Abstract
Despite the improvement in clinical outcomes derived by the introduction of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (EGFR-TKIs) in the treatment of patients with advanced non-small cell lung cancer (NSCLC) whose tumours harbour EGFR-activating mutations, prognosis remains unfavourable because of the occurrence of either intrinsic or acquired resistance. We reviewed the published literature and abstracts of oral and poster presentations from international conferences addressing EGFR-TKIs resistance mechanisms discovered in preclinical models and in patients with NSCLC. The molecular heterogeneity of lung cancer has several implications in terms of possible mechanisms of either intrinsic or acquired resistance to EGFR-targeted inhibitors. Several mechanisms of resistance have been described to EGFR-TKIs, such as the occurrence of secondary mutation (T790M, C797S), the activation of alternative signalling (Met, HGF, AXL, Hh, IGF-1R), the aberrance of the downstream pathways (AKT mutations, loss of PTEN), the impairment of the EGFR-TKIs-mediated apoptosis pathway (BCL2-like 11/BIM deletion polymorphism) and histological transformation. Although some of the mechanisms of resistance have been identified, much additional information is needed to understand and overcome resistance to EGFR-TKI agents. The majority of resistance mechanisms described are the result of a selection of pre-existing clones; thus, studies on the mechanisms by which subclonal alterations have an impact on tumour biology and influence cancer progression are extremely important in order to define the best treatment strategy.
Keywords: EGFR TKIs
Introduction
Non-small cell lung cancer (NSCLC) is the major cause of cancer-related deaths worldwide.1
Platinum-based combination chemotherapy, which has represented the only therapeutic option for patients with advanced NSCLC until a few years ago, has yielded a limited outcome improvement with a median overall survival (OS) <12 months and a 5-year survival rate <1%. Treatment of selected patients with advanced NSCLC has been revolutionised by the discovery and subsequent targeting of the epidermal growth factor receptor (EGFR) pathway.
EGFR is a member of the HER family, which also includes HER2 (ErbB2), HER3 (ErbB3), HER4 (ErbB4). When the EGFR extracellular domain binds to its ligands, such as epidermal growth factor (EGF) and transforming growth factor-α (TGF-α), it forms dimers with other EGFR or other HER family members and undergoes autophosphorylation at the key tyrosine residues, thus activating several downstream signalling pathways such as protein kinase B (AKT/PKB) and mitogen-activated protein kinases (MAPK), which regulate multiple cellular processes, including proliferation, survival and apoptosis. The constitutive activation of EGFR signalling, caused by gene mutations or by gene amplification or both, has been demonstrated to have close connection with the initiation, progression and poor prognosis of NSCLC. The two most common EGFR-activating mutations are small in-frame deletions in exon 19 (particularly E746-A750del) and amino acid substitution in exon 21 (leucine to arginine at codon 858 (L858R)), which collectively account for >90% of known activating EGFR mutations.2 3 These two alterations are the best-characterised mutations conferring sensitivity to EGFR-tyrosine kinase inhibitor (EGFR-TKI) therapy, resulting in higher response rates (RR) (up to 70%) and longer median survival (up to 24–30 months) than those observed in patients with wild-type (WT) EGFR. The higher sensitivity of these mutations relays in an increased affinity of the ATP-binding pocket for EGFR-TKIs as compared with WT EGFR.
Thus, mutations in EGFR play a role as both biomarkers and rational targets for targeted therapy. First-generation EGFR-TKIs, gefitinib and erlotinib, were designed to reversibly combine with the ATP-binding sites, thus blocking EGFR-induced activation of downstream signalling, whereas the second-generation EGFR-TKIs, such as afatinib and dacomitinib, are irreversible inhibitors with greater affinity for the EGFR kinase domain also inhibiting other members of the EGFR family (ErbB2, ErbB3 and ErbB4).
Eight randomised controlled phase III trials (table 1) have demonstrated that first-generation or second-generation EGFR-TKIs represent the best first-line treatment option in patients with advanced NSCLC whose tumours harbour EGFR mutations, when compared with chemotherapy, because they significantly improved the RR and progression-free survival (PFS).4–11 The lack of OS improvement is due to treatment crossover at progression, although a pooled analysis of two studies with afatinib demonstrated an OS improvement in those patients harbouring the exon 19 deletion.12
Table 1.
Phase III studies of EGFR-TKI as first-line treatment of patients with EGFR mutated NSCLC
| Study | EGFR-TKI | Chemotherapy | Mutation | Median PFS (months) | RR (%) | Median OS (months) |
|---|---|---|---|---|---|---|
| IPASS4 | Gefitinib | Carboplatin–paclitaxel | All | 9.5 vs 6.3 | 71.2 vs 47.3 | 21.6 vs 21.9 |
| WJTOG34055 | Gefitinib | Cisplatin–docetaxel | L858R Ex19D |
9.2 vs 6.3 | 62.1 vs 32.2 | 36 vs 39 |
| NEJ0026 | Gefitinib | Carboplatin–paclitaxel | L858R Ex19D |
10.8 vs 5.4 | 73.7 vs 30.7 | 27.7 vs 26.6 |
| OPTIMAL7 | Erlotinib | Gemcitabine–carboplatin | L858R Ex19D |
13.1 vs 4.6 | 83 vs 36 | 22.7 vs 28.9 |
| First-Signal8 | Gefitinib | Gemcitabine–cisplatin | All | 8.0 vs 6.3 | 84.6 vs 37.5 | 27.2 vs 25.6 |
| EURTAC9 | Erlotinib | Cisplatin–docetaxel/gemcitabine | L858R Ex19D |
9.7 vs 5.2 | 58 vs 15 | 19.3 vs 19.5 |
| LUX-Lung 310 12 | Afatinib | Cisplatin–pemetrexed | All | 11.1 vs 6.9 | 56 vs 23 | 31.6 vs 28.2 |
| LUX-Lung 611 | Afatinib | Gemcitabine–cisplatin | All | 11.0 vs 5.6 | 66.9 vs 23 | 23.6 vs 23.511 |
EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitor; NSCLC, non-small cell lung cancer; OS, overall survival; PFS, progression-free survival; RR, response rate.
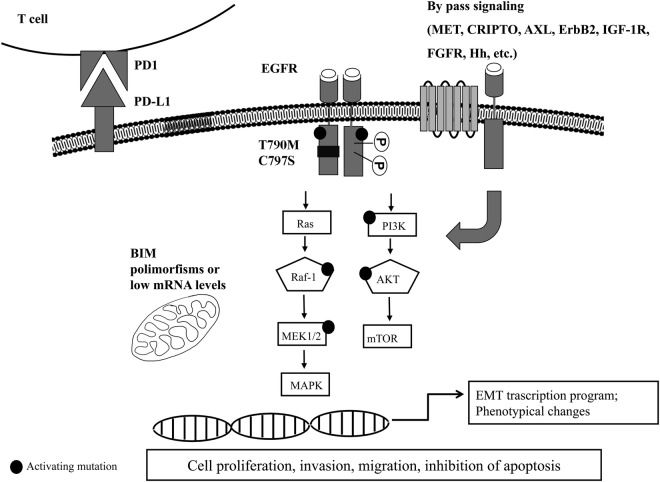
However, most patients with EGFR-mutant NSCLC and treated with EGFR-TKIs develop resistance within 9–14 months. Consequently, it is critical to establish mechanisms by which drug resistance occurs and to apply that knowledge to the development of further generation drugs or of combination strategies to overcome resistance. Different mechanisms of acquired resistance to first-generation EGFR-TKIs have been reported (figure 1).13 The major mechanism of acquired resistance to first-generation EGFR-TKIs is the occurrence of secondary EGFR kinase domain mutation in exon 20, the T790M substitution, which accounts for about half of the cases. Other abnormalities in tumour cells that may contribute to resistance to anti-EGFR agents include constitutive activation of transducers downstream to EGFR, overexpression of other cell surface receptors, perturbation of the apoptotic machinery or phenotypic transformation (table 2).
Figure 1.
Schematic representation of main EGFR-TKIs resistance mechanisms. Resistance to EGFR-TKIs can occur through different mechanisms either intrinsic or acquired. Known mechanisms are secondary resistance mutations occurring in the ATP-binding domain (such as T790M and C797S), mutation or amplification of bypass signallings (such as AXL, Hh, ERBb2, CRIPTO, etc), activating mutations in the downstream pathways (PI3K, AKT, MEK, RAF), low levels of mRNA or polymorphisms of the pro-apoptotic protein BIM, induction of a transcription programme for EMT and phenotypical changes, or induction of elevated tumour PD-L1 levels. EGFR, epidermal growth factor receptor; EMT, epithelial-to-mesenchymal transition; mRNA, messenger RNA; PD-1, programmed death receptor-1; PD-L1, programmed death ligand-1; TKI, tyrosine kinase inhibitor.
Table 2.
| Criteria of acquired resistance to EGFR-TKIs in lung cancer by Jackman et al27 | |
|---|---|
| |
| Clinical subtyping of acquired resistance to EGFR-directed TKI therapy in patients with NSCLC according to PD by Gandara et al28 | |
| Type of PD | Management |
| (1) CNS sanctuary PD | Local therapy (eg, surgery, radiotherapy or both) with continuation of the present EGFR-TKI |
| (2) Oligo-PD | Local therapy (eg, surgery, radiotherapy or both) with continuation of the present EGFR-TKI |
| (3) Systemic PD | If slowly progressing lesions, or lesions smaller than pretreatment, or progression without worsening of systemic symptoms and/or signs, continuation of the present EGFR-TKI may be considered |
CNS, central nervous system; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PD, progression disease; RECIST, Response Evaluation Criteria in Solid Tumors; TKI, tyrosine kinase inhibitor.
A recent breakthrough in the treatment of EGFR T790M mutant cancers occurred with the development of mutant selective pyrimidine-based third-generation EGFR-TKIs, which irreversibly block T790M mutant EGFR.8–12 The third-generation EGFR-TKIs include the WZ4002, CO-1686, AZD9291 and HM61713 inhibitors, which have demonstrated tumour responses in >50% of patients with the EGFR T790M mutation.8–12 These agents are designed to specifically inhibit mutant EGFR (ie, 19del-EGFR, L858R-EGFR and/or T790M+EGFR), sparing the WT receptor.14–18 Therefore, since they have reduced affinity for WT EGFR, the patients will not suffer from the ‘traditional’ EGFR side effects such as skin rash, diarrhoea, hypomagnesaemia. However, it is fully anticipated that resistance will also occur to this class of EGFR inhibitors.
It is now clear that EGFR-TKIs are superior to chemotherapy in EGFR-mutated NSCLC; thus, patients will be treated with multiple lines of EGFR-targeted therapies with increasing frequency, although the definition of what constitutes the optimum treatment after disease progression is not yet clear and several different treatment strategies are considered. Additionally, the choice of the best EGFR-TKI in the first-line setting is still an object of debate and results from currently recruiting clinical studies will help to define the best algorithm of treatment.
In this review, we will analyse the most significant and recently reported mechanisms of resistance to EGFR-targeted therapies. We need to distinguish between primary resistance, referring to patients who experience an immediate inefficacy of EGFR-TKIs, and secondary or acquired resistance, which is usually defined as progression of the disease after a period of clinical benefit. However, despite the clear scholastic differentiation between these two mechanisms, the reality is quite different; some of the mechanisms, such as the coexpression of other ErbB receptors or the constitutive activation of other downstream pathways, are unlikely to be located in one of the two types of resistance.
Source and selection criteria
We analysed several publications investigating EGFR-TKIs resistance mechanisms in NSCLC models and patients published in peer-reviewed scientific journals and listed in PubMed since the discovery of EGFR-activating mutations in 2004 until the most recent publications. We also searched for relevant abstracts of oral and poster presentations submitted to the American Society of Clinical Oncology and European Society for Medical Oncology from 2010 to 2015. The search strategy included combined keywords for ‘NSCLC’, ‘EGFR mutations’ and ‘resistance’. Articles or abstracts published in a language other than English were excluded.
Mechanisms of primary resistance to EGFR-TKIs in NSCLC
Intrinsic resistance is usually defined as an immediate inefficacy of EGFR-TKIs. Although mechanisms of intrinsic resistance are not fully understood, several cases on non-response to EGFR-TKIs have been described in the presence of non-classical sensitising EGFR mutations and rarely in classical EGFR mutations (deletion in exon 19 and L858R). The most common mutations found in the EGFR gene among patients with NSCLC involve point mutations in exon 18, insertions or deletions (indels) in exon 19 (44% of all EGFR-activating mutations), insertions/duplications and point mutations in exon 20 and point mutations in exon 21 (41% of all EGFR-activating mutations). These mutations result in destabilisation of the equilibrium between the active and inactive states of EGFR kinase activity.19 20
Intrinsic resistance is often the consequence of the presence of a non-sensitive EGFR mutation. The most important and frequent drug-resistant EGFR mutations are represented by an exon 20 insertion, whose frequency ranges from 1% to 10% of the total number of EGFR mutations.
Exon 20 insertions add residues at the N-lobe of EGFR (M766 to C775) and their preferential location is the C-helix (A767 to C775). This region is essential in orienting the kinase into a state that controls ATP and EGFR-TKI binding and may indeed regulate the kinase domain conformation into an active position.19
The majority of exon 20 insertion mutations present a reduced affinity for EGFR-TKIs, although some insertion mutations have demonstrated prolonged periods of disease control with reversible EGFR-TKIs suggesting at least intermediate sensitivity, such as the insertion EGFR-A763_Y764insFQEA, which is highly sensitive to EGFR-TKIs in vitro.20 21 Although the T790M mutation is the most common mechanism of acquired resistance to first-generation EGFR-TKIs, rarely has it been identified in tumours before exposure to EGFR-TKIs concurrently with other more common sensitising mutations.20 This gatekeeper T790M point mutation increases the affinity of EGFR for ATP and consequently attenuates the binding efficacy of EGFR-TKIs. The reported frequency of baseline EGFR T790M mutations varies widely in the literature as a consequence of the detection method used and the population tested. The clinical implication of the baseline EGFR T790M mutation varies among published studies, although it is commonly associated with poor clinical outcomes in patients treated with EGFR-TKIs.22 The impact on responsiveness to EGFR-TKI therapy of the pre-existing T790M mutation may depend on the proportion of pretreatment EGFR T790M-mutant alleles within a tumour that may range from a small subclone to one clonally dominant.
A distinct EGFR mutation is represented by the variant III (vIII) in-frame deletion of exons 2–7 in the extracellular domain that prevents EGFRvIII from binding EGF and other ligands. The constitutive signalling by EGFRvIII and the resistance to EGFR-targeted therapy are thought to be the consequence of structural changes in the EGFR protein that could affect the intracellular domain conformation and the ATP pocket.23 It is present in 5% of analysed human lung squamous cell carcinoma (SCC) and has been associated with TKI resistance in vitro. Indeed, gefitinib can reduce EGFRvIII phosphorylation after several days of treatment, but it does not influence cell growth.24
Intrinsic resistance may also be the consequence of concurrent molecular or genetic alterations that could potentially decrease the sensitivity of patients with sensitising EGFR mutations to EGFR-TKIs treatment. One example is represented by the ability of tumours to evade TKI-induced apoptosis as a consequence of deletion polymorphisms or low-to-intermediate levels of messenger RNA (mRNA) of the proapoptotic Bcl-2 family member, BIM, that is a critical mediator of EGFR-TKIs-induced apoptosis in EGFR-mutant NSCLC.25 Patients harbouring BIM deletion polymorphisms23 or patients with low-to-intermediate levels of BIM mRNA18 are associated with reduced clinical efficacy when treated with EGFR-TKIs.25
Another example is represented by high basal levels of CRIPTO1, also known as teratocarcinoma-derived growth factor 1 (TDGF1), which is a glycosylphosphatidylinositol-linked cell membrane-anchored protein that belongs to the EGF-CFC family, and is able to reduce sensitivity to EGFR-TKIs through activation of both ZEB1 and SRC, thus promoting epithelial-to-mesenchymal transition (EMT) and stimulation of AKT and MEK signalling, respectively.26
Mechanisms of acquired resistance to EGFR-TKIs in NSCLC
Secondary or acquired resistance typically occurs after prolonged treatment, and several molecular mechanisms have been suggested to contribute to the resistance phenotype.
The entity of progression differs among cases in terms of the extent and/or sites of progressive disease and treatment options may vary widely based on the type of progression.
In 2010, Jackman et al27 proposed a clinical definition of acquired resistance to EGFR-TKIs in patients with NSCLC. These criteria aimed to benefit both practising oncologists and research undertaken in patients who had acquired resistance from first-line EGFR-TKIs, but needed further clinical validation. Gandara et al28 proposed a clinical subtyping of acquired resistance to EGFR-directed TKI therapy in patients with NSCLC according to a progression disease (PD) occurring as (1) central nervous system (CNS) sanctuary PD, (2) oligo-PD and (3) systemic PD. Although the optimal therapeutic strategy for patients experiencing acquisition of resistance during treatment with EGFR-TKIs is not yet defined, this classification actually helps physicians in the management according to progression patterns. For patients with slowly progressing lesions and with lesions smaller than pretreatment and progression, as documented by Response Evaluation Criteria in Solid Tumors (RECIST), and without the worsening of systemic symptoms and/or signs, the continuation of the present EGFR-TKI can be suggested. Similarly, for patients with CNS PD or oligo-PD, some data have shown that local therapy (eg, surgery, radiotherapy or both) to the site of progression might be appropriate, with continuation of EGFR-TKI treatment thereafter.28
Since EGFR antagonists interfere with the activation of several intracellular pathways that control cell proliferation, survival, apoptosis, metastatic capability, invasion and angiogenesis, the molecular mechanisms of acquired resistance can be due to several processes.
The comprehensive analysis of resistance mechanisms in patients at progression on EGFR-TKIs by repeated tumour biopsy has led to the definition of a mechanism of resistance which has been defined in around 60–70% of cases and classified in one of the following categories:
Insurgence of secondary mutations in the EGFR gene.
Phenotypic transformation.
Activation of alternative pathways.
1. Insurgence of secondary mutations in the EGFR gene
The most common secondary mutation responsible for acquisition of resistance occurs in exon 20 (T790M).14–18 The presence of the T790M mutation was observed in approximately 50% of the cases in which biopsy was obtained at the time of relapse following gefitinib or erlotinib treatment in patients with the exon 19 deletion or the L858R EGFR mutation.
The crystallographic structure of the EGFR protein and its kinase domain shows that this mutation involves a substitution of the threonine residue, located in the hydrophobic ATP-binding pocket of the catalytic domain, where it forms a critical hydrogen bond with the drug with a large methionine residue, thus resulting in a steric conflict with the drugs. Additionally, the T790M mutation alters the affinity of EGFR to ATP, rendering ATP as the favoured substrate compared with ATP-competitive EGFR-TKIs.29 Interestingly, patients whose tumours harbour the T790M mutation might experience a more indolent natural history and more favourable prognosis than do patients whose tumours do not harbour the T790M mutation.30 However, even patients with acquired resistance to gefitinib, erlotinib and afatinib with the T790M mutation can potentially have a rapid clinical decline and short survival.
Although the occurrence of the T790M mutation at acquisition of resistance is consolidated information, little is known about how resistant clones evolve during drug therapy. A recent work by Hata et al31 studied the development of resistance caused by the EGFR T790M gatekeeper mutation and tried to answer the question of whether resistance depends on selection of pre-existing clones or on acquisition of validated genetic resistance mechanisms after a period of drug tolerance. By the monitoring of the development of large numbers of resistant clones in parallel, authors were able to identify patterns characterised by pre-existing drug-resistant EGFRT790M-positive clones as well as the de novo acquisition of the EGFRT790M mutation within initially EGFRT790M-negative drug-tolerant cells. The evolution from drug-tolerant cells to resistant ones seems to impact the biology of the resistant clone; epigenetic hallmarks of the drug-tolerant state coexist with a diminished apoptotic response to third-generation EGFR inhibitors that target EGFRT790M. However, treatment with navitoclax, an inhibitor of the antiapoptotic factors BCL-xL and BCL-2, restored sensitivity.31 These findings provide important evidence that drug-resistant cancer cells bearing the identical clinically relevant genetic resistance mechanism can both pre-exist or evolve from drug-tolerant cells, suggesting that cancer cells that survive initial therapy may serve as an important reservoir from which acquired resistance can emerge in the clinic.
Rare EGFR point mutations (<10% of patients) that result in resistance include Asp761Tyr,39 Thr854Ala,40 and Leu747Ser. The mechanism (or mechanisms) underlying resistance conferred by these mutations is still unclear.
Similar to early-generation EGFR inhibitors, insurgence of secondary mutation has been described as a mechanism of acquired resistance also to third-generation TKIs.32 33 The first report of an acquired EGFR mutation after therapy with third-generation EGFR-TKI was identified in a lung cancer sample from a patient experiencing resistance to AZD9291.32 The EGFR C797S is a ‘tertiary’ substitution mutation at the binding site, changing cysteine 797 into serine (EGFR C797S), which is essential for the covalent bond with the drugs, and therefore confers cross-resistance to all third-generation inhibitors. Subsequent studies have described several mechanisms of acquired resistance to AZD9291 and CO1686 in vitro and in the clinical setting. In a study analysing cell-free DNA of 15 patients with resistance to AZD9291 by next-generation sequencing (NGS), distinct EGFR genotypes before and after AZD9291 treatment were defined: acquired C797S together with a T790M mutation (40%), T790M mutation without a C797S mutation (33%) and loss of the T790M mutation without a C797S mutation (27%).33 In these models, the tumour growth is still dependent on EGFR signalling and under the strong selective pressure of EGFR-TKIs, the tumour developed secondary and tertiary mutations in the EGFR gene (T790M and C797S, respectively).33 Whether these tertiary mutations derive from the expansion of a pre-existing clone is still an object of investigation.
2. Phenotypic transformation
Repeated bioptic sampling from patients with EGFR-mutant NSCLCs have shown a rare but consistent observation of histological transformation from adenocarcinoma to small cell lung cancer (SCLC).34 This plasticity to switch histologies raises the possibility of a shared cell of origin between adenocarcinoma and SCLC. No exact mechanism underlying this phenomenon has been launched. Probably, SCLC cells originate from the minor pre-existent cells under the selection pressure of EGFR-TKIs, or transdifferentiate from the adenocarcinoma cells, or arise from the multipotent stem cells. In particular, there is preclinical evidence that type II alveolar cells have the potential to differentiate into SCLC after the targeted disruption of Tp53 and Rb1.35 Genomic sequencing of EGFR from both the baseline and repeated biopsy samples shows that a transformed SCLC tumour sample retained the original EGFR-activating mutation, suggesting that these were not de novo clones, but rather a transformed phenotype of pre-existing cancer cells. However, patients with adenocarcinoma-SCLC transformation presented mixed responses to EGFR inhibitors, despite the persistency of the activating mutation, probably due to the loss of EGFR expression at the protein level.34
Another aspect, more common, in the context of phenotypical transformation is the EMT, which is a process characterised by a loss of polarity and cell–cell contacts by the epithelial cell layers, which undergo a dramatic remodelling of their cytoskeleton.36 Along with a loss of epithelial cell adhesion and alterations in their cytoskeletal component, cells undergoing EMT acquire expression of mesenchymal components. A main feature of EMT is the loss of E-cadherin expression37 and the upregulation of mesenchymal proteins such as vimentin, fibronectin and N-cadherin. In the Tarceva Responses in conjunction with Paclitaxel and Carboplatin (TRIBUTE) trial,38 among patients receiving erlotinib and chemotherapy, time to progression was longer for those with E-cadherin-positive staining.39 EMT plays an important role in multiple physiological and pathological processes of human biology by regulating the transcription of genes involved in embryonic development, inflammatory response, tissue regeneration, organ fibrosis, tumour invasion and metastasis.
In the context of an EMT, AXL upregulation appears as a novel mechanism of acquired EGFR-TKI resistance in EGFR-mutant NSCLCs. Recently, an integrated analysis in human EGFR-mutant NSCLC models and in a large clinical cohorts of paired NSCLC specimens from EGFR-TKI-treated patients demonstrated an upregulation of AXL tyrosine kinase receptor or of its ligand, GAS6, in resistant samples. Pharmacological inhibition of AXL significantly decreased the proliferative and invasive abilities of cancer cells and increased their chemosenstivity through inhibition of AKT and MAPK pathways. Therefore, an EMT-associated transcriptional programme involving upregulation of vimentin may, in part, drive AXL overexpression in EGFR-mutant lung cancer cells with acquired EGFR-TKI resistance.40
Another pathway recently identified in the EMT setting and responsible for acquisition of resistance to first-generation EGFR-TKIs is the Hedgehog (Hh) pathway,41 whose activation has been implicated in tumourigenesis, metastatisation and progression along with cancer stem-like cell maintenance and treatment resistance in several types of human cancer. More deeply, gene amplification of the Hh receptor, SMO, concomitantly with MET activation, has been recently identified, for the first time, as a novel mechanism of acquired resistance to EGFR-TKI in EGFR-mutant NSCLC cells. Hh-mediated acquisition of EGFR-TKI resistance was concomitant with the mesenchymal shift of EGFR-mutated NSCLC cells, which displayed higher invasive and metastatic abilities. These preclinical results are in agreement with the results of a cohort of patients with EGFR-mutant NSCLC that were treated with EGFR-TKIs.42 Gianikopoulos et al demonstrated the presence of SMO gene amplification in tumour biopsies that were taken at the clinical occurrence of resistance to EGFR-TKIs in 2 of the 16 patients. In both cases, the MET gene was also amplified. In this respect, the combined inhibition of both SMO and MET exerted a significant antiproliferative and proapoptotic effect concomitantly with the loss of mesenchymal features in preclinical models of acquired resistance to EGFR-TKIs in EGFR-mutated NSCLC cells, suggesting new combination strategies at the occurrence of resistance. Consistently with these results, Bai et al43 found that the Hh signalling pathway was inappropriately activated in EGFR-TKI-resistant NSCLC cells, accompanied by EMT induction and ATP-binding cassette subfamily G member 2 (ABCG2) overexpression. The combined inhibition of Hh and EGFR pathways markedly inhibited tumourigenesis and proliferation, reverted mesenchymal phenotype by restoring E-cadherin expression and downregulated Snail and ABCG2 in EGFR-TKI-resistant cells.
These findings confirmed that upregulation of Hh signalling resulted in EGFR-TKI resistance, by EMT induction, and inhibition of Hh signalling increased sensitivity to EGFR-TKI.43
3. Activation of alternative pathways
Activation of alternative pathways represents the second most common resistance mechanism to EGFR-TKIs. In particular, amplification of the MET oncogene, described for the first time in 2007,44 accounts for 5–20% of acquired resistance causes. MET is a transmembrane tyrosine kinase receptor that, once activated by its ligand, the hepatocyte growth factor (HGF, also known as the scatter factor), promotes the activation of the downstream AKT pathway, which is the key signalling pathway for cell proliferation, survival and antiapoptosis. Uncontrolled activation of MET is oncogenic and facilitates the invasive and metastatic behaviour of EGFR-TKI resistant cells.44
MET overactivation in most EGFR-TKI acquired resistant tumours occurs via increased transcription and expression of MET protein while MET gene amplification is detected in 22% of cases. In addition, over 20 oncogenic mutations have been identified in MET and the majority of them were found to be germline mutations. The most frequent mutations in NSCLC are in the semaphorin domain (affecting HGF binding), the juxtamembrane domain (affecting the actin cytoskeleton, cell motility and migration) and the TK domain (activating MET even in the absence of HGF).
The overexpressed MET receptor leads to a persistent ErbB3-AKT signalling by maintaining ErbB phosphorylation despite the presence of the EGFR blockade. ErbB3 is a tyrosine kinase receptor of the ErbB family, which can form homodimers or heterodimers with ErbB2 to transduce growth signals. Overexpression of the HGF has also been shown to induce resistance to EGFR-TKIs. The efficacy of MET signalling inhibitors, TKIs or monoclonal antibodies against the receptor or the ligand, is still an object of investigation as the most informative method to define MET amplification is not established yet.45
Similarly, while amplification of the ErbB2 gene is a rare event in untreated adenocarcinoma (1% of the cases), it has been responsible for acquisition of resistance in 12% of cases.46 Moreover, ErbB2 mutations also occurred in about 2% of patients with NSCLC, more frequently in never smokers with adenocarcinoma histology, oriental ethnicity and female gender; they are located in exon 20, encoding for the kinase domain of the ErbB2 protein. Different from the other family members, ErbB2 has strong kinase activity but has no identified ligand-binding domain. Thus, it has to form heterodimers with the other family members to activate. The dependence of ErbB2 activation on the transphosphorylation of EGFR determines the strong inhibition by EGFR-TKIs on the WT ErbB2. However, when ErbB2 mutates in the kinase domain, it becomes EGFR-independent and induces resistance to EGFR-TKIs. Previous studies have postulated conflicting data on the role of EGFR heterodimers in mediating sensitivity to EGFR-TKIs in EGFR-mutant lung cancer: immunoprecipitation studies suggest that mutant EGFRs, especially the L858R/T790M variant, have a propensity to heterodimerise with ErbB2, which allows for evasion of CBL-mediated ubiquitinylation and the subsequent lysosomal degradation.47 Therefore, a hypothesis still to demonstrate, might be that mutant cancer cells can become resistant either by acquiring the T790M mutation which enhances ErbB2 heterodimerisation in the absence of ErbB2 amplification, or by acquiring ErbB2 amplification in the absence of a second-site mutation.
Both amplifications of MET and ErbB2 have been described as mechanisms of acquired resistance to the third-generation inhibitor AZD9291, concomitantly with the loss of T790M.48
Another alternative pathway involved in EGFR-TKI resistance is the insulin-like growth factor 1 receptor (IGF-1R), whose activation has been detected in multiple gefitinib or erlotinib resistant lung cancer lines.49 The interplay between the IGF-1R and EGFR pathways seems to be not completely understood. A known mechanism is that IGF-1R could be activated by heterodimerisation with EGFR after erlotinib treatment49 transmitting extracellular survival signals to downstream mediators such as AKT and MAPK. Co-treatment of IGF-1R inhibitors such as α-IR3, AG1024 or R1507 with EGF-TKIs-enhanced TKI-induced growth inhibition and apoptosis, offering a potential new approach to overcome the resistance of EGFR-TKIs in NSCLC.50
Activation of other cell receptors such as FGFR1,2,351 or of cell signalling pathways such as BRAF (Val600Glu, Gly469Ala) mutation (1%)52 and PIK3CA mutation (5%)34, that play a key role in promoting the proliferation, survival, drug resistance of cancer cells, have been described. In particular, AKT activation can be tied to AKT gene mutation, mutations and amplifications of PIK3CA (the gene encoding the main catalytic subunit of PI3K) as well as loss or reduced expression of PTEN. Preclinical data confirmed that P110α E545K, a PIK3CA oncogenic mutation, resulted in dramatically suppressed sensitivity to gefitinib.53
Early translational studies demonstrated that a mutant EGFR receptor drives expression of programmed death ligand-1 (PD-L1) and that blockade of the programmed death receptor-1 (PD-1) improved survival of mice with EGFR-mutant tumours.54
Therefore, another potential strategy is the use of immune checkpoint inhibitors such as PD-1 pathway inhibitors. Indeed, preliminary results of a study investigating the combination of nivolumab (anti-PD-1 monoclonal antibody) and erlotinib reported an overall reposnse rate (ORR) of 19% with the majority of responders having previously progressed while receiving erlotinib.55 Nivolumab has been recently approved by the Food and Drug Administration (FDA) for the treatment of patients with squamous NSCLC after failure of chemotherapy. Further investigations of nivolumab as monotherapy or in combination with EGFR-TKI in patients with NSCLC and EGFR mutations will provide further insight into the role of immunotherapy (NCT02323126).
Conclusions
Heterogeneous tumours, such as NSCLC, are composed of multiple subclones and under selection pressures, such as the EGFR inhibition, clones with either intrinsic or acquired resistance can be selected and drive disease progression. The analysis of multiple biopsies from the same tumour during the time and treatments reveal the evolutionary trajectory of these subclones, where clonal mutations present in all tumour regions, and eventually persisting during treatments, such as activating EGFR mutations, occur early in tumourigenesis representing the most recent common ancestor (truncal events on the evolutionary tree), whereas subclonal mutations present in only a subset of regions, or cells within a single biopsy, occur later in tumourigenesis (branched events on the evolutionary tree) and can be lost after specific therapies (such as the T790M mutation). Further studies on the mechanisms by which subclonal alterations have an impact on tumour biology and phenotype and influence progression suggest challenges for predictive and prognostic implications. However, serial tumour sampling to monitor clonal evolution poses practical challenges and is currently not standard practice. An alternative approach may be the use of ‘liquid biopsies’, whereby circulating cell-free tumour DNA (cfDNA) or circulating tumour cells (CTCs) are analysed in the peripheral blood of patients with cancer. Liquid biopsies have the potential to inform early detection of cancer, to detect minimal residual disease, to mirror the heterogeneity of tumour and track evolution of resistant disease and therefore detect early relapse.
Since introduction of third generation EGFR TKIs requires monitoring the occurrence of T790M mutation for the pianification of treatment at resistance, this noninvasive approach will be useful for the clinical practice treatment at resistance.53 56 Of interest, cfDNA has also applied to explore novel mechanism of acquired resistance to third-generation EGFR-TKI. Identification of the C797S mutation in patients with lung cancer, whose tumours had developed resistance to AZD9291, was identified by Thress et al57 using NGS in cfDNA samples. Thus, sequencing analysis of cfDNA after the initiation of EGFR-TKI, either early generation or third generation, can ultimately provide information in the dynamic mutation profile. Since deeper knowledge on the subclonal composition of NSCLC will be obtained, different treatment approaches can be considered.
Adoptive therapy concept has been proposed by Gatenby et al,58 whereby treatment-sensitive cells are initially targeted, resulting in the likely expansion of treatment-resistant cells that are subsequently targeted having predicted beforehand their likely mechanism of resistance.
Another possible strategy in order to maximise the survival benefit from first-line treatment in patients with NSCLC and EGFR mutations and delay the occurrence of resistance is an upfront EGFR-TKI-based combination therapy, including combinations of EGFR inhibitors with various targeted agents, chemotherapy or even immunotherapy.
In any case, to obtain meaningful results, careful selection of patients and design of randomised clinical trials is of primary importance.
Footnotes
Twitter: Follow Carminia Della Corte at @Carminia Della Corte
Funding: This work has been supported by Associazione Italiana Per La Ricerca Sul Cancro (AIRC)-Project MFAG 2013-N.14392.
Competing interests: None declared.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1. SEER Stat Fact Sheets: Lung and Bronchus Cancer. National Cancer Institute. http://seer.cancer.gov/statfacts/html/lungb.html.
- 2.Lynch TJ, Bell DW, Sordella R et al. . Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–39. 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- 3.Paez JG, Jänne PA, Lee JC et al. . EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–500. 10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu YL, Thongprasert S et al. . Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T, Morita S, Yatabe Y et al. . West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8. [DOI] [PubMed] [Google Scholar]
- 6.Maemondo M, Inoue A, Kobayashi K et al. . North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Wu YL, Chen G et al. . Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 8.Han JY, Park K, Kim SW et al. . First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122–8. 10.1200/JCO.2011.36.8456 [DOI] [PubMed] [Google Scholar]
- 9.Rosell R, Carcereny E, Gervais R et al. . Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46. [DOI] [PubMed] [Google Scholar]
- 10.Sequist LV, Yang JC, Yamamoto N et al. . Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34. [DOI] [PubMed] [Google Scholar]
- 11.Wu YL, Zhou C, Hu CP et al. . Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213–22. 10.1016/S1470-2045(13)70604-1 [DOI] [PubMed] [Google Scholar]
- 12.Yang JC, Wu YL, Schuler M et al. . Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141–51. 10.1016/S1470-2045(14)71173-8 [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi S, Boggon TJ, Dayaram T et al. . EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786–92. 10.1056/NEJMoa044238 [DOI] [PubMed] [Google Scholar]
- 14.Zhou W, Ercan D, Chen L et al. . Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 2009;462:1070–4. 10.1038/nature08622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross DAE, Ashton SE, Ghiorghiu S et al. . AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046–61. 10.1158/2159-8290.CD-14-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soria J, Sequist LV, Goldman JW et al. . Interim phase 2 results of study CO-1686-008: a phase 1/2 study of the irreversible, mutant selective, EGFR inhibitor rociletinib (CO-1686) in patients with advanced non small cell lung cancer. Eur J Cancer 2014;50:199 10.1016/S0959-8049(14)70731-2 [DOI] [Google Scholar]
- 17.Tan CS, Cho BC, Soo RA. Next-generation epidermal growth factor receptor tyrosine kinase inhibitors in epidermal growth factor receptor-mutant non-small cell lung cancer. Lung Cancer 2016;93:59–68. [DOI] [PubMed] [Google Scholar]
- 18.Murakami H, Nokihara H, Shimizu T et al. . 9LBA Antitumor activity of ASP8273, an irreversible mutant selective EGFR-TKI, in NSCLC patients with tumors harboring EGFR activating mutations and T790M resistance mutation. Eur J Cancer 2014;50:198. [Google Scholar]
- 19.Eck MJ, Yun CH. Structural and mechanistic underpinnings of the differential drug sensitivity of EGFR mutations in non-small cell lung cancer. Biochim Biophys Acta 2010;1804:559–66. 10.1016/j.bbapap.2009.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa DB, Yasuda H, Sng NY. Sensitivity to EGFR inhibitors based on location of EGFR exon 20 insertion mutations within the tyrosine kinase domain of EGFR. J Clin Oncol 2012;30:110–12. 10.1200/JCO.2011.39.4486 [DOI] [PubMed] [Google Scholar]
- 21.De Pas T, Toffalorio F, Manzotti M et al. . Activity of epidermal growth factor receptor-tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring rare epidermal growth factor receptor mutations. J Thorac Oncol 2011;6:1895–901. 10.1097/JTO.0b013e318227e8c6 [DOI] [PubMed] [Google Scholar]
- 22.Costa C, Molina MA, Drozdowskyj A et al. . The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or 35 chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res 2014;20:2001–10. 10.1158/1078-0432.CCR-13-2233 [DOI] [PubMed] [Google Scholar]
- 23.Ji H, Zhao X, Yuza Y et al. . Epidermal growth factor receptor variant III mutations in lung tumorigenesis and sensitivity to tyrosine kinase inhibitors. Proc Natl Acad USA 2006;103:7817–22. 10.1073/pnas.0510284103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan J, Wang Z, Bai H et al. . Epidermal growth factor receptor variant III mutation in Chinese patients with squamous cell cancer of the lung. Thorac Cancer 2015;6:319–26. 10.1111/1759-7714.12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faber AC, Corcoran RB, Ebi H et al. . BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov 2011;1:352–65. 10.1158/2159-8290.CD-11-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park KS, Raffeld M, Moon YW et al. . CRIPTO1 expression in EGFR-mutant NSCLC elicits intrinsic EGFR-inhibitor resistance. J Clin Invest 2014;124:3003–15. 10.1172/JCI73048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackman D, Pao W, Riely GJ et al. . Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 2010;28:357–60. 10.1200/JCO.2009.24.7049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandara DR, Li T, Lara PN et al. . Acquired resistance to targeted therapies against oncogene-driven non-small-cell lung cancer: approach to subtyping progressive disease and clinical implications. Clin Lung Cancer 2014;15:1–6. 10.1016/j.cllc.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun C-H, Mengwasser KE, Toms A V et al. . The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci USA 2008;105:2070–5. 10.1073/pnas.0709662105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oxnard GR, Arcila ME, Sima CS et al. . Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616–22. 10.1158/1078-0432.CCR-10-2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hata AN, Niederst MJ, Archibald HL et al. . Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med 2016;22:262–9. 10.1038/nm.4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu HA, Tian SK, Drilon AE et al. . Acquired resistance of EGFR-mutant lung cancer to a T790M-specific EGFR inhibitor: emergence of a third mutation (C797S) in the EGFR tyrosine kinase domain. JAMA Oncol 2015;1:982–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niederst MJ, Hu H, Mulvey HE et al. . The allelic context of the C797S mutation acquired upon treatment with third generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res 2015;21:3924–33. 10.1158/1078-0432.CCR-15-0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sequist LV, Waltman BA, Dias-Santagata D et al. . Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26 10.1126/scitranslmed.3002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutherland KD, Proost N, Brouns I et al. . Cell of origin of small lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 2011;19:754–64. 10.1016/j.ccr.2011.04.019 [DOI] [PubMed] [Google Scholar]
- 36.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 2003;15:740–6. 10.1016/j.ceb.2003.10.006 [DOI] [PubMed] [Google Scholar]
- 37.Kang Y, Massagué J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 2004;118:277–9. [DOI] [PubMed] [Google Scholar]
- 38.Herbst RS, Prager D, Hermann R et al. . TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2005;23:5892–9. [DOI] [PubMed] [Google Scholar]
- 39.Yauch RL, Januario T, Eberhard DA et al. . Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res 2005;11:8686–98. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z, Lee JC, Lin L et al. . Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 2012;44:852–60. 10.1038/ng.2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Della Corte CM, Bellevicine C, Vicidomini G et al. . SMO gene amplification and activation of the hedgehog pathway as novel mechanisms of resistance to anti-epidermal growth factor receptor drugs in human lung cancer. Clin Cancer Res 2015;21:4686–97. 10.1158/1078-0432.CCR-14-3319 [DOI] [PubMed] [Google Scholar]
- 42.Gianikopoulos B, Wellde H, Polkinghorn P et al. . Integrated genomic analysis by whole exome and transcriptome sequencing of tumor samples from EGFR-mutant non-small-cell lung cancer (NSCLC) patients with acquired resistance to erlotinib [abstract]. Proceedings of the 15th World Conference on Lung Cancer; 2013 Oct 27–31; Sydney, Australia; 2013. Abstract nr 1426. [Google Scholar]
- 43.Bai XY, Zhang XC, Yang SQ et al. . Blockade of Hedgehog signaling synergistically increases sensitivity to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer cell lines. PLoS ONE 2016;11:e0149370 10.1371/journal.pone.0149370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engelman JA, Zejnullahu K, Gale CM et al. . PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 2007;67:11924–32. 10.1158/0008-5472.CAN-07-1885 [DOI] [PubMed] [Google Scholar]
- 45.Fan W, Tang Z, Yin L et al. . MET-independent lung cancer cells evading EGFR kinase inhibitors are therapeutically susceptible to BH3 mimetic agents. Cancer Res 2011;71:4494–505. 10.1158/0008-5472.CAN-10-2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takezawa K, Pirazzoli V, Arcila ME et al. . HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov 2012;2:922–33. 10.1158/2159-8290.CD-12-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shtiegman K, Kochupurakkal BS, Zwang Y et al. . Defective ubiquitinylation of EGFR mutants of lung cancer confers prolonged signaling. Oncogene 2007;26:6968–78. 10.1038/sj.onc.1210503 [DOI] [PubMed] [Google Scholar]
- 48.Oxnard GR, Thress K, Paweletz C et al. . Mechanisms of acquired resistance to AZD9291 in EGFR T790M positive lung cancer. J Thorac Oncol 2015;10(Suppl 2):ORAL17.07. [Google Scholar]
- 49.Morgillo F, Woo JK, Kim ES et al. . Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res 2006; 66:10100–11. 10.1158/0008-5472.CAN-06-1684 [DOI] [PubMed] [Google Scholar]
- 50.Choi YJ, Rho JK, Jeon BS et al. . Combined inhibition of IGFR enhances the effects of gefitinib in H1650: a lung cancer cell line with EGFR mutation and primary resistance to EGFR-TK inhibitors. Cancer Chemother Pharmacol 2010;66:381–8. 10.1007/s00280-009-1174-7 [DOI] [PubMed] [Google Scholar]
- 51.Ware KE, Marshall ME, Heasley LR et al. . Rapidly acquired resistance to EGFR tyrosine kinase inhibitors in NSCLC cell lines through de-repression of FGFR2 and FGFR3 expression. PLoS ONE 2010;5:e14117 10.1371/journal.pone.0014117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohashi K, Sequist LV, Arcila ME et al. . PNAS plus: lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci USA 2012;109:E2127–33. 10.1073/pnas.1203530109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taniguchi K, Uchida J, Nishino K et al. . Quantitative detection of EGFR mutations in circulating tumor DNA derived from lung adenocarcinomas. Clin Cancer Res 2011;17:7808–15. [DOI] [PubMed] [Google Scholar]
- 54.Akbay EA, Koyama S, Carretero J et al. . Activation of the PD-1 pathway contributes to immune escape in EGFR- driven lung tumors. Cancer Discov 2013;3:1355–63. 10.1158/2159-8290.CD-13-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rizvi NA, Chow LQM, Borghaei H et al. . Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC (abstract). J Clin Oncol 2014;32(Suppl):802283. [Google Scholar]
- 56.Murtaza M, Dawson SJ, Tsui DW et al. . Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 2013;497:108–12. [DOI] [PubMed] [Google Scholar]
- 57.Thress KS, Paweletz CP, Felip E et al. . Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21: 560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer 2012;12:487–93. 10.1038/nrc3298 [DOI] [PMC free article] [PubMed] [Google Scholar]