Abstract
Background
The progesterone receptor (PR) is expressed by ∼70% of early breast tumours and is implicated in the progression of breast cancer. In cancerous tissues PR may be activated in the absence of a ligand, or when ligand concentrations are very low, resulting in aberrantly activated PR (APR). The presence of APR may indicate that patients with breast cancer are more likely to respond to antiprogestins. The aims of this study were to describe and classify the histological subnuclear morphology of active and inactive PR in archival breast cancer samples.
Methods
Archived tumour specimens from 801 women with invasive breast cancer were collected. Tissue samples (n=789) were analysed for PR isoforms A and B (PRA and PRB), Ki67 and estrogen receptors (ERα) status, using immunohistochemistry. Medical records were used to determine human epidermal growth factor 2 (HER2) status, tumour stage and grade.
Results
A total of 79% of tumours stained positive for either PRA or PRB, and of these 25% of PRA-positive and 23% of PRB-positive tumours had PR present in the activated form. APRA was associated with higher tumour grade (p=0.001). APRB was associated with a higher tumour grade (p=0.046) and a trend for a more advanced stage. Patients with PR-positive tumours treated with antiestrogens had better disease-free survival (DFS) than those with PR-negative tumours (p<0.0001). Cumulative progression rate and DFS were similar irrespective of APR status. Both APRA and APRB were independent of HER2, ERα and Ki67 expression.
Conclusions
APR had a binary mode of expression in the breast cancer specimens tested, allowing separation into two tumour subsets. APR is an independent target at the cellular and tumour level and may therefore be a suitable predictive marker for antiprogestins, such as onapristone. Using the described technique, a companion diagnostic is under development to identify APR in solid tumours.
Keywords: breast cancer, onapristone, activated, progesterone receptor
Key questions.
What is already known about this subject?
There is a lack of sufficiently sensitive diagnostics to identify patients with breast cancer who may respond to antiprogestin therapy.
What does this study add?
Immunohistochemistry (IHC) was used to classify the histological subnuclear morphology of activated progesterone receptors (APR) in archival primary breast cancer samples.
How might this impact on clinical practice?
This routine diagnostic IHC technique has the potential to identify patients with APR who may be responsive to antiprogestin therapies, such as onapristone.
Introduction
Estrogen receptors (ERα) are expressed by ∼75% of human breast cancers.1–3 Hormonal therapies, which act by blocking ERα binding or depriving the tumour of estrogen, have become the mainstay of treatment of patients with breast cancer with ERα-positive tumours.1 2 Progesterone receptor(PR), which is expressed in ∼70% of early breast cancers, has also been implicated in the progression of breast cancer.2 4 5 Antiprogestins have been shown to have antiproliferative activity in vitro: examples from animal models have shown both antiproliferative and proliferative effects but until now only limited antitumoural activity has been reported in a clinic setting.5–10 Gaining a more complete understanding of the actions of PR in breast cancer is of considerable clinical importance for the optimisation of treatment. Despite investigations into the use of antiprogestins to target PR, only onapristone, which is currently in clinical development, has demonstrated substantial activity in patients with breast cancer.9–11
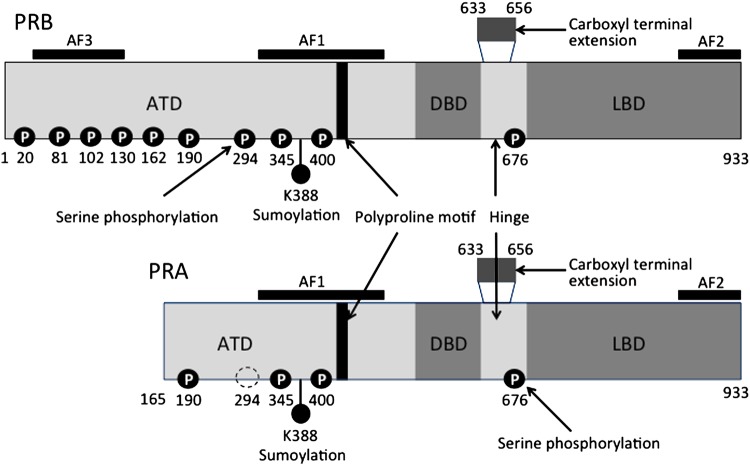
PR exists in two structurally distinct primary isoforms, PRA and PRB. Both isoforms are transcribed from a single gene but are regulated by two distinct tandem promoters.12 PRA (97 kDA) is smaller than PRB (120 kDA) because it lacks 164 amino acids at the N-terminus (figure 1).12–14 In normal human tissue, the levels of expression of PRA and PRB, within the same cells, are usually comparable.15 However, the progression of tissue from normal to malignant is reported to be associated with imbalances in the expression of PR isoforms.15 16
Figure 1.
Progesterone receptor (PR)A and PRB isoform receptor domains and post-translational modifications. PRs exist as either A (97 kDa) or B (120 kDa) translated from the same gene by the use of alternate promoters. Each isoform contains a C-terminal ligand-binding domain (LBD), a DNA-binding domain (DBD) and at least two transcriptional activation function (AF) domains. AF1 and AF2 are located within the aminoterminal domain (ATD) and the LBD, respectively. PRB contains an additional AF (AF3) within the unique 164-amino acid B-upstream segment. Adapted from Lange;13 Hill KK, et al.14
Ligand binding causes PR to undergo a sequence of conformational changes: it dimerises and translocates into the nucleus where it forms a complex with other cofactors.17–19 The functional PR complex binds to specific DNA promoter sequences of PR-dependent genes, termed progesterone response elements (PREs).17–19
Two subnuclear morphological PR distribution patterns have been previously reported in breast tumours, and are indicative of transcriptional activation status.20 Using standard, high-magnification microscopy techniques, activated ligand-bound PR can be seen as distinctive foci or in an aggregated (A) pattern.20 When not bound to a ligand, inactive PR is distributed in a diffuse (D) pattern across the nucleus. This same phenomenon has been observed in a healthy endometrial tissue and endometrial cancer.21
Post-translational modifications, which include phosphorylation, acetylation, ubiquitination and sumoylation, play roles in PR activation and regulation.4 9 Phosphorylation promotes PR binding to PREs and association with transcription specificity protein (Sp) factors. PR is known to contain at least 14 phosphorylation sites, which are serine residues concentrated at the N-terminus (figure 1).13 Kinases such as mitogen activated protein kinase, cyclin A/cyclin-dependent protein kinase 2 and casein kinase II are known to be involved in the phosphorylation process.13 In cancerous tissues, where kinase activities are often high, PR may be phosphorylated in the absence of a ligand or when ligand concentrations are very low.4 In addition to increased kinase activity, growth factors may reduce or supplant the need for a progestin ligand, resulting in aberrantly activated PR (APR).4 Studies have shown that APR is present in a significant number of postmenopausal breast and endometrial tumours.20 21 Thus, the presence of APR foci/aggregates in breast cancer cells provides a strong rationale for the use of antiprogestins.
We hypothesise that determination of APR could be developed as a diagnostic to identify patients with PR-positive (PRpos) cancers that are more likely to respond to antiprogestin treatment. Immunohistochemistry (IHC) diagnostic tests to identify the activated forms of steroid receptors were previously described in breast and endometrial cancers.22–24 We have developed an IHC-based technique to determine APR status, which is potentially applicable as a routine diagnostic.
In this study we described and classified the histological subnuclear morphology of APR and inactive PR in archival breast cancer samples, using IHC. We also analysed the relationships between APR and antiestrogen treatment outcomes.
Methods
Ethics consent and permissions
Archived specimens with previously determined ERα and PR status from 801 women with early invasive breast cancer were collected from Oscar Lambret Cancer Center, Lille, France. Informed consent for the research use of archived specimens had been previously obtained and was compliant with local legislation. This was standard practice at Oscar Lambret Cancer. Ethics Committee approval was not necessary for this type of retrospective study in France at the time.
Immunohistochemistry
IHC was carried out on 10% formalin fixed paraffin embedded tissue sections using a Tissue-Tek DRS 2000 system, Sakura Fine-Tek Europe BV Alphen aan den Rijn, the Netherlands. For antigen retrieval, dewaxed, 3–4 μm tissue sections were autoclaved in citrate buffer (pH 6.1) for 20 min at 95–99°C. After washing in wash buffer at room temperature for 5–10 min, the sections were processed to quench the endogenous peroxidase activity with 200 μL EnVision +/HRP mouse or rabbit (3,30-diaminobenzidine tetrahydrochloride (DAB)+) peroxidase block for 5 min, and then rinsed in fresh wash buffer for 5 min. The sections were incubated with each primary antibody, antimouse monoclonal PRA antibody (clone 16, 1:500, Novocastra 35033), antimouse monoclonal PRB antibody (clone SAN27, 1:500, Novocastra, 31146) and antimouse monoclonal Ki67 (clone MIB-1, 1:100, Dako M7240), or antirabbit monoclonal ERα antibody (clone SP1, 1:500, Thermo Scientific, MA1-39540) for 60 min at room temperature. Negative controls were obtained by substitution of the primary antibodies with isoform control mouse IgG1 (DAK-GO1, 1:125) or rabbit polyclonal IgG fraction (Dako X0936, 1:3500) with antibody diluent alone (wash buffer negative control) in the IHC staining procedure. Secondary labelled-polymer-HRP antimouse or antirabbit antibody was applied for 30 min at room temperature followed by 5 min in wash buffer. The PR, ERα and Ki67 were visualised by immersing the sections in 1 mL of DAB substrate buffer containing 25–30 μL of DAB+ chromogen for 5 min, and washed with wash buffer for 5 min. Each section was counterstained using the Tissue-Tek DRS 2000 autostainer light counterstain programme.
Stained specimens were assessed using a Zeiss Axioscope microscope. Human epidermal growth factor 2 (HER2) status, stage, grade, date of diagnosis, treatments and follow-up were obtained from pathological or medical records. Two different PR staining patterns were observed: a D pattern characterised by diffuse and homogeneous fine granular staining and an A pattern by mottled and heterogeneous staining of aggregates or foci. In the first set of analyses, both IHC and immunofluorescence and confocal microscopy were employed. Both the techniques were found to be concordant (data not shown) so only IHC was used for the remaining analyses.
Analyses
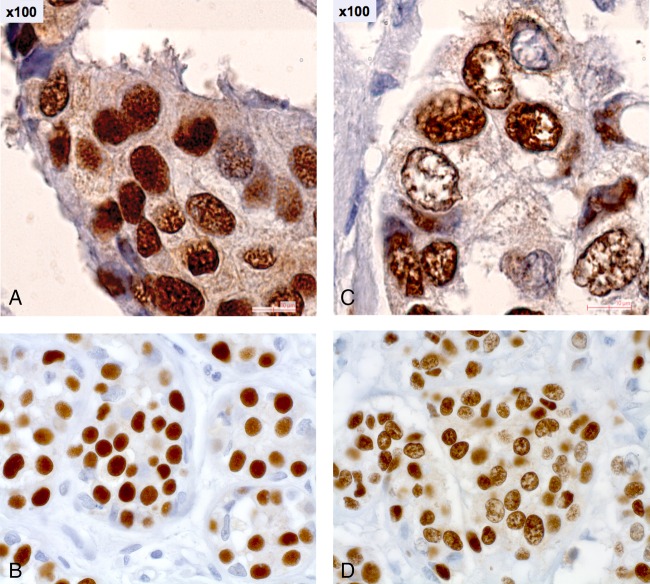
Tumours were deemed PRpos if >1% of tumour cells were stained positive with either PRA or PRB. Tumours with >1% of tumour cells that stained positive with Thermo Scientific SP1 were classified as being ERα-positive (ERαpos). Ki67 positivity was determined as at least one staining-positive cell using the Dako M7240 reagent, using an internal control (epithelial cells or lymphocytes) to assess the reliability of the Ki67 expression. Staining intensity was ranked on a scale from 0 to 3 (0: no staining; 1: weak intensity, partly specific; 2: moderate intensity; 3: strong intensity). Note that APR-negative (APRneg) tumours are composed of cells that only exhibit the D pattern, while APR-positive (APRpos) tumours include the cases with more than 5% of tumour cells with the A pattern (figure 2). Therefore, APRpos tumours may have cellular targets of antiprogestins whereas APRneg tumours, by definition, do not.
Figure 2.
Progesterone receptor nuclear morphology patterns in breast cancer. (A) Diffuse pattern: homogeneous and diffuse fine granular distribution in the nuclei of tumour cells (×100). (B) Diffuse pattern: homogeneous and diffuse fine granular distribution in the nuclei of tumour cells (×40). (C) Aggregated pattern: heterogeneous distribution of moderate and large spots in the nuclei of tumour cells (×100). (D) Aggregated pattern: heterogeneous distribution of moderate and large spots in the nuclei of tumour cells (×40).
The total number of samples in each analysis was based on those patients for whom data were available. Descriptive statistics included Fisher's exact test for categories and Kruskal-Wallis test for continuous variables. Cox modelling analysis for disease-free survival (DFS) used ERα and PR isoform and HER2 status as factors.
Results
Of the 789 primary breast cancer cases evaluable for staging, 376 (48%) were stage I, 356 (45%) stage II and 57 (7%) were stage III. Analyses populations differed due to missing data. A total of 776 available breast cancer tumour specimens were analysed. A total of 623 (90%) patients had received antiestrogen/aromatase inhibitor (AI) therapy. Median follow-up for DFS was 41 months. The mean age of the evaluable patients was 57 years (17–89 years). The ERα status and PRA and/or PRB status of the samples is summarised in table 1. Overall, 72% (552/764) of tumours were both ERαpos and PRpos (table 1). A total of 79% (600/764) of tumours stained positive for either PRA (PRApos) or PRB (PRBpos; table 1). Overall, 13.1% of specimens only expressed one PR isoform: PRA 5.1% and PRB 8.0% of all PRpos cases (table 2). Eight per cent of all PRpos cases were ERα-negative (ERαneg).
Table 1.
Cross tabulation of ER and PR positivity in BC
| PR (PRA or PRB) n (%) |
Total | ||
|---|---|---|---|
| ERα | PRneg | PRpos | Total |
| ERαneg | 93 (12.2) | 48 (6.3) | 141 (18.5) |
| ERαpos | 71 (9.3) | 552 (72.3) | 623 (81.5) |
| Total | 164 (21.5) | 600 (78.5) | 764 (100) |
BC, breast cancer; ER, estrogen receptor; PR, progesterone receptor; PRneg, PR-negative; PRpos, PR-positive.
Table 2.
Activated progesterone receptor (PR) status by PR isoform
| PRB status n (%) |
||||
|---|---|---|---|---|
| PRA status | Aggregated pattern | Diffuse pattern | Negative | Total |
| Aggregated pattern | 90 (15.7) | 41 (7.2) | 3 (0.5) | 134 (23.4) |
| Diffuse pattern | 34 (5.9) | 332 (58.0) | 26 (4.6) | 392 (68.5) |
| Negative | 2 (0.4) | 44 (7.7) | 0 (0.0) | 46 (8.0) |
| Total | 126 (22.0) | 417 (72.9) | 29 (5.1) | 572 (100) |
Using both specific antibodies was justified to fully characterise APR due to the incomplete overlap of PRA and PRB expression in the tumours (table 2). Of all the PRpos tumours, 30% (170/572) stained positive for APR (APRpos; table 2). Of the PRApos tumours, 25% (134/538) were APRpos (table 2). APRpos was associated with a higher percentage of PRApos (p=0.0003) and higher tumour grade (p=0.001; table 3). APRpos was independent of HER2, ERα, Ki67 and staining intensity (data not shown). Note: Ki67 was available only in 467 cases.
Table 3.
Tumour characteristics by PR isoform, nuclear pattern
| PRA n (%) |
PRB n (%) |
|||||
|---|---|---|---|---|---|---|
| Aggregated pattern | Diffuse pattern | Total | Aggregated pattern | Diffuse pattern | Total | |
| Stage | p=0.18 | p=0.040* | ||||
| I | 58 (10.9) | 193 (36.3) | 251 (47.2) | 50 (9.1) | 216 (39.3) | 266 (48.4) |
| II | 65 (12.2) | 181 (34.0) | 246 (46.2) | 64 (11.6) | 181 (32.9) | 245 (44.6) |
| III | 13 (2.4) | 22 (4.1) | 35 (6.6) | 13 (2.4) | 26 (4.7) | 39 (7.1) |
| Total | 136 (25.6) | 396 (74.4) | 532 (100.00) | 127 (23.1) | 423 (76.9) | 550 (100.0) |
| Histology | p=1 | p=0.477 | ||||
| Ductal | 118 (22.0) | 345 (64.3) | 463 (86.2) | 108 (19.4) | 373 (67.1) | 481 (86.5) |
| Lobular | 16 (3.0) | 49 (9.1) | 65 (12.1) | 19 (3.4) | 48 (8.6) | 67 (12.1) |
| Other | 2 (0.4) | 7 (1.3) | 9 (1.7) | 1 (0.2) | 7 (1.3) | 8 (1.4) |
| Total | 136 (25.3) | 401 (74.7) | 537 (100.0) | 128 (23.0) | 428 (77.0) | 556 (100.0) |
| SBR† grade | p=0.001 | p=0.046 | ||||
| I | 21 (4.0) | 125 (23.8) | 146 (27.8) | 24 (4.4) | 123 (22.7) | 147 (27.2) |
| II | 85 (16.2) | 206 (39.2) | 291 (55.3) | 82 (15.2) | 225 (41.6) | 307 (56.8) |
| III | 28 (5.3) | 61 (11.6) | 89 (16.9) | 21 (3.9) | 66 (12.2) | 87 (16.1) |
| Total | 134 (25.5) | 392 (74.5) | 526 (100.0) | 127 (23.5) | 414 (76.5) | 541 (100.0) |
| HER2 | p=0.776 | p=0.778 | ||||
| Negative | 109 (22.0) | 311 (62.8) | 420 (84.9) | 102 (19.8) | 331 (64.2) | 433 (83.9) |
| Positive | 18 (3.6) | 57 (11.5) | 75 (15.2) | 18 (3.5) | 65 (12.6) | 83 (16.1) |
| Total | 127 (25.7) | 368 (74.3) | 495 (100.0) | 120 (23.3) | 396 (76.7) | 516 (100.0) |
Analyses populations differed due to missing data.
*Trend APR—more advanced stage.
†Scarff-Bloom-Richardson.
APR, activated progesterone receptor; HER2, human epidermal growth factor 2; PR, progesterone receptor.
A total of 23% (126/543) of PRBpos tumours were classified as activated (table 2). Activated PRBpos (APRpos) was associated with a higher percentage of PRpos (p=0.001; figure 3), a higher tumour grade (p=0.046) and a trend for more advanced stage (table 3). APRpos was associated with a lower staining intensity (p=0.003) and was independent of HER2 (table 3), ERα and Ki67 (data not shown).
Figure 3.
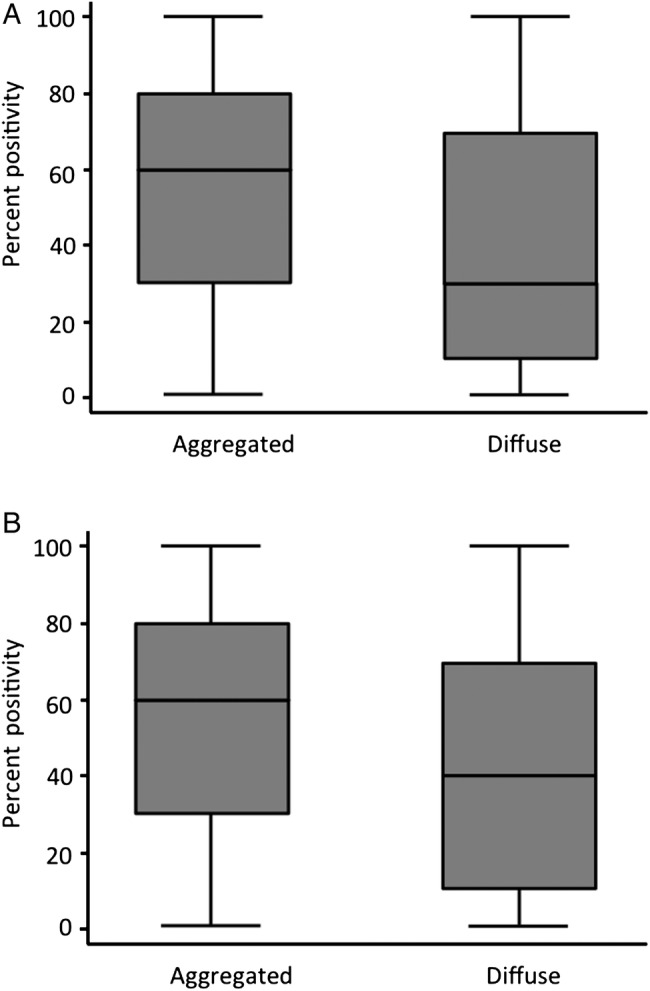
Per cent progesterone receptor (PR) positivity as a function of activated PR pattern. Each box corresponds to the range of percentage values of PRA (A) or PRB (B) stained tumour cells. The limits of the boxes show the SD of the distribution, and the line the extremes, while the line inside the box is the average. The left panel compared the distribution of the percentages for aggregated pattern (activated) and diffuse pattern (inactive). The same is shown on the right for PRA. It is apparent that the boxes for each PR are not at the same levels, and in particular that the average lines are at different levels, for example, the average percentages are different. The tests used in a Kruskal-Wallis test confirm that the distributions are different between aggregated and diffuse. (A) p=0.0003; (B) p=0.001.
APR was associated with higher grade tumours (table 3). APR was not predictive of outcome with antiestrogens and AIs (p=0.4). PRpos tumours treated with hormonal therapy had better DFS than the PR-negative tumours (PRneg; p<0.0001). The results were the same with PRA and PRB (data not shown). This effect was independent of the prognostic effect of ERα. DFS and cumulative progression rate were similar, irrespective of APR status.
Discussion
Most breast cancers express both ERα and PR. In this series of primary breast cancers, stained using specific antibodies for PRA, PRB and ERα, we found that 79% of tumours were PRpos. Of the tumours that were PRpos a significant proportion (30%) were APRpos. APR had a binary mode of expression in the breast cancer specimens tested, which allows the classification of tumours into two subsets. One subset corresponded morphologically to tumours containing cells that express the transcriptionally active form of PR and may indicate tumours suitable for treatment with antiprogestins, such as onapristone.
Previous observations have shown that PRneg tumours are associated with poorer outcomes.25 As expected, we found that patients with PRpos tumours had a better prognosis than those with PRneg tumours.
When individual PR isoforms were examined, we found that the percentage of APRpos tumours were 25% for PRA and 23% for PRB. As reported previously, imbalanced expression of PR isoforms is a common trait of breast and endometrial tumours.15 16 26–28 The loss of one isoform has been reported in higher histological grades of endometrial cancer.27 The loss of PRB expression in breast cancer may be explained by phosphorylation-dependent turnover of transcriptionally active PRB compared with the less active and more stable PRA.4 We observed a small imbalance in tumour expression of PRA and PRB with 5.1% of tumours expressing PRA only and 8.0% of tumours expressing PR (table 2). These results highlight the importance of using two antibodies instead of one single bispecific antibody, as this has consequences for biomarker positivity.28 29 There was a trend towards more APR positivity among patients with more advanced stages of breast cancer. This trend was more evident with the PRB isoform compared with PRA (p=0.040 vs p=0.18).
APRpos was also associated with both the per cent and intensity of PR cell-positive staining. In this series of breast cancer samples, APR status was independent of the per cent of tumour cells expressing ERα, Ki67 and HER2. This suggests that targeting APR, using a therapy with a distinct mechanism of action compared with the currently available therapies, has the potential for independent treatment benefits and should not be cross-resistant.
The development of therapies that target tumours expressing hormones and other receptors including ERα and HER2 has transformed treatment outcomes for patients with breast cancer. Recent studies have renewed interest in the potential of PR as an independent target for treatment with antiprogestins, such as onapristone. A routine diagnostic technique for identifying patients who are likely to respond to antiprogestin therapy would be a major advantage. Regular immunofluorescence and confocal microscopy are not practical for identifying APR on a routine clinical basis. We have developed a generalisable diagnostic technique to identify APR in endometrial and breast tumours. The diagnostic development plan has been reviewed by the regulatory authorities and will be moving forward as part of the registration strategy for the development of onapristone. This technology has now been migrated onto a commercial platform (Leica Biosystems' Bond-III) and is currently undergoing analytical validation.
Conclusion
Breast cancer tumour classification is essential for choosing treatments and optimising outcomes. Despite being correlated with a prognostic factor like tumour grade, APR is not independently prognostic. APR is a novel target at the cellular and tumour level and may therefore be a suitable marker for tailoring treatment with antiprogestins, such as onapristone. A companion diagnostic is under development to identify APR in endometrial, breast, prostate and other PRpos tumours. Further studies are required to assess the efficacy of antiprogestins in patients with breast cancer with tumours expressing APR.
Acknowledgments
The authors would like to thank Alice Bexon, Matthew Reid and Karen Rittweger for medical writing, and Emilie Hutt for reviewing the patients' clinical files.
Footnotes
Contributors: JBon provided archived tumour samples and clinical information from the clinical centre. JBos provided central pathology review and read development. PJ provided technical method development and execution. AV provided technical method development and execution. EMG was the concept originator and provided scientific direction and statistical analyses. AAZ provided sponsorship, strategic scientific input and direction of companion diagnostic development (APR test). SAWF provided scientific guidance in design, data review and interpretation. CAL provided design input, data review, data interpretation and guidance on presentation. JO provided disease area expertise in design and execution, advice and guidance during execution, data review and interpretation, and guidance on presentation.
Funding: ARNO Therapeutics.
Competing interests: JBon is an investigator of an ARNO Therapeutics study and has received honoraria payments, research credits and trip fees for presentations at various congresses from Invivis Pharmaceuticals Inc. PJ has received travel fees for presentations at various congresses from Invivis Pharmaceuticals Inc. EMG holds a leadership position at Invivis Pharmaceuticals Inc. AAZ is the CEO of ARNO Therapeutics Inc. JO has received honoraria payments from ARNO Therapeutics Inc.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Zhao M, Ramaswamy B. Mechanisms and therapeutic advances in the management of endocrine-resistant breast cancer. World J Clin Oncol 2014;5:248–62. 10.5306/wjco.v5.i3.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Purdie CA, Quinlan P, Jordan LB et al. . Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study. Br J Cancer 2014;110:565–72. 10.1038/bjc.2013.756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadji M, Gomez-Fernandez C, Ganjei-Azar P et al. . Immunohistochemistry of estrogen and progesterone receptors reconsidered experience with 5,993 breast cancers. Am J Clin Pathol 2005;123:21–7. 10.1309/4WV79N2GHJ3X1841 [DOI] [PubMed] [Google Scholar]
- 4.Hagan CR, Lange CA. Molecular determinants of context-dependent progesterone receptor action in breast cancer. BMC Med 2014;12:32 10.1186/1741-7015-12-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanari C, Wargon V, Rojas P et al. . Antiprogestins in breast cancer treatment: are we ready? Endocr Relat Cancer 2012;19:R35–50. 10.1530/ERC-11-0378 [DOI] [PubMed] [Google Scholar]
- 6.Zukiwski A, Gilles EM, Serin G et al. . Comparative assessment of in vitro activity and activated progesterone receptor (APR) biomarker predictivity for multiple antiprogestins [abstract]. Cancer Res 2015;75(Suppl 15):P3471 10.1158/1538-7445.AM2015-3471 [DOI] [Google Scholar]
- 7.Vanzulli SI, Soldati R, Meiss R et al. . Estrogen or antiprogestin treatment induces complete regression of pulmonary and axillary metastases in an experimental model of breast cancer progression. Carcinogenesis 2005;26:1055–63. 10.1093/carcin/bgi060 [DOI] [PubMed] [Google Scholar]
- 8.Sartorius CA, Tung L, Takimoto GS et al. . Antagonist-occupied human progesterone receptors bound to DNA are functionally switched to transcriptional agonists by cAMP. J Biol Chem 1993;268:9262–6. [PubMed] [Google Scholar]
- 9.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev 2013;34:130–62. 10.1210/er.2012-1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonat W, Giurescu M, Robertson JFR. The clinical efficacy of progesterone antagonists in breast cancer. In: Robertson JFR, Nicholson RI, Hayes DF, eds. Endocrine therapy of breast cancer. London: Martin Dunitz Ltd, 2002:117–24. [Google Scholar]
- 11.Robertson JFR, Willsher PC, Winterbottom L et al. . Onapristone, a progesterone receptor antagonist as first-line therapy in primary breast cancer. Eur J Cancer 1999;35:214–18. 10.1016/S0959-8049(98)00388-8 [DOI] [PubMed] [Google Scholar]
- 12.Kastner P, Krust A, Turcotte B et al. . Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO 1990;9:1603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange CA. Making sense of cross-talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol Endocrinol 2004;18:269–78. 10.1210/me.2003-0331 [DOI] [PubMed] [Google Scholar]
- 14.Hill KK, Roemer SC, Churchill MEA et al. . Structural and functional analysis of domains of the progesterone receptor. Mol Cell Endocrinol 2012;348:418–29. 10.1016/j.mce.2011.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mote PA, Bartow S, Tran N et al. . Loss of co-ordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat 2002;72:163–72. 10.1023/A:1014820500738 [DOI] [PubMed] [Google Scholar]
- 16.Graham JD, Yeates C, Balleine RL et al. . Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Res 1995;55:5063–8. [PubMed] [Google Scholar]
- 17.Lange CA. Challenges to defining a role for progesterone in breast cancer. Steroids 2008;73:914–21. 10.1016/j.steroids.2007.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards DP, Altmann M, DeMarzo A et al. . Progesterone receptor and the mechanism of action of progesterone antagonists. J Steroid Biochem Mol Biol 1995;53:449–58. 10.1016/0960-0760(95)00091-D [DOI] [PubMed] [Google Scholar]
- 19.Scarpin KM, Graham JD, Mote PA et al. . Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nucl Recept Signal 2009;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnett-Mansfield RL, Graham JD, Hanson AR et al. . Focal subnuclear distribution of progesterone receptor is ligand dependent and associated with transcriptional activity. Mol Endocrinol 2007;21:14–29. 10.1210/me.2006-0041 [DOI] [PubMed] [Google Scholar]
- 21.Arnett-Mansfield RL, DeFazio A, Mote PA et al. . Subnuclear distribution of progesterone receptors A and B in normal and malignant endometrium. J Clin Endocrinol Metab 2004; 89:1429–42. 10.1210/jc.2003-031111 [DOI] [PubMed] [Google Scholar]
- 22.Graham D, Bosq J, Caillaud JM et al. . Determination of the activated form of the progesterone receptor (PR) in endometrial cancer (EC) [abstract]. ASCO University, 2013. http://meetinglibrary.asco.org/content/82578 (accessed 1 Feb 2016). [Google Scholar]
- 23.Bosq J, Caillaud JM, Lange CA et al. . Identification of the activated form of the progesterone receptor (PR) in breast cancer (BC) [abstract]. ASCO University, 2013. http://meetinglibrary.asco.org/content/85461?media=vm (accessed 1 Feb 2016). [Google Scholar]
- 24.Hutt E, Bosq J, Gilles EM et al. . Clinical and pathological correlation of the activated form of the estrogen receptor (ERβ) in breast cancer (BC) [abstract]. ASCO University, 2014. http://meetinglibrary.asco.org/content/96030?media=vm&poster=1 (accessed 1 Feb 2016). [Google Scholar]
- 25.Bardou VJ, Arpino G, Elledge RM et al. . Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases . J Clin Oncol 2003;21:1973–9. 10.1200/JCO.2003.09.099 [DOI] [PubMed] [Google Scholar]
- 26.Hopp TA, Weiss HL, Hilsenbeck SG et al. . Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res 2004;10:2751–60. 10.1158/1078-0432.CCR-03-0141 [DOI] [PubMed] [Google Scholar]
- 27.Arnett-Mansfield RL, deFazio A, Wain GV et al. . Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Res 2001;61:4576–82. [PubMed] [Google Scholar]
- 28.Mote PA, Johnston JF, Manninen T et al. . Detection of progesterone receptor forms A and B by immunohistochemical analysis . J Clin Pathol 2001;54:624–30. 10.1136/jcp.54.8.624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kariagina A, Aupperlee MD, Haslam SZ. Progesterone receptor isoform functions in normal breast development and breast cancer. Crit Rev Eukaryot Gene Expr 2008;18:11–33. 10.1615/CritRevEukarGeneExpr.v18.i1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]