Abstract
Objective
To assess the correlation between presurgery neutrophil to lymphocyte ratio (NLR) and distant metastasis-free survival (DMFS) in patients with early breast cancer.
Design
Retrospective analysis.
Participants
300 Caucasian patients with early (T1–2, N0–1, non-metastatic) breast cancer who were followed from July 1999 to June 2015 at our Institution.
Main outcome measures
Distant metastasis-free survival (DMFS).
Results
Of whole populations (300 patients), 134 and 166 patients were grouped as low and high NLR, respectively, on the basis of NLR value of 1.97, as established by receiver operating characteristic (ROC) curve analysis (area under curve (AUC)=0.625, p=0.0160). The DMFS rates for 1, 3, 6, 9, 12 and 15 years were better in low NLR patients (100%, 98.9%, 91.7%, 82.7%, 82.7%, 82.7%, respectively), than in high NLR patients (99.4%, 94.3%, 84.5%, 69.2%, 66.0%, 51.4%, respectively), with a statistically significant association. On multivariate analysis, premenopausal status (HR=2.78, 95% CI 1.36 to 5.67, p=0.0049), N1 stage (HR=2.31, 95% CI 1.16 to 4.60, p=0.0167) and a high NLR value (HR=2.64, 95% CI 1.22 to 5.638, p=0.0133) were shown to be independent prognostic factors related to poor recurrence rate. To avoid risk of confounding bias, a propensity score-matched analysis was performed and multivariate analysis according to the Cox model confirmed premenopausal status (HR=2.94, 95% CI 1.25 to 6.93, p=0.0136), N1 stage (HR=2.77, 95% CI 1.25 to 6.12, p=0.0117) and high NLR values (HR=2.52, 95% CI 1.11 to 5.73, p=0.0271), as independent prognostic variables of worse outcome.
Conclusions
This is the first study, to our knowledge, to show a significant correlation between high NLR and worse prognosis in Caucasian patients with early breast cancer by means of propensity score-matched analysis. Further well designed prospective trials with a large sample size are needed to verify our findings and to justify introducing NLR assessment in clinical practice for prediction of cancer recurrence.
Keywords: early breast cancer, neutrophil to lymphocyte ratio, prognosis
Key questions.
What is already known about this subject?
Several studies have reported neutrophil to lymphocyte ratio (NLR) as an unfavourable prognostic indicator for patients with gastrointestinal, lung, renal and gynaecological cancers.
In the breast cancer setting, the results of published trials evaluating the relationship between NLR and outcome are controversial.
Evidence of a prognostic role for NLR has been obtained mainly from studies on women of Asian race, whereas only three papers have included patients of Europe race.
What does this study add?
To the best of our knowledge, this is the first study to show a significant correlation between high NLR and worse prognosis in early breast cancer Caucasian patients by means of propensity score-matched analysis.
We analysed the prognostic significance of NLR in a highly selected population of patients with breast cancer, specifically, stage I and IIA breast cancer.
The propensity procedure was chosen by virtue of its ability to yield robust and scientifically sound results.
How might this impact on clinical practice?
Our study suggests that presurgery NLR is strongly associated with distant metastasis-free survival in a series of 300 Italian patients with early breast cancer. Prospective studies are needed to validate the introduction of NLR assessment in everyday clinical practice for prediction of cancer recurrence in order to guide decision-making for adjuvant therapy.
Introduction
Breast cancer is a commonly diagnosed malignancy and the leading cause of cancer death in women worldwide.1 Despite the widespread adoption of adjuvant treatments having resulted in improved survival, nearly 20% of patients with breast cancer still suffer from recurrence of disease.2–4
The validated clinical and molecular prognostic factors used in treatment decision-making for breast cancer include tumour stage, age, endocrine receptor status, Her-2 status, Ki67 value, number of involved regional lymph nodes, tumour histology, grade and presence of vascular invasion; additional biomarkers, namely, tumour infiltrating lymphocytes, urokinase plasminogen activator inhibitors and multigene signatures, have recently been investigated, but few of these met the requirements for ideal markers to justify their routine clinical use.5
Increasing evidence suggests that cancer-related inflammatory response plays a role in the development and progression of several malignancies. For instance, impairment of adaptive immune responses during chronic inflammation may favour promotion of tumour growth, angiogenesis and cancer cell survival.6–10
Changes in blood parameters reflecting systemic inflammation, such as C reactive protein, proinflammatory cytokines, white cell counts and platelet-to-lymphocyte ratio, have been linked to poor outcome in patients with cancer.11 12 In this regard, cell-mediated immunity may be reflected by lymphocyte count, whereas systemic inflammation may be suggested by neutrophilia. Consequently, neutrophil to lymphocyte ratio (NLR), calculated as the neutrophil count divided by the lymphocyte count, may represent an easily measurable and inexpensive marker of systemic inflammation. Several studies and two meta-analyses consistently reported NLR as an unfavourable prognostic indicator for patients with gastrointestinal, lung, renal and gynaecological cancers.13–21
In the breast cancer setting, the results of published trials evaluating the relationship between NLR and outcome are controversial, and a recent meta-analysis including eight trials published between 2012 and 2014 has shown that elevated NLR is strongly associated with poor survival. Of note, the available data mainly concern women of Asian race.22–24
The purpose of our study was to clarify the correlation between presurgery NLR and distant metastasis-free survival (DMFS) in a series of 300 Caucasian patients with early breast cancer. The propensity score-matched analysis was chosen for statistical evaluation to avoid risk of confounding bias.25
Patients and methods
A total of 300 female patients with histologically proven early (T1–2, N0–1, non-metastatic) breast cancer treated at our Institution from July 1999 to June 2015 were considered for this retrospective study. Three hundred and twenty-seven patients, whose clinical records were lacking data relevant to this study or because of preoperative chemotherapy, and 24 patients with abnormal white cell counts possibly due to concomitant infectious diseases, autoimmune diseases, or other recognisable inflammatory conditions, thus possibly causing misinterpretation of results, were primarily excluded. The following data were collected: age, menopausal status, histological tumour type, tumour size, tumour-node-metastasis stage, degree of histological differentiation, expression of oestrogen and/or progesterone receptor, HER2 status, Ki67 levels, recurrence rate and DMFS rates. Patient and tumour characteristics of the series are summarised in table 1. HER2 status was defined using the HercepTest, integrated by a FISH confirmatory test in all cases of 2+ level of staining.
Table 1.
Characteristics of the series (300 patients)
| Number (%) | |
|---|---|
| Age (years) | |
| ≤35 | 9 (3) |
| >35 | 291 (97) |
| Menopausal status | |
| Premenopausal | 139 (46.3) |
| Postmenopausal | 161 (53.7) |
| Histological type | |
| Ductal | 270 (90) |
| Lobular | 20 (6.7) |
| Others | 10 (3.3) |
| Tumour size | |
| T1 | 190 (63.3) |
| T2 | 110 (36.7) |
| Nodal status | |
| N0 | 192 (64) |
| N1 | 108 (36) |
| Grading | |
| Low | 34 (11.3) |
| Intermediate | 172 (57.3) |
| High | 94 (31.3) |
| Oestrogen receptor | |
| Positive | 250 (83.3) |
| Negative | 50 (16.7) |
| Progesterone receptor | |
| Positive | 235 (78.3) |
| Negative | 65 (21.7) |
| HER2 status | |
| Negative | 228 (76) |
| Positive | 72 (24) |
| Molecular subtype | |
| Luminal A | 77 (25.7) |
| Luminal B HER2– | 124 (41.3) |
| Luminal B Her2+ | 51 (17) |
| HER2-enriched | 21 (7) |
| Basal-like | 27 (9) |
| Ki-67 | |
| ≤20 | 102 (34) |
| > 20 | 198 (66) |
| NLR | |
| Low (≤1.97) | 134 (44.7) |
| High (> 1.97) | 166 (55.3) |
NLR, neutrophil to lymphocyte ratio.
Preoperative blood cell counts were obtained within 1 week of planned surgery. In 59 (19.6%) patients, a modified radical mastectomy was performed, whereas the remaining 241 patients received breast-conservative surgery, followed by standard radiation therapy. One hundred and fifty-eight patients (52.7%) were treated with adjuvant chemotherapy plus hormonal therapy; chemotherapy or hormonal therapy alone were administered in 55 (18.3%) and 87 (29.0%) patients, respectively, according to ESMO guidelines. Patients were followed-up according to our standardised protocol, which includes a three-monthly clinical assessment for the first 2 years, with subsequent bi-annual radiological surveillance; in case of suspicion for recurrence, further diagnostic methods, always complemented by routine histopathological examination of a biopsy specimen, are carried out as appropriate.
No patient was lost to follow-up and the study was completed by 31 December 2015. The study was approved by the Institutional Review Board at the Department of Clinical and Experimental Medicine of the Second University of Naples.
Statistical analysis
Since the primary end point of the study was to individuate factors related to tumour relapse, the DMFS rate, defined as the time from breast cancer diagnosis to the date of evidence of tumour relapse, constituted the study primary end point. Patients who died of causes other than breast cancer—without experiencing tumour recurrence—were regarded as censored events at the date of death when computing the DMFS rate. Secondary end points included the identification of the best cut-off value for NLR and possible correlations between NLR and other clinicopathological characteristics. The NLR was calculated before surgery using values derived from standard laboratory blood test results.
Statistical analysis was carried out using the SPSS statistical package (SPSS Inc, Chicago, Illinois, USA) and integrated using Medcalc software V.9.4.2.0 (Mariakerke, Belgium). In all analyses, the significance level was specified as p<0.05. Continuous data were expressed as mean±SD, range and median value. Multiple regression was performed to analyse correlations between NLR and the following prognostic variables: age, menopausal status, histology, tumour size, nodal involvement, grade of differentiation, hormonal receptor expression, HER2 status, Ki67 level and triple negativity. Receiver operating characteristic (ROC) curve analysis was used to investigate whether NLR could distinguish between recurrent and non-recurrent patients (area under the ROC curve, area under curve (AUC)). The NLR value with the best accuracy (the highest sensitivity and specificity) was selected as the NLR cut-off value. Univariate statistical analysis was determined by log-rank test (Mantel-Cox); curves were plotted using the Kaplan-Meier method, and p values and HRs with 95% CI were obtained. The independent significance of each factor was determined by the Cox proportional hazards model, following inclusion of prognostic variables showing a significant p value on univariate analysis. Moreover, Cox models were used to identify possible interactions in treatment effect between subgroups, with and without adjustment for prognostic factors. Subgroups were defined by factors showing significant value on univariate analysis or directly correlated with NLR values, namely, menopausal status, tumour size and nodal and HER2 status. All available variables (ie, age, menopausal status, histology, tumour size, nodal involvement, grade of differentiation, hormonal receptor expression, HER2 status, Ki67 level, triple negativity) were introduced in a multivariate logistic regression to calculate a propensity score for each patient. Finally, a propensity score-matched analysis, using the 1:1 nearest neighbour technique with a small caliper of 0.15 to ensure better balance, was performed to re-evaluate univariate and multivariate analyses in the matched couples.25 26
Results
NLR ranged from 0.21 to 30.00 (mean 2.67±2.52, median 2.09) in the whole population (300 patients). A significant NLR increase was observed only with T2 stage (r=0.11; p=0.043). On the contrary, NLR values did not correlate with all other prognostic variables. NLR had the ability to distinguish between relapsing and non-relapsing patients, as established by ROC curve analysis (AUC=0.625, p=0.0160), which selected the value of 1.97 as that with the highest sensitivity and specificity (figure 1). Consequently, 134 and 166 patients were grouped as low and high NLR, respectively. At the end of the study, 37 (12%) patients experienced distant metastases. Less than 10% of patients had a follow-up time shorter than 1 year; the DMFS rates for 1, 3, 6, 9, 12 and 15 years were 99.7%, 96.3%, 87.5%, 74.5%, 72.5% and 64.5%, respectively, having a statistically significant correlation with menopausal status, tumour size, nodal and HER2 status and elevated NLR values (table 2). The DMFS rates for 1, 3, 6, 9, 12 and 15 years were, respectively, 100%, 98.9%, 91.7%, 82.7%, 82.7% and 82.7% in low NLR patients, and 99.4%, 94.3%, 84.5%, 69.2%, 66.0% and 51.4% in high NLR patients, with a statistically significant association (figure 2).
Figure 1.
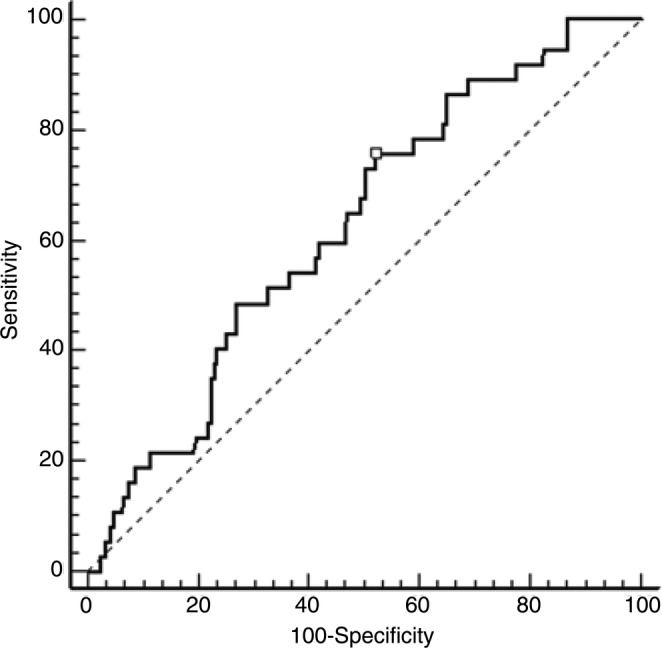
ROC analysis based on NLR for DMFS. In this model, sensitivity was 75.7% and specificity was 47.9%; AUC was 0.625 (95% CI 0.56 to 0.6), p=0.0160. AUC, area under curve; DMFS, distant metastasis-free survival; NLR, neutrophil to lymphocyte ratio; ROC, receiver operating characteristics.
Table 2.
Univariate analysis related to distant metastasis-free survival in 300 early stage patients with breast cancer
| Patients, (n) | Recurrence | 7-year DFS %* | HR for Recurrence | 95% CI *HR | p Value | |
|---|---|---|---|---|---|---|
| Age (≤35/>35 years) | 9/291 | 0/37 | 100/84 | 0.00 | 0.03 to 4.14 | 0.4123 |
| Pre/post Menopausal | 139/161 | 24/13 | 78/91 | 0.46 | 0.23 to 0.88 | 0.0194 |
| Histological type | ||||||
| Ductal | 270 | 30 | 85 | // | // | 0.4852 |
| Lobular | 20 | 7 | 77 | |||
| Others | 10 | 0 | 100 | |||
| Tumour size | ||||||
| T1 | 190 | 17 | 90 | 0.54 | 0.26 to 1.02 | 0.0577 |
| T2 | 110 | 20 | 76 | |||
| Nodal status | ||||||
| N0 | 192 | 15 | 90 | 0.41 | 0.19 to 0.75 | 0.0054 |
| N1 | 108 | 22 | 76 | |||
| Grading | ||||||
| Low | 34 | 2 | 100 | |||
| Intermediate | 172 | 21 | 86 | // | // | 0.0812 |
| High | 94 | 14 | 74 | |||
| Oestrogen receptor | ||||||
| Positive | 250 | 30 | 86 | 1.20 | 0.50 to 2.93 | 0.6561 |
| Negative | 50 | 7 | 78 | |||
| Progesterone receptor | ||||||
| Positive | 235 | 28 | 85 | 1.17 | 0.53 to 2.59 | 0.6785 |
| Negative | 65 | 9 | 81 | |||
| HER2 status | ||||||
| Negative | 228 | 22 | 89 | |||
| Positive | 72 | 15 | 76 | 0.56 | 0.23 to 1.06 | 0.0711 |
| Triple negativity | ||||||
| Yes | 30 | 3 | 85 | 0.67 | 0.25 to 1.95 | 0.5056 |
| No | 270 | 34 | 85 | |||
| Ki-67 | ||||||
| ≤20 | 102 | 18 | 90 | 1.01 | 0.52 to 1.97 | 0.9587 |
| > 20 | 198 | 19 | 82 | |||
| NLR | ||||||
| Low (≤1.97) | 134 | 9 | 89 | 0.45 | 0.25 to 0.94 | 0.0337 |
| High (> 1.97) | 166 | 28 | 81 | |||
p Values highlighted in bold are statistically significant.
*Median follow-up time.
NLR, neutrophil to lymphocyte ratio.
Figure 2.
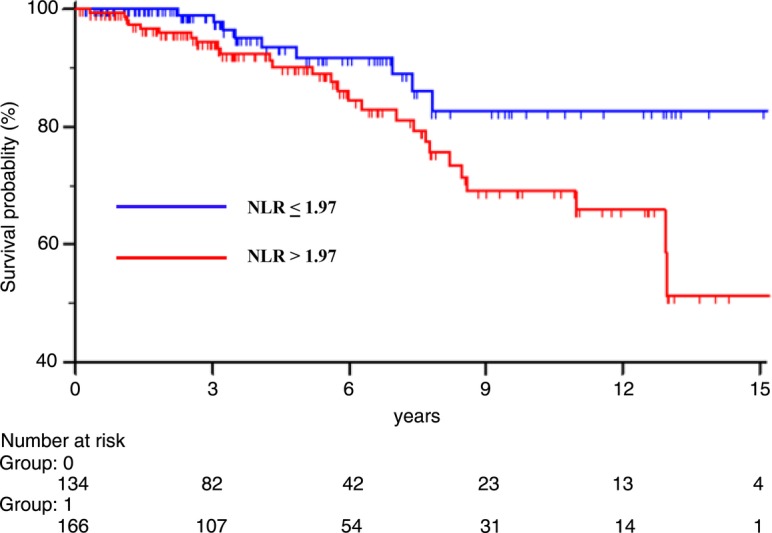
DMFS of 300 early patients with breast cancer based on NLR value. DMFS, distant metastasis-free survival; NLR, neutrophil to lymphocyte ratio.
On multivariate analysis, premenopausal status, N1 stage and a high NLR value were shown to be independent prognostic factors related to poor recurrence rate (table 3).
Table 3.
Cox proportional hazards model related to distant metastasis-free survival in 300 early stage patients with breast cancer
| Cox proportional hazards model |
|||||
|---|---|---|---|---|---|
| Prognostic factor | Coefficient | SE coefficient | HR | HR 95% CI | p Value |
| Premenopausal | 1.0252 | 0.3645 | 2.78 | 1.36/5.67 | 0.0049 |
| Tumour size (T2) | 0.3609 | 0.3448 | 1.43 | 0.73/2.81 | 0.2953 |
| Nodal status (N1) | 0.8408 | 0.3515 | 2.31 | 1.16/4.60 | 0.0167 |
| HER2 status (positive) | 0.3795 | 0.3543 | 1.46 | 0.73/2.91 | 0.2841 |
| NLR (>1.97) | 0.9718 | 0.3928 | 2.64 | 1.22/5.638 | 0.0133 |
p Values highlighted in bold are statistically significant.
NLR, neutrophil to lymphocyte ratio.
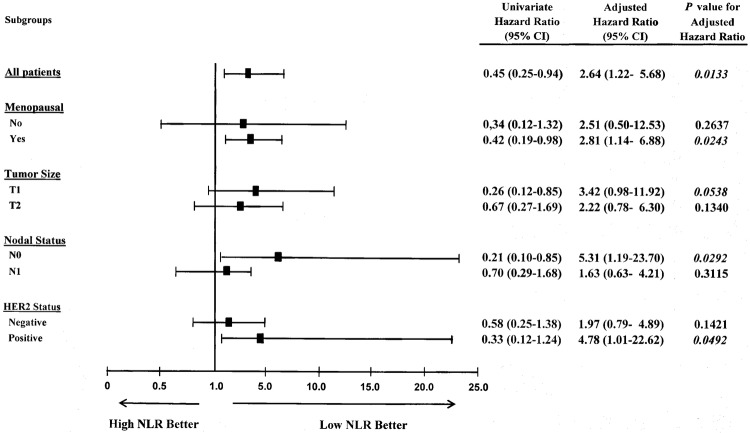
After adjusting for interfering factors related to NLR and/or probability of tumour recurrence, low NLR patients continued to display a better outcome. Particularly, significant differences were shown in postmenopausal patients (HR=2.81, 95% CI 1.14 to 6.88, p=0.0243), node negative cancers (HR=5.31, 95% CI 1.19 to 23.70, p=0.0292) and HER2-positive tumours (HR=4.78, 95% CI 1.01 to 22.62, p=0.0492) (figure 3).
Figure 3.
Forest plot showing adjusted HRs (oblongs) and 95% CIs (bars) for distant metastasis-free survival rate in 300 early patients with breast cancer undergoing potentially curative surgery, according to subgroup effects. Univariate HRs, as well as HRs adjusted for baseline covariates and related p values, are listed on the right side. Subgroups were defined by factors showing significant correlation with NLR and recurrence rate. NLR, neutrophil to lymphocyte ratio.
Propensity score-matched analysis
To further corroborate the results observed in the whole series, a propensity score-matched analysis was performed. One hundred and thirteen couples were matched, except 21 and 53 patients in the low and high NLR group, respectively. The overall χ2 balance test was not significant (p=1.0000), and the λ1 measure was larger in the unmatched (0.540) than in the matched sample (0.478), indicating improved overall balance with matching.26 In addition, before matching, the propensity score was 0.53±0.09 and 0.56±0.08, in the low and high NLR group (p=0.002), respectively. After matching, the difference between the two groups was not significant (0.55±0.08 and 0.55±0.09, respectively, p=1.000).
On univariate analysis, premenopausal status, and T2 and N1 stages as well, were confirmed to be negatively correlated with recurrence rate. Conversely, a low NLR was still significantly associated with longer disease-free survival (HR=0.40, 95% CI 0.20 to 0.89, p=0.0235 (table 4)). The multivariate analysis according to the Cox model excluded tumour stage, while confirming premenopausal status, N1 stage and high NLR values, as independent prognostic variables of poor outcome (table 5).
Table 4.
Univariate analysis related to distant metastases-free survival in propensity score-matched 226 early stage breast cancers
| Patients, (n) | Recurrence | 7-year DFS %* | HR for Recurrence | 95% CI HR | p Value | |
|---|---|---|---|---|---|---|
| Age (≤35/>35 years) | 9/217 | 0/28 | 100/84 | 0.0000 | 0.03 to 3.85 | 0.3961 |
| Pre/post menopausal | 121/105 | 21/7 | 77/92 | 0.32 | 0.16 to 0.73 | 0.0058 |
| Histological type | − | − | 0.5773 | |||
| Ductal | 205 | 235 | 85 | |||
| Lobular | 15 | 5 | 77 | |||
| Others | 6 | 0 | 100 | |||
| Tumour size | 0.48 | 0.21 to 1.00 | 0.0512 | |||
| T1 | 145 | 13 | 89 | |||
| T2 | 81 | 15 | 76 | |||
| Nodal status | 0.32 | 0.13 to 0.64 | 0.0023 | |||
| N0 | 148 | 11 | 92 | |||
| N1 | 78 | 17 | 70 | |||
| Grading | − | − | 0.1151 | |||
| Low | 28 | 2 | 100 | |||
| Intermediate | 130 | 15 | 86 | |||
| High | 68 | 11 | 72 | |||
| Oestrogen receptor | 1.44 | 0.54 to 4.17 | 0.4240 | |||
| Positive | 187 | 22 | 86 | |||
| Negative | 39 | 6 | 74 | |||
| Progesterone receptor | 1.40 | 0.59 to 3.54 | 0.4121 | |||
| Positive | 177 | 20 | 86 | |||
| Negative | 49 | 8 | 78 | |||
| HER2 status | 0.61 | 0.24 to 1.37 | 0.2151 | |||
| Negative | 174 | 18 | 87 | |||
| Positive | 52 | 10 | 76 | |||
| Triple negativity | 1.01 | 0.30 to 3.41 | 0.9750 | |||
| Yes | 22 | 3 | 78 | |||
| No | 204 | 25 | 85 | |||
| Ki-67 | 1.01 | 0.47 to 2.14 | 0.9798 | |||
| ≤20 | 73 | 13 | 88 | |||
| >20 | 153 | 15 | 82 | |||
| NLR | 0.40 | 0.20 to 0.89 | 0.0235 | |||
| Low (≤1.97) | 113 | 8 | 88 | |||
| High (> 1.97) | 113 | 20 | 81 | |||
p Values highlighted in bold are statistically significant.
*Median follow-up time.
NLR, neutrophil to lymphocyte ratio.
Table 5.
Cox proportional hazards model related to distant metastasis-free survival in propensity score-matched early stage patients with breast cancer (number 226)
| Cox proportional hazards model | |||||
|---|---|---|---|---|---|
| Prognostic factor | Coefficient | SE coefficient | Hazard rate | Hazard rate 95% CI | p Value |
| Premenopausal | 1.0816 | 0.4387 | 2.94 | 1.25 to 6.93 | 0.0136 |
| Tumour size (T2) | 0.2619 | 0.4013 | 1.29 | 0.59 to 2.84 | 0.5141 |
| Nodal status (N1) | 1.0217 | 0.4054 | 2.77 | 1.25 to 6.12 | 0.0117 |
| NLR (>1.97) | 0.9274 | 0.4197 | 2.52 | 1.11 to 5.73 | 0.0271 |
NLR, neutrophil to lymphocyte ratio.
Discussion
To the best of our knowledge, this is the first study to show a significant correlation between high NLR and worse prognosis in Caucasian patients with early breast cancer by means of propensity score-matched analysis.
Despite looking apparently simple, the relationship between NLR and outcome in patients with cancer is probably a complex and multifactorial process, still poorly understood. In simple terms, a high NLR may reflect the key role of systemic inflammation in enhancing angiogenesis, tumour growth and development of metastasis.27 Cancer-associated neutrophilia has been shown to promote remodelling of the extracellular matrix, which, in turn, leads to release of basic fibroblast growth factor, migration of endothelial cells and dissociation of tumour cells; additionally, neutrophil-derived reactive oxygen species are known to inhibit the cytotoxic activity of lymphocytes, to reduce the adhesion-promoting properties of the extracellular matrix, to suppress apoptosis of cancer cells and to prolong neutrophil life itself in the tumour microenvironment.28–30 When coupled with lymphopaenia, which seems also to correlate with poor prognosis in patients with cancer, the predictive effect on cancer prognosis may be enhanced. Indeed, the role of lymphocytes in cancer control is exemplified by the strong association between high densities of tumour-infiltrating lymphocytes and better responses to both, cytotoxic treatments and outcome in patients with breast cancer.31–35 Thus, NLR may quickly combine the prognostic strength of the above two indicators.
Actually, a high NLR has been unequivocally associated with adverse prognosis in many cancers; with regard to breast cancer, evidence of a prognostic role for NLR has been obtained mainly from studies on women of Asian race,22 36 whereas only three papers have included patients of Europe race. Besides, the survival of Asian patients with breast cancer is known to be longer than that of Caucasian women, probably owing more to lifestyle habits than to genetic susceptibility; however, this matter needs to be clarified by further studies.37–43
Another issue concerns the best approach to define the optimal cut-off value for LNR, as median, quartiles and ROC curve analysis have been variably used by different authors.
While the use of means, medians and quartiles allows division of a group or a continuous variable into two or more groups according to a purely mathematical procedure, ROC curve analysis is built by probing individual values to the final result (in this case, the presence or absence of recurrence). The result of ROC analysis, that is, the cut-off value, is the figure that proves to possess the best sensitivity and specificity in predicting the result (ie, the best prediction). For these reasons, ROC analysis, in our opinion, is the most appropriate approach.
With regard to published evidence concerning non-Asiatic patients with breast cancer, women in the highest NLR quartile (>3.3) had a significantly shorter survival in a series of 316 cases referred to Staten Island University Hospital of NY.44 In a recently published Italian trial, in which the significance of NLR was explored in 90 triple negative patients with breast cancer by means of ROC analysis, NLR >3 was associated with worse survival.45 Finally, in two cohorts of 363 and 147 patients, respectively, treated with conservative breast surgery (and intraoperative non-steroidal anti-inflammatory drugs) at Louvain University, disease-free survival and overall survival were shown to be significantly correlated with high NLR (>3.3), as calculated by ROC analysis.46
It should be noted that the NLR cut-off value selected by ROC curve analysis in our study (1.97) is lower than that used in the abovementioned papers. An explanation for this difference may be represented by the fact that our patients had a limited stage of disease. However, to further confirm the predictive value of NLR, we carried out an additional analysis using a cut-off equal to 3, and NLR again turned out to be a strong predictor of recurrence; in fact, the 7-year DMFS in 220 low and 80 high NLR breast patients with cancer was 87.3% and 77.1%, respectively (HR=0.53; 95% CI 00.23 to 1.02; p=0.0587).
Interestingly, in the whole population (300 patients) and in the 226 score-matched patients, the only variables showing prognostic significance were premenopausal status, N1stage and high NLR; in the subgroup analysis (Cox model), the prognostic value of NLR in node negative patients (HR=5.31, p=0.0292) emerged as the most intriguing finding. Indeed, in clinical practice, the latter patients are not always treated with postoperative chemotherapy, thus raising the issue as to whether early oestrogen-receptor positive patients with breast cancer should be more aggressively managed.
In conclusion, we have, for the first time, analysed the prognostic significance of NLR in a highly selected population of patients with breast cancer, specifically, stage I and IIA breast cancer, by means of propensity score analysis. This statistical procedure was chosen by virtue of its ability to yield robust and scientifically sound results. One limitation of our work lies in the retrospective nature of the study; thus, prospective studies are needed to validate its accuracy prior to introducing NLR assessment in every-day clinical practice for prediction of cancer recurrence.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Globocan 2012. Fast Stats. Most frequent cancers: both sexes. http://globocan.iarc.fr/old/bar_sex_site_prev.asp?selection=3152&title=Breast&statistic=3&populations=6&window=1&grid=1&color1=5&color1e=&color2=4&color2e=&submit=%C2%A0Execute%C2%A0 (accessed 12 Sep 2015).
- 2.Peto R, Davies C, Godwin J et al. , Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432–44. 10.1016/S0140-6736(11)61625-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies C, Godwin J, Gray R et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378:771–84. 10.1016/S0140-6736(11)60993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darby S, McGale P, Correa C, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707–16. 10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Senkus E, Kyriakides S, Ohno S et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26(Suppl 5):v8–v30. 10.1093/annonc/mdv298 [DOI] [PubMed] [Google Scholar]
- 6.Elinav E, Nowarski R, Thaiss CA et al. Inflammation-induced cancer: cross talk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013;13:759–71. [DOI] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Callaghan DS, O'Donnell D, O'Connell F et al. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol 2010;5:2024–36. 10.1097/JTO.0b013e3181f387e4 [DOI] [PubMed] [Google Scholar]
- 9.Galizia G, Orditura M, Romano C et al. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol 2002;102:169–78. 10.1006/clim.2001.5163 [DOI] [PubMed] [Google Scholar]
- 10.Mei Z, Liu Y, Liu C et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer 2014;110:1595–605. 10.1038/bjc.2014.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce BL, Ballard-Barbash R, Bernstein L et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol 2009;27:3437–44. 10.1200/JCO.2008.18.9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMillan DC. The systemic inflammation-based Glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–40. 10.1016/j.ctrv.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 13.Templeton AJ, McNamara MG, Šeruga B et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:124 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 14.Guthrie GJ, Charles KA, Roxburgh CS et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218–30. 10.1016/j.critrevonc.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 15.Gomez D, Farid S, Malik HZ et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg 2008;32:1757–62. 10.1007/s00268-008-9552-6 [DOI] [PubMed] [Google Scholar]
- 16.Shimada H, Takiguchi N, Kainuma O et al. High preoperative neutrophil–lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer 2010;13:170–6. 10.1007/s10120-010-0554-3 [DOI] [PubMed] [Google Scholar]
- 17.Tomita M, Shimizu T, Ayabe T et al. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res 2011;31: 2995–8. [PubMed] [Google Scholar]
- 18.Lee YY, Choi CH, Kim HJ et al. Pretreatment neutrophil: lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res 2012;32:1555–61. [PubMed] [Google Scholar]
- 19.Sarraf KM, Belcher E, Raevsky E et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:425–8. 10.1016/j.jtcvs.2008.05.046 [DOI] [PubMed] [Google Scholar]
- 20.Ohno Y, Nakashima J, Ohori M et al. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol 2012;187:411–17. 10.1016/j.juro.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 21.Galizia G, Lieto E, Zamboli A et al. Neutrophil to lymphocyte ratio is a strong predictor of tumor recurrence in early colon cancers: a propensity score-matched analysis. Surgery 2015;158:112–20. 10.1016/j.surg.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Deng Q, Pan Y et al. Prognostic value of neutrophil-to-lymphocyte ratio in breast cancer 2015. FEBS Open Bio 2015;5:502–7. 10.1016/j.fob.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dirican A, Kucukzeybek BB, Alacacioglu A et al. Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? Int J Clin Oncol 2015;20:70–81. 10.1007/s10147-014-0672-8 [DOI] [PubMed] [Google Scholar]
- 24.Cihan YB, Arslan A, Cetindag MF et al. Lack of prognostic value of blood parameters in patients receiving adjuvant radiotherapy for breast cancer. Asian Pac J Cancer Prev 2014;15:4225–31. 10.7314/APJCP.2014.15.10.4225 [DOI] [PubMed] [Google Scholar]
- 25.Lonjon G, Boutron I, Trinquart L et al. Comparison of treatment effect estimates from prospective nonrandomized studies with propensity score analysis and randomized controlled trials of surgical procedures. Ann Surg 2014;259:18–25. 10.1097/SLA.0000000000000256 [DOI] [PubMed] [Google Scholar]
- 26.West SG, Cham H, Thoemmes F et al. Propensity scores as a basis for equating groups: basic principles and application in clinical treatment outcome research. J Consult Clin Psychol 2014;82:906–19. 10.1037/a0036387 [DOI] [PubMed] [Google Scholar]
- 27.Mantovani A, Allavena P, Sica A et al. Cancer-related inflammation. Nature 2008;454:436–44. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 28.De Larco JE, Wuertz BRK, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 2004;10:4895–900. 10.1158/1078-0432.CCR-03-0760 [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez PC, Ernstoff MS, Hernandez C et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 2009;69:1553–60. 10.1158/0008-5472.CAN-08-1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller I, Munder M, Kropf P et al. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol 2009;30:522–30. 10.1016/j.it.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 31.Loi S, Sirtaine N, Piette F et al. Prognostic and predictive value of tumor infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J ClinOncol 2013;31:860–7. 10.1200/JCO.2011.41.0902 [DOI] [PubMed] [Google Scholar]
- 32.Gooden MJ, de Bock GH, Leffers N et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105:93–103. 10.1038/bjc.2011.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denkert C, Loibl S, Noske A et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J ClinOncol 2009;28:105–13. 10.1200/JCO.2009.23.7370 [DOI] [PubMed] [Google Scholar]
- 34.Mahmoud SMA, Paish EC, Powe DG et al. Tumor-Infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J ClinOncol 2011;29:1949–55. 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 35.West NR, Milne K, Truong PT et al. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res 2011;13:R126 10.1186/bcr3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia W, Wu J, Jia H et al. The peripheral blood neutrophil-to-lymphocyte ratio is superior to the lymphocyte-to-monocyte ratio for predicting the long-term survival of triple-negative breast cancer patients. PLoS ONE 2015;10:e0143061 10.1371/journal.pone.0143061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maskarinec G, Sen C, Koga K et al. Ethnic differences in breast cancer survival: status and determinants. Womens Health (Lond Engl) 2011;7:677–87. 10.2217/whe.11.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat 2011;127:729–38. 10.1007/s10549-010-1191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med 2003;163:49–56. 10.1001/archinte.163.1.49 [DOI] [PubMed] [Google Scholar]
- 40.Huang X, Dugo M, Callari M et al. Molecular portrait of breast cancer in China reveals comprehensive transcriptomic likeness to Caucasian breast cancer and low prevalence of luminal A subtype. Cancer Med 2015;4:1016–30. 10.1002/cam4.442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vona-Davis L, Rose DP. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J Womens Health (Larchmt) 2009;18:883–93. 10.1089/jwh.2008.1127 [DOI] [PubMed] [Google Scholar]
- 42.Wang JH, Adams IF, Tucker-Seeley R et al. A mixed method exploration of survivorship among Chinese American and non-Hispanic White breast cancer survivors: the role of socioeconomic well-being. Qual Life Res 2013;22:2709–20. 10.1007/s11136-013-0374-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen DN, Song CG, Ouyang QW et al. Differences in breast cancer characteristics and outcomes between Caucasian and Chinese women in the US. Oncotarget 2015;6:12774–82. 10.18632/oncotarget.3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azab B, Bhatt VR, Phookan J et al. Usefulness of the Neutrophil-to-Lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Sur Oncol 2012;19:217–24. 10.1245/s10434-011-1814-0 [DOI] [PubMed] [Google Scholar]
- 45.Pistelli M, De Lisa M, Ballatore Z et al. Pre-treatment neutrophil to lymphocyte ratio May be a useful tool in predicting survival in early triple negative breast cancer patients. BMC Cancer 2015;15:195 10.1186/s12885-015-1204-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forget P, Bentin C, Machiels JP et al. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. Br J Anaesth 2014;113(Suppl 1):i82–7. 10.1093/bja/aet464 [DOI] [PubMed] [Google Scholar]