkable response in cSCC with EGFR inhibitors such as cetuximab, geftinib and erlotinib.17 To the best of our knowledge, lapatinib has not been used in treatment for cSCC so far, but several phase II and III studies are under investigation for lapatinib treatment in different HNSCC settings.12 25 26 In our study, we choose a high dosage of lapatinib with 1500 mg/day as used in treatment for HNSCC. For advanced cSCC and metastasised cSCC treatment, options remain limited and are associated with high toxicities. At the moment, anti-EGFR-targeted therapies are the only option beyond radiotherapy and conventional chemotherapeutics.
Furthermore, EGFR inhibition could benefit patients in the settings of primary and secondary prevention. There is an unmet medical need in the highly prevalent condition of SCC of the skin. The need is highest in high-risk groups such as OTR and in chronic lymphatic leukaemia and for high-risk tumours such as SCC thicker than 5 mm. Also, patients with the occurrence of SCC with a known high risk of metastasis (ie, tumour thickness above 5 mm, localisation on the ear or lips, histologically low differentiation, perineural invasion, rapid growth, involvement of regional lymph nodes) could benefit from early introduction of systemic EGFR inhibitors to slow or halt the disease progression to distant metastasis.
Footnotes
Funding: University of Zürich.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The study protocol (developed by RD and MK) was approved by the institutional board (national ethical committee KEK-ZH-Nr. 2011–0416 and Swissmedic, reference 2012DR2064) and registered on clinical trial.gov (number NCT0166431)..
Provenance and peer review: Not commissioned; internally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol 2002;146(Suppl 61):1–6. 10.1046/j.1365-2133.146.s61.2.x [DOI] [PubMed] [Google Scholar]
- 2.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med 2003;348:1681–91. 10.1056/NEJMra022137 [DOI] [PubMed] [Google Scholar]
- 3.Brantsch KD, Meisner C, Schonfisch B et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol 2008;9:713–20. 10.1016/S1470-2045(08)70178-5 [DOI] [PubMed] [Google Scholar]
- 4.Bonerandi JJ, Beauvillain C, Caquant L et al. , French Dermatology Recommendations Association (aRED). Guidelines for the diagnosis and treatment of cutaneous squamous cell carcinoma and precursor lesions. J Eur Acad Dermatol Venereol 2011;25(Suppl 5):1–51. 10.1111/j.1468-3083.2011.04296.x [DOI] [PubMed] [Google Scholar]
- 5. NCCN Clinical Practice Guidelines in Oncology. Basal cell and squamous cell skin cancers. National Comprehensive Cancer Network; Version 2. 2012. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. [DOI] [PubMed]
- 6.Barysch MJ, Eggmann N, Beyeler M et al. Long-term recurrence rate of large and difficult to treat cutaneous squamous cell carcinomas after superficial radiotherapy. Dermatology (Basel) 2012;224:59–65. 10.1159/000337027 [DOI] [PubMed] [Google Scholar]
- 7.Cranmer LD, Engelhardt C, Morgan SS. Treatment of unresectable and metastatic cutaneous squamous cell carcinoma. Oncologist 2010;15:1320–8. 10.1634/theoncologist.2009-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horn L, Lovly C. Update on HER1–3 in advanced non-small-cell lung cancer. J Thorac Oncol 2012;7:S369–71. 10.1097/JTO.0b013e31826defaa [DOI] [PubMed] [Google Scholar]
- 9.Hong L, Han Y, Brain L. The role of epidermal growth factor receptor in prognosis and treatment of gastric cancer. Expert Rev Gastroenterol Hepatol 2014;8:111–17. 10.1586/17474124.2014.844648 [DOI] [PubMed] [Google Scholar]
- 10.Baselga J. The EGFR as a target for anticancer therapy—focus on cetuximab. Eur J Cancer 2001;37(Suppl 4):S16–22. 10.1016/S0959-8049(01)00233-7 [DOI] [PubMed] [Google Scholar]
- 11.Ciardiello F, Caputo R, Bianco R et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin Cancer Res 2001;7:1459–65. [PubMed] [Google Scholar]
- 12.Cohen RB. Current challenges and clinical investigations of epidermal growth factor receptor (EGFR)- and ErbB family-targeted agents in the treatment of head and neck squamous cell carcinoma (HNSCC). Cancer Treat Rev 2014;40:567–77. 10.1016/j.ctrv.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 13.Uribe P, Gonzalez S. Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: molecular bases for EGFR-targeted therapy. Pathol Res Pract 2011;207:337–42. 10.1016/j.prp.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 14.Nelson MH, Dolder CR. Lapatinib: a novel dual tyrosine kinase inhibitor with activity in solid tumors. Ann Pharmacother 2006;40:261–9. 10.1345/aph.1G387 [DOI] [PubMed] [Google Scholar]
- 15.Nolting M, Schneider-Merck T, Trepel M. Lapatinib. Recent Results Cancer Res 2014;201:125–43. 10.1007/978-3-642-54490-3_7 [DOI] [PubMed] [Google Scholar]
- 16.Xia W, Mullin RJ, Keith BR et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 2002;21:6255–63. 10.1038/sj.onc.1205794 [DOI] [PubMed] [Google Scholar]
- 17.Bejar C, Maubec E. Therapy of advanced squamous cell carcinoma of the skin. Curr Treat Options Oncol 2014;15:302–20. 10.1007/s11864-014-0280-x [DOI] [PubMed] [Google Scholar]
- 18.Widmer DS, Hoek KS, Cheng PF et al. Hypoxia contributes to melanoma heterogeneity by triggering HIF1alpha-dependent phenotype switching. J Invest Dermatol 2013;133:2436–43. 10.1038/jid.2013.115 [DOI] [PubMed] [Google Scholar]
- 19.Schad K, Baumann Conzett K, Zipser MC et al. Mitogen-activated protein/extracellular signal-regulated kinase inhibition results in biphasic alteration of epidermal homeostasis with keratinocytic apoptosis and pigmentation disorders. Clin Cancer Res 2010;16:1058–64. 10.1158/1078-0432.CCR-09-1766 [DOI] [PubMed] [Google Scholar]
- 20.Fogarty GB, Conus NM, Chu J et al. Characterization of the expression and activation of the epidermal growth factor receptor in squamous cell carcinoma of the skin. Br J Dermatol 2007;156:92–8. 10.1111/j.1365-2133.2006.07603.x [DOI] [PubMed] [Google Scholar]
- 21.Peuvrel L, Bachmeyer C, Reguiai Z et al. Semiology of skin toxicity associated with epidermal growth factor receptor (EGFR) inhibitors. Support Care Cancer 2012;20:909–21. 10.1007/s00520-012-1404-0 [DOI] [PubMed] [Google Scholar]
- 22.Nardone B, Nicholson K, Newman M et al. Histopathologic and immunohistochemical characterization of rash to human epidermal growth factor receptor 1 (HER1) and HER1/2 inhibitors in cancer patients. Clin Cancer Res 2010;16:4452–60. 10.1158/1078-0432.CCR-10-0421 [DOI] [PubMed] [Google Scholar]
- 23.Lucchini E, Pilotto S, Spada E et al. Targeting the epidermal growth factor receptor in solid tumors: focus on safety. Expert Opin Drug Saf 2014;13:535–49. 10.1517/14740338.2014.904283 [DOI] [PubMed] [Google Scholar]
- 24.Lacouture ME, Anadkat MJ, Bensadoun RJ et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer 2011;19:1079–95. 10.1007/s00520-011-1197-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Campo JM, Hitt R, Sebastian P et al. Effects of lapatinib monotherapy: results of a randomised phase II study in therapy-naive patients with locally advanced squamous cell carcinoma of the head and neck. Br J Cancer 2011;105: 618–27. 10.1038/bjc.2011.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandhi MD, Agulnik M. Targeted treatment of head and neck squamous-cell carcinoma: potential of lapatinib. Onco Targets Ther 2014;7:245–51. 10.2147/OTT.S46933 [DOI] [PMC free article] [PubMed] [Google Scholar]