Abstract
Understanding the early evolution of cancer heterogeneity during the initial steps of tumorigenesis can uncover vulnerabilities of cancer cells that may be masked at later stages. We describe a comprehensive approach employing gene expression analysis in early lesions to identify novel therapeutic targets and the use of mouse models to test synthetic lethal drug combinations to treat human Kirsten rat sarcoma viral oncogene homologue (KRAS)-driven lung adenocarcinoma.
Keywords: KRAS, Lung adenocarcinoma
Introduction
Understanding the early evolution of cancer heterogeneity during the initial steps of tumorigenesis can uncover vulnerabilities of cancer cells that may be masked at later stages. We describe a comprehensive approach employing gene expression analysis in early lesions to identify novel therapeutic targets and the use of mouse models to test synthetic lethal drug combinations to treat human Kirsten rat sarcoma viral oncogene homologue (KRAS)-driven lung adenocarcinoma.
Non-small cell lung cancer classification
Lung cancer is the leading cause of cancer-related mortality worldwide, with an average 5-year survival of 15%.1 Non-small cell lung carcinoma (NSCLC), the most common type of lung cancer, encompasses three distinct histological subtypes: adenocarcinoma, squamous carcinoma and large cell carcinoma. During the previous decade, lung adenocarcinomas have been further subclassified based on the presence of driver mutations occurring in oncogenes such as KRAS, BRAF and EGFR or gene rearrangements in the ALK and ROS1 loci.2 Though KRAS mutations were the first genetic lesions identified in lung adenocarcinoma, having been discovered over 30 years ago,3 the clinical value of determining the mutational status of KRAS in tumours is still controversial. Several studies have shown that lung adenocarcinomas harbouring KRAS mutations have worse overall survival.4–6 However, in a large study based on 1500 resected NSCLC, KRAS mutations were neither prognostic nor predictive for outcome after adjuvant chemotherapy.7 More recent studies indicate that KRAS mutations are not predictive for therapeutic benefit for either docetaxel or erlotitinib.8 Given the lack of consistent prognostic value associated with KRAS mutational status alone, there is an urgent need to identify additional genetic alterations and therapeutic targets in patients with KRAS-mutant lung adenocarcinoma.
KRAS-mutant lung adenocarcinoma
KRAS is the most commonly mutated oncogene in lung adenocarcinoma, with mutations detected in about 30% of patients. Though the recent development of KRASG12C allosteric inhibitors offers promise, significant previous efforts to fully develop drugs that directly target mutant KRAS have largely failed, highlighting the need for alternative therapeutic approaches.9 10 Studies employing genetically engineered mouse (GEM) tumour models11 have recently identified novel targets in Kras-mutated adenocarcinomas and inhibitors for some of these targets have already entered clinical trials, though their clinical efficacy remains to be established.12 One such study using GEMs demonstrated that lung adenocarcinomas with activated Kras were very sensitive to the MEK inhibitor selumetinib in combination with docetaxel treatment. However, tumours with mutated Kras and Lkb1 loss did not benefit from the addition of selumetinib to docetaxel.13 In a phase II clinical trial on patients with lung cancer, selumetinib plus docetaxel significantly improved progression-free survival in patients with KRAS-mutant lung adenocarcinoma resulting in a trend towards increased overall survival.14 However, there was greater toxicity, as indicated by febrile neutropenia, diarrhoea, nausea, vomiting and rash in patients treated with combination therapy compared to those treated with single agents. Another ongoing randomised phase III clinical trial is currently being carried out in patients with NSCLC with KRAS mutations to compare the efficacy of abemaciclib (a potent CDK4/6 inhibitor) with the best supportive care (BSC) versus erlotinib with BSC.15 This study is based on the synthetic lethality observed between oncogenic KRAS and CDK4 inhibition in murine preclinical models.16
Lung tumour heterogeneity
The results summarised above collectively suggest that human KRAS mutant tumours have diverse treatment responses, which depend on their genetic background. Indeed, advanced lung adenocarcinomas carry a substantial degree of genetic heterogeneity, which represents a major obstacle to therapeutic success, and a potential source of relapse.17 During the past few years, next-generation sequencing has uncovered a large number of previously unknown alterations in lung adenocarcinoma, and this number will undoubtedly grow as more tumours are analysed.12 A fundamental challenge that remains is how to differentiate aberrations that are functionally relevant for lung carcinogenesis and/or treatment response from non-relevant passenger mutations. Although genetic heterogeneity exists in the majority of solid tumours, it presents even greater difficulties in lung adenocarcinoma, which has the highest burden of somatic mutations following malignant melanoma.18 This genetic heterogeneity in tumours is likely to impact gene transcription and may well have hindered previous attempts to clearly identify oncogene-specific gene expression signatures in human lung cancers carrying KRAS mutations, which only became apparent when compared to signatures present in Kras-driven mouse tumours.19 Additionally, the fact that gene expression signatures from KRAS wild-type tumours have more prognostic power than those from KRAS mutant tumours most likely reflects increased genetic heterogeneity in the latter,20 presenting a particular challenge for the identification of therapeutic targets in lung adenocarcinomas with activated KRAS.21
It was recently proposed that future clinical trials could target early founder events in tumour evolution,22 which would presumably affect a greater majority of cells within the tumour and decrease chances of relapse. Such early molecular aberrations could represent tumour-driving alterations that would be difficult to identify within the complex heterogeneity of late stage tumours. This approach would therefore require molecular analysis of premalignant lesions, an approach that is not feasible in humans for obvious reasons. In this overview, we discuss the utility of analysing early neoplastic lesions in mouse models in order to discover new oncogenic drivers in Kras-driven lung adenocarcinoma.
Early lung lesions analysis
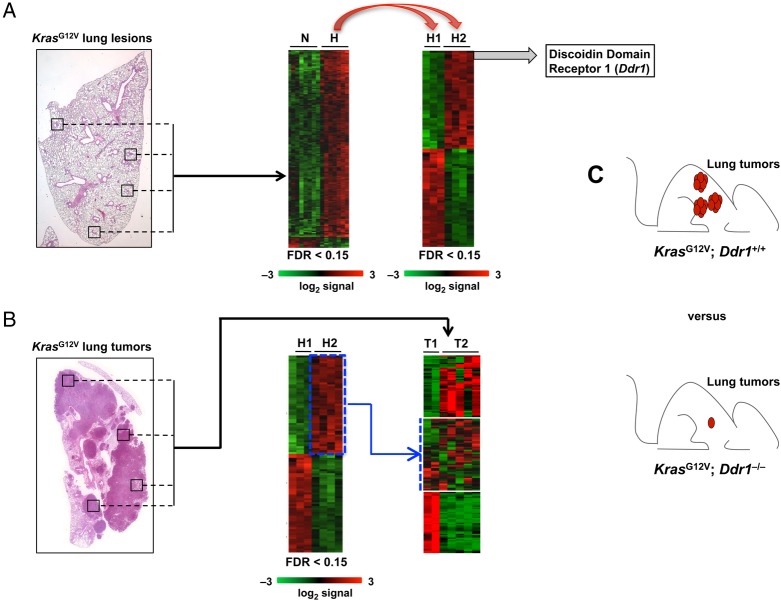
The molecular events occurring immediately after activation of most endogenous oncogenic drivers in vivo are mostly unknown. We reasoned that the study of the early steps of lung tumour progression in our Cre-inducible KrasG12V mouse model23 could uncover novel oncogenic drivers which might be obscured by complex heterogeneity in full-blown tumours and which might represent novel therapeutic targets. Following expression of the KrasG12V oncogene in alveolar type 2 (AT2) cells in mice, we isolated early lung hyperplastic lesions not larger than 500 cells by laser capture microdissection and analysed gene expression by standard Affymetrix array technology.24 Interestingly, although all of these early hyperplastic lesions had identical gross histology, we identified two distinct molecular subgroups. One subgroup displayed substantial homology with a previously described gene expression signature present in human and mouse advanced lung adenocarcinomas19 (figure 1A). We designated the tumours identified by this molecular signature as H2. The second subgroup (H1), displayed a signature similar to that obtained from the neighbouring normal alveolar parenchyma. We next compared the expression signatures of H1 and H2 to those of full-blown adenocarcinomas 10 months after activation of Kras by Ad-Cre infection. Our advanced adenocarcinomas exhibited an expression profile similar to that previously reported in adenocarcinomas with KRAS activation across species.19 We additionally found our advanced adenocarcinomas could be clustered into two different subgroups that we designated as T1 and T2. According to Gene Set Enrichment Analysis (GSEA), one expression signature correlated with the profile of H1 and was designated as T1. It is reasonable to argue that this signature is associated with lesions that did not undergo major transcriptional changes during tumour progression. While the second signature, designated as T2, still maintained a significant correlation with the profiling observed in advanced human and murine lung adenocarcinomas19 the top 50 up-regulated genes that defined H2 were not shared by the T2 signature, suggesting that the major molecular aberrations defining the H2 signature were obscured by the increasing molecular complexity in T2 adenocarcinomas (figure 1B). Taken together, these findings suggest that the aggressive nature of advanced tumours is determined early regardless of the mutations and transcriptional changes that may occur during tumour progression. These findings additionally highlight the advantage of analysing early hyperplastic lesions to identify tumour-driving events that might be masked by the molecular heterogeneity that is present in more advanced KRAS-driven lung.
Figure 1.
Early lesion analysis to identify potential targets for therapeutic intervention. (A). KrasG12V-driven early lung lesions (≤500 cells) were microdissected and analysed by standard Affymetrix technology. Tumour signatures clustered in two distinct groups, one of which resembled normal lung alveolar cells (H1) and the other one aggressive murine and human lung adenocarcinoma (H2). The top-scoring gene of the H2 signature was the Discoidin Domain Receptor 1 (DDR1). (B). KrasG12V-driven lung tumours (10 months after Ad-Cre infection) were isolated and analysed by standard Affymetrix technology. Tumour signatures clustered in two distinct groups, T1 and T2. H2 signature was diluted in the T2 signature. (C). KrasG12V;Ddr1−/− mice survived longer and have reduced tumour size/number compared to control KrasG12V; Ddr1+/+ mice.
Target validation
If early Kras oncogenic mediators are important for both tumour onset and tumour progression, then the majority of cells in full-blown lung tumours should depend on these mediators for growth and survival. One such candidate mediator identified by our analysis was the Discoidin Domain Receptor 1 (Ddr1) gene, which was the top-scoring upregulated gene in our ‘early aggressive’ hyperplastic H2 signature, and was also a particularly interesting therapeutic target because it encodes for a receptor tyrosine kinase (thus, potentially druggable). DDR1 becomes activated on collagen binding which allows it to control the remodelling of the extracellular matrix and cell migration. Following binding to collagen, Ddr1 triggers the activation of several downstream signalling pathways such as MAPK, PI3K and Notch pathways25—which have been linked to the development of a variety of cancers, including lung, breast, brain, prostate, liver and pancreas as well as lymphoma and leukaemia.25–27 Intriguingly, DDR1 has already been identified by a quantitative phosphoproteomic screening as the most abundant phosphorylated protein in human NSCLC28 and has been shown to correlate with poor prognosis in human lung adenocarcinoma.29 However, the role of DDR1 in lung cancer progression and its potential as a therapeutic target is currently unknown. In order to investigate the role of Ddr1 in KrasG12V-driven lung adenocarcinoma we crossed our inducible KrasG12V knocked-in model with Ddr1−/− mice.30 KrasG12V; Ddr1−/− mice exhibited longer survival, displayed decreased tumour number and size and had a higher adenoma to adenocarcinoma ratio than KrasG12V; Ddr1+/+ control mice, suggesting that the absence of Ddr1 impairs adenocarcinoma progression (figure 1C).
To explore the possibility that pharmacological Ddr1 inhibition in vivo is a feasible strategy to treat lung adenocarcinomas in humans, we carried out preclinical studies in mice. To do this, we treated mice bearing KrasG12V-driven lung tumours over a period of two months with the recently developed oral Ddr1 inhibitor 7rh31 and measured tumour progression by CT scanning. Interestingly, tumours demonstrating the most significant responses at the end of the treatment were those expressing high levels of the Ddr1 receptor by immunohistochemistry staining.24 In order to look for synthetic lethal drug combinations that could boost the therapeutic efficacy of the Ddr1 inhibitor alone we considered the pathways known to be regulated by Ddr1 in cancer cells. We chose the Notch pathway given that it is required for Ddr1-mediated prosurvival signalling32 and is also necessary for Kras-driven lung adenocarcinoma progression.33 Combined treatment with Ddr1 and Notch inhibitors was more potent than Ddr1 inhibitor alone in inducing apoptosis and impaired tumour growth even in the more aggressive KrasG12V; Trp53-null lung adenocarcinomas. Hence, both genetic and pharmacological inhibition of Ddr1 in Kras-driven lung adenocarcinoma impedes tumour progression.
Orthotopic lung PDX
Patients carrying KRAS-mutant lung adenocarcinoma are currently treated with platinum-doublet chemotherapy (eg, carboplatin and paclitaxel plus bevacizumab or cisplatin plus pemetrexed) as first-line treatment. In order to compare the efficacy of our therapy with the current standard of care treatment, mice bearing KrasG12V; Trp53-null lung tumours were treated in parallel with either Ddr1/Notch targeted therapy or standard chemotherapy. CT scan imaging revealed that the combined targeted therapy is at least comparable to standard chemotherapy in terms of tumour volume reduction, but histopathological analysis showed that Ddr1/Notch therapy is more potent in inducing tumour necrosis.24
Finally, we extrapolated our findings in mice to human disease by developing an orthotopic model of patient-derived lung xenografts (ortho-PDX). Of note, this therapeutic validation was accomplished with a combination of drugs that are currently either Food and Drug Administration (FDA) approved (dasatinib to target DDR1) or in advanced clinical evaluation (demcizumab, an anti-DLL4 antibody to interfere with Notch signalling), which would permit for rapid testing in the clinical trials.
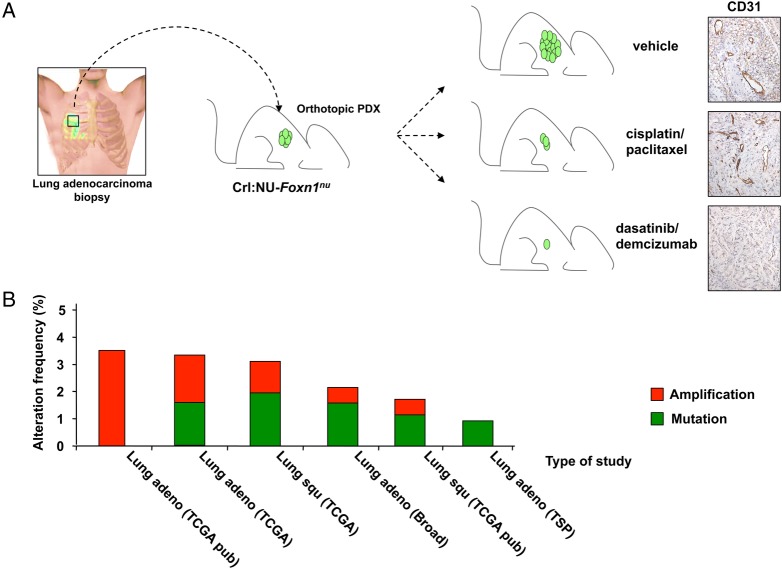
In three ortho-PDXs, each derived from different patients carrying concomitant KRAS mutations and TP53 deletions, we observed that combined inhibition of DDR1/Notch1 signalling dampened important signalling pathways required for tumour progression and survival to a greater extent than that achieved by standard chemotherapy. This was accompanied by increased apoptosis and necrosis resulting in a substantial reduction in tumour volume. Furthermore, follow-up assessment by positron emission tomography (PET) demonstrated a long-lasting response to dasatinib/demcizumab compared to standard chemotherapy (figure 2A). Importantly, the treatment with dasatinib/demcizumab significantly delayed the re-emergence of tumour growth when compared with standard chemotherapy following discontinuation of both regimens. This observation supports the notion that targeting oncogenic events present in early aggressive lesions, such as DDR1 upregulation, can be an efficacious therapeutic strategy in advanced tumours that prevents disease relapse.
Figure 2.
Therapeutic validation in orthotopic lung PDX. (A). Biopsies from patients carrying KRAS-mutant;TP53-deficient lung adenocarcinomas were orthotopically implanted in Crl:NU-Foxn1nu mice and subjected to treatment with either vehicle, cisplatin/paclitaxel chemotherapy or dasatinib/demcizumab. Positron emission tomography (PET) follow-up demonstrated a better response when compared with chemotherapy. CD31 immunostaining showed a substantial decrease of the endothelial compartment in tumours subjected to the combined therapy. (B). Percentages of alteration frequencies identified in different NSCLC studies obtained from the TCGA database (http://www.cbioportal.org). Red=gene amplification; green=mutation. PDX, patient-derived xenograft; NSCLC, non-small cell lung carcinoma.
Discussion
By analysing early hyperplastic lesions in AT2 cells with activation of a resident KrasG12V oncogene, we uncovered two distinct transcriptional signatures that arise early during tumorigenesis that we have designated as H1 and H2. Whereas the H1 signature closely resembles that of normal lung cells, that of H2 is highly related to a signature previously identified in advanced lung adenocarcinomas of both mouse and human origin.19 These distinct transcriptional profiles are not due to histological differences within the early hyperplastic areas, supported by the fact that all cells within the foci unambiguously expressed the AT2-specific marker surfactant protein C (SPC).34 It has been recently reported that a minor subpopulation of adult progenitor AT2 cells function as lung stem cells, which self-renew on oncogenic Kras expression.35 Our aggressive H2 signature displayed significant overlap with that of this progenitor AT2 cell population, whereas H1 did not. Interestingly, we found early hyperplastic lesions with the progenitor-like H2 signature at a similar frequency to those with an H1 signature. Given the rare frequency of the progenitor population in normal lung, this could be explained by a greater susceptibility of progenitors to Ras-mediated transformation. However, we cannot exclude the possibility that AT2 cells, conventionally described as a single SPC-positive cell population, might comprise different, yet unidentified subpopulations of cells with variable responses on oncogenic Kras activation. Indeed, preliminary data from our laboratory using a Ddr1 probe suggest that not all AT2 cells express Ddr1 mRNA, thus raising the possibility that SPC+ AT2 cells do not represent a homogeneous population.
Cancer heterogeneity arises from the selective outgrowth of distinct subclones within a tumour that have acquired distinct molecular profiles that confer a proliferative and survival advantage.22 36 In our study we utilised an unbiased gene expression profiling approach to formally demonstrate that inter-tumour heterogeneity, at least in KrasG12V-driven lung adenocarcinoma, appears early during tumour evolution. Of the two signatures identified, the H1 profile remained stable over time and was detectable even in late stage adenocarcinomas months after oncogene expression. The stability of this H1 signature, which probably represents a less aggressive form of the disease, suggests that the progression towards an aggressive phenotype is not merely a time-dependent process that invariably occurs following oncogene activation. In contrast, the H2 signature became masked as early hyperplastic lesions progressed to yield advanced adenocarcinomas so much so that Ddr1, the top marker in early H2 hyperplastic lesions, was no longer recognised as a highly up-regulated gene in advanced T2 tumours. This is probably due to the high degree of variability of Ddr1 levels in late stage tumours and underscores the necessity to perform molecular profiling in early hyperplastic lesions due to the limitations posed by tumour heterogeneity in full-blown tumours. In addition to being the most highly upregulated gene in early aggressive lesions, the fact that Ddr1 is the most hyper-phosphorylated protein in advanced human lung tumours28 suggested that it could be a valid therapeutic target. To test this hypothesis, we genetically and pharmacologically inhibited Ddr1 in murine models of lung cancer. Ddr1 deficiency significantly impaired tumour development and progression of KrasG12V-driven lung adenocarcinomas in a Trp53-proficient background, leading to prolonged overall survival. Pharmacological inhibition of Ddr1 with the selective Ddr1 inhibitor 7rh also yielded a favourable therapeutic response. Importantly, Ddr1 seems to exert a dose-dependent effect on tumour growth as suggested by the fact that even Ddr1+/− mice showed intermediate improved survival between that of Ddr1+/+ and Ddr1−/− mice, indicating that even partial pharmacological inhibition of Ddr1 is likely to reduce tumour growth. Other selective Ddr1 inhibitors including receptor-blocking antibodies have been recently described, reflecting an increasing interest in targeting this tyrosine kinase receptor for cancer treatment.31 37–39
Though genetic and pharmacological inhibition of Ddr1 alone in KrasG12V/Trp53-null tumours extended survival and slowed tumour growth, the therapeutic effects were not complete. Thus, in order to increase the therapeutic efficacy of Ddr1 inhibition, we treated mice with a combination of 7rh and a γ-secretase inhibitor, which interferes with Notch activation. This strategy was based on the fact that Notch activity is required for lung adenocarcinoma progression.32 33 40 41 Additionally, Notch has been reported to mediate cellular survival downstream of Ddr1 in a ligand-independent fashion,32 which would permit for residual Notch signalling in the setting or 7rh-mediated Ddr1 inhibition, which targets the extracellular domain. Notably, the combination of 7rh and γ-secretase inhibitors displayed a significant inhibitory effect on the growth of aggressive KrasG12V-driven/Trp53-null lung adenocarcinomas, whereas neither inhibitor used alone exerted appreciable therapeutic activity. This additive therapeutic effect suggests that, in addition to the known role of Notch signalling downstream of DDR1, Notch and DDR1 also have non-redundant roles in driving lung adenocarcinoma. Of further note, tumours treated with 7rh alone displayed a robust reduction of Hes1, a canonical downstream target of the Notch pathway, an effect which was not seen in the surrounding normal tissue.24 The reason for tumour-specific (7rh-mediated) inhibition of Notch1 is uncertain but deserves further investigation and has obvious advantageous implications in terms of minimising toxic side effects in healthy tissue.
This work suggests that DDR1 and HES1 levels can be used as biomarkers in human lung adenocarcinoma to stratify patients who more likely can benefit from combined Notch and DDR1 inhibitor treatment. We probed lung adenocarcinoma Tissue Microarrays and we found that ∼75% of KRAS-mutant lung adenocarcinoma expressed high levels of DDR1, whereas ∼60% coexpressed high levels of both DDR1 and HES1. Unfortunately, pan-Notch inhibitors have limited clinical utility due to gastrointestinal toxicity as a consequence of Notch1/2 inhibition in intestinal crypts.42 43 While Notch1 has been shown to play an important role in lung adenocarcinoma progression, Notch3 has also been reported to regulate lung cancer stem cell self-renewal and propagation.40 44 Identification and more selective therapeutic targeting of the individual Notch family member that most significantly mediates Ddr1's effects will lead to combined drug regimens for adenocarcinoma patients with tolerable side effects.
To extrapolate the applicability of our findings to human tumours, we assessed the efficacy of DDR1/Notch inhibition in a human lung orthotopic PDX (orthoxenograft) model, using human lung adenocarcinomas mutant for KRAS treated with demcizumab to block Notch signalling or dasatinib for DDR1 inhibition. Dasatinib is an already-FDA approved small molecule tyrosine kinase inhibitor that was initially isolated as a dual SRC/ABL inhibitor and was later found to have broader inhibitory activity against other targets such as DDR1, cKIT and PDGFR.45 Though lack of specificity is generally undesirable for kinase inhibitors, this feature of dasatinib most likely has beneficial effects in the context of DDR1 inhibition, since DDR1 and SRC are engaged in a regulatory loop46 whereby dasatinib-mediated inhibition of SRC leads to vertical inhibition of the pathway. Demcizumab is a monoclonal antibody that selectively targets Delta-like ligand 4 (DLL4), an activator of the Notch signalling pathway and a mediator that promotes tumour angiogenesis. This antibody is currently in phase II clinical trials and is intended to have both anticancer stem cell and antiangiogenic activity. The dasatinib/demcizumab combination treatment of PDXs resulted in potent inhibition of key signalling pathways that drive tumour progression and survival, reducing the intratumoral fluorodeoxyglucose-PET signal and inducing tumour shrinkage more effectively than chemotherapy. We further observed a substantial decrease of the endothelial compartment in tumours subjected to the combined therapy as measured by CD31 immunostaining, presumably due to the antiangiogenic effects of demcizumab (figure 2A). Given these studies employing primary human tumour samples, further investigation is warranted into dual DDR1/Notch inhibition as a therapeutic strategy for KRAS-driven lung adenocarcinoma expressing DDR1, regardless of the TP53 status.
Finally, several DDR1 mutations have been identified in various independent studies involving human NSCLC (figure 2B), with ∼50% of these alterations being located within the kinase domain. Given their clustering within this region, it is tempting to speculate that these mutations may be functionally relevant (and selected for) during disease progression. It would be of interest to investigate the presence of such mutations specifically in tumours from patients after relapse following chemotherapy. The presence of DDR1 mutations in these cases would support using DDR1 inhibitors to treat chemoresistant individuals or patients with residual disease following chemotherapy, who currently lack therapeutic options.
Highlights
The study of early lesions as a new approach to look for valuable therapeutic targets could, in principle, be extended to other types of solid tumour in relevant mouse models.
The aggressive nature of Kirsten rat sarcoma viral oncogene homologue (KrasG12V)-driven lung adenocarcinoma is determined early during tumour development.
The receptor tyrosine kinase Ddr1 is required for KrasG12V-driven lung adenocarcinoma progression in vivo.
Dual pharmacological inhibition of Discoidin Domain Receptor 1 DDR1 and Notch pathways hampers the growth of murine and human KRAS-mutant; TP53-deficient lung adenocarcinoma.
Preclinical tests in lung orthotopic patient-derived xenografts performed with Food and Drug Administration-approved drug dasatinib, together with demcizumab (which is currently in phase II as a first-line therapy in combination with carboplatin/pemetrexed chemotherapy for non-small cell lung carcinoma) show promising outcomes.
Footnotes
Contributors: CA and DS wrote the manuscript with contributions from all other authors.
Funding: This work was supported by AECC (Asociación Española Contra el Cáncer) fellowship to CA. MB is the recipient of an Endowed Chair from the AXA Research Fund and was supported by grants from the European Research Council (ERC-AG/250297-RAS AHEAD), EU-Framework Programme (HEALTH-F2-2010-259770/LUNGTARGET and HEALTH-2010-260791/EUROCANPLATFORM), Spanish Ministry of Economy and Competitiveness (SAF2011-30173 and SAF2014-59864-R) and Autonomous Community of Madrid (S2011/BDM-2470/ONCOCYCLE). Ernest Nadal was supported by a Juan Rodés fellowship from the Carlos III Health Institute (JR13/0002).
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Ettinger DS, Akerley W, Borghaei H et al. . Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013;11:645–53; quiz 653. [DOI] [PubMed] [Google Scholar]
- 2.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:29S–55S. 10.1378/chest.07-1347 [DOI] [PubMed] [Google Scholar]
- 3.Santos E, Martin-Zanca D, Reddy EP et al. . Malignant activation of a K-ras oncogene in lung carcinoma but not in normal tissue of the same patient. Science 1984;223:661–4. 10.1126/science.6695174 [DOI] [PubMed] [Google Scholar]
- 4.Califano R, Landi L, Cappuzzo F. Prognostic and predictive value of K-RAS mutations in non-small cell lung cancer. Drugs 2012;72:28–36. 10.2165/1163012-S0-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 5.Meng D, Yuan M, Li X et al. . Prognostic value of K-RAS mutations in patients with non-small cell lung cancer: a systematic review with meta-analysis. Lung Cancer 2013;81:1–10. [DOI] [PubMed] [Google Scholar]
- 6.Nadal E, Chen G, Prensner JR et al. . KRAS-G12C mutation is associated with poor outcome in surgically resected lung adenocarcinoma. J Thorac Oncol 2014;9:1513–22. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA, Domerg C, Hainaut P et al. . Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvant chemotherapy. J Clin Oncol 2013;31:2173–81. 10.1200/JCO.2012.48.1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rulli E, Marabese M, Torri V et al. . Value of KRAS as prognostic or predictive marker in NSCLC: results from the TAILOR trial. Ann Oncol 2015;26:2079–84. 10.1093/annonc/mdv318 [DOI] [PubMed] [Google Scholar]
- 9.Ostrem JM, Peters U, Sos ML et al. . K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 2013;503:548–51. 10.1038/nature12796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westover KD, Janne PA, Gray NS. Progress on Covalent Inhibition of KRASG12C. Cancer Discov 2016;6:233–4. 10.1158/2159-8290.CD-16-0092 [DOI] [PubMed] [Google Scholar]
- 11.Kwon MC, Berns A. Mouse models for lung cancer. Mol Oncol 2013;7:165–77. 10.1016/j.molonc.2013.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, Fillmore CM, Hammerman PS et al. . Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014;14:535–46. 10.1038/nrc3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Cheng K, Walton Z et al. . A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature 2012;483:613–17. 10.1038/nature10937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jänne PA, Shaw AT, Pereira JR et al. . Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol 2013;14:38–47. 10.1016/S1470-2045(12)70489-8 [DOI] [PubMed] [Google Scholar]
- 15.Goldman JW, Shi P, Reck M et al. . Treatment Rationale and Study Design for the JUNIPER Study: a Randomized Phase III Study of Abemaciclib With Best Supportive Care Versus Erlotinib With Best Supportive Care in Patients With Stage IV Non-Small-Cell Lung Cancer With a Detectable KRAS Mutati. Clin Lung Cancer 2016;17:80–4. 10.1016/j.cllc.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 16.Puyol M, Martín A, Dubus P et al. . A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 2010;18:63–73. 10.1016/j.ccr.2010.05.025 [DOI] [PubMed] [Google Scholar]
- 17.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer 2012;12:323–34. 10.1038/nrc3261 [DOI] [PubMed] [Google Scholar]
- 18.Alexandrov LB, Nik-Zainal S, Wedge DC et al. . Signatures of mutational processes in human cancer. Nature 2013;500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweet-Cordero A, Mukherjee S, Subramanian A et al. . An oncogenic KRAS2 expression signature identified by cross-species gene-expression analysis. Nat Genet 2005;37:48–55. 10.1038/ng1490 [DOI] [PubMed] [Google Scholar]
- 20.Starmans MHW, Pintilie M, Chan-Seng-Yue M et al. . Integrating RAS status into prognostic signatures for adenocarcinomas of the lung. Clin Cancer Res 2015;21:1477–86. 10.1158/1078-0432.CCR-14-1749 [DOI] [PubMed] [Google Scholar]
- 21.Skoulidis F, Byers LA, Diao L et al. . Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov 2015;5:861–78. 10.1158/2159-8290.CD-14-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burrell RA, Swanton C. The evolution of the unstable cancer genome. Curr Opin Genet Dev 2014;24:61–7. 10.1016/j.gde.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 23.Guerra C, Mijimolle N, Dhawahir A et al. . Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell 2003;4:111–20. 10.1016/S1535-6108(03)00191-0 [DOI] [PubMed] [Google Scholar]
- 24.Ambrogio C, López-Gómez G, Falcone M et al. . Combined inhibition of DDR1 and Notch signaling is a therapeutic strategy for KRAS-driven lung adenocarcinoma. Nat Med 2016;22:270–7. 10.1038/nm.4041 [DOI] [PubMed] [Google Scholar]
- 25.Valiathan RR, Marco M, Leitinger B et al. . Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev 2012;31:295–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borza CM, Pozzi A. Discoidin domain receptors in disease. Matrix Biol 2014;34:185–92. 10.1016/j.matbio.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valencia K, Ormazabal C, Zandueta C et al. . Inhibition of collagen receptor discoidin domain receptor-1 (DDR1) reduces cell survival, homing, and colonization in lung cancer bone metastasis. Clin Cancer Res 2012;18:969–80. 10.1158/1078-0432.CCR-11-1686 [DOI] [PubMed] [Google Scholar]
- 28.Rikova K, Guo A, Zeng Q et al. . Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190–203. 10.1016/j.cell.2007.11.025 [DOI] [PubMed] [Google Scholar]
- 29.Miao L, Zhu S, Wang Y et al. . (2013) Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung cancer and promotes cell invasion via epithelial-to-mesenchymal transition. Med Oncol 2013;30:626 10.1007/s12032-013-0626-4 [DOI] [PubMed] [Google Scholar]
- 30.Vogel WF, Aszódi A, Alves F et al. . Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol 2001;21:2906–17. 10.1128/MCB.21.8.2906-2917.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao M, Duan L, Luo J et al. . Discovery and optimization of 3-(2-(Pyrazolo[1,5-a]pyrimidin-6-yl)ethynyl)benzamides as novel selective and orally bioavailable discoidin domain receptor 1 (DDR1) inhibitors. J Med Chem 2013;56:3281–95. 10.1021/jm301824k [DOI] [PubMed] [Google Scholar]
- 32.Kim HG, Hwang SY, Aaronson SA et al. . DDR1 receptor tyrosine kinase promotes prosurvival pathway through Notch1 activation. J Biol Chem 2011;286:17672–81. 10.1074/jbc.M111.236612 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Maraver A, Fernandez-Marcos PJ, Herranz D et al. . Therapeutic effect of γ-secretase inhibition in Kras G12V-driven non-small cell lung carcinoma by derepression of DUSP1 and inhibition of ERK. Cancer Cell 2012;22:222–34. 10.1016/j.ccr.2012.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mainardi S, Mijimolle N, Francoz S et al. . Identification of cancer initiating cells in K-Ras driven lung adenocarcinoma. Proc Natl Acad Sci USA 2014;111:255–60. 10.1073/pnas.1320383110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014;507:190–4. 10.1038/nature12930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature 2013;501:328–37. 10.1038/nature12624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canning P, Tan L, Chu K et al. . Structural mechanisms determining inhibition of the collagen receptor DDR1 by selective and multi-targeted type II kinase inhibitors. J Mol Biol 2014;426:2457–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carafoli F, Mayer MC, Shiraishi K et al. . Structure of the discoidin domain receptor 1 extracellular region bound to an inhibitory Fab fragment reveals features important for signaling. Structure 2012;20:688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H-G, Tan L, Weisberg EL et al. . Discovery of a potent and selective DDR1 receptor tyrosine kinase inhibitor. ACS Chem Biol 2013;8:2145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Licciulli S, Avila JL, Hanlon L et al. . (2013) Notch1 is required for Kras-induced lung adenocarcinoma and controls tumor cell survival via p53. Cancer Res 2013;73:5974–84. 10.1158/0008-5472.CAN-13-1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer 2011;11:338–51. 10.1038/nrc3035 [DOI] [PubMed] [Google Scholar]
- 42.Van Es JH, van Gijn ME, Riccio O et al. . Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005;435:959–63. [DOI] [PubMed] [Google Scholar]
- 43.Staal FJT, Langerak AW. Signaling pathways involved in the development of T-cell acute lymphoblastic leukemia. Haematologica 2008;93:493–7. 10.3324/haematol.12917 [DOI] [PubMed] [Google Scholar]
- 44.Zheng Y, de la Cruz CC, Sayles LC et al. . A rare population of CD24(+)ITGB4(+)Notch(hi) cells drives tumor propagation in NSCLC and requires Notch3 for self-renewal. Cancer Cell 2013;24:59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karaman MW, Herrgard S, Treiber DK et al. . A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol 2008;26:127–32. [DOI] [PubMed] [Google Scholar]
- 46.Leitinger B. Discoidin domain receptor functions in physiological and pathological conditions. Int Rev Cell Mol Biol 2014;310:39–87. [DOI] [PMC free article] [PubMed] [Google Scholar]