Abstract
Immune checkpoint inhibitors, such as ipilimumab (an anti-CTLA4 antibody), have become a commonly used therapy in cancer. To date, safety data of patients with underlying autoimmune disease is limited. We present a case of a patient with rheumatoid arthritis who was diagnosed of a BRAF-mutant metastatic melanoma. The patient was treated with ipilimumab and presented with high-grade colitis requiring immunosuppressors. Despite of the immune-related adverse event, no exacerbation of the rheumatoid arthritis was observed and the patient achieved a complete response. This case report contributes to the scarce literature on the use of immune checkpoint inhibitors in patients with an underlying autoimmune condition.
Keywords: melanoma, autoimmune disease, ipilimumab, rheumatoid arthritis, immune-related adverse event
Key questions.
What is already known about this subject?
To date, safety data of immune checkpoint inhibitors in patients with an underlying autoimmune disease are scarce. Only case reports of patients are available with different outcomes.
What does this study add?
This report provides information of the use of immune checkpoints inhibitors in real-world clinical practice, such in patients with autoimmune disorders. Although our patient presented with a grade 4 immuno-related colitis, a complete response was observed after two infusions of ipilimumab.
How might this impact on clinical practice?
Autoimmune diseases may not be an absolute contraindication for immune checkpoints inhibitors in those patients that can potentially benefit from them. Each patient should be individually assessed depending on the therapeutic options available and the status of the underlying autoimmune condition.
Introduction
Ipilimumab, a fully human antibody against CTLA-4 (cytotoxic T-lymphocyte antigen 4), is an immune-checkpoint receptor inhibitor approved for the treatment of metastatic melanoma. As a result of its mechanism of action and subsequent activation of the immune system, ipilimumab is associated with immune-related adverse events (irAEs), being dermatitis and colitis the most frequently reported.1 Clinical trials with ipilimumab and other immune-checkpoint inhibitors have excluded patients with underlying autoimmune diseases because of the concern of the activation of the immune system and the possibility of induction or exacerbation of the disease, and even the development of ipilimumab-related adverse side effects.
We report the case of a patient with a BRAF-mutant metastatic melanoma and a medical history of rheumatoid arthritis (RA). Ipilimumab induced a complete response, with no exacerbation of her RA but the patient developed a life-threatening diarrhoea-colitis.
Case report
A 51-year-old woman presented in August 2010 with pain and swelling of small joints of hands and feet and morning stiffness. Blood tests showed elevated acute phase reactants, a negative rheumatoid factor and a highly positive anticyclic citrullinated peptide antibody. She was diagnosed with seropositive RA. Treatment with low-dose prednisone (10 mg/day) and methotrexate, with rapid increase to 20 mg orally of methotrexate once a week, was started. The patient persisted with high disease activity and 8 months later biological therapy with rituximab (an anti-CD20 monoclonal antibody) was initiated. The patient presented a good response to rituximab (cycles of 2 doses of 1000 mg separated by 2 weeks, every 6 months), achieving a sustained clinical remission since April 2012.
Almost simultaneously to the diagnosis of the RA, she was diagnosed with a superficial spreading melanoma on her left lower limb, Breslow 1.57 mm, Clark level IV, in July 2010. She underwent wide excisional surgery and selective sentinel node dissection with evidence of micrometastases in the two biopsied lymph nodes. Left groin lymphadenectomy was indicated with evidence of a total of five affected nodes (AJCC stage IIIA, pT2aN2a). Interferon α was discussed but was finally ruled out as an adjuvant option because of the underlying RA. The patient continued clinical follow-up. Two years later she experienced several non-surgical locoregional recurrences in left lower limb. Those cutaneous metastases were treated initially with topic imiquimod. After 3 months she initiated intralesional interleukine-2 (9 million international units (MIU) weekly) because of progressive skin nodules, until January 2013 when multiple skin metastases appeared involving most of the left lower limb. Subsequently, she underwent an isolated hyperthermic perfusion of the left leg with melfalan (100 mg, 41°C) and tumour necrosis factor (TNF) (2 mg) with no benefit.
As molecular analysis demonstrated a BRAF V600 mutation, she started systemic treatment with vemurafenib (960 mg orally twice daily) plus cobimetinib/placebo (60 mg once daily for 21 days, followed by 7 days off) within the context of a clinical trial in September 2013. Radiological partial response was achieved. Nine months later, BRAF inhibitor therapy was stopped because of disease progression with the appearance of liver metastases by CT scan.
The limited treatment options and adequate RA symptomatic control, with no need for treatment during the previous year (ie, rituximab had been withheld due to persistent disease remission) were taken into account. After considering potential risks related to her underlying autoimmune disorder or the development of severe irAEs ipilimumab (3 mg/kg every 3 weeks, planned for a total of 4 doses) was initiated on 3rd October.
On 25th October 2014, the patient was admitted to hospital after two doses of ipilimumab due to grade 4 diarrhoea and hypovolemic shock from gastrointestinal losses. She was started on methylprednisolone 1 mg/kg IV (intravenous), empirical piperacillin/tazobactam and parenteral fluid replacement without improvement of grade 3 diarrhoea. A full colonoscopy showed erythematous mucosa, loss of normal vascular pattern and multiple ulcers (figure 1). Blood and stool microbiological cultures and detection of Clostridium difficile toxin were negative. Infection by cytomegalovirus was also ruled out in colonic biopsy. Owing to the lack of response to steroids, treatment with infliximab (5 mg/kg IV) was initiated. After 7 days diarrhoea improved, the patient tolerated oral diet and was finally discharged 1 month later on tapering oral prednisone 1 mg/kg. No RA reactivation was observed despite immunosupressive therapies targeted to control the irAE described.
Figure 1.
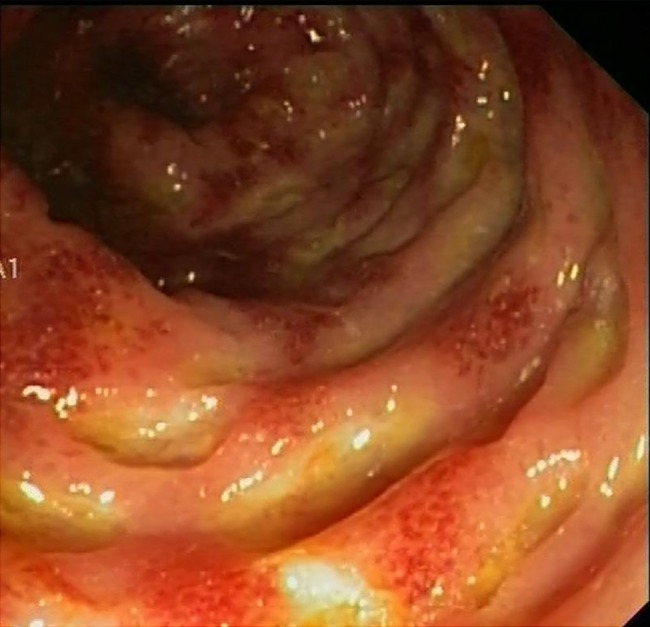
The fibrocolonoscopy performed in October 2014 showed multiple ulcers on the colonic mucosa.
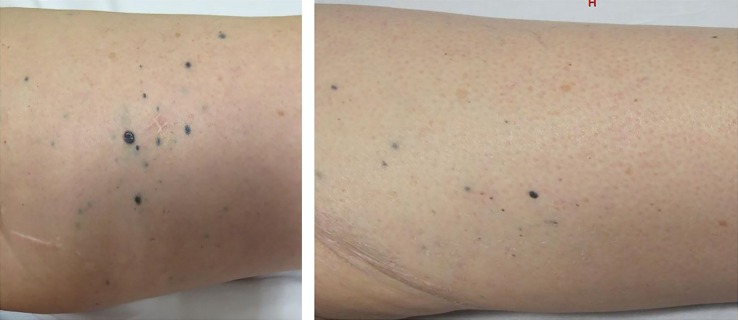
During admission a CT scan performed at week 12 post-ipilimumab showed a complete response of metastatic liver disease (figure 2). Moreover a skin examination revealed flat blue-pigmented lesions on the left limb (figure 3) that were biopsied, confirming a complete regression of melanoma with presence of melanophages in the dermis.
Figure 2.
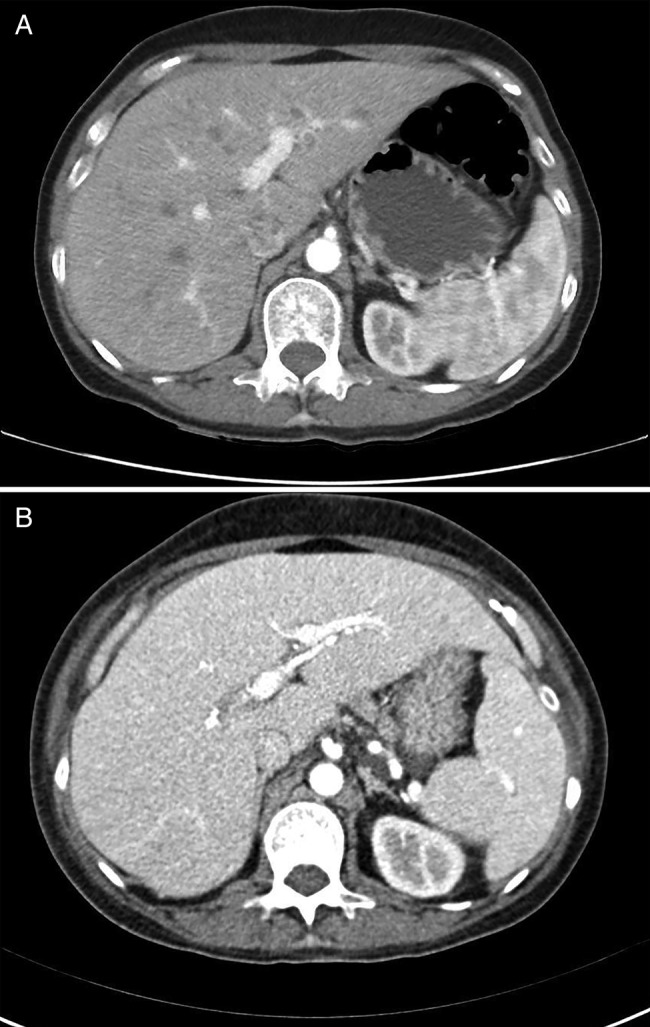
CT scan showing a radiological complete response. (A) was taken on 2 September, 2014 and (B) was taken 12 weeks after first dose of ipilimumab, on 5 November, 2014.
Figure 3.
Skin examinations previous and at week 12 after initiation of ipilimumab. We can observe regression in number and size of multiples skin metastases on the left limb and less swelling.
Two weeks after discharge, the patient was readmitted with an exacerbation of her colitis symptoms with grade 3 diarrhoea when she was still on oral prednisone 30 mg daily. Metilprednisolone (1 mg/kg/day IV) was immediately initiated. Repeated stool cultures were negative for bacterial growth and the toxin test for C. difficile was positive. She was also started on oral vancomycin (250 mg every 6 h) with partial resolution of symptoms. After negativisation of toxin with at least two confirmatory tests, a second dose of infliximab 5 mg/kg was administered with gradual improvement of diarrhoea. During the hospitalisation, a second colonoscopy was suggestive of chronic ipilimumab-mediated colitis with numerous infiltrating T cells. The patient continued with oral tapering steroid therapy and was finally discharged 3 weeks post second admission.
By June 2015, after 9 months after the first dose of ipilimumab, the patient remains in complete response, maintaining an ECOG Performance Status of 0 and her RA remains in clinical remission.
Discussion
We present the case of a patient with an autoimmune disease and metastatic BRAF-mutant melanoma, who achieved a remarkable response with ipilimumab with no reactivation of her RA, though experienced a life-threatening immune-mediated enterocholitis. After progression on several therapies, including BRAF inhibitors, we considered and discussed extensively with the patient the administration of ipilimumab because of the lack of alternatives and effective therapies at that time and stability of clinical activity of her RA.
The role of CTLA-4 in tumour cells evasion from the immune system has been proved thoroughly. CTLA-4 is a cell surface coreceptor strongly associated with attenuation of T-cell activation, and is an essential component of regulatory systems implied in peripheral immune tolerance. Furthermore, T-cells have shown to have a relevant role in RA. Binding of CTLA-4 to CD80/CD86 provides a control signal that suppresses ongoing T-cell activation. Based on this rational a CTLA4-Ig, abatacept, was developed for the treatment of RA. Its efficacy and safety were demonstrated in multiple trials and is currently approved for the treatment of moderate to severe RA after failure to methotrexate or other disease-modifying antirheumatic drugs including anti-TNF.2
Autoimmune diseases affect approximately 5% of population. Clinical trials involving immunotherapies for the treatment of cancer systematically excluded patients with these disorders because drugs targeting molecules affecting mechanisms of self-tolerance could result in the development of autoimmune disease symptoms. However, advances in immunobiology have entailed the incorporation of immunotherapies, such as anti-CTLA4 and anti-PD1 antibodies, into the daily activity of oncologists in a broad sort of cancers and experience in the management and decision-making process for patients with autoimmune diseases is lacking. The case herein reported shows how these patients can benefit from immunooncological treatments without worsening the underlying autoimmune disease.
To our knowledge, only case reports of patients affected by autoimmune disorders are available. These autoimmune diseases comprise RA (one case), multiple sclerosis (one case), ulcerative colitis (UC) (two cases) and Behcet disease (one case).3–5 Although most of these patients seemed to benefit from immunotherapies, only in one of the cases reported, authors described a severe steroid-refractory colitis in a patient with UC, being unable to distinguish between an aggravation of the UC or an irAEs. In the case of RA, the patient maintained treatment with weekly metotrexate 15 mg and low-dose prednisone through the ipilimumab course and experienced no disease reactivation (only slight increase of bilateral knee pain that was consistent with known osteoarthrosis rather than RA and was effectively treated with celecoxib). However, our patient presented a high-grade enterocolitis without worsening of her RA. Although gastrointestinal adverse effects such as diarrhoea and colitis are among the most frequent irAEs described in the literature for ipilimumab and this event may be completely independent of the underlying diagnosis of RA, we cannot exclude an immune-based predisposition.
Until specific trials and immunobiology of cancer and autoimmune diseases shed light into the role of the immune checkpoint inhibitors in both situations, clinicians should be cautious. In the meantime, the clinical experience of single cases should be taken into account and autoimmune diseases may not be considered an absolute contraindication but a special condition in which risks and benefits must be thoroughly evaluated.
Footnotes
Competing interests: A. Arance: advisory board and lecture honorarium from Novartis, Roche, BMS and MSD. J Malvehy: advisory board and honorarium from Novartis, Roche, BMS, MSD and Amgen.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Bertrand A, Kostine M, Barnetche T et al. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 2015;13:211 10.1186/s12916-015-0455-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruderman EM, Pope RM. Drug Insight: abatacept for the treatment of rheumatoid arthritis. Nat Clin Pract Rheumatol 2006;2:654–60. 10.1038/ncprheum0345 [DOI] [PubMed] [Google Scholar]
- 3.Kyi C, Carvajal RD, Wolchok JD et al. Ipilimumab in patients with melanoma and autoimmune disease. J Immunother Cancer 2014;2:35 10.1186/s40425-014-0035-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedersen M, Andersen R, Nørgaard P et al. Successful treatment with Ipilimumab and Interleukin-2 in two patients with metastatic melanoma and systemic autoimmune disease. Cancer Immunol Immunother 2014;63:1341–6. 10.1007/s00262-014-1607-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostwick AD, Salama AK, Hanks BA. Rapid complete response of metastatic melanoma in a patient undergoing ipilimumab immunotherapy in the setting of active ulcerative colitis. J Immunother Cancer 2015;3:19 10.1186/s40425-015-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]