Abstract
Endoscopic submucosal dissection (ESD) is an innovative advance in the treatment of early gastrointestinal (GI) cancer without lymph node metastases and precancerous lesions as it is an effective and safe therapeutic method. ESD has also been a promising therapeutic option for removal of submucosal tumors (SMTs) for improving the completeness of resection of a large lesion. Endoscopic ultrasonography (EUS) can be used to detect the depth of invasion during the preoperative evaluation because of its close proximity to the lesion. EUS-guided fine-needle aspiration can be used to increase the diagnostic accuracy of EUS in determining the malignant lymph node. EUS is considered to be a useful imaging procedure to characterize early GI cancer, which is suspicious for submucosal invasion, and the most accurate procedure for detecting and diagnosing SMTs for further treatment. In the process of ESD, EUS can also be used to detect surrounding blood vessels and the degree of fibrosis; this may be helpful for predicting procedure time and decreasing the risk of bleeding and perforation. EUS-guided injection before ESD renders the endoscopic resection safe and accurate. Therefore, EUS plays an important role in the use of ESD. However, compared to conventional endoscopic staging, EUS sometimes can under or overstage the lesion, and the diagnostic accuracy is controversial. In this review, we summarize the latest research findings regarding the role of EUS in ESD.
Keywords: Early gastrointestinal cancer, endoscopic submucosal dissection, endoscopic ultrasonography, submucosal tumor
INTRODUCTION
Endoscopic submucosal dissection (ESD) is an advanced endoscopic technique that allows for curative resection of superficial neoplasms and submucosal tumors (SMTs) originating from deep layers in the gastrointestinal (GI) tract. ESD can achieve en bloc margin-negative resection of tumors while avoiding invasive surgery. Lymph node metastasis is the major influencing factor for choosing the rational therapeutic procedure. The risk of lymph node metastases is largely based on the depth of tumor invasion. Thus, estimating the depth of invasion is a focus during the preoperative evaluation. Lesions limited to the mucosa (m1 or m2) or <500 μm of submucosa (sm1) can be resected endoscopically. Deep invasion of the submucosa (sm2 or sm3) requires surgical treatment. For deep mucosa (m3) or superficial submucosal invasion (sm1), the choice between endoscopic and surgical treatment depends on the type of cancer, size of the tumor, macroscopic endoscopic characteristics, and patient performance status. The ESD complication rate, including bleeding and perforation, is considered to be high because of tumor size and the relatively long procedure time.
Endoscopic ultrasonography (EUS) was introduced into clinical practice due to its unique ability to examine tumors from within the GI lumen with extremely close proximity. Accurate locoregional cancer staging and diagnostic information about SMTs provide necessary information to differentiate patients who will be better candidates for ESD instead of surgical resection. Thus, before ESD, EUS has evolved into an important and widely accepted diagnostic tool for the diagnosis and staging of various GI lesions; however, the diagnostic accuracy of EUS is controversial. Under- or over-staging the lesion makes it difficult to choose as an appropriate treatment for patients. Even when high frequency (12–20 MHz), miniprobe EUS is used instead of standard EUS, the results have been shown to be no better than standard resolution endoscopy in local staging of early cancer (mucosal vs. submucosal invasion).[1]
SUPERFICIAL ESOPHAGEAL NEOPLASIA
ESD is indicated for early esophageal cancer with no risk of lymph node invasion. According to the Japan Esophageal Society Guidelines for treatment of esophageal cancer, the absolute indication for endoscopic resection is defined as flat lesions (Paris 0–II), with m1–m2 invasion, and circumferential extent less than or equal to two-third while the relative indication is defined as m3–sm1 esophageal cancer in which endoscopic resection would leave a mucosal defect of circumferential extent greater than or equal to three-fourth.[2] The depth of invasion and lymph node involvement are important factors which influence whether or not to select endoscopic resection. EUS enables the acquisition of clear images of the GI tract wall and the surrounding structures. Thosani et al.[3] published the results of a meta-analysis on the use of EUS in staging early esophageal cancer and reported the sensitivity and specificity as 85% and 87% for T1a staging, respectively, and 86% for both sensitivity and specificity for T1b staging. EUS understaged 15.05% T1b superficial esophageal cancer and overstaged 4.30% T1a lesion about the evaluation for submucosal invasion. Locoregional invasion in esophageal cancer can also be predicted by positron emission tomography/computed tomography (PET/CT), PET/magnetic resonance (PET/MR), and CT. According to Lee et al.,[4] the accuracy of determining T1 lesions was 86.7%, 80.0%, and 46.7% for EUS, PET/MR imaging, and CT, respectively; for lymph node staging, the accuracy was 83.3%, 75.0%, 66.7%, and 50.0% for PET/MR imaging, EUS, PET/CT, and CT, respectively. Pech et al.[5] analyzed 179 consecutive patients for staging, and the overall accuracy for EUS in identifying the correct T stage was 74% while the sensitivity and specificity of EUS were 82% and 91% for T1, respectively. Initial evidence suggests that EUS plays an important role in the choice of candidates for ESD, and EUS is recommended as a routine procedure before endoscopic resection. However, Bergeron et al.[6] demonstrated in their study that EUS was not sufficient to distinguish mucosal from submucosal invading lesions. They reviewed 107 patients with superficial carcinoma (T1a or T1b) and found that tumor depth was correctly staged by EUS in only 39% of T1a tumors and 51% of T1b tumors. The majority (69.2%) of their clinically staged T1a tumors were understaged. The reason for understaging or overstaging the T1 tumor is still unclear.
With the development of endoscopic technique, some advances were made in substaging T1 tumor. There was the report that the accuracy of the combination of submucosal saline injection + EUS for substaging early esophageal cancer increased significantly.[7] However, only 15 patients were included in that study.
On presurgical EUS, the sensitivity and specificity for N staging are 60%–97% and 40%–100%, respectively.[8,9] The accuracy of CT and EUS on average is 63% and 66%, respectively, when EUS-guided fine-needle aspiration (EUS-FNA) is used, the accuracy increases.[10,11] Although identification of the N stage is more accurate with EUS, the role of EUS remains limited before ESD. In superficial esophageal cancer, invasion of the lymph nodes surrounding the cervical area and the celiac trunk is possible without invasion of the lymph nodes around the lesion itself. This significant characteristic of esophageal cancer makes it difficult to select appropriate patients for endoscopic resection. Although a negative EUS finding is not very helpful, a positive EUS finding can modify management. The European Society for Gastrointestinal Endoscopy suggests that EUS should be considered for superficial esophageal carcinomas with suspicious features (submucosal invasion or lymph node metastases).[12]
ESOPHAGEAL SUBMUCOSAL TUMORS
ESD has been proven useful in the management of large esophageal SMTs arising from deep layers of the esophagus. For esophageal tumors originating from the muscularis propria, endoscopic submucosal tunneling dissection (ESTD) has been confirmed to be feasible and useful.
EUS can provide information about the character, originating layer, size, and extramural extension of a SMT. Such information determines whether or not an endoscopic method can be performed. Leiomyomas are predominantly found in the esophagus derived from the muscularis mucosae and muscularis propria. Leiomyomas generally appear as a homogenous hypoechoic mass arising from the fourth or second layer with a regular, well-defined outline. During the ESTD procedure, tumors are sometimes difficult to identify and differentiate from other physiologic protrusions (e.g., aorta compression) based on the endoscopic view in the tunnel. EUS can be performed to identify the tumor during the endoscopic dissection procedure.[13] EUS can also be used to evaluate the healing quality of the submucosal tunnel after the ESTD procedure.[14]
GASTRIC SUPERFICIAL NEOPLASTIC LESIONS
ESD should be considered for the treatment of gastric superficial neoplastic lesions (low- or high-grade noninvasive neoplasia and adenocarcinomas with no evidence of deep submucosal invasion). Conventional endoscopy has an accuracy of 72%–84% for evaluating the depth of invasion (mucosa vs. submucosa) in early gastric cancer (EGC).[15,16] EUS is regarded as the best available method for locoregional staging of gastric cancer;[17] the total accuracy of EUS, particularly for superficial gastric lesions, is rather low (41.4%–87%).[18,19,20,21,22,23,24,25,26,27,28,29] Reports comparing the accuracy of EUS with conventional endoscopy have produced inconsistent results. Mouri et al.[30] evaluated the usefulness of EUS in determining the applicability of ESD and visualizing the depth of invasion in EGC. The accuracy of EUS for determining the depth of invasion was as follows: EUS-mucosa (M), 99% were M and SM1 lesions; and EUS-M/submucosa (SM) border, 87% were M and SM1 lesions. They concluded that EUS-M and EUS-M/SM border lesions were good indicators for ESD. Yanai et al.[29] reported that EUS was useful in combination with conventional endoscopy for evaluating the depth of invasion of EGC. Okada et al.[23] evaluated the accuracy of EUS in identifying lesions meeting expanded indication criteria for ESD. For patients with EGC, differentiated adenocarcinoma (D-type) lesions <30 mm in diameter and undifferentiated adenocarcinoma (UD-type) lesions <20 mm in diameter could be diagnosed with high accuracy by EUS. In most Western nations, EUS is generally recommended before treatment.[31] Other studies, however, showed that EUS failed to improve the accuracy of EGC invasion depth assessment.[24,26,32] Moreover, a comparative study of EUS versus endoscopic evaluation for predicting endoscopic resectability clearly favored endoscopy because EUS findings indicated gastrectomy for many lesions that did not require surgery.[18] The depth of invasion is most likely exaggerated when ulcers or fibrotic lesions are present. In one Western ESD series that systematically used EUS before endoscopic resection, the feasibility of ESD was comparable and even slightly inferior to that of another Western ESD series in which EUS was not performed (93% vs. 97%).[33,34] Lee et al.[18] reviewed 393 EGCs with well-differentiated histology and found that the appropriate treatment selection rates were 75.3% (296/393) in the endoscopy-based plan and 71.5% (281/393) in the EUS-based plan (P = 0.184). EUS examination did not increase the appropriate selection of treatment in well-differentiated EGCs. For this reason, EUS before ESD is not considered for lesions amenable to endoscopic resection in many Eastern nations. Park et al.[21] analyzed the inaccurate evaluation of lesion infiltration by EUS and concluded that of lesions located in the upper stomach, 62.5% cases were underestimated, as to nondepressed macroscopic morphology, 77.6% cases were underestimated. When tumors were located in the body or lower area, 60% and 69.6% were overestimated, respectively. In cases with nondepressed macroscopic morphology, 77.6% cases were overestimated. In cases in which the histologic findings were of the differentiated type, 70.7% cases were overestimated. As a result, EUS should be considered for lesions located in the upper one-third of the stomach with a depressed morphology. Tsuzuki et al.[22] arrived at a similar conclusion. Type 0–I lesions tended to be overstaged and upper one-third lesions tended to be understaged. The accuracy was significantly less in differentiated adenocarcinomas with massive submucosal invasion. The findings of the 12 studies involving EGC are summarized in Table 1.
Table 1.
Summary of therapeutic decision-making using endoscopic ultrasonography in staging and treatment of early gastric cancer
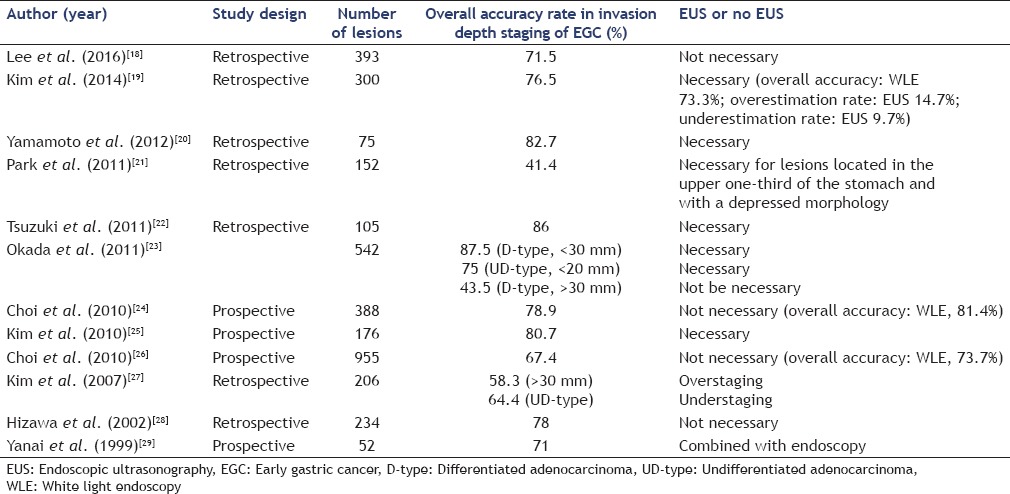
EUS is useful for choosing a treatment approach for lesions in which invasion to the submucosa is suspected on the examination of the gross endoscopic appearance or for gastric adenomas or mucosal cancers detected by pathologic examination.[10] Moreover, EUS may be used before ESD to predict gastric ESD safety. The question becomes: is it actually effective? 106 patients with gastric neoplasms who underwent EUS before ESD were compared retrospectively with respect to procedure time, degree of anemia, and frequency of clip use in the rich vascular group and the nonrich vascular group. The results suggested that identification of submucosal vascular structure by EUS might allow prediction of intraoperative bleeding during ESD.[35] Nevertheless, in another study, a total of 110 patients with EGC were divided into the following two groups based on EUS findings: Group P, few blood vessels in the submucosa or ≤4 small vessels per field of view; and Group R, the remaining patients. The mean decrease in hemoglobin did not differ significantly between the two groups. The authors concluded that EUS was not helpful in predicting the risk of exacerbation of anemia or occurrence of perforation. The mean procedure time was significantly longer in Group R (105.4 min) than Group P (65.5 min; P < 0.001). The incidence of muscle injury and clip use was significantly higher in Group R (25.6% and 48.7%, P = 0.02) than Group P (8.0% and 20.0%; P = 0.004).[36] With respect to large lesions, EUS can predict the regions where blood vessels are abundant and guide the approach to the region accordingly as well as predict bleeding.
EUS can be used to detect submucosal fibrosis, which may predict incomplete ESD. Hirasawa et al.[37] analyzed 26 lesions in their center, which were incompletely resected because of severe ulcer scarring, and considered surgery to be more preferable than ESD for EGC with an interruption of >5 mm of the third layer.
GASTRIC SUBMUCOSAL TUMORS
ESD can be used for the resection of intraluminal gastric tumors.[38] ESD can achieve similar oncologic outcomes when compared with surgery for treatment of gastric SMTs <50 mm in size.[39] EUS is the main procedure for detecting and diagnosing gastric SMTs. Information pertaining to the location, size, echo pattern, and originating layer of the SMTs can be provided by EUS. Gastric SMTs include a diverse array of benign, potentially malignant and malignant lesions, including GI stromal tumors (GISTs), leiomyomas, neuroendocrine tumors, lipomas; granular cell tumors, heterotopic pancreas, lymphangiomas, and endometriosis. Therapeutic approaches for SMTs include endoscopic and surgical resection depending on the characteristics of the tumors.
SMTs >50 mm in size, SMTs increasing in size, and SMTs with high-risk features including irregular borders, heterogeneous internal echoes, and heterogeneous enhancement by contrast media may be removed by surgery.[40] GISTs have well-recognized malignant potential. The majority of GISTs present in the stomach (50%–70%). Unfortunately, EUS is not able to reliably differentiate GISTs from other benign hypoechoic lesions from the fourth layer, such as leiomyomas. Therefore, EUS-FNA plays an important role in the correct diagnosis of SMTs. Sepe et al.[41] reported the sensitivity of EUS-FNA cytology for the diagnosis of GISTs to be 78.4%.
Only well-marginated SMTs arising from the muscularis propria, which show the underlying muscle layer under EUS, appear to be endoscopically resectable.[42] Bialek et al.[43] reported that EUS findings could predict complete tumor resections. Successful R0 resections were predicted by the observation of no, or only narrow, tumor connections with the underlying muscle layer during EUS.
An EUS-guided injection before ESD can render the endoscopic resection safe and accurate. Fujii et al.[44] described an EUS-assisted injection into the muscularis propria to provide a deeper safety cushion for ESD of a subepithelial lesion with broad attachment to the muscularis propria. In this case, EUS was useful for precise real-time imaging guidance during injection, and confirmation of an adequate lift of complex lesions. In an ESD procedure, small subepithelial lesions cannot be found by endoscopy alone because of tissue edema and injection, resulting in terminating of ESD. At this point, the EUS plays an important role in locating and marking the lesion, which enables continuation of ESD.
COLORECTAL NEOPLASMS
Neoplasms of the colorectum are subdivided into low-grade dysplasia, high-grade dysplasia (HGD), and carcinoma. The depth of infiltration should additionally be measured before ESD and the limit for sm1 is defined as <1000 μm.
Although the accuracy of EUS for presurgical invasion depth evaluation is 80%–95%, the sensitivity is not ideal for early cancer in identifying the T stage. A recent meta-analysis, which included 90 studies involving T1 and T2 lesions, concluded that while EUS and MRI had similar sensitivities, the specificity of EUS was higher.[45] Hurlstone et al.[46] reported that high-frequency EUS (high-frequency ultrasound probes sonography [HFUPS]) was superior to chromoendoscopy for determining the depth of invasion in staging for early colorectal neoplasia, with an accuracy of 93% versus 59% (P < 0.0001) and a high positive predictive value for sm3 differentiation, which was associated with nodal metastasis. A systematic review and meta-analysis by Gall et al.[47] confirmed that HFUPS was a highly effective procedure for clinical staging in colon and rectal cancers, with a pooled sensitivity of 0.91 and specificity of 0.98 for T1 tumors. This result is necessary in identifying patients who may be suitable for nonsurgical treatment. Urban et al.[48] reported that the impact of HFUPS on treatment of superficial colorectal neoplasia depended on the endoscopic characteristics. Low-risk lesions could be treated on the basis of endoscopic appearance alone. HFUPS changed the subsequent therapeutic approach in a positive way for up to 42% of high-risk lesions, including those with a depressed component and an invasive pit pattern; however, this technique had the risk of recommending surgery for some patients who could have been treated by endoscopic resection. If the endoscopist feels the lesion is endoscopically resectable, there probably is no need for EUS.
EUS accuracy is low for nodal staging because EUS cannot detect benign lymph nodes around the rectum. In rectal lesions with endoscopic features suspicious for submucosal invasion, EUS or MRI can be considered because finding suspicious lymph nodes could be an indication for neoadjuvant treatment.
Colorectal ESD is specifically complicated by the thinness of the intestinal wall and by lesions with severe fibrosis. Severe submucosal fibrosis can lead to lengthy treatment and unexpected complications such as perforation. A recent clinical study concluded that preoperative EUS before colorectal ESD successfully predicted the degree of fibrosis in a number of cases.[49] Therefore, submucosal fibrosis detected by EUS may predict incomplete ESD, and surgery is preferred over ESD with an interruption of >5 mm of the third layer.
Rectal endoscopic resection may occasionally identify high-risk histopathologic features, including HGD, adenocarcinomas, or carcinoid tumors. Is EUS useful for residual disease after endoscopic resection? Luz et al.[50] assessed the incremental yield of EUS compared with WLE for evaluation of residual disease after endoscopic resection of high-risk rectal lesions. The results were limited, especially in patients with benign disease.
CONCLUSIONS
The exclusion of nodal involvement, evaluation of the depth of tumor penetration within the GI wall, and prevention of complications are major advantages for ESD procedures. EUS plays an important role in the use of ESD. EUS can facilitate selection of patients suitable for ESD treatment, predict the safety of ESD, and reduce postoperative complications. Compared to conventional endoscopic staging, however, EUS can also under- or over-stage the lesion. EUS before ESD for HGD or superficial cancer may not be a routine procedure, but EUS can be a critical diagnostic tool for superficial GI neoplasia with suspicious features for submucosal invasion or lymph node metastasis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pech O, Günter E, Dusemund F, et al. Value of high-frequency miniprobes and conventional radial endoscopic ultrasound in the staging of early Barrett's carcinoma. Endoscopy. 2010;42:98–103. doi: 10.1055/s-0029-1243839. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Japanese classification of esophageal cancer, 10 th edition: Part I. Esophagus. 2009;6:1–25. [Google Scholar]
- 3.Thosani N, Singh H, Kapadia A, et al. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: A systematic review and meta-analysis. Gastrointest Endosc. 2012;75:242–53. doi: 10.1016/j.gie.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Lee G, Hoseok I, Kim SJ, et al. Clinical implication of PET/MR imaging in preoperative esophageal cancer staging: Comparison with PET/CT, endoscopic ultrasonography, and CT. J Nucl Med. 2014;55:1242–7. doi: 10.2967/jnumed.114.138974. [DOI] [PubMed] [Google Scholar]
- 5.Pech O, Günter E, Dusemund F, et al. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: Results from a referral center for early esophageal cancer. Endoscopy. 2010;42:456–61. doi: 10.1055/s-0029-1244022. [DOI] [PubMed] [Google Scholar]
- 6.Bergeron EJ, Lin J, Chang AC, et al. Endoscopic ultrasound is inadequate to determine which T1/T2 esophageal tumors are candidates for endoluminal therapies. J Thorac Cardiovasc Surg. 2014;147:765–71. doi: 10.1016/j.jtcvs.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Li JJ, Shan HB, Gu MF, et al. Endoscopic ultrasound combined with submucosal saline injection for differentiation of T1a and T1b esophageal squamous cell carcinoma: A novel technique. Endoscopy. 2013;45:667–70. doi: 10.1055/s-0033-1344024. [DOI] [PubMed] [Google Scholar]
- 8.Sgourakis G, Gockel I, Lyros O, et al. Detection of lymph node metastases in esophageal cancer. Expert Rev Anticancer Ther. 2011;11:601–12. doi: 10.1586/era.10.150. [DOI] [PubMed] [Google Scholar]
- 9.Kelly S, Harris KM, Berry E, et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut. 2001;49:534–9. doi: 10.1136/gut.49.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi J, Kim SG, Kim JS, et al. Comparison of endoscopic ultrasonography (EUS), positron emission tomography (PET), and computed tomography (CT) in the preoperative locoregional staging of resectable esophageal cancer. Surg Endosc. 2010;24:1380–6. doi: 10.1007/s00464-009-0783-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine needle aspiration is superior to lymph node echofeatures: A prospective evaluation of mediastinal and peri-intestinal lymphadenopathy. Am J Gastroenterol. 2004;99:628–33. doi: 10.1111/j.1572-0241.2004.04064.x. [DOI] [PubMed] [Google Scholar]
- 12.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829–54. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]
- 13.Sharma M, Rai P, Mehta V, et al. Techniques of imaging of the aorta and its first order branches by endoscopic ultrasound (with videos) Endosc Ultrasound. 2015;4:98–108. doi: 10.4103/2303-9027.156722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge N, Sun S, Wang S, et al. Endoscopic ultrasound-assisted tunnel-type endoscopic submucosal dissection for the treatment of esophageal tumors arising in the muscularis propria (with video) Endosc Ultrasound. 2013;2:11–5. doi: 10.7178/eus.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi J, Kim SG, Im JP, et al. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc. 2011;73:917–27. doi: 10.1016/j.gie.2010.11.053. [DOI] [PubMed] [Google Scholar]
- 16.Hizawa K, Iwai K, Esaki M, et al. Is endoscopic ultrasonography indispensable in assessing the appropriateness of endoscopic resection for gastric cancer? Endoscopy. 2002;34:973–8. doi: 10.1055/s-2002-35851. [DOI] [PubMed] [Google Scholar]
- 17.Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: A meta-analysis. Gastrointest Endosc. 2011;73:1122–34. doi: 10.1016/j.gie.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Lee JY, Choi IJ, Kim CG, et al. Therapeutic decision-making using endoscopic ultrasonography in endoscopic treatment of early gastric cancer. Gut Liver. 2016;10:42–50. doi: 10.5009/gnl14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SJ, Choi CW, Kang DH, et al. Efficacy of endoscopic ultrasonography compared to conventional endoscopy in decision making of early gastric cancer treatment. Gastrointest Endosc. 2014;79(Suppl 5):AB404. [Google Scholar]
- 20.Yamamoto S, Nishida T, Kato M, et al. Evaluation of endoscopic ultrasound image quality is necessary in endosonographic assessment of early gastric cancer invasion depth. Gastroenterol Res Pract 2012. 2012 doi: 10.1155/2012/194530. 194530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JM, Ahn CW, Yi X, et al. Efficacy of endoscopic ultrasonography for prediction of tumor depth in gastric cancer. J Gastric Cancer. 2011;11:109–15. doi: 10.5230/jgc.2011.11.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuzuki T, Okada H, Kawahara Y, et al. Usefulness and problems of endoscopic ultrasonography in prediction of the depth of tumor invasion in early gastric cancer. Acta Med Okayama. 2011;65:105–12. doi: 10.18926/AMO/45269. [DOI] [PubMed] [Google Scholar]
- 23.Okada K, Fujisaki J, Kasuga A, et al. Endoscopic ultrasonography is valuable for identifying early gastric cancers meeting expanded-indication criteria for endoscopic submucosal dissection. Surg Endosc. 2011;25:841–8. doi: 10.1007/s00464-010-1279-4. [DOI] [PubMed] [Google Scholar]
- 24.Choi J, Kim SG, Im JP, et al. Is endoscopic ultrasonography indispensable in patients with early gastric cancer prior to endoscopic resection? Surg Endosc. 2010;24:3177–85. doi: 10.1007/s00464-010-1112-0. [DOI] [PubMed] [Google Scholar]
- 25.Kim GH, Park DY, Kida M, et al. Accuracy of high-frequency catheter-based endoscopic ultrasonography according to the indications for endoscopic treatment of early gastric cancer. J Gastroenterol Hepatol. 2010;25:506–11. doi: 10.1111/j.1440-1746.2009.06111.x. [DOI] [PubMed] [Google Scholar]
- 26.Choi J, Kim SG, Im JP, et al. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy. 2010;42:705–13. doi: 10.1055/s-0030-1255617. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Song KS, Youn YH, et al. Clinicopathologic factors influence accurate endosonographic assessment for early gastric cancer. Gastrointest Endosc. 2007;66:901–8. doi: 10.1016/j.gie.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Hizawa K, Iwai K, Esaki M, et al. Is endoscopic ultrasonography indispensable in assessing the appropriateness of endoscopic resection for gastric cancer? Endoscopy. 2002;34:973–8. doi: 10.1055/s-2002-35851. [DOI] [PubMed] [Google Scholar]
- 29.Yanai H, Noguchi T, Mizumachi S, et al. A blind comparison of the effectiveness of endoscopic ultrasonography and endoscopy in staging early gastric cancer. Gut. 1999;44:361–5. doi: 10.1136/gut.44.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouri R, Yoshida S, Tanaka S, et al. Usefulness of endoscopic ultrasonography in determining the depth of invasion and indication for endoscopic treatment of early gastric cancer. J Clin Gastroenterol. 2009;43:318–22. doi: 10.1097/MCG.0b013e3181775966. [DOI] [PubMed] [Google Scholar]
- 31.Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2. 2013: Featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531–46. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 32.Tsujii Y, Kato M, Inoue T, et al. Integrated diagnostic strategy for the invasion depth of early gastric cancer by conventional endoscopy and EUS. Gastrointest Endosc. 2015;82:452–9. doi: 10.1016/j.gie.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Pimentel-Nunes P, Mourão F, Veloso N, et al. Long-term follow-up after endoscopic resection of gastric superficial neoplastic lesions in Portugal. Endoscopy. 2014;46:933–40. doi: 10.1055/s-0034-1377348. [DOI] [PubMed] [Google Scholar]
- 34.Probst A, Pommer B, Golger D, et al. Endoscopic submucosal dissection in gastric neoplasia - Experience from a European center. Endoscopy. 2010;42:1037–44. doi: 10.1055/s-0030-1255668. [DOI] [PubMed] [Google Scholar]
- 35.Kikuchi D, Iizuka T, Hoteya S, et al. Usefulness of endoscopic ultrasound for the prediction of intraoperative bleeding of endoscopic submucosal dissection for gastric neoplasms. J Gastroenterol Hepatol. 2011;26:68–72. doi: 10.1111/j.1440-1746.2010.06412.x. [DOI] [PubMed] [Google Scholar]
- 36.Kikuchi D, Iizuka T, Hoteya S, et al. Prospective study about the utility of endoscopic ultrasound for predicting the safety of endoscopic submucosal dissection in early gastric cancer (T-HOPE 0801) Gastroenterol Res Pract 2013. 2013 doi: 10.1155/2013/329385. 329385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirasawa D, Maeda Y. Submucosal fibrosis detected by endoscopic ultrasonography may predict incomplete endoscopic submucosal dissection. Dig Endosc. 2015;27(Suppl 1):24. doi: 10.1111/den.12452. [DOI] [PubMed] [Google Scholar]
- 38.Lu J, Zheng M, Jiao T, et al. Transcardiac tunneling technique for endoscopic submucosal dissection of gastric fundus tumors arising from the muscularis propria. Endoscopy. 2014;46:888–92. doi: 10.1055/s-0034-1377442. [DOI] [PubMed] [Google Scholar]
- 39.Meng FS, Zhang ZH, Hong YY, et al. Comparison of endoscopic submucosal dissection and surgery for the treatment of gastric submucosal tumors originating from the muscularis propria layer: A single-center study (with video) Surg Endosc. 2016 doi: 10.1007/s00464-016-4860-7. doi:10.1007/s00464-016-4860-7. [DOI] [PubMed] [Google Scholar]
- 40.Nishida T, Kawai N, Yamaguchi S, et al. Submucosal tumors: Comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013;25:479–89. doi: 10.1111/den.12149. [DOI] [PubMed] [Google Scholar]
- 41.Sepe PS, Moparty B, Pitman MB, et al. EUS-guided FNA for the diagnosis of GI stromal cell tumors: Sensitivity and cytologic yield. Gastrointest Endosc. 2009;70:254–61. doi: 10.1016/j.gie.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 42.Hwang JC, Kim JH, Kim JH, et al. Endoscopic resection for the treatment of gastric subepithelial tumors originated from the muscularis propria layer. Hepatogastroenterology. 2009;56:1281–6. [PubMed] [Google Scholar]
- 43.Bialek A, Wiechowska-Kozlowska A, Pertkiewicz J, et al. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video) Gastrointest Endosc. 2012;75:276–86. doi: 10.1016/j.gie.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 44.Fujii LL, Gomez V, Song LM, et al. Endoscopic ultrasound-assisted endoscopic submucosal dissection of a gastric subepithelial tumor. Endoscopy. 2013;45(Suppl 2):E225–6. doi: 10.1055/s-0033-1344157. [DOI] [PubMed] [Google Scholar]
- 45.Bipat S, Glas AS, Slors FJ, et al. Rectal cancer: Local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging – A meta-analysis. Radiology. 2004;232:773–83. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 46.Hurlstone DP, Brown S, Cross SS, et al. High magnification chromoscopic colonoscopy or high frequency 20 MHz mini probe endoscopic ultrasound staging for early colorectal neoplasia: A comparative prospective analysis. Gut. 2005;54:1585–9. doi: 10.1136/gut.2005.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gall TM, Markar SR, Jackson D, et al. Mini-probe ultrasonography for the staging of colon cancer: A systematic review and meta-analysis. Colorectal Dis. 2014;16:1–8. doi: 10.1111/codi.12445. [DOI] [PubMed] [Google Scholar]
- 48.Urban O, Kliment M, Fojtik P, et al. High-frequency ultrasound probe sonography staging for colorectal neoplasia with superficial morphology: Its utility and impact on patient management. Surg Endosc. 2011;25:3393–9. doi: 10.1007/s00464-011-1737-7. [DOI] [PubMed] [Google Scholar]
- 49.Makino T, Kanmura S, Sasaki F, et al. Preoperative classification of submucosal fibrosis in colorectal laterally spreading tumors by endoscopic ultrasonography. Endosc Int Open. 2015;3:E363–7. doi: 10.1055/s-0034-1391782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luz LP, Cote GA, Al-Haddad MA, et al. Utility of EUS following endoscopic polypectomy of high-risk rectosigmoid lesions. Endosc Ultrasound. 2015;4:137–44. doi: 10.4103/2303-9027.156744. [DOI] [PMC free article] [PubMed] [Google Scholar]


