Abstract
Diagnosis and treatment of bone metastasis requires various types of measures, specialists and caregivers. To provide better diagnosis and treatment, a multidisciplinary team approach is required. The members of this multidisciplinary team include doctors of primary cancers, radiologists, pathologists, orthopaedists, radiotherapists, clinical oncologists, palliative caregivers, rehabilitation doctors, dentists, nurses, pharmacists, physical therapists, occupational therapists, medical social workers, etc. Medical evidence was extracted from published articles describing meta-analyses or randomised controlled trials concerning patients with bone metastases mainly from 2003 to 2013, and a guideline was developed according to the Medical Information Network Distribution Service Handbook for Clinical Practice Guideline Development 2014. Multidisciplinary team meetings are helpful in diagnosis and treatment. Clinical benefits such as physical or psychological palliation obtained using the multidisciplinary team approaches are apparent. We established a guideline describing each specialty field, to improve understanding of the different fields among the specialists, who can further provide appropriate treatment, and to improve patients’ outcomes.
Keywords: bone metastasis, multidisciplinary team approach, a diagnosis and treatment guideline
Find the ESMO Clinical Practive Guidelines on bone health in cancer pateints here: http://www.esmo.org/Guidelines/Supportive-Care/Bone-Health-in-Cancer-Patients
Introduction
Bone metastasis is a devastating condition that can have a negative impact on the lives of patients with advanced cancer in many ways. Patients may experience limitations in the activities of daily living (ADL), decreases in quality of life (QOL), threat of survival and increases in medical expenses. Large-scale aetiological studies on the prevalence or incidence of bone metastasis have not been conducted in Japan or in other countries. A smaller study on autopsy cases over the period from 1959 to 1997 recorded by the Shikoku Cancer Center in Japan indicated that the frequencies of bone metastasis varied among cancers; they were as high as 75% in cancers such as of the breast and prostate, and as low as 22% in stomach and colon cancers.
Recently, the number of cancer survivors pertaining to breast and colorectal cancers has globally increased, and the 5-year survival rates are ≥60% and ≥85%, respectively. In many countries, the 5-year survival rate for prostate cancer is ≥95%.1 An increase in the survival time may increase the incidence of bone metastasis.
Recently, cancer chemotherapy has also made considerable progress in increasing survival of patients with far-advanced cancer. For example, gefitinib improved disease-free survival of epidermal growth factor receptor (EGFR) mutant non-small cell lung cancer (NSCLC).2 Pertuzumab and trastuzumab increased the median overall survival (OS) of patients with EGFR 2-positive metastatic breast cancer.3
In addition to these therapeutic measures, agents that target bone metastatic lesions have provided clinical benefits to patients. Medicines targeting bone metastasis include bone-modifying agents (BMAs) and radiopharmaceuticals. To date, zoledronic acid (ZA) is the most promising of the bisphosphonates (BPs). Denosumab (D-mab), another potent BMA, which targets the receptor activator of nuclear factor κ-B ligand, has also been approved for skeletal-related events (SREs). β Emitters such as strontium-89 (89Sr) and samarium-153 (153Sm) have been shown to be effective for palliation of cancer pain induced by bone metastases.
Furthermore, surgical and interventional measures such as vertebroplasty and ablation have been developed. Caregivers should consider comprehensive strategies to treat patients with bone metastasis, using multimodal measures.
Methods
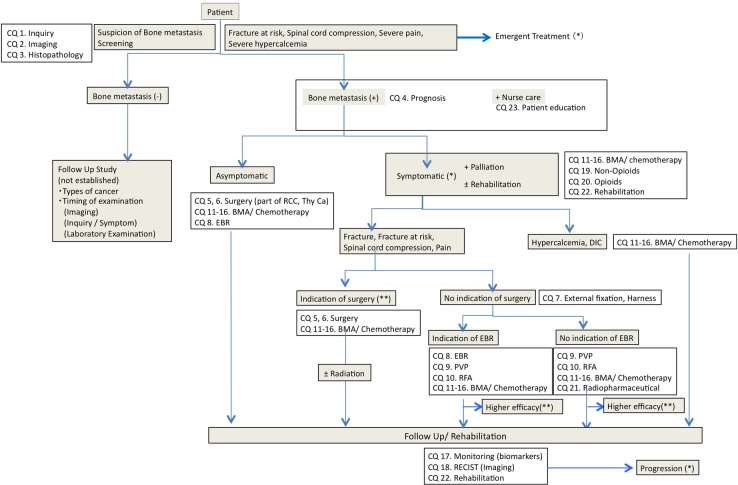
The guideline was developed according to the Medical Information Network Distribution Service (MINDS) Handbook for Clinical Practice Guideline Development 2014. The clinical algorithm is depicted in figure 1, and clinical questions (box 1) were derived on the basis of this algorithm.
Figure 1.
Clinical algorithm of diagnosis and treatment of bone metastasis guideline. BMA, bone-modifying agent; CQ, Clinical Question; DIC, disseminated intravascular coagulation; EBR, external beam radiation; PVP, percutaneous vertebroplasty; RCC, renal cell carcinoma; RECIST, Response Evaluation Criteria in Solid Tumor; RFA, radiofrequency ablation; thy CA, thyroid cancer.
Box 1. Table Clinical Questions and Answers.
CQ 1. What are the symptoms induced by bone metastasis that necessitate emergent treatment?
A. Spinal cord compression and hypercalcemia need to be treated emergently.
CQ 2. What kinds of imaging are useful to diagnose bone metastasis?
A. Bone scintigraphy, 18F-fluorodeoxyglucose positron emission tomography/CT and MRI are useful to diagnose bone metastasis.
CQ 3. When is pathological examination needed?
A. It is needed when diagnosis is difficult, in primary unknown cancers, or in cases with multiple cancers.
CQ 4. Does the presence of bone metastasis affect the patient's prognosis?
A. Bone metastasis at diagnosis might affect the prognosis.
CQ 5. Is surgery beneficial to treat spinal metastasis with spinal cord compression?
A. Surgery is effective for functional improvement.
(Weak, Evidence B)
CQ 6. Is surgery beneficial to treat long bone metastasis with pathological fracture or those at risk?
A. Surgery is beneficial for pain relief and/or functional improvement.
(Strong, Evidence C)
CQ 7. Is the use of instrumentation beneficial in bone metastasis?
A. Instruments are beneficial for treatment and prevention of pathological fracture.
(Strong, Evidence C)
CQ 8. Is external beam radiation beneficial in bone metastasis?
A. External beam radiation is beneficial for relief from pain.
(Strong, Evidence A)
CQ 9. Is vertebroplasty beneficial in bone metastasis?
A. Vertebroplasty is beneficial for inoperable cases to sooner relieve pain on movement.
(Weak, Evidence C)
CQ 10. Is ablation is beneficial in bone metastasis?
A. Ablation is beneficial to relieve pain.
(not approved in Japan, Evidence C)
CQ 11. Are bone-modifying agents (BMAs) beneficial in bone metastasis of lung cancer?
A. Zoledronic acid (ZA) and denosumab (D-mab) are beneficial to SREs, regardless of symptoms.
(Strong, Evidence A)
CQ 12. Are BMAs beneficial in bone metastasis of breast cancer?
A. ZA, pamidronic acid and D-mab are beneficial in treating SREs.
(Strong, Evidence A)
CQ 13. Are BMAs beneficial in bone metastasis of prostate cancer?
A. ZA and D-mab are beneficial in treating SREs among castration resistant cases.
(Strong, Evidence A)
CQ 14. Are BMAs beneficial in bone lesions of multiple myeloma?
A. Bisphosphonates are beneficial in treating SREs.
(Strong, Evidence A)
CQ 15. Are BMAs beneficial in bone metastasis of the other cancers?
A. BMAs are beneficial in treating SREs of the other cancers.
(Weak, Evidence C)
CQ 16. What are the adverse events with BMAs to be cautious of?
A. Osteonecrosis of the jaw, renal toxicity, hypocalcaemia and flu-like illness should raise caution.
CQ 17. What kinds of biomarkers are useful to monitor the effects?
A. No biomarkers are recommended for practice.
CQ 18. What kinds of imaging are useful to monitor the effects of therapeutic measures to bone metastasis?
A. Osteolytic or mixed bone metastases with soft tissue components can be monitored with CT or MRI.
CQ 19. Are non-opioids effective to relieve pain of bone metastasis?
A. Non-opioids are effective to relieve pain.
(Strong, Evidence C)
CQ 20. Are opioids effective to relieve pain of bone metastasis?
A. Opioids are effective to relieve pain.
(Strong, Evidence C)
CQ 21. Are radiopharmaceuticals effective to relieve pain of bone metastasis?
A. Radiopharmaceuticals are beneficial to relieve pain in valid cases with the other measures.
(Weak, Evidence B).
CQ 22. Is Rehabilitation beneficial in bone metastasis?
A. Rehabilitation is beneficial to improve activities of daily living and quality of life, and to prevent disuse syndrome.
(Weak, Evidence C).
CQ 23. Is patient education beneficial in bone metastasis?
A. Patient education is beneficial in bone metastasis.
Medical evidence was extracted from published articles describing meta-analyses or randomised controlled trials (RCTs) in PubMed, the Cochrane Library, CINAHL and the Japan Medical Abstracts Society. A systematic literature search was performed by Japan Library Association, mainly from 2003 to 2013. Medical evidence was evaluated critically and divided into four levels, A–D, covering estimated effects with higher reliability (A) to those that were mere speculations (D). On the basis of these levels, preliminary recommendations were elicited. Forward questions were fundamentally written in Patient, Intervention, Comparison, Outcome style. For forward questions, the synopsis of recommendations was assessed as strong or weak. To achieve consensus, majority voting (>70%) of the conference was adopted according to the Delphi technique.
Results
Diagnostic procedures
Symptoms (CQ 1)
‘Spinal cord compression (SpCC) and hypercalcaemia need to be treated emergently’. Among SREs, SpCC and malignant hypercalcaemia (MHC) should be emergently treated. The frequencies of SpCC and MHC are reported to be 3% and 13% in breast cancer, 8% and 1% in prostate cancer, and 4% and 4% in lung cancer, and other cancers, respectively.4–6 These cancers are asymptomatic in the very early stage; therefore, careful examination is required. Symptoms of SpCC are back pain, listlessness of the lower limb and cauda equina syndrome.7 8 Symptoms of MHC are anorexia, nausea, fatigue, polyuria, muscle weakness, hyporeflexia, confusion, tremor, torpor, etc.9
Imaging (CQ 2)
‘Bone scintigraphy (BS), 18F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are useful to diagnose bone metastasis’. BS, 18F-fluorodeoxyglucose PET, and MRI are recommended as practical imaging measures to detect bone metastasis. A meta-analysis of BS combined with single-photon emission CT (SPECT) indicated that the sensitivity was 86% and specificity was 81%.10 A meta-analysis showed that the sensitivity of PET combined with CT (PET/CT) was 90% and the specificity of this combination was 97%.10 For cancers with a high risk of metastasis, including breast cancer, PET/CT is recommended in the National Comprehensive Cancer Network (NCCN) guideline.11 PET is better than CT in diagnosing osteolytic lesions; however, when combined with CT, its ability to detect osteoplastic lesions increases.12 MRI, particularly whole-body MRI, can detect bone metastasis with sensitivity and specificity of 91% and 95%, respectively.13 Using diffusion-weighted MRI (DW-MRI), the specificity increases to 96%.13 Radiography and CT are useful in evaluating the size of lesions or the rigidity of skeletal structures;14 sodium 18F-fluoride has high diagnostic potential and accuracy.15
Histopathology (CQ 3)
‘Histopathology is needed when diagnosis is difficult, and in primary unknown cancers or in cases with multiple cancers’. In many cases where bone metastasis is confirmed based on the patient's clinical course, histopathological analysis is often omitted; however, in case of an unknown primary and ≥2 synchronous or metachronous cancers, it is necessary.16 Tissue materials can be obtained using CT-guided percutaneous needle or open biopsy; aspiration cytology, despite the lower accuracy, is an alternative.17 18
Prognosis (CQ 4)
‘Bone metastasis at diagnosis might affect the prognosis’. In the Danish National Patient Registry (DNPR; 1997–2007), the 5-year survival rates of patients with prostate cancer with and without bone metastasis at diagnosis were 3% and 56%, respectively.19 Among registrants in the Surveillance Epidemiology, and End Results programme in the USA (1999–2005), the HRs for risk of death in patients with prostate cancer with and without SREs were 10.2 and 6.6, respectively.20 In DNPR, the 5-year survival rates of patients with breast cancer with and without bone metastasis at diagnosis were 8.3% and 75.8%, respectively.21 Katagiri et al22 proposed a scoring system that correlated with prognosis.
Treatments
Surgery
Metastatic spinal tumour (CQ 5)
‘It is weakly suggested that surgery is effective for functional improvement’. Surgery for spinal metastasis is beneficial for tumour resection as well as for relieving pain and improving neurological manifestations. Significant improvement pertaining to walking was observed in patients treated with surgery plus radiation compared with that in patients treated with radiation alone;23 however, a report by Rades et al24 did not confirm these results. Surgery for spinal metastases because of radiosensitive tumours such as multiple myeloma, malignant lymphoma and leukaemia, should be avoided.23–25 Surgery is not recommended in cases where ≥48 h are passed since complete paralysis or when prognosis is predicted within 6 months.23 26 For spinal metastasis of breast or prostate cancer, hormonal treatment or radiation is the first choice before surgery.23 27 Operative methods for spinal metastasis differ according to the sites and sizes of metastases. A posterior approach, decompression by laminectomy and spinal fixation, are the most common procedures. Total en bloc spondylectomy may be beneficial when the lesion is single and long survival is expected.26
Metastasis to long bones (CQ 6)
‘It is strongly suggested that surgery is beneficial for pain relief and/or functional improvement’. Surgery is beneficial to repair mechanical ruptures, relieve pain, and improve diseased limb function and QOL during pathological fractures or at risk fractures.28–30 The outcomes of surgery for at risk fractures are better than those of surgery for pathological fractures in many aspects such as those concerning blood loss, period of hospitalisation and functional recovery.31 32 Mirels reported a scoring system for risk of fracture.33–35 Linden claimed that axial cortical involvement >30 mm and circumferential cortical involvement >50% are predictive of fracture.36 Operative methods are divided into two types: internal fixation and prosthesis replacement.
External fixation (CQ 7)
‘It is strongly suggested that instruments are beneficial for treatment and prevention of pathological fractures’. Braces are used for various purposes such as relief from pain, and preservation and stabilisation of diseased bone. Braces are appropriate for postoperative fixation. For pathological and at risk fractures, surgery is superior to non-surgical conservative treatment in increasing QOL. Among conservative approaches such as resting and rehabilitation, braces and body casts, braces are best for thoracic and lumbar spinal compression (compression rate <50%) without neurological symptoms.37 Emergently, external fixation using splint and weight-bearing orthoses such as crutches are suitable for long-bone metastasis.
External beam radiotherapy (CQ 8)
‘It is strongly suggested that external beam radiation (EBR) is beneficial for relief from pain’. EBR can relieve pain caused by bone metastasis without SpCC or pathological fracture in 59–73% of cases,38–40 and neuropathic pain in 53–61% of cases.41 Dose fractionation is performed using multifractionated radiation (MFR) such as 30 Gy divided into 10 fractions (30 Gy/10 Fr or 20 Gy/5 Fr). Single-dose irradiation (SDI) such as 8 Gy is also performed. In some studies, the effects on pain using either MFR or SDI were identical; pain relief was achieved in 60–73% patients using SDI and in 59–73% patients using MFR.38–40 Pain relief was achieved within 3 weeks in half the total number of cases where the treatment was effective. Neuropathic pain was relieved in 53% of patients treated using SDI and in 61% of patients treated using MFR.41 Regarding the duration of pain relief, there was no significant difference between SDI and MFR; the median time for recurrence was 2.4 months after SDI and 3.7 months after MFR.41 The average duration of pain relief was 29 weeks after SDI and 30 weeks after MFR.42 Additional radiation is performed in 7–8% of MFR patients and in 20–22% of SDI patients.38–40 Additional radiation relieved pain in 58% of patients in another study comprising 33–66% of patients in whom the first radiation treatment was not effective.43 Additional SDI and MFR relieved pain in 66–70% and 33–57% of patients, respectively.43 It is not clear whether EBR can completely prevent pathological fracture. The incidence of fracture was identical by both methods (SDI=3.3% vs MFR=3.0%).40 Thus, fixation of the damaged cortex of a femoral metastasis >30 mm in longitudinal length is necessary before irradiation.44 The frequency of SpCC after EBR is reduced to 2.8–3.0% using SDI, and 1.6–1.9% using MFR.39 40
Vertebroplasty (CQ 9)
‘It is weakly suggested that vertebroplasty is beneficial for inoperable cases to sooner relieve pain on movement’. Percutaneous vertebroplasty (PVP) can relieve pain associated with movement of weighted vertebrae or relieve neuropathic pain when surgery is not indicated. Complications such as acute phase of infection, haemorrhagic diathesis and severe heart disease, are contraindicative.45 PVP is applicable for radio-resistant patients, and an additive effect is obtained by combining it with radiotherapy. PVP relieves pain within 1–3 days.46 Polymethyl methacrylate is commonly used as cement. Extreme care should be taken to not leak cement out of the targeted vertebral body. As the therapeutic effect does not correlate with cement volume, it is recommended that only the minimum amount needed should be used. Balloon kyphoplasty is performed to attempt kyphosis of the diseased vertebra to avoid leakage.47
Ablation (CQ 10)
‘Ablation is beneficial for pain relief’. Radiofrequency ablation (RFA) is used to kill tumours by heating, using image-guided needle centesis. RFA is one measure to relieve pain from bone metastasis, based on results from two RCTs.47 48 RFA is used to treat resistant patients or patients unresponsive to radiotherapy; however, in Japan, it is not covered by medical insurance. Cryoablation is an alternative method to relieve pain.49
Bone modifying agents
Lung cancer (CQ 11)
‘It is strongly suggested that zoledronic acid (ZA) and denosumab (D-mab) are beneficial to SREs, regardless of symptoms’. RCTs comparing ZA with placebo that target NSCLC (50% of total participants) and small cell lung cancer (8%) have shown the occurrence rate of SRE treated with ZA to be 38.9% and that with placebo to be 48.0% (p=0.039).50 51 RCT comparing ZA with D-mab showed that the duration of the first SRE was 16.3 months using ZA and 20.6 months using D-mab (40% were NSCLC).52 Non-inferiority of D-mab to ZA is proven (p=0.06). For SREs that needed radiotherapy, the grade of pain and dosage of opioids were significantly suppressed in the D-mab group.53 Exploratory analysis of this trial indicated that OS was prolonged in the D-mab group.54
Breast cancer (CQ 12)
‘It is strongly suggested that ZA, pamidronic acid (PA) and D-mab are beneficial to SREs’. An RCT comparing PA with placebo showed that PA could significantly decrease SREs of breast cancer from 4.0 to 2.5 per person-year. PA was effective with chemotherapy or hormonal treatment.55 In the breast cancer subgroup, ZA significantly decreased SREs by 20%.56 Improvements in QOL score, progression free survival (PFS) and OS were not achieved. Ibandronate decreased SREs and improved pain and QOL scores.57–60 An RCT comparing ZA with D-mab showed that D-mab significantly decreased the first SREs by 18% and the second SREs by 23%.61 62 Improvement in QOL score was achieved in 37.1% of the D-mab group and in 31.4% of the ZA group.61 62 No improvements were observed in PFS and OS. A meta-analysis showed 8 of 10 papers reporting BPs to significantly reduce SREs (relative risk (RR) = 0.85). ZA significantly reduced SREs compared with PA (RR=0.80).63 D-mab significantly reduced SREs compared with ZA (RR=0.78).
Prostate cancer (CQ13)
‘It is strongly suggested that ZA and D-mab are beneficial to SREs of castration resistant cases’. Hormonal treatment is effective in most prostate cancers, regardless of bone metastasis. In many cases, combined androgen blockade with lutenising hormone-releasing hormone analogue, antagonist and a non-steroidal antiandrogen agent, is used.64 Prostate cancer is sensitive to hormonal treatment in the first 2 years on average, but finally turns into castration-resistant prostate cancer (CRPC).65 66 Clodronate combined with hormonal treatment prolongs survival compared with placebo (RR=0.77).67 The survival benefit of ZA or D-mab with hormonal treatment is controversial. An RCT comparing ZA with placebo against CRPC indicated that the frequency of SREs was 33.2% in the ZA group and 44.2% in the placebo group (p=0.021). OS was longer in the ZA group than in the placebo group (546 vs 464 days, p=0.094).5 68 An RCT comparing D-mab with ZA against CRPC showed that the duration to the first SRE was 20.7 months in the D-mab group and 17.1 months in the ZA group (p=0.0085).69
Multiple myeloma (CQ 14)
‘It is strongly suggested that BPs are beneficial to SREs’. Combination therapy of PA with chemotherapy for bone lesions reduced the occurrence of SREs compared with chemotherapy alone (24% vs 41%, p<0.001).70 Clodronate with chemotherapy inhibited the progression of osteolytic lesions compared with chemotherapy alone (12% vs 24%, p=0.026).71 An RCT comparing PA with ZA to treat breast cancer and multiple myeloma showed that they had similar effects.72 An RCT comparing the first-line treatment using ZA plus chemotherapy with that of clodronate plus chemotherapy showed that SREs were significantly suppressed in the ZA group (HR=0.74, p=0.0004).73 A meta-analysis comparing BPs with placebo showed that BPs significantly suppressed pathological fracture of vertebrae (RR=0.74) and occurrence of SREs (RR=0.80), and improved pain (RR=0.75).74 A better survival benefit with ZA was achieved compared with clodronate (HR=0.84, p=0.0118).73 A meta-analysis comparing all types of BPs to placebo showed that BPs had no survival benefits. However, ZA showed a survival benefit (HR=0.61).74 An RCT comparing ZA with D-mab showed that D-mab significantly prolonged the duration to the first SRE (14.4 vs 19.0 months, p=0.022).75 However, the survival benefit with D-mab was inferior to that with ZA (HR=2.26).
An RCT comparing melphalan plus prednisone (MP) with MP plus bortezomib (VMP) showed that VMP suppressed the progression of bone lesions and decreased the need for radiation.76 77
Other cancers (CQ 15)
‘It is weakly suggested that BMAs are beneficial to SREs of the other cancers’. In an RCT comparing ZA with placebo, other cancers such as gastrointestinal cancer accounted for 10% of the total.50 Subset analysis showed that ZA had a similar effect on SREs. A meta-analysis of three RCTs comparing ZA with D-mab showed that D-mab significantly suppressed SREs.75 No survival benefit of D-mab was observed.
Combination with radiotherapy
There are no meta-analyses of combination treatment with BMAs and radiation; however, >200 retrospective studies have been reported,78–82 where radiation with BPs has been effective. The American Society of Clinical Oncology (ASCO) guideline for bone metastasis of breast cancer recommends radiation with BPs for SREs.83 84
Adverse events (CQ 16)
‘Osteonecrosis of the jaw (ONJ), renal toxicity, hypocalcaemia and flu-like illness should be cautious’.
Osteonecrosis of the jaw
The frequency of ONJ induced by BPs varies from 1% to 10%.85 D-mab induces ONJ with the same frequency.61 Longer duration of BP injection increases the risk; the incidence at 4–12 months is 1.5%, whereas that at 27–48 months is 7.7%.85 Appropriate oral hygiene decreases ONJ.86 87 Patients should maintain good oral hygiene, and have dental examinations and preventive dental treatment prior to initiating therapy.83 Tooth extraction, oral infection and artificial dentures are risk factors.88 Extraction should be completed before administration and takes 14–21 days to recover from.89
Renal toxicities
The frequency of renal toxicities with ZA is 4.9–44.5%.6 51 52 61 69 90–93 However, many of these toxicities remain within grade 1 or 2 and are reversible. Risk factors are older age (>65 years), combination use with non-steroidal anti-inflammatory drugs (NSAIDs) or cis-platinum, diabetes mellitus and multiple myeloma. Multiplicity and longer duration (>2 years) increase the risk.93 The median time to occurrence is 4.7–5.4 months.94 95 The risk for acute renal failure increases in low creatinine clearance rates (CCR, <60 mL/min).52 61 Dose modification according to CCR is recommended. The frequency of renal toxicities with D-mab is 3.3–14.7% for all grades and 0.4% for grades >3.52 61 69
Hypocalcaemia
The frequency of hypocalcaemia with ZA is 3.3–9.0%.53 61 69 75 94 The frequency of clinical symptoms or for grades >3 is 1.0–4.7%.53 69 74 96 The frequency with D-mab is 1.7–10.8% for all grades and 1.3–5.1% for grades >3.53 61 68 75 Administration of BMA without vitamin D and oral calcium elevates the frequency of hypocalcaemia by 5–6 times.53 61 68 In case of D-mab, a daily supply of vitamin D (natural form; 400 IU) and oral calcium (500 mg) is necessary. Risk factors are low serum calcium before treatment, and renal dysfunction.97 98 The onset is ≤10 days in most cases and early monitoring is important.
Others
Other adverse events include flu-like reactions, which occur within 3 days; their frequencies are 17.7–22.0% with ZA and 8.4–10.4% with D-mab.61 68 92 Atypical femoral fractures are rare severe adverse events associated with ZA.99
Monitoring of treatment
Biomarkers (CQ 17)
‘No biomarkers are suggested for practice’. Broun reported that elevation of type I collagen cross-linked N-telopeptide (NTx) or bone-specific alkaline phosphatase during treatment indicated poor prognosis of lung and prostate cancers with bone metastases.100 Coleman et al101 reported that, in cases with high urine NTx (uNTx), SRE or disease progression risks were 4–6 times higher than those with low uNTx. The prognostic value of uNTx is apparent, but the predictive value for therapeutic effects is not. Longer survival was achieved in cases where uNTx was normalised using ZA compared with cases where uNTx was not normalised (RR=0.52).102 Clinical events such as death or SREs were preceded by elevation of bone turnover markers (BTMs) in >90% of cases. Inverse phenomena were observed only in 5.6% of breast cancers and 5.9% of prostate cancers.103 The ASCO guideline does not recommend measurement of BTMs to monitor BMA effects.87
Imaging (CQ 18)
‘Osteolytic or mixed bone metastases with soft tissue components can be monitored with CT or MRI’. Evaluation using radiography, BS, or PET is not suitable for bone metastasis, according to the Response Evaluation Criteria in Solid Tumor V.1.1.104 Osteolytic and mixed lesions can be evaluated by CT or MRI. There are no imaging devices for evaluating osteoplastic lesions. The Prostate Cancer Working Group 2 in the USA set the criteria for progressive disease of osteoplastic new lesions using BS.105 The bone scan index (BSI) is a quantitative method for detecting possible metastatic lesions as a percentage of total bone quantity. Changes in BSI before and after treatment show better correlation with prognosis than changes in prostate specific antigen (PSA).106 DW-MRI can measure water diffusion, which reflects cellularity as an apparent diffusion coefficient (ADC).107 The possibility of ADC to predict tumour response clinically is now under verification.
Others
The patient-reported outcome (PRO) measures a patients’ own health condition by self-reporting. PRO has recently been considered to provide a real benefit to patients; for example, McGill-Melzack reported benefits of using the pain intensity scale and brief pain inventory scale.108 109
Palliation
Non-opioids (CQ 19)
‘It is strongly suggested that non-opioids are effective to relieve pain’. The WHO has developed a three-step ladder for cancer pain, and non-opioids are the first choice. No RCTs have compared non-opioids on a large scale.110 111 Joishy and Walsh112 reported that the dosage of morphine could be reduced by ketorolac. Combined effects of opioids and non-opioids are still controversial.110 111 Other reviews have concluded that acetaminophen, NSAIDs and steroids are effective. Steroids are not analgaesics but are effective for reducing pain flare induced by radiation.113
Opioids (CQ 20)
‘It is strongly suggested that opioids are effective to relieve pain’. Many observational studies indicate that opioids are effective for pain of bone metastases.114 115 Comparison between the fentanyl patch and codeine–acetaminophen combination indicated that fentanyl had significantly superior effects.116 Bone metastatic pains are divided into two types: continuous pain at rest and breakthrough pain. The treatment strategy differs for each type. A systemic review found that the utility of rescue drugs for breakthrough pain and the dosage should be individually adjusted.117 For neuropathic pain, when not fully relieved by opioids, combination use with adjuvant analgaesics should be considered.
Radiopharmaceuticals (CQ 21)
‘It is weakly suggested that radiopharmaceuticals are beneficial to relieve pain in valid cases with the other measures’. A meta-analysis showed that radiopharmaceuticals were effective for 1–6 months.118 Two-thirds of patients treated using 89Sr showed pain relief.119 120 89Sr contributed to reducing the dosage of analgaesics and improving patients’ QOL.118 In Japan, 153Sm and rhenium-186 are not covered by medical insurance. 89Sr is particularly effective for prostate cancer because it is highly taken up by osteoplastic lesions as calcium mimetics.118 120 In many reports, the effect of 89Sr is identical to that of EBR, but the incidences of nausea and vomiting are lower using 89Sr.119 The effect of a combination of 89Sr and EBR remains controversial.118 119 The combination of 89Sr and ZA was effective for prostate cancer.121 An RCT comparing the combination of 89Sr and ZA with 89Sr alone for asymptomatic bone metastasis of NSCLC showed that the combination reduced the occurrence of SREs and improved OS.122 The antitumour effects of 89Sr, including reduction of PSA levels or improved survival in prostate cancer, are reported in a few cases.123 89Sr can be administered every 3 months. Reported adverse events include bone marrow suppression; thrombocytopaenia is most common (15–50%), but the grade is <2 in most cases.118–120 Leucocytopaenia is less frequent; however, caution is required when 89Sr is combined with chemotherapy. An RCT showed that 223Ra significantly increased OS and the time to occurrence of SREs with low toxicities compared with placebo.124
Rehabilitation and patient education (CQ 22, 23)
‘It is weakly suggested that rehabilitation is beneficial to improve ADL and QOL, and to prevent disuse syndrome’. Rehabilitation is beneficial in terms of providing pain relief, prevention of degeneration, improvement of ADL and QOL, and increased survival. An RCT comparing treatment plus rehabilitation with treatment alone of symptomatic spinal metastasis showed significant improvements in pain score (p<0.001), dosage of analgaesics (p<0.001) and depression status.125 Tang retrospectively showed that rehabilitation for spinal metastasis significantly improved the scores of functional independence measures and recovered function.126 Resting in bed is good for preventing SREs but leads to reduction of ADL and QOL, and results in disuse syndrome, which can cause sepsis or respiratory failure and increase the risk of death.
‘Patient education is beneficial to bone metastasis’. PRO-SELF is an education programme provided in the patient's home and by telephone. An RCT comparing PRO-SELF with normal care showed that knowledge of cancer pain reduced pain scores.127–129 A systematic review of educational intervention showed that it could reduce pain scores but could not improve patient's QOL.130
Conclusion
Diagnosis to treatment for bone metastasis needs various types of measures, specialists and caregivers. Consideration of the status of diseases, as well as of the background of patients, should be taken. For this purpose, a multidisciplinary team is necessary. In treating, multidisciplinary meetings are helpful. To collaborate with other specialists, a guideline describing each specialty field is necessary. The clinical benefits, such as physical or psychological palliation, obtained by the multidisciplinary team approaches, are described in some papers.131–133 Further, registration in addition to multidisciplinary team approaches could be advantageous to monitor the therapeutic outcomes according to the guideline.
Acknowledgments
The authors thank the members of the guideline committee and the JSMO guideline evaluation committee for their reviews. In particular, they acknowledge Kei Muro, Takayuki Yoshino and Yutaka Fujiwara, for their critical reviews of the final manuscript in Japanese. They also thank Chiharu Tada and Hiromi Nishizawa, secretaries of the JSMO, and Yuji Tatsugami, for their help with editing the Japanese version of the guideline, and ENAGO for their English language review.
Footnotes
Twitter: Follow Hiroyuki Shibata at @Shibacchi
Funding: The total cost of developing this guideline was borne by JSMO.
Competing interests: S Kato, sponsored research (Chugai Pharmaceutical Co, Ltd); IO, honoraria (Taiho Pharmaceutical Co, Ltd); S Kinuya, data and safety monitoring board (Bayer Yakuhin, Ltd); NS, honoraria (Novartis Pharma); ST, honoraria (Astellas Pharma Inc, AstraZeneca plc, Daiichi Sankyo Co, Ltd, Novartis Pharma), sponsored research (Sanofi, Zenyaku Kogyo Co, Ltd, Taiho Pharmaceutical Co, Ltd, Boehringer Ingelheim Japan, Chugai Pharmaceutical Co, Ltd, Novartis Pharma); YT, honoraria (Janssen Pharma); K Takayama20, consultation and honoraria (Clinical Research Support Center Kyushu, AstraZeneca plc, Chugai Pharmaceutical Co, Ltd, Eli Lilly Japan, PfizerJapan Inc), sponsored research (Kyowa Hakko Kirin Co, Ltd, plc, Daiichi Sankyo Co, Ltd, Chugai Pharmaceutical Co, Ltd, Eli Lilly Japan, Novartis Pharma, Bristol-Myers Squibb); UT, consultation and honoraria (Micron Inc, SymBio Pharmaceutical Co, Ltd, Chugai Pharmaceutical Co, Ltd, Nihon Medi-Physics Co, Ltd); HM, sponsored research (Eisai Co, Ltd, Taiho Pharmaceutical Co, Ltd); T Yuasa, honoraria (Novartis Pharma, PfizerJapan Inc).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Allemani C, Weir HK, Carreira H et al. , CONCORD Working Group. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977–1010. 10.1016/S0140-6736(14)62038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CK, Brown C, Gralla RJ et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595–605. 10.1093/jnci/djt072 [DOI] [PubMed] [Google Scholar]
- 3.Swain SM, Baselga J, Kim SB et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724–34. 10.1056/NEJMoa1413513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipton A, Theriault RL, Hortobagyi GN et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer 2000;88:1082–90. [DOI] [PubMed] [Google Scholar]
- 5.Saad F, Gleason DM, Murray R et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst 2004;96:879–82. 10.1093/jnci/djh141 [DOI] [PubMed] [Google Scholar]
- 6.Rosen LS, Gordon D, Tchekmedyian NS et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer 2004;100:2613–21. 10.1002/cncr.20308 [DOI] [PubMed] [Google Scholar]
- 7. Acute low back problems in adults: Assessment and treatment, AHCPR Quick Reference Guides. http://www.silcom.com/~dwsmith/lbpqtxt.html.
- 8.Suarez-Almazor ME, Belseck E, Russell AS et al. Use of lumbar radiographs for the early diagnosis of low back pain: proposed guidelines would increase utilization. JAMA 1997;277:1782–6. 10.1001/jama.1997.03540460046031 [DOI] [PubMed] [Google Scholar]
- 9.Papadakis MA, McPhee SJ. Current medical diagnosis and treatment 2014. 53rd edn McGraw-Hill Education, 2013. [Google Scholar]
- 10.Yang HL, Liu T, Wang XM et al. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol 2011;21:2604–17. 10.1007/s00330-011-2221-4 [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network (NCCN) Breast Cancer NCCN Practice Guidelines in Oncology Fort Washington, PA: NCCN, 2014. Ver. 2. http://www.nccn.org/%20professionals/physician_gls/recently_updated.asp [Google Scholar]
- 12.Nakai T, Okuyama C, Kubota T et al. Pitfalls of FDG-PET for the diagnosis of osteoblastic bone metastases in patients with breast cancer. Eur J Nucl Med Mol Imaging 2005;32:1253–8. 10.1007/s00259-005-1842-8 [DOI] [PubMed] [Google Scholar]
- 13.Wu LM, Gu HY, Zheng J et al. Diagnostic value of whole-body magnetic resonance imaging for bone metastases: a systematic review and meta-analysis. J Magn Reson Imaging 2011;34:128–35. 10.1002/jmri.22608 [DOI] [PubMed] [Google Scholar]
- 14.Chow E, Finkelstein JA, Sahgal A et al. Metastatic cancer to the bone. In DeVita VT Jr, Lawrence TS, Rosenberg SA, eds. Cancer: principles and practice of oncology. 9th edn Philadelphia, PA: Lippincott Williams & Wilkins, 2011;2192–204. [Google Scholar]
- 15.Tateishi U, Morita S, Taguri M et al. A meta-analysis of 18F-Fluoride positron emission tomography for assessment of metastatic bone tumor. Ann Nucl Med 2010;24:523–31. 10.1007/s12149-010-0393-7 [DOI] [PubMed] [Google Scholar]
- 16.Tehranzadeh J, Tao C, Browning CA. Percutaneous needle biopsy of the spine. Acta Radiol 2007;48:860–8. 10.1080/02841850701459783 [DOI] [PubMed] [Google Scholar]
- 17.Dupuy DE, Rosenberg AE, Punyaratabandhu T et al. Accuracy of CT-guided needle biopsy of musculoskeletal neoplasms. Am J Radiol 1998;171:759–62. [DOI] [PubMed] [Google Scholar]
- 18.Hau MA, Kim JI, Kattapuram S et al. Accuracy of CT-guided biopsies in 359 patients with musculoskeletal lesions. Skeletal Radiol 2002;31:349–53. 10.1007/s00256-002-0474-3 [DOI] [PubMed] [Google Scholar]
- 19.Nørgaard M, Jensen AØ, Jacobsen JB et al. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol 2010;184:162–7. 10.1016/j.juro.2010.03.034 [DOI] [PubMed] [Google Scholar]
- 20.Yong M, Jensen AÖ, Jacobsen JB et al. Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999–2007). Breast Cancer Res Treat 2011;129:495–503. 10.1007/s10549-011-1475-5 [DOI] [PubMed] [Google Scholar]
- 21.Sathiakumar N, Delzell E, Morrisey MA et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis 2011;14:177–83. 10.1038/pcan.2011.7 [DOI] [PubMed] [Google Scholar]
- 22.Katagiri H, Okada R, Takagi T et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med 2014;3:1359–67. 10.1002/cam4.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patchell RA, Tibbs PA, Regine WF et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366:643–8. 10.1016/S0140-6736(05)66954-1 [DOI] [PubMed] [Google Scholar]
- 24.Rades D, Huttenlocher S, Dunst J et al. Matched pair analysis comparing surgery followed by radiotherapy and radiotherapy alone for metastatic spinal cord compression. J Clin Oncol 2010;28:3597–604. 10.1200/JCO.2010.28.5635 [DOI] [PubMed] [Google Scholar]
- 25.Tokuhashi Y, Matsuzaki H, Oda H et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005;30:2186–91. 10.1097/01.brs.0000180401.06919.a5 [DOI] [PubMed] [Google Scholar]
- 26.Tomita K, Kawahara N, Kobayashi T et al. Surgical strategy for spinal metastases. Spine 2001;26:298–306. 10.1097/00007632-200102010-00016 [DOI] [PubMed] [Google Scholar]
- 27.Feiz-Erfan I, Rhines LD, Weinberg JS. The role of surgery in the management of metastatic spinal tumors. Semin Oncol 2008;35:108–17. 10.1053/j.seminoncol.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 28.Talbot M, Turcotte RE, Isler M et al. Function and health status in surgically treated bone metastases. Clin Orthop Relat Res 2005;438:215–20. 10.1097/01.blo.0000170721.07088.2e [DOI] [PubMed] [Google Scholar]
- 29.Toliusis V, Kalesinskas RJ, Kiudelis M et al. Surgical treatment of metastatic tumors of the femur. Medicina (Kaunas) 2010;46:323–8. [PubMed] [Google Scholar]
- 30.Wedin R, Hansen BH, Laitinen M et al. Complications and survival after surgical treatment of 214 metastatic lesions of the humerus. J Shoulder Elbow Surg 2012;21:1049–55. 10.1016/j.jse.2011.06.019 [DOI] [PubMed] [Google Scholar]
- 31.Ward WG, Holsenbeck S, Dorey FJ et al. Metastatic disease of the femur: surgical treatment. Clin Orthop Relat Res 2003;(415 Suppl):S230–44. 10.1097/01.blo.0000093849.72468.82 [DOI] [PubMed] [Google Scholar]
- 32.Johnson SK, Knobf MT. Surgical interventions for cancer patients with impending or actual pathologic fractures. Orthop Nurs 2008;27:160–71. 10.1097/01.NOR.0000320543.90115.d5 [DOI] [PubMed] [Google Scholar]
- 33.Damron TA, Morgan H, Prakash D et al. Critical evaluation of Mirels’ rating system for impending pathologic fractures. Clin Orthop Relat Res 2003;(415 Suppl):S201–7. 10.1097/01.blo.0000093842.72468.73 [DOI] [PubMed] [Google Scholar]
- 34.Evans AR, Bottros J, Grant W et al. Mirels’ rating for humerus lesions is both reproducible and valid. Clin Orthop Relat Res 2008;466:1279–84. 10.1007/s11999-008-0200-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jawad MU, Scully SP. In brief: classifications in brief: Mirels’ classification: metastatic disease in long bones and impending pathologic fracture. Clin Orthop Relat Res 2010;468:2825–7. 10.1007/s11999-010-1326-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Linden YM, Dijkstra PD, Kroon HM et al. Comparative analysis of risk factors for pathological fracture with femoral metastases. J Bone Joint Surg Br 2004;86:566–73. [PubMed] [Google Scholar]
- 37.Stadhouder A, Buskens E, Vergroesen DA et al. Nonoperative treatment of thoracic and lumbar spine fractures: a prospective randomized study of different treatment options. J Orthop Trauma 2009;23:588–94. 10.1097/BOT.0b013e3181a18728 [DOI] [PubMed] [Google Scholar]
- 38.Wu JS, Wong R, Johnston M et al. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys 2003;55:594–605. 10.1016/S0360-3016(02)04147-0 [DOI] [PubMed] [Google Scholar]
- 39.Sze WM, Shelley M, Held I et al. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy: a systematic review of the randomised trials. Cochrane Database Syst Rev 2004;(2):CD004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chow E, Zeng L, Salvo N et al. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol 2012;24:112–24. 10.1016/j.clon.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 41.Roos DE, Turner SL, O'Brien PC et al. , Trans-Tasman Radiation Oncology Group, TROG 96.05. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05). Radiother Oncol 2005;75:54–63. 10.1016/j.radonc.2004.09.017 [DOI] [PubMed] [Google Scholar]
- 42.van der Linden YM, Steenland E, van Houwelingen HC et al. Patients with a favourable prognosis are equally palliated with single and multiple fraction radiotherapy: results on survival in the Dutch Bone Metastasis Study. Radiother Oncol 2006;78:245–53. 10.1016/j.radonc.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 43.van der Linden YM, Lok JJ, Steenland E et al. Single fraction radiotherapy is efficacious: a further analysis of the Dutch Bone Metastasis Study controlling for the influence of retreatment. Int J Radiat Oncol Biol Phys 2004;59:528–37. 10.1016/j.ijrobp.2003.10.006 [DOI] [PubMed] [Google Scholar]
- 44.van der Linden YM, Kroon HM, Dijkstra SP et al. Simple radiographic parameter predicts fracturing in metastatic femoral bone lesions: results from a randomised trial. Radiother Oncol 2003;69:21–31. 10.1016/S0167-8140(03)00232-9 [DOI] [PubMed] [Google Scholar]
- 45.Saliou G, Kocheida el M, Lehmann P et al. Percutaneous vertebroplasty for pain management in malignant fractures of the spine with epidural involvement. Radiology 2010;254:882–90. 10.1148/radiol.09081698 [DOI] [PubMed] [Google Scholar]
- 46.Itagaki MW, Talenfeld AD, Kwan SW et al. Percutaneous vertebroplasty and kyphoplasty for pathologic vertebral fractures in the Medicare population: safer and less expensive than open surgery. J Vasc Interv Radiol 2012;23:1423–9. 10.1016/j.jvir.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 47.Mendel E, Bourekas E, Gerszten P et al. Percutaneous techniques in the treatment of spine tumors: what are the diagnostic and therapeutic indications and outcomes? Spine 2009;34:S93–S100. 10.1097/BRS.0b013e3181b77895 [DOI] [PubMed] [Google Scholar]
- 48.Goetz MP, Callstrom MR, Charboneau JW et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol 2004;22:300–6. 10.1200/JCO.2004.03.097 [DOI] [PubMed] [Google Scholar]
- 49.Callstrom MR, Atwell TD, Charboneau JW et al. Painful metastases involving bone: percutaneous image-guided cryoablation: prospective trial interim analysis. Radiology 2006;241:572–80. 10.1148/radiol.2412051247 [DOI] [PubMed] [Google Scholar]
- 50.Rosen LS, Gordon D, Tchekmedyian S et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial-the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol 2003;21:3150–7. 10.1200/JCO.2003.04.105 [DOI] [PubMed] [Google Scholar]
- 51.Rosen LS, Gordon D, Kaminski M et al. Long term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinomas: a randomized, double-blind, multicenter, comparative trial. Cancer 2003;98:1735–44. 10.1002/cncr.11701 [DOI] [PubMed] [Google Scholar]
- 52.Henry DH, Costa L, Goldwasser F et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011;29:1125–32. 10.1200/JCO.2010.31.3304 [DOI] [PubMed] [Google Scholar]
- 53.Vadhan-Raj S, von Moos R, Fallowfield LJ et al. Clinical benefit in patients with metastatic bone diseases: results of a phase 3 study of denosumab versus zoledronic acid. Ann Oncol 2012;23:3045–51. 10.1093/annonc/mds175 [DOI] [PubMed] [Google Scholar]
- 54.Scagliotti GV, Hirsh V, Siena S et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol 2012;7:1823–9. 10.1097/JTO.0b013e31826aec2b [DOI] [PubMed] [Google Scholar]
- 55.Hortobagyi GN, Theriault RL, Lipton A et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate: protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol 1998;16:2038–44. [DOI] [PubMed] [Google Scholar]
- 56.Rosen LS, Gordon DH, Dugan W Jr et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 2004;100:36–43. 10.1002/cncr.11892 [DOI] [PubMed] [Google Scholar]
- 57.Body JJ, Diel IJ, Lichinitzer M et al. Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phaseIII studies. Br J Cancer 2004;90:1133–7. 10.1038/sj.bjc.6601663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Body JJ, Diel IJ, Bell R et al. Oral ibandronate improves bone pain and preserves quality of life in patients with skeletal metastases due to breast cancer. Pain 2004;111:306–12. 10.1016/j.pain.2004.07.011 [DOI] [PubMed] [Google Scholar]
- 59.Body JJ, Diel IJ, Belle R et al. Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol 2003;14:1399–405. 10.1093/annonc/mdg367 [DOI] [PubMed] [Google Scholar]
- 60.Diel IJ, Body JJ, Lichinitser MR et al. Improved quality of life after long-term treatment with the bisphosphonate ibandronate in patients with metastatic bone disease due to breast cancer. Eur J Cancer 2004;40:1704–12. 10.1016/j.ejca.2004.03.025 [DOI] [PubMed] [Google Scholar]
- 61.Stopeck AT, Lipton A, Body JJ et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010;28:5132–9. 10.1200/JCO.2010.29.7101 [DOI] [PubMed] [Google Scholar]
- 62.Martin M, Bell R, Bourgeois H et al. Bone-related complications and quality of life in advanced breast cancer: results from a randomized phase III trial of denosumab versus zoledronic acid. Clin Cancer Res 2012;18:4841–9. 10.1158/1078-0432.CCR-11-3310 [DOI] [PubMed] [Google Scholar]
- 63.Wong MHF, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev 2012;2:CD003474. [DOI] [PubMed] [Google Scholar]
- 64.Samson DJ, Seidenfield J, Schmitt B et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer 2002;95:361–76. 10.1002/cncr.10647 [DOI] [PubMed] [Google Scholar]
- 65.Tannock IF, de Wit R, Berry WR et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502–12. 10.1056/NEJMoa040720 [DOI] [PubMed] [Google Scholar]
- 66.Petrylak DP, Tangen CM, Hussain MH et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351:1513–20. 10.1056/NEJMoa041318 [DOI] [PubMed] [Google Scholar]
- 67.Dearnaley DP, Mason MD, Parmar MK et al. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol 2009;10:872–6. 10.1016/S1470-2045(09)70201-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saad F, Gleason DM, Murray R et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002;94:1458–68. 10.1093/jnci/94.19.1458 [DOI] [PubMed] [Google Scholar]
- 69.Fizazi K, Carducci M, Smith M et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomized, double-blind study. Lancet 2011;377:813–22. 10.1016/S0140-6736(10)62344-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berenson JR, Lichtenstein A, Porter L et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma: Myeloma Aredia Study Group. N Engl J Med 1996;334:488–93. 10.1056/NEJM199602223340802 [DOI] [PubMed] [Google Scholar]
- 71.Lahtinen R, Laakso M, Palva I et al. Randomised, placebo-controlled multicentre trial of clodronate in multiple myeloma: Finnish Leukaemia Group. Lancet 1992;340:1049–52. 10.1016/0140-6736(92)93075-X [DOI] [PubMed] [Google Scholar]
- 72.Morgan GJ, Davies FE, Gregory WM et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet 2010;376:1989–99. 10.1016/S0140-6736(10)62051-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morgan GJ, Child JA, Gregory WM et al. Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): secondary outcomes from a randomised controlled trial. Lancet Oncol 2011;12:743–52. 10.1016/S1470-2045(11)70157-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mhaskar R, Redzepovic J, Wheatley K et al. Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev 2012;5:CD003188. [DOI] [PubMed] [Google Scholar]
- 75.Lipton A, Fizazi K, Stopeck AT et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 2012;48:3082–192. 10.1016/j.ejca.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 76.San Miguel JF, Schlag R, Khuageva NK et al. , VISTA Trial Investigators. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008;359:906–17. 10.1056/NEJMoa0801479 [DOI] [PubMed] [Google Scholar]
- 77.Delforge M, Terpos E, Richardson PG et al. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. Eur J Haematol 2011;86:372–84. 10.1111/j.1600-0609.2011.01599.x [DOI] [PubMed] [Google Scholar]
- 78.Rades D, Hakim SG, Bajrovic A et al. Impact of zoledronic acid on control of metastatic spinal cord compression. Strahlenther Onkol 2012;188:910–16. 10.1007/s00066-012-0158-4 [DOI] [PubMed] [Google Scholar]
- 79.Takeda N, Isu K, Hiraga H et al. Zoledronic acid enhances the effect of radiotherapy for bone metastases from renal cell carcinomas: more than a 24-month median follow-up. J Orthop Sci 2012;17:770–4. 10.1007/s00776-012-0294-9 [DOI] [PubMed] [Google Scholar]
- 80.Vassiliou V, Kalogeropoulou C, Christopoulos C et al. Combination ibandronate and radiotherapy for the treatment of bone metastases: clinical evaluation and radiologic assessment. Int J Radiat Oncol Biol Phys 2007;67:264–72. 10.1016/j.ijrobp.2006.08.022 [DOI] [PubMed] [Google Scholar]
- 81.Kouloulias EV, Kouvaris RJ, Antypas C et al. An intra-patient dose-escalation study of disodium pamidronate plus radiotherapy versus radiotherapy alone for the treatment of osteolytic metastases. Monitoring of recalcification using image-processing techniques. Strahlenther Onkol 2003;179:471–9. [DOI] [PubMed] [Google Scholar]
- 82.Atahan L, Yildiz F, Cengiz M et al. Zoledronic acid concurrent with either high- or reduced-dose palliative radiotherapy in the management of the breast cancer patients with bone metastases: a phase IV randomized clinical study. Support Care Cancer 2010;18:691–8. 10.1007/s00520-009-0663-x [DOI] [PubMed] [Google Scholar]
- 83.Van Poznak CH, Temin S, Yee GC et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol 2011;29:1221–7. 10.1200/JCO.2010.32.5209 [DOI] [PubMed] [Google Scholar]
- 84.Hillner BE, Ingle JN, Chlebowski RT et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 2003;21:4042–57. 10.1200/JCO.2003.08.017 [DOI] [PubMed] [Google Scholar]
- 85.Bamias A, Kastritis E, Bamia C et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 2005;23:8580–7. 10.1200/JCO.2005.02.8670 [DOI] [PubMed] [Google Scholar]
- 86.Ripamonti CI, Maniezzo M, Campa T et al. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of The National Cancer Institute of Milan. Ann Oncol 2009;20:137–45. 10.1093/annonc/mdn526 [DOI] [PubMed] [Google Scholar]
- 87.Kyrgidis A, Vahtsevanos K, Koloutsos G et al. Bisphosphonate-related osteonecrosis of the jaws: a case-control study of risk factors in breast cancer patients. J Clin Oncol 2008;26:4634–8. 10.1200/JCO.2008.16.2768 [DOI] [PubMed] [Google Scholar]
- 88.Vahtsevanos K, Kyrgidis A, Verrou E et al. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol 2009;27:5356–62. 10.1200/JCO.2009.21.9584 [DOI] [PubMed] [Google Scholar]
- 89.Ruggiero SL, Dodson TB, Assael LA et al. , American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J Oral Maxillofac Surg 2009;67(5 Suppl):2–12. 10.1016/S0278-2391(09)01877-1 [DOI] [PubMed] [Google Scholar]
- 90.Guarneri V, Donati S, Nicolini M et al. Renal safety and efficacy of i.v. bisphosphonates in patients with skeletal metastases treated for up to 10 Years. Oncologist 2005;10:842–8. 10.1634/theoncologist.10-10-842 [DOI] [PubMed] [Google Scholar]
- 91.Diel IJ, Weide R, Köppler H et al. Risk of renal impairment after treatment with ibandronate versus zoledronic acid: a retrospective medical records review. Support Care Cancer 2009;17:719–25. 10.1007/s00520-008-0553-7 [DOI] [PubMed] [Google Scholar]
- 92.Carteni G, Bordonaro R, Giotta F et al. Efficacy and safety of zoledronic acid in patients with breast cancer metastatic to bone: a multicenter clinical trial. Oncologist 2006;11:841–8. 10.1634/theoncologist.11-7-841 [DOI] [PubMed] [Google Scholar]
- 93.Aguiar Bujanda D, Bohn Sarmiento U, Cabrera Suárez MA et al. Assessment of renal toxicity and osteonecrosis of the jaws in patients receiving zoledronic acid for bone metastasis. Ann Oncol 2007;18:556–60. 10.1093/annonc/mdl408 [DOI] [PubMed] [Google Scholar]
- 94.Zuradelli M, Masci G, Biancofiore G et al. High incidence of hypocalcemia and serum creatinine increase in patients with bone metastases treated with zoledronic acid. Oncologist 2009;14:548–56. 10.1634/theoncologist.2008-0227 [DOI] [PubMed] [Google Scholar]
- 95.Shah SR, Jean GW, Keisner SV et al. Risk of renal failure in cancer patients with bone metastasis treated with renally adjusted zoledronic acid. Support Care Cancer 2012;20:87–93. 10.1007/s00520-010-1067-7 [DOI] [PubMed] [Google Scholar]
- 96.Smith MR, Saad F, Coleman R et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 2012;379:39–46. 10.1016/S0140-6736(11)61226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Block GA, Bone HG, Fang L et al. A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 2012;27:1471–9. 10.1002/jbmr.1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hanamura M, Iwamoto T, Soga N et al. Risk factors contributing to the development of hypocalcemia after zoledronic acid administration in patients with bone metastases of solid tumor. Biol Pharm Bull 2010;33:721–4. 10.1248/bpb.33.721 [DOI] [PubMed] [Google Scholar]
- 99.Black DM, Kelly MP, Genant HK et al. Fracture Intervention Trial Steering Committee; HORIZON Pivotal Fracture Trial Steering Committee: bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med 2010;362:1761–71. 10.1056/NEJMoa1001086 [DOI] [PubMed] [Google Scholar]
- 100.Brown JE, Cook RJ, Major P et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst 2005;97:59–69. 10.1093/jnci/dji002 [DOI] [PubMed] [Google Scholar]
- 101.Coleman RE, Major P, Lipton A et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 2005;23:4925–35. 10.1200/JCO.2005.06.091 [DOI] [PubMed] [Google Scholar]
- 102.Lipton A, Cook R, Saad F et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer 2008;113:193–201. 10.1002/cncr.23529 [DOI] [PubMed] [Google Scholar]
- 103.Lipton A, Cook R, Brown J et al. Skeletal-related events and clinical outcomes in patients with bone metastases and normal levels of osteolysis: exploratory analyses. Clin Oncol (R Coll Radiol) 2013;25:217–26. 10.1016/j.clon.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 104.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 105.Scher HI, Halabi S, Tannock I et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008;26:1148–59. 10.1200/JCO.2007.12.4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dennis ER, Jia X, Mezheritskiy IS et al. Bone scan index: a quantitative treatment response biomarker for castration-resistant metastatic prostate cancer. J Clin Oncol 2012;30:519–24. 10.1200/JCO.2011.36.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Heijmen L, Verstappen MC, Ter Voert EE et al. Tumour response prediction by diffusion-weighted MR imaging: ready for clinical use? Crit Rev Oncol Hematol 2012;83:194–207. 10.1016/j.critrevonc.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 108.Reuben DB, Tinetti ME. Goal-oriented patient care—an alternative health outcomes paradigm. N Engl J Med 2012;366:777–9. 10.1056/NEJMp1113631 [DOI] [PubMed] [Google Scholar]
- 109.Frampton CL, Hughes-Webb P. The measurement of pain. Clin Oncol (R Coll Radiol) 2011;23:381–6. 10.1016/j.clon.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 110.McNicol E, Strassels S, Goudas L et al. Nonsteroidal anti-inflammatory drugs, alone or combined with opioids, for cancer pain: a systematic review. J Clin Oncol 2004;22:1975–92. 10.1200/JCO.2004.10.524 [DOI] [PubMed] [Google Scholar]
- 111.Nabal M, Librada S, Redondo MJ et al. The role of paracetamol and nonsteroidal anti-inflammatory drugs in addition to WHO Step III opioids in the control of pain in advanced cancer: a systematic review of the literature. Palliat Med 2012;26:305–12. 10.1177/0269216311428528 [DOI] [PubMed] [Google Scholar]
- 112.Joishy SK, Walsh D. The opioid-sparing effects of intravenous ketorolac as an adjuvant analgesic in cancer pain: application in bone metastases and opioid bowel syndrome. J Pain Symptom Manage 1998;16:334–9. 10.1016/S0885-3924(98)00081-5 [DOI] [PubMed] [Google Scholar]
- 113.Slatkin N. Cancer-related pain and its pharmacologic management in the patient with bone metastasis. J Support Oncol 2006;4:15–21. [PubMed] [Google Scholar]
- 114.Ventafridda V, Tamburini M, Caraceni A et al. A validation study of the WHO method for cancer pain relief. Cancer 1987;59:850–6. [DOI] [PubMed] [Google Scholar]
- 115.Mercadante S. Pain treatment and outcomes for patients with advanced cancer who receive follow-up care at home. Cancer 1999;85:1849–58. [DOI] [PubMed] [Google Scholar]
- 116.Mercadante S, Villari P, Ferrera P et al. Optimization of opioid therapy for preventing incident pain associated with bone metastases. J Pain Symptom Manage 2004;28:505–10. 10.1016/j.jpainsymman.2004.02.024 [DOI] [PubMed] [Google Scholar]
- 117.Zeppetella G, Davies AN. Opioids for the management of breakthrough pain in cancer patients. Cochrane Database Syst Rev 2013;10:CD004311. [DOI] [PubMed] [Google Scholar]
- 118.Roqué I Figuls M, Martinez-Zapata MJ, Scott-Brown M et al. Radioisotopes for metastatic bone pain. Cochrane Database Syst Rev 2011;(7):CD003347. [DOI] [PubMed] [Google Scholar]
- 119.Bauman G, Charette M, Reid R et al. Radiopharmaceuticals for the palliation of painful bone metastasis: a systemic review. Radiother Oncol 2005;75:258–70. 10.1016/j.radonc.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 120.D'angelo G, Sciuto R, Salvatori M et al. Targeted ‘bone-seeking’ radiopharmaceuticals for palliative treatment of bone metastases: a systematic review and meta-analysis. Q J Nucl Med Mol Imaging 2012;56:538–43. [PubMed] [Google Scholar]
- 121.Storto G, Klain M, Paone G et al. Combined therapy of Sr-89 and zoledronic acid in patients with painful bone metastases. Bone 2006;39:35–41. 10.1016/j.bone.2005.12.004 [DOI] [PubMed] [Google Scholar]
- 122.Wang Y, Tao H, Yu X et al. Clinical significance of zoledronic acid and strontium-89 in patients with asymptomatic bone metastases from non-small-cell lung cancer. Clin Lung Cancer 2013;14:254–60. 10.1016/j.cllc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 123.Kuroda I. Strontium-89 for prostate cancer with bone metastases: the potential of cancer control and improvement of overall survival. Ann Nucl Med 2014;28:11–16. 10.1007/s12149-013-0775-8 [DOI] [PubMed] [Google Scholar]
- 124.Parker C, Nilsson S, Heinrich D et al. , ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213–23. 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- 125.Ruff RL, Ruff SS, Wang X. Persistent benefits of rehabilitation on pain and life quality for nonambulatory patients with spinal epidural metastasis. J Rehabil Res Dev 2007;44:271–8. 10.1682/JRRD.2007.01.0006 [DOI] [PubMed] [Google Scholar]
- 126.Tang V, Harvey D, Park Dorsay J et al. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord 2007;45:671–7. 10.1038/sj.sc.3102024 [DOI] [PubMed] [Google Scholar]
- 127.Kim JE, Dodd M, West C et al. The PRO-SELF pain control program improves patients’ knowledge of cancer pain management. Oncol Nurs Forum 2004;31:1137–43. 10.1188/04.ONF.1137-1143 [DOI] [PubMed] [Google Scholar]
- 128.Rustøen T, Valeberg BT, Kolstad E et al. The PRO-SELF(©) Pain Control Program improves patients’ knowledge of cancer pain management. J Pain Symptom Manage 2012;44: 321–30. 10.1016/j.jpainsymman.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 129.Miaskowski C, Dodd M, West C et al. Randomized clinical trial of the effectiveness of a self-care intervention to improve cancer pain management. J Clin Oncol 2004;22:1713–20. 10.1200/JCO.2004.06.140 [DOI] [PubMed] [Google Scholar]
- 130.Ling CC, Lui LY, So WK. Do educational interventions improve cancer patients’ quality of life and reduce pain intensity? Quantitative systematic review. J Adv Nurs 2012;68:511–20. 10.1111/j.1365-2648.2011.05841.x [DOI] [PubMed] [Google Scholar]
- 131.Vieillard MH, Thureau S. Multidisciplinary meetings dedicated to bone metastases: a historical perspective and rationale. Bull Cancer 2013;100:1135–9. [DOI] [PubMed] [Google Scholar]
- 132.Blum RH, Novetsky D, Shasha D et al. The multidisciplinary approach to bone metastases. Oncology (Williston Park) 2003;17:845–57. [PubMed] [Google Scholar]
- 133.Ibrahim T, Flamini E, Fabbri L et al. Multidisciplinary approach to the treatment of bone metastases: Osteo-Oncology Center, a new organizational model. Tumori 2009;95:291–7. [DOI] [PubMed] [Google Scholar]