Abstract
Introduction
Metastatic colorectal cancer is rarely curable. Improving quality of life is therefore a key treatment goal. We report quality of life for patients with RAS wild-type metastatic colorectal cancer in the PRIME study.
Methods
A randomised phase 3 open-label study of first-line panitumumab+FOLFOX4 vs FOLFOX4 enrolled adults with untreated metastatic colorectal cancer and an Eastern Cooperative Oncology Group performance status of 0–2. This analysis includes patients with wild-type RAS tumours (n=505). Quality of life (prespecified end point) was assessed using the EuroQoL 5-domain health state index and overall health rating in all patients and by early tumour shrinkage status (≥30% reduction in size by week 8; exploratory end point). Differences in quality of life were assessed using analysis of covariance and a mixed-effect piecewise linear model, and were also analysed by skin toxicity severity.
Results
There were no statistically significant differences between treatment arms from baseline to progression or to discontinuation. Grade 3+ skin toxicity was reported by 38% of patients receiving panitumumab+FOLFOX4 and 2% receiving FOLFOX4 alone. There were no significant differences in quality of life between patients with grade 0–2 skin toxicity and those with grade 3+ skin toxicity. More patients receiving panitumumab+FOLFOX4 vs FOLFOX4 had early tumour shrinkage (p<.001). In patients with tumour symptoms at baseline, there were statistically significant improvements in quality of life in those with early tumour shrinkage versus those without early tumour shrinkage.
Conclusions
Addition of panitumumab to FOLFOX4 in first-line therapy for metastatic colorectal cancer prolongs survival and has no negative effect on overall quality of life compared with FOLFOX4 alone. Specific quality of life assessments for skin toxicity should be included in study designs to better define the direct effect of these adverse events.
Trial registration number
Keywords: chemotherapy, tumour shrinkage, quality of life, EGFR inhibitors, metastatic colorectal cancer
Find all abstract addressing studies on panitumumab at ESMO 2014 here: http://oncologypro.esmo.org/Science-Education/Drug-Index-of-ESMO-2014-Abstracts
Key questions.
What is already known about this subject?
Panitumumab in first-line therapy can improve survival in patients with wild-type RAS metastatic colorectal cancer, but is associated with skin toxicities characteristic of epidermal growth factor receptor (EGFR) inhibitors.
What does this study add?
Addition of panitumumab to FOLFOX4 chemotherapy in first-line therapy for RAS wild-type metastatic colorectal cancer has no negative effect on overall quality of life compared with FOLFOX4 alone.
How might this impact on clinical practice?
Physicians can be reassured that the survival benefits associated with first-line panitumumab in RAS wild-type metastatic colorectal cancer do not come at the expense of impaired quality of life.
Introduction
Optimal anticancer treatment involves a balance between efficacy and safety,1 as adverse events can have a negative effect on quality of life (QoL).2 3 Despite recent advances, the curative treatment of metastatic colorectal cancer (mCRC) is limited to a subgroup of patients undergoing complete resection, and improvement of QoL is therefore an important treatment goal in patients with mCRC.4 5
Panitumumab is a fully human monoclonal antibody (mAb) targeting the epidermal growth factor receptor (EGFR),6 which is indicated in combination with FOLFOX or FOLFIRI for the first-line treatment of patients with RAS wild-type (WT) mCRC in Europe.7 The efficacy and safety of first-line panitumumab in combination with FOLFOX4 were evaluated in the PRIME study,8 9 in which panitumumab significantly improved overall survival (OS 26.0 months) versus FOLFOX4 alone (20.2 months; p=.04) in patients with mCRC WT for KRAS and NRAS exons 2–4 (RAS WT).9 In addition, more patients receiving panitumumab+FOLFOX4 vs FOLFOX4 had ≥30% tumour shrinkage at week 8, while objective response rate, duration of response and depth of response were also improved in the panitumumab group.10 Panitumumab treatment was well tolerated, but was associated with adverse events typical of EGFR inhibitors, including skin toxicity and diarrhoea.8 Notably, skin toxicity has been linked with improved survival outcomes in patients with mCRC receiving panitumumab+FOLFOX4.11 This is, however, an area of debate, with analyses suggesting that skin toxicity alone cannot be used as a surrogate marker for efficacy. For example, in the analysis of data from the PRIME study, duration of treatment was a confounding factor, with patients who received panitumumab remaining on treatment longer than those receiving FOLFOX4 alone. We report prespecified tertiary QoL end points from the PRIME study for patients with RAS WT mCRC, including exploratory analyses of the impact of early tumour shrinkage (ETS) and skin toxicity on QoL.
Methods
Study design and patients
PRIME (ClinicalTrials.gov: NCT00364013) was a randomised (1:1), open-label phase 3 study of first-line panitumumab+FOLFOX4 vs FOLFOX4 alone in patients with mCRC. The study was conducted in 133 study centres in 18 countries (Argentina, Australia, Belgium, Brazil, Canada, Chile, Costa Rica, the Czech Republic, France, Hungary, Italy, Latvia, Mexico, Poland, South Africa, Spain, Switzerland, the UK). The first patient was enrolled in August 2006 (first participant enrolled), and the final data cut-off date was August 2010.
Panitumumab was given as an intravenous infusion of 6.0 mg/kg on the first day of each 14-day cycle. Participants were randomised using an interactive voice response system, and randomisation was stratified by geographic region (Western Europe, Canada and Australia vs rest of the world) and Eastern Cooperative Oncology Group (ECOG) performance status (PS 0 or 1 vs 2). Each patient was assigned a unique identification number used to identify that patient throughout the study. Details of the study design and inclusion criteria have been published previously.8
The primary end point was progression-free survival (PFS), with secondary end points including OS, objective tumour response and safety. Although the study was originally designed to test treatment effect in all randomised patients, it was amended to compare PFS and OS according to KRAS status prior to any efficacy analyses. Sample size was originally set at 900 patients, assuming that all time-to-event end points are exponentially distributed. The sample size was revised to 1150 when the protocol was revised to assess the primary end point in patients with WT KRAS tumours only (assumed prevalence, 55%). This is based on a PFS HR of 0.714 and a median PFS of 12 months in the control arm.
Eligible patients were aged ≥18 years with previously untreated metastatic adenocarcinoma of the colon or rectum, and had an ECOG PS of 0–2. The protocol was approved by the ethics committees at participating sites and adhered to all ethical guidelines, and all patients signed informed consent before any study-related procedures were performed. The present analysis focuses on patients with RAS WT mCRC (ie, tumours WT for KRAS/NRAS exon 2 (codons 12 and 13), exon 3 (codons 59 and 61) and exon 4 (codons 117 and 146)).9 Mutation analysis was performed using Sanger sequencing and SURVEYOR/WAVE laboratory-developed testing, as described previously.12
QoL end points and analysis
QoL was assessed as a prespecified tertiary end point during PRIME, using the EuroQoL 5-domain (EQ-5D) health state index (HSI) and overall health rating (OHR) measures. HSI scores range from −0.594 to 1.0 (higher scores represent better health, with 1.0 equivalent to perfect health). The OHR comprises a 0–100 visual analogue scale, with 0 representing the ‘worst imaginable health state’ and 100 representing the ‘best imaginable health state’. QoL was assessed ≤7 days before randomisation and every 4 weeks until disease progression, with a final assessment at a safety follow-up visit.
Between-treatment differences in QoL were assessed from baseline to disease progression, and to discontinuation of first-line treatment, using analysis of covariance (ANCOVA). Treatment-by-covariate interactions (between treatment and baseline QoL score, between treatment and baseline ECOG score, and between treatment and region) was tested at the 5% level and those that were found to be significant were retained in the final model. Changes in QoL from baseline to discontinuation of first-line treatment, and between-treatment differences, were also assessed using a mixed-effect model adjusted for worst grade skin toxicity (ie, nail changes, erythema, pruritus, acne, rash and ulceration; grade 0–2 vs grade 3+), baseline QoL score and time (QoL assessment week). Random effects for intercept and time were also included. In the HSI model, the five subscales (anxiety/depression, mobility, pain/discomfort, self-care and usual activities) were evaluated separately.
The primary QoL analyses were conducted on the subset of patients in the intent-to-treat analysis set who had a baseline QoL assessment and at least one postbaseline QoL assessment. For this analysis, the minimally important differences (MIDs) were defined as 0.08 for HSI and 7 for OHR.13 Changes in QoL were also assessed in subgroups of patients with and without ETS, both in the overall population and in patients with tumour-related symptoms at baseline (defined as EQ-5D pain/discomfort scale score >1). Tumour size and response were measured as described previously,10 and ETS was defined as a decrease of ≥30% in tumour size at week 8.
Results
Patients
Of the 1183 patients enrolled in PRIME, 505 had RAS WT mCRC (patient disposition shown in online supplementary figure S1). Baseline demographics, disease characteristics and QoL scores were similar between treatment groups (see online supplementary table S1). Overall rates of compliance with the QoL assessments (expressed as evaluable vs expected assessments) were 57% for HSI and OHR.
esmoopen-2016-000041supp.pdf (157.8KB, pdf)
Quality of life
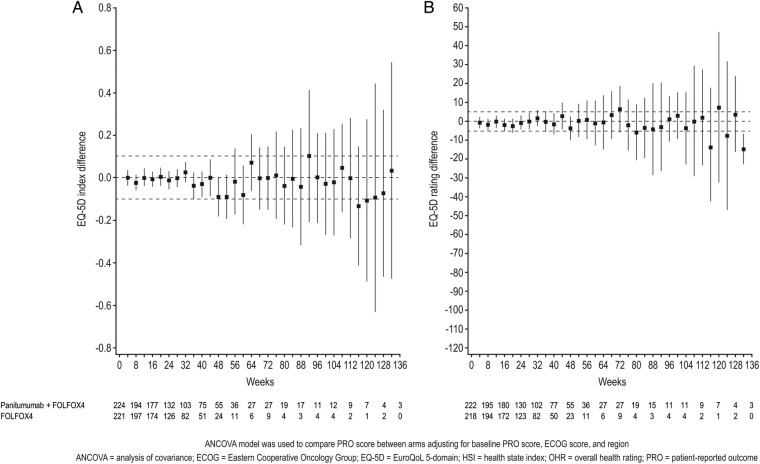
When analysed by ANCOVA, there were no statistically significant differences between the panitumumab+FOLFOX4 and FOLFOX4 arms in HSI or OHR scores from baseline to progression or to discontinuation (table 1 and figure 1). In total, 450 patients (89%) were included in the mixed-effect model. In this model, there were no statistically significant differences between panitumumab+FOLFOX4 and FOLFOX4 alone in terms of HSI or OHR scores from baseline to discontinuation, or on any subscale of the HSI (table 2). There were, however, individual change scores for specific HSI items in both treatment arms that were greater than the MID, including improvements in mobility and decreases in anxiety/depression in both treatment arms (table 2).
Table 1.
Analysis of change in EuroQoL 5-domain (EQ-5D) scores from baseline
| Panitumumab+FOLFOX4 | FOLFOX4 | Difference | |
|---|---|---|---|
| Disease progression | |||
| n | 223 | 221 | |
| Health state index | 0.74 (0.70 to 0.78) | 0.76 (0.73 to 0.80) | −0.02 (−0.06 to 0.01) |
| n | 222 | 216 | |
| Overall health rating | 72.5 (69.6 to 75.4) | 74.0 (71.1 to 76.9) | −1.48 (−3.96 to 1.01) |
| Discontinuation | |||
| n | 222 | 220 | |
| Health state index | 0.75 (0.72 to 0.79) | 0.77 (0.74 to 0.81) | −0.02 (−0.05 to 0.01) |
| n | 221 | 215 | |
| Overall health rating | 73.0 (70.2 to 75.8) | 74.5 (71.7 to 77.3) | −1.48 (−3.87 to 0.91) |
Analysis of covariance (ANCOVA) analysis of weighted mean EQ-5D health state index and overall health rating scores from baseline to disease progression and from baseline to discontinuation of first-line therapy.
Values are least squares means (95% CIs).
Figure 1.
Analysis of treatment difference by study week. ANCOVA analysis of treatment difference (95% CIs) by study week in (A) EQ-5D health state index and (B) overall health rating scores, from baseline to discontinuation of first-line therapy.
Table 2.
Change from baseline to discontinuation of treatment in EuroQoL 5-domain (EQ-5D) scores by treatment arm
| Panitumumab+FOLFOX4 (n=232)* |
FOLFOX4 (n=224)* |
Difference | p Value | |
|---|---|---|---|---|
| Health state index | −0.005 (−0.027 to 0.017) | 0.006 (−0.022 to 0.034) | −0.011 (−0.042 to 0.020) | 0.50 |
| Anxiety/depression | −0.117 (−0.167 to −0.066) | −0.115 (−0.181 to −0.049) | −0.001 (−0.075 to 0.073) | 0.98 |
| Mobility | 0.123 (0.076 to 0.171) | 0.145 (0.086 to 0.204) | −0.0214 (−0.083 to 0.041) | 0.50 |
| Pain/discomfort | −0.009 (−0.059 to 0.042) | −0.037 (−0.103 to 0.028) | 0.029 (−0.044 to 0.102) | 0.44 |
| Self-care | 0.098 (0.059 to 0.137) | 0.055 (0.006 to 0.103) | 0.0431 (−0.008 to 0.094) | 0.10 |
| Usual activities | 0.078 (0.023 to 0.132) | 0.015 (−0.056 to 0.086) | 0.062 (−0.017 to 0.142) | 0.12 |
| Overall health rating | −0.906 (−2.773 to 0.960) | 0.734 (−1.674 to 3.142) | −1.640 (−4.257 to 0.976) | 0.22 |
Mixed-effect model of change from baseline to discontinuation of treatment in EQ-5D health state index and overall health rating scores.
Values are least squares means (95% CIs).
*Actual patient numbers differed for each scale/subscale.
Analysis of QoL by skin toxicity
Overall, 94 patients (38%) receiving panitumumab+FOLFOX4 and 6 patients (2%) receiving FOLFOX4 alone experienced grade 3+ skin toxicity. The most common skin toxicity adverse event was rash (panitumumab+FOLFOX4, 56%; FOLFOX4, 8%; see online supplementary table S2), which led to discontinuation in 10 patients (4%) in the panitumumab+FOLFOX4 arm and 3 patients (1%) in the FOLFOX4 arm. Other skin toxicities that led to discontinuation in more than one patient were dermatitis acneiform (n=6; 3%) and paronychia (n=5; 2%), all in the panitumumab+FOLFOX4 arm.
In the mixed-effect model of QoL by skin toxicity, 79% of patients overall had a worst-grade skin toxicity of <3. There were no significant differences in QoL outcomes in patients with grade 0–2 skin toxicity and those with grade 3+ skin toxicity (table 3). Again, there were improvements in mobility and decreases in anxiety/depression greater than the MID in both treatment arms.
Table 3.
Change from baseline to discontinuation of treatment in EuroQoL 5-domain (EQ-5D) scores by worst skin toxicity grade
| Worst skin toxicity grade <3 (n=360)* |
Worst skin toxicity grade ≥3 (n=96)* |
Difference | p Value | |
|---|---|---|---|---|
| Health state index | 0.007 (−0.012 to 0.025) | −0.006 (−0.040 to 0.029) | 0.0123 (−0.026 to 0.050) | 0.52 |
| Anxiety/depression | −0.125 (−0.167 to −0.082) | −0.107 (−0.187 to −0.027) | −0.018 (−0.108 to 0.072) | 0.70 |
| Mobility | 0.130 (0.088 to 0.171) | 0.138 (0.068 to 0.209) | −0.009 (−0.085 to 0.067) | 0.82 |
| Pain/discomfort | −0.025 (−0.067 to 0.018) | −0.021 (−0.101 to 0.058) | −0.003 (−0.092 to 0.086) | 0.94 |
| Self-care | 0.077 (0.043 to 0.110) | 0.076 (0.018 to 0.134) | 0.001 (−0.062 to 0.063) | 0.98 |
| Usual activities | 0.059 (0.013 to 0.105) | 0.034 (−0.052 to 0.120) | 0.025 (−0.071 to 0.121) | 0.61 |
| Overall health rating | −0.022 (−1.609 to 1.565) | −0.150 (−3.037 to 2.737) | 0.128 (−3.034 to 3.289) | 0.94 |
Mixed-effect model of change from baseline to discontinuation of treatment in EQ-5D health state index and overall health rating scores.
Values are least squares means (95% CIs).
*Actual patient numbers differed for each scale/subscale.
Analysis of QoL by ETS
More patients receiving panitumumab+FOLFOX4 (59%) versus FOLFOX4 (38%) had ETS of ≥30% (p<.001). In patients with tumour symptoms at baseline, there were statistically significant improvements in QoL in those with ETS versus those without ETS (table 4). In addition, improvement from baseline in HSI score in symptomatic patients with ETS (+0.096) was greater than the MID. In the overall population (ie, irrespective of symptomatic disease at baseline), there was no difference in QoL for those with ETS versus those without ETS.
Table 4.
Change from baseline to discontinuation of treatment in EQ-5D Health scores by ETS
| Overall population |
Symptomatic patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| ≥30% ETS | <30% ETS | Difference | p Value | ≥30% ETS | <30% ETS | Difference | p Value | |
| n | 202 | 206 | 84 | 109 | ||||
| Health state index | 0.005 (−0.019 to 0.029) | −0.017 (−0.042 to 0.008) | 0.022 (−0.006 to 0.050) | 0.12 | 0.096 (0.049 to 0.142) | 0.025 (−0.020 to 0.071) | 0.070 (0.013 to 0.127) | 0.02 |
| n | 198 | 205 | 82 | 109 | ||||
| Overall health rating | 0.534 (−1.556 to 2.624) | −1.411 (−3.587 to 0.764) | 1.945 (−0.510 to 4.401) | 0.12 | 4.614 (1.405 to 7.824) | 0.300 (−2.822 to 3.422) | 4.314 (0.636 to 7.993) | 0.02 |
Mixed-effect model of change from baseline to discontinuation of treatment in EQ-5D health state index and overall health rating scores in patients who achieved ETS and those who did not.
Values are least squares means (95% CIs).
EQ-5D, EuroQoL 5-domain; ETS, early tumour shrinkage.
Discussion
The maintenance of QoL is an important component of cancer management, as codified in the recent European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS).5 Indeed, studies have shown that QoL is important, not just for the patient's well-being, but also because it can influence survival and response to therapy.14 The relationship between ETS and QoL is, however, unknown. We therefore conducted an analysis of QoL in patients receiving first-line treatment for mCRC with FOLFOX4 with or without panitumumab in the phase 3 PRIME study.
While addition of panitumumab to FOLFOX4 in first-line RAS WT mCRC is associated with a high incidence of skin toxicity, there was no negative impact on global QoL. While skin toxicity might be expected to have a negative impact on QoL, our results are consistent with previous studies of the EGFR inhibitors panitumumab and cetuximab in mCRC. For example, similar results were seen in an earlier analysis of data from patients with WT KRAS mCRC in the PRIME study,15 while studies of first-line FOLFIRI combined with panitumumab or cetuximab have shown no negative effect on QoL or social functioning during treatment.16–18 Similarly, addition of panitumumab to second-line FOLFIRI treatment resulted in a significant improvement in PFS without compromising QoL.15 In a study of the psychological effects associated with cetuximab treatment (n=80), psychological distress was present in 41% of patients.19 Distress was linked with overall QoL, but not with rash, which did not affect psychological status or social life. Furthermore, a recent quality-adjusted time without symptoms of disease or toxicity (Q-TWiST) analysis from the PRIME study showed that panitumumab+FOLFOX4 significantly improved quality-adjusted survival time compared with FOLFOX4 alone (20.5 vs 18.2 months, respectively; p=0.025).20 The ESMO-MCBS is a standardised and validated approach to stratify the magnitude of clinical benefit that can be expected from anticancer therapies. A recent analysis of therapies for first-line mCRC provides additional confirmation of the overall clinical benefit of panitumumab: panitumumab scored 4/5, representing ‘a high level of proven clinical benefit’ in terms of both prolonged survival and improved QoL.5
The QoL scales used in these studies, including the EQ-5D, are measures of global QoL and are not specific for skin toxicity. In patients treated with panitumumab in the PRIME study, therefore, the impact of rash-related events may have been balanced by favourable antitumour treatment-related effects. For example, patients with advanced cancer may consider skin rash to be part of their overall condition, and they may feel that skin rash is a marker of treatment efficacy.19 It is also possible that the beneficial effects of treatment may outweigh skin-related side effects.3 This is supported by our analysis of ETS, in which patients with tumour-related symptoms at baseline who experienced ETS showed a statistically meaningful improvement in QoL compared with those who did not have ETS. These important data add to the idea that achieving early reductions in tumour load is associated with symptomatic benefit for patients.
The strengths of this study include its randomised design and international recruitment, as well as the use of planned QoL assessments with internationally validated tools available in local languages. The main weakness of the study is the use of global QoL instruments. The lack of a QoL difference associated with different grades of adverse events suggests that tools specific for skin-related events are required to assess the direct effect of skin toxicity on QoL for patients receiving treatment with EGFR inhibitors. One such instrument is the Dermatology Life Quality Index, which has previously been used to assess the impact of toxicities such as hair loss, hyperpigmentation and dry skin, in patients receiving cancer chemotherapy.21 It should also be noted that other drug-related adverse events may also affect QoL. For example, hypomagnesaemia affects QoL by causing muscle cramps and spasms, both directly and indirectly as a result of secondary hypocalcaemia. An analysis of QoL by muscle-related symptoms (with or without magnesium levels) may therefore also be of interest. Finally, disease progression itself may affect QoL, which was not systematically analysed in the present study.
In conclusion, the addition of panitumumab to FOLFOX4 in first-line RAS WT mCRC improves OS9 with no negative effect on overall QoL, despite the high incidence of skin toxicity. Skin toxicity of grade 3+ appeared to have a similar impact on QoL outcomes as skin toxicity of grade 0–2. In patients with tumour-related symptoms, those with ETS experienced significant improvements in QoL compared with those who did not achieve ETS. In future studies, QoL tools specific for skin-related events may be required to assess the direct effect of skin toxicity on QoL for patients receiving EGFR inhibitors.
Acknowledgments
The authors wish to acknowledge all the patients who participated in the PRIME (20050203) study, as well as the study investigators and their study staff, and the study team at Amgen, for their participation in the conduct and reporting of this study. DC is supported by the National Institute for Health Research Biomedical Research Centre based at the Royal Marsden Hospital and the Institute of Cancer Research (both in London, UK). Medical writing support (funded by Amgen (Europe) GmbH) was provided by Dan Booth, PhD (Bioscript Medical Ltd).
Footnotes
Contributors: SS, JT, GB, DC, FR, PR, JLC, GD, GH and J-YD were involved in the acquisition, analysis or interpretation of data, drafting of the manuscript and its critical revision for important intellectual content. RK was involved in the acquisition, analysis or interpretation of data, drafting of the manuscript and its critical revision for important intellectual content, and statistical analysis. All authors approved the final manuscript ahead of submission.
Funding: Amgen sponsored the PRIME study and was involved in design, data collection, analysis and data interpretation, including the analyses presented here.
Competing interests: SS is a member of advisory boards or steering committees, or is a principal investigator, for Amgen, Bayer, Boehringer Ingelheim, Celgene, Eli Lilly, Genentech, Ignyta, Merck, Merrimack, Novartis, Pfizer, Roche, Sanofi and Taiho. JT is a consultant or advisory board member for Amgen, Imclone, Lilly, Merck KGaA, Millennium, Novartis, Roche, Sanofi, Celgene, Chugai and Taiho. GB is a consultant, advisory board member or speaker's bureau member for Bayer, Roche, Lilly, Novartis, GSK and Taiho. DC has received research funding from Amgen, AstraZeneca, Bayer, Celgene, Medimmune, Merck Serono, Merrimack, Roche and Sanofi. FR has received honoraria for advisory activities (including travel and accommodation expenses) and research funding from Amgen, Roche, Merck-Serono, Celgene, Sanofi-Aventis and Bayer. PR has received institutional funding and honoraria related to his work from Amgen, Roche and Sanofi, but has received no personal funding. JLC is a consultant/advisor for Roche and Amgen. RK is an employee and stockholder of Amgen Ltd, Uxbridge, UK. GD is an employee and stockholder of Amgen (Europe) GmbH. GH is an employee and stockholder of Amgen (Europe) GmbH. J-YD has received honoraria for consulting/advisory activities (including travel and accommodation expenses) from Amgen, Bayer, Roche and Merck, as well as research funding from Merck Serono.
Patient consent: Obtained.
Ethics approval: Ethics committees at all participating sites.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.American Cancer Society. Chemotherapy principles. 2015. http://www.cancer.org/acs/groups/cid/documents/webcontent/002995-pdf.pdf (accessed Jun 2015).
- 2.Boyd KA, Briggs AH, Paul J et al. Analysis of adverse events and quality of life data for an economic evaluation of adjuvant chemotherapy in colorectal cancer: when can we stop collecting? Trials 2011;12(Suppl 1):A41 (Abstract). [Google Scholar]
- 3.Russi EG, Moretto F, Rampino M et al. Acute skin toxicity management in head and neck cancer patients treated with radiotherapy and chemotherapy or EGFR inhibitors: literature review and consensus. Crit Rev Oncol Hematol 2015;96:167–82. 10.1016/j.critrevonc.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Nordlinger B, Cervantes A. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann Oncol 2010;21 5):v93–7. 10.1093/annonc/mdq222 [DOI] [PubMed] [Google Scholar]
- 5.Cherny NI, Sullivan R, Dafni U et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol 2015;26:1547–73. 10.1093/annonc/mdv249 [DOI] [PubMed] [Google Scholar]
- 6.Keating GM. Panitumumab: a review of its use in metastatic colorectal cancer. Drugs 2010;70:1059–78. 10.2165/11205090-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 7.Amgen Europe B.V. Vectibix. EPAR product information. Breda: Amgen Europe B.V., 2015. [Google Scholar]
- 8.Douillard JY, Siena S, Cassidy J et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697–705. 10.1200/JCO.2009.27.4860 [DOI] [PubMed] [Google Scholar]
- 9.Douillard JY, Oliner KS, Siena S et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–34. 10.1056/NEJMoa1305275 [DOI] [PubMed] [Google Scholar]
- 10.Douillard JY, Siena S, Peeters M et al. Impact of early tumour shrinkage and resection on outcomes in patients with wild-type RAS metastatic colorectal cancer. Eur J Cancer 2015;51:1231–42. 10.1016/j.ejca.2015.03.026 [DOI] [PubMed] [Google Scholar]
- 11.Douillard JY, Rong A, Sidhu R. RAS mutations in colorectal cancer. N Engl J Med 2013;369:2159–60. 10.1056/NEJMc1312697 [DOI] [PubMed] [Google Scholar]
- 12.Douillard JY, Siena S, Cassidy J et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346–55. 10.1093/annonc/mdu141 [DOI] [PubMed] [Google Scholar]
- 13.Peeters M, Price TJ, Cervantes A et al. Final results from a randomized phase 3 study of FOLFIRI {+/-} panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:107–16. 10.1093/annonc/mdt523 [DOI] [PubMed] [Google Scholar]
- 14.Marventano S, Forjaz M, Grosso G et al. Health related quality of life in colorectal cancer patients: state of the art. BMC Surg 2013;13 2):S15 10.1186/1471-2482-13-S2-S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett L, Zhao Z, Barber B et al. Health-related quality of life in patients with metastatic colorectal cancer treated with panitumumab in first- or second-line treatment. Br J Cancer 2011;105:1495–502. 10.1038/bjc.2011.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thaler J, Karthaus M, Mineur L et al. Skin toxicity and quality of life in patients with metastatic colorectal cancer during first-line panitumumab plus FOLFIRI treatment in a single-arm phase II study. BMC Cancer 2012;12:438 10.1186/1471-2407-12-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Láng I, Köhne CH, Folprecht G et al. Quality of life analysis in patients with KRAS wild-type metastatic colorectal cancer treated first-line with cetuximab plus irinotecan, fluorouracil and leucovorin. Eur J Cancer 2013;49:439–48. 10.1016/j.ejca.2012.08.023 [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi K, Ando M, Ooki I et al. Quality of life analysis in patients with RAS wild-type metastatic colorectal cancer treated with first-line FOLFIRI+cetuximab in the CRYSTAL study. Poster presented at European Cancer Congress; Vienna, Austria, 25–29 September 2015 (Abstract #2120). [Google Scholar]
- 19.Romito F, Giuliani F, Cormio C et al. Psychological effects of cetuximab-induced cutaneous rash in advanced colorectal cancer patients. Support Care Cancer 2010;18:329–34. 10.1007/s00520-009-0656-9 [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Hechmati G, Dong J et al. Q-TWiST analysis of panitumumab plus FOLFOX4 versus FOLFOX4 alone in patients with previously untreated wild-type RAS metastatic colorectal cancer. Curr Med Res Opin 2016;32:459–65. [DOI] [PubMed] [Google Scholar]
- 21.Ra HS, Shin SJ, Kim JH et al. The impact of dermatological toxicities of anti-cancer therapy on the dermatological quality of life of cancer patients. J Eur Acad Dermatol Venereol 2013;27:e53–9. 10.1111/j.1468-3083.2012.04466.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2016-000041supp.pdf (157.8KB, pdf)