Abstract
Introduction
The contribution of programmed cell death ligand-1 (PD-L1) immune checkpoint molecule toward progression of non-small cell lung cancer (NSCLC) has not yet been elucidated, in part, because of lack of a standardised method to evaluate PD-L1 expression. In this study, we developed a novel method for the evaluation of PD-L1 expression on NSCLC cells and examined its correlation with clinicopathological characteristics.
Methods
After immunohistochemical examination of PD-L1 expression for surgically resected pulmonary adenocarcinomas (n=106), based on the findings that PD-L1 are consistently expressed on alveolar macrophages, PD-L1 staining intensity of tumour cells was classified into four levels relative to PD-L1 staining intensity in alveolar macrophages; PD-L1 expression scores (range, 0–300) were semiquantitatively assessed. An analysis of statistical association between PD-L1 expression score and clinicopathological characteristics was performed.
Results
Almost all of the alveolar macrophages in the specimens were moderately to strongly stained with PD-L1, serving as an internal positive control in the immunohistochemistry of PD-L1. PD-L1 expression score (median, 52.3) was significantly higher in tumours with G2/3 differentiation than in those with G1 (p=0.022) and higher in those with lymphatic invasion than in those without invasion (p=0.032). Postoperative relapse-free survival was significantly shorter in patients with a high PD-L1 expression score than in those with low PD-L1 expression score (p=0.035). Smoking habits, histological subtype, and epidermal growth factor receptor mutation status were not associated with PD-L1 expression score.
Conclusions
Given the heterogeneous distribution of PD-L1 expression in pulmonary adenocarcinoma cells, the scoring of PD-L1 expression on tumour cells relative to that in alveolar macrophages appears to be a valid indicator of PD-L1 status of patients with pulmonary adenocarcinomas, demonstrating a significant correlation with several factors associated with tumour progression.
Keywords: Alveolar macrophage, Expression score, Immunohistochemistry, Programmed cell death-ligand 1, Pulmonary adenocarcinoma
Key questions.
What is already known about this subject?
Immune checkpoint mechanisms, such as the programmed cell death 1 (PD-1) and programmed cell death ligand-1 (PD-L1) axis, is a promising therapeutic target in non-small cell lung cancer (NSCLC).
Several studies have reported a correlation between PD-L1 expression on NSCLC cells and clinicopathological features of the patients; however the methods to evaluate PD-L1 expression on NSCLC cells are different among those studies and has not yet been standardised.
Inappropriate handling of surgical tumour samples would influence the PD-L1 staining of tumour cells on immunohistochemistry. Thus, an internal control or standard for PD-L1 staining is needed when we try to compare the PD-L1 staining intensity of tumour cells between patients.
What does this study add?
Alveolar macrophages are proven to be consistently stained with anti-PD-L1 antibody, serving as a positive control on PD-L1 immunohistochemistry.
A novel method to evaluate PD-L1 expression on NSCLC cells was developed, where PD-L1 staining intensity on tumour cells was classified into four levels relative to that of alveolar macrophages and scored.
The scoring method for PD-L1 expression appears to be a valid indicator of PD-L1 status of patients with pulmonary adenocarcinoma, showing significant correlation with low-grade differentiation, lymphatic invasion and postoperative relapse-free survival.
How might this impact on clinical practice?
Through the scoring method, the tumour status with heterogeneous PD-L1 expression can be semiquantitatively evaluated.
The method to evaluate PD-L1 expression on NSCLC cells is standardised, contributing to development of biomarkers for clinical responses of immune checkpoint inhibitors.
Introduction
Non-small cell lung cancer (NSCLC) currently is a leading cause of cancer death worldwide.1 Therapeutic strategies, cytotoxic chemotherapy, radiotherapy and surgery have improved the survival of patients with NSCLC over the past few decades.2–5 Furthermore, breakthroughs in molecular-targeted therapy directed at mutations of driver oncogenes, such as epidermal growth factor receptor (EGFR), has improved prognosis of patients with NSCLC.6–9 However, novel therapeutic strategies must be developed to help prolong the survival of patients with NSCLC.
Immune checkpoint mechanisms in the functional reaction between tumour and immune cells are novel therapeutic targets in NSCLC and other malignant tumours.10 Tumour cells are known to evade immunological surveillance of effector immune cells through immune checkpoint mechanisms such as, the programmed cell death 1 (PD-1)-programmed cell death-ligand 1 (PD-L1) axis.11 12 PD-L1 expressed on tumour cells interacts with PD-1 expressed on activated effector immune cells like cytotoxic T lymphocytes, which induces apoptosis, anergy and exhaustion of effector immune cells.13 14 Research on the immune escape system of tumour cells has led to the development of PD-1-targeted therapies and PD-L1-targeted therapies; several clinical trials of anti-PD-1 and anti-PD-L1 antibodies have revealed their antitumour effects with significant durable responses in patients with NSCLC.15 16 Inhibition of the PD-1–PD-L1 axis is a promising therapeutic target for NSCLC. However, therapeutic biomarkers in these molecular-targeted therapies remain to be established, and an actual role of PD-L1 in the progression of NSCLC is yet to be elucidated.
Several studies have demonstrated a correlation between PD-L1 expression on NSCLC cells and clinicopathological characteristics of patients with NSCLC.17–21 However, these results have not been consistent and definitive conclusions have yet to be drawn. These discrepancies may be due to the lack of standardised methods to evaluate PD-L1 expression on NSCLC cells.
The goal of this study was to establish a standard evaluation method for PD-L1 expression in NSCLC. We conducted a semiquantitative evaluation of PD-L1 expression of pulmonary adenocarcinomas, and compared the staining intensity with that of alveolar macrophages; an analysis of the correlation between PD-L1 expression intensity and clinicopathological characteristics is presented.
Materials and methods
NSCLC tissue samples
Tumour tissue samples were obtained from surgically resected specimens from patients with pulmonary adenocarcinoma at the Shiga University of Medical Science Hospital, between January 2008 and December 2013. Those patients did not receive anticancer therapy such as neo-adjuvant chemotherapy prior to the surgery for their pulmonary adenocarcinoma. Data on clinicopathological variables of patients with EGFR mutation status was obtained from their medical records. The study design was approved by the Ethical Committee of Shiga University of Medical Science; written informed consent was obtained from all patients.
Immunohistochemistry
Whole tissue sections rather than tissue microarrays were used for immunohistochemistry in this study. The 4 μm thick sections of formalin-fixed paraffin-embedded tissue specimens were stained by standard indirect immunoperoxidase procedures, according to the manufacturer's protocol (Cell Signaling Technology, Danvers, Massachusetts, USA). Briefly, each tissue section was deparaffinised in xylene, and rehydrated in ethanol and distilled water. Antigen retrieval was performed by microwave treatment in 10 mM sodium citrate buffer (pH 6.0) for 10 min; endogenous peroxidase activity was blocked by treatment with 3% H2O2 for 10 min. After blocking with 5% normal goat serum in Tris-buffered saline with Tween 20 for 1 hour at room temperature, the sections were incubated overnight with anti-human PD-L1 monoclonal antibody (clone: E1L3N, diluted at 1:200) (Cell Signaling Technology) at 4°C. On the following day, the sections were incubated with SignalStain boost IHC detection reagent (Cell Signaling Technology), and visualised using the SignalStain DAB substrate kit (Cell Signaling Technology) for 1 min, followed by counterstaining with hematoxylin.
We confirmed by flow-cytometric analysis that lung cancer cell line H-1975 cells (American Type Culture Collection, Manassas, Virginia, USA) are positive and A549 cells (American Type Culture Collection) are negative for PD-L1 (data not shown). Based on the finding, paraffin-embedded cell-blocks of H-1975 and A549 cells were utilised for positive and negative controls of PD-L1 immunohistochemistry, respectively. Rabbit IgG monoclonal antibody (Cell Signaling Technology) was used as a negative control of anti-human PD-L1 monoclonal antibody.
PD-L1 expression intensity scoring
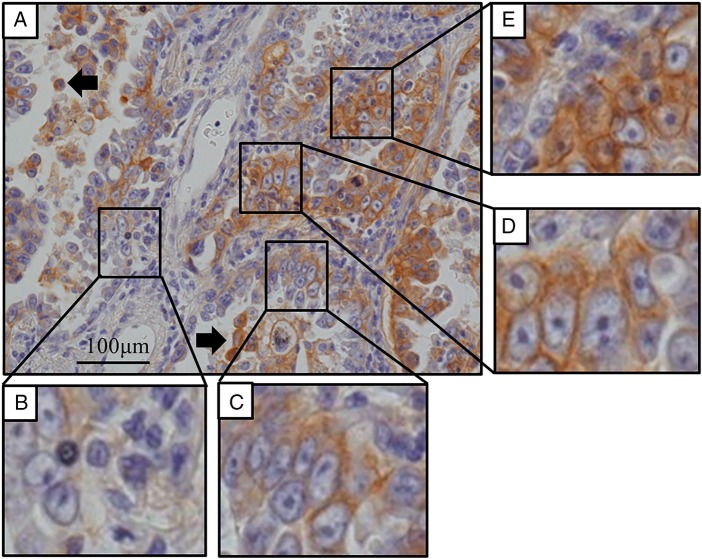
Following PD-L1 immunohistochemistry, tumour tissue sections were independently examined by two researchers, including a pathologist. PD-L1 staining intensity of each tumour cell was classified into four levels relative to that of alveolar macrophages (AMs) in the same section (figure 1A). Level 0, non-stained tumour cell (figure 1B); level 1: weakly stained tumour cell (staining intensity of tumour cell lower than that of AMs) (figure 1C); level 2: moderately stained tumour cell (staining intensity of tumour cell similar to that of AMs) (figure 1D); and level 3: strongly stained tumour cells (staining intensity of tumour cells stronger than that of AMs) (figure 1E). The number of tumour cells in three randomly selected fields was counted under 200-fold magnification; the number (%) of tumour cells in each PD-L1 staining level was counted. PD-L1 expression score (H score) was calculated for each case according to the following formula:
Figure 1.
Programmed cell death ligand-1 (PD-L1) immunohistochemistry for pulmonary adenocarcinoma tissues. (A) Heterogeneous distribution of PD-L1 staining intensity of adenocarcinoma cells. PD-L1 staining intensity was classified relative to that of alveolar macrophages (arrow) into four levels: (B) non-stained, (C) weakly stained, (D) moderately stained and (E) strongly stained (magnification, ×200).
PD-L1 expression score (H score) (range, 0–300)=0×% of non-stained tumour cells +1×% of weakly stained tumour cells +2×% of moderately stained tumour cells +3×% of strongly stained tumour cells.
Statistical analysis
Correlation between PD-L1 expression score of tumour cells and clinicopathological characteristics of patients were statistically analysed by Mann-Whitney U test. Receiver operating characteristics (ROC) curve analysis was performed to determine the optimal cut-off level of PD-L1 expression score (H score) associated with postoperative recurrence of pulmonary adenocarcinoma. The cut-off level was determined as the point closest to (0, 1) on the ROC curve; this was calculated as ‘(1−sensitivity)2+(1−specificity)2,’ or the point associated with maximum Youden index (calculated as ‘sensitivity+specificity−1’).22 Median relapse-free survival after surgery was calculated using Kaplan-Meier analysis; the relapse-free survival in different groups was compared with log-rank test. p Values of less than 0.05 were considered statistically significant. All analyses were performed using SPSS Statistics V.22.0 software (IBM, Armonk, New York, USA).
Results
Patients' characteristics
A total of 106 (62 men (58.5%) and 44 women (41.5%)) patients with pulmonary adenocarcinoma who underwent surgical resection were included in this study (table 1). The median age of patients at the time of surgery was 66 years (range, 36–87 years). Sixty-two patients (58.5%) were habitual smokers. The most frequent histological subtype was papillary adenocarcinoma (N=38, 35.8%), followed by adenocarcinoma in situ or minimally invasive adenocarcinoma (AIS/MIA) (N=25, 23.6%), acinar adenocarcinoma (N=15, 14.2%), micropapillary adenocarcinoma (N=12, 11.3%), lepidic adenocarcinoma (N=10, 9.4%) and solid adenocarcinoma (N=6, 5.7%).
Table 1.
Patients' characteristics
| Characteristic | |
|---|---|
| Total, N | 106 |
| Median age (range) | 66 (36−87) |
| Gender, N (%) | |
| Male | 62 (58.5) |
| Female | 44 (41.5) |
| Smoking status, N (%) | |
| Current/former | 62 (58.5) |
| Never | 42 (39.6) |
| Unknown | 2 (1.9) |
| Histology, N (%) | |
| AIS/MIA | |
| Lepidic | 25 (23.6) |
| Papillary | 10 (9.4) |
| Acinar | 38 (35.8) |
| Solid | 6 (5.7) |
| Micropapillary | 12 (11.3) |
| Pathological stage, N (%) | |
| IA | 45 (42.5) |
| IB | 14 (13.2) |
| IIA | 10 (9.4) |
| IIB | 3 (2.8) |
| IIIA | 22 (20.8) |
| IIIB | 5 (4.7) |
| IV | 8 (7.5) |
| EGFR status, N (%) | |
| Mutation | 37 (34.9) |
| Wild-type | 69 (65.1) |
AIS, adenoma in situ; MIA, minimally invasive adenocarcinoma.
Postoperative pathological staging of pulmonary adenocarcinoma was IA in 45 cases (42.5%), IB in 14 (13.2%), IIA in 10 (9.4%), IIB in 3 (2.8%), IIIA in 22 (20.8%), IIIB in 5 (4.7%), and IV in 8 (6.6%). Among these, 46 patients (43.3%) had received postoperative adjuvant chemotherapy (uracil/tegafur or platinum-based doublet) ∼1 month after surgery. Thirty-seven patients (34.9%) exhibited EGFR mutations including L858R point mutation on exon 21 or deletion mutation in exon 19.
PD-L1 expression on adenocarcinoma cells and AMs
On preliminary PD-L1 immunohistochemical analysis, we confirmed that PD-L1 was usually expressed at the membrane of pulmonary adenocarcinoma cells, and in some cases, in the cytoplasm. However, variability was observed in the levels of PD-L1 staining intensity in tumour cells. Heterogeneous distribution of PD-L1 staining intensity was observed within a single section of tumour tissue (figure 1A), with some areas being dominated by cells with strong PD-L1 staining intensity, whereas other areas by cells lacking PD-L1 expression. In some cases, tumour cells with various levels of PD-L1 staining intensity coexisted within one area of the tissue section. These findings suggest that all areas in a tissue section should be observed to appropriately evaluate PD-L1 expression on NSCLC cells. In addition, we observed that AMs in pulmonary adenocarcinoma tissues consistently stained positive for PD-L1 (figure 2). Tumour-associated macrophages were also found to be positive for PD-L1 staining, therefore we need to morphologically discriminate tumour-associated macrophages from tumour cells and AMs. AMs usually existed in the air space of pulmonary alveoli around the tumour cells, which suggests that PD-L1 staining intensity of AMs could serve as an internal positive control in immunohistochemical studies of PD-L1 expression.
Figure 2.
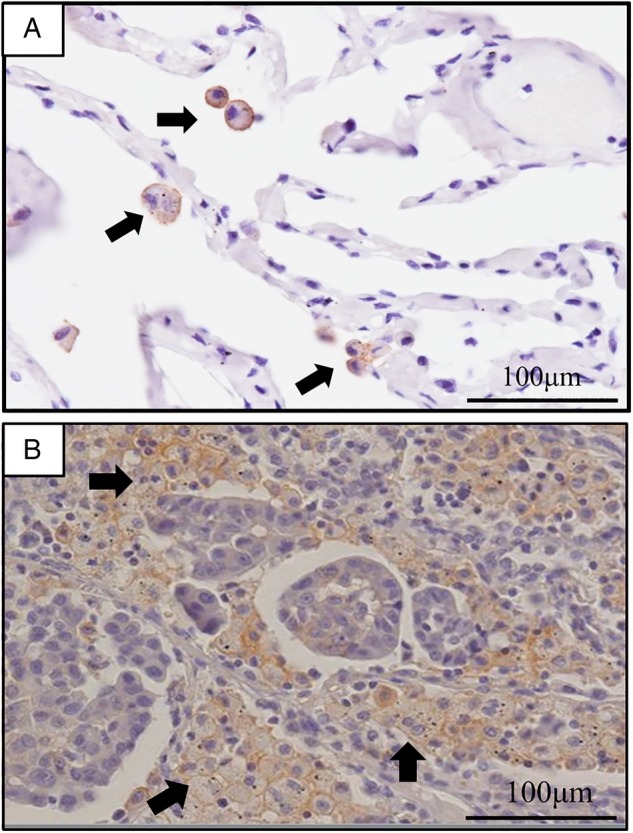
Programmed cell death ligand-1 (PD-L1) expression of alveolar macrophages. (A) Alveolar macrophages in the normal lung tissue are expressing PD-L1 on their membrane and/or cytoplasm. (B) Alveolar macrophages in the pulmonary adenocarcinoma tissue are expressing PD-L1 on their membrane and/or cytoplasm (arrows). Some multinucleated giant cells are also observed around the PD-L1-expressing tumour cells. (magnification, ×200).
Semiquantitative analyses of PD-L1 expression
Based on these preliminary findings, PD-L1 staining intensity of each pulmonary adenocarcinoma cell was classified into four levels by comparing it with the staining intensity of AMs in the same tissue section (figure 1). A semiquantitative PD-L1 expression score (H-score) was obtained for each case as described in the Methods section. The median PD-L1 expression score of pulmonary adenocarcinomas was 52.3 (range, 0.1–273.3). When both ‘moderately stained’ and ‘strongly stained’ tumour cells were considered as ‘PD-L1-positive’, the median percentage of ‘PD-L1-positive’ tumour cells was 6.6%. The frequency of cases in which more than 1%, 5%, 10% and 50% of tumour cells were ‘PD-L1-positive’ was 94.3% (100/106), 82.1% (87/106), 73.5% (78/106), 48.1% (51/106), respectively.
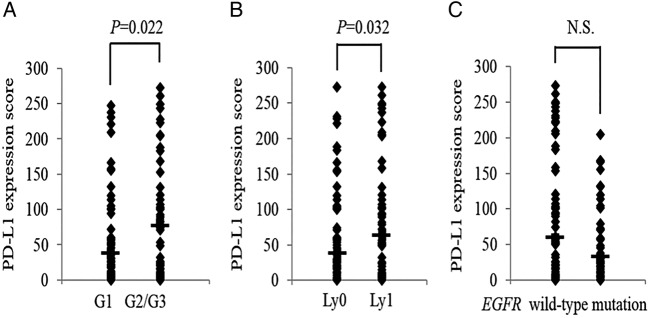
Correlation between PD-L1 expression score and clinicopathological characteristics
The PD-L1 expression score of pulmonary adenocarcinomas with grade G2 and G3 differentiation was significantly higher than those with grade G1 differentiation (101.0 vs 36.7; p=0.022) (figure 3A). In addition, PD-L1 expression score of those with lymphatic vessel invasion was higher than those without invasion (36.7 vs 64.0; p=0.032) (figure 3B). Patient gender, age, smoking habits, histological subtype, pathological stage, pleural invasion and vascular invasion were not associated with PD-L1 expression score (table 2). Further, no significant correlation was observed between PD-L1 expression score and EGFR mutation status (p=0.140; figure 3C).
Figure 3.
(A) Programmed cell death ligand-1 (PD-L1) expression score was significantly higher in pulmonary adenocarcinomas with grade G2/G3 differentiation as compared to those with grade G1 differentiation (36.7 vs 101.0, p=0.022) and (B) pulmonary adenocarcinomas with lymphatic invasion as compared with those without lymphatic vessels invasion (36.7 vs 64.0, p=0.032). (C) No correlation was observed between the score and EGFR mutation status of tumour cells (30.8 vs 58.8, p=0.140). EGFR, epidermal growth factor receptor; NS, not significant.
Table 2.
Correlation between PD-L1 expression score and clinicopathological characteristics
| N (%) | H-score±SD | p Value | |
|---|---|---|---|
| Gender | |||
| Male | 62 (58.5) | 47.6±83.0 | NS |
| Female | 44 (41.5) | 56.5±72.4 | |
| Smoking status | |||
| Current/former | 62 (58.5) | 57.6±84.4 | NS |
| Never | 42 (39.6) | 49.2±69.7 | |
| Unknown | 2 (1.9) | ||
| Histology | |||
| AIS/MIA | 25 (23.6) | 50.1±67.6 | |
| Lepidic | 10 (9.4) | 33.6±38.6 | |
| Papillary | 38 (35.8) | 55.9±87.6 | |
| Acinar | 15 (14.2) | 48.6±66.1 | |
| Solid | 6 (5.7) | 84.2±106.0 | |
| Micropapillary | 12 (11.3) | 107.0±88.4 | |
| EGFR status | |||
| mutation | 37 (34.9) | 30.8±57.3 | NS |
| wild-type | 69 (65.1) | 58.8±86.0 | |
| Cellular differentiation | |||
| G0 | 53 (50.0) | 36.7±68.7 | 0.022 |
| G2/G3 | 52 (49.1) | 101.0±93.0 | |
| Unknown | 1 (0.9) | ||
| Microblood vessels invasion | |||
| v0 | 45 (42.5) | 50.1±74.6 | NS |
| v1 | 61 (57.5) | 53.6±81.6 | |
| Lymphatic vessels invasion | |||
| ly0 | 55 (51.9) | 36.7±70.7 | 0.032 |
| ly1 | 50 (47.2) | 64.0±86.4 | |
| Unknown | 1 (0.9) | ||
| Pleural invasion | |||
| p0 | 87 (82.1) | 51.3±77.9 | NS |
| p1 | 18 (17.0) | 51.7±84.9 | |
| Unknown | 1 (0.9) | ||
| Pathological stage | |||
| IA | 45 (42.5) | 51.0±62.0 | |
| IB | 14 (13.2) | 29.3±78.1 | |
| IIA | 10 (9.4) | 36.8±62.0 | |
| IIB | 3 (2.8) | 158.0±16.1 | |
| IIIA | 22 (20.8) | 93.4±105.0 | |
| IIIB | 5 (4.7) | 52.5±63.8 | |
| IV | 8 (7.5) | 76.3±86.5 | |
AIS, adenoma in situ; MIA, minimally invasive adenocarcinoma; NS, not significant; PD-L1, programmed cell death-ligand 1.
PD-L1 expression score and relapse-free survival
Of the 106 patients, 12 patients were excluded from the analysis of relapse-free survival time (eight patients had pathological stage IV after surgery; lack of clinical records for four patients). In the 94 patients included in this analysis, median postoperative follow-up duration was 27.2 months (range, 0.1–83.7 months). Using 71.5 as the cut-off level for PD-L1 expression score, median PD-L1 expression in patients with PD-L1 expression score above the cut-off level (N=54) was 131.5 (range, 72.2–272.8), while those with PD-L1 expression score below the cut-off level (N=40) was 17.8 (range, 0.1–70.9). Relapse-free survival after surgery was 24.1 months (95% CI 19.7 to 28.4) in patients with PD-L1 expression score above the cut-off level, and 36.4 months (95% CI 29.1 to 39.3) in those below the cut-off level. The findings demonstrate that postoperative relapse-free survival in patients with high PD-L1 expression score was significantly shorter than that in patients with low PD-L1 expression score (p=0.035; figure 4).
Figure 4.
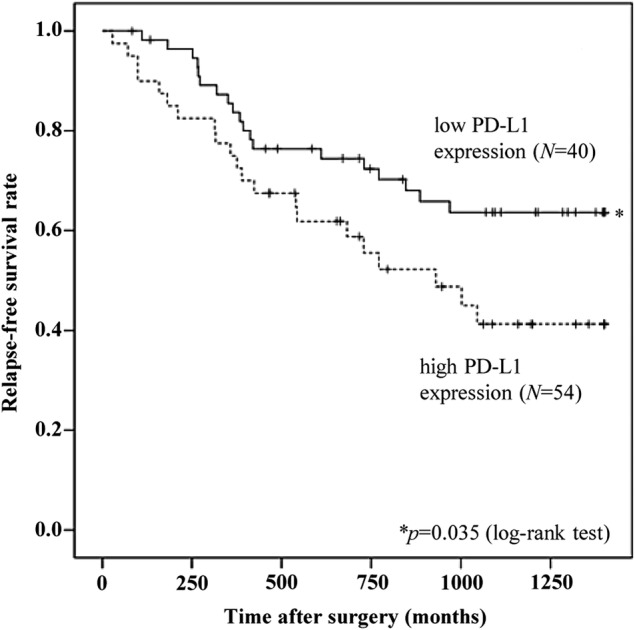
Kaplan-Meier analysis showing postoperative relapse-free survival. Patients in pulmonary adenocarcinomas with high Programmed cell death ligand-1 (PD-L1) expression (N=54) had a significantly shorter relapse-free survival time (N=40) (24.1 vs 36.4 months for those with low expression; p=0.032).
Discussion
Given the heterogeneous distribution of PD-L1 expression in pulmonary adenocarcinomas cells, we established a scoring method to evaluate PD-L1 expression status in patients with pulmonary adenocarcinomas. PD-L1 staining intensity of tumour cells was semiquantitatively scored relative to that of AMs; high PD-L1 expression score of tumour cells significantly correlated with low grade tumour differentiation, lymph vessel invasion, and postoperative relapse-free survival.
Several studies have investigated the correlation between PD-L1 expression on NSCLC cells and clinicopathological characteristics of patients.17–21 However, the reagents used for immunohistochemical analyses, such as anti-PD-L1 antibodies, have varied among the studies. Standardised evaluation of PD-L1 expression on NSCLC cells requires positive and negative controls for PD-L1 staining.23 In this study, we aimed to determine a positive control for PD-L1 immunohistochemical examination for NSCLC tissues.
In our preliminary study, we noticed that AMs in NSCLC tissues were strongly stained with anti-PD-L1 antibody. AMs are localised in the air space of pulmonary alveoli, and are relatively larger in size (20–50 μm diameter) than other types of leukocytes. Given, both, the role of AMs and the functional nature of PD-L1 in the immune system, it is consistent that PD-L1 is expressed on AMs to converge immune responses.24 25 We observed around 20 AMs on average per field of view at 200-fold magnification, and were able to detect them easily due to their typical localisation and size of the cells. In addition, PD-L1 staining intensity of AMs was stable, and did not vary between cells. In PD-L1 immunohistochemistry, inappropriate handling of surgical tumour samples would influence the PD-L1 staining intensity of tumour cells. Thus, an internal control or standard for PD-L1 staining is required, especially when we try to compare PD-L1 staining intensity of tumour cells between patients. Our findings demonstrate that PD-L1 staining intensity of AMs can serve as a valid positive internal control in PD-L1 immunohistochemistry, and that PD-L1 staining intensity of NSCLC cells can be assessed relative to that of AMs. Our findings also suggest that automatic analysis of PD-L1 expression on NSCLC cells using image analysing software is liable to count PD-L1-expressing AMs as PD-L1-positive cells, which amounts to a miscount of PD-L1-positive NSCLC cells.
In the preliminary study, we also observed variability in PD-L1 staining intensity among NSCLC cells.26 In some cases, cells with various levels of PD-L1 staining intensity coexisted in a single section of NSCLC tissue. These findings suggest that all areas in a tissue section should be observed for evaluation of PD-L1 expression. Therefore, in this study, we used a whole-tissue section of NSCLC specimens for PD-L1 immunohistochemistry, and randomly selected the fields of view for assessment of PD-L1 staining intensity of pulmonary adenocarcinoma cells. In 89 of the 106 cases (83.9%), PD-L1-expressing tumour cells and PD-L1-non-expressing cells were mixed in a single tissue section, with different areas tending to be dominated by either PD-L1-expressing cells or PD-L1-non-expressing cells. This finding indicates that a case can be categorised as PD-L1-positive or PD-L1-negative case according to the field of view selected for evaluation. In addition, in terms of evaluation of PD-L1 expression on NSCLC cells, a small fragment of tissue section, such as small specimen obtained by needle biopsy and tissue microarray, is unlikely to be representative of the tumour issue. Given the heterogeneous distribution of NSCLC cells with various levels of PD-L1 staining intensity, we consider that PD-L1 expression on NSCLC cells needs to be evaluated using whole-tissue sections.
In most previous studies, an NSCLC case was defined as PD-L1-positive when more than 5% of NSCLC cells were positive for PD-L1 staining.15 27 28 However, we questioned the validity of this cut-off level because of lack of a rationale for its setting. In the present study, PD-L1 staining intensity was classified into four levels. When NSCLC cells with PD-L1 staining intensity other than ‘non-staining’ were defined as ‘PD-L1-positive’ NSCLC cells, the median percentage of ‘PD-L1-positive’ NSCLC cells was calculated to be 47.6%. If a case with more than 5% of ‘PD-L1-positive’ NSCLC cells was defined as ‘PD-L1-positive’ , 82.1% of cases (87/106) would have been categorised as ‘PD-L1-positive’ NSCLC cases in our study. In some studies, the cut-off level of PD-L1 positivity was set as more than 10% or 50% of PD-L1-positive NSCLC cells;16 29 30 however, the rationale for this cut-off level is not clear. In other studies, only moderate-to-strong ‘PD-L1-positive’ staining NSCLC cells were considered while weakly stained cells were excluded.20 However, the reason why ‘weakly stained cells’ were excluded in the analyses is unclear. Given the heterogeneous expression levels, the frequency of ‘PD-L1-positive’ NSCLC cells is likely to vary according to the field of view observed, even if only moderately-to-strongly stained cells are counted as ‘PD-L1-positive’ NSCLC cells. Based on these findings, we suggest that PD-L1-positivity in NSCLC should be determined by semiquantitative analysis through observation of whole-tissue sections.
We propose that the correlation between PD-L1 expression and clinicopathological characteristics should be disaggregated by histological type, given the variability in clinicopathological characteristics between pulmonary adenocarcinomas, squamous cell carcinomas and other pathological types of NSCLC. Therefore, distinct from previous papers,18 20 21 in the present study, we used a single histological type of NSCLC, pulmonary adenocarcinomas, for investigating the association between clinicopathological features and PD-L1 expression score. Based on our findings, PD-L1 expression intensity of tumour cells does not appear to be associated with gender, age or smoking habits of the patients, which indicates that these variables may not contribute to the regulation of PD-L1 expression responsible for immune checkpoint mechanisms. In addition, histological subtypes of pulmonary adenocarcinomas, pathological stages, pleural invasion and blood vessel invasion were not associated with PD-L1 expression intensity of tumour cells, which indicates that PD-L1 expression may not necessarily contribute to the aggressive property of adenocarcinoma cells. On the other hand, we observed significant correlation between PD-L1 expression intensity and lymphatic invasion (p=0.032) and low-grade differentiation (G2 and 3) (p=0.022). These data are partly consistent with a recent study.17
In contrast to previously reported findings,20 21 we did not observe any correlation between PD-L1 expression intensity and frequency of EGFR mutation in pulmonary adenocarcinoma. For cancer cells with EGFR mutation, PD-L1 expression should, theoretically, be upregulated via activation of stat-3, PI3K-AKT or RAS-RAF-MAPK pathways by constant signals from EGFR.31–37 Therefore, the case with up-regulated PD-L1 expression by EGFR mutation would be included in pulmonary adenocarcinomas. However, our findings appear to negate the main contribution of EGFR mutation of tumour cells towards PD-L1 expression intensity of pulmonary adenocarcinomas, although a relationship between PD-L1 expression and oncogene addiction including various signal pathway cascades remains to be elucidated in future studies. In addition, additional immunohistochemical analysis and/or more sensitive techniques for PD-L1 expression using larger sets of lung adenocarcinomas with EGFR mutation could clarify this discrepancy.
In conclusion, this is the first report to establish a positive control for immunohistochemical examination of PD-L1 expression and semiquantitative assessment of PD-L1 expression intensity of pulmonary adenocarcinoma cells. Given the heterogeneous distribution of PD-L1 expression in pulmonary adenocarcinomas cells, assessment of PD-L1 expression relative to that of AMs appears to be valid for the evaluation of PD-L1 expression status in patients with pulmonary adenocarcinomas. High PD-L1 expression score of tumour cells showed a significant correlation with low-grade differentiation, lymphatic invasion and postoperative relapse-free survival.
Acknowledgments
The authors thank the staff at the Central Research Laboratory in Shiga University of Medical Science for their technical support and Dr Takashi Hisamatsu (Shiga University of Medical Science) for his assistance in statistical analyses.
Footnotes
Contributors: KT and YD took part in concept and study design. TI, MI and JH acquired the data for study. TI and KT analysed and interpreted the data, and drafted the manuscript. YD critically revised the manuscript.
Funding: This work was supported in part by Grant-in-Aid for Scientific Research (B) and Grant-in-Aid for Scientific Research on Innovative Areas from The Japan Society for the Promotion of Science (JSPS KAKENHI grant number JP: 15H04761 and 16H06277). YD. and KT. are members of Shiga Cancer Treatment Project supported by Shiga Prefecture (Japan).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The Institutional Review Board of Shiga University of Medical Science.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2.Sawabata N, Miyaoka E, Asamura H et al. . Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol 2011;6:1229–35. 10.1097/JTO.0b013e318219aae2 [DOI] [PubMed] [Google Scholar]
- 3.Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev 2010;5:Cd007309 10.1002/14651858.CD007309.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baggstrom MQ, Stinchcombe TE, Fried DB et al. . Third-generation chemotherapy agents in the treatment of advanced non-small cell lung cancer: a meta-analysis. J Thorac Oncol 2007;2:845–53. 10.1097/JTO.0b013e31814617a2 [DOI] [PubMed] [Google Scholar]
- 5.Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer. A meta-analysis. Cancer 1995;76:593–601. [DOI] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Thongprasert S et al. . Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 7.Wu YL, Zhou C, Hu CP et al. . Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22. 10.1016/S1470-2045(13)70604-1 [DOI] [PubMed] [Google Scholar]
- 8.Shaw AT, Kim DW, Nakagawa K et al. . Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–94. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wu YL, Chen G et al. . Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 10.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 2005;54:307–14. 10.1007/s00262-004-0593-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keir ME, Butte MJ, Freeman GJ et al. . PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H, Strome SE, Salomao DR et al. . Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793–800. 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- 14.Hansen JD, Du Pasquier L, Lefranc MP et al. . The B7 family of immunoregulatory receptors: a comparative and evolutionary perspective. Mol Immunol 2009;46:457–72. 10.1016/j.molimm.2008.10.007 [DOI] [PubMed] [Google Scholar]
- 15.Topalian SL, Hodi FS, Brahmer JR et al. . Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brahmer JR, Tykodi SS, Chow LQ et al. . Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang CY, Lin MW, Chang YL et al. . Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014;50:1361–9. 10.1016/j.ejca.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 18.Mu CY, Huang JA, Chen Y et al. . High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011;28:682–8. 10.1007/s12032-010-9515-2 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Wang L, Li Y et al. . Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther 2014;7:567–73. 10.2147/OTT.S59959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Incecco A, Andreozzi M, Ludovini V et al. . PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 2015;112:95–102. 10.1038/bjc.2014.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azuma K, Ota K, Kawahara A et al. . Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 2014;25:1935–40. 10.1093/annonc/mdu242 [DOI] [PubMed] [Google Scholar]
- 22.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 2006;163:670–5. 10.1093/aje/kwj063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr KM, Tsao MS, Nicholson AG et al. . Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thorac Oncol 2015;10:985–9. 10.1097/JTO.0000000000000526 [DOI] [PubMed] [Google Scholar]
- 24.Qu QX, Huang Q, Shen Y et al. . The increase of circulating PD-L1-expressing CD68 macrophage in ovarian cancer. Tumour Biol 2015;37:5031–7. 10.1007/s13277-015-4066-y [DOI] [PubMed] [Google Scholar]
- 25.Bloch O, Crane CA, Kaur R et al. . Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res 2013;19:3165–75. 10.1158/1078-0432.CCR-12-3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin J, Han G, Schalper KA et al. . Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol 2016;2:46–54. 10.1001/jamaoncol.2015.3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson RH, Kuntz SM, Leibovich BC et al. . Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006;66:3381–5. 10.1158/0008-5472.CAN-05-4303 [DOI] [PubMed] [Google Scholar]
- 28.Taube JM, Anders RA, Young GD et al. . Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra137 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borghaei H, Paz-Ares L, Horn L et al. . Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cha E, Wallin J, Kowanetz M. PD-L1 inhibition with MPDL3280A for solid tumors. Semin Oncol 2015;42:484–7. 10.1053/j.seminoncol.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 31.Akbay EA, Koyama S, Carretero J et al. . Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355–63. 10.1158/2159-8290.CD-13-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto R, Nishikori M, Tashima M et al. . B7-H1 expression is regulated by MEK/ERK signaling pathway in anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci 2009;100:2093–100. 10.1111/j.1349-7006.2009.01302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marzec M, Zhang Q, Goradia A et al. . Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc Natl Acad Sci USA 2008;105:20852–7. 10.1073/pnas.0810958105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang WB, Yen ML, Liu KJ et al. . Interleukin-25 mediates transcriptional control of PD-L1 via STAT3 in multipotent human mesenchymal stromal cells (hMSCs) to suppress Th17 responses. Stem Cell Reports 2015;5:392–404. 10.1016/j.stemcr.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atefi M, Avramis E, Lassen A et al. . Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res 2014;20:3446–57. 10.1158/1078-0432.CCR-13-2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang X, Zhou J, Giobbie-Hurder A et al. . The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res 2013;19:598–609. 10.1158/1078-0432.CCR-12-2731 [DOI] [PubMed] [Google Scholar]
- 37.Gao SP, Mark KG, Leslie K et al. . Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest 2007;117:3846–56. 10.1172/JCI31871 [DOI] [PMC free article] [PubMed] [Google Scholar]