Abstract
Introduction
Recent studies have shown that the presence of systemic inflammation correlates with worse outcomes in many types of cancers. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have been proposed as indicators of systemic inflammatory response. The aim of the study was to assess the prognostic value of NLR and PLR before starting chemotherapy among patients with newly diagnosed ovarian cancer.
Methods
We conducted a retrospective analysis of medical documentation of 315 patients with newly diagnosed epithelial ovarian cancer, treated in Maria Skłodowska-Curie Memorial Cancer Center and Institute of Oncology, Gliwice Branch, between 2007 and 2013. 31 (12.1%) patients had metastatic disease at the time of diagnosis. Receiver-operating characteristic (ROC) curves for progression free survival (PFS) and overall survival (OS) prediction were plotted to verify cut-off points for NLR and PLR. PFS and OS were analysed for correlation with NLR and PLR, using the Cox regression model. Other potential prognostic variables included in multivariate analysis were: patient's age at diagnosis (<65 vs ≥65 years), Eastern Cooperative Oncology Group performance status (ECOG-PS) ≥2, FIGO stage of the disease and baseline Ca-125 level.
Results
In multivariate analysis, higher pretreatment NLR (p=0.002), poor ECOG-PS (p=0.0002), higher disease stage (p<0.0001) and baseline Ca-125 (p=0.03) level were independent negative prognostic factors for PFS. However, only ECOG-PS ≥2 (p<0.0001), high stage of the disease (p<0.0001) and high baseline Ca-125 level (p=0.0003) were independent negative prognostic factors for OS.
Conclusions
Advanced stage of the disease with high Ca-125 level and poor patient performance status are the most important prognostic factors in ovarian cancer. Higher pretreatment value of NLR was an independent negative prognostic factor for PFS, with no significant impact on OS.
Keywords: ovarian cancer, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio
Key questions.
What is already known about this subject?
The negative prognostic role of neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) was proven in patients with breast, colorectal and lung cancer. Data regarding NLR and PLR among patients with ovarian cancer are sparse and conflicting.
What does this study add?
In this paper, we seek novel prognostic factors in woman with epithelial ovarian cancer in light of immunology. As far as we are concerned, this is the first analysis of the prognostic role of NLR and PLR among patients with ovarian cancer. NLR and PLR are easily available and relatively inexpensive parameters that can be implemented into prognostic scores for patients with epithelial ovarian cancer. Moreover, they might also be predictive for immunotherapy use.
How might this impact on clinical practice?
In light of our results, NLR and PLR seem to add little value to well-known prognostic factors in patients with ovarian cancer. However, due to a strong relationship between angiogenesis and immune response, it would be worth analysing the prognostic role of NLR and PLR among patients treated with bevacizumab.
Introduction
Ovarian cancer is the second most common gynaecological cancer in the world. Nevertheless, it is responsible for the highest mortality rate among woman with gynaecological malignancies.1 2 Disease stage is considered the most important prognostic marker and approximately 70% of woman with ovarian cancer are diagnosed at stage III or IV.1 The gold standard in the first-line treatment of ovarian cancer is chemotherapy doublet with a platinum and taxane regimen, however, the survival rates for the advanced stage of the disease remain poor.2 Recently, bevacizumab, a monoclonal antibody targeting vascular endothelial growth factor (VEGF), was introduced into clinical practice, as angiogenesis is believed to play a crucial role in ovarian cancer development and progression.2–4 However, we do not have any strong, easily assessable biomarkers to define the subgroup of woman with ovarian cancer who will benefit most from antiangiogenic treatment.5–7
It has been reported that many types of cancers, such as lung cancer, gastrointestinal malignancies or breast cancer, are linked with systemic inflammation.8–12 It should be noted that immune response and angiogenesis are linked to each other. VEGF, beyond angiogenesis, also regulates dendric cell function.13 14 Inhibition of dendric cell maturation results in impairment of antigen presentation and type 1 cellular immunity.13 14
Changes in systemic inflammatory response to tumour cell manifestation or systemic inflammation can be easily measured by blood parameter examination.15 Usually, neutrophilia, thromocytosis and relative lymphocytopaenia are observed.16 Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have been assumed to be easily available and promising biomarkers in the abovementioned types of cancer.8–12
There is a paucity of data regarding the prognostic influence of systemic inflammatory response in ovarian cancer. Scant and conflicting data are available concerning the role of NLR and PLR in distinguishing benign from malignant ovarian masses.17 18 The aim of our study was to assess the prognostic value of NLR and PLR before starting chemotherapy in newly diagnosed patients with ovarian cancer.
Material and methods
Patients
We conducted a retrospective analysis of medical records of 315 patients with newly diagnosed epithelial ovarian cancer. The patients were treated in Maria Skłodowska-Curie Memorial Cancer Center and Institute, Gliwice Branch, between 2007 and 2013. Inclusion criteria were: patient's age above 18 years, pathologically confirmed epithelial ovarian cancer and first-line treatment with a platinum-taxane regimen. We have not performed any additional morphological, immunohistochemical or molecular analysis to find out if carcinomas of the ovary originate from the fallopian tube.19
The date of diagnosis was defined as the date of initial surgery. Optimal cytoreduction was considered as <1 cm of remaining tumour. Disease stage was evaluated according to International Federation of Gynaecology and Obstetrics (FIGO) 2009 Criteria, and response to treatment, according to RECIST 1.0 criteria. Recurrence/progression was diagnosed by CT scans, regardless of the Ca-125 rise.
NLR and PLR were evaluated on the basis of blood counts before initiation of chemotherapy. Blood counts before operation were not noted, as the patients were operated on in different hospitals and we did not have access to full medical data at the time. The time interval between surgery and blood analysis at our hospital was 4–8 weeks. NLR was calculated as the absolute neutrophil count divided by the absolute lymphocyte count. PLR was calculated as the absolute platelet count divided by the absolute lymphocyte count.
Statistical analysis
The statistical assessment of the data was performed using PQStat V.1.6.
Overall survival (OS) was calculated from the date of diagnosis until death of any cause or the date the patient was last seen alive. Progression free survival (PFS) was calculated from the date of diagnosis to the date of disease progression or patient's death. We used the Kaplan-Meier method for PFS and OS analysis.
We used the receiver-operating characteristic (ROC) curve for the determination of the appropriate cut-off value of the NLR and PLR for survival prediction.
Cox proportional hazard models were applied to explore predictors of treatment outcomes and survival in univariate and multivariate analysis. Potential prognostic variables were: patient's age at diagnosis (<65 vs ≥65 years), Eastern Cooperative Oncology Group performance status (ECOG-PS) ≥2, FIGO stage of the disease and baseline Ca-125 level.
A p value less than 0.05 was considered statistically significant and p<0.01—highly significant.
Results
The median age of patients was 54 years (22–77 years). Most patients were diagnosed with ovarian cancer at stage III—186 (59%), 61 (19.4%)—at stage I, 30 (9.5%)—at stage II and 38 (12.1%)—at stage IV. One hundred and eighty-two (57.8%) women underwent optimal surgery. The most common pathological type of disease was serous carcinoma—201 (63.8%) patients. The median PFS in our group of patients was 21.7 months (1.7–79.7 months) and the median OS was 31.8 months (3.7–93.7 months).
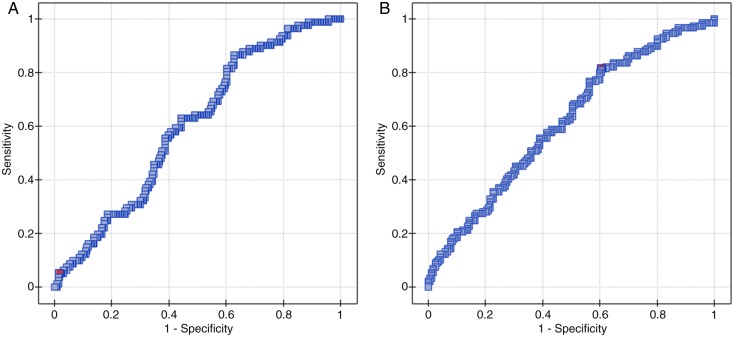
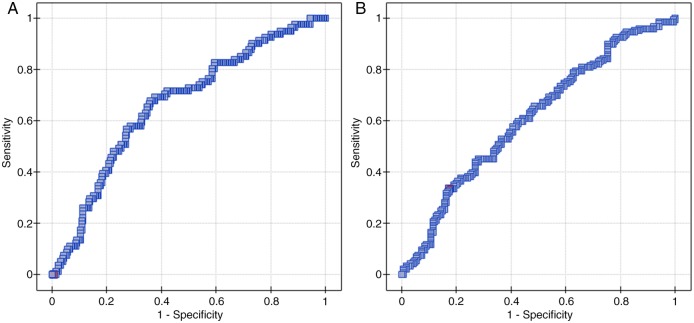
The median NLR was 2.2 (0.56–21.46) and the median PLR reached 165.19 (62.31–3414.29). By using ROC curve analysis we determined cut-off values of NLR and PLR to predict 33 months PFS and OS (figures 1 and 2). ROC curve analysis suggested that the optimum cut-off value of 0.89 for NLR was the best to discriminate between patient's PFS and 2.96—OS (area under the curve (AUC): 0.601, 95% CI (0.53 to 0.67), p=0.007 and AUC: 0.614, 95% CI (0.55 to 0.68), p=0.0005, respectively). A cut-off value of 62.31 for PLR was the best to discriminate between patient's PFS and 129.78—OS (AUC: 0.665, 95% CI (0.59 to 0.73), p<0.0001 and AUC: 0.610, 95% CI (0.55 to 0.67), p=0.0008, respectively).
Figure 1.
ROC curve analysis to determine cut-off value of NLR (A) and PLR (B) to predict 33 months PFS. PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio ROC, receiver-operating characteristic.
Figure 2.
ROC curve analysis to determine cut-off value of NLR (A) and PLR (B) to predict 33 months OS. PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; ROC, receiver-operating characteristic.
In univariate analysis, patients with higher pretreatment NLR reached significantly shorter PFS and OS (p=0.0002 and p=0.02, respectively).
In multivariate analysis, independent negative prognostic factors for 3 years PFS were: high pretreatment NLR (p=0.002), ECOG-PS ≥2 (p=0.0002), high baseline Ca-125 level (p=0.03) and advanced disease stage (p<0.0001). High pretreatment PLR was associated with longer PFS (p=0.034). Patient's age ≥65 years was not predictive for PFS. Independent negative prognostic factors for 3 years OS were: ECOG-PS ≥2 (p<0.0001), higher baseline Ca-125 level (p=0.0003) and advanced stage of disease (p<0.0001). The results of multivariate analysis are detailed in tables 1 and 2.
Table 1.
Multivariate analysis of prognostic factors for PFS
| 95% CI | HR | p Value | |
|---|---|---|---|
| NLR | 1.075 to 1.393 | 1.22 | 0.002 |
| PLR | 0.997 to 0.999 | 0.99 | 0.034 |
| ECOG-PS ≥2 | 1.428 to 3.144 | 2.12 | 0.0002 |
| Patient's age ≥65 years | 0.553 to 1.757 | 0.98 | 0.96 |
| Baseline Ca-125 level | 1.000 to 1.0001 | 1.00 | 0.03 |
| Disease stage | 1.217 to 1.774 | 1.47 | <0.0001 |
PFS, progression free survival; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio.
Table 2.
Multivariate analysis of prognostic factors for OS
| 95% CI | HR | p Value | |
|---|---|---|---|
| NLR | 0.979 to 1.217 | 1.09 | 0.11 |
| PLR | 0.999 to 1.000 | 0.99 | 0.54 |
| ECOG-PS ≥2 | 1.485 to 3.064 | 2.13 | <0.0001 |
| Patient's age ≥65 years | 0.684 to 1.536 | 1.02 | 0.9 |
| Baseline Ca-125 level | 1.000 to 1.0001 | 1.00 | 0.0003 |
| Disease stage | 1.275 to 1.569 | 1.41 | <0.0001 |
OS, overall survival; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio.
We also performed univariate and multivariate analysis for each disease stage separately.
Among patients with disease stage I, in neither univariate nor multivariate analysis were the analysed parameters prognostic for PFS. High pretreatment PLR was associated with better OS in this subgroup (HR=1.00, 95% CI 0.99 to 1.02, p=0.05).
Higher pretreatment PLR was associated with longer PFS and OS in univariate (p=0.04 and p=0.02, respectively) and multivariate analysis (HR=0.99, 95% CI 0.99 to 1.01, p=0.04 and HR=0.98, 95% CI 0.97 to 0.99, p=0.01, respectively) among patients with stage II ovarian cancer.
Among patients with stage III disease, higher pretreatment NLR (p<0.0001, p=0.0002), PLR (p<0.0001, p=0.001), poor ECOG-PS (p=0.0008, p=0.002) and high baseline Ca-125 level (p=0.0001, p=0.0009) were negative prognostic factors for PFS and OS in univariate analysis. However, the only independent negative prognostic factors for PFS and OS were ECOG-PS ≥2 (HR=1.55, 95% CI 1.02 to 2.37, p=0.04 and HR=1.58, 95% CI 1.05 to 2.37, p=0.03, respectively) and high baseline Ca-125 level (HR=1.00, 95% CI 1.000 to 1.0001, p=0.005 and HR=1.00, 95% CI 1.000 to 1.0001, p=0.03, respectively). We found no cases with only nodal involvement without peritoneum spread in this subgroup to perform further analysis on.
In patients with metastatic ovarian cancer, poor ECOG-PS and high baseline Ca-125 level were negative prognostic factors for PFS in univariate analysis (p=0.007 and p=0.02, respectively). In univariate analysis, ECOG-PS ≥2 was the only negative prognostic factor for OS (p=0.02). None of the analysed parameters were independent prognostic factors for PFS and OS in this subgroup.
Discussion
There is a strong link between inflammation and cancer. Cancer cells produce many cytokines and chemokines such as granulocyte colony stimulating factor, interleukin-1, interleukin-6 and tumour necrosis factor α, which lead to leucocytosis and neutrophilia.20 21 Neutrophils and lymphocytes play different roles in inflammatory response. Activated neutrophils produce angiogenetic and grow factors linked with tumour progression.22 23 Moreover, they are responsible for cytotoxic T-cell and natural killer cell suppression, and regulatory T-cell activation, which create the immunosuppressive milieu.23 Tumour-related inflammation is also associated with thrombocytosis, but this dependence is not clearly established.24 25 On the other hand, lymphocytopaenia was shown to be a poor prognostic factor in advanced cancer patients and presence of tumour infiltrating lymphocytes (TIL) is of prognostic value in many types of cancer, including ovarian cancer.26 However, there are no strong data supporting the contention that blood parameters resemble information on the inflammatory microenvironment of the tumour.
Owing to its asymptomatic course in the early stage, ovarian cancer is usually diagnosed at an advanced stage with high mortality rate.2 Until the introduction of bevacizumab for epithelial ovarian cancer treatment, there was no targeted agent approved in this diagnosis and carboplatin-paclitaxel regimen remained the gold standard in first-line treatment.2 Adding bevacizumab to chemotherapy led to significant improvement of PFS and OS in some studies, especially among patients with advanced disease.3 4 Nevertheless, treatment outcomes in patients with advanced ovarian cancer are still unsatisfactory. Recently, clinical trials with another antiangiogenic drug, pazopanib, were conducted, with conflicting results.6 27 However, we still do not have any predictive markers for the choice of antiangiogenic treatment. Angiogenesis and immune response are linked to each other, since VEGF affects dendric cell differentiation.13 Activation of dendric cells and their interaction with T-cells creates a powerful antitumour milieu.13 14 Further studies should probably be aimed at assessing the prognostic role of baseline NLR and PLR among patients with ovarian cancer receiving antiangiogenic treatment. As bevacizumab has only been available in Poland since 2013, earlier analysis was impossible, but we are planning to perform it in the future.
NLR and PLR are easily accessed and relatively inexpensive parameters that reflect host immune response. They have been shown to be independent prognostic factors in many types of cancers, and have helped in choosing optimal treatment, for example, adjuvant chemotherapy.28 29 Among patients with pathological ovarian masses, NLR and PLR have been shown to be markers of malignancy, but these data are sparse and conflicting.17 18 The aim of our study was to evaluate the prognostic role of NLR and PLR among woman with newly diagnosed epithelial ovarian cancer. Owing to lack of any defined NLR and PLR thresholds, we distinguished them by ROC curve analysis in our patient population. In our analysis, higher rates of NLR were associated with significantly shorter PFS, however, neither NLR nor PLR had significant impact on OS in multivariate analysis. Poor patient PS and advanced disease with high Ca-125 levels remained strong, evident negative prognostic factors for treatment outcomes. Importantly, a patient's age being ≥65 years was not predictive for survival outcomes. It is also worth noting that high pretreatment PLR was associated with better outcomes among patients with early stage of the disease (I and II).
There are some limitations in our study. The study is retrospective, from a single institution and the number of patients included is relatively small. Although the data are from a single institution, patients were operated on by different surgeons in different hospitals, so there may be a lack of uniformity. We evaluated pretreatment variables, as the use of chemotherapy limits analysis of changes in blood counts. Other markers associated with systemic inflammation, such as albumin or C reactive protein, are also included in some prognostic scores.30 31 We do not routinely check these before chemotherapy, therefore they were not included in our analysis. Moreover, it should be remembered that the number of blood cells depends on a wide range of factors such as acute or chronic infection or inflammatory disease and lifestyle habits (smoking). These factors were difficult to elucidate in retrospective analysis.
In conclusion, according to data in the available literature, advanced stage disease with high Ca-125 level and poor patient performance status are the most important prognostic factors in ovarian cancer. Higher pretreatment value of NLR was an independent negative prognostic factor for PFS, with no significant impact on OS. Larger prospective trials are needed to verify these preliminary results.
Footnotes
Twitter: Follow Agnieszka Badora-Rybicka at @agbad
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jemal A, Siegel R, Ward E et al. . Cancer statistics, 2009. CA Cancer J Clin 2009;59:225–49. 10.3322/caac.20006 [DOI] [PubMed] [Google Scholar]
- 2.Ledermann JA, Raja FA, Fotopoulou C et al. . Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24(Suppl 6):vi24–32. 10.1093/annonc/mdt333 [DOI] [PubMed] [Google Scholar]
- 3.Burger RA, Brady MF, Bookman MA et al. . Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011;365:2473–83. 10.1056/NEJMoa1104390 [DOI] [PubMed] [Google Scholar]
- 4.Perren TJ, Swart AM, Pfisterer J et al. . A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011;365:2484–96. 10.1056/NEJMoa1103799 [DOI] [PubMed] [Google Scholar]
- 5.du Bois A, Floquet A, Kim JW et al. . Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol 2014;32:3374–82. 10.1200/JCO.2014.55.7348 [DOI] [PubMed] [Google Scholar]
- 6.Floquet A, Vergote I, Colombo N et al. . Progression-free survival by local investigator versus independent central review: comparative analysis of the AGO-OVAR16 Trial. Gynecol Oncol 2015;136:37–42. 10.1016/j.ygyno.2014.11.074 [DOI] [PubMed] [Google Scholar]
- 7.Schneider BP, Wang M, Radovich M et al. . ECOG 2100; association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol 2008;26:4672–8. 10.1200/JCO.2008.16.1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halazun KJ, Hardy MA, Rana AA et al. . Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg 2009;250:141–51. 10.1097/SLA.0b013e3181a77e59 [DOI] [PubMed] [Google Scholar]
- 9.Bhatti I, Peacock O, Lloyd G et al. . Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg 2010;200:197–203. 10.1016/j.amjsurg.2009.08.041 [DOI] [PubMed] [Google Scholar]
- 10.Aizawa M, Gotohda N, Takahashi S et al. . Predictive value of baseline neutrophil/lymphocyte ratio for T4 disease in wall-penetrating gastric cancer. World J Surg 2011;35:2717–22. 10.1007/s00268-011-1269-2 [DOI] [PubMed] [Google Scholar]
- 11.Tomita M, Shimizu T, Ayabe T et al. . Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res 2011;31:2995–8. [PubMed] [Google Scholar]
- 12.Lee YY, Choi CH, Kim HJ et al. . Pretreatment neutrophil: lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Res 2012;32:1555–61. [PubMed] [Google Scholar]
- 13.Gabrilovich DI, Chen HL, Girgis KR et al. . Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 1996;2:1096–103. 10.1038/nm1096-1096 [DOI] [PubMed] [Google Scholar]
- 14.Gabrilovich D, Ishida T, Oyama T et al. . Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998;92:4150–66. [PubMed] [Google Scholar]
- 15.Ueno H, Hawrylowicz CM, Banchereau J. Immunological intervention in human diseases. J Transl Med 2007;5:59 10.1186/1479-5876-5-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–54. 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- 17.Yildirim MA, Seckin KD, Togrul C et al. . Roles of neutrophil/lymphocyte and platelet/lymphocyte ratios in the early diagnosis of malignant ovarian masses. Asian Pac J Cancer Prev 2014;15:6881–5. 10.7314/APJCP.2014.15.16.6881 [DOI] [PubMed] [Google Scholar]
- 18.Topcu HO, Guzel AI, Ozer I et al. . Comparison of neutrophil/lymphocyte and platelet/lymphocyte ratios for predicting malignant potential of suspicious ovarian masses in gynecology practice. Asian Pac J Cancer Prev 2014;15:6239–41. 10.7314/APJCP.2014.15.15.6239 [DOI] [PubMed] [Google Scholar]
- 19.Li J, Fadare O, Xiang L et al. . Ovarian serous carcinoma: recent concepts on its origin and carcinogenesis. J Hematol Oncol 2012;5:8 10.1186/1756-8722-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res 2002;22:913–22. 10.1089/10799900260286623 [DOI] [PubMed] [Google Scholar]
- 21.Kusumanto YH, Dam WA, Hospers GA et al. . Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 2003;6:283–7. 10.1023/B:AGEN.0000029415.62384.ba [DOI] [PubMed] [Google Scholar]
- 22.Yamanaka T, Matsumoto S, Teramukai S et al. . The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology 2007;73:215–20. 10.1159/000127412 [DOI] [PubMed] [Google Scholar]
- 23.Azab B, Bhatt VR, Phookan J et al. . Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol 2012;19:217–24. 10.1245/s10434-011-1814-0 [DOI] [PubMed] [Google Scholar]
- 24.Tamussino KF, Gucer F, Reich O et al. . Pretreatment hemoglobin, platelet count, and prognosis in endometrial carcinoma. Int J Gynecol Cancer 2001;11:236–40. 10.1046/j.1525-1438.2001.01024.x [DOI] [PubMed] [Google Scholar]
- 25.Crasta JA, Premlatha TS, Krishnan SM et al. . Significance of preoperative thrombocytosis in epithelial ovarian cancer. Indian J Pathol Microbiol 2010;53:54–6. 10.4103/0377-4929.59184 [DOI] [PubMed] [Google Scholar]
- 26.Lo Presti E, Dieli F, Meraviglia S. Tumor-Infiltrating γδ T Lymphocytes: pathogenic role, clinical significance, and differential programing in the tumor microenvironment. Front Immunol 2014;5:607 10.3389/fimmu.2014.00607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hainsworth JD, Firdaus ID, Earwood CB et al. . Pazopanib and liposomal Doxorubicin in the treatment of patients with relapsed/refractory epithelial ovarian cancer: a phase ib study of the sarah cannon research institute. Cancer Invest 2015;33:47–52. 10.3109/07357907.2014.998833 [DOI] [PubMed] [Google Scholar]
- 28.Jankova L, Dent OF, Chan C et al. . Preoperative neutrophil/lymphocyte ratio predicts overall survival but does not predict recurrence or cancer-specific survival after curative resection of node-positive colorectal cancer. BMC Cancer 2013;13:442 10.1186/1471-2407-13-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei Y, Jiang YZ, Qian WH. Prognostic role of NLR in urinary cancers: a meta-analysis. PLoS ONE 2014;9:e92079 10.1371/journal.pone.0092079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Car 2009;12:223–6. 10.1097/MCO.0b013e32832a7902 [DOI] [PubMed] [Google Scholar]
- 31.Buyukkaya E, Karakas MF, Karakas E et al. . Correlation of neutrophil to lymphocyte ratio with the presence and severity of metabolic syndrome. Clin Appl Thromb Hemost 2014;20:159–63. 10.1177/1076029612459675 [DOI] [PubMed] [Google Scholar]