Abstract
Small cell lung cancer (SCLC) is a very aggressive disease, characterised by rapid growth, high response rates to both chemotherapy and radiotherapy and subsequent development of treatment resistance in the vast majority of patients. In the past 30 years, little progress has been made in systemic treatments and the established management paradigm of platinum-based chemotherapy has reached an efficacy plateau. Several clinical trials have investigated targeted therapies, without producing clinically significant benefits. Recently presented early phase clinical trials with immune checkpoint inhibitors (blockade of the cytotoxic T-lymphocyte antigen-4 (CTLA-4) and blockade of the programmed cell death-1 (PD-1) receptor) have shown promising results. In this review, we present the emerging evidence on immune checkpoint blockade for SCLC.
Keywords: small cell lung cancer, nivolumab, ipilimumab, pembrolizumab, immune checkpoint
Introduction
Lung cancer represents the most common cause of cancer-related deaths worldwide with more than 1.2 million new cases diagnosed each year and 1.3 million deaths annually.1
Small cell lung cancer (SCLC) accounts for ∼13–15% of all bronchogenic carcinomas. Smoking is the primary cause of this tumour type and 90% of SCLC arise in current or former smokers.2 SCLC is an aggressive disease characterised by rapid growth, early metastasis and early sensitivity to chemotherapy and radiotherapy (RT). Unfortunately, at the time of diagnosis, only 30% of patients with SCLC have tumours confined to the hemithorax of origin, the mediastinum or the supraclavicular lymph nodes. On the basis of the anatomical extent of the disease, as proposed originally by the Veterans Administration Lung Study Group, these patients are designated as having limited-stage disease (LS).3 Patients with tumours that have spread beyond the supraclavicular areas are considered to have extensive-stage disease (ES). This staging system is accepted in clinical practice, but the Tumor Nodes Metastasis (TNM) staging system should also be applied to SCLC.4 In the TNM system, LS includes stages I–IIIB, whereas ES is applied to patients with distant metastases. Regardless of stage, despite improvements in diagnosis and therapy made during the past 25 years, the current prognosis for patients with SCLC is poor. Without treatment, median survival is about 2–4 months. Patients with LS have a median survival of 18–24 months; about 20% of them can achieve long-term remissions with concurrent chemoradiotherapy, and in this setting, a 5-year survival of 14% has been reported.5 On the other hand, median survival of patients with ES-SCLC ranges from 8 to 10 months. Surgery has a limited role in the current management of LS-SCLC and it is considered only for stage I patients (<5% of total) with T1-2 N0-1 M0 disease. These patients should then be considered for adjuvant chemotherapy and, in case of unforeseen N2 or N1 or in patients who have not undergone systematic nodal dissection, postoperative RT should be discussed on a case-by-case basis. In patients with completely resectable stage I disease, the largest study evaluating surgical resection with curative intent followed by adjuvant platinum chemotherapy reported an impressive 5-year survival rate of 86%.6 The gold standard treatment for T1-4 N0-3 M0 tumours and good performance status (PS) is concurrent chemotherapy and thoracic RT. Standard first-line chemotherapy is platinum and etoposide, producing an objective response rate of 70–80%. This is better tolerated than the non-platinum-containing regimen of cyclophosphamide, vincristine plus an anthracycline (doxorubicin or epirubicin as in cyclophosphamide/doxorubicin/vincristine or cyclophosphamide/epirubicin/vincristine).7 Concurrent chemoradiotherapy has been shown to be superior to sequential chemoradiotherapy in terms of overall survival (OS) and accelerated hyperfractionation is also preferred over daily radiation dosing.8 Turrisi et al5 showed that 45 Gy administered twice daily, in fractions of 1.5 Gy over 3 weeks, conferred a significant survival improvement of 10% at 5 years compared with daily fractions (once daily) of 1.8 Gy over 5 weeks (26% vs 16%, respectively). Guidelines from the European Society for Medical Oncology (ESMO) suggest that patients with LS-SCLC with good PS should be treated with four cycles of platinum–etoposide with concurrent accelerated hyperfractionation RT (1.5 Gy twice daily for 30 fractions for a total dose of 45 Gy) starting with the second cycle of chemotherapy.9 The CONVERT study, presented at the American Society of Clinical Oncology (ASCO) meeting 2016, compared OS and toxicity of twice daily with once daily RT, using modern conformal RT techniques given concurrently with chemotherapy. The primary end point was 2-year survival and 547 patients were randomised 1:1 to receive 45 Gy in 30 twice daily fractions over 3 weeks or 66 Gy in 33 once daily fractions over 6.5 weeks starting on day 22 of cycle 1 chemotherapy (4–6 cycles of cisplatin 25 mg/m2 days 1–3 or 75 mg/m2 day 1 with etoposide 100 mg/m2 days 1–3), followed by prophylactic cranial irradiation (PCI) if indicated. RT was planned using three-dimensional conformal or intensity-modulated RT. At a median follow-up of 45 months, 2-year OS rate was 56% vs 51% and median OS was 30 vs 25 months (HR 1.17, 95% CI 0.95 to 1.45; p=0.15) for twice daily and once daily treatment, respectively. Toxicities were comparable, except for a significantly higher incidence of grade 3/4 neutropenia (74% twice daily vs 65% once daily, p=0.03). There was no statistical difference, between twice daily and once daily, respectively, in rates of febrile neutropenia (23.4% vs 18%), grade 2 oesophagitis (63% vs 55%), grade 3/4 oesophagitis (19% vs 19%) and grade 3/4 radiation pneumonitis (2.5% vs 2.2%), which was rare. In conclusion, using modern RT techniques, twice daily RT did not result in a superior survival or worse toxicity than once daily RT, supporting the use of both regimens for standard of care treatment of LS-SCLC with good PS. Interestingly, in this study, the survival rates for both regimens were higher than previously reported and radiation toxicities were lower than expected.10
Regarding stage IV SCLC (ES), the aim of the treatment is palliative and the standard of care is 4–6 cycles of platinum/etoposide chemotherapy. A recent meta-analysis of seven randomised studies showed that irinotecan plus platinum regimens improved OS but not progression-free survival (PFS), compared with platinum and etoposide. Irinotecan led to more gastrointestinal toxicity, while more haematological toxicity was observed with etoposide.11 It is important to highlight that pharmacogenomic differences between Asian and Western populations may contribute to these differential outcomes and that, until now, no chemotherapy doublet has yet been shown to be superior to intravenous platinum/etoposide in the western population.12 In patients with poor PS and/or severe comorbidities, carboplatin as a single agent could be considered and produces an objective response rate (ORR) of about 40%.13 PCI should be considered in all patients with LS-SCLC or patients with ES-SCLC with good PS, who have achieved partial response or stable disease to first-line chemotherapy. A meta-analysis of trials in LS demonstrated a 5.4% improvement in OS at 3 years and a significant decrease (relative risk 0.46; 95% CI 0.38 to 0.57; p<0.001) in the incidence of brain metastases at 3 years.14 The study by Slotman et al15 evaluating the role of PCI in patients with ES-SCLC not progressing after first-line platinum–etoposide showed a reduction in the risk of symptomatic brain metastases at 1-year (40.4% vs 14.6%; p<0.001) and also longer OS (p=0.003) in the PCI group. The CREST study investigated the role of palliative RT to the chest in patients with ES-SCLC who benefited from first-line platinum–etoposide. Despite the lack of a significant benefit in OS at 1-year, the primary end point (33% vs 28%; p=0.066), this trial showed significant improvements in terms of OS at 2 years (13% vs 3%, p=0.004) and PFS at 6 months (24% vs 7%; p=0.001) in the group of patients treated with palliative RT.16 Unfortunately, the vast majority of patients with SCLC will develop disease relapse and, according to the time in which the recurrence occurs, these patients have varying prognosis and treatment options. For patients, progressing during first-line treatment (platinum-refractory) or within 3 months since completion of first-line (platinum-resistant) prognosis is very poor and benefit from second-line chemotherapy is uncertain, and consequently, according to their PS, these patients should be assessed for clinical trials or best supportive care. If disease progression occurs later than 3 months since completion of chemotherapy (platinum-sensitive), these patients can be rechallenged with platinum–etoposide or treated with topotecan or an anthracycline-containing regimen such as vincristine/doxorubicin/cyclophosphamide or vincristine/epidoxorubicin/cyclophosphamide.17–19 Several biological agents have been investigated and, until now, none has shown a significant benefit in OS over standard treatment. The aim of this review is to describe recent advances in the field of immune checkpoint blockade in SCLC.
Cancer and the immune system
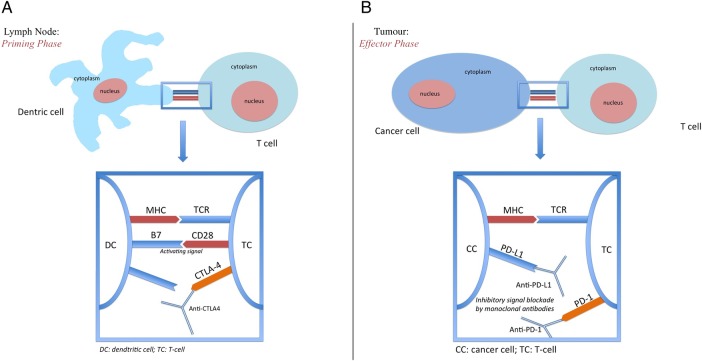
Cancer is characterised by genetic mutations that can lead to the expression of various tumour-related antigens. These antigens are presented to cytotoxic T-lymphocytes by antigen-presenting cells (APCs) and T-lymphocytes have the potential to recognise cancer-related antigens as ‘non-self’ and eradicate these cancer cells.20 The level of control that the immune system is able to exert against cancer is crucial and, based on the effectiveness of this control, three different conditions can be established. The first condition is that of ‘elimination’ in which the cancer cells are eliminated by the immune system. The second is the condition of ‘equilibrium’ in which the immune system, unable to eliminate cancer cells, prevents their growth and spread. Finally, in the third condition ‘escape’, the tumour cells have been successful in evading immune-related destruction.21 22 The activation of the immune system is recognised as an important treatment strategy against cancer. However, the mechanisms underlying the functioning of the immune system are complex and, in order to achieve an effective immune response against cancer, a series of steps must be carried out. First, the tumour-related antigen must be picked up and processed by the APCs (eg, macrophages, B-lymphocytes and dendritic cells). In the so-called priming phase, the APCs travel to the lymph nodes to present the processed antigens bound to major histocompatibility complex (MHC) molecules to the T-lymphocytes. Two signals are required to activate and prime the T-cell for its effector phase (ie, to respond against the cancer-related antigens). The first signal is represented by the binding of the T-cell receptor (TCR) to the MHC-bound antigen. The second signal involves the interaction between costimulatory molecules, such as B7 on activated APCs and CD28 expressed on T-lymphocytes.23 There are multiple costimulatory and coinhibitory pathways that act in order to regulate this process. Between the most important of these, for its relevance as an immune checkpoint, there is the upregulation of cytotoxic T-lymphocyte antigen-4 (CTLA-4) after T-cell activation that inhibits the T-cell response in order to maintain immune homoeostasis and avoid complications from immune overactivation (figure 1A). Once T-lymphocytes have been activated, they migrate to peripheral tissue to carry out the effector phase of the immune response. The effector T-lymphocyte will recognise the tumour cell through interaction between the TCR and MHC on the tumour cell. After the immune response has been mounted, the tumour is able to express programmed death-ligand 1 (PD-L1) on its surface. The subsequent binding between PD-L1 and programmed cell death-1 (PD-1) (on T-cell) will shut down the immune response and allow the tumour cells to escape death (figure 1B).
Figure 1.
(A) Lymph node priming phase: recognition of major histocompatibility complex (MHC) by T-cell receptor (TCR), coactivating CD 28/B7 pathway activation and subsequent intracellular transduction leading to T-cell activity. On the other hand, cytotoxic T-lymphocyte antigen-4 (CTLA-4) mediated downregulation of T-cell priming can be reverted with anti-CTLA-4 antibodies. (B) Effector phase: programmed cell death-1 (PD-1)/programmed death-ligand 1 inhibitory interaction in the tumour microenvironment that can be dampened by therapy with immune checkpoint blockade.
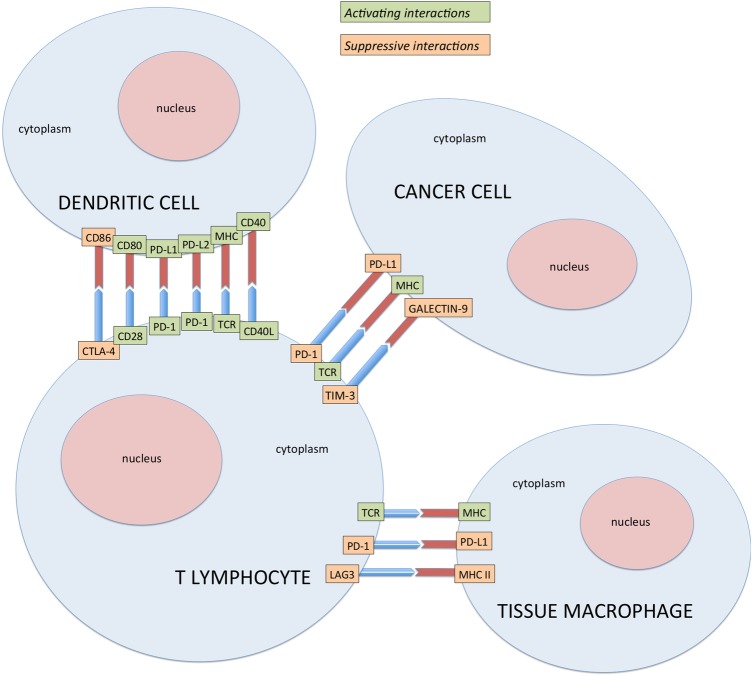
Immune checkpoint proteins are coinhibitory factors which reduce the antigen-specific immune response by limiting their magnitude and duration and include CTLA-4, PD-1, B7-H3, B7x, T-cell immunoglobulin and mucin-domain-containing molecule-3 (TIM-3), B-cell and T-cell lymphocyte attenuator (BTLA) lymphocyte-activation gene 3 (LAG3), killer cell immunoglobulin-like receptor (KIR) and V-domain Ig suppressor of T cell activation (VISTA) (figure 2).
Figure 2.
Activating and suppressive interactions between T-lymphocytes, tissue macrophages and dendritic cells in tumour microenvironment at molecular level. CTLA-4, cytotoxic T-lymphocyte antigen-4; MHC, major histocompatibility complex; PD-1, programmed cell death-1; TCR, T-cell receptor; TIM-3, T-cell immunoglobulin and mucin-domain-containing molecule-3.
In common solid tumours, such as colon, breast, brain or pancreatic cancer, an average of 33–66 genes display subtle somatic mutations that would be expected to alter their protein products. As a consequence, these tumours express neoantigens becoming recognisable by immune cells. Certain tumour types may have a lower or higher mutational load than average. Notably, among these outliers, SCLC has one of the highest mutational loads with ∼200 non-synonymous mutations per tumour. About 95% of these mutations are single-base substitutions and the mutational spectrum is dominated by C→A mutations, consistent with the exposure to the polycyclic aromatic hydrocarbons in tobacco smoke.24 The high mutational burden in SCLC reflects the involvement of potent mutagens in the pathogenesis of these tumour types (tobacco smoke) and, accordingly, other types of lung cancers arising in smokers also have 10 times as many somatic mutations as those from non-smokers.25 Whole-exome sequencing (WES) has enabled the comprehensive characterisation of somatic mutations in tumour samples and an ongoing effort is to employ a mutational landscape to identify patients who will be more likely to benefit from immune checkpoint blockade.26 For example, data from 64 patients with melanoma treated with CTLA-4 blockade indicate that a high mutational burden correlated with a sustained clinical benefit.27 WES was also used to unveil the genomic determinants of response to pembrolizumab in non-small cell lung cancer (NSCLC). A higher somatic non-synonymous mutation burden has been found to be associated with improved ORR, durable clinical benefit (DCB) (partial or stable response lasting >6 months) and PFS. The sensitivity and specificity using a non-synonymous mutation burden larger than 178 for DCB are 100% and 77% in the discovery cohort and 86% and 75% in the validation cohort, respectively. These results suggest that the mutational landscape shapes the response to anti-PD-1 therapy also in NSCLC. A possible explanation for the association between mutational burden and efficacy of checkpoint blockade is that tumour antigens, as a consequence of somatic mutations, may function as the target of T-cells activated by checkpoint blockade immunotherapy.28 On the basis of these data, SCLC may be an optimal target for immune checkpoint blockade and monoclonal antibodies that inhibit CTLA-4, PD-1 and PD-L1 are currently being investigated as treatment options for this disease.
Anti-CTLA-4 blockade
CTLA-4 has been the first immune checkpoint receptor to be targeted by a therapeutic agent. Ipilimumab (a fully human monoclonal IgG1) blocks the interaction between CTLA-4 and its ligands CD80 and CD86. Ipilimumab was studied in combination with carboplatin and paclitaxel as first-line treatment for extensive stage (ES) SCLC in a randomised phase II clinical trial.29
One hundred and thirty patients, with untreated ES-SCLC, were randomised to receive either carboplatin/paclitaxel/placebo or phased or concurrent carboplatin/paclitaxel/ipilimumab. Phased ipilimumab was associated with an improved immune-related PFS compared with chemotherapy/placebo (HR 0.64; p=0.03). There were no differences in PFS but there was a trend towards improved OS in the patients treated with phased ipilimumab (HR 0.75; p=0.13). A randomised double-blind phase 3 trial comparing the efficacy of ipilimumab plus etoposide/platinum versus etoposide/platinum in patients with newly diagnosed ES-SCLC (NCT01450761) has completed accrual and results are awaited. Ipilimumab is currently being investigated in the STIMULI study as a consolidation treatment, in combination with nivolumab, after concurrent chemotherapy for patients with LS-SCLC (NCT02046733).
PD1 and PD-L1 blockade
A phase I/II open-label study (CheckMate 032) of nivolumab with or without ipilimumab for treatment of recurrent SCLC was recently presented.30 In this study, 128 patients with progressive disease after at least one platinum-based chemotherapy were enrolled regardless of PD-L1 status and allocated to four different cohorts where induction treatment was given for four cycles, followed by maintenance nivolumab (3 mg/kg intravenously once every 2 weeks) until PD. In the first arm, patients received nivolumab 3 mg/kg intravenously once every 2 weeks as monotherapy. In the second and third arms, patients received nivolumab 1 mg/kg in combination with either 1 mg/kg or 3 mg/kg of ipilimumab Q3W for four cycles. The fourth arm included nivolumab 3 mg/kg plus ipilimumab 1 mg/kg Q3W. More than 40% of the patients had received ≥2 previous lines of treatment and about one-third of patients had platinum refractory/resistant disease. The ORR was 18% for nivolumab and 32.6% for nivolumab+ipilimumab, with a median time to response of 1.6 and 2.1 months for nivolumab and nivolumab+ipilimumab, respectively. Median duration of response was not reached in the nivolumab arm and was 6.9 months in the nivolumab+ipilimumab arm. OS was 4.4 vs 8.2 months in the nivolumab arm and the nivolumab+ipilimumab arm, respectively. Analysis of survival data highlights that ∼20% of patients show durable response to therapy. The adverse event (AE) profile of nivolumab monotherapy and nivolumab+ipilimumab combination therapy was consistent with what was previously reported. The most common any grade drug-related AEs in the nivolumab monotherapy arm were fatigue (18%), diarrhoea (13%), nausea (10%) and reduced appetite (10%). With nivolumab+ipilimumab, the most common any grade drug-related AEs were fatigue (29%), diarrhoea (17%), pruritus (14%), rash, nausea and endocrine disorders (11% each). The rate of grade 3–4 AEs was 15% vs 34% for nivolumab and nivolumab plus ipilimumab, respectively. Drug-related pneumonitis occurred in two patients (1 in the monotherapy arm and 1 with the combination). There was one treatment-related death (myasthenia gravis) in the combination arm receiving the higher dose of ipilimumab. There was no correlation between PD-L1 expression (1% cut-off) and ORR. Nivolumab is currently being investigated as a second-line treatment versus topotecan or amrubicin (NCT02481830) or as a maintenance treatment after standard platinum/etoposide (NCT02538666).
KEYNOTE-028 is an ongoing phase Ib multicohort study evaluating pembrolizumab in PD-L1-positive patients with ES-SCLC with failure or inability to receive standard therapy.31 Twenty-four patients with PS 0–1 and PD-L1-positive tumour (membranous PD-L1 expression in ≥1% of tumour and associated inflammatory cells or positive staining in stroma using the 22C3 antibody clone) received pembrolizumab at the dose of 10 mg/kg intravenously once every 2 weeks for up to 2 years, until progression or unacceptable toxicity. Primary end points were overall response rate (ORR) and safety and tolerability. PD-L1 expression analysis was performed in 157 patients; 147 samples were evaluable and 42 (28.6%) were found to have PD-L1-positive tumours. Of these, only 20 patients were treated in the study, with a median follow-up of 21 weeks (range 2–48). ORR was 35% with median time to response of 8.6 weeks. Six of the seven patients had ongoing response at data cut-off. The safety profile was consistent with previous experience of pembrolizumab in other tumour types. The most common treatment-related AEs were arthralgia 15%, asthenia 15%, nausea 10% and rash 10%. There was one case of grade 2 autoimmune thyroiditis resulting in treatment interruption. Two patients had grade ≥3 AEs including one treatment-related death from colitis. There were no reported cases of drug-related pneumonitis. The authors looked at the potential relationship between level of PD-L1 expression and frequency of response and there was no significant difference found (p=0.235). A phase II trial by European Organization for research and Treatment of Cancer will evaluate platinum/etoposide±pembrolizumab (NCT02580994) in patients with ES-SCLC. Another phase II trial is currently ongoing and investigates maintenance pembrolizumab after four cycles of induction platinum/etoposide (NCT02359019). Other immune checkpoint inhibitors that are being studied for the treatment of SCLC include durvalumab (MEDI4736), a PD-L1 antibody and tremelimumab, a CTLA-4 antibody. These agents are being investigated in an open-label non-randomised phase I safety study of patients with advanced solid malignancies including SCLC. The trial is currently open to recruitment (NCT02537418). Ongoing trials with immune checkpoint inhibitors in SCLC are summarised in table 1.
Table 1.
Other ongoing phase II/III immunotherapy trials in small cell lung cancer
| Trial identifier | Immunotherapy agent | Phase | Study design | Treatment arms | Primary end point(s) |
|---|---|---|---|---|---|
| CTLA-4 antibodies | |||||
| NCT01331525 | Ipilimumab | II | Open-label, single group | Ipilimumab+carboplatin/etoposide | 1-year PFS |
| NCT01450761 | Ipilimumab | III | Randomised, double-blind | Ipilimumab+platinum/etoposide vs placebo+platinum/etoposide | OS |
| PD-1 antibodies | |||||
| NCT02472977 | Nivolumab | I/II | Randomised, open-label | Nivolumab+ulocuplumab | ORR, DLT |
| NCT02359019 | Pembrolizumab | II | Open-label, single group | Pembrolizumab monotherapy | PFS |
| NCT02580994 | Pembrolizumab | II | Randomised, open-label | Pembrolizumab+cisplatin/carboplatin+etoposide vs cisplatin/carboplatin+etoposide | PFS |
| NCT02481830 CheckMate 331 | Nivolumab | III | Randomised, open-label | Nivolumab vs topotecan vs amrubicin | OS |
| Checkpoint inhibitor combinations | |||||
| NCT02046733 | Nivolumab Ipilimumab |
II | Randomised, open-label | Nivolumab+ipilimumab | OS, PFS |
| NCT02538666 | Nivolumab Ipilimumab |
III | Randomised, multicentre, double-blind | Nivolumab vs nivolumab+ipilimumab vs placebo | OS, PFS |
CTLA-4, cytotoxic T-lymphocyte antigen-4; DLT, dose limiting toxicity; OS, overall survival; PD-1, programmed cell death-1; PFS, progression-free survival.
Conclusion
SCLC is a very aggressive form of lung cancer and there is a desperate need for new agents that are able to impact on patients’ outcome. SCLC is a genetically complex disease encompassing a wide variety of genetic alterations. The recent advances in molecular technology with Next Generation Sequencing (NGS) have allowed scientists to better understand the biology of this disease with a view to find and address targetable mutations. The most frequently mutated genes in SCLC are TP53 and RB1, which, as tumour suppressor genes, are difficult to target.32 In addition, deletions in chromosome 3p, which contains several tumour suppressor genes, often occur and gene copy number gains have also been described in JAK2, FGFR1 and MYC genes.33 A prospective molecular evaluation on biopsies from patients with ES-SCLC has been performed using a comprehensive mutation analysis program at Memorial Sloan Kettering Cancer Center.34 In this study, recurrent mutations in RB1 (N=7), TP53 (N=8), amplifications of FGFR1 (N=2) and MET (N=1) were observed. Subsequent testing with NGS technology of 25 patient samples showed: RB1 mutations/deletions (N=18/4), TP53 mutations (N=24), MLL3 (N=9) and EPHA 5 mutations (N=9), and amplifications of CDKN2C (N=5), MYCL1 (N=3), SOX2 (N=2) and FGFR1 (N=1), indicating a high-molecular diversity of SCLC.
Although major improvements in understanding biology of SCLC have been made, the underlying mechanism by wich SCLC rapidly progresses remain to be defined. The identification of these mechanisms could promote the development of novel therapeutic agents and improve the management of this challenging disease. In order to further understand these mechanisms, rebiopsying at progression is very important but at the same time very difficult to be obtained. Detection of circulating tumour DNA in the plasma of patients with cancer, the so-called ‘liquid biopsy’, represents an interesting field of investigation also in SCLC, where the serial monitoring of circulating tumour DNA revealed altered prevalence of mutations in patients with emerging resistance to targeted therapies.
In this contest, the use of circulating tumour cells (CTCs) can help in investigating molecular alterations occurring within tumour cells and understanding drug resistance mechanisms. Hou et al35 demonstrated that the number of CTCs detected by the CellSearch technology (expressing EpCAM and cytokeratins) is an independent prognostic factor for SCLC. Recently, Hodgkinson et al36 developed an in vivo model of SCLC from CTCs obtained from patients with SCLC (CTC-derived explant) and demonstrated, for the first time, that this model shows preserved morphological and genetic characteristics, faithfully mirroring responses of donor patients to platinum and etoposide chemotherapy. These fascinating new findings open up the possibility of developing and routinely implementing personalised medicine strategies for patients with SCLC based on simple blood collection with subsequent and rapidly reported molecular analysis of CTCs.
Immune checkpoint inhibitors have, until now, made the greatest advances in clinical research for SCLC. Preliminary data for pembrolizumab, nivolumab and ipilimumab show promising antitumour activity, with durable responses and manageable AE profiles. The role of PD-L1 as a potential biomarker for patient selection still remains unclear and further research is necessary to ascertain the long-term safety and efficacy of immune checkpoint blockade.
There is a lot of excitement in the oncology community for the potential impact of these drugs on patients’ outcome and results of ongoing phase II/III trials are eagerly awaited.
Footnotes
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Siegel RL, Miller KD, Jemal A et al. . Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Govindan R, Page N, Morgensztern D et al. . Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic and results database. J Clin Oncol 2006;24:4539–44. 10.1200/JCO.2005.04.4859 [DOI] [PubMed] [Google Scholar]
- 3.Mountain CF. Revisions in the international system for staging lung cancer. Chest 1997;111:1710–17. 10.1378/chest.111.6.1710 [DOI] [PubMed] [Google Scholar]
- 4.Giroux DJ, Rami-Porta R, Chansky K et al. . The IASLC Lung Cancer Staging Project: data elements for the prospective project. J Thorac Oncol 2009;4:679–83. 10.1097/JTO.0b013e3181a52370 [DOI] [PubMed] [Google Scholar]
- 5.Turrisi AT III, Kim K, Blum R et al. . Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 1999;340:265–71. 10.1056/NEJM199901283400403 [DOI] [PubMed] [Google Scholar]
- 6.Brock MV, Hooker CM, Syphard JE et al. . Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: its time has come. J Thorac Cardiovasc Surg 2005;129:64–72. 10.1016/j.jtcvs.2004.08.022 [DOI] [PubMed] [Google Scholar]
- 7.Sundstrøm S, Bremnes RM, Kaasa S et al. . Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol 2002;20:4665–72. 10.1200/JCO.2002.12.111 [DOI] [PubMed] [Google Scholar]
- 8.Takada M, Fukuoka M, Kawahara M et al. . Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002;20:3054–60. 10.1200/JCO.2002.12.071 [DOI] [PubMed] [Google Scholar]
- 9.Früh M, De Ruysscher D, Popat S et al. . Small-cell lung cancer: ESMO clinical practice guidelines. Ann Oncol 2013;24(Suppl 6):vi99–105. 10.1093/annonc/mdt178 [DOI] [PubMed] [Google Scholar]
- 10.Faivre-Finn C, Snee M, Ashcroft L et al. . CONVERT: an international randomised trial of concurrent chemo-radiotherapy (cCTRT) comparing twice-daily (BD) and once-daily (OD) radiotherapy schedules in patients with limited stage small cell lung cancer (LS-SCLC) and good performance status (PS). J Clin Oncol 2016;34(suppl; abstr 8504). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shao N, Jin S, Zhu W et al. . An updated meta-analysis of randomized controlled trials comparing irinotecan/platinum with etoposide/platinum in patients with previously untreated extensive-stage small cell lung cancer. J Thorac Oncol 2012;7:470–2. 10.1097/JTO.0b013e31823c5a23 [DOI] [PubMed] [Google Scholar]
- 12.Lara PN, Natale R, Crowley J et al. . Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530–5. 10.1200/JCO.2008.20.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White SC, Lorigan P, Middleton MR et al. . Randomized phase II study of cyclophosphamide, doxorubicin, and vincristine compared with single-agent carboplatin in patients with poor prognosis small cell lung carcinoma. Cancer 2001;92:601–8. [DOI] [PubMed] [Google Scholar]
- 14.Aupérin A, Arriagada R, Pignon JP et al. . Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476–84. 10.1056/NEJM199908123410703 [DOI] [PubMed] [Google Scholar]
- 15.Slotman B, Faivre-Finn C, Kramer G et al. . Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664–72. 10.1056/NEJMoa071780 [DOI] [PubMed] [Google Scholar]
- 16.Slotman BJ, van Tinteren H, Praag JO et al. . Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36–42. 10.1016/S0140-6736(14)61085-0 [DOI] [PubMed] [Google Scholar]
- 17.Albain KS, Crowley JJ, Hutchins L et al. . Predictors of survival following relapse or progression of small cell lung cancer. Southwest Oncology Group Study 8605 report and analysis of recurrent disease database. Cancer 1993;72:1184–91. [DOI] [PubMed] [Google Scholar]
- 18.von Pawel J, Schiller JH, Shepherd FA et al. . Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999;17:658–67. [DOI] [PubMed] [Google Scholar]
- 19.Genestreti G, Tiseo M, Kenmotsu H et al. . Outcomes of platinum-sensitive small-cell lung cancer patients treated with platinum/etoposide rechallenge: a multi-institutional retrospective analysis. Clin Lung Cancer 2015;16:e223–8. 10.1016/j.cllc.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 20.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1–10. 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 21.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329–60. 10.1146/annurev.immunol.22.012703.104803 [DOI] [PubMed] [Google Scholar]
- 22.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest 2007;117:1137–46. 10.1172/JCI31405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schreiber RD, Old LJ, Smyth MJ et al. . Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565–70. 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 24.Lawrence MS, Stojanov P, Polak P et al. . Mutational heterogeneity in cancer and the search for new cancer genes. Nature 2013;499:214–18. 10.1038/nature12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogelstein B, Papadopoulos N, Velculescu VE et al. . Cancer genome landscapes. Science 2013;339:1546–58. 10.1126/science.1235122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson IR, Takahashi K, Futreal PA et al. . Emerging patterns of somatic mutations in cancer. Nat Rev Genet 2013;14:703–18. 10.1038/nrg3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder A, Makarov V, Merghoub T et al. . Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189–99. 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizvi NA, Hellmann MD, Snyder A et al. . Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reck M, Bondarenko I, Luft A et al. . Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75–83. 10.1093/annonc/mds213 [DOI] [PubMed] [Google Scholar]
- 30.Antonia SJ, Bendell JC, Taylor MH et al. . Phase I/II study of nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer (SCLC): CA209-032. J Clin Oncol 2015;33(suppl; abstr 7503) [Google Scholar]
- 31.Ott PA, Fernandez ME, Hiret S et al. . Pembrolizumab (MK-3475) in patients (pts) with extensive-stage small cell lung cancer (SCLC): preliminary safety and efficacy results from KEYNOTE-028. J Clin Oncol 2015;33(suppl; abstr 7502). [Google Scholar]
- 32.Peifer M, Fernández-Cuesta L, Sos ML et al. . Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104–10. 10.1038/ng.2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voortman J, Lee JH, Killian JK et al. . Array comparative genomic hybridization-based characterization of genetic alterations in pulmonary neuroendocrine tumours. Proc Natl Acad Sci USA 2010;107:13040–5. 10.1073/pnas.1008132107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pietanza MC, Varghese AM, Won HH et al. . Prospective molecular evaluation of small cell lung cancer (SCLC) utilizing the comprehensive mutation analysis program at Memorial Sloan-Kettering Cancer Center (MSKCC). J Clin Oncol 2013;Suppl:Abstract 7600. [Google Scholar]
- 35.Hou JM, Krebs MG, Lancashire L et al. . Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 2012;30:525–32. 10.1200/JCO.2010.33.3716 [DOI] [PubMed] [Google Scholar]
- 36.Hodgkinson CL, Morrow CJ, Li Y et al. . Tumorigenicity and genetic profiling of circulating tumour cells in small-cell lung cancer. Nat Med 2014;20:897–903. 10.1038/nm.3600 [DOI] [PubMed] [Google Scholar]